Abstract
Almost 2 decades after linking LRRK2 to Parkinson’s disease, a vibrant research field has developed around the study of this gene and its protein product. Recent studies have begun to elucidate molecular structures of LRRK2 and its complexes, and our understanding of LRRK2 has continued to grow, affirming decisions made years ago to therapeutically target this enzyme for PD. Markers of LRRK2 activity, with potential to monitor disease progression or treatment efficacy, are also under development. Interestingly, there is a growing understanding of the role of LRRK2 outside of the central nervous system in peripheral tissues such as gut and immune cells that may also contribute to LRRK2 mediated pathology. In this perspective, our goal is to take stock of LRRK2 research by discussing the current state of knowledge and critical open questions in the field.
Subject terms: Parkinson's disease, Parkinson's disease
Introduction
The leucine-rich repeat kinase 2 (LRRK2) gene was identified in 2004 as the gene responsible for the PARK8 locus that had itself previously been linked to Parkinson’s disease (PD) in 20021–3 (see timeline of key milestones in the LRRK2 field in Fig. 1). Almost 20 years later, a PubMed search using LRRK2 as search term results in >3000 publications and a thriving research field has developed around the study of LRRK2. One of the reasons for this is that from the moment of its discovery LRRK2 showed that it was potentially a major determinant in the pathophysiology of PD. The study of neurodegenerative diseases of aging are a priority area for research worldwide, as the number of individuals with these diseases is dramatically increasing due to the aging population, and LRRK2 has received significant attention. What research on LRRK2 has revealed thus far confirms that this attention is well deserved and may ultimately lead to improvements in the diagnosis of PD and novel therapies for the patients.
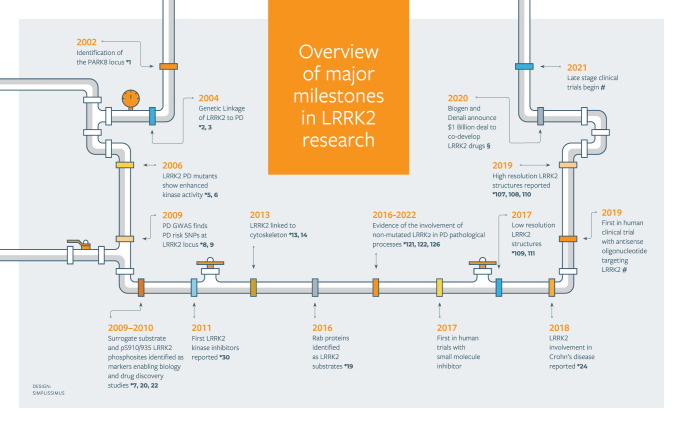
Fig. 1. Timeline of key milestones in the advancement of the field of LRRK2 research.
Numbers next to the stars (*) denote the citation references pertaining to the milestone. #See text above for the ClinicalTrials.gov identifiers (NCT numbers) for the clinical milestones. §https://investors.biogen.com/news-releases/news-release-details/biogen-and-denali-collaborate-lrrk2-program-parkinsons-disease.
The LRRK2 gene is located on chromosome 12, contains 51 exons spanning 144 kb and encodes for a large 286 kDa multidomain protein of the same name. As its name suggests, LRRK2 contains a leucine-rich repeat (LRR) domain and a kinase domain. LRRK2 was identified as a member of the Ras of complex proteins (ROCO) family4 and thus it also contains a Ras-like GTPase domain (ROC) followed by a C-terminal of ROC (COR) domain. In addition, sequence homology revealed the presence of additional domains, including an armadillo repeat domain (ARM) and ankyrin repeat domain (ANK) in the N-terminus and a WD40-repeat at the C-terminus (Fig. 2). In the first years after its discovery, work on LRRK2 genetics revealed several missense mutations that segregated with disease, many located in LRRK2’s catalytic core, constituted of the ROC, COR and Kinase domains. Mutations had an autosomal dominant mode of transmission and when biochemical analysis showed that many mutations led to an increase in LRRK2 autophosphorylation activity5,6 as well as an increase in phosphorylation of surrogate substrates7, the notion of a gain of toxic kinase function was born to explain LRRK2’s pathophysiological mechanism(s) in PD. Although LRRK2 mutations were found to be relatively common compared to mutations in other PD genes, these are still only affecting a small proportion of all PD patients suggesting LRRK2 may only be a relevant gene for a subset of patients8. However, in 2009 and following, PD genome-wide association studies (GWAS) identified common genetic variance at and near the LRRK2 locus as a risk factor for PD9,10, pointing to the possibility that LRRK2 dysfunction may be involved in PD pathomechanisms in patients who do not harbour a missense mutation. These findings of genetic evidence that LRRK2 is involved in PD and that the kinase hyperactivation may be a primary culprit were strong drivers of research programmes in academia and industry to understand LRRK2 dysfunction and develop inhibitors of LRRK2 kinase activity.
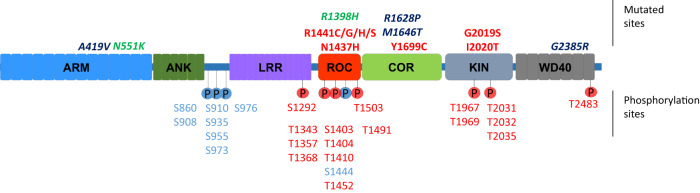
Fig. 2. Schematic of the LRRK2 domain structure.
Above the schematic, key amino acid substitutions are indicated that alter risk for PD, including mutations that increase risk for PD such as pathogenic mutations (red) and risk factor mutations (blue) as well as mutations that confer reduced risk for PD (green). Phosphorylated residues are given below the schematic, with heterologous phosphosites give in blue and autophosphorylation sites in red. Figure adapted from ref. 22.
Studies on LRRK2 have extensively explored the toxic gain of function hypothesis for LRRK2 suggesting that expression of a disease mutant form of LRRK2 would lead, in cellular or in vivo models, to PD phenotypes, notably cell death, the presence of intracellular inclusions or loss of synaptic connections. However, research has shown that LRRK2 mutants are relatively well tolerated, in cells and in animals, and they do not induce the severe disease phenotypes that were predicted (reviewed in11,12). What has emerged through these efforts are specific cellular functions that are modulated by LRRK2 activity. For instance, several reports have described the involvement of LRRK2 in the cytoskeleton, including interactions with microtubules or actin13,14, as well as the modulation of cell morphology15. Another series of reports points to a role of LRRK2 in the endolysosomal system16–18. One key finding in this regard is that a subset of Rab proteins are physiological substrates of LRRK219, coinciding with several observations showing the involvement of LRRK2 in intracellular trafficking or endolysosomal functions, such as its recruitment to lysosomes or Golgi apparatus or its regulation of receptor trafficking20. Another major feature of LRRK2 is that it is multiphosphorylated (Fig. 2), including heterologous phosphorylation sites and autophosphorylation sites that are observed to be inversely regulated in disease (reduction for the heterologous sites clustering around S910/S93521 and increase for the autophosphorylation sites5,6) while both are reduced after pharmacological treatments with type I kinase inhibitors22,23. Another significant development over the past decade is the increased understanding that LRRK2 is important in several peripheral functions such as the immune system or the gut, and that these functions may also contribute to the PD pathomechanisms. Examples of this are the observations that LRRK2 expression and phosphorylation is upregulated in B-cells and microglia upon immune challenge and the identification of disease linked mutations for LRRK2 in Crohn’s disease24, a type of immune-mediated inflammatory bowel disease, as well as observations that pathological α-synuclein recruits LRRK2 expressing pro-inflammatory monocytes from the periphery to the brain25. Several recent reviews discuss in more detail the roles of LRRK2 in the periphery26–29.
In terms of LRRK2 as a therapeutic target, several inhibitors of the LRRK2 kinase have been developed in the past decade or more, including initial non-brain penetrant compounds LRRK2-IN130 and GSK2578215A31, followed by several brain penetrant compounds GNE7915, GNE-087732, PF-47533, PF-36034, MLi-235, DNL20136, and antisense oligonucleotides37, the best of which demonstrate potency in the low nanomolar range and very good selectivity. These tools have allowed the advancement of LRRK2 kinase inhibitors through preclinical testing, with thee small molecule inhibitors (DNL201, DNL151, ClinicalTrials.gov Identifiers NCT04557800, NCT05119790, NCT04056689, NCT05005338, NCT05418673, NCT05348785, NCT05229562, NCT04551534, NCT03710707, and NEU-723 ClinicalTrials.gov Identifier NCT05633745, note BIIB122 is an alternate name for DNL151) and one anti-sense oligonucleotide (BIIB094, ClinicalTrials.gov Identifier NCT03976349) in clinical trials as potential treatments for PD.
The research around LRRK2 also led to initiatives to facilitate discussion and scientific exchanges, such as the creation, in 2008, of a specific international LRRK2 consortium hosted by the Michael J. Fox Foundation and the organisation of an International Scientific Conference on LRRK2 in 2012 by the Biochemical Society. Both of these exchange initiatives are still active, in the form of a PD Research Exchange forum (including LRRK2 as well as several other areas of PD research) and a Biennial International LRRK2 conference, the fourth and latest edition of which was held in June-July of 2022. In this paper, our goal is to take stock of the current state of advances in the field of LRRK2 research by discussing current key questions in the field and what perspectives these point to for the future.
What is the normal vs pathological function of LRRK2?
Since the identification and implication of LRRK2 in PD, much attention has been paid to how LRRK2 pathogenic mutations alter specific functions of this enzyme relative to the wild type LRRK2, while not yet having a detailed picture of the normal function of LRRK2. Hence, a further understanding of the phenotypes associated to the loss of LRRK2 for instance by the study of LRRK2 KO animals under basal conditions, has proven insightful. Indeed, as described above, contours are emerging of the normal function of LRRK2, however several questions remain. For instance, is LRRK2 an agent of the cytoskeleton, or an agent of the endolysosomal system? Primary neurons isolated from LRRK2 KO mice are reported to develop longer and more complex neurites14, suggesting a role for LRRK2 in the cytoskeleton and cell morphology. By contrast, several findings point to a role of LRRK2 in membrane trafficking or subcellular organization, such as the ability of LRRK2 to phosphorylate several members of the family of Rab-GTPases19, the ability of LRRK2 to be recruited to specific subcellular compartments such as Golgi network (via Rab29 overexpression)38 or lysosomes (under influence of lysosomotropic agents)39. The link between LRRK2 and Rabs are potentially also relevant to a role for LRRK2 in centrosome cohesion40 and ciliogenesis41,42. Besides involvement in the cytoskeleton and in membrane trafficking, additional cellular functions are reported to be affected by LRRK2, including iron homoeostasis43 and mitochondrial function44.
Aside from normal function, work with LRRK2 deficient models would also be helpful in discerning whether native unmutated LRRK2 may contribute to disease. Mice and rat LRRK2 knockouts (KO) are viable and do not display strong changes in outward signs of health45–47. Although initial work did not demonstrate changes in toxicity induced in transgenic α-synuclein overexpression or MPTP in LRRK2 KO animals45,48, several other studies report results consistent with the conclusion that LRRK2 is a modulator of toxicity. Some relevant examples are reports that toxicity induced by nigral α-synuclein overexpression, LPS, HIV-1 Tat or Mycobacterium tuberculosis in rodent models is abrogated in LRRK2 KO animals49–51. If LRRK2 is a toxicity modifier, is this the primary toxic mechanism for LRRK2 or might LRRK2 also act as a toxic agent in its own right?
The study of LRRK2 KO animals may also help in answering additional key questions, such as is the toxic function of LRRK2 in the brain or in the periphery? Observations of LRRK2 KO animals show that peripheral tissues such as kidneys or lungs display a microvacuolation phenotype that is not observed in the brain47. Another phenomenon observed in the periphery and not in the CNS is that LRRK2 KO mice and rats show an age dependent darkening of the kidneys46, a phenomenon which is reported to be linked to haemoglobin and lipofuscin accumulation in renal tubules52 although little is known on how this may be involved in pathological processes. LRRK2 is also expressed in the gastrointestinal (GI) tract53 and LRRK2 KO mice show reduced symptoms in an experimental colitis model54, suggesting that LRRK2 affects GI pathologies. Further, taking these data together with the findings that patients with Crohn’s disease (inflammatory bowel disease, IBD) have a higher risk of developing PD (meta-analysis55) may also point to a role for LRRK2 in the GI in the development of PD. Other peripheral tissues/systems are also impacted in LRRK2 KO animals as observed by vacuolation of type-2 pneumocytes in the lungs34,56 or altered function of immune cells57,58. Therefore, the question arises: should we be looking more at immune function and GI function of LRRK2 rather than brain function?
Besides the fundamental scientific goal of fully understanding LRRK2, improving our understanding of the normal function of LRRK2 may teach us how this is altered in PD. This, in turn, would point to possibilities to identify and stratify patients, among others by the identification of LRRK2 based biomarkers of disease in urine, blood, CSF or other patient based samples59. Developing the potential of the LRRK2 pathway for use as biomarkers (examples given below in the section on therapeutics) would be a welcome addition to the panel of potential measures included in clinical testing, given the concern that trials may fail because we do not have the right measures of LRRK2 disease-involvement in an individual or of target engagement in those receiving targeted therapy.
What about LRRK2 mutations and penetrance?
For many diseases with genetic causes, the simple presence of a disease linked genetic mutation does not coincide 100% with the presence of the disease. This is also the case for LRRK2 (an overview of LRRK2 mutations in given in Table 1). For instance, many individuals carrying the LRRK2 G2019S mutation will never develop PD, even at very old ages60,61. The estimates of the lifetime penetrance of LRRK2 G2019S is wide, from 17 to 80%62–64, for instance 25% at the age of 80 among Ashkenazi Jews62 and around 40% among non-Ashkenazi Jews65. Other mutations, such as G2385R, may have even lower penetrance, for example: 10% at 80 years of age63,66, yet early estimates of R1441G penetrance were higher, reaching 80% at 80 years of age67 with a potentially more homogenous phenotype68, which could be important for clinical trials. These observations beg the question: why do some individuals carrying LRRK2 mutations develop PD and others do not? Which additional factors contribute to the penetrance of LRRK2 mutations and are they genetic or are they environmental? There may in fact be evidence for both. For instance, caffeine consumption is observed to have a greater protective effect in LRRK2 G2019S carriers compared to non-mutation carriers69. Also, genetic epistasis has been observed between risk SNPs at the PARK16 (with RAB29 as nominated gene responsible for this locus) and LRRK2 loci70, and LRRK2 G2019S penetrance is influenced by a polygenic risk score71.
Table 1.
Overview of LRRK2 amino-acid substitutions that alter disease risk.
| Mutation | Domain where mutation is located | Nature of the mutation | Reported prevalence in specific populations |
|---|---|---|---|
| A419V | ARM | Increased risk for PD | East Asian |
| N551K | ARM | Reduced risk for PD | East Asian, Ashkenazi Jewish, non-Jewish European |
| R1398H | ROC | Reduced risk for PD | European, East Asian, Ashkenazi Jewish, non-Jewish European |
| N1437H | ROC | Increased risk for PD | European |
| R1441C | ROC | Increased risk for PD | European |
| R1441G | ROC | Increased risk for PD | European Basque |
| R1441H | ROC | Increased risk for PD | European |
| R1628P | COR | Increased risk for PD | East Asian |
| Y1699C | COR | Increased risk for PD | European |
| G2019S | Kinase | Increased risk for PD | European/West Asian, Mixed populations |
| I2020T | Kinase | Increased risk for PD | East Asian |
| N2081D | Kinase | Increased risk for CD and potentially PD | Ashkenazi Jewish, non-Jewish European |
| G2385R | WD40 | Increased risk for PD | East Asian |
| M2397T | WD40 | Increased Risk for CD and Leprosy | East Asian, Mixed populations |
While there are several LRRK2 pathogenic mutations associated with PD, G2019S LRRK2 has been extensively studied given the prevalence of this mutation over other disease-causing mutations64. The clinical phenotype of G2019S LRRK2-PD can be described as a typical PD syndrome with both motor and non-motor symptoms and a clear response to levodopa. However, several unique characteristics have emerged: a higher percent of gait manifestations and lower extremity onset72,73 and slower motor progression74. Better cognitive performance75 and slower cognitive decline compared to sporadic cases74 have been described as well. In addition, less non-motor involvement including lower rates of hyposmia and REM sleep behaviour disorder75–77 have been reported. While some structural imaging studies managed to detect increased cerebral volume among LRRK2-PD compared with iPD78, others did not and reported decreased cortical thickness between LRRK2-PD and healthy controls, with no difference between LRRK2-PD and iPD79. Dopamine transporter SPECT studies indicate higher striatal binding ratios (SBR) in the contralateral caudate and putamen among LRRK2-PD compared with iPD80. Interestingly, while the age of onset of PD in LRRK2 G2019S carriers is comparable to sporadic PD, the disease progression of LRRK2 G2019S PD patients is slower than sporadic PD74,81,82. Thus, it seems that LRRK2-PD has a more favourable clinical phenotype83 and perhaps even longer survival than iPD patients84,85. While these group differences remain to be defined unambiguously, it should be emphasized that these clinical phenotypes cannot distinguish, on an individual basis, G2019S from sporadic cases. Interestingly, mutations in the GBA gene are the strongest risk factors for PD86, and like the G2019S mutation in the LRRK2 gene, are common among Ashkenazi Jews. Harbouring a mutation in the GBA gene has been associated with a more severe and rapidly deteriorating phenotype of PD87,88. Yet, dual mutation carriers (GBA-LRRK2-PD) have been found to have a milder disease phenotype compared to GBA-PD89–91, raising the possibility of a protective mechanism for LRRK2 on the GBA disease phenotype.
These observations raise a controversial point: if LRRK2-PD subjects do indeed have a milder phenotype than iPD, then perhaps treating LRRK2 might worsen instead of improving the disease. It remains to be demonstrated whether the LRRK2 mutation status of a patient changes the potential benefit of these patients for specific PD therapies and it will certainly be necessary to factor in this aspect when planning clinical studies and analysing clinical study data.
LRRK2 pathology: distinct from idiopathic PD?
In pathological studies performed to date in LRRK2-PD, variable changes have been described including Lewy bodies, tau positive inclusions, TDP-43 aggregates, ubiquitin inclusions and pure nigral cell loss with no inclusions92,93. Recent advances in aggregation assays have enabled the assessment of α-synuclein from cerebrospinal fluid (CSF) and other tissues from patients with PD. LRRK2-PD carriers have been found to have lower rates of α-synuclein seed amplification on these assays as compared to both idiopathic PD and GBA-PD94,95. These differences in pathological findings, together with the clinical and imaging studies, could explain the existence of different subgroups within LRRK2-PD93. In fact, a recent study assessing olfactory functions in LRRK2-PD identified a subgroup of patients with earlier age of onset and faster olfactory loss76. Multi-omics profiling of human biofluids from LRRK2 mutation carriers are beginning to identify additional pathways that are altered in LRRK2-PD and LRRK2 non-manifesting carriers when compared to iPD and healthy controls96,97. These data suggest that multiple mechanisms may exist in LRRK2 carriers that lead to PD pathogenesis and progression. Translating our understanding of these mechanisms into better biomarkers to diagnose at-risk individuals, monitor progression and enrich patients for clinical trials will be critical- especially given the heterogeneity in clinical, pathological and biological endpoints in LRRK2 cohorts. Future studies should be aimed at correlating biological endpoints with clinical, imaging and pathological measures of LRRK2-PD to better understand the biological basis of the subgroups within LRRK2. Such analyses will be critical for patient selection into clinical trials as different LRRK2 mutation carriers may benefit from different treatment strategies98.
It is noteworthy that with distinct LRRK2 mutations, I2020T99 and G2019S93,100,101, the reported pathology differs from idiopathic PD. In the I2020T mutation carriers there was dopaminergic neuron loss in the substantia nigra in 6 of the 8 cases, with no Lewy bodies, nor other marked pathology e.g. Tau99. The first case study with G2019S100 showed no Lewy bodies but did show tau-immunoreactive neurofibrillary tangles and an argyrophilic grain disease–like pathology. More recently Kalia et al. showed that 30% of G2019S mutations carriers and 70% of other mutations carriers (including I2020T, R1441C, R1441G and Y1699C) did not have Lewy bodies, but did have dopaminergic neuron degeneration93. Kalia et al. did not report on Tau pathology, but this has been addressed by Henderson et al.101. These investigators found that α-synuclein pathology was present in 63.6% of LRRK2 mutation carriers, but tau pathology was found in 100% of carriers and is abundant in 91% of carriers. They used an antibody which selectively binds Alzheimer’s disease (AD)-type tau and used quantitative analysis of this tau pathology to demonstrate that AD tau is the prominent type of tau present in LRRK2 mutation carriers. They also make the important point that AD tau staging in LRRK2 PD follows a similar distribution to iPD and iPD with dementia and is accompanied by abundant concurrent Aβ pathology in most cases. They suggest that tau is not an independent disease factor in LRRK2 PD, but is associated with the degree of α-synuclein pathology and progression to dementia101. Moreover, there is recent data that goes one step further, suggesting that the initiation of dopaminergic neurodegeneration occurs independently of α-synuclein aggregation and is likely tau mediated102. The lower incidence of α-synuclein pathology has recently been addressed in ante-mortem CSF samples using α-synuclein seeding amplification assays94,103, where positive signal was observed in only 40–78% of LRRK2 mutation carriers. However, this may be dependent on the techniques used and the forms of α-synuclein measured (Sekiya et al. 2022, Ann Neurol, 92: S1-S243, M177. 10.1002/ana.26484). These studies all point to subgroups of LRRK2 PD patients based on whether they harbour α-synuclein or Lewy body deposits.
Overall, the prevalence of Tau pathology and the reduced incidence of pronounced α-synuclein pathology in certain LRRK2 mutation carriers does raise important considerations with respect to inclusion criteria in clinical trials, endpoints (biomarkers) measured and ultimately the therapeutic strategies. It could be beneficial to stratify the mutation carriers based on these pathologies with respect to future clinical trials, particularly if additional data were to emerge that provides clearer links between disease progression and one or more co-pathologies. The availability of Tau imaging agents102 and the potential utility of blood-based assays to measure specific Tau species104, could enable this, indeed there are already efforts to image Tau in G2019S subjects (ClinicalTrials.gov Identifier: NCT04557865).
The ongoing clinical trials will hopefully be able to perform post-hoc analysis of their data to increase our understanding of this complex interplay between LRRK2 mutations and pathophysiology. Future trials, using combination therapies e.g. reducing LRRK2 and Tau, may be required to alter disease progression, employing a precision medicine strategy enabled by biomarkers.
LRRK2 as a therapeutic target
LRRK2 is a therapeutic target for Parkinson’s disease (PD). Indeed, findings of LRRK2 hyperactivation in disease mutants and that deleterious effects of LRRK2 mutants in preclinical models can be blocked by inhibiting LRRK2 expression or kinase activity provided an initial validation of LRRK2 as a therapeutic target105,106. Efforts to develop LRRK2 targeting agents have met a certain degree of success as potent and selective agents targeting LRRK2 have been developed, both small molecule inhibitors of LRRK2 kinase activity and antisense oligonucleotides to reduce LRRK2 levels, and these targeting approaches are currently under evaluation in early phases of clinical trials. While this is very encouraging in the path to PD modifying therapies, it should be noted that the end goal of validating LRRK2 as a PD therapeutic target has yet to be reached. For validation of a therapeutic target, it is necessary to demonstrate significant and reproducible disease modification in human patients following pharmacological targeting, i.e. after successful phase III clinical trials. One of the potential pitfalls in this process is that specific targeting agents are selected to be evaluated, and it may be that these display shortcomings or secondary effects that can lead to inconclusive clinical results. It is therefore important to test more than 1 agent and/or more than one targeting modality for a given target.
The study of LRRK2 biology has revealed that LRRK2 itself can be targeted in more ways than one. Besides LRRK2 kinase activity, LRRK2 function is also regulated by its GTPase domain and its quaternary structure, both of which are potentially targetable. We have also advanced in our understanding of key cellular partners of LRRK2 that participate in generating the ‘output’ of the LRRK2 pathway that is deregulated in disease. In as far as these partners are crucial to the functioning of the LRRK2 pathway, they may constitute valuable therapeutic targets for PD in their own right.
Recent advances in the elucidation of the 3D domain organisation and atomic structure of LRRK2107–111 are a very big boost to the field on several levels. For instance, we can now integrate rational design steps in the therapeutic development process. This can be at the level of molecules targeting catalytic pockets of LRRK2 enzymatic functions, as well as to understand molecules targeting at allosteric sites or even at sites that would mediate the quaternary structure or protein-protein interactions with LRRK2 partners in the event of the design of protein-protein interaction modulators. The potential of pursuing additional 3D structural information for LRRK2 is big. Indeed, structures that have been identified thus far are reflecting LRRK2 in specific states depending on the experimental conditions. By studying additional conditions such as pH, binding partners or mutations, additional structures are likely to emerge, that will shed light on the structural dynamics of LRRK2. It should also not be excluded that specific conformations of LRRK2 may be identified that are reflective of healthy or pathological states112. In this case, this phenomenon may be useful as a screening tool for compounds promoting a healthy conformation, but also can open a path for developing conformation specific probes for use in biomarker testing. Conformation specific antibodies have already been developed to identify different α-synuclein species, suggesting a similar approach could be feasible for LRRK2113.
Concerning the targeting strategies themselves, LRRK2 kinase inhibition has taken a very significant lead ahead of other targeting options for LRRK2. Undoubtedly, this was influenced by significant existing expertise in drug discovery organisations on developing kinase inhibitors. Those inhibitors that have been most extensively tested are type I inhibitors, i.e. inhibitors that bind to the active conformation of the kinase. Interestingly, recent data suggests that this class of inhibitor is responsible for inducing the recruitment of LRRK2 to microtubules107. These same inhibitors may display a potential unwanted side effect as they are reported at high doses to generate a vacuolation phenotype in kidneys and type 2 pneumocytes upon chronic treatment in rodents and non-human primates34,56. Understanding the link between type I kinase inhibitors of LRRK2 and these undesired phenotypes would be most useful to pre-empt potential failure of this class of compounds. Alternative solutions could be to develop type II kinase inhibitors for LRRK2, i.e. inhibitors that target and stabilize an inactive form of LRRK2 or a knock down approach using anti-sense oligonucleotides (ASO) to LRRK2 that are in clinical development (ClinicalTrials.gov Identifier: NCT03976349) with intrathecal administration. The ASOs are poorly taken up by type 2 pneumocytes and may provide differentiation from the small molecules if the lung phenotype proves to be an issue. Further work will be needed to determine whether targeting the LRRK2 kinase or expression is indeed sufficient and/or required to stop disease without strong side effects.
By analogy with the questions on LRRK2 function in the CNS compared to peripheral tissues, it is yet to be determined whether targeting LRRK2 in the periphery would be beneficial or detrimental. While the vacuolation observed in the kidneys or lungs in certain conditions after LRRK2 kinase inhibition is not considered as a protective marker, it can be hypothesized that targeting LRRK2 in the GI tract or immune system may be protective, for instance by positively affecting intestinal inflammation114 or by blocking transmission of toxic α-synuclein species from the GI tract to the CNS through the vagus nerve. In addition, further work is warranted to verify whether therapeutic targeting should be focused specifically for the central nervous system or whether targeting of LRRK2 in periphery should be preferred, or whether it is best to target both central and peripheral LRRK2.
Treatment of iPD with LRRK2 targeting agents?
Aside from PD-causative mutations, PD risk has also been identified in genomic variants at the LRRK2 locus, raising the possibility that LRRK2 may be involved in the pathogenesis of iPD independent of coding mutations. Given that pathogenic mutations of LRRK2 are associated with aberrantly enhanced kinase activity and, consequently, that kinase inhibition may be useful therapeutically, it is worth investigating the LRRK2 kinase activation state in iPD. In this context, preclinical rodent models showed that genetic ablation or pharmacological kinase inhibition of endogenous wild-type LRRK2 protects against α-synuclein-induced neurodegeneration49. Implicit in such a conclusion is the assumption that wild-type LRRK2 kinase must impact nigrostriatal dopamine neurons under these experimental conditions. Similarly, in an ‘environmental’ model of PD in wild-type rats, it was demonstrated that exposure to rotenone (which increased LRRK2 kinase activity as assessed by pThr73-Rab10 levels) could reproduce endolysosomal deficits seen in human post-mortem brains—and treatment with a LRRK2 kinase inhibitor could prevent them and thereby avert α-synuclein pathology and associated neurodegeneration115.
Of crucial relevance to starting LRRK2 targeting therapies in general and for iPD in particular, is the availability of biomarker tests to probe the activity of the LRRK2 pathway in clinical samples. Several assays exist for this, primarily measuring auto- and substrate phosphorylation to assess LRRK2 kinase activity or measuring LRRK2 phosphorylation at heterologous sites known to impact LRRK2 subcellular distributions and complex formation (reviewed in Rideout et al.59). For instance, by measuring levels of the autophosphorylation site, pSer1292-LRRK2, and the LRRK2 substrate, pThr73-Rab10, it was demonstrated that LRRK2 kinase activity is elevated in nigrostriatal dopamine neurons in post-mortem brain tissue from individuals with iPD116. Moreover, a recent study developed a cytometry based assessment of peripheral blood mononuclear cells (PBMCs) for LRRK2, Rab and GBA, and observed elevated levels of LRRK2 and phosphorylation of Rab10 in PBMC samples from subjects with iPD117. In urinary extracellular vesicles (EVs), several changes have also been observed in patients. Increased pSer1292-LRRK2 in urinary EVs has been observed in specific iPD cohorts118,119, although additional studies did not replicate this120, suggesting that this increase may be linked to a subset of patients. In addition, reduced phosphorylation of the heterologous phosphosites S910-LRRK2 and S935-LRRK2 and increased Rab8 and EV abundance is observed in urinary EVs in iPD120. There is still a gap in the data with respect to CSF measurements of (elevated) pRabs in disease (idiopathic and mutation carriers), which may be, at least in part, due to technical limitations in the measurements of the phosphorylated Rab proteins or in the processing of this sample type. Thus, there is abundant experimental and human data indicating that LRRK2 kinase activity is abnormally increased in iPD and models thereof—and the experimental studies strongly suggest that blocking LRRK2 kinase may be neuroprotective. It should also be noted that a PD risk SNP at the LRRK2 locus has been associated with dysregulated microglial function121.
In sum, these results implicate wild-type LRRK2 in iPD and, for the most part, indicate that LRRK2 kinase activity may be a viable therapeutic target. However, unanswered questions remain. For example, since pre-clinical LRRK2 therapeutics have generally only been tested before or coincident with the toxic insults that form the basis of parkinsonian models, it remains to be determined whether such strategies will also be effective if started once the degenerative process is already well underway, such as at the time of clinical diagnosis of PD.
Another outstanding question in the field is what level of LRRK2 kinase inhibition is required to drive efficacy in the clinic, especially as it pertains to treating patients with iPD. In LRRK2 mutation carriers, the elevation in kinase activity is ~2–4 fold higher than non-mutation carriers, thus is has been hypothesised that inhibiting LRRK2 kinase activity by ~50% would effectively normalise the overactive kinase activity. However, it is not known if this level of inhibition over the duration of a clinical trial will be sufficient. Moreover, it is not known if this level of inhibition will similarly be effective in iPD patients who do not have an activating mutation but have elevated LRRK2 kinase activity. Further compounding this is that the field still does not have a good way to assess CNS LRRK2 target engagement in the clinic. For example efforts to develop a LRRK2 PET tracer or a CSF biomarker have largely been unsuccessful with exception of the recently described CSF assay for assessing total LRRK2 levels122. This lack of understanding around the level of CNS target engagement presents a significant challenge when designing clinical trials for LRRK2 kinase inhibitors where, given the number of subjects required for adequate statistical power, challenges with patient requirement (e.g. LRRK2 G2019S mutation carriers) and potential cost, the number of dose levels of a given compound that can be practically tested in the clinic may be limited, potentially to only a single dose level vs placebo. In the case where an IC50 level of inhibition were to be tested, a negative outcome in the clinic could be interpreted as a need to push to higher levels of target engagement which would require additional clinical trials. One approach to mitigate this challenge could be target higher levels of LRRK2 kinase inhibition from the outset, i.e. IC75 or greater, however, this strategy increases the potential risk of running into treatment emergent adverse events which may impact the overall outcome of the study. Also, given that if LRRK2 therapeutics do modify the course of PD, and would therefore presumably be used for a lifetime after diagnosis, the long-term safety of the specific intervention will be of utmost importance. Based on studies in rodents and non-human primates, and the fact that humans with LRRK2 loss of function mutations are not known to have deleterious health effects123, blockade of LRRK2 kinase activity seems to be reasonably safe, although this remains an area of active investigation. Ultimately, the decision to move forward or not will depend on an informed collaboration between biopharma, regulatory agencies and people with PD.
Conclusion
The LRRK2 field is at an exciting juncture with studies on the biology of this protein that can rely on two decades of groundwork that is increasingly verified and robustly built upon, resulting in clinical trials with LRRK2 targeting agents that are in their early phases. While the temptation may exist to leave the fundamental biology work for what it is and wait out the results of clinical trials that may confirm new clinical solutions for patients, history tells us that work on the fundamental biology of this target is more important than ever in order to be poised to interpret clinical data and prepare the next generation of targeting strategies. We know that a clinical trial may fail with only a small portion of responders, however this may provide the opportunity to work out why a small number respond and others do not.
Acknowledgements
The authors would like to acknowledge fruitful discussions with Prof. Dario Alessi (University of Dundee) and Prof. Suzanne Pfeffer (Stanford University) that were helpful in developing this manuscript. The authors also thank the graphics development team of the Michael J. Fox Foundation for help in designing and generating Fig. 2. Funding support is acknowledged of the Michael J. Fox Foundation (grant 022431) and the National Institutes of Health (grants NS110188 and AG077269).
Author contributions
Specific writing contributions include J.M.T., W.H., and A.M. for the introduction; T.G., W.H., I.P., A.M., and J.M.T. for the section ‘What is the normal vs pathological function of LRRK2?’; A.T., I.P., T.G., J.M.T., and S.P. for the section ‘What about LRRK2 mutations and penetrance?’; T.G., A.T., I.P., W.H., J.M.T., and S.P. for the section ‘LRRK2 pathology: distinct from idiopathic PD?’; T.G., W.H., M.F., H.R., and J.M.T. for the section ‘LRRK2 as a therapeutic target’; T.G., W.H., M.F., and H.R. for the section ‘Treatment of iPD with LRRK2 targeting agents?’; J.M.T., W.H. for the conclusion. S.P., W.H., and J.M.T. contributed to Fig. 1, J.M.T. contributed Fig. 2; A.T., I.P., and J.M.T. contributed Table 1. J.M.T. ensured the coordination of the writing contributions. All authors approved the final manuscript.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Competing interests
Authors J.M.T., T.G., A.M., S.P., I.P., and H.R. declare no financial or non-financial competing interests. Author M.F. is an employee of Merck & Co. Author W.D.H. is an employee of Biogen. Author A.T. declares honoraria from Abbvie and consultation fees from Capsida. Authors M.F., W.D.H. and A.T. declare no non-financial competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Funayama M, et al. A new locus for Parkinson’s disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann. Neurol. 2002;51:296–301. doi: 10.1002/ana.10113. [DOI] [PubMed] [Google Scholar]
- 2.Paisán-Ruíz C, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Zimprich A, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Bosgraaf L, Van Haastert PJM. Roc, a Ras/GTPase domain in complex proteins. Biochim. Biophys. Acta. 2003;1643:5–10. doi: 10.1016/j.bbamcr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 5.West AB, et al. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc. Natl Acad. Sci. USA. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greggio E, et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol. Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Nichols RJ, et al. Substrate specificity and inhibitors of LRRK2, a protein kinase mutated in Parkinson’s disease. Biochem. J. 2009;424:47–60. doi: 10.1042/BJ20091035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paisán-Ruiz C, Lewis PA, Singleton AB. LRRK2: cause, risk, and mechanism. J. Parkinson’s Dis. 2013;3:85–103. doi: 10.3233/JPD-130192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satake W, et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat. Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- 10.Simón-Sánchez J, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat. Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seegobin SP, et al. Progress in LRRK2-associated parkinson’s disease animal models. Front. Neurosci. 2020;14:674. doi: 10.3389/fnins.2020.00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniëls V, Baekelandt V, Taymans J-M. On the road to leucine-rich repeat kinase 2 signalling: evidence from cellular and in vivo studies. Neuro-Signals. 2011;19:1–15. doi: 10.1159/000324488. [DOI] [PubMed] [Google Scholar]
- 13.Kett LR, et al. LRRK2 Parkinson disease mutations enhance its microtubule association. Hum. Mol. Genet. 2012;21:890–899. doi: 10.1093/hmg/ddr526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parisiadou L, et al. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J. Neurosci. 2009;29:13971–13980. doi: 10.1523/JNEUROSCI.3799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drolet RE, Sanders JM, Kern JT. Leucine-rich repeat kinase 2 (LRRK2) cellular biology: a review of recent advances in identifying physiological substrates and cellular functions. J. Neurogenet. 2011;25:140–151. doi: 10.3109/01677063.2011.627072. [DOI] [PubMed] [Google Scholar]
- 16.Erb ML, Moore DJ. LRRK2 and the endolysosomal system in Parkinson’s disease. J. Parkinson’s Dis. 2020;10:1271–1291. doi: 10.3233/JPD-202138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kluss JH, Bonet-Ponce L, Lewis PA, Cookson MR. Directing LRRK2 to membranes of the endolysosomal pathway triggers RAB phosphorylation and JIP4 recruitment. Neurobiol. Dis. 2022;170:105769. doi: 10.1016/j.nbd.2022.105769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonet-Ponce, L. & Cookson, M. R. LRRK2 recruitment, activity, and function in organelles. FEBS J.10.1111/febs.16099 (2021). [DOI] [PMC free article] [PubMed]
- 19.Steger M, et al. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. eLife. 2016;5:1–28. doi: 10.7554/eLife.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonet-Ponce, L. & Cookson, M. R. LRRK2 recruitment, activity, and function in organelles. FEBS J.10.1111/febs.16099 (2021). [DOI] [PMC free article] [PubMed]
- 21.Nichols RJ, et al. 14-3-3 binding to LRRK2 is disrupted by multiple Parkinson’s disease-associated mutations and regulates cytoplasmic localization. Biochem. J. 2010;430:393–404. doi: 10.1042/BJ20100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchand A, Drouyer M, Sarchione A, Chartier-Harlin MC, Taymans JM. LRRK2 phosphorylation, more than an Epiphenomenon. Front. Neurosci. 2020;14:4646–4656.e4. doi: 10.3389/fnins.2020.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dzamko N, et al. Inhibition of LRRK2 kinase activity leads to dephosphorylation of Ser 910 /Ser 935, disruption of 14-3-3 binding and altered cytoplasmic localization. Biochem. J. 2010;430:405–413. doi: 10.1042/BJ20100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui KY, et al. Functional variants in the LRRK2 gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci. Transl. Med. 2018;10:4646–4656.e4. doi: 10.1126/scitranslmed.aai7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu E, et al. Pathological α-synuclein recruits LRRK2 expressing pro-inflammatory monocytes to the brain. Mol. Neurodegener. 2022;17:7. doi: 10.1186/s13024-021-00509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsafaras G, Baekelandt V. The role of LRRK2 in the periphery: link with Parkinson’s disease and inflammatory diseases. Neurobiol. Dis. 2022;172:105806. doi: 10.1016/j.nbd.2022.105806. [DOI] [PubMed] [Google Scholar]
- 27.Wallings RL, Herrick MK, Tansey MG. LRRK2 at the interface between peripheral and central immune function in Parkinson’s. Front. Neurosci. 2020;14:4646–4656.e4. doi: 10.3389/fnins.2020.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tansey, M. G. et al. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol.22, 657–673 (2022). [DOI] [PMC free article] [PubMed]
- 29.Herrick MK, Tansey MG. Is LRRK2 the missing link between inflammatory bowel disease and Parkinson’s disease? NPJ Parkinsons Dis. 2021;7:26. doi: 10.1038/s41531-021-00170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng X, et al. Characterization of a selective inhibitor of the Parkinson’s disease kinase LRRK2. Nat. Chem. Biol. 2011;7:203–205. doi: 10.1038/nchembio.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reith AD, et al. GSK2578215A; a potent and highly selective 2-arylmethyloxy-5-substitutent-N-arylbenzamide LRRK2 kinase inhibitor. Bioorg. Med. Chem. Lett. 2012;22:5625–5629. doi: 10.1016/j.bmcl.2012.06.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Estrada AA, et al. Discovery of highly potent, selective, and brain-penetrant aminopyrazole leucine-rich repeat kinase 2 (LRRK2) small molecule inhibitors. J. Med. Chem. 2014;57:921–936. doi: 10.1021/jm401654j. [DOI] [PubMed] [Google Scholar]
- 33.Henderson, J. L. et al. Discovery and preclinical profiling of 3-[4-(morpholin-4-yl)−7H-pyrrolo[2,3-d]pyrimidin-5-yl]benzonitrile (PF-06447475), a highly potent, selective, brain penetrant, and in vivo active LRRK2 kinase inhibitor. J. Med. Chem.58, 419–432 (2015). [DOI] [PubMed]
- 34.Baptista MAS, et al. LRRK2 inhibitors induce reversible changes in nonhuman primate lungs without measurable pulmonary deficits. Sci. Transl. Med. 2020;12:eaav0820. doi: 10.1126/scitranslmed.aav0820. [DOI] [PubMed] [Google Scholar]
- 35.Fell MJ, et al. MLi-2, a potent, selective, and centrally active compound for exploring the therapeutic potential and safety of LRRK2 kinase inhibition. J. Pharmacol. Exp. Ther. 2015;355:397–409. doi: 10.1124/jpet.115.227587. [DOI] [PubMed] [Google Scholar]
- 36.Jennings D, et al. Preclinical and clinical evaluation of the LRRK2 inhibitor DNL201 for Parkinson’s disease. Sci. Transl. Med. 2022;14:eabj2658. doi: 10.1126/scitranslmed.abj2658. [DOI] [PubMed] [Google Scholar]
- 37.Zhao HT, et al. LRRK2 antisense oligonucleotides ameliorate α-synuclein inclusion formation in a Parkinson’s disease mouse model. Mol. Ther. Nucleic Acids. 2017;8:508–519. doi: 10.1016/j.omtn.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beilina A, et al. Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc. Natl Acad. Sci. USA. 2014;111:2626–2631. doi: 10.1073/pnas.1318306111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eguchi T, et al. LRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasis. Proc. Natl Acad. Sci. USA. 2018;115:E9115–E9124. doi: 10.1073/pnas.1812196115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madero-Pérez J, et al. Parkinson disease-associated mutations in LRRK2 cause centrosomal defects via Rab8a phosphorylation. Mol. Neurodegen. 2018;13:4646–4656.e4. doi: 10.1186/s13024-018-0235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steger M, et al. Systematic proteomic analysis of LRRK2-mediated rab GTPase phosphorylation establishes a connection to ciliogenesis. eLife. 2017;6:4646–4656.e4. doi: 10.7554/eLife.31012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhekne HS, et al. A pathway for Parkinson’s disease LRRK2 kinase to block primary cilia and Sonic hedgehog signaling in the brain. eLife. 2018;7:1–26. doi: 10.7554/eLife.40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mamais A, et al. Mutations in LRRK2 linked to Parkinson disease sequester Rab8a to damaged lysosomes and regulate transferrin-mediated iron uptake in microglia. PLOS Biol. 2021;19:e3001480. doi: 10.1371/journal.pbio.3001480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mortiboys H, Johansen KK, Aasly JO, Bandmann O. Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2. Neurology. 2010;75:2017–2020. doi: 10.1212/WNL.0b013e3181ff9685. [DOI] [PubMed] [Google Scholar]
- 45.Andres-Mateos E, et al. Unexpected lack of hypersensitivity in LRRK2 knock-out mice to MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) J. Neurosci.: Off. J. Soc. Neurosci. 2009;29:15846–15850. doi: 10.1523/JNEUROSCI.4357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herzig MC, et al. LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum. Mol. Genet. 2011;20:4209–4223. doi: 10.1093/hmg/ddr348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baptista MaS, et al. Loss of Leucine-Rich Repeat Kinase 2 (LRRK2) in rats leads to progressive abnormal phenotypes in peripheral organs. PloS one. 2013;8:e80705. doi: 10.1371/journal.pone.0080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herzig MC, et al. High LRRK2 levels fail to induce or exacerbate neuronal alpha-synucleinopathy in mouse brain. PLoS One. 2012;7:e36581. doi: 10.1371/journal.pone.0036581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daher JPL, Volpicelli-Daley LA, Blackburn JP, Moehle MS, West AB. Abrogation of α-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc. Natl Acad. Sci. USA. 2014;2014:1–6. doi: 10.1073/pnas.1403215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puccini JM, et al. Leucine-rich repeat kinase 2 modulates neuroinflammation and neurotoxicity in models of human immunodeficiency virus 1-associated neurocognitive disorders. J. Neurosci. 2015;35:5271–5283. doi: 10.1523/JNEUROSCI.0650-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Härtlova A, et al. LRRK2 is a negative regulator of Mycobacterium tuberculosis phagosome maturation in macrophages. EMBO J. 2018;37:1–17. doi: 10.15252/embj.201798694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boddu R, et al. Leucine-rich repeat kinase 2 deficiency is protective in rhabdomyolysis-induced kidney injury. Hum. Mol. Genet. 2015;24:1–16. doi: 10.1093/hmg/ddv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Derkinderen P. LRRK2 expression in the enteric nervous system: ENSuring its significance. Dig. Dis. Sci. 2017;62:826–827. doi: 10.1007/s10620-017-4500-7. [DOI] [PubMed] [Google Scholar]
- 54.Yan J, et al. LRRK2 deficiency mitigates colitis progression by favoring resolution of inflammation and restoring homeostasis of gut microbiota. Genomics. 2022;114:110527. doi: 10.1016/j.ygeno.2022.110527. [DOI] [PubMed] [Google Scholar]
- 55.Zhu Y, et al. Association between inflammatory bowel diseases and Parkinson’s disease: systematic review and meta-analysis. Neural Regen. Res. 2022;17:344–353. doi: 10.4103/1673-5374.317981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bryce DK, et al. Characterization of the onset, progression, and reversibility of morphological changes in mouse lung after pharmacological inhibition of Leucine-rich kinase 2 kinase activity. J. Pharm. Exp. Ther. 2021;377:11–19. doi: 10.1124/jpet.120.000217. [DOI] [PubMed] [Google Scholar]
- 57.Cook DA, et al. LRRK2 levels in immune cells are increased in Parkinson’s disease. NPJ Parkinson’s Dis. 2017;3:11. doi: 10.1038/s41531-017-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wallings RL, Tansey MG. LRRK2 regulation of immune-pathways and inflammatory disease. Biochem. Soc. Trans. 2019;47:1581–1595. doi: 10.1042/BST20180463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rideout HJ, et al. The current state-of-the art of LRRK2-based biomarker assay development in Parkinson’s disease. Front. Neurosci. 2020;14:4646–4656.e4. doi: 10.3389/fnins.2020.00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kay DM, Kramer P, Higgins D, Zabetian CP, Payami H. Escaping Parkinson’s disease: a neurologically healthy octogenarian with the LRRK2 G2019S mutation. Mov. Disord. 2005;20:1077–1078. doi: 10.1002/mds.20618. [DOI] [PubMed] [Google Scholar]
- 61.Carmine Belin A, et al. Leucine-rich repeat kinase 2 (LRRK2) mutations in a Swedish Parkinson cohort and a healthy nonagenarian. Mov. Disord. 2006;21:1731–1734. doi: 10.1002/mds.21016. [DOI] [PubMed] [Google Scholar]
- 62.Marder, K. et al. Age-specific penetrance of LRRK2 G2019S in the Michael J. Fox Ashkenazi Jewish LRRK2 Consortium. Neurology85, 89–95 (2015). [DOI] [PMC free article] [PubMed]
- 63.Trinh J, et al. Molecular mechanisms defining penetrance of LRRK2-associated Parkinson’s disease. Med. Genet. 2022;34:103–116. doi: 10.1515/medgen-2022-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simpson C, et al. Prevalence of ten LRRK2 variants in Parkinson’s disease: a comprehensive review. Parkinsonism Relat. Disord. 2022;98:103–113. doi: 10.1016/j.parkreldis.2022.05.012. [DOI] [PubMed] [Google Scholar]
- 65.Lee AJ, et al. Penetrance estimate of LRRK2 p.G2019S mutation in individuals of non-Ashkenazi Jewish ancestry. Mov. Disord. 2017;32:1432–1438. doi: 10.1002/mds.27059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trinh J, Guella I, Farrer MJ. Disease penetrance of late-onset parkinsonism: a meta-analysis. JAMA Neurol. 2014;71:1535–1539. doi: 10.1001/jamaneurol.2014.1909. [DOI] [PubMed] [Google Scholar]
- 67.Ruiz-Martínez J, et al. Penetrance in Parkinson’s disease related to the LRRK2 R1441G mutation in the Basque country (Spain) Mov. Disord. 2010;25:2340–2345. doi: 10.1002/mds.23278. [DOI] [PubMed] [Google Scholar]
- 68.Vinagre-Aragón A, et al. A more homogeneous phenotype in Parkinson’s disease related to R1441G mutation in the LRRK2 Gene. Front. Neurol. 2021;12:635396. doi: 10.3389/fneur.2021.635396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crotty GF, et al. Association of caffeine and related analytes with resistance to Parkinson disease among LRRK2 mutation carriers: a metabolomic study. Neurology. 2020;95:e3428–e3437. doi: 10.1212/WNL.0000000000010863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MacLeod DA, et al. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron. 2013;77:425–439. doi: 10.1016/j.neuron.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iwaki H, et al. Penetrance of Parkinson’s disease in LRRK2 p.G2019S carriers is modified by a polygenic risk score. Mov. Disord. 2020;35:774–780. doi: 10.1002/mds.27974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mirelman A, et al. Fall risk and gait in Parkinson’s disease: the role of the LRRK2 G2019S mutation. Mov. Disord. 2013;28:1683–1690. doi: 10.1002/mds.25587. [DOI] [PubMed] [Google Scholar]
- 73.Alcalay RN, et al. Parkinson disease phenotype in Ashkenazi Jews with and without LRRK2 G2019S mutations. Mov. Disord. 2013;28:1966–1971. doi: 10.1002/mds.25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saunders-Pullman R, et al. Progression in the LRRK2-asssociated Parkinson disease population. JAMA Neurol. 2018;75:312–319. doi: 10.1001/jamaneurol.2017.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alcalay RN, et al. Neuropsychological performance in LRRK2 G2019S carriers with Parkinson’s disease. Parkinsonism Relat. Disord. 2015;21:106–110. doi: 10.1016/j.parkreldis.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saunders-Pullman R, et al. Association of olfactory performance with motor decline and age at onset in people with Parkinson disease and the LRRK2 G2019S variant. Neurology. 2022;99:e814–e823. doi: 10.1212/WNL.0000000000200737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pont-Sunyer C, et al. Sleep disorders in Parkinsonian and nonparkinsonian LRRK2 mutation carriers. PloS One. 2015;10:e0132368. doi: 10.1371/journal.pone.0132368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brockmann K, et al. Clinical and brain imaging characteristics in leucine-rich repeat kinase 2-associated PD and asymptomatic mutation carriers. Mov. Disord. 2011;26:2335–2342. doi: 10.1002/mds.23991. [DOI] [PubMed] [Google Scholar]
- 79.Thaler A, et al. Cerebral imaging markers of GBA and LRRK2 related Parkinson’s disease and their first-degree unaffected relatives. Brain Topogr. 2018;31:1029–1036. doi: 10.1007/s10548-018-0653-8. [DOI] [PubMed] [Google Scholar]
- 80.Simuni T, et al. Clinical and dopamine transporter imaging characteristics of Leucine Rich Repeat Kinase 2 (LRRK2) and Glucosylceramidase Beta (GBA) Parkinson’s disease participants in the Parkinson’s progression markers initiative: a cross-sectional study. Mov. Disord. 2020;35:833–844. doi: 10.1002/mds.27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahamadi M, et al. A disease progression model to quantify the nonmotor symptoms of Parkinson’s disease in participants with Leucine-rich repeat kinase 2 mutation. Clin. Pharmacol. Ther. 2021;110:508–518. doi: 10.1002/cpt.2277. [DOI] [PubMed] [Google Scholar]
- 82.Ahamadi M, et al. Development of a disease progression model for Leucine-rich repeat kinase 2 in Parkinson’s disease to inform clinical trial designs. Clin. Pharmacol. Ther. 2020;107:553–562. doi: 10.1002/cpt.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kozlovski T, et al. Hierarchical data-driven analysis of clinical symptoms among patients with Parkinson’s disease. Front. Neurol. 2019;10:531. doi: 10.3389/fneur.2019.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thaler A, et al. Survival rates among Parkinson’s disease patients who carry mutations in the LRRK2 and GBA genes. Mov. Disord. 2018;33:1656–1660. doi: 10.1002/mds.27490. [DOI] [PubMed] [Google Scholar]
- 85.Lanore A, et al. Differences in survival across monogenic forms of Parkinson’s disease. Ann. Neurol. 2023 doi: 10.1002/ana.26636. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, et al. A meta-analysis of GBA-related clinical symptoms in Parkinson’s disease. Parkinson’s Dis. 2018;2018:3136415. doi: 10.1155/2018/3136415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gan-Or Z, et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008;70:2277–2283. doi: 10.1212/01.wnl.0000304039.11891.29. [DOI] [PubMed] [Google Scholar]
- 88.Cilia R, et al. Survival and dementia in GBA-associated Parkinson’s disease: the mutation matters. Ann. Neurol. 2016;80:662–673. doi: 10.1002/ana.24777. [DOI] [PubMed] [Google Scholar]
- 89.Yahalom G, et al. Carriers of both GBA and LRRK2 mutations, compared to carriers of either, in Parkinson’s disease: risk estimates and genotype-phenotype correlations. Parkinsonism Relat. Disord. 2019;62:179–184. doi: 10.1016/j.parkreldis.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 90.Omer N, et al. A possible modifying effect of the G2019S mutation in the LRRK2 gene on GBA Parkinson’s disease. Mov. Disord. 2020;35:1249–1253. doi: 10.1002/mds.28066. [DOI] [PubMed] [Google Scholar]
- 91.Ortega RA, et al. Association of dual LRRK2 G2019S and GBA variations with Parkinson disease progression. JAMA Netw. open. 2021;4:e215845. doi: 10.1001/jamanetworkopen.2021.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wider C, Dickson DW, Wszolek ZK. Leucine-rich repeat kinase 2 gene-associated disease: redefining genotype-phenotype correlation. Neurodegener. Dis. 2010;7:175–179. doi: 10.1159/000289232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kalia LV, et al. Clinical correlations with Lewy body pathology in LRRK2-related Parkinson disease. JAMA Neurol. 2015;72:100–105. doi: 10.1001/jamaneurol.2014.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garrido A, et al. synuclein RT-QuIC in cerebrospinal fluid of LRRK2-linked Parkinson’s disease. Ann. Clin. Transl. Neurol. 2019;6:1024–1032. doi: 10.1002/acn3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Siderowf A, et al. Assessment of heterogeneity among participants in the Parkinson’s progression markers initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 2023;22:407–417. doi: 10.1016/S1474-4422(23)00109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Virreira Winter S, et al. Urinary proteome profiling for stratifying patients with familial Parkinson’s disease. EMBO Mol. Med. 2021;29:4646–4656.e4. doi: 10.15252/emmm.202013257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hadisurya, M. et al. Quantitative proteomics and phosphoproteomics of urinary extracellular vesicles define diagnostic and prognostic biosignatures for Parkinson’s Disease. medRxiv3, 64 (2022). [DOI] [PMC free article] [PubMed]
- 98.Lang AE, et al. Trial of cinpanemab in early Parkinson’s disease. N. Engl. J. Med. 2022;387:408–420. doi: 10.1056/NEJMoa2203395. [DOI] [PubMed] [Google Scholar]
- 99.Hasegawa K, et al. Familial parkinsonism: Study of original Sagamihara PARK8 (I2020T) kindred with variable clinicopathologic outcomes. Parkinsonism Relat. Disord. 2009;15:300–306. doi: 10.1016/j.parkreldis.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rajput A, et al. Parkinsonism, Lrrk2 G2019S, and tau neuropathology. Neurology. 2006;67:1506–1508. doi: 10.1212/01.wnl.0000240220.33950.0c. [DOI] [PubMed] [Google Scholar]
- 101.Henderson MX, Sengupta M, Trojanowski JQ, Lee VMY. Alzheimer’s disease tau is a prominent pathology in LRRK2 Parkinson’s disease. Acta Neuropathol. Commun. 2019;7:4646–4656.e4. doi: 10.1186/s40478-019-0836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chu Y, et al. Is Tau the initial pathology in dopaminergic nigrostriatal degeneration? Studies in Parkinsonism and Parkinson’s disease. bioRxiv. 2022 doi: 10.1101/2022.08.04.502831. [DOI] [Google Scholar]
- 103.Brockmann K, et al. Association between CSF alpha-synuclein seeding activity and genetic status in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol. Commun. 2021;9:1–11. doi: 10.1186/s40478-021-01276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park SA, Jang YJ, Kim MK, Lee SM, Moon SY. Promising blood biomarkers for clinical use in Alzheimer’s disease: a focused update. J. Clin. Neurol. (Korea) 2022;18:401–409. doi: 10.3988/jcn.2022.18.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Daher JPL, et al. Leucine-rich Repeat Kinase 2 (LRRK2) pharmacological inhibition Abates α-Synuclein gene-induced neurodegeneration. J. Biol. Chem. 2015;290:19433–19444. doi: 10.1074/jbc.M115.660001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Obergasteiger J, et al. Kinase inhibition of G2019S-LRRK2 enhances autolysosome formation and function to reduce endogenous alpha-synuclein intracellular inclusions. Cell Death Discov. 2020;6:45. doi: 10.1038/s41420-020-0279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deniston CK, et al. Structure of LRRK2 in Parkinson’s disease and model for microtubule interaction. Nature. 2020;588:344–349. doi: 10.1038/s41586-020-2673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Watanabe R, et al. The in situ structure of Parkinson’s disease-linked LRRK2. Biophys. J. 2020;118:486a. doi: 10.1016/j.bpj.2019.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sejwal K, et al. Cryo-EM analysis of homodimeric full-length LRRK2 and LRRK1 protein complexes. Sci. Rep. 2017;7:8667. doi: 10.1038/s41598-017-09126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Myasnikov, A. et al. Structural analysis of the full-length human LRRK2. Cell10.1016/j.cell.2021.05.004 (2021). [DOI] [PMC free article] [PubMed]
- 111.Guaitoli, G. et al. Structural model of the dimeric Parkinson’s protein LRRK2 reveals a compact architecture involving distant interdomain contacts. Proc. Natl Acad. Sci. USA10.1073/pnas.1523708113 (2016). [DOI] [PMC free article] [PubMed]
- 112.Vaikath NN, et al. Generation and characterization of novel conformation-specific monoclonal antibodies for α-synuclein pathology. Neurobiol. Dis. 2015 doi: 10.1016/j.nbd.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 113.Kumar ST, et al. How specific are the conformation-specific α-synuclein antibodies? Characterization and validation of 16 α-synuclein conformation-specific antibodies using well-characterized preparations of α-synuclein monomers, fibrils and oligomers with distinct struct. Neurobiol. Dis. 2020;146:105086. doi: 10.1016/j.nbd.2020.105086. [DOI] [PubMed] [Google Scholar]
- 114.Takagawa T, et al. An increase in LRRK2 suppresses autophagy and enhances Dectin-1-induced immunity in a mouse model of colitis. Sci. Transl. Med. 2018;10:eaan8162. doi: 10.1126/scitranslmed.aan8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rocha EM, et al. LRRK2 inhibition prevents endolysosomal deficits seen in human Parkinson’s disease. Neurobiol. Dis. 2020;134:104626. doi: 10.1016/j.nbd.2019.104626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Di Maio R, et al. LRRK2 activation in idiopathic Parkinson’s disease. Sci. Transl. Med. 2018;10:eaar5429. doi: 10.1126/scitranslmed.aar5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wallings RL, et al. WHOPPA enables parallel assessment of Leucine-rich repeat kinase 2 and glucocerebrosidase enzymatic activity in Parkinson’s disease monocytes. Front. Cell Neurosci. 2022;16:892899. doi: 10.3389/fncel.2022.892899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fraser KB, et al. Ser(P)−1292 LRRK2 in urinary exosomes is elevated in idiopathic Parkinson’s disease. Mov. Disord. 2016;31:1543–1550. doi: 10.1002/mds.26686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fraser KB, Moehle MS, Alcalay RN, West AB. & LRRK2 Cohort Consortium. Urinary LRRK2 phosphorylation predicts parkinsonian phenotypes in G2019S LRRK2 carriers. Neurology. 2016;86:994–999. doi: 10.1212/WNL.0000000000002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Taymans, J.-M. et al. Alterations in the LRRK2-Rab pathway in urinary extracellular vesicles as Parkinson’ s disease and pharmacodynamic biomarkers. npj Parkinsons Dis.9, 21 (2022). [DOI] [PMC free article] [PubMed]
- 121.Langston RG, et al. Association of a common genetic variant with Parkinson’s disease is mediated by microglia. Sci. Transl. Med. 2022;14:eabp8869. doi: 10.1126/scitranslmed.abp8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mabrouk OS, et al. Quantitative measurements of LRRK2 in human cerebrospinal fluid demonstrates increased levels in G2019S patients. Front. Neurosci. 2020;14:4646–4656.e4. doi: 10.3389/fnins.2020.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Whiffin N, et al. The effect of LRRK2 loss-of-function variants in humans. Nat. Med. 2020;26:869–877. doi: 10.1038/s41591-020-0893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.