Abstract
Due to the limited capacity of the adult mammalian brain to self-repair and regenerate, neurological diseases, especially neurodegenerative disorders and stroke, characterized by irreversible cellular damage are often considered as refractory diseases. Neural stem cells (NSCs) play a unique role in the treatment of neurological diseases for their abilities to self-renew and form different neural lineage cells, such as neurons and glial cells. With the increasing understanding of neurodevelopment and advances in stem cell technology, NSCs can be obtained from different sources and directed to differentiate into a specific neural lineage cell phenotype purposefully, making it possible to replace specific cells lost in some neurological diseases, which provides new approaches to treat neurodegenerative diseases as well as stroke. In this review, we outline the advances in generating several neuronal lineage subtypes from different sources of NSCs. We further summarize the therapeutic effects and possible therapeutic mechanisms of these fated specific NSCs in neurological disease models, with special emphasis on Parkinson’s disease and ischemic stroke. Finally, from the perspective of clinical translation, we compare the strengths and weaknesses of different sources of NSCs and different methods of directed differentiation, and propose future research directions for directed differentiation of NSCs in regenerative medicine.
Subject terms: Neuroscience, Neural stem cells
Facts
Neural stem cells from different sources can be induced to differentiate into mature and functional neurons or glial cells in vitro.
Transplantation of pre-differentiated neural stem cells can differentiate and mature into a specific type of cells, promoting the recovery of neurodegenerative disease or stroke models.
Currently, dopaminergic neurons derived from human embryonic stem cells that undergo the neural stem cells stage are being tested in clinical trials in patients with Parkinson’s disease.
Open questions
For a neurodegenerative disease or stroke, which source of neural stem cells and which directed differentiation method will enable the transplanted cells to meet good manufacturing practices guideline?
What is the optimal time window of the differentiation of neural stem cells for transplantation?
What is the underlying mechanism of cell replacement in transplanted predifferentiated neural stem cells?
Introduction
Neurodegenerative diseases (NDs) are a heterogeneous group of disorders that characterized by progressive and selective losses of neurons [1, 2], resulting in loss of sensation, movement and memory impairment, which are represented by Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS) [3]. Ischemic stroke, the most common type of stroke, causes neuronal and non-neuronal death in the ischemic core due to decreased blood flow to part of the brain. Given enough time, reversible loss of tissue function in the ischemic penumbra can be permanent [4, 5]. These diseases directly threaten the lives of patients and bring a heavy economic burden to family and society [6]. However, current treatments involved in these diseases are not curative and relatively limited, most of which can relieve symptoms and delay the course of diseases [7, 8].
Nerve repair and regeneration therapy is an ideal way to treat neurological diseases. A great deal of work has been done in this area, mainly from both endogenous and exogenous aspects to promote nerve repair and regeneration [9]. Neural stem cells (NSCs) are a class of multipotent cells defined on the basis of their robust self-renewal capacity and ability to differentiate into various central nervous system (CNS) neuronal and glial cell types [10, 11]. Endogenous neurogenesis mediated by NSCs has been shown in several pathological conditions, such as epilepsy, MS, ischemic stroke, and AD [12], but endogenous repair alone is insufficient. NSCs transplantation strategy, as a type of regenerative medicine, has attracted increasing attention in the treatment of NDs [13]. Moreover, as the field of stem cells advances, the source of NSCs for transplantation has expanded from direct isolation of brain tissue initially to differentiation from pluripotent stem cells (PSCs) and transdifferentiation of somatic cells. So far, NSC-based therapies have been implemented in many rodent models of NDs and ischemic stroke, and several studies proposed potential mechanisms to explain the disease-improving effects of NSCs, including neuroinflammation inhibition, neuronal replacement, immunomodulation and neurotrophic support, which promotes the recovery of ND and stroke models [14–18]. Now clinical trials exploring the feasibility of NSCs treatment for neurological diseases are being conducted. Most studies based on NSCs therapy involve direct transplantation of NSCs from different sources into animal disease models.
However, non-negligible challenges of the directly transplanted NSCs are the low survival and irrational differentiation [19–22]. In both NDs and ischemic stroke, chronic or acute activation of innate immune cells in the CNS can be observed [23, 24]. The host micro-environment induced by a neuro-inflammatory response may play a critical role in the survival and differentiation of transplanted NSCs [25–27]. In addition, autophagy, which is involved in inflammatory pathways, has been demonstrated to regulate the differentiation of transplanted NSCs [28]. In animal models of spinal cord injury, transplanted NSCs were influenced by the neurotoxic inflammatory microenvironment and most of them differentiated into astrocytes, resulting in further aggravation [29]. Thus, the inflammatory response may adversely affect the ability of transplanted NSCs to participate in functional recovery. Further, the pathology of NDs is characterized by the selective loss of specific neurons or glial cells in restricted brain regions [30], such as midbrain dopaminergic (DAergic) neuron death in PD, medium spiny γ-aminobutyric acid–mediated (GABAergic) neurons (MSNs) loss in HD, degeneration of cholinergic motor neurons in ALS, and oligodendrocytes loss in MS. Compared with direct transplantation of NSCs, induced differentiation into specific phenotypes may be more amenable to replace lost cells in the CNS.
To overcome the limitations of direct transplanted NSCs and given the pathological features of loss of a specific cell type in some NDs, great efforts have been devoted to explore the feasibility of manipulation of NSCs fate prior to transplantation to control the terminal lineage so as to replace lost cells in NDs [31, 32]. Currently, by using chemical-defined systems or ectopic overexpression of critical lineage-specific transcription factors, NSCs from different sources can be directed to differentiate into a specific type of neural lineage cells in vitro, such as DAergic neurons, GABAergic neurons, cholinergic motor neurons, oligodendrocytes, glutamatergic neurons. And subsequent studies have performed in vivo transplantation of predifferentiated cells to investigate their therapeutic role in neurological diseases (Fig. 1).
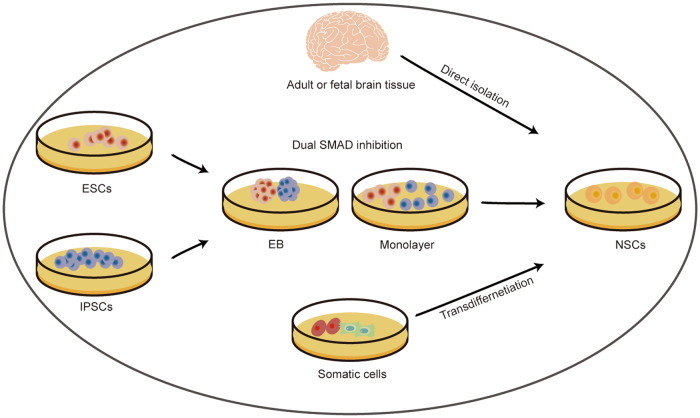
Fig. 1. The directional differentiation of neural stem cells from different sources.
Currently, NSCs can be obtained from three ways: isolate from primary CNS tissues, mainly including adult and fetal brain tissue; differentiate from pluripotent stem cells, including iPSCs and ESCs; transdifferentiate from somatic cells, such as blood cells and fibroblasts. NSCs derived from these three sources can be further processed in vitro to control their fate after transplantation in NDs models, thus replacing the lost cells. In NDs, NSCs can be induced to differentiate into DA neurons after transplantation in a PD model, MSNs after transplantation in a HD model, cholinergic motor neurons after transplantation in an ALS model, and oligodendrocytes after transplantation in a MS model. In acute neurodegeneration, NSCs can be induced to differentiate into cortical glutaminergic neurons or oligodendrocytes after transplantation in ischemic stroke models. After transplantation, pretreated NSCs can mature in the host, stably express specific phenotypes, and integrate into neural circuits to improve the symptoms of ND models. ALS Amyotrophic lateral sclerosis, CNS central nervous system, DA dopamine, ESC embryonic stem cell, HD Huntington’s disease, IPSC induced pluripotent stem cell, MS multiple sclerosis, MSN medium spiny γ-aminobutyric acid–mediated neurons, ND neurodegenerative disease, NSC neural stem cell, PD Parkinson’s disease.
In this review, we summarized strategies for inducing differentiation of NSCs from different sources in vitro. Then, we outlined the functional improvements and underlying mechanisms of the transplanted preconditioned NSCs in PD and ischemic stroke models. What’s more, we also discussed the limitations of the directional induction of NSCs for clinical translation in NDs and ischemic stroke.
The directional differentiation of NSCs from different sources in vitro
At present, NSCs can be derived in three different ways: direct extraction from primary CNS tissues, differentiation of PSCs and transdifferentiation from somatic cells [33] (Fig. 2). NSCs are present throughout the developing brain. And in the adult mammalian brain, NSCs can be found in the subgranular zone of hippocampus, the subventricular zone, and even multiple sites along the entire ventricular system [16, 34, 35]. PSCs, including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), can be induced to differentiate into NSCs in vitro via two main methods: embryoid body (EB) formation and adherent monolayer culture [36, 37]. Specifically, the process of neural differentiation of PSCs is multistep, first triggering differentiation toward all the three embryonic germ layers by removing mediators that promote self-renewal, and subsequently inhibiting extraembryonic and meso-endoderm differentiation and favoring neural differentiation by culturing the cells in serum-free medium [38]. In addition, dual SMAD inhibition, which simultaneously inhibits transforming growth factor β and BMP signaling pathways, can reduce cultural variability and improve the efficiency of neural induction [38, 39]. Intriguingly, both of these neural induction methods of human PSCs closely resemble the neural induction processes in vivo, giving rise to NSCs with dorsal forebrain identity [38–40]. In addition, neural induction of PSCs can also be achieved by coculture with stromal cell feeder layers, which can provide clues to restrict the fate of PSCs towards neural lineage [41–43]. Induced NSCs can be directly reprogrammed from somatic cells, such as peripheral blood mononuclear cells (PBMNCs), fibroblasts and other cell types [44, 45]. NSCs from different sources can be induced to differentiate into desired neural lineage cells.
Fig. 2. Sources of neural stem cells.
Currently, NSCs can be obtained from three ways: 1) isolate from primary CNS tissues, mainly including adult and fetal brain tissue; 2) differentiate from pluripotent stem cells (iPSCs and ESCs) via EB formation or monolayer culture, dual SMAD inhibition can boost the neural induction process; 3) transdifferentiate from somatic cells, such as blood cells and fibroblasts. CNS central nervous system, EB embryoid body, ESC embryonic stem cell, IPSC induced pluripotent stem cell.
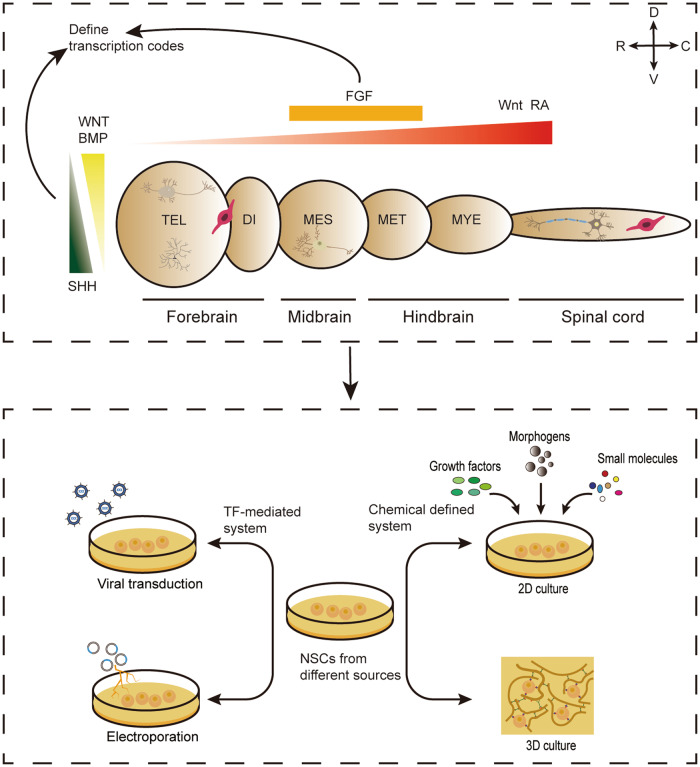
Understanding the natural development of the nervous system is paramount to manipulating the targeted differentiation of NSCs. The embryonic neural tube undergoes a precise patterning process along the dorso-ventral and antero-posterior axes, resulting in the generation of specific neuronal and glial cell subtypes from NSCs. These intricate developmental events are predominantly orchestrated by organizers, i.e., small groups of cells that release patterning molecules to regulate the fate of NSCs small groups of cells that release patterning molecules to regulate cell fate [38, 46]. Patterning molecules involved in the antero-posterior patterning include fibroblast growth factors (FGF), wingless-type MMTV integration site family (WNT), and retinoic acid (RA), while those affecting dorso-ventral mode include WNTs, bone morphogenic proteins (BMPs), and sonic hedgehog (SHH) [38]. The gradients of morphogens can regulate the intrinsic signaling pathways that define transcription codes [38, 47]. Consequently, it is possible to induce differentiation of NSCs from different sources in vitro by mimicking the regional patterning principles of neural development in vivo, So far, two main approaches of induced differentiation have been developed: chemically defined system and intrinsic transcription factor-mediated method, by which the desired neural lineage cell types can be generated, such as DAergic neurons, MSNs, cholinergic motor neurons, oligodendrocytes, and cortical glutaminergic neurons (Fig. 3). Here, we provide an overview on the progress that has been made in generating several neuronal subtypes as well as oligodendrocytes from different sources of NSCs in vitro (Table 1).
Fig. 3. Neurodevelopmental principle for neural lineage subtype specification that guide the directional differentiation of NSCs from different sources in vitro.
A Morphogen gradients, including BMP, WNT, FGF, SHH and RA, define transcription codes of various neural lineage subtypes in corresponding brain regions during early neural development both along the rostral-caudal and dorsal-ventral axes. The depicted neural lineage subtypes include the MSN in ventral TEL, the cortical glutaminergic neuron in dorsal TEL, the DAergic in ventral MES, the motor neuron in spinal cord, the OPC in forebrain and spinal cord. B By using the same chemical or TF patterning principles as seen in vivo, NSCs from different sources can be directional differentiated towards neural lineage subtypes in vitro. The methods of inducing differentiation of NSCs in vitro mainly include external chemical defined system and TF-mediated system. Changes in culture environment include two-dimensional and three-dimensional culture. Overexpression of TFs through viral transduction or non-viral mediated transfection, such as electroporation. BMP bone morphogenic protein, DI diencephalon, FGF fibroblast growth factor, MES mesencephalon, MET metencephalon, MSN medium spiny γ-aminobutyric acid–mediated neurons, MYE myelencephalon, NSC neural stem cell, OPC oligodendrocyte precursor cell, RA retinoic acid, SHH sonic hedgehog, TEL telencephalon, TF transcription factor, WNT wingless-type MMTV integration site family, 2D two-dimensional, 3D three-dimensional.
Table 1.
In vitro differentiation protocols for per neural lineage phenotype and their application in models of neurological diseases.
| Phenotypes | Source of NSCs | Differentiation Protocol | Differentiation factors | Phenotypic markers (% cells) in vitro/vivo | Models | Functional outcome | Reference |
|---|---|---|---|---|---|---|---|
| DAergic neurons | Human fetal VM tissue | Chemical-defined system | BDNF, AA, low oxygen |
40–50% MAP2+ 15% TH+/MAP2+ |
NA | NA | [55] |
| NA | |||||||
| DAergic neurons | Human fetal VM tissue (passage 2) | Chemical-defined system | WNT5 (SHH, FGF8, FGF2 for proliferation) | 35%TH+ | NA | NA | [62] |
| NA | |||||||
| DAergic neurons | Rat embryonic VM tissue | Transfected by electroporation | Nurr1, Brn4 | NA | 6-OHDA PD rats | Increased DA level; Improved rotational behavior | [67] |
|
18%TH+ 14%DAT+ | |||||||
| DAergic neurons | Rat embryonic VM tissue | Transfected by lentivirus | TH, Brn4 |
65.71 ± 5.18%TH+ 32.28 ± 4.39% DAT+ |
NA | NA | [66] |
| NA | |||||||
| DAergic neurons | Rat embryonic VM tissue | Chemical-defined system and transfected by lipofectamine | SHH, FGF8 and Wnt5a | a 20-fold TH+ cells increase | 6-OHDA PD mice | Increased DA level, improved rotational behavior | [60] |
| 9.5% TH+ | |||||||
| DAergic neurons | Rodents embryonic cortical tissue | Transfected by retroviruses | Foxa2, Nurr1 |
37.1% TH+ 55.1% PITX3+/TH+ >78% VMAT2+/TH+ |
6-OHDA PD rats | Exhibited a mature midbrain DAergic neuronal morphology, improved rotational behavior | [73] |
| about 14-fold TH+ cells increase | |||||||
| DAergic neurons | Rats embryonic cortical tissue | Transfected by retroviruses with appropriate vectors and promoters | Foxa2, Nurr1, ca-PKA |
60% TH+/TUJ1+ 80–90% PITX3+/TH+ VMAT2+/TH+ DAT+/TH+ |
6-OHDA PD rats | Exhibited an extremely mature midbrain DAergic neuronal morphology, no rotational behavior improvement | [74] |
|
few TH+ cells <100 cells | |||||||
| DAergic neurons | Primate ESCs (Co-culture with PA6) | Chemical-defined system | NA |
25 ± 6% TUJ1+ 35 ± 6% TH+/TUJ1+ |
6-OHDA PD mice | NA | [43] |
| 0.7% TH+ | |||||||
| DAergic neurons | Human ESCs (Co-culture with PA6) | Chemical-defined system | SHH, FGF8 |
46 ± 8% MAP+ 80 ± 11% TH+/MAP+ 32% TH+ |
NA | NA | [75] |
| DAergic neurons | Mouse ESCs (Co-culture with MS5) | Chemical-defined system | SHH, FGF8 | 50 ± 10% TH+/TUJ1+ | 6-OHDA PD mice | improved rotational behavior | [42] |
| 10–20% TH+ | |||||||
| DAergic neurons | Human ESCs (EB) | Chemical-defined system | SHH, FGF8 |
50–60% TH+/TUJ1+ 31.8 ± 3.1% TH+ |
NA | NA | [77, 79] |
| NA | |||||||
| DAergic neurons | Human PESCs (EB/Dual SMAD inhibition) | Chemical-defined system | SHH C25II, FGF8, PUR and CHIR99021 |
60–80%/70-100% TUJ1+ 20–40%/30-40% TH+ |
MPTP PD primates | Increased DA level, improved rotational behavior | [76] |
| 5.2–8.1% TH+ | |||||||
| DAergic neurons | Human iPSC (EB) | Chemical-defined system | SHH, FGF8 |
30 ± 5% TH+ 100% GIRK2+/TH+ |
6-OHDA PD rats | Improved rotational behavior | [78] |
| ~2% TH+ | |||||||
| DAergic neurons | Human ESCs/iPSCs (Dual SMAD- inhibition) | Chemical-defined system | CHIR99021, FGF8, PUR and SHH-C25II |
±75% TH+ ±50% NURR1+ ±80% FOXA2+ ±60% LMX1A+ |
6-OHDA PD mice/rats MPTP PD primates |
Exhibited excellent DA neuron survival, improved motor deficits. | [84] |
| 6% TH+ (rats) | |||||||
| DAergic neurons | Human ESCs (Dual SMAD inhibition with EB) | Chemical-defined system | CHIR99021, SHH- C24II | NA | 6-OHDA PD rats | Increased DA level, improved motor deficits, showed similar efficacy and potency to fetal DAergic neurons | [82, 178] |
|
54.2 ± 2.5% TH+ 81% LMX1A+/FOXA2+ | |||||||
| DAergic neurons | Human/primate ESCs/iPSCs (Dual SMAD- inhibition) | Chemical-defined system | CHIR99021, FGF8b and SHH- C25II |
43.6 ± 6.2% TH+ 95.3 ± 2.4% NURR1+/TH+ 96.7 ± 1.8% FOXA2+/TH+ 96.5 ± 2.3% LMX1A+/TH+ 56.3 ± 6.7% GIRK2+/TH+ |
NA | NA | [83] |
| DAergic neurons | Human iPSCs (Dual SMAD- inhibition) | Chemical-defined system | CHIR99021, FGF8, and PUR |
42 ± 4.4% TH+ 19.9 ± 6.9% NURR1+ 70–75% FOXA2+ |
6-OHDA PD rats /MPTP PD primates | Improved rotational behavior(rats) increased spontaneous movement, extended dense neurites into the host striatum, increased DA synthesis | [81, 85] |
|
±17% TH+ ±28%TH+/NEUN+(rats) 33.3 ± 24.4% TH+(primates) | |||||||
| DAergic neurons | Human ESCs (Dual SMAD- inhibition) | Chemical-defined system | CHIR99021, FGF8b, SHH- C25II and SAG |
69% TH+ 84% TH+/TUJ1+ >85% GIRK2+/ TH+ |
6-OHDA PD mice | Displayed A9 characteristics, restored functionality of the reconstructed nigrostriatal circuit, improved motor deficits. | [179] |
| 68% TH+/survived | |||||||
| DAergic neurons | Human iPSC (Dual SMAD- Inhibition with EB) Human iNSC | Chemical-defined system | CHIR 99021, FGF8, PUR, BMP5 and BMP7 | 30–50% TH+/TUJ1+ | NA | NA | [86] |
| NA | |||||||
| DAergic neurons | Human ESCs/iPSC (dual SMAD- Inhibition) | Chemical-defined system (3D) | CHIR99021, FGF8b and PUR | 47% TH+ | Fischer rats | NA | [87] |
|
8.12% TH+/transplanted 46.7% FOXA2/ TH+ | |||||||
| DAergic neurons | INSCs reprogrammed from PBMNCs | Chemical-defined system | SAG1, FGF8 |
57.23% TH+ 62.87% TH+/FOXA2 58.69% TH+/NURR1+ 13.84% TH+ 86.78% FOXA2+/TH+ 91.72% NURR1+/TH+ 98.77% GIRK2+/TH+ |
6-OHDA PD mice | Improved rotational behavior | [88] |
| GABAergic neurons | Immortalized striatal human NSC line (STROC05) | Chemical-defined system | PUR |
6.3% DARPP-32+ 46% TUJ+ 27%+ MAP2+ |
NA | NA | [99] |
| GABAergic neurons | Immortalized striatal human NSC line (ST14A) | Chemical-defined system | RA, KCl | 74% GABA+ | QA HD rats | maintained neuronal GABAergic phenotype, established pre- and postsynaptic contacts with endogenous striatal cells, improved motor deficits | [100] |
| GABAergic neurons | Immortalized human NSC line (ReNcell VM) | Chemical-defined system | VPA |
68 ± 4% MAP2+ 90% GABA+/MAP2+ 54% CALB1+/MAP2+ |
NA | NA | [101] |
| DKK1, SHH |
63 ± 4% MAP2+ 96% GABA+/MAP2+ 84% CALB1+/MAP2+ |
||||||
| GABAergic neurons | Human ESCs (EB) | Chemical-defined system | SHH/PUR |
90.2 ± 4.2% GABA+/TUJ1+ 89.7 ± 8.3% DARPP32+/TUJ1+ |
QA HD mice | Projected to the anterior substantia nigra and potentially form connections with DAergic neurons, improved motor deficits | [102] |
|
62.8 ± 2.6% GABA+ 58.6 ± 3% DARPP-32+/ GABA+ | |||||||
| GABAergic neurons | Human iPSCs (Co-culture with PA6) | Chemical-defined system | BDNF |
34.1 ± 4.5% DLX2 27.0 ± 1.7%DARPP-32+ 19.1 ± 2.1% CALB1+ |
QA HD rat | Improved motor deficits | [41] |
| GABAergic neurons | Human ESCs/iPSC (Dual SMAD- Inhibition) | Chemical-defined system | DKK1, SHH-C25II |
±51% MAP2+ ±78% GABA+/MAP2+ ±60.3% CTIP2+/MAP2+ ±86% GABA+/CTIP2+/MAP2+ ±53% CALB1+/MAP2+ ±70.6% CTIP2+/CALB1+/MAP2+ |
QA HD rat | Improved rotational behavior | [103] |
| GABAergic neurons | Human ESCs (Dual SMAD- Inhibition with EB) | Chemical-defined system | XAV939, SAG |
±87% DARPP32+/MAP2+ ±89.5% GABA+/TUJ1+ 80–100% DARPP-32+/GABA+ 80–100% CALB1+/TUJ1+ |
QA HD mice | Improved motor deficits | [104] |
| 48.7 ± 2.8% DARPP32+/hN+ | |||||||
| GABAergic neurons | Human ESCs/iPSC (Dual SMAD- Inhibition) | Chemical-defined system (3D) | PUR, DKK1 |
78%MAP2+ 61% GABA+/MAP2+ 55%DARPP-32+/MAP2+ 70%CTIP2+/MAP2+ 46%CALB1+/MAP2+ 100%CTIP2+/DARPP-32+ |
R6/2 HD mice | Innervated substantia nigra, improved motor deficits. | [105] |
| GABAergic neurons | Human ESCs/iPSCs (Dual SMAD- Inhibition) | Chemical-defined system | Activin A | 20–50%DARPP-32+ | QA HD rats | no motor improvement | [106] |
|
49 ± 5% DARPP-32+/hN+ 86 ± 4.6%GABA+/hN+ 35 ± 8%CALB1+/hN+ | |||||||
| GABAergic neurons | Human ESCs/iPSCs (Dual SMAD- Inhibition) | Chemical-defined system | IWR1 |
±6%DARPP-32+/Map2b+ ±6%DARPP-32+/CTIP2+ ±60 %CTIP2+ |
NA | NA | [107] |
| NA | |||||||
| Cholinergic motor neurons | Human fetal cortical NSCs | Chemical-defined system | FGF2 |
61% HB9+ 50% H9+/ChAT+ |
NA | NA | [114] |
| NA | |||||||
| Cholinergic motor neurons | HB1.F3 human NSC line | Chemical-defined system and transfected by vector | Olig2, SHH | NA | SOD1G93A mutant mice | Migrated into ventral horn, and replaced lost host motor neurons, delayed clinical onset and extended life span. | [233] |
| Cholinergic motor neurons | Mouse ESCs (Co-culture with MS5) | Chemical-defined system | SHH, RA and FGF2 | ±60%HB9+/TUJ1 | NA | NA | [42] |
| NA | |||||||
| Cholinergic motor neurons | Human ESCs, primate ESCs (Co-culture with MS5) | Chemical-defined system | SHH, RA |
20% HB9+(human) 43% HB9+(primate) |
NA | NA | [116] |
| NA | |||||||
| Cholinergic motor neurons | Human ESCs (EB) | Chemical-defined system | FGF2, RA and SHH |
>50% ISL1+/TUJ1+/MAP2+ ±50% HB9+/ISL1/2+ ±21% HB9+ |
NA | NA | [120] |
| NA | |||||||
| Cholinergic motor neurons | Human iPSCs (EB) | Chemical-defined system | PUR, RA |
±60%OLIG2+/SOX3+ ±30%ISL1+/TUJ1+ |
NA | NA | [118] |
| NA | |||||||
| Cholinergic motor neurons | Human iPSCs (EB) | Chemical-defined system | RA, SHH agonist |
20%HB9+ >90%ISL1/2+/HB9+ >50%ChAT+/ISL1/2+/HB9+ |
NA | NA | [119] |
| NA | |||||||
| Cholinergic motor neurons | Human ESCs and iPSCs (EB) | Chemical-defined system | PUR, RA and SAG, |
83 ± 1% TUJ1+ 30 ± 6% ISL1+ 16 ± 5% HB9+ 37 ± 2% ISL1+and HB9+ |
NA | NA | [124] |
| NA | |||||||
| Cholinergic motor neurons |
Human ESCs and iPSCs (Dual SMAD Inhibition with EB) |
Chemical-defined system | BIO, PUR and RA | 40–50%HB9+ | NA | NA | [122] |
| NA | |||||||
| Cholinergic motor neurons | Human ESCs and iPSCs | Chemical-defined system (Dual SMAD inhibition) | SAG, RA and CHIR99021 | 74% HB9+/ISL1+ | NA | NA | [125] |
| NA | |||||||
| Cholinergic motor neurons | Human iPSCs (Dual SMAD inhibition) | Chemical-defined system | CHIR99021, PUR and RA |
90 ± 9% MNX1 + 95 ± 3% ISL1+ 91 ± 6%ChAT+/MAP2+ |
NA | NA | [126] |
| NA | |||||||
| Cholinergic motor neurons | Human iPSCs (Dual SMAD inhibition) | Transfected by lentivirus | NGN2, ISL1, LHX3 |
88.2 ± 3.5% HB9+ 86.5 ± 4.1%ChAT+ |
NA | NA | [121] |
| NA | |||||||
| Cholinergic motor neurons | Human iNSCs (Reprogrammed from PBMNCs) | Chemical-defined system | RA, SAG1 |
14.80 ± 0.90% HB9+ 14.40 ± 1.29% ISL1+ |
NA | NA | [130] |
| NA | |||||||
| Cholinergic motor neurons | Rat iNSCs (Reprogrammed from astrocytes) | Chemical-defined system | RA, SHH | 34.1% ± 2.9% HB9+ | NA | NA | [131] |
| NA | |||||||
| oligodendrocytes | Human fetal diencephalic/telencephalic tissue | Chemical-defined system | FGF2, NT3 and PDGF-AA |
15–20% O4+ 15–20%GalC+ |
Lysolecithin MS mice | Showed limited myelinating capacity | [141] |
| NA | |||||||
| oligodendrocytes | Human fetal brain tissue | Chemical-defined system | FGF2, NT3 and PDGF-AA |
80.5 ± 2.1%A2B5+ 85.4 ± 3.9%O4+ 90%GalC+ |
NA | NA | [140] |
| NA | |||||||
| oligodendrocytes | Human ESCs (EB) | Chemical-defined system | RA, SHH, FGF2, NT3, PDGF-AA and IGF1 |
83.95% PDGFRα+ 91.3%NGN2+ |
Shiverer MS mice | expressed MBP and formed myelin sheaths around nerve fibers | [135, 142] |
| NA | |||||||
| oligodendrocytes | Human ESCs (EB) | Chemical-defined system | RA, PUR/SAG, FGF2, PDGF-AA, T3, low oxygen |
Spinal cord 77 ± 13% NGN2+ 38.5 ± 9.0%O4+ 29.9 ± 5.5%MBP+/O4+ Ventral forebrain 91% ± 7% NGN2+ 43% ± 5% O4+ 29.9 ± 5.5%MBP+/O4+ |
NA | NA | [143] |
| NA | |||||||
| oligodendrocytes | Human ESCs and iPSCs (Dual SMAD inhibition) | Chemical-defined system | RA, SAG, NT3, PDGF-AA and T3 | 44–70% O4 + | Shiverer MS mice | Achieved mature oligodendrocyte differentiation and formed dense compact myelin. | [145] |
| NA | |||||||
| oligodendrocytes | Human iPSCs (Dual SMAD inhibition) | Transfected by lentivirus | SOX10, OLIG2, NKX6.2 |
62.1 ± 9.5%-79.0 ± 14.8% O4 + 30.37 ± 7.87% MBP+/O4 + |
Shiverer MS mice | myelinated the forebrain, remyelinated the demyelinated spinal cord | [146] |
| oligodendrocytes | Human iPSCs (Dual SMAD inhibition) | Transfected by lentivirus | SOX10 | 50–65% O4 + | Shiverer MS mice | myelinated neurons | [147, 234] |
| 48.13 ± 4.15%MBP+ | |||||||
| oligodendrocytes | Human ESCs and iPSCs (Dual SMAD inhibition) | Chemical-defined system | XAV939, PUR, PDGFRα, IGF-1, cAMP and T3 | 35% O4+ | NA | NA | [149] |
| NA | |||||||
| oligodendrocytes | Human ESCs | Transfected by lentivirus | SOX10, OLIG2 |
19.24 ± 3.18% O4+ 81.58 ± 3.94% FOXG1+/O4+ |
[148] | ||
| Cortical glutamatergic neurons | Human ESCs and iPSCs (Monolayer) | Chemical-defined system | Noggin |
<65% TUJ1+ ±60% VGLUT1+/TUJ1+ <75% TBR1+/TUJ1+ <72% CTIP2+/TUJ1+ <18% CTIP2+/TBR1+/TUJ1+ |
NA | NA | [155] |
| NA | |||||||
| Cortical glutamatergic neurons | Human ESCs and iPSCs (Dual SMAD inhibition with monolayer) | Chemical-defined system | FGF2, Vitamin A |
22–29% TBR1+ 25–30% CTIP2+ 28–36% BRN2+ |
NA | NA | [164, 165] |
| NA | |||||||
| Cortical glutamatergic neurons | Human iPSCs (EB) | Chemical-defined system | BMP4, WNT3A and cyclopamine |
62.2 ± 2.1% TBR1+ ±80% VGLUT1+/TUJ1+ |
MCAO rats | Alleviated sensorimotor deficits, differentiated to glutamatergic neurons and form excitatory, glutamatergic synapses | [166, 168, 169] |
| 2.5 ± 0.3% TBR1+ | |||||||
| Cortical glutamatergic neurons | Human ESCs and iPSCs (EB) | Chemical-defined system (3D) | None |
30-40% TBR1+ ±30% CTIP2+ ±10%SATB2 |
NA | NA | [170] |
| NA |
The phenotypes of neural lineages, sources of neural stem cells, differentiation protocols, drivers of differentiation, representative phenotypic markers (in vitro) for evaluating the differentiation efficiency and culture homogeneity, expression of representative phenotypic markers after transplantation into corresponding neurological disease model, and improvement of functional outcomes after transplantation are broadly reviewed.
+ represents the percentage of cells stained positive for a specific marker in the differentiation system (in vitro) or in the transplanted population.
AA ascorbic acid, BDNF brain derived neurotrophic factor, BIO GSK3β inhibitor 6-bromoindirubin-3′-oxime, BMP5 bone morphogenic protein 5, BMP7 bone morphogenic protein 7, BRN2 brain-specific homeobox/POU domain protein 2 (POU3F2), Brn4 brain-specific homeobox/POU domain protein 4, CALB1 calbindin 1, Ca-PKA constitutively active protein kinase A, CHAT choline acetyltransferase, CHIR99021 GSK3β inhibitor, CTIP2 b-cell CLL/lymphoma 11b(BCL11B)/COUP-TF-interacting protein 2 (COUP-TFII), 3D three-dimensional, DA dopamine, DARPP-32 dopamine and cAMP-regulated neuronal phosphoprotein 32, DAT dopamine transporter, DKK1 dickkopf-1, DLX2 distal-less homeobox 2, ESCs embryonic stem cells, EB embryoid body, EGF epidermal growth factor, FGF2 fibroblast growth factor 2/basic fibroblast growth factor (bFGF), FGF8 fibroblast growth factor 8, FGF8b fibroblast growth factor 8 isoform b, FOXA2 forkhead box protein A2, FOXG1 forkhead box protein G1, GABA γ-aminobutyric acid, GalC Galactocerebrosides, GIRK2 G protein-activated inward rectifier potassium channel 2 (KCNJ6), HB9 homeobox HB9/motor neuron and pancrease homeobox 1 (MNX1), HD Huntington’s disease, hN human nucleus, IGF-1 insulin-like growth factor 1, iNSC induced neural stem cells, iPSCs induced pluripotent stem cells, IWR1 a tankyrase/Wnt inhibitor, ISL1 ISL LIM homeobox 1, ISL1/2 ISL LIM homeobox 1/2, LHX3 LIM homeobox 3, MAP2 microtubule-associated protein 2, MBP myelin basic protein, MPTP 1-methyl-4-phenyl-1236-tetrahydropyridine, MS multiple sclerosis, MS-5 stromal cell line derived from irradiated murine bone marrow cultures, NGN2 neurogenin 2, NKX6-2 NK6 homeobox 2, NSCs neural stem cells, NURR1 nuclear receptor related 1 protein, NT3 neurotrophin-3, 6-OHDA 6-hydroxydopamine, OLIG2 oligodendrocyte transcription factor 2, PA6 stromal cell line derived from newborn calvaria tissue of the C57BL/6 mice, PBMNCs peripheral blood mononuclear cells, PD Parkinson’s disease, PGDF-AA platelet-derived growth factor AA, PGDFα platelet-derived growth factor -alpha receptor, PUR purmorphamine, PITX3 paired-like homeodomain 3, QA quinolinic acid, RA retinoic acid, SAG smoothened agonist, SATB2 special AT-rich sequence-binding protein 2, SHH sonic hedgehog, SHH-C24II recombinant human SHH, SHH-C25II recombinant mouse SHH, SMAD transcription factor and member of the BMP and TGF-β signaling pathways, T3 triiodothyronine, TBR1 T-box brain 1, SOX3 SRY box 3, SOX10 SRY box 10, TH tyrosine hydroxylase, TUJ1 neuron-specific class III beta-tubulin (TUBB3), VGLUT vesicular glutamate transporter, VM ventral midbrain, VPA valproic acid, VMAT2 vesicular monoamine transporter 2, WNT5 wingless-type MMTV integration site family 5, WNT5a wingless-type MMTV integration site family 5a, XAV939 WNT/β-catenin inhibitor.
Induction of DAergic neurons from NSCs
Differentiation protocols for DAergic neurons, particularly those targeting midbrain DAergic neurons, have garnered considerable interest in the field of regenerative medicine, owing to their potential to treat PD. Midbrain DAergic neurons are thought to originate from mesencephalic floor plate in embryonic development [48, 49]. The correct establishment of midbrain DAergic precursor domains and the subsequent terminal differentiation of ventral midbrain (VM) DAergic neurons are partly attributed to the synergistic action of regulatory networks controlled by SHH, WNT and FGF [50–52]. In more detail, WNT1 represses the transcription factor Nkx2.2 via the upregulation of Otx2 and the WNT1-Lmx1a autoregulatory loop induces the expression of Lmx1a thus repressing Nkx6-1, both of which promotes the establishment of the midbrain DAergic progenitor domain from ventral mesencephalic NSCs. In addition, the two autoregulatory loop (WNT1-Lmx1a and SHH-Foxa2) induce downstream targets, Pitx3 and Nurr1, which are important factors in the terminal differentiation/survival of midbrain DAergic neurons [51, 53]. FGF8 also provides positional information for the development of midbrain DA neurons [51].
Induction of DAergic neurons from NSCs derived from primary CNS tissues
Initially, NSCs were extracted from embryonic or adult brain tissue for targeted differentiation of DAergic neurons. NSCs emanating from the mouse or human VM have been shown to naturally develop into DAergic neurons in vitro [54], and the addition of neurotrophins, such as brain derived neurotrophic factor (BDNF) and glial cell-line derived neurotrophic factor (GDNF) [55, 56], cyclic adenosine monophosphate [57], BMP2 [58], or cytokines [56] has been demonstrated to facilitated the yield of DAergic neurons. In addition, mitogenic factors, such as FGF2 or epidermal growth factor can amplify NSCs to increase the initial number of NSCs used for differentiation [54, 59]. Unfortunately, it has long been reported that the number of rodents VM-derived NSCs differentiated into DAergic neurons decreased after subculture [60, 61], but unlike their rodent counterparts, human VM tissue exhibits a greater ability to expand and differentiate into DAergic neurons [62]. To overcome the reduced ability of dopamine differentiation after passages, modifications of culture conditions such as lowering oxygen levels to mimic the hypoxic conditions of brain development, or the addition of ascorbic acid (AA) has been shown to be useful measures to increase the differentiation of human DAergic neurons after passages [63, 64]. A study adjusted the culture conditions of long-term expanded human VM NSCs, and increased the generation of tyrosine hydroxylase (TH)-positive cells by around 40 times (7% of total cell) through the combined application of BDNF, AA, low oxygen, and prolonged differentiation time [55]. Furthermore, another study revealed that by applying midbrain-specific instructive signals, SHH, FGF8, and FGF2 to proliferating human VM NSCs, these cells maintained the ability to generating midbrain DAergic neurons and extended differentiation in the presence of WNT5 [62]. In addition to manipulation in external culture conditions, the expression of internal key transcription factors can also be regulated to promote dopamine neuronal production from VM NSCs [65], for example, NSCs were transfected with Nurr1/TH and Brn4 by electroporation or lentivirus [66, 67]. And one of the first approaches to boost the yield of DAergic neurons from VM NSCs was based on the both external and internal manipulation [60]. Several studies have revealed that DAergic neuron-inducing activity is specific to VM derived NSCs [68]. Compared with VM NSCs, NSCs from other brain regions seem to be hardly to differentiate into functional DA neurons and lack the ability to release DA [69, 70], suggesting that VM NSCs and non-midbrain NSCs differ significantly in their responses to dopamine-induced signals, possibly due to non-midbrain NSCs lacking appropriate “priming” epigenetic states [71]. Lee et al. demonstrated that by co-expression of Nurr1 and Foxa2 via retrovirus transfection, non-midbrain NSCs gave rise to midbrain DA neuron phenotypes at late stages of midbrain development [72, 73]. The Nurr1+Foxa2 project has been modified in cortical-derived NSCs to mimic the physiological expression pattern of developmental factors of VM NSCs via selecting appropriate vectors and promoters, thus inducing the generation of completely mature midbrain DAergic neurons [74]. Despite extensive efforts, the quest for efficient differentiation of DAergic neurons from tissue-derived NSCs remains elusive.
Induction of DAergic neurons from NSCs derived from PSCs
In contrast to the CNS-derived NSCs, the targeted differentiation of PSCs derived NSCs has progressed rapidly, especially the generation of DAergic neurons. It was initially reported that co-cultured with PA6 or MS-5 feeder cells, mouse or primate ESCs can be induced to differentiate into NSCs and further TH-expressing neurons effectively [42, 43]. Furthermore, SHH and FGF8 can provide lineage-specific instructions to enhance the generation of DAergic neurons [42, 75]. Gradually, studies have shown that based on the EBs formation coupled with the action of SHH and FGF8, TH-positive DAergic neurons can be induced successfully from human PSCs [76–79]. With a better understanding of the pattern molecules and transcriptional networks involved in the generation of DAergic neurons in the midbrain during embryonic development, the differentiation protocol has been modified over time. The activation of WNT signaling involved in early caudalization of the cells in the neural plate, was mimicked by the glycogen synthase kinase 3β inhibitor CHIR99021 in vitro, which resulted in an improved midbrain specification reliably and efficiently [76, 80–85]. Compared with DAergic differentiation via a neural rosette intermediate (i.e., the differentiation protocol using SHH and FGF8 only), the DAergic neurons generated from the floor plate were more efficient, both in number and midbrain markers, that is, the number of cells co-expressing TH and fox2 accounted for about 75% of the culture [84]. It is well known that the BMP/SMAD inhibition is used to promote the neural induction of PSCs, but more recently BMP activation has been found to be helpful in the specification of DAergic neurons [86]. A study has revealed that in vitro application of BMP5/7 during the maturation phase can effectively promote the generation of VM DA neurons [86]. Furthermore, the culture system has expanded from two dimensional (2D) to three dimensional (3D) platforms, where cells can be embedded in biomaterials for 3D culture. Schaffer and colleagues have shown that in a 3D thermoresponsive biomaterial platform, by applying the same small molecules used to induce differentiation as in the 2D system, a higher number of TH-positive neurons (~40%) could be generated rapidly after 25 days of differentiation than in their 2D culture control (20%) [87] or other 2D culture systems (15–30%) [84], and these cells exhibited temporal marker expression profiles that resemble natural VM DAergic development [87].
Induction of DAergic neurons from NSCs derived from somatic transdifferentiation
DAergic differentiation of induced NSCs (iNSCs) is infancy and there are not as many DAergic differentiation protocols as the other two types of NSCs s. Induced NSCs from PBMNCs can be induced into mature DAergic neurons through a two-stage method. The first stage mediated the generation of DA progenitors mainly through FGF8 and SAG1, a SHH pathway agonist, and the second stage promoted the maturation of DA neurons through a combination of BDNF, GDNF, TGF-β3, AA and other soluble factors [44, 88, 89] . At the end of differentiation, about 60% of cultured cells co-expressed Foxa2 and TH. Moreover, unlike the characteristics of tissue derived NSCs, induced NSCs retained their dopamine differentiation ability after multiple passages [88]. In addition to NSCs derived from PBMNCs, NSCs transdifferentiated from fibroblasts have also been used for DAergic neuron induction, and the DAergic induction protocols of both were similar. The difference is that the addition of BMP5/7 in the maturity phase further increased the generation of DAergic neurons [86].
Induction of MSNs from NSCs
MSNs in the striatum are the most affected cell type in HD, making them the most suitable target cell type for cell replacement therapy [90]. During embryonic development, the lateral ganglionic eminence (LGE) of the ventral telencephalon gives birth to the MSNs [91]. The concentration gradient of SHH, WNT and BMP can affect the pattern of the dorsal ventral axis, that is, SHH promotes ventral localization of the neural tube and WNT and BMP promote dorsal localization [46]. And the LGE specification is patterned under the impact of antagonistic morphogen gradients. In detail, the repression of WNT together with SHH can efficiently induce the ventral fate of the telencephalic precursors. Furthermore, based on the expression of activin receptors and phosphorylated SMAD2 (an activin signaling pathway component) in the developing LGE, it is speculated that the activation of TGF-β pathway is also involved in the generation of striatum MSN [92]. DLX2 and GSX2 are markers of LGE [93], and MSNs are further characterized by expressed dopamine and cAMP-regulated neuronal phosphoprotein 32 (DARPP-32) and a range of other subtype-specific markers [94, 95]. So far, the generation of MSNs has been primary induced from NSCs from the first two sources, and there is no induction protocol for obtaining MSNs from iNSCs.
Induction of MSNs from NSCs derived from primary CNS tissues
NSCs isolated from fetal ganglion eminences can generate up to 25% of DARPP-32 positive neurons in vitro [96, 97], but similar to midbrain DAergic differentiation, this characteristic declines with culture time [98]. Possibly because of previous exposure to a favorable microenvironment, primary tissue-derived NSCs can be predisposed to adopt a specific phenotype, and these predispositions may be largely lost or offset by in vitro cell expansion. Therefore, it may be necessary to provide external cues to support or even increase the MSN differentiation ability of NSCs after passage, such as morphogens [99] and growth factors [97, 99]. A study explored the MSN differentiation conditions of immortalized striatal human NSC line. They compared the chemical induction systems of SHH, SHH/dickkopf-1 (DKK1)/BDNF, Dibutyryl cAMP/valproic acid (VPA)/BDNF, RA, and Purmorphamine, and found that the hedgehog agonist Purmorphamine most remarkably increased the MSN differentiation of NSCs, doubling the number of MSN in the short-term differentiation and tripling the number of MSN in the long-term differentiation [99]. In addition, sequential RA treatment and KCl depolarization can effectively yield 74% functional GABAergic neurons from the immortalized striatum NSCs [100]. Recently, another study found that striatal GABAergic neurons could be reliably induced from immortalized VM NSCs under hypoxic culturing conditions using two- or three-step differentiation protocol based on VPA or SHH and DKK1, respectively [101]. A majority of cultured cells expressed MSN markers and functional glutamate receptors, in addition to releasing GABA on stimulation [101].
Induction of MSNs from NSCs derived from PSCs
It is acknowledged that neural induction via EB formation and subsequent exposure to SHH could drive ventral telencephalic fate in human ESCs. Further exploration found that medium dosage of SHH can pattern NSCs into LGE-like progenitor cells, which generate predominantly DARPP32-expressing GABA neurons, ~75% of the total number of cells in culture [102]. Jeon and colleagues induced the generation of NSCs by co-culturing ESCs and iPSCs derived from a patient with juvenile HD with PA6 stromal cells and subsequently producing 27% of DARPP-32 neurons in the presence of BDNF. Additionally, DARPP-32 can be co-localized with LGE markers DLX2 and GSX2, indicating successful generation of the MSN-like cells [41]. In another protocol, neural induction of human ESC and iPSCs was achieved via dual SMAD inhibition, followed by exposure to SHH and WNT inhibitor DKK1, which patterned NSC towards LGE progenitors [103]. A recent study has optimized this protocol by using small molecules to replace protein components, using a chemical cocktail to quickly and efficiently generate GABAergic MSNs from human ESCs [104]. Similar to the improved differentiation protocol of DAergic neurons, a study used a 3D culture method for neural induction and neural specification of ESCs, then matured on 2D laminin-coated plates. The MSNs generated by this 3D-2D method showed electrophysiological activity compared with those generated by 2D method [105]. Interestingly, the addition of Activin rather than SHH also induced the LGE-like progenitor fate after neural induction via dual SMAD inhibition, and the data showed that activin-mediated LGE fate was independent of SHH signaling [106]. Furthermore, another differentiation protocol did not apply SHH or Activin to induce the LGE-like progenitor fate, but continued to inhibit the BMP and WNT signaling pathways via dual SMAD inhibition and the use of IWR1 to regionalize NSCs after neural induction in ESCs [107].
Induction of cholinergic motor neurons from NSCs
Motor neurons can generally be divided into two categories, depending on the location of the cell body: (I) Upper motor neurons that are located in the cerebral cortex, and (II) lower motor neurons that exist in the brainstem and spinal cord [108, 109]. The differences between the two types of motor cells are not limited to their location, but also manifest in neurotransmitters, targeting, and characteristics upon lesion (reviewed in refs. [108, 109]). Spinal MNs are patterned in the highly restricted foci of ventral neural tube in response to morphogens RA, FGFs, and SHH [110]. In more detail, caudalization of the neural tube is primarily facilitated by RA, produced via the activity of retinaldehyde dehydrogenase 2 [111]. And SHH allows specification of ventral part in the neural tube [112]. The temporal and spatial action of these extrinsic morphogens induce the upregulation of the basic helio-loop-helix (bHLH) transcription factor Olig2, which together with another bHLH transcription factor neurogenin 2 directs the expression of MN fate determining genes such as Islet1 and Hb9 [109, 110]. ALS and other motor neuron diseases characterized by motor neuron injury often result in muscle wasting and even paralysis, and the desire to protect and eventually regenerate motor circuits has prompted attempts to generate motor neurons for translational applications [112]. Here we focus our attention exclusively on the induction protocols for NSCs-derived spinal motor neurons, namely cholinergic motor neurons.
Induction of cholinergic motor neurons from NSCs derived from primary CNS tissues
To date, there has been little exploration of spinal motor neurons differentiation protocols from CNS tissue-derived NSCs. FGF2 is well recognized as a mitogen in the CNS, but it has been shown that FGF2 can direct the differentiation of NSCs into spinal motor neurons [113, 114]. In induction medium supplemented with FGF2, about 60% of human fetal forebrain-derived NSCs differentiated into H9 immunopositive cells on day 10, which supports the dual functions of FGF2, i.e., at high concentrations FGF2 primarily serves as a mitogen for NSCs, while at low concentrations it promotes neurogenesis [114]. Furthermore, immortalized telencephalic NSCs transduced Olig2 via retroviral vector expressed motor neuron-specific phenotypes following treatment with SHH, such as Hb9, Islet1 and choline acetyltransferase [115].
Induction of cholinergic motor neurons from NSCs derived from PSCs
Compared with CNS tissue-derived NSCs, the motor neuron differentiation protocol of PSC derived NSCs has been widely investigated. Initial protocols for the differentiation of functional cholinergic motor neurons from ESCs have also heavily relied on the use of stromal feeder cells [42, 116]. Furthermore, studies have differentiated NSCs from PSCs via EB formation, followed by treatment with RA and SHH to induce cholinergic motor neuron generation successfully [117–120]. Gradually, most studies have used dual SMAD inhibition or combined with EB formation to accelerate the neuralization of PSCs [121–124]. In addition, some studies optimized ventral and caudal signaling molecules to promote the induction efficiency of motor neurons, for example, Maury et al. activated WNT signal via exposure to appropriate concentration of CHIR to cooperate with RA in caudal optimization, resulting in 80% of cells expressing the MN progenitor cell marker Olig2 [125]. Similarly, a single concentration of ventral morphogen SHH results in a mix of Olig2-expressing motor neuron progenitors with NKX2.2 -expressing interneuron progenitors residing in the adjacent domains. Du et al. used a combination of SHH (induced the Nkx2.2- and Olig2-expressing progenitors) and CHIR (antagonized the induction of Nkx2.2 expression by SHH) to enrich Olig2+/Nkx2.2−MN progenitors, resulting in a purity of more than 90% of motor neurons [126]. Patani et al. described a retinoid-independent protocol for the cadualization of human ESCs based on activin/nodal signaling inhibition, which resulted in the bias to medial motor columnar pools [117]. In addition to exposure to different combinations of patterning molecules that regulate intrinsic transcription factors to induce the generation of motor neurons, some studies have directly transfected transcription factors Neurog2, Islet1, and Lhx3 into human PSCs-derived NSCs via retroviral vectors to promote motor neuron production, which is simple, reliable and efficient [121, 127–129].
Induction of cholinergic motor neurons from iNSCs
Currently, few studies have reported cholinergic motor neurons differentiation protocols starting from iNSCs. INSCs can be reprogrammed from astrocytes or PBMCs, and then patterned by RA and SHH in a chemical defined system to confer caudal and ventral anatomical identities, respectively, finally gave rise to Olig2 expressing progenitors. Finally, these progenitors mature into motor neurons under the action of growth factors [130, 131]. But neither protocol was efficient, producing about 15% and 35% HB9-positive cells, respectively [130, 131].
Induction of oligodendrocytes from NSCs
Oligodendrocytes are glial cells that form myelin sheaths around axons in the CNS, supporting rapid nerve conduction and providing trophic and metabolic support to neuronal cells [132]. During neural development, NSCs give rise to oligodendrocyte precursor cells (OPCs), which are patterned in different regions of the neural tube, such as the ventral and dorsal sides of both spinal cord and forebrain [132–134]. Caudalization of the neural tube is modulated by RA, followed by the generation of Olig2-expressing spinal progenitors in response to ventral signal SHH, which are a source of both motoneurons and OPC [132, 135]. After the generation of motor neurons, Olig2-expressing spinal progenitors downregulate neurogenic transcription factors, and give rise to OPCs that express the oligodendroglial transcription factors Nkx2.2 and Sox10 and the surface markers, such as A2B5, platelet-derived growth factor receptor alpha (PDGFRα) and membrane proteoglycan NG2 [132]. These OPCs differentiate into immature oligodendrocytes expressing marker O4 and further become mature oligodendrocytes expressing myelin marker myelin basic protein [132, 134]. Not only the ventral source, a small number of oligodendrocytes also originate from the dorsal neural tube that is independently of SHH, but Olig2 expression is requisite for dorsal OPC specification [136, 137].
Induction of oligodendrocytes from NSCs derived from primary CNS tissues
Genetic modification and culture environmental modification of human NSCs has been tested for the sake of obtaining cell populations enriched in oligodendroglia. One of the first approaches to induce oligodendrocytes from human fetal NSCs was based on the overexpression of the bHLH transcription factor Olig2 via lentiviral vectors. This protocol allowed an increased number of A2B5-positive oligodendroglial precursors in vitro, but fully committed O4-positive oligodendrocytes were not detected after 7 days of differentiation [138]. In a chemically defined system, it has been reported that a combination of FGF2, neurotrophin-3 (NT3) and platelet-derived growth factor-AA (PDGF-AA) successfully increased the proportion of oligodendrocytes expressing O4 and GalCer to 15–20% of the total culture cells from embryonic forebrain-derived NSCs [139]. And another protocol used a similar cocktail combination, resulting in highly pure OPCs from human fetal NSCs. In this study, up to 80–90% of culture cells expressed OPC markers O4, Sox10 A2B5, and PDGF-αR, and about 90% of the cells expressed GalCer with further differentiation [140]. The difference in the efficiency of oligodendrocyte production between the two protocols may be due to the origin of NSCs, as well as the difference in the concentration and duration of action of these factors [139, 140]. In addition, a study committed fetal forebrain-derived NSCs to oligodendrocyte phenotypes by adding PDGF-AA, FGF2, SHH, triiodothyronine, and NT-3, followed by the removal of four factors (PDGF-AA, FGF2, SHH, and NT-3) that promoted the expression of final markers of oligodendrocyte differentiation, with more than half of cultured cells expressing myelin basic protein [141].
Induction of oligodendrocytes from NSCs derived from derived from PSCs
Thus far, there have been many attempts to generate oligodendrocytes from human PSCs-derived NSCs. One of the first protocols to induce oligodendrocytes from human ESCs was based on the EBs formation in combination with the activation of RA, SHH and FGF2 signaling [133, 142]. Specifically, ESCs-derived NSCs were patterned to progenitor cells expressing Olig2 and Nkx2.2 in the presence of RA and SHH, and subsequent treatment with FGF2 can inhibit motor neuron differentiation to increase pre-OPCs during the neurogenic phase. Finally, the removal of FGF2 and the addition of PDGF-AA, insulin growth factor 1 and NT3 promotes the transition of pre-OPCs to OPCs, and ~80% of cultured cells expressed OPC markers, such as PDGFRα and NG2 [133, 135, 142]. But unfortunately, this induction protocol takes a long time, at least 3 months, to generate OPCs from human PSCs [133, 142]. To address the limitation of long differentiation time, some groups have modified the induction protocol. Franklin et al developed a physiological oxygen tension protocol to generate oligodendrocytes from human ESCs under low oxygen conditions, mimicking the environment of the developing brain [143]. And the results indicated that hypoxic conditions could not only accelerate the overall differentiation process, but also significantly improve oligodendrocyte production [143]. Fossati and colleagues induced the generation of NSCs by using dual SMAD inhibition rapidly and modified the previous oligodendrocyte differentiation conditions slightly, thus speeding up the timetable of glial induction and resulting in most of cultured cells displaying the late OPC marker O4 [144, 145]. However, these optimized protocols shorten the differentiation cycle to 70 days at most, and primary rate-limiting steps are oligodendroglial specification and differentiation, some studies have accelerated the generation of oligodendrocytes by overexpression of transcription factors. More recently, an effective strategy that facilitated the generation of O4- expressing oligodendrocytes to 70% within 28 days of differentiation by using a combination of three transcription factors, Olig2, Sox10, and Nkx6.2 has been reported [146]. Verfaillie and colleagues described that overexpression of a single transcription factor, Sox10, was sufficient to generate similar levels of O4+ cells from human PSCs derived NSCs within 22 days [147]. In addition to the generation of spinal cord OPCs and oligodendrocytes via the use of caudal morphogen RA, several RA-independent approaches that favor telencephalic OPC generation have been reported [143, 148, 149]. A study accelerated the production of RA-independent telencephalic oligodendrocytes by enhancing neural induction using dual SMAD inhibition in conjunction with the tankase inhibitor XAV 939 (antagonizing WNT signaling) [149]. More recently, Xiong and colleagues first promoted the generation of ventral forebrain NSCs of ESCs by dual SMAD inhibition and activation SHH signals, and subsequently demonstrated that overexpression of Sox10 and Olig2 in these cells was sufficient to generate forebrain mature oligodendrocytes at day 40 of differentiation [148].
Induction of cortical glutamatergic neurons from NSCs
Glutamatergic pyramidal neurons are the vast majority of excitatory nerve cells in the cerebral cortex that mediate myriad information processing streams and output channels [150]. In brain development, all cortical glutamatergic neurons originate from the embryonic dorsal telencephalon [151], which is patterned by WNTs and BMPs that are derived from the cortical hem [152, 153]. Early dorsal forebrain primordium co-express Pax6 and Otx1/2, and over time, these cells differentiated into cortical glutamatergic neurons, displaying unipolar and pyramidal morphology, and expressing TBR1, CTIP2, and vesicular glutamate transporters [154, 155]. Glutamatergic neurogenesis is also present in adult neurogenic niches, SVZ and SGZ [156, 157]. Interestingly, the pattern of transcription factor expression during adult glutamatergic neurogenesis is akin to the sequential expression of transcription factors in cortical glutamatergic neurons during the embryonic period, indicating that the genetic program specifying the fate of glutamate is spatially and temporally conserved [156].
Induction of cortical glutamatergic neurons from NSCs derived from primary CNS tissues
Existing differentiation protocols tend to generate a mixture of cortical neurons from primary CNS tissue derived NSCs, rather than differentiating specifically into cortical glutamate neurons. NSCs isolated from cortex of human fetuses retained their regional identity and differentiated primarily into cortical GABAergic interneurons and glutamatergic neurons after the removal of the mitogen [158]. In addition, immortal fetal cortical NSCs lines also showed similar differentiation characteristics [159, 160], and a cortical human NSCs line CTX0E16 generated about 40% CTIP2-positive cells with typical pyramidal neuron morphology in vitro [159]. More recently, a study indicated that embryonic mouse dorsal cortical derived NSCs developed towards cortical glutaminergic neurons under FGF at below proliferative concentrations, possibly due to the endogenous and transient wave of BMP signals induced by low FGF2 [161].
Induction of cortical glutamatergic neurons from NSCs derived from derived from PSCs
As we mentioned earlier, forebrain identity is the default procedure after neural induction of PSCs, and it has been shown that human PSCs predominantly differentiate into dorsal telencephalic NSCs after neural induction without the need for additional patterning morphogens, which is attributed to endogenous WNT signaling [153, 162]. In addition, several differentiation protocols, involving either EB formation or monolayer culture, enhanced neural induction of human PSCs through the use of the BMP inhibitor Noggin, thereby increasing the yield of cortical glutamate-like neuron production [155, 163], and most of these cells generated here exhibited an identity corresponding to deep layers rather than upper layers, which was determined by the expression of layer-specific markers during the process of differentiation [155]. In contrast, another differentiation protocol described that the equivalent proportions of deep and upper layer neurons can be generated from human PSCs when combined with dual inhibition of SMAD signaling and retinoic acid signaling [164, 165]. Nevertheless, recent studies have shown that the use of cyclopamine (an inhibitor of SHH) can promote the population of cortical glutamate neurons by inhibiting ventral differentiation from iPSCs [166–169]. Compared with 2D induction protocols, the generation and maturation of cortical glutamatergic neurons were further promoted by cultivating human PSCs derived NSCs in the PDMS-based 3D culture system [170].
Therapeutic potential of induced directed differentiation of NSCs in neurological disease models
As mentioned above, NSCs have been directed to differentiate into specific lineages of cells expressing corresponding transcription factors and markers (Table 1), displaying cellular morphology, as well as manifested by electrophysiological properties in vitro [171, 172]. Furthermore, whether predifferentiated NSCs can survive, stably express the desired cell subtypes, and functionally integrate into the host brain of neurological models are increasingly being investigated (Table 1). The main neurological disease models currently involved include PD, HD, MS, ALS, and ischemic stroke. Here we primarily outlined the therapeutic potential of NSCs-derived specific phenotypes in chronic neurodegenerative disease PD and acute neurodegeneration ischemic stroke.
Parkinson’s disease
PD is a progressive neurodegenerative disorder characterized pathologically by the degeneration of DAergic neurons in the substantia nigra pars compacta, with a subsequent loss of DAergic axon terminals innervating the striatum, which results in motor disorder [173]. Currently, the main treatment for PD is dopamine replacement therapy. However, it can only relieve the symptoms of PD without delaying the progression of PD [174]. Regarding to the success of targeted differentiation of VM DAergic neurons in vitro, some studies have transplanted these cells into different models of PD to observe their therapeutic effects at the behavioral, cellular, and molecular levels.
Most of the studies mentioned above implanted pre-differentiated NSCs into the striatum of 6-OHDA or MPTP-induced PD models, resulting in sensorimotor improvements, such as increased spontaneous activity and reduced circling behavior [60, 67, 73, 76, 78, 81, 82, 84, 85, 88]. Ectopic implantation is considered given that VM tissue grafts placed in the substantia nigra are unable to extend axons long enough to reach their target area, the striatum, to form complex neural circuits [42, 175–177]. After implantation, some of these preconditioned NSCs could survive in the PD animal model and exhibit a phenotype of nigra DAergic neurons, with increased dopamine levels [60, 67, 73, 76, 81, 82, 84, 85, 178]. The development of experimental techniques over the past decade has led to a refined understanding of how transplanted cells integrate with circuitry of the host nervous system [179]. Previous optogenetic and electrophysiological studies have demonstrated that ectopically transplanted NSCs-derived midbrain DAergic neurons are spontaneously active and receive appropriate presynaptic input from the host [180, 181], and the recent availability of the monosynaptic rabies tracing technique allowed us to further investigate the sources and extent of host synaptic inputs comprehensively [182]. For example, it has been shown that despite the ectopic intrastriatal location of NSCs-derived midbrain DAergic neurons, they can receive excitatory and inhibitory inputs from host cortical, striatal and pallidum neuronal subtypes, which are acknowledged to modulate the function of endogenous DAergic neurons in the substantia nigra, and this host afferent pattern helped to explain the proper regulation of DA release in “ectopic” intrastriatal VM-patterned grafts [183]. But the ectopic location of grafted cells may hinder the maximization of their function due to incompatibility with the physiological anatomy. To achieve a more complete circuit repair, homotopic transplantation to the substantia nigra is gradually being performed in rodent PD models [178, 179, 183]. Using VM DAergic cells derived from green fluorescent protein transgenic mouse embryos, earlier studies demonstrated the anatomical and functional reconstruction of nigrostriatal pathway after homotopic transplantation [175, 184]. Subsequently, it was observed that by using species-specific antibodies or genetic labeling, VM DAergic cells derived from NSCs innervated caudate putamen or prefrontal cortex, the main target of endogenous substantia nigra pars compacta (A9) or ventral tegmental area (A10) VM DAergic neurons, indicating that the grafted human VM DAergic neurons are the mixture of A9 and A10 phenotypes [179, 185, 186]. Interestingly, the innervated areas of intrastriatal VM DAergic neuron grafts were almost identical to those of the intranigral grafts, suggesting that the graft path-finding and target projection were largely determined by the cell-intrinsic factors [179, 183]. Therefore, the present studies showed that both ectopic and homotopic transplanted VM DA neurons can perform presynaptic and postsynaptic integration, and it was natural to assume that the reconstructed functional nigra-striatal circuit resulted in motor recovery in PD models, as demonstrated by the use of optogenetic and chemogenetic tools [179, 180, 187].
In addition to the transplant location, the optimal time window of DAergic neuron differentiation for transplantation is also important for the reconstruction of the nigra-striatal circuit [188, 189]. Using VM tissue as the donor, the maturity of the donor cells at transplantation significantly affected the grafts composition and functional outcomes. Specifically, donor tissue isolated before the peak of DA neurogenesis, embryonic day (E) 12, produced more DAergic neurons in the graft, which was attributed to increased DA neuroblasts survival and proliferation at the time of implantation [190–192]. Further studies on embryonic development showed that different DAergic subtypes originated from different progenitor pools and had different birth dates, with A9 DAergic neurons emerging earlier during DA neurogenesis [193–195], thus grafting of younger ventral midbrain donor tissue (E10) enriched A9 population and enhanced motor recovery [189, 193]. This may also be the reason why some studies have selected younger donor tissues for DAergic differentiation of ventral midbrain derived NSCs, and these pretreated VM NSCs grafts showed a higher TH-positive cell survival rate than fetal VM tissue grafts [60]. Currently, despite advanced protocols for the targeted differentiation of NSCs from two other sources into DAergic progenitor cells suitable for transplantation [42, 76, 78, 82, 84, 85, 88, 178, 179] and their rapid transition to clinical trials [196], the optimal stage of differentiation for transplantation has rarely been explored. On the one hand, the more mature the cells are in vitro, the more fragile they are and the more difficult they are to survive after transplantation. On the other hand, a higher degree of stemness is responsible for a greater chance of survival, but may result in insufficient regional regulation to generate mature DAergic neurons in vivo [188]. Therefore, most studies have focused on transplanting their derived VM progenitor cells at intermediate stages of differentiation, aiming to balance the ability to survive and mature [82, 84, 88, 186, 197]. A recent study attempted to transplant progenitor cells at different times of DAergic differentiation to determine the most appropriate transplantation time window, and it was surprising to find that grafts derived from younger progenitor cells consisted of the highest proportion of VM DAergic neurons and the lowest proportion of non-target cell types, showing intensive innervation ability as well as increased DA levels [188]. Although the donor age effect was observed in both human PSC lines, there was some variability across cell lines, highlighting the significance of characterizing cells in vivo on the basis of different cell lines or standardizing differentiation protocols [188]. Furthermore, in this study, some commonly used mesencephalic floor plate markers were used to determine the optimal differentiation time window of transplanted cells, such as FOXA2 and OTX2 [188]. But by using RNA sequencing, another study found that the high DAergic yield and their functional maturation in vivo positively correlated with a specific group of markers associated with the caudal midbrain, rather than the levels of those commonly used markers. According to these markers, a good manufacturing practice differentiation protocol for VM DAergic progenitor cell production was developed through the use timed delivery of FGF8 and a number of other adjustments [197]. In conclusion, the ability of these markers to more precisely predict graft outcome will accelerate the clinical application of stem cells. In the future, a panel of markers can be refined and identified at the progenitor stage in vitro to predict more functional mature A9 DAergic neurons in vivo.
Ischemic stroke
Ischemic stroke is a cerebrovascular event that, although not classified as a neurodegenerative disease, also presents pathological cell death in the infarct area, including different types of neurons and glial cells [198]. In recent years, the limited treatments for ischemic stroke include endorvascular surgery (called thrombectomy) or intravenous administration of alteplase (called thrombolysis) for the purpose of restoring blood flow [199]. However, due to the narrow therapeutic window, some contraindications, low efficacy to recanalize the large artery via thrombolysis, and even the reperfusion injury after recanalization, only a small percentage of ischemic patients can benefit from these two treatments [200–203]. Possibly inspired by replacement therapy for specific cells lost in NDs, recently there have been studies targeting different lost cell types in ischemic stroke to transplant specific cells for replacement to reconstruct damaged neural circuits [166, 168, 169].
A clinical and imaging study showed that the distribution of damaged cells under the most severe symptoms in stroke patients was often not in the striatum, suggesting that cell replacement strategies should emphasize reconstructing the damaged cortex rather than the striatum [204]. Therefore, some studies have implanted progenitor cells with a cortical glutaminergic phenotype derived from PSCs into the cortex of rat models of ischemic stroke and found that these cells could alleviate sensorimotor impairment at 2 or 6 months after transplantation [166, 169]. Behavioral improvements at the early time point of 2 months post-transplantation were most likely not attributable to neuronal replacement and circuitry integration. The use of human-specific cytoplasmic markers combined with green protein immunostaining revealed that axonal projection of transplanted cells extended to both ipsilateral and contralateral hemispheres [169]. Further by using rabies virus–based transsynaptic tracing and optogenetics, it was found that the contralateral somatosensory cortex received functional monosynaptic input from the transplanted neurons [166]. In addition to functional efferent integration, transplanted cells also received synaptic inputs from the thalamocortex and were able to modulate their own activity in response to physiological sensory stimuli [168]. The functional circuit reconstruction is responsible for the recovery of motor function at late time points after transplantation [166]. Except for cortical glutamatergic excitatory neurons, another study identified the phenotype of transplanted predifferentiated NSCs as GABAergic inhibitory neurons. NSCs isolated from human fetal SVZ were predifferentiated in the presence of BDNF, and the predifferentiated cells were subsequently transplanted into the striatum and cortex of cerebral ischemic rats. Histopathology 28 days-post transplant indicated that these cells stably expressed the GABA phenotype, increased GABA levels, and exerted their trophic effects to promote endogenous neurogenesis, which may lead to faster functional recovery in cerebral ischemic rats than those treated with undifferentiated NSCs [205]. In addition to sensorimotor dysfunction, most stroke survivors suffer from cognitive impairment, which may be related to demyelination of the brain’s white matter, resulting from oligodendrocyte death [206, 207]. Xu et al. proposed a two-step protocol to derive NG2-positive OPCs stably and rapidly from iPSCs, first inhibiting SHH to generate NPC and then overexpressing Olig2 [208]. After transplanting OPCs into the cerebral ventricles of ischemic rats, it was found that these cells could protect host neurons from death under the ischemic environment by suppressing inflammatory and immune responses. Furthermore, these cells rescued learning and memory loss to some extent by facilitating the remyelination process in ischemic stroke rats [208].
Discussion and future directions
Based on numerous studies of cell transplantation, it can be inferred that both pre-transplantation history and source of donor cells are critical factors that affect the outcome of transplantation. In terms of pre-transplant history, most of the protocols for targeted differentiation of NSCs from different sources involve external chemical-defined system through patterning cues or intrinsic ectopic overexpression of lineage-specific transcription factors. Currently the external culture system is gradually moving from the initial co-culture or use of poorly defined xenogeneic factors toward a fully chemical-defined, and xeno-free condition, which encourages the establishment of the more robust differentiation protocol [209]. However, most existing targeted differentiation methods tend to generate heterogeneous cultures containing the cell type of interest as well as other undesirable phenotypes. Consequently, further exploration of the use of morphogens, growth factors, and small molecules in concentration, sequence, and duration is warranted. On the other hand, when manipulating gene overexpression, full consideration should be given to their expression patterns during neural development in order to achieve adequate maturation of differentiated cells and long-term phenotypic maintenance [74]. but virus-mediated multigene transduction is somewhat cytotoxic leading to poor implantation of donor cells, and another concern is that viral integration could disrupt normal gene expression, thus may not be suitable for clinical scenarios. In addition to the two approaches to orchestrate the directed induction differentiation of NSCs, increasingly, it has been shown that epigenetic machinery can regulate the interaction between activation and inhibition of various developmental signaling pathways in neural differentiation [210], such as finely tuning genetic programs to coordinate distinct neural lineage differentiation [211]. With the current advances in RNA interference, they could also be used to guide the targeted differentiation of NSCs [212–215]. In addition, Traditional two-dimensional induction methods provide basic soluble regulators to control the fate of NSCs. However, stem cell behaviors are regulated by different physiological, physico-chemical and physico-mechanical cues, so three-dimensional induction involving biological materials is increasingly being emphasized to effectively control the fate of stem cells [216–218]. With the rise of interdisciplinary of medicine and engineering, conducting polymers have been proved to induce the directional differentiation of NSCs through electrical stimulus in vitro [219]. All these efforts are aimed at promoting the targeted differentiation of NSCs and obtaining specific neural lineage cells in vitro.
The selection of primary CNS tissues and cell types for the generation of specific neuronal lineage phenotype also requires to be taken into account, as it may affect the efficiency of neuronal lineage differentiation and the effectiveness of transplantation. The potential of primary CNS tissue-derived NSCs varies depending on the developmental stage at which they are obtained and the site from where they were isolated [220]. For instance, under identical culture conditions, NSCs derived from ventral mesencephalic produced more DAergic neurons than those from striatum [68], and A9 neurons are produced earlier than A10 neurons during neural development [189], so NSCs derived from VM at an early stage is more conducive to the generation of A9 DAergic neurons. In contrast, NSCs derived from neural induction of PSCs tend to be at an earlier stage, and they can actively respond to multiple patterning molecules to differentiate into different neural lineages. However, it is gradually recognized that epigenetic differences exist between different PSC strains, leading to deviations in lineage differentiation or different generation efficiencies of the same lineage [163, 188, 221]. These differences highlight the need to further compare the results of directed differentiation of neural lineages across different PSC lines. In contrast to tissue-derived NSCs or ESCs, iPSCs and iNSCs are not subject to ethical concerns. In particular, patient-specific iPSCs or iNSCs are targeted to differentiate into neural lineage cells that will match individual immunity—a goal long pursed in regenerative medicine, but before they can be reasonably used for cell therapy, it is critical to understand and correct any intrinsic defects in these cells [119]. Neural grafts derived from human iNSCs are less likely to produce fast-growing tumors following grafting compared with grafts of PSCs [222, 223]. Currently, several strategies are actively being taken to address the possibility of potentially pathological growth of grafted cells from various sources, such as the use of cell-sorting techniques to remove Off-target contaminating cell types prior to transplantation [81, 224, 225] or the transduction of ligand-activated suicide genes to ablate proliferating cells in vivo [226, 227]. With the rapid development of induced neuron (iN) technology in recent years, it is possible to directly reprogram somatic cells to obtain functional neurons [93]. However, the iN method converts somatic cells directly into non-dividing neurons rather than fate committed neuronal progenitors, and these non-dividing neurons often tend to survive and integrate poorly in the host brain after transplantation [228].
In addition, the specific pathological states presented by different neurological diseases should be fully considered before donor cell transplantation, which may affect the cell transplantation strategy in a degree. In neurodegenerative disorders, the outcome of cell replacement relies on the complexity and precision of the connection patterns that need to be restored [229]. In the case of Parkinson’s disease, only a partial pattern repair can result in significant functional recovery. Ectopic transplantation of DAergic cells, also known as paracrine strategy, can restore the efficient release of dopamine to regulate the substantia nigrostriatal circuitry [60, 67, 73, 76]. Unlike Parkinson’s disease, for local neuronal degeneration due to HD or ALS and global neurodegeneration due to ischemic stroke, it is necessary to re-establish the specific afferent–efferent connections between the graft and host, emphasizing the importance of homotopic transplantation [229]. So far, based on advances in nanotechnology, molecular biology and imaging techniques, transplanted cell tracing technology has shifted from ex vitro detection to in vivo imaging [230], and further combining with viral or genetic labeling strategies can help researchers explore the synaptic connection between transplanted cells and host brain [179].
In addition to transplantation of directionally induced neural stem cells, in vivo cell reprogramming, the in-situ conversion of glial cells to functional new neurons is also a promising neural regeneration strategy [9]. Furthermore, transplanted exogenous stem cells can be genetically engineered to steadily produce growth factors that support the repair of dysfunctional endogenous neurons [231]. For example, human iPSC-derived neural progenitor cells genetically engineered to stably produce GDNF can delivery GDNF after transplantation to protect degenerated neurons in PD models and ALS models [232]. As a result, researchers have poured a great deal of effort in different ways, towards the same goal: to promote nerve repair or regeneration.
Recent preclinical studies have demonstrated the safety and efficacy of DAergic neurons derived from PSCs. Based on these promising results, clinical trials are being conducted in different countries [76, 224]. PD clinical trials provide important guidelines for other derived neural lineage cells venturing into these uncharted territories, and we believe that neural lineage cells derived from different sources of NSCs, especially PSCs, will be tested in clinical trials in the near future to develop treatments for related neurological diseases.
Author contributions
NL and ZT: conceptualization; Luwei Nie: initial draft preparation, wrote the main manuscript text, with the help of DY, SC, JW, CP, and DW; LN prepared table and figures; all authors: literature search, review, commentary, and final approval of the manuscript.
Funding
This work was funded by National Natural Science Foundation of China (No. 82071330) and the Natural Science Foundation of Hubei Province, China (No. 2019CFB113).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Na Liu, Email: liuna_2003abc@163.com.
Zhouping Tang, Email: ddjtzp@163.com.
References
- 1.Kahroba H, Ramezani B, Maadi H, Sadeghi MR, Jaberie H, Ramezani F. The role of Nrf2 in neural stem/progenitors cells: from maintaining stemness and self-renewal to promoting differentiation capability and facilitating therapeutic application in neurodegenerative disease. Ageing Res Rev. 2021;65:101211. doi: 10.1016/j.arr.2020.101211. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Jangili P, Son S, Ji MS, Won M, Kim JS. Fluorescent diagnostic probes in neurodegenerative diseases. Adv Mater. 2020;32:e2001945. doi: 10.1002/adma.202001945. [DOI] [PubMed] [Google Scholar]
- 3.Yaribeygi H, Panahi Y, Javadi B, Sahebkar A. The underlying role of oxidative stress in neurodegeneration: a mechanistic review. CNS Neurol Disord Drug Targets. 2018;17:207–15. doi: 10.2174/1871527317666180425122557. [DOI] [PubMed] [Google Scholar]
- 4.Tuo QZ, Zhang ST, Lei P. Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med Res Rev. 2022;42:259–305. doi: 10.1002/med.21817. [DOI] [PubMed] [Google Scholar]
- 5.Back T. Pathophysiology of the ischemic penumbra—revision of a concept. Cell Mol Neurobiol. 1998;18:621–38. doi: 10.1023/a:1020629818207. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Huang L, Lan J, Feng X, Li P, Wu L, et al. Molecular mechanisms of mitophagy and its roles in neurodegenerative diseases. Pharm Res. 2021;163:105240. doi: 10.1016/j.phrs.2020.105240. [DOI] [PubMed] [Google Scholar]
- 7.Pasteuning-Vuhman S, de Jongh R, Timmers A, Pasterkamp RJ. Towards advanced iPSC-based drug development for neurodegenerative disease. Trends Mol Med. 2021;27:263–79. doi: 10.1016/j.molmed.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 8.McCrary MR, Jesson K, Wei ZZ, Logun M, Lenear C, Tan S, et al. Cortical transplantation of brain-mimetic glycosaminoglycan scaffolds and neural progenitor cells promotes vascular regeneration and functional recovery after ischemic stroke in mice. Adv Health Mater. 2020;9:e1900285. doi: 10.1002/adhm.201900285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker RA, Gotz M, Parmar M. New approaches for brain repair-from rescue to reprogramming. Nature. 2018;557:329–34. doi: 10.1038/s41586-018-0087-1. [DOI] [PubMed] [Google Scholar]
- 10.Andreotti JP, Silva WN, Costa AC, Picoli CC, Bitencourt FCO, Coimbra-Campos LMC, et al. Neural stem cell niche heterogeneity. Semin Cell Dev Biol. 2019;95:42–53. doi: 10.1016/j.semcdb.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 12.Bellenchi GC, Volpicelli F, Piscopo V, Perrone-Capano C, di Porzio U. Adult neural stem cells: an endogenous tool to repair brain injury? J Neurochem. 2013;124:159–67. doi: 10.1111/jnc.12084. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B, Yan W, Zhu Y, Yang W, Le W, Chen B, et al. Nanomaterials in neural-stem-cell-mediated regenerative medicine: imaging and treatment of neurological diseases. Adv Mater. 2018;30:e1705694. doi: 10.1002/adma.201705694. [DOI] [PubMed] [Google Scholar]
- 14.Qin C, Wang K, Zhang L, Bai L. Stem cell therapy for Alzheimer’s disease: an overview of experimental models and reality. Anim Model Exp Med. 2022;5:15–26. doi: 10.1002/ame2.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Gioia R, Biella F, Citterio G, Rizzo F, Abati E, Nizzardo M, et al. Neural stem cell transplantation for neurodegenerative diseases. Int J Mol Sci. 2020;21. 10.3390/ijms21093103. [DOI] [PMC free article] [PubMed]
- 16.Huang L, Zhang L. Neural stem cell therapies and hypoxic-ischemic brain injury. Prog Neurobiol. 2019;173:1–17. doi: 10.1016/j.pneurobio.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corey S, Bonsack B, Heyck M, Shear A, Sadanandan N, Zhang H, et al. Harnessing the anti-inflammatory properties of stem cells for transplant therapy in hemorrhagic stroke. Brain Hemorrhages. 2020;1:24–33. doi: 10.1016/j.hest.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monsour M, Borlongan CV. Emerging regenerative medicine for hemorrhagic stroke: an update on stem cell therapies. Brain Hemorrhages. 2023;4:22–26. doi: 10.1016/j.hest.2022.07.001. [DOI] [Google Scholar]
- 19.Benner EJ, Luciano D, Jo R, Abdi K, Paez-Gonzalez P, Sheng H, et al. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature. 2013;497:369–73. doi: 10.1038/nature12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pous L, Deshpande SS, Nath S, Mezey S, Malik SC, Schildge S, et al. Fibrinogen induces neural stem cell differentiation into astrocytes in the subventricular zone via BMP signaling. Nat Commun. 2020;11:630. doi: 10.1038/s41467-020-14466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tejeda G, Ciciriello AJ & Dumont, CM. Biomaterial strategies to bolster neural stem cell-mediated repair of the central nervous system. Cells Tissues Organs. 2021;1–15. 10.1159/000515351. [DOI] [PubMed]
- 22.Bruggeman KF, Moriarty N, Dowd E, Nisbet DR, Parish CL. Harnessing stem cells and biomaterials to promote neural repair. Br J Pharm. 2019;176:355–68. doi: 10.1111/bph.14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambertsen KL, Finsen B, Clausen BH. Post-stroke inflammation-target or tool for therapy? Acta Neuropathol. 2019;137:693–714. doi: 10.1007/s00401-018-1930-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon HS, Koh SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020;9:42. doi: 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathieu P, Battista D, Depino A, Roca V, Graciarena M, Pitossi F. The more you have, the less you get: the functional role of inflammation on neuronal differentiation of endogenous and transplanted neural stem cells in the adult brain. J Neurochem. 2010;112:1368–85. doi: 10.1111/j.1471-4159.2009.06548.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang K, Lu WC, Zhang M, Zhang Q, Xian PP, Liu FF, et al. Reducing host aldose reductase activity promotes neuronal differentiation of transplanted neural stem cells at spinal cord injury sites and facilitates locomotion recovery. Neural Regen Res. 2022;17:1814–20. doi: 10.4103/1673-5374.330624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ideguchi M, Shinoyama M, Gomi M, Hayashi H, Hashimoto N, Takahashi J. Immune or inflammatory response by the host brain suppresses neuronal differentiation of transplanted ES cell-derived neural precursor cells. J Neurosci Res. 2008;86:1936–43. doi: 10.1002/jnr.21652. [DOI] [PubMed] [Google Scholar]
- 28.He Z, Lang L, Hui J, Ma Y, Yang C, Weng W, et al. Brain extract of subacute traumatic brain injury promotes the neuronal differentiation of human neural stem cells via autophagy. J Clin Med. 2022;11. 10.3390/jcm11102709. [DOI] [PMC free article] [PubMed]
- 29.Yang Y, Fan Y, Zhang H, Zhang Q, Zhao Y, Xiao Z, et al. Small molecules combined with collagen hydrogel direct neurogenesis and migration of neural stem cells after spinal cord injury. Biomaterials. 2021;269:120479. doi: 10.1016/j.biomaterials.2020.120479. [DOI] [PubMed] [Google Scholar]
- 30.Adams KV, Morshead CM. Neural stem cell heterogeneity in the mammalian forebrain. Prog Neurobiol. 2018;170:2–36. doi: 10.1016/j.pneurobio.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Y, Huang R, Wu Z, Song S, Cheng L, Zhu R. Deep learning-based predictive identification of neural stem cell differentiation. Nat Commun. 2021;12:2614. doi: 10.1038/s41467-021-22758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HR, Farhanullah, Lee J, Jajoo R, Kong SY, Shin JY, et al. Discovery of a small molecule that enhances astrocytogenesis by activation of STAT3, SMAD1/5/8, and ERK1/2 via induction of cytokines in neural stem cells. ACS Chem Neurosci. 2016;7:90–99. doi: 10.1021/acschemneuro.5b00243. [DOI] [PubMed] [Google Scholar]
- 33.Tang Y, Yu P, Cheng L. Current progress in the derivation and therapeutic application of neural stem cells. Cell Death Dis. 2017;8:e3108. doi: 10.1038/cddis.2017.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kempermann G, Song H, Gage FH. Neurogenesis in the Adult Hippocampus. Cold Spring Harb Perspect Biol. 2015;7:a018812. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin R, Iacovitti L. Classic and novel stem cell niches in brain homeostasis and repair. Brain Res. 2015;1628:327–42. doi: 10.1016/j.brainres.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 36.Muratore CR, Srikanth P, Callahan DG, Young-Pearse TL. Comparison and optimization of hiPSC forebrain cortical differentiation protocols. PLoS One. 2014;9:e105807. doi: 10.1371/journal.pone.0105807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan Y, Shin S, Jha BS, Liu Q, Sheng J, Li F, et al. Efficient and rapid derivation of primitive neural stem cells and generation of brain subtype neurons from human pluripotent stem cells. Stem Cells Transl Med. 2013;2:862–70. doi: 10.5966/sctm.2013-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao Y, Zhang SC. Neural subtype specification from human pluripotent stem cells. Cell Stem Cell. 2016;19:573–86. doi: 10.1016/j.stem.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–80. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–33. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 41.Jeon I, Lee N, Li JY, Park IH, Park KS, Moon J, et al. Neuronal properties, in vivo effects, and pathology of a Huntington’s disease patient-derived induced pluripotent stem cells. Stem Cells. 2012;30:2054–62. doi: 10.1002/stem.1135. [DOI] [PubMed] [Google Scholar]
- 42.Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21:1200–7. doi: 10.1038/nbt870. [DOI] [PubMed] [Google Scholar]
- 43.Kawasaki H, Suemori H, Mizuseki K, Watanabe K, Urano F, Ichinose H, et al. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc Natl Acad Sci USA. 2002;99:1580–5. doi: 10.1073/pnas.032662199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng W, Chen Z. Generation of induced neural stem cells from peripheral mononuclear cells and differentiation toward dopaminergic neuron precursors for transplantation studies. J Vis Exp. 2019. 10.3791/59690. [DOI] [PubMed]
- 45.Thier M, Worsdorfer P, Lakes YB, Gorris R, Herms S, Opitz T, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–9. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Kiecker C, Lumsden A. The role of organizers in patterning the nervous system. Annu Rev Neurosci. 2012;35:347–67. doi: 10.1146/annurev-neuro-062111-150543. [DOI] [PubMed] [Google Scholar]
- 47.Christie KJ, Emery B, Denham M, Bujalka H, Cate HS, Turnley AM. Transcriptional regulation and specification of neural stem cells. Adv Exp Med Biol. 2013;786:129–55. doi: 10.1007/978-94-007-6621-1_8. [DOI] [PubMed] [Google Scholar]
- 48.Ono Y, Nakatani T, Sakamoto Y, Mizuhara E, Minaki Y, Kumai M, et al. Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development. 2007;134:3213–25. doi: 10.1242/dev.02879. [DOI] [PubMed] [Google Scholar]
- 49.Placzek M, Briscoe J. The floor plate: multiple cells, multiple signals. Nat Rev Neurosci. 2005;6:230–40. doi: 10.1038/nrn1628. [DOI] [PubMed] [Google Scholar]
- 50.Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–66. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- 51.Chung S, Leung A, Han BS, Chang MY, Moon JI, Kim CH, et al. Wnt1-lmx1a forms a novel autoregulatory loop and controls midbrain dopaminergic differentiation synergistically with the SHH-FoxA2 pathway. Cell Stem Cell. 2009;5:646–58. doi: 10.1016/j.stem.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mesman S, Smidt MP. Acquisition of the midbrain dopaminergic neuronal identity. Int J Mol Sci. 2020;21. 10.3390/ijms21134638. [DOI] [PMC free article] [PubMed]
- 53.Prakash N, Brodski C, Naserke T, Puelles E, Gogoi R, Hall A, et al. A Wnt1-regulated genetic network controls the identity and fate of midbrain-dopaminergic progenitors in vivo. Development. 2006;133:89–98. doi: 10.1242/dev.02181. [DOI] [PubMed] [Google Scholar]
- 54.Hovakimyan M, Haas SJ, Schmitt O, Gerber B, Wree A, Andressen C. Mesencephalic human neural progenitor cells transplanted into the neonatal hemiparkinsonian rat striatum differentiate into neurons and improve motor behaviour. J Anat. 2006;209:721–32. doi: 10.1111/j.1469-7580.2006.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maciaczyk J, Singec I, Maciaczyk D, Nikkhah G. Combined use of BDNF, ascorbic acid, low oxygen, and prolonged differentiation time generates tyrosine hydroxylase-expressing neurons after long-term in vitro expansion of human fetal midbrain precursor cells. Exp Neurol. 2008;213:354–62. doi: 10.1016/j.expneurol.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 56.Jin G, Tan X, Tian M, Qin J, Zhu H, Huang Z, et al. The controlled differentiation of human neural stem cells into TH-immunoreactive (ir) neurons in vitro. Neurosci Lett. 2005;386:105–10. doi: 10.1016/j.neulet.2005.04.065. [DOI] [PubMed] [Google Scholar]
- 57.Sanchez-Pernaute R, Studer L, Bankiewicz KS, Major EO, McKay RD. In vitro generation and transplantation of precursor-derived human dopamine neurons. J Neurosci Res. 2001;65:284–8. doi: 10.1002/jnr.1152. [DOI] [PubMed] [Google Scholar]
- 58.Yan W, Chen ZY, Chen JQ, Chen HM. BMP2 promotes the differentiation of neural stem cells into dopaminergic neurons in vitro via miR-145-mediated upregulation of Nurr1 expression. Am J Transl Res. 2016;8:3689–99. [PMC free article] [PubMed] [Google Scholar]
- 59.Studer L, Tabar V, McKay RD. Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nat Neurosci. 1998;1:290–5. doi: 10.1038/1105. [DOI] [PubMed] [Google Scholar]
- 60.Parish CL, Castelo-Branco G, Rawal N, Tonnesen J, Sorensen AT, Salto C, et al. Wnt5a-treated midbrain neural stem cells improve dopamine cell replacement therapy in parkinsonian mice. J Clin Investig. 2008;118:149–60. doi: 10.1172/JCI32273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung S, Shin BS, Hwang M, Lardaro T, Kang UJ, Isacson O, et al. Neural precursors derived from embryonic stem cells, but not those from fetal ventral mesencephalon, maintain the potential to differentiate into dopaminergic neurons after expansion in vitro. Stem Cells. 2006;24:1583–93. doi: 10.1634/stemcells.2005-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribeiro D, Laguna Goya R, Ravindran G, Vuono R, Parish CL, Foldi C, et al. Efficient expansion and dopaminergic differentiation of human fetal ventral midbrain neural stem cells by midbrain morphogens. Neurobiol Dis. 2013;49:118–27. doi: 10.1016/j.nbd.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 63.He XB, Kim M, Kim SY, Yi SH, Rhee YH, Kim T, et al. Vitamin C facilitates dopamine neuron differentiation in fetal midbrain through TET1- and JMJD3-dependent epigenetic control manner. Stem Cells. 2015;33:1320–32. doi: 10.1002/stem.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan J, Studer L, McKay RD. Ascorbic acid increases the yield of dopaminergic neurons derived from basic fibroblast growth factor expanded mesencephalic precursors. J Neurochem. 2001;76:307–11. doi: 10.1046/j.1471-4159.2001.00073.x. [DOI] [PubMed] [Google Scholar]
- 65.Roybon L, Hjalt T, Christophersen NS, Li JY, Brundin P. Effects on differentiation of embryonic ventral midbrain progenitors by Lmx1a, Msx1, Ngn2, and Pitx3. J Neurosci. 2008;28:3644–56. doi: 10.1523/JNEUROSCI.0311-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan X, Zhang L, Zhu H, Qin J, Tian M, Dong C, et al. Brn4 and TH synergistically promote the differentiation of neural stem cells into dopaminergic neurons. Neurosci Lett. 2014;571:23–28. doi: 10.1016/j.neulet.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 67.Tan X, Zhang L, Qin J, Tian M, Zhu H, Dong C, et al. Transplantation of neural stem cells co-transfected with Nurr1 and Brn4 for treatment of Parkinsonian rats. Int J Dev Neurosci. 2013;31:82–87. doi: 10.1016/j.ijdevneu.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 68.Okano H, Yoshizaki T, Shimazaki T, Sawamoto K. Isolation and transplantation of dopaminergic neurons and neural stem cells. Parkinsonism Relat Disord. 2002;9:23–28. doi: 10.1016/s1353-8020(02)00041-x. [DOI] [PubMed] [Google Scholar]
- 69.Yang H, Wang J, Wang F, Liu X, Chen H, Duan W, et al. Dopaminergic neuronal differentiation from the forebrain-derived human neural stem cells induced in cultures by using a combination of BMP-7 and pramipexole with growth factors. Front Neural Circuits. 2016;10:29. doi: 10.3389/fncir.2016.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jain M, Armstrong RJ, Tyers P, Barker RA, Rosser AE. GABAergic immunoreactivity is predominant in neurons derived from expanded human neural precursor cells in vitro. Exp Neurol. 2003;182:113–23. doi: 10.1016/s0014-4886(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 71.Rossler R, Boddeke E, Copray S. Differentiation of non-mesencephalic neural stem cells towards dopaminergic neurons. Neuroscience. 2010;170:417–28. doi: 10.1016/j.neuroscience.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 72.Yi SH, He XB, Rhee YH, Park CH, Takizawa T, Nakashima K, et al. Foxa2 acts as a co-activator potentiating expression of the Nurr1-induced DA phenotype via epigenetic regulation. Development. 2014;141:761–72. doi: 10.1242/dev.095802. [DOI] [PubMed] [Google Scholar]
- 73.Lee HS, Bae EJ, Yi SH, Shim JW, Jo AY, Kang JS, et al. Foxa2 and Nurr1 synergistically yield A9 nigral dopamine neurons exhibiting improved differentiation, function, and cell survival. Stem Cells. 2010;28:501–12. doi: 10.1002/stem.294. [DOI] [PubMed] [Google Scholar]
- 74.Kim T, Song JJ, Puspita L, Valiulahi P, Shim JW, Lee SH. In vitro generation of mature midbrain-type dopamine neurons by adjusting exogenous Nurr1 and Foxa2 expressions to their physiologic patterns. Exp Mol Med. 2017;49:e300. doi: 10.1038/emm.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vazin T, Chen J, Lee CT, Amable R, Freed WJ. Assessment of stromal-derived inducing activity in the generation of dopaminergic neurons from human embryonic stem cells. Stem Cells. 2008;26:1517–25. doi: 10.1634/stemcells.2008-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang YK, Zhu WW, Wu MH, Wu YH, Liu ZX, Liang LM, et al. Human clinical-grade parthenogenetic esc-derived dopaminergic neurons recover locomotive defects of nonhuman primate models of Parkinson’s disease. Stem Cell Rep. 2018;11:171–82. doi: 10.1016/j.stemcr.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma L, Liu Y, Zhang SC. Directed differentiation of dopamine neurons from human pluripotent stem cells. Methods Mol Biol. 2011;767:411–8. doi: 10.1007/978-1-61779-201-4_30. [DOI] [PubMed] [Google Scholar]
- 78.Swistowski A, Peng J, Liu Q, Mali P, Rao MS, Cheng L, et al. Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells. 2010;28:1893–904. doi: 10.1002/stem.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–90. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gantner CW, Cota-Coronado A, Thompson LH, Parish CL. An optimized protocol for the generation of midbrain dopamine neurons under defined conditions. STAR Protoc. 2020;1:100065. doi: 10.1016/j.xpro.2020.100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doi D, Samata B, Katsukawa M, Kikuchi T, Morizane A, Ono Y, et al. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Rep. 2014;2:337–50. doi: 10.1016/j.stemcr.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, et al. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1:703–14. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 83.Xi J, Liu Y, Liu H, Chen H, Emborg ME, Zhang SC. Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cells. 2012;30:1655–63. doi: 10.1002/stem.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–51. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kikuchi T, Morizane A, Doi D, Magotani H, Onoe H, Hayashi T, et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature. 2017;548:592–6. doi: 10.1038/nature23664. [DOI] [PubMed] [Google Scholar]
- 86.Jovanovic VM, Salti A, Tilleman H, Zega K, Jukic MM, Zou H, et al. BMP/SMAD pathway promotes neurogenesis of midbrain dopaminergic neurons in vivo and in human induced pluripotent and neural stem cells. J Neurosci. 2018;38:1662–76. doi: 10.1523/JNEUROSCI.1540-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adil MM, Rodrigues GM, Kulkarni RU, Rao AT, Chernavsky NE, Miller EW, et al. Efficient generation of hPSC-derived midbrain dopaminergic neurons in a fully defined, scalable, 3D biomaterial platform. Sci Rep. 2017;7:40573. doi: 10.1038/srep40573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yuan Y, Tang X, Bai YF, Wang S, An J, Wu Y, et al. Dopaminergic precursors differentiated from human blood-derived induced neural stem cells improve symptoms of a mouse Parkinson’s disease model. Theranostics. 2018;8:4679–94. doi: 10.7150/thno.26643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li M, Wang Z, Zheng T, Huang T, Liu B, Han D, et al. Characterization of human-induced neural stem cells and derivatives following transplantation into the central nervous system of a nonhuman primate and rats. Stem Cells Int. 2022;2022:1396735. doi: 10.1155/2022/1396735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yan S, Tu Z, Liu Z, Fan N, Yang H, Yang S, et al. A Huntingtin Knockin Pig Model recapitulates features of selective neurodegeneration in Huntington’s disease. Cell. 2018;173:989–1002 e1013. doi: 10.1016/j.cell.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Waclaw RR, Wang B, Pei Z, Ehrman LA, Campbell K. Distinct temporal requirements for the homeobox gene Gsx2 in specifying striatal and olfactory bulb neuronal fates. Neuron. 2009;63:451–65. doi: 10.1016/j.neuron.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maira M, Long JE, Lee AY, Rubenstein JL, Stifani S. Role for TGF-beta superfamily signaling in telencephalic GABAergic neuron development. J Neurodev Disord. 2010;2:48–60. doi: 10.1007/s11689-009-9035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fjodorova M, Noakes Z, Li M. How to make striatal projection neurons. Neurogenesis. 2015;2:e1100227. doi: 10.1080/23262133.2015.1100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arlotta P, Molyneaux BJ, Jabaudon D, Yoshida Y, Macklis JD. Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J Neurosci. 2008;28:622–32. doi: 10.1523/JNEUROSCI.2986-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ouimet CC, Langley-Gullion KC, Greengard P. Quantitative immunocytochemistry of DARPP-32-expressing neurons in the rat caudatoputamen. Brain Res. 1998;808:8–12. doi: 10.1016/s0006-8993(98)00724-0. [DOI] [PubMed] [Google Scholar]
- 96.Ostenfeld T, Joly E, Tai YT, Peters A, Caldwell M, Jauniaux E, et al. Regional specification of rodent and human neurospheres. Brain Res Dev Brain Res. 2002;134:43–55. doi: 10.1016/s0165-3806(01)00291-7. [DOI] [PubMed] [Google Scholar]
- 97.Ivkovic S, Ehrlich ME. Expression of the striatal DARPP-32/ARPP-21 phenotype in GABAergic neurons requires neurotrophins in vivo and in vitro. J Neurosci. 1999;19:5409–19. doi: 10.1523/JNEUROSCI.19-13-05409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kelly CM, Precious SV, Penketh R, Amso N, Dunnett SB, Rosser AE. Striatal graft projections are influenced by donor cell type and not the immunogenic background. Brain. 2007;130:1317–29. doi: 10.1093/brain/awm053. [DOI] [PubMed] [Google Scholar]
- 99.El-Akabawy G, Medina LM, Jeffries A, Price J, Modo M. Purmorphamine increases DARPP-32 differentiation in human striatal neural stem cells through the Hedgehog pathway. Stem Cells Dev. 2011;20:1873–87. doi: 10.1089/scd.2010.0282. [DOI] [PubMed] [Google Scholar]
- 100.Bosch M, Pineda JR, Sunol C, Petriz J, Cattaneo E, Alberch J, et al. Induction of GABAergic phenotype in a neural stem cell line for transplantation in an excitotoxic model of Huntington’s disease. Exp Neurol. 2004;190:42–58. doi: 10.1016/j.expneurol.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 101.Lin L, Yuan J, Sander B, Golas MM. In vitro differentiation of human neural progenitor cells into striatal GABAergic neurons. Stem Cells Transl Med. 2015;4:775–88. doi: 10.5966/sctm.2014-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ma L, Hu B, Liu Y, Vermilyea SC, Liu H, Gao L, et al. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell. 2012;10:455–64. doi: 10.1016/j.stem.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Delli Carri A, Onorati M, Lelos MJ, Castiglioni V, Faedo A, Menon R, et al. Developmentally coordinated extrinsic signals drive human pluripotent stem cell differentiation toward authentic DARPP-32+ medium-sized spiny neurons. Development. 2013;140:301–12. doi: 10.1242/dev.084608. [DOI] [PubMed] [Google Scholar]
- 104.Wu M, Zhang D, Bi C, Mi T, Zhu W, Xia L, et al. A chemical recipe for generation of clinical-grade striatal neurons from hESCs. Stem Cell Rep. 2018;11:635–50. doi: 10.1016/j.stemcr.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adil MM, Gaj T, Rao AT, Kulkarni RU, Fuentes CM, Ramadoss GN, et al. hPSC-derived striatal cells generated using a scalable 3D hydrogel promote recovery in a Huntington disease mouse model. Stem Cell Rep. 2018;10:1481–91. doi: 10.1016/j.stemcr.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arber C, Precious SV, Cambray S, Risner-Janiczek JR, Kelly C, Noakes Z, et al. Activin A directs striatal projection neuron differentiation of human pluripotent stem cells. Development. 2015;142:1375–86. doi: 10.1242/dev.117093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Comella-Bolla A, Orlandi JG, Miguez A, Straccia M, Garcia-Bravo M, Bombau G, et al. Human pluripotent stem cell-derived neurons are functionally mature in vitro and integrate into the mouse striatum following transplantation. Mol Neurobiol. 2020;57:2766–98. doi: 10.1007/s12035-020-01907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zayia LC, Tadi P. in StatPearls. 2023.
- 109.Stifani N. Motor neurons and the generation of spinal motor neuron diversity. Front Cell Neurosci. 2014;8:293. doi: 10.3389/fncel.2014.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patani R. Generating diverse spinal motor neuron subtypes from human pluripotent stem cells. Stem Cells Int. 2016;2016:1036974. doi: 10.1155/2016/1036974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sances S, Bruijn LI, Chandran S, Eggan K, Ho R, Klim JR, et al. Modeling ALS with motor neurons derived from human induced pluripotent stem cells. Nat Neurosci. 2016;19:542–53. doi: 10.1038/nn.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davis-Dusenbery BN, Williams LA, Klim JR, Eggan K. How to make spinal motor neurons. Development. 2014;141:491–501. doi: 10.1242/dev.097410. [DOI] [PubMed] [Google Scholar]
- 113.Natarajan R, Singal V, Benes R, Gao J, Chan H, Chen H, et al. STAT3 modulation to enhance motor neuron differentiation in human neural stem cells. PLoS One. 2014;9:e100405. doi: 10.1371/journal.pone.0100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jordan PM, Ojeda LD, Thonhoff JR, Gao J, Boehning D, Yu Y, et al. Generation of spinal motor neurons from human fetal brain-derived neural stem cells: role of basic fibroblast growth factor. J Neurosci Res. 2009;87:318–32. doi: 10.1002/jnr.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee HJ, Kim KS, Ahn J, Bae HM, Lim I, Kim SU. Human motor neurons generated from neural stem cells delay clinical onset and prolong life in ALS mouse model. PLoS One. 2014;9:e97518. doi: 10.1371/journal.pone.0097518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, et al. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25:1931–9. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- 117.Patani R, Hollins AJ, Wishart TM, Puddifoot CA, Alvarez S, de Lera AR, et al. Retinoid-independent motor neurogenesis from human embryonic stem cells reveals a medial columnar ground state. Nat Commun. 2011;2:214. doi: 10.1038/ncomms1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27:806–11. doi: 10.1002/stem.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–21. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 120.Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–21. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 121.Ren J, Li C, Zhang M, Wang H, Xie Y, Tang Y. A Step-by-step refined strategy for highly efficient generation of neural progenitors and motor neurons from human pluripotent stem cells. Cells. 2021;10. 10.3390/cells10113087. [DOI] [PMC free article] [PubMed]
- 122.Shimojo D, Onodera K, Doi-Torii Y, Ishihara Y, Hattori C, Miwa Y, et al. Rapid, efficient, and simple motor neuron differentiation from human pluripotent stem cells. Mol Brain. 2015;8:79. doi: 10.1186/s13041-015-0172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kiskinis E, Sandoe J, Williams LA, Boulting GL, Moccia R, Wainger BJ, et al. Pathways disrupted in human ALS motor neurons identified through genetic correction of mutant SOD1. Cell Stem Cell. 2014;14:781–95. doi: 10.1016/j.stem.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Amoroso MW, Croft GF, Williams DJ, O’Keeffe S, Carrasco MA, Davis AR, et al. Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. J Neurosci. 2013;33:574–86. doi: 10.1523/JNEUROSCI.0906-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maury Y, Come J, Piskorowski RA, Salah-Mohellibi N, Chevaleyre V, Peschanski M, et al. Combinatorial analysis of developmental cues efficiently converts human pluripotent stem cells into multiple neuronal subtypes. Nat Biotechnol. 2015;33:89–96. doi: 10.1038/nbt.3049. [DOI] [PubMed] [Google Scholar]
- 126.Du ZW, Chen H, Liu H, Lu J, Qian K, Huang CL, et al. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat Commun. 2015;6:6626. doi: 10.1038/ncomms7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu J, Tang Y. Transcription factor-mediated differentiation of motor neurons from human pluripotent stem cells. Methods Mol Biol. 2023;2593:245–58. doi: 10.1007/978-1-0716-2811-9_16. [DOI] [PubMed] [Google Scholar]
- 128.Akter M, Cui H, Sepehrimanesh M, Hosain MA, Ding B. Generation of highly pure motor neurons from human induced pluripotent stem cells. STAR Protoc. 2022;3:101223. doi: 10.1016/j.xpro.2022.101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sepehrimanesh M, Ding B. Generation and optimization of highly pure motor neurons from human induced pluripotent stem cells via lentiviral delivery of transcription factors. Am J Physiol Cell Physiol. 2020;319:C771–C780. doi: 10.1152/ajpcell.00279.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tang X, Yu M, Liao C, Lan S, Fan Y. Induced neural stem cells-derived spinal cord progenitor cells as stable stage for rapid and efficient generation of human oligodendrocytes and motor neurons. Connect Tissue Res. 2021;62:206–14. doi: 10.1080/03008207.2019.1670651. [DOI] [PubMed] [Google Scholar]
- 131.Shao Z, Luo Q, Liu D, Mi Y, Zhang P, Ju G. Induced differentiation of neural stem cells of astrocytic origin to motor neurons in the rat. Stem Cells Dev. 2011;20:1163–70. doi: 10.1089/scd.2010.0262. [DOI] [PubMed] [Google Scholar]
- 132.Cristobal CD, Lee HK. Development of myelinating glia: an overview. Glia. 2022;70:2237–59. doi: 10.1002/glia.24238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Goldman SA, Kuypers NJ. How to make an oligodendrocyte. Development. 2015;142:3983–95. doi: 10.1242/dev.126409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bergles DE, Richardson WD. Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol. 2015;8:a020453. doi: 10.1101/cshperspect.a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hu BY, Du ZW, Li XJ, Ayala M, Zhang SC. Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development. 2009;136:1443–52. doi: 10.1242/dev.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fogarty M, Richardson WD, Kessaris N. A subset of oligodendrocytes generated from radial glia in the dorsal spinal cord. Development. 2005;132:1951–9. doi: 10.1242/dev.01777. [DOI] [PubMed] [Google Scholar]
- 137.Cai J, Qi Y, Hu X, Tan M, Liu Z, Zhang J, et al. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron. 2005;45:41–53. doi: 10.1016/j.neuron.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 138.Maire CL, Buchet D, Kerninon C, Deboux C, Baron-Van Evercooren A, Nait-Oumesmar B. Directing human neural stem/precursor cells into oligodendrocytes by overexpression of Olig2 transcription factor. J Neurosci Res. 2009;87:3438–46. doi: 10.1002/jnr.22194. [DOI] [PubMed] [Google Scholar]
- 139.Neri M, Maderna C, Ferrari D, Cavazzin C, Vescovi AL, Gritti A. Robust generation of oligodendrocyte progenitors from human neural stem cells and engraftment in experimental demyelination models in mice. PLoS One. 2010;5:e10145. doi: 10.1371/journal.pone.0010145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang C, Luan Z, Yang Y, Wang Z, Wang Q, Lu Y, et al. High purity of human oligodendrocyte progenitor cells obtained from neural stem cells: suitable for clinical application. J Neurosci Methods. 2015;240:61–66. doi: 10.1016/j.jneumeth.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 141.Monaco MC, Maric D, Bandeian A, Leibovitch E, Yang W & Major EO Progenitor-derived oligodendrocyte culture system from human fetal brain. J Vis Exp. 2012. 10.3791/4274. [DOI] [PMC free article] [PubMed]
- 142.Hu BY, Du ZW, Zhang SC. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc. 2009;4:1614–22. doi: 10.1038/nprot.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stacpoole SR, Spitzer S, Bilican B, Compston A, Karadottir R, Chandran S, et al. High yields of oligodendrocyte lineage cells from human embryonic stem cells at physiological oxygen tensions for evaluation of translational biology. Stem Cell Rep. 2013;1:437–50. doi: 10.1016/j.stemcr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Douvaras P, Fossati V. Generation and isolation of oligodendrocyte progenitor cells from human pluripotent stem cells. Nat Protoc. 2015;10:1143–54. doi: 10.1038/nprot.2015.075. [DOI] [PubMed] [Google Scholar]
- 145.Douvaras P, Wang J, Zimmer M, Hanchuk S, O’Bara MA, Sadiq S, et al. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Rep. 2014;3:250–9. doi: 10.1016/j.stemcr.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ehrlich M, Mozafari S, Glatza M, Starost L, Velychko S, Hallmann AL, et al. Rapid and efficient generation of oligodendrocytes from human induced pluripotent stem cells using transcription factors. Proc Natl Acad Sci USA. 2017;114:E2243–E2252. doi: 10.1073/pnas.1614412114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Garcia-Leon JA, Garcia-Diaz B, Eggermont K, Caceres-Palomo L, Neyrinck K, Madeiro da Costa R, et al. Generation of oligodendrocytes and establishment of an all-human myelinating platform from human pluripotent stem cells. Nat Protoc. 2020;15:3716–44. doi: 10.1038/s41596-020-0395-4. [DOI] [PubMed] [Google Scholar]
- 148.Ma L, Mei Y, Xu P, Cheng Y, You Z, Ji X, et al. Fast generation of forebrain oligodendrocyte spheroids from human embryonic stem cells by transcription factors. iScience. 2022;25:105172. doi: 10.1016/j.isci.2022.105172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Piao J, Major T, Auyeung G, Policarpio E, Menon J, Droms L, et al. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell. 2015;16:198–210. doi: 10.1016/j.stem.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Matho KS, Huilgol D, Galbavy W, He M, Kim G, An X, et al. Genetic dissection of the glutamatergic neuron system in cerebral cortex. Nature. 2021;598:182–7. doi: 10.1038/s41586-021-03955-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rakic P. The radial edifice of cortical architecture: from neuronal silhouettes to genetic engineering. Brain Res Rev. 2007;55:204–19. doi: 10.1016/j.brainresrev.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.MuhChyi C, Juliandi B, Matsuda T, Nakashima K. Epigenetic regulation of neural stem cell fate during corticogenesis. Int J Dev Neurosci. 2013;31:424–33. doi: 10.1016/j.ijdevneu.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 153.Li XJ, Zhang X, Johnson MA, Wang ZB, Lavaute T, Zhang SC. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136:4055–63. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sperandeo A, Tamburini C, Noakes Z, de la Fuente DC, Keefe F, Petter O, et al. Cortical neuronal hyperexcitability and synaptic changes in SGCE mutation-positive myoclonus dystonia. Brain. 2022 doi: 10.1093/brain/awac365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Espuny-Camacho I, Michelsen KA, Gall D, Linaro D, Hasche A, Bonnefont J, et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–56. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 156.Hodge RD, Kahoud RJ, Hevner RF. Transcriptional control of glutamatergic differentiation during adult neurogenesis. Cell Mol Life Sci. 2012;69:2125–34. doi: 10.1007/s00018-011-0916-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sequerra EB, Miyakoshi LM, Froes MM, Menezes JR, Hedin-Pereira C. Generation of glutamatergic neurons from postnatal and adult subventricular zone with pyramidal-like morphology. Cereb Cortex. 2010;20:2583–91. doi: 10.1093/cercor/bhq006. [DOI] [PubMed] [Google Scholar]
- 158.Kallur T, Darsalia V, Lindvall O, Kokaia Z. Human fetal cortical and striatal neural stem cells generate region-specific neurons in vitro and differentiate extensively to neurons after intrastriatal transplantation in neonatal rats. J Neurosci Res. 2006;84:1630–44. doi: 10.1002/jnr.21066. [DOI] [PubMed] [Google Scholar]
- 159.Anderson GW, Deans PJ, Taylor RD, Raval P, Chen D, Lowder H, et al. Characterisation of neurons derived from a cortical human neural stem cell line CTX0E16. Stem Cell Res Ther. 2015;6:149. doi: 10.1186/s13287-015-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cacci E, Villa A, Parmar M, Cavallaro M, Mandahl N, Lindvall O, et al. Generation of human cortical neurons from a new immortal fetal neural stem cell line. Exp Cell Res. 2007;313:588–601. doi: 10.1016/j.yexcr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 161.Micali N, Kim SK, Diaz-Bustamante M, Stein-O’Brien G, Seo S, Shin JH, et al. Variation of human neural stem cells generating organizer states in vitro before committing to cortical excitatory or inhibitory neuronal fates. Cell Rep. 2020;31:107599. doi: 10.1016/j.celrep.2020.107599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–7. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- 163.Kim JE, O’Sullivan ML, Sanchez CA, Hwang M, Israel MA, Brennand K, et al. Investigating synapse formation and function using human pluripotent stem cell-derived neurons. Proc Natl Acad Sci USA. 2011;108:3005–10. doi: 10.1073/pnas.1007753108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shi Y, Kirwan P, Livesey FJ. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc. 2012;7:1836–46. doi: 10.1038/nprot.2012.116. [DOI] [PubMed] [Google Scholar]
- 165.Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15:477–86. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Palma-Tortosa S, Tornero D, Hansen G, Monni E, Hajy M, Kartsivadze S, et al. Activity in grafted human iPS cell-derived cortical neurons integrated in stroke-injured rat brain regulates motor behavior. Proc Natl Acad Sci USA. 2020;117:9094–100. doi: 10.1073/pnas.2000690117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cao SY, Hu Y, Chen C, Yuan F, Xu M, Li Q, et al. Enhanced derivation of human pluripotent stem cell-derived cortical glutamatergic neurons by a small molecule. Sci Rep. 2017;7:3282. doi: 10.1038/s41598-017-03519-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tornero D, Tsupykov O, Granmo M, Rodriguez C, Gronning-Hansen M, Thelin J, et al. Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain. 2017;140:692–706. doi: 10.1093/brain/aww347. [DOI] [PubMed] [Google Scholar]
- 169.Tornero D, Wattananit S, Gronning Madsen M, Koch P, Wood J, Tatarishvili J, et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain. 2013;136:3561–77. doi: 10.1093/brain/awt278. [DOI] [PubMed] [Google Scholar]
- 170.Chen C, Dong X, Fang KH, Yuan F, Hu Y, Xu M, et al. Develop a 3D neurological disease model of human cortical glutamatergic neurons using micropillar-based scaffolds. Acta Pharm Sin B. 2019;9:557–64. doi: 10.1016/j.apsb.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vergano-Vera E, Diaz-Guerra E, Rodriguez-Traver E, Mendez-Gomez HR, Solis O, Pignatelli J, et al. Nurr1 blocks the mitogenic effect of FGF-2 and EGF, inducing olfactory bulb neural stem cells to adopt dopaminergic and dopaminergic-GABAergic neuronal phenotypes. Dev Neurobiol. 2015;75:823–41. doi: 10.1002/dneu.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ghareghani M, Sadeghi H, Zibara K, Danaei N, Azari H, Ghanbari A. Melatonin increases oligodendrocyte differentiation in cultured neural stem cells. Cell Mol Neurobiol. 2017;37:1319–24. doi: 10.1007/s10571-016-0450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397:2284–303. doi: 10.1016/s0140-6736(21)00218-x. [DOI] [PubMed] [Google Scholar]
- 174.Han F, Hu B. Stem cell therapy for Parkinson’s disease. Adv Exp Med Biol. 2020;1266:21–38. doi: 10.1007/978-981-15-4370-8_3. [DOI] [PubMed] [Google Scholar]
- 175.Gaillard A, Decressac M, Frappe I, Fernagut PO, Prestoz L, Besnard S, et al. Anatomical and functional reconstruction of the nigrostriatal pathway by intranigral transplants. Neurobiol Dis. 2009;35:477–88. doi: 10.1016/j.nbd.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 176.Mendez I, Sanchez-Pernaute R, Cooper O, Vinuela A, Ferrari D, Bjorklund L, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005;128:1498–510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mukhida K, Baker KA, Sadi D, Mendez I. Enhancement of sensorimotor behavioral recovery in hemiparkinsonian rats with intrastriatal, intranigral, and intrasubthalamic nucleus dopaminergic transplants. J Neurosci. 2001;21:3521–30. doi: 10.1523/JNEUROSCI.21-10-03521.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Grealish S, Diguet E, Kirkeby A, Mattsson B, Heuer A, Bramoulle Y, et al. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell. 2014;15:653–65. doi: 10.1016/j.stem.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xiong M, Tao Y, Gao Q, Feng B, Yan W, Zhou Y, et al. Human stem cell-derived neurons repair circuits and restore neural function. Cell Stem Cell. 2021;28:112–126.e116. doi: 10.1016/j.stem.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Steinbeck JA, Choi SJ, Mrejeru A, Ganat Y, Deisseroth K, Sulzer D, et al. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat Biotechnol. 2015;33:204–9. doi: 10.1038/nbt.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tonnesen J, Parish CL, Sorensen AT, Andersson A, Lundberg C, Deisseroth K, et al. Functional integration of grafted neural stem cell-derived dopaminergic neurons monitored by optogenetics in an in vitro Parkinson model. PLoS One. 2011;6:e17560. doi: 10.1371/journal.pone.0017560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bjorklund A, Parmar M. Dopamine cell therapy: from cell replacement to circuitry repair. J Parkinsons Dis. 2021;11:S159–S165. doi: 10.3233/JPD-212609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Adler AF, Cardoso T, Nolbrant S, Mattsson B, Hoban DB, Jarl U, et al. hESC-Derived dopaminergic transplants integrate into Basal Ganglia circuitry in a preclinical model of Parkinson’s disease. Cell Rep. 2019;28:3462–3473.e3465. doi: 10.1016/j.celrep.2019.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Thompson LH, Grealish S, Kirik D, Bjorklund A. Reconstruction of the nigrostriatal dopamine pathway in the adult mouse brain. Eur J Neurosci. 2009;30:625–38. doi: 10.1111/j.1460-9568.2009.06878.x. [DOI] [PubMed] [Google Scholar]
- 185.Aldrin-Kirk P, Akerblom M, Cardoso T, Nolbrant S, Adler AF, Liu X, et al. A novel two-factor monosynaptic TRIO tracing method for assessment of circuit integration of hESC-derived dopamine transplants. Stem Cell Rep. 2022;17:159–72. doi: 10.1016/j.stemcr.2021.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Niclis JC, Gantner CW, Hunt CPJ, Kauhausen JA, Durnall JC, Haynes JM, et al. A PITX3-EGFP reporter line reveals connectivity of dopamine and non-dopamine neuronal subtypes in grafts generated from human embryonic stem cells. Stem Cell Rep. 2017;9:868–82. doi: 10.1016/j.stemcr.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chen Y, Xiong M, Dong Y, Haberman A, Cao J, Liu H, et al. Chemical control of grafted human PSC-derived neurons in a mouse model of Parkinson’s disease. Cell Stem Cell. 2016;18:817–26. doi: 10.1016/j.stem.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.de Luzy IR, Pavan C, Moriarty N, Hunt CPJ, Vandenhoven Z, Khanna A, et al. Identifying the optimal developmental age of human pluripotent stem cell-derived midbrain dopaminergic progenitors for transplantation in a rodent model of Parkinson’s disease. Exp Neurol. 2022;358:114219. doi: 10.1016/j.expneurol.2022.114219. [DOI] [PubMed] [Google Scholar]
- 189.Kauhausen J, Thompson LH, Parish CL. Cell intrinsic and extrinsic factors contribute to enhance neural circuit reconstruction following transplantation in Parkinsonian mice. J Physiol. 2013;591:77–91. doi: 10.1113/jphysiol.2012.243063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Torres EM, Monville C, Gates MA, Bagga V, Dunnett SB. Improved survival of young donor age dopamine grafts in a rat model of Parkinson’s disease. Neuroscience. 2007;146:1606–17. doi: 10.1016/j.neuroscience.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 191.Gates MA, Torres EM, White A, Fricker-Gates RA, Dunnett SB. Re-examining the ontogeny of substantia nigra dopamine neurons. Eur J Neurosci. 2006;23:1384–90. doi: 10.1111/j.1460-9568.2006.04637.x. [DOI] [PubMed] [Google Scholar]
- 192.Freeman TB, Sanberg PR, Nauert GM, Boss BD, Spector D, Olanow CW, et al. The influence of donor age on the survival of solid and suspension intraparenchymal human embryonic nigral grafts. Cell Transpl. 1995;4:141–54. doi: 10.1177/096368979500400118. [DOI] [PubMed] [Google Scholar]
- 193.Bye CR, Thompson LH, Parish CL. Birth dating of midbrain dopamine neurons identifies A9 enriched tissue for transplantation into parkinsonian mice. Exp Neurol. 2012;236:58–68. doi: 10.1016/j.expneurol.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 194.Blaess S, Bodea GO, Kabanova A, Chanet S, Mugniery E, Derouiche A, et al. Temporal-spatial changes in Sonic Hedgehog expression and signaling reveal different potentials of ventral mesencephalic progenitors to populate distinct ventral midbrain nuclei. Neural Dev. 2011;6:29. doi: 10.1186/1749-8104-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Joksimovic M, Anderegg A, Roy A, Campochiaro L, Yun B, Kittappa R, et al. Spatiotemporally separable Shh domains in the midbrain define distinct dopaminergic progenitor pools. Proc Natl Acad Sci USA. 2009;106:19185–90. doi: 10.1073/pnas.0904285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schweitzer JS, Song B, Herrington TM, Park TY, Lee N, Ko S, et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N. Engl J Med. 2020;382:1926–32. doi: 10.1056/NEJMoa1915872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kirkeby A, Nolbrant S, Tiklova K, Heuer A, Kee N, Cardoso T, et al. Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC-based therapy for Parkinson’s disease. Cell Stem Cell. 2017;20:135–48. doi: 10.1016/j.stem.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Datta A, Sarmah D, Mounica L, Kaur H, Kesharwani R, Verma G, et al. Cell death pathways in ischemic stroke and targeted pharmacotherapy. Transl Stroke Res. 2020;11:1185–202. doi: 10.1007/s12975-020-00806-z. [DOI] [PubMed] [Google Scholar]
- 199.Campbell BCV, Khatri P. Stroke. Lancet. 2020;396:129–42. doi: 10.1016/s0140-6736(20)31179-x. [DOI] [PubMed] [Google Scholar]
- 200.Patel P, Yavagal D, Khandelwal P. Hyperacute management of ischemic strokes: JACC focus seminar. J Am Coll Cardiol. 2020;75:1844–56. doi: 10.1016/j.jacc.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 201.Stoll G, Pham M. Beyond recanalization—a call for action in acute stroke. Nat Rev Neurol. 2020;16:591–2. doi: 10.1038/s41582-020-00417-0. [DOI] [PubMed] [Google Scholar]
- 202.Chen J, Duris K, Yang X. Effect of cerebral microbleeds on hemorrhagic transformation and functional prognosis after intravenous thrombolysis of cerebral infarction. Brain Hemorrhages. 2022;3:117–9. doi: 10.1016/j.hest.2021.05.004. [DOI] [Google Scholar]
- 203.Jiang Y, Han J, Spencer P, Li Y, Vodovoz SJ, Ning M-M, et al. Diabetes mellitus: a common comorbidity increasing hemorrhagic transformation after tPA thrombolytic therapy for ischemic stroke. Brain Hemorrhages. 2021;2:116–23. doi: 10.1016/j.hest.2020.11.004. [DOI] [Google Scholar]
- 204.Delavaran H, Sjunnesson H, Arvidsson A, Lindvall O, Norrving B, van Westen D, et al. Proximity of brain infarcts to regions of endogenous neurogenesis and involvement of striatum in ischaemic stroke. Eur J Neurol. 2013;20:473–9. doi: 10.1111/j.1468-1331.2012.03877.x. [DOI] [PubMed] [Google Scholar]
- 205.Abeysinghe HC, Bokhari L, Quigley A, Choolani M, Chan J, Dusting GJ, et al. Pre-differentiation of human neural stem cells into GABAergic neurons prior to transplant results in greater repopulation of the damaged brain and accelerates functional recovery after transient ischemic stroke. Stem Cell Res Ther. 2015;6:186. doi: 10.1186/s13287-015-0175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang Y, Liu G, Hong D, Chen F, Ji X, Cao G. White matter injury in ischemic stroke. Prog Neurobiol. 2016;141:45–60. doi: 10.1016/j.pneurobio.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shi L, Sun Z, Su W, Xu F, Xie D, Zhang Q, et al. Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke. Immunity. 2021;54:1527–1542 e1528. doi: 10.1016/j.immuni.2021.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xu J, Zhao J, Wang R, Zhang Y, Shen L, Xiao Q, et al. Shh and Olig2 sequentially regulate oligodendrocyte differentiation from hiPSCs for the treatment of ischemic stroke. Theranostics. 2022;12:3131–49. doi: 10.7150/thno.69217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Niclis JC, Gantner CW, Alsanie WF, McDougall SJ, Bye CR, Elefanty AG, et al. Efficiently specified ventral midbrain dopamine neurons from human pluripotent stem cells under xeno-free conditions restore motor deficits in parkinsonian rodents. Stem Cells Transl Med. 2017;6:937–48. doi: 10.5966/sctm.2016-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vieira MS, Santos AK, Vasconcellos R, Goulart VAM, Parreira RC, Kihara AH, et al. Neural stem cell differentiation into mature neurons: Mechanisms of regulation and biotechnological applications. Biotechnol Adv. 2018;36:1946–70. doi: 10.1016/j.biotechadv.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 211.Cacci E, Negri R, Biagioni S, Lupo G. Histone methylation and microRNA-dependent regulation of epigenetic activities in neural progenitor self-renewal and differentiation. Curr Top Med Chem. 2017;17:794–807. doi: 10.2174/1568026616666160414124456. [DOI] [PubMed] [Google Scholar]
- 212.Shi Z, Zhou H, Lu L, Pan B, Wei Z, Liu J, et al. MicroRNA-29a regulates neural stem cell neuronal differentiation by targeting PTEN. J Cell Biochem. 2018;119:5813–20. doi: 10.1002/jcb.26768. [DOI] [PubMed] [Google Scholar]
- 213.Roese-Koerner B, Stappert L, Koch P, Brustle O, Borghese L. Pluripotent stem cell-derived somatic stem cells as tool to study the role of microRNAs in early human neural development. Curr Mol Med. 2013;13:707–22. doi: 10.2174/1566524011313050003. [DOI] [PubMed] [Google Scholar]
- 214.Stappert L, Borghese L, Roese-Koerner B, Weinhold S, Koch P, Terstegge S, et al. MicroRNA-based promotion of human neuronal differentiation and subtype specification. PLoS One. 2013;8:e59011. doi: 10.1371/journal.pone.0059011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Low WC, Yau WW, Stanton LW, Marcy G, Goh E, Chew SY. Directing neuronal differentiation of primary neural progenitor cells by gene knockdown approach. DNA Cell Biol. 2012;31:1148–60. doi: 10.1089/dna.2011.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Karimi M, Bahrami S, Mirshekari H, Basri SM, Nik AB, Aref AR, et al. Microfluidic systems for stem cell-based neural tissue engineering. Lab Chip. 2016;16:2551–71. doi: 10.1039/c6lc00489j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Darvishi M, Ghasemi Hamidabadi H, Sahab Negah S, Moayeri A, Tiraihi T, Mirnajafi-Zadeh J, et al. PuraMatrix hydrogel enhances the expression of motor neuron progenitor marker and improves adhesion and proliferation of motor neuron-like cells. Iran J Basic Med Sci. 2020;23:431–8. doi: 10.22038/ijbms.2020.39797.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zimmermann JA, Schaffer DV. Engineering biomaterials to control the neural differentiation of stem cells. Brain Res Bull. 2019;150:50–60. doi: 10.1016/j.brainresbull.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 219.Chao KY, Huang WY, Ho CY, Wan D, Wang HC, Yang CY, et al. Biodegradable aniline-derived electroconductive film for the regulation of neural stem cell fate. J Mater Chem B. 2021;9:1325–35. doi: 10.1039/d0tb02171g. [DOI] [PubMed] [Google Scholar]
- 220.Temple S. The development of neural stem cells. Nature. 2001;414:112–7. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 221.Nishizawa M, Chonabayashi K, Nomura M, Tanaka A, Nakamura M, Inagaki A, et al. Epigenetic variation between human induced pluripotent stem cell lines is an indicator of differentiation capacity. Cell Stem Cell. 2016;19:341–54. doi: 10.1016/j.stem.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 222.Gao M, Yao H, Dong Q, Zhang H, Yang Z, Yang Y, et al. Tumourigenicity and immunogenicity of induced neural stem cell grafts versus induced pluripotent stem cell grafts in syngeneic mouse brain. Sci Rep. 2016;6:29955. doi: 10.1038/srep29955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chen Z. Cell therapy for Parkinson’s disease: new hope from reprogramming technologies. Aging Dis. 2015;6:499–503. doi: 10.14336/AD.2014.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Doi D, Magotani H, Kikuchi T, Ikeda M, Hiramatsu S, Yoshida K, et al. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nat Commun. 2020;11:3369. doi: 10.1038/s41467-020-17165-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Samata B, Doi D, Nishimura K, Kikuchi T, Watanabe A, Sakamoto Y, et al. Purification of functional human ES and iPSC-derived midbrain dopaminergic progenitors using LRTM1. Nat Commun. 2016;7:13097. doi: 10.1038/ncomms13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Itakura G, Kawabata S, Ando M, Nishiyama Y, Sugai K, Ozaki M, et al. Fail-safe system against potential tumorigenicity after transplantation of iPSC derivatives. Stem Cell Rep. 2017;8:673–84. doi: 10.1016/j.stemcr.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tieng V, Cherpin O, Gutzwiller E, Zambon AC, Delgado C, Salmon P, et al. Elimination of proliferating cells from CNS grafts using a Ki67 promoter-driven thymidine kinase. Mol Ther Methods Clin Dev. 2016;6:16069. doi: 10.1038/mtm.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Thompson LH, Andersson E, Jensen JB, Barraud P, Guillemot F, Parmar M, et al. Neurogenin2 identifies a transplantable dopamine neuron precursor in the developing ventral mesencephalon. Exp Neurol. 2006;198:183–98. doi: 10.1016/j.expneurol.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 229.Rossi F, Cattaneo E. Opinion: neural stem cell therapy for neurological diseases: dreams and reality. Nat Rev Neurosci. 2002;3:401–9. doi: 10.1038/nrn809. [DOI] [PubMed] [Google Scholar]
- 230.Xue CR, Wang K, Zhang MZ, Wang Z, Song YY, Yu HJ, et al. Tracking neural stem cells in vivo: achievements and limitations. Stem Cell Rev Rep. 2022;18:1774–88. doi: 10.1007/s12015-022-10333-z. [DOI] [PubMed] [Google Scholar]
- 231.Gowing G, Svendsen S, Svendsen CN. Ex vivo gene therapy for the treatment of neurological disorders. Prog Brain Res. 2017;230:99–132. doi: 10.1016/bs.pbr.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 232.Akhtar AA, Gowing G, Kobritz N, Savinoff SE, Garcia L, Saxon D, et al. Inducible expression of GDNF in transplanted iPSC-derived neural progenitor cells. Stem Cell Rep. 2018;10:1696–704. doi: 10.1016/j.stemcr.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hwang DH, Kim BG, Kim EJ, Lee SI, Joo IS, Suh-Kim H, et al. Transplantation of human neural stem cells transduced with Olig2 transcription factor improves locomotor recovery and enhances myelination in the white matter of rat spinal cord following contusive injury. BMC Neurosci. 2009;10:117. doi: 10.1186/1471-2202-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Garcia-Leon JA, Kumar M, Boon R, Chau D, One J, Wolfs E, et al. SOX10 single transcription factor-based fast and efficient generation of oligodendrocytes from human pluripotent stem cells. Stem Cell Rep. 2018;10:655–72. doi: 10.1016/j.stemcr.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]