Abstract
Purpose
Advances in therapy of metastatic castration-refractory prostate cancer (mCRPC) resulted in more therapeutic options and led to a higher need of predictive/prognostic biomarkers. Systemic inflammatory biomarkers could provide the basis for personalized treatment selection. This study aimed to assess the modified Glasgow Prognostic Score (mGPS), the neutrophile-to-lymphocyte ratio (NLR), the platelet-to-lymphocyte ratio (PLR) and the systemic immune-inflammation index (SII) in men with mCRPC under docetaxel.
Methods
Patients with mCRPC and taxane chemotherapy at a tertiary care centre between 2010 and 2019 were screened retrospectively. The biomarkers mGPS, NLR, PLR and SII were assessed and analyzed for biochemical/radiologic response and survival.
Results
We included 118 patients. Of these, 73 (61.9%) had received docetaxel as first-line, 31 (26.2%) as second-line and 14 (11.9%) as third-line treatment. For biochemical response, mGPS (odds ratio (OR) 0.54, p = 0.04) and PLR (OR 0.63, p = 0.04) were independent predictors in multivariable analysis. SII was significant in first-line cohort only (OR 0.29, p = 0.02). No inflammatory marker was predictive for radiologic response. In multivariable analysis, mGPS and NLR (hazard ratio (HR) 1.71 and 1.12, both p < 0.01) showed significant association with OS in total cohort and mGPS in the first-line cohort (HR 2.23, p < 0.01). Haemoglobin (Hb) and alkaline phosphatase (AP) showed several significant associations regarding 1 year, 3 year, OS and biochemical/radiologic response.
Conclusions
Pre-treatment mGPS seems a promising prognostic biomarker. A combination of mGPS, NLR and further routine markers (e.g., Hb and AP) could yield optimized stratification for treatment selection. Further prospective and multicentric assessment is needed.
Keywords: Biomarker, Pre-treatment, Prognosis, Systemic inflammatory markers, Metastatic castration-refractory prostate cancer
Introduction
As the most common cancer in Europe and the second most common cancer among the male population worldwide, prostate cancer (PC) depicts a huge burden for the individual patient as well as for the health care system (Kreis et al. 2021; Michaeli and Michaeli 2022). While 74% of patients are diagnosed in a localized and curable stage, 13% present lymph node metastases and 7% already show distant metastases at time of diagnosis (Cancer Stat Facts 2021). Once metastasized, PC usually becomes resistant to luteinizing hormone-releasing antagonist or agonist therapy within 12 to 24 months leading to the stage of metastatic castration-resistant prostate cancer (mCRPC). In recent years, development and research has led to a broader field of treatment options in patients with mCRPC including docetaxel, abiraterone, enzalutamide, cabazitaxel, Olaparib, radium-223 and others (Cornford et al. 2021). With more therapeutic options available, the clinical decision-making process to choose the best treatment and the best sequence for the individual patient has become more difficult.
Docetaxel chemotherapy is a treatment that is recommended for fit patients only according to the EAU guidelines. For docetaxel chemotherapy, anaemia is the only laboratory prognostic biomarker for overall survival (OS), next to visceral metastases, pain, bone scan progression and prior estramustine (Armstrong et al. 2010). In addition to these clinical characteristics, the inflammatory response of patients has been described as predictor in cancer disease. To facilitate treatment decisions, different biomarkers have been developed and evaluated. These markers include a variety of routine laboratory markers, alterations in circulating cell-free DNA or genomic sequencing of tumor tissues (Stangl-Kremser et al. 2019; Neeb et al. 2021). However, the latter are cost- and time-expensive. The abundance of (inflammatory) biomarkers studied has led to the combination of biomarkers and combined prognostic scores like the modified Glasgow Prognostic Score (mGPS), the neutrophile-to-lymphocyte ratio (NLR), the platelet-to-lymphocyte ratio and the systemic immune-inflammation index (SII) as well in urological as in non-urological malignancies (Lee et al. 2015; Dolan et al. 2018; Wang et al. 2021). Recently, several studies provided evidence on the prognostic value of inflammatory markers in patients with mCRPC under various treatments including docetaxel (Donate-Moreno et al. 2020; Stangl-Kremser et al. 2020; Yamada et al. 2020).
This study’s aim was to investigate pre-treatment inflammatory biomarkers in a real-world cohort and add evidence on their suitability as predictors for treatment response and prognostic factors for survival in men with mCRPC receiving docetaxel chemotherapy in general and as first-line treatment.
Materials and methods
Study population and data collection
All patients who had received taxane-based chemotherapy at a tertiary university care centre (University Medical Centre Mannheim, Heidelberg University) in Germany between March 2010 and September 2019 were screened for docetaxel treatment in an mCRPC setting. All patients had continuous androgen-deprivation therapy. Laboratory routine markers were recorded for all cycles received at the centre. MGPS, NLR, PLR and SII were calculated as shown in Table 1.
Table 1.
The modified Glasgow prognostic score (mGPS), neutrophile-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and systemic immune-inflammation index (SII)
| mGPS | |
| C-reactive protein ≤ 10 mg/l and any albumin value | 0 |
| C-reactive protein > 10 mg/l and albumin ≥ 35 g/l | 1 |
| C-reactive protein > 10 mg/l and albumin < 35 g/l | 2 |
| NLR | |
| PLR | |
| SII | |
Biochemical response was defined in two separate ways: PSA reduction of 30% and 50% comparing PSA value at initial administration and the value after the last received cycle or after cycle 6. Radiologic response to docetaxel was assessed by comparison of baseline staging and available imaging 4–6 weeks after last docetaxel application and was categorized in complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD). CR, PR and SD were analyzed individually and grouped. To assess survival status, death register query was carried out in April 2020, which marks the end date of survival analyses. Overall survival (OS), 1-year, 3-year and 5-year survival as well as radiologic response were assessed as binary parameters. Additionally, survival time or time to death was calculated. Demographic and clinical information were extracted from the medical records in the centre.
Statistical analyses
Descriptive characteristics were performed for cohort characterization: for categorial variables, frequencies and proportions were determined, whereas medians and interquartile ranges (IQR) were computed for continuous variables. To assess the inflammatory markers as predictors for biochemical and radiologic response as well as survival Cochran-Armitage Trend Test was used. Furthermore, univariable logistic regression was used to test the impact of laboratory and clinical variables on the endpoints biochemical and radiologic response. Thereafter, multivariable logistic regression (backward selection) was used to evaluate for independent prognostic markers. For survival analysis, Kaplan–Meier analysis, log rank test and uni- and multivariable Cox-regression was conducted. All tests comparing two groups were two-sided. Statistical significance level was set at α = 0.05. Calculations were performed using the software SAS®(SAS Institute Inc., Cary, North Carolina, USA), release 9.4. For illustration GraphPad Prism9 (GraphPad Software, Inc, San Diego, California, USA) was used.
Results
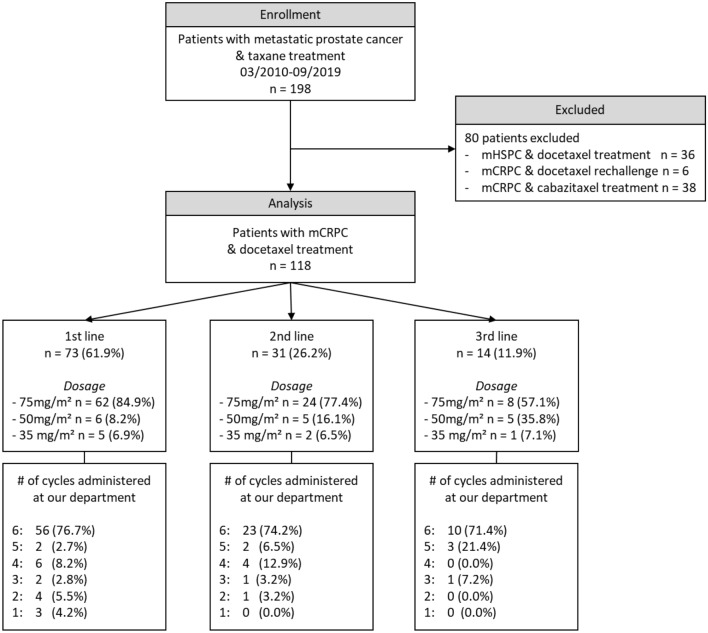
A total of 118 patients were included in the analysis, of whom 73 (61.9%) received first-line docetaxel. A detailed clinical characterization of the cohort is shown in Fig. 1 and Table 2. Median survival was 18.5 (IQR 10.8–36.5) months in the total and 26.0 (IQR 12.0–49.5) months in first-line cohort.
Fig. 1.
Flow-diagram and docetaxel treatment information of study cohort
Table 2.
Baseline characteristics of study cohort
| Characteristic | Cohort (n = 118) |
|---|---|
| Age [years], median [IQR] | 72 [65–76] |
| Gleason score ≥ 8 (n, %)a | 67 (69.8%) |
| PSA [ng/ml] at 1st cycle, median [IQR]b | 82.0 [23.4–266.5] |
| Lymphatic metastases (n, %) | 68 (57.6) |
| Osseous metastases (n, %) | 101 (85.6) |
| Visceral metastases (n, %) | 29 (24.6) |
| Hb [g/dl], median [IQR]c | 12.2 [10.2–13.3] |
| mGPSd | |
| − 0 (n, %) | 55 (57.9) |
| − 1 (n, %) | 13 (13.7) |
| − 2 (n, %) | 27 (28.4) |
| NLR, median [IQR]e | 3.9 [2.74–5.82] |
| PLR, median [IQR]e | 233.5 [141.5–312.4] |
| SII, median [IQR]f | 160 [114.6–202.8] |
| AP [U/l], median [IQR]g | 111.5 [74.8–230.0] |
| Albumin [g/dl], median [IQR]e | 35.6 [31.8–38.7] |
| CRP [mg/l], median [IQR]h | 15.4 [7.2–53.9] |
aData of 22 patient missing
bData of 9 patients missing
cData of 3 patients missing
dData of 23 patients missing
eData of 21 patients missing
fData of 34 patients missing
gData of 12 patients missing
hData of 38 patients missing
Total cohort
In the total cohort, mGPS (OR 0.54, 95% CI 0.30–0.98, p = 0.04) and PLR (OR 0.63, 95% CI 0.41–0.97, p = 0.04) remained independent predictors for biochemical response in multivariable logistic regression analysis. Regarding radiologic response, only Hb showed a significant association (OR 1.66, 95% CI 1.19–2.34, p < 0.01). As prognostic factors NLR, PLR and mGPS showed significant association with OS and 3-year survival in univariable Cox-regression analysis (all p < 0.01). However, of the four inflammatory markers examined, only mGPS showed significant association with OS (HR 1.71, 95% CI 1.25–2.36, p < 0.01) and 3-year survival (HR 1.63, 95% CI 1.17–2.27, p < 0.01) in multivariable Cox-regression analysis. Of notice, also Hb showed significant association with 3-year survival (0.77, 95% CI 0.66–0.91, p < 0.01) and remained the only significant prognostic factor for 1-year survival (0.54, 95% CI 0.43–0.68, p < 0.01) in multivariable analysis. Results are shown in Table 3.
Table 3.
Uni- and multivariable logistic and Cox-regression in the total cohort (n = 118) to detect variables associated with A the biochemical response, B the radiologic response, C the overall survival (OS), D the 3-year survival and E the 1-year survival
| A | Biochemical response (outcome: PSA reduction by 30%) | |||||
|---|---|---|---|---|---|---|
| Logistic regression: | Univariable | Multivariablea | ||||
| HR | 95% CI | p | OR | 95% CI | p | |
| Age (per year) | 0.98 | 0.93–1.03 | 0.45 | |||
| Visceral disease (yes vs. no) | 0.50 | 0.20–1.26 | 0.14 | |||
| Gleason Score ≥ 8 (yes vs. no) | 1.09 | 0.45–2.68 | 0.85 | |||
| AP (per 100 units) | 1.00 | 0.91–1.09 | 0.91 | |||
| Hb (per unit) | 1.44 | 1.15–1.80 | < 0.01 | 0.79 | ||
| PSA (per 100 units) | 0.89 | 0.78–1.01 | 0.08 | 0.28 | ||
| NLR (per unit) | 0.86 | 0.74–1.00 | 0.06 | 0.49 | ||
| PLR (per 100 units) | 0.61 | 0.43–0.87 | < 0.01 | 0.63 | 0.41–0.97 | 0.04 |
| SII (per 100 units) | 0.63 | 0.32–1.23 | 0.17 | |||
| mGPS (per unit) | 0.55 | 0.34–0.91 | 0.02 | 0.54 | 0.30–0.98 | 0.04 |
| B | Radiologic Response (outcome: radiologic response) | |||||
|---|---|---|---|---|---|---|
| Logistic regression: | Univariable | Multivariablea | ||||
| HR | 95% CI | p | OR | 95% CI | p | |
| Age (per year) | 1.00 | 0.94–1.07 | 0.85 | |||
| Visceral disease (yes vs. no) | 0.82 | 0.24–2.77 | 0.75 | |||
| Gleason Score ≥ 8 (yes vs. no) | 1.01 | 0.31–3.28 | 0.98 | |||
| AP (per 100 units) | 0.84 | 0.61–1.15 | 0.27 | |||
| Hb (per unit) | 1.66 | 1.19–2.34 | < 0.01 | 1.66 | 1.19–2.34 | < 0.01 |
| PSA (per 100 units) | 0.93 | 0.78–1.11 | 0.41 | |||
| NLR (per unit) | 0.79 | 0.60–1.03 | 0.08 | 0.38 | ||
| PLR (per 100 units) | 0.71 | 0.48–1.11 | 0.13 | |||
| SII (per 100 units) | 0.66 | 0.25–1.77 | 0.41 | |||
| mGPS (per unit) | 1.00 | 0.56–1.80 | 1.00 | |||
| C | Overall Survival (outcome: death) | |||||
|---|---|---|---|---|---|---|
| Cox-regression: | Univariable | Multivariablea | ||||
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (per year) | 1.01 | 0.98–1.04 | 0.67 | |||
| Visceral disease (yes vs. no) | 1.67 | 1.04–2.66 | 0.03 | 0.24 | ||
| Gleason Score ≥ 8 (yes vs. no) | 0.94 | 0.57–1.57 | 0.82 | |||
| AP (per 100 units) | 1.05 | 1.02–1.08 | < 0.01 | 0.14 | ||
| Hb (per unit) | 0.77 | 0.68–0.86 | < 0.01 | 0.44 | ||
| PSA (per 100 units) | 1.08 | 1.03–1.12 | < 0.01 | 0.11 | ||
| NLR (per unit) | 1.10 | 1.02–1.17 | 0.01 | 1.12 | 1.03–1.22 | < 0.01 |
| PLR (per 100 units) | 1.25 | 1.07–1.45 | < 0.01 | 0.50 | ||
| SII (per 100 units) | 1.20 | 0.84–1.72 | 0.32 | |||
| mGPS (per unit) | 1.82 | 1.39–2.39 | < 0.01 | 1.71 | 1.25–2.36 | < 0.01 |
| D | 3-year survival (outcome: death after 3 years) | |||||
|---|---|---|---|---|---|---|
| Cox-regression: | Univariable | Multivariablea | ||||
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (per year) | 1.00 | 0.97–1.03 | 0.92 | |||
| Visceral disease (yes vs. no) | 2.14 | 1.32–3.47 | < 0.01 | 0.29 | ||
| Gleason Score ≥ 8 (yes vs. no) | 1.03 | 0.59–1.79 | 0.92 | |||
| AP (per 100 units) | 1.05 | 1.02–1.08 | < 0.01 | 0.29 | ||
| Hb (per unit) | 0.73 | 0.65–0.83 | < 0.01 | 0.77 | 0.66–0.91 | < 0.01 |
| PSA (per 100 units) | 1.08 | 1.03–1.12 | < 0.01 | 0.25 | ||
| NLR (per unit) | 1.11 | 1.03–1.12 | < 0.01 | 0.05 | ||
| PLR (per 100 units) | 1.28 | 1.01–1.50 | < 0.01 | 0.51 | ||
| SII (per 100 units) | 1.11 | 0.75–1.65 | 0.60 | |||
| mGPS (per unit) | 2.05 | 1.15–2.75 | < 0.01 | 1.63 | 1.17–2.27 | < 0.01 |
| E | 1-year survival (outcome: death after 1 year) | |||||
|---|---|---|---|---|---|---|
| Cox-regression: | Univariable | Multivariablea | ||||
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (per year) | 0.98 | 0.94–1.03 | 0.45 | |||
| Visceral disease (yes vs. no) | 3.26 | 1.69–6.30 | < 0.01 | 0.28 | ||
| Gleason Score ≥ 8 (yes vs. no) | 2.23 | 0.85–5.87 | 0.10 | |||
| AP (per 100 units) | 1.06 | 1.01–1.10 | 0.01 | 0.38 | ||
| Hb (per unit) | 0.57 | 0.47–0.69 | < 0.01 | 0.54 | 0.43–0.68 | < 0.01 |
| PSA (per 100 units) | 1.08 | 1.03–1.13 | < 0.01 | 0.21 | ||
| NLR (per unit) | 1.11 | 1.02–1.22 | 0.02 | 0.20 | ||
| PLR (per 100 units) | 1.44 | 1.21–1.71 | < 0.01 | 0.08 | ||
| SII (per 100 units) | 1.55 | 0.91–2.66 | 0.11 | |||
| mGPS (per unit) | 3.85 | 2.30–6.44 | < 0.01 | 0.59 | ||
HR hazard ratio, OR odds ratio, CI confidence interval
aBackward selection
Subgroup of patients with docetaxel as first-line therapy
In the subgroup that received first-line docetaxel, SII (p = 0.05) and PLR (p = 0.02) showed significant association with biochemical response. SII remained the only independent and significant predictor in multivariable logistic regression analysis (OR 0.29, 95% CI 0.10.-0.82, p = 0.02). Matching the results from the total cohort, none of the four examined inflammatory markers but only Hb (OR 1.48, 95% CI 1.01–2.14, p = 0.04) showed significant prediction for radiologic response. As prognostic factor for survival, mGPS as the only one of the four inflammatory markers showed significant association in univariable Cox-regression with OS, 3-year and 1-year survival (all p < 0.01) and remained an independent prognostic marker in multivariable Cox-regression analysis for OS (HR 2.24, 95% CI 1.50–3.36, p < 0.01), 3 year (HR 2.74, 95% CI 1.74–4.37, p < 0.01) and 1-year survival (HR 5.24, 95% CI 2.39–11.51, p < 0.01). Of the other variables studied, AP (p = 0.02) and Hb (p < 0.01) showed a significant association with OS, but AP only remained significant in multivariable analysis (p = 0.01). Furthermore, for 3-year survival, visceral disease (p < 0.01), AP (0.02) and Hb (< 0.01) showed significant association as did visceral disease and Hb (both p < 0.01) for 1-year survival prediction. In multivariable Cox-regression analysis, only AP remained an independent prognostic factor for 3-year survival (HR 1.07 (per 100 units), 95% CI 1.02–1.12, p < 0.01). Detailed results are shown in Appendix 1.
Survival analysis
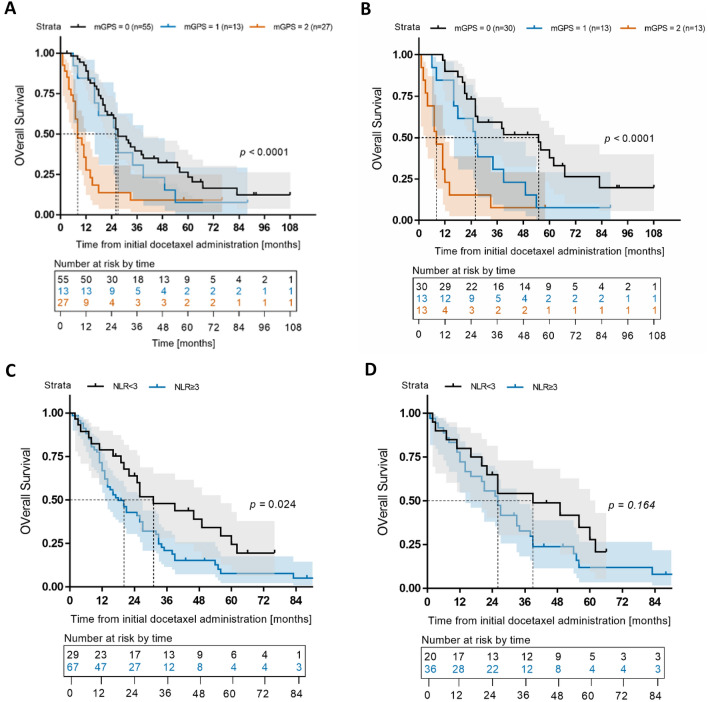
Kaplan–Meier analysis revealed longer OS in patients with lower mGPS than those with higher mGPS in the total cohort (median survival: mGPS 0 = 27 months, mGPS 1 = 26 months, mGPS 2 = 8 months, p < 0.01) and in the subgroup of patients with first-line docetaxel treatment (median survival: mGPS 0 = 55 months, mGPS 1 = 26 months, mGPS 2 = 8 months, p < 0.01). Using the commonly accepted NLR cut-off of 3, Kaplan–Meier analysis of the total cohort additionally showed a significantly poorer survival for patients with an NLR ≥ 3 (median survival 18 vs. 31 months, HR 1.74, 95% CI 1.08–2.82, p = 0.02). In the subgroup of patients receiving docetaxel as first-line treatment, the NLR cut-off of 3 did not reach significance (median survival 26 vs. 39 months, HR 1.54, 95% CI 0.84–2.88, p = 0.16). Results are shown in Fig. 2.
Fig. 2.
Kaplan–Meier analysis of overall survival (OS) [months] depending on A mGPS in the total cohort B mGPS in the docetaxel first-line subgroup C NLR in the total cohort and D NLR in the docetaxel first-line subgroup
Discussion
The recent advances in therapy of mCRPC with more therapeutic options becoming available have led to a higher need of predictive and prognostic biomarkers for personalized treatment selection. Inflammation is one of cancers’ hallmarks and systemic inflammatory biomarkers represent the body’s reaction to disease. Furthermore, they are easily available and inexpensive. Even though there has been more evidence on inflammatory biomarkers in mCRPC lately (Stangl-Kremser et al. 2020; Yamada et al. 2020), the available guidelines on PC (e.g., EAU, AUA, German S3) do not contain any statement in this regard and recommend Hb, AP and LDH as baseline laboratory biomarkers only (Cornford et al. 2021; Leitlinienprogramm Onkologie 2021; Lowrance et al. 2021). In this study, we aimed to provide further evidence for systemic inflammatory markers as predictive and prognostic factors.
Regarding biochemical response, the results yielded in this study do not coincide between the total cohort and the subgroup with docetaxel as first-line therapy: whereas PLR and mGPS remained independent predictors for biochemical response multivariable analysis in the total cohort, SII was the only predictor in the cohort that received docetaxel as first-line treatment. As predictive biomarkers for radiologic response, none of the inflammatory markers but only Hb showed an independent predictive value as assessed in multivariable analysis. Regarding survival, the results where conclusive: mGPS remained the only independent prognostic factor in both cohorts for OS and 3-year survival. In the subgroup treated with docetaxel as first-line treatment, mGPS additionally showed significant association with 1-year survival. NLR and PLR showed significant results as prognostic factors regarding 1 year, 3 year, and OS in uni- but not in multivariable analyses in the total cohort but not the first-line subgroup. Survival analysis revealed a longer survival for patients with lower mGPS in both groups as well as for patients with an NLR > 3 in the total cohort. SII did not reach significance as a prognostic marker in any analysis. Of the non-inflammatory markers/variables studied, AP and Hb were identified as independent prognostic markers for survival, which matches existing evidence. Taken together, our results particularly underline the additional value of mGPS and NLR as pragmatic prognostic biomarkers next to Hb.
In a prospective cohort-study including 80 patients, Donate-Moreno et al. in 2020 investigated inflammatory markers in mCRPC under various treatment and could show a negative correlation of NLR, PLR and SII with survival time (Donate-Moreno et al. 2020). Yamada et al. in 2020 retrospectively analyzed 196 patients with mCRPC from multiple institutions and built an inflammation index based on derived neutrophiles/(leukocytes minus neutrophils) ratio (dNLR) and LDH. They could show that stratification by their inflammation index led to longer OS in the “Good inflammatory index” group (Yamada et al. 2020). In their 2019 meta-analysis on pretreatment systemic inflammatory markers, Peng et al. included 32 studies. Sub-analysis of the 13 studies investigating patients with mCRPC and undergoing chemotherapy revealed NLR as possible effective predictive biomarker (Peng and Luo 2019). Fan et al. could show that a high SII remained a significant predictor of OS, radiologic and biochemical progression free survival in their 2018 publication including 104 patients that had received either abiraterone followed by docetaxel or vice versa (Fan et al. 2018). The mGPS has been shown to be correlated with OS (Linton et al. 2013, Ando et al. 2021), progression of mCRPC (Stangl-Kremser et al. 2020) and poorer relative survival independent of age as well as 5-year survival (Shafique et al. 2012). For patients with metastatic hormone-sensitive PC, mGPS could be shown as a predictive and prognostic biomarker for radiologic response and OS (Neuberger et al. 2022). In metastatic penile cancer, the mGPS was associated significantly with treatment response (Draeger et al. 2021) and in pretreated advanced urinary tract cancer the combination of SII, programme death-ligand 1(PD-L1) and LDH showed itself useful as a prognostic tool (Fornarini et al. 2021).
Next to urological cancers, systemic inflammatory markers have been evaluated in multiple other malignancies: For example, a recent meta-analysis showed high levels of NLR, GPS and CRP to be associated with worsened prognosis in patients with osteosarcoma (Song et al. 2021). Another systematic review and meta-analysis showed, that among others, mGPS, NLR, PLR, SII have a moderate predictive ability in OS, disease-free survival and cancer-specific survival in oesophageal cancer (Jiang et al. 2021). In colorectal cancer, NLR could be confirmed as prognostic biomarker for OS (Naszai et al. 2021) and in gastric cancer poor survival was associated with CRP, NLR and GPS/mGPS (Kim et al. 2020) in other systematic reviews and meta-analyses.
Furthermore, the results of this study add to the existing evidence for the predictive and prognostic value of systemic inflammatory markers in mCRPC. From the four markers that we examined, mGPS seems to be the most promising one as it allows a stratification and yielded significant results associated with patient survival as well in the total cohort as in the subgroup of patients with docetaxel as first-line therapy. Additionally, mGPS combines an inflammatory component (CRP) with a surrogate nutritional assessment (albumin). A recent study showed that the combination of the body mass index and albumin in patients with mCRPC treated with abiraterone is predictive of OS (Pan et al. 2021) regardless of previous chemotherapies. This underlines the importance of the assessment and intervention of nutritional status in terms of supportive therapy in this patient group. Other studies could show that low albumin levels correlate with poor prognosis in metastatic renal cell carcinoma (Zhou et al. 2022). Next to mGPS, NLR shows promising results regarding prediction of survival in patients with mCRPC, which has been shown in various studies (Peng and Luo 2019). Considering Hb, which also showed a significant association with patients’ survival, a combined score of mGPS, NLR and Hb could be a promising tool for survival prediction and thereby help to assess the patient for a more personalized treatment selection.
Limitations
The retrospective design of the study and the fact that it is monocentric limit the significance of this study. Furthermore, the rather small sample size and the fact that one of the necessary variables for mGPS was missing in 23 patients limits the statistical power and generalisability. Additionally, there was no assessment of other diseases (e.g., secondary malignancies, infections, bleeding) as only disease-specific characteristics were collected. Of note, the performed death register query did not assess the reason of death, which means, that patients could have died from PC unspecific causes. Furthermore, the cohort is heterogeneous in terms of received cancer therapies: not only has docetaxel been given as first-, second- or third-line therapy, but also the numbers of administered cycles as well as the difference in existence and numbers of previous and following PC therapies differ among these patients. Considering these limitations, this could also strengthen our findings and make them more robust and pragmatic.
Another limitation is the fact that the imaging was not evaluated according to Response Evaluation Criteria In Solid Tumors (RECIST).
Conclusion
This study evaluated mGPS, NLR, PLR and SII as predictive and prognostic biomarkers in patients with mCRPC who receive docetaxel. Pre-treatment mGPS seems the most promising independent and pragmatic biomarker regarding survival prediction. A combination of mGPS, NLR and Hb could yield an optimized stratification. The potential of mGPS, PLR, and SII as predictors for biochemical response remains unclear. Further assessment in prospective and multicentric studies is needed.
Data availability statement
Data are available for bona fide researchers who request it from the authors.
Acknowledgements
We thank the patients who participated in the study.
Appendix 1
Uni- and multivariable logistic and Cox-regression in the first-line docetaxel subgroup (n = 73) to detect variables associated with A the biochemical response B the radiologic response C the overall survival (OS) D the 3-year survival and D the 1-year survival
| A | Biochemical response (outcome: PSA reduction by 30%) | |||||
|---|---|---|---|---|---|---|
| Logistic regression: | Univariable | Multivariablea | ||||
| HR | 95% CI | p | OR | 95% CI | p | |
| Age (per year) | 0.98 | 0.92–1.06 | 0.67 | |||
| Visceral disease (yes vs. no) | 0.50 | 0.16–1.65 | 0.26 | |||
| Gleason Score ≥ 8 (yes vs. no) | 0.77 | 0.22–2.69 | 0.68 | |||
| AP (per 100 units) | 1.04 | 0.90–1.20 | 0.60 | |||
| Hb (per unit) | 1.36 | 1.03–1.80 | 0.03 | 0.13 | ||
| PSA (per 100 units) | 0.92 | 0.74–1.15 | 0.48 | |||
| NLR (per unit) | 0.93 | 0.77–1.13 | 0.47 | |||
| PLR (per 100 units) | 0.62 | 0.38–1.00 | 0.05 | 0.83 | ||
| SII (per 100 units) | 0.29 | 0.10–0.82 | 0.02 | 0.29 | 0.10–0.82 | 0.02 |
| mGPS (per unit) | 0.53 | 0.26–1.10 | 0.09 | 0.56 | ||
| B | Radiologic response (outcome: radiologic response) | |||||
|---|---|---|---|---|---|---|
| logistic regression: | Univariable | Multivariablea | ||||
| HR | 95% CI | p | OR | 95% CI | p | |
| Age (per year) | 1.00 | 0.92–1.09 | 0.97 | |||
| Visceral disease (yes vs. no) | 1.09 | 0.25–4.86 | 0.91 | |||
| Gleason Score ≥ 8 (yes vs. no) | 0.85 | 0.18–4.01 | 0.84 | |||
| AP (per 100 units) | 0.92 | 0.71–1.18 | 0.51 | |||
| Hb (per unit) | 1.48 | 1.01–2.14 | 0.04 | 1.48 | 1.01–2.14 | 0.04 |
| PSA (per 100 units) | 0.98 | 0.74–1.28 | 0.87 | |||
| NLR (per unit) | 0.91 | 0.69–1.19 | 0.47 | |||
| PLR (per 100 units) | 0.96 | 0.53–1.74 | 0.88 | |||
| SII (per 100 units) | 0.71 | 0.19–2.64 | 0.60 | |||
| mGPS (per unit) | 0.97 | 0.40–2.35 | 0.95 | |||
| C | Overall Survival (outcome: death) | |||||
|---|---|---|---|---|---|---|
| Cox-regression: | Univariable | Multivariablea | ||||
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (per year) | 1.01 | 0.97–1.05 | 0.68 | |||
| Visceral disease (yes vs. no) | 1.73 | 0.96–3.14 | 0.07 | 0.31 | ||
| Gleason Score ≥ 8 (yes vs. no) | 1.04 | 0.52–2.07 | 0.92 | |||
| AP (per 100 units) | 1.05 | 1.00–1.09 | 0.02 | 1.06 | 1.01–1.11 | 0.01 |
| Hb (per unit) | 0.80 | 0.70–0.92 | < 0.01 | 0.44 | ||
| PSA (per 100 units) | 1.08 | 0.98–1.18 | 0.14 | |||
| NLR (per unit) | 1.06 | 0.96–1.12 | 0.23 | |||
| PLR (per 100 units) | 1.08 | 0.85–1.36 | 0.55 | |||
| SII (per 100 units) | 1.13 | 0.72–1.77 | 0.60 | |||
| mGPS (per unit) | 2.30 | 1.57–3.37 | < 0.01 | 2.24 | 1.50–3.36 | < 0.01 |
| D | 3-year survival (outcome: death after 3 years) | |||||
|---|---|---|---|---|---|---|
| Cox-regression: | Univariable | Multivariablea | ||||
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (per year) | 1.00 | 0.96–1.04 | 0.99 | |||
| Visceral disease (yes vs. no) | 2.36 | 1.26–4.43 | < 0.01 | 0.83 | ||
| Gleason Score ≥ 8 (yes vs. no) | 1.30 | 0.56–3.05 | 0.54 | |||
| AP (per 100 units) | 1.05 | 1.01–1.09 | 0.02 | 1.07 | 1.02–1.12 | < 0.01 |
| Hb (per unit) | 0.77 | 0.66–0.90 | < 0.01 | 0.61 | ||
| PSA (per 100 units) | 1.05 | 0.94–1.18 | 0.38 | |||
| NLR (per unit) | 1.08 | 0.97–1.19 | 0.17 | |||
| PLR (per 100 units) | 1.09 | 0.84–1.43 | 0.52 | |||
| SII (per 100 units) | 0.96 | 0.57–1.63 | 0.89 | |||
| mGPS (per unit) | 2.65 | 1.73–4.06 | < 0.01 | 2.74 | 1.74–4.37 | < 0.01 |
| E | 1-year survival (outcome: death after 1 year) | |||||
|---|---|---|---|---|---|---|
| Cox-regression: | Univariable | Multivariablea | ||||
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (per year) | 1.01 | 0.95–1.08 | 0.66 | |||
| Visceral disease (yes vs. no) | 3.39 | 1.37–8.37 | < 0.01 | 0.90 | ||
| Gleason Score ≥ 8 (yes vs. no) | 2.30 | 0.51–10.3 | 0.28 | |||
| AP (per 100 units) | 1.04 | 0.98–1.10 | 0.19 | |||
| Hb (per unit) | 0.68 | 0.53–0.86 | < 0.01 | 0.37 | ||
| PSA (per 100 units) | 1.10 | 0.95–1.29 | 0.21 | |||
| NLR (per unit) | 1.08 | 0.93–1.26 | 0.32 | |||
| PLR (per 100 units) | 1.38 | 0.94–2.04 | 0.10 | |||
| SII (per 100 units) | 1.38 | 0.64–2.96 | 0.41 | |||
| mGPS (per unit) | 5.24 | 2.39–11.51 | < 0.01 | 5.24 | 2.39–11.51 | < 0.01 |
HR hazard ratio, OR odds ratio, CI confidence interval
abackward selection
Author contributions
All authors contributed to the study conception and design. MN. conceptualization, formal analysis, methodology, writing—original draft, writing—review and editing, visualization, project administration. N.G.: investigation, data curation, writing: review and editing. JS. investigation, data curation, writing—review and editing. VM. data curation. CW. methodology, formal analysis, visualization, writing—review and editing. FW. visualization, writing—review and editing. Philipp Erben: writing—review and editing. KN. writing—review and editing, data acquisition, resources. BG.: material support, writing—review and editing. CMH. writing—review and editing. FH. Writing—review and editing. JH. writing—review and editing. JJ. material support, writing—review and editing. KFK. writing—review and editing. FW. resources, writing—review and editing. M.CM. writing—review and editing. NW. resources, writing—review and editing. T.S.W. writing—review and editing. PN. conceptualization, resources, supervision, writing—review and editing, project administration.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study has been conducted according to the Declaration of Helsinki; all patients gave their written informed consent. This study was approved by the local ethics committee (University of Heidelberg’s Ethics Committee II, Medical Faculty Mannheim, reference number 2015-549 N-MA).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Cancer Stat Facts: Prostate Cancer. Retrieved 06.11.2021, from https://seer.cancer.gov/statfacts/html/prost.html.
- Ando K, Sakamoto S, Saito S, Maimaiti M, Imamura Y, Sazuka T, Sato N, Komiya A, Anzai N, Ichikawa T. Prognostic value of high-sensitivity modified Glasgow prognostic score in castration-resistant prostate cancer patients who received docetaxel. Cancers. 2021;13(4):773. doi: 10.3390/cancers13040773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong AJ, Garrett-Mayer E, de Wit R, Tannock I, Eisenberger M. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2010;16(1):203–211. doi: 10.1158/1078-0432.CCR-09-2514. [DOI] [PubMed] [Google Scholar]
- Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N, Gandaglia G, Gillessen S, Grivas N, Grummet J, Henry AM, der Kwast THV, Lam TB, Lardas M, Liew M, Mason MD, Moris L, Oprea-Lager DE, der Poel HGV, Rouvière O, Schoots IG, Tilki D, Wiegel T, Willemse PM, Mottet N. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79(2):263–282. doi: 10.1016/j.eururo.2020.09.046. [DOI] [PubMed] [Google Scholar]
- Dolan RD, Laird BJA, Horgan PG, McMillan DC. The prognostic value of the systemic inflammatory response in randomised clinical trials in cancer: a systematic review. Crit Rev Oncol Hematol. 2018;132:130–137. doi: 10.1016/j.critrevonc.2018.09.016. [DOI] [PubMed] [Google Scholar]
- Donate-Moreno MJ, Lorenzo-Sánchez MV, Díaz I, de Mera-Sánchez ML, Esper-Rueda HRJA, Legido-Gómez O, Rico-Marco S, Salinas-Sánchez AS. Inflammatory markers as prognostic factors in metastatic castration-resistant prostate cancer. Actas Urol Esp. 2020;44(10):692–700. doi: 10.1016/j.acuro.2020.08.001. [DOI] [PubMed] [Google Scholar]
- Draeger DL, Groh S, Buchholz T, Woehl M, Nolting J, Hakenberg OW. Prediction of treatment response and survival with chemotherapy for metastatic penile cancer by the modified Glasgow prognostic score. Urol Int. 2021;5:1–7. doi: 10.1159/000519358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Wang R, Chi C, Cai W, Zhang Y, Qian H, Shao X, Wang Y, Xu F, Pan J, Zhu Y, Shangguan X, Zhou L, Dong B, Xue W. Systemic immune-inflammation index predicts the combined clinical outcome after sequential therapy with abiraterone and docetaxel for metastatic castration-resistant prostate cancer patients. Prostate. 2018;78(4):250–256. doi: 10.1002/pros.23465. [DOI] [PubMed] [Google Scholar]
- Fornarini G, Rebuzzi SE, Banna GL, Calabrò F, Scandurra G, De Giorgi U, Masini C, Baldessari C, Naglieri E, Caserta C, Manacorda S, Maruzzo M, Milella M, Buttigliero C, Tambaro R, Ermacora P, Morelli F, Nolè F, Astolfi C, Sternberg CN. Immune-inflammatory biomarkers as prognostic factors for immunotherapy in pretreated advanced urinary tract cancer patients: an analysis of the Italian SAUL cohort. ESMO Open. 2021;6(3):100118. doi: 10.1016/j.esmoop.2021.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Xu D, Song H, Qiu B, Tian D, Li Z, Ji Y, Wang J. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: a systematic review and meta-analysis. BMJ Open. 2021;11(9):e048324. doi: 10.1136/bmjopen-2020-048324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MR, Kim AS, Choi HI, Jung JH, Park JY, Ko HJ. Inflammatory markers for predicting overall survival in gastric cancer patients: a systematic review and meta-analysis. PLoS ONE. 2020;15(7):e0236445. doi: 10.1371/journal.pone.0236445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis K, Horenkamp-Sonntag D, Schneider U, Zeidler J, Glaeske G, Weissbach L. Treatment-related healthcare costs of metastatic castration-resistant prostate cancer in germany: a claims data study. Pharmacoecon Open. 2021;5(2):299–310. doi: 10.1007/s41669-020-00219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Russell A, Hellawell G. Predictive value of pretreatment inflammation-based prognostic scores (neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio) for invasive bladder carcinoma. Korean J Urol. 2015;56(11):749–755. doi: 10.4111/kju.2015.56.11.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton A, Pond G, Clarke S, Vardy J, Galsky M, Sonpavde G. Glasgow prognostic score as a prognostic factor in metastatic castration-resistant prostate cancer treated with docetaxel-based chemotherapy. Clin Genitourin Cancer. 2013;11(4):423–430. doi: 10.1016/j.clgc.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Lowrance WT, Breau RH, Chou R, Chapin BF, Crispino T, Dreicer R, Jarrard DF, Kibel AS, Morgan TM, Morgans AK, Oh WK, Resnick MJ, Zietman AL, Cookson MS. Advanced prostate cancer: AUA/ASTRO/SUO guideline PART II. J Urol. 2021;205(1):22–29. doi: 10.1097/JU.0000000000001376. [DOI] [PubMed] [Google Scholar]
- Michaeli T, Michaeli D. Prostate cancer follow-up costs in Germany from 2000 to 2015. J Cancer Surviv. 2022;16(1):86–94. doi: 10.1007/s11764-021-01006-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naszai M, Kurjan A, Maughan TS. The prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio (NLR) in colorectal cancer: a systematic review and meta-analysis. Cancer Med. 2021;10(17):5983–5997. doi: 10.1002/cam4.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeb A, Herranz N, Arce-Gallego S, Miranda S, Buroni L, Yuan W, Athie A, Casals T, Carmichael J, Rodrigues DN, Gurel B, Rescigno P, Rekowski J, Welti J, Riisnaes R, Gil V, Ning J, Wagner V, Casanova-Salas I, Cordoba S, Castro N, Fenor de la Maza MD, Seed G, Chandran K, Ferreira A, Figueiredo I, Bertan C, Bianchini D, Aversa C, Paschalis A, Gonzalez M, Morales-Barrera R, Suarez C, Carles J, Swain A, Sharp A, Gil J, Serra V, Lord C, Carreira S, Mateo J, de Bono JS. Advanced prostate cancer with ATM Loss: PARP and ATR Inhibitors. Eur Urol. 2021;79(2):200–211. doi: 10.1016/j.eururo.2020.10.029. [DOI] [PubMed] [Google Scholar]
- Neuberger M, Skladny J, Goly N, Wessels F, Weiß C, Egen L, Erben P, Grüne GRWMB, Hartung F, Herrmann J, Honeck P, Jarczyk J, Kowalewski KF, Mühlbauer J, Nitschke K, Nientiedt M, Walach MT, Waldbillig F, Westhoff N, Kriegmair VONHJM, Worst TS, Nuhn P. Baseline modified Glasgow prognostic score predicts radiologic response and overall survival in metastatic hormone sensitive prostate cancer treated with docetaxel chemotherapy. Anticancer Res. 2022;42(4):1911–1918. doi: 10.21873/anticanres.15668. [DOI] [PubMed] [Google Scholar]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, D. K., AWMF) 2021 "Interdisziplinäre Leitlinie der Qualität S3 zur Früherkennung, diagnose und therapie der verschiedenen stadien des Prostatakarzinoms, Langversion 6.2, Oktober 2021, AWMF Registernummer: 043/022OL." from http://www.leitlinienprogramm-onkologie.de/leitlinien/prostatakarzinom/ (Access date: 20.03.2022).
- Pan J, Wang J, Wei Y, Zhang T, Zhang S, Ye D, Zhu Y. Combination of body mass index and albumin predicts the survival in metastatic castration-resistant prostate cancer patients treated with abiraterone: a post hoc analysis of two randomized trials. Cancer Med. 2021;10(19):6697–6704. doi: 10.1002/cam4.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Luo X. Prognostic significance of elevated pretreatment systemic inflammatory markers for patients with prostate cancer: a meta-analysis. Cancer Cell Int. 2019;19:70. doi: 10.1186/s12935-019-0785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafique K, Proctor MJ, McMillan DC, Qureshi K, Leung H, Morrison DS. Systemic inflammation and survival of patients with prostate cancer: evidence from the Glasgow inflammation outcome study. Prostate Cancer Prostatic Dis. 2012;15(2):195–201. doi: 10.1038/pcan.2011.60. [DOI] [PubMed] [Google Scholar]
- Song X, Zhang H, Yin F, Guo P, Yang X, Liu J, Han Y, Ren Z. Systemic inflammatory markers for predicting overall survival in patients with osteosarcoma: a systematic review and meta-analysis. Mediators Inflamm. 2021;2021:3456629. doi: 10.1155/2021/3456629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangl-Kremser J, Lemberger U, Hassler MR, Bruchbacher A, Ilijazi D, Garstka N, Kramer G, Haitel A, Abufaraj M, Shariat SF. Prevalence and prognostic value of the polymorphic variant 1245A>C of HSD3B1 in castration-resistant prostate cancer. Clin Genitourin Cancer. 2019;17(5):389–394. doi: 10.1016/j.clgc.2019.06.012. [DOI] [PubMed] [Google Scholar]
- Stangl-Kremser J, Mari A, Suarez-Ibarrola R, D'Andrea D, Korn SM, Pones M, Kramer G, Karakiewicz P, Enikeev DV, Glybochko PV, Briganti A, Shariat SF. Development of a prognostic model for survival time prediction in castration-resistant prostate cancer patients. Urol Oncol. 2020;38(6):600.e609–600.e615. doi: 10.1016/j.urolonc.2019.11.005. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhu SR, Huang XP, Liu XQ, Liu JB, Tian G. Prognostic value of systemic immune-inflammation index in patients with urinary system cancers: a meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25(3):1302–1310. doi: 10.26355/eurrev_202102_24834. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Sakamoto S, Rii J, Yamamoto S, Kamada S, Imamura Y, Nakamura K, Komiya A, Nakatsu H, Ichikawa T. Prognostic value of an inflammatory index for patients with metastatic castration-resistant prostate cancer. Prostate. 2020;80(7):559–569. doi: 10.1002/pros.23969. [DOI] [PubMed] [Google Scholar]
- Zhou X, Fu G, Zu X, Xu Z, Li HT, D'Souza A, Tulpule V, Quinn DI, Bhowmick NA, Weisenberger DJ, Liang G, Chen J. Albumin levels predict prognosis in advanced renal cell carcinoma treated with tyrosine kinase inhibitors: a systematic review and meta-analysis. Urol Oncol. 2022;40(1):12.e13–12.e22. doi: 10.1016/j.urolonc.2021.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available for bona fide researchers who request it from the authors.