Abstract
INTRODUCTION:
Empathy relies on fronto-cingular and temporal networks that are selectively vulnerable in behavioral variant frontotemporal dementia (bvFTD). This study modeled when in the disease process empathy changes begin, and how they progress.
METHODS:
431 individuals with asymptomatic genetic FTD (n=114), genetic and sporadic bvFTD (n=317), and 163 asymptomatic non-carrier controls were enrolled. In subsamples, we investigated empathy measured by the informant-based Interpersonal Reactivity Index (IRI) at each disease stage and over time (n=91), and its correspondence to underlying atrophy (n=51).
RESULTS:
Empathic concern (estimate=4.38, 95%CI=[2.79, 5.97], p<0.001) and perspective taking (estimate=5.64, 95%CI=[3.81, 7.48], p<0.001) scores declined between the asymptomatic and very mild symptomatic stages regardless of pathogenic variant status. More rapid loss of empathy corresponded with subcortical atrophy.
DISCUSSION:
Loss of empathy is an early and progressive symptom of bvFTD that is measurable by IRI informant-ratings and can be used to monitor behavior in neuropsychiatry practice and treatment trials.
Keywords: Behavioral variant frontotemporal dementia, emotional empathy, cognitive empathy, Interpersonal Reactivity Index, volumetric MRI, clinical trials
BACKGROUND
Striking loss of empathy is a well-known key feature of behavioral variant frontotemporal dementia (bvFTD),1,2 which manifests early in the course of illness in reduced interest in, and emotional response to, other people’s feelings. While bvFTD patients often show poor self-awareness of their socioemotional deficits,3 caregivers of patients with loss of empathy show heightened levels of burden, loneliness, and depression.4 Loss of empathy also has a negative impact on relationship status, including frequency of relationship dissolution and infidelity.5 While numerous smaller, cross-sectional studies have examined various aspects of empathy in bvFTD,2,6,7 until recently large longitudinal patient cohorts have not been available with which to model more precise empathy estimates or show the rate of empathy change over the whole course of the disease, including at the very earliest prodromal stages. The focus of this study is to perform this modeling by using for the first time the very comprehensive empathy dataset from the cohort of bvFTD patients in the large longitudinal multisite ALLFTD study.
Empathy involves a complex set of emotional and cognitive processes: an affective response that may include affect sharing, perspective taking, assignment of agency, suppression of one’s viewpoint, and a prosocial motivation or the desire to help.8,9 Emotional empathy engages mainly regions of two brain networks that are affected in early bvFTD: the salience network (SN) underlying homeostatically-guided attention,10 and the semantic-appraisal network (SAN) that links stored social concepts with their hedonic valence, including reward value.11 When empathy involves greater levels of cognitive perspective taking, regions of the default-mode network (DMN) that are involved in higher-order executive aspects of social cognition such as predicting outcomes and imagining others’ intentions are recruited.12,13
We used the Interpersonal Reactivity Index (IRI)14 to examine the trajectory of loss of empathy in bvFTD. Specifically, we investigated whether empathy changes as a function of disease stage from asymptomatic to very mild and more advanced bvFTD, both in carriers of pathogenic variants in the main FTD genes (C9orf72 that is technically a hexanucleotide expansion and we will refer to simply as a gene; GRN; MAPT) and in non-carriers. In a true longitudinal subsample, we examined whether empathy declines with progression, and whether rate of change in empathy corresponds to rate of atrophy in the SN, SAN, and DMN. Based on cross-sectional evidence showing that emotional and cognitive empathy is affected in bvFTD,1,2,15 we expected that both aspects of empathy would deteriorate over time. In addition, we hypothesized that rate of decline in emotional empathy would correspond to rate of atrophy in the SN and SAN, whereas rate of decline in cognitive empathy would additionally be associated with rate of atrophy in the DMN.
METHODS
Participants
We enrolled 594 participants from the UCSF FTD PPG and the multisite ALLFTD (previously ARTFL and LEFFTDS consortia) studies between 1999 and 2018. The sample consisted of 307 patients with clinical bvFTD16 (88 carried a pathogenic variant in one of the three autosomal dominant FTD genes C9orf72, MAPT, GRN), 10 pathogenic variant carriers with behavioral Mild Cognitive Impairment (MCI), and 277 asymptomatic pathogenic variant positive (n=114) and negative (n=163) individuals. Patients with behavioral MCI had one or two of the key features as required for possible bvFTD:17 disinhibition, apathy or inertia, loss of sympathy/empathy, ritualistic/compulsive behavior, or hyperorality and appetite changes, and no cognitive domain impaired other than behavior. The asymptomatic pathogenic variant negative individuals were noncarrier family members who served as controls for the asymptomatic pathogenic variant positive group because of their similar demographics, background, and environment. Participants’ diagnoses were based on thorough neurological, neuropsychological, neuroimaging, and genetic examination. Each participant was required to have a spouse/partner, first-degree family member or friend who had known the participant for five or more years, and to have at least one timepoint of informant ratings on the Empathic Concern (EC) and Perspective Taking (PT) subscales of the IRI14 available. For our first set of analyses examining the EC and PT subscale scores at each level of disease severity, we included all 594 participants and all timepoints. For our second (true longitudinal) set of behavioral analyses in which we examined change in IRI subscale scores in bvFTD over time, we included only the 91 patients (212 observations) from the above sample (40 sporadic bvFTD, 37 genetic bvFTD, 7 genetic MCI, 6 asymptomatic pathogenic variant positive, 1 asymptomatic pathogenic variant negative) who had at least two timepoints of valid IRI data. The subsample with both longitudinal behavioral and structural imaging data available consisted of 51 participants (124 observations). The average time interval between IRI data collection and MRI scanning was 2.7±8.4 (M±SD) days. To compare patients’ longitudinal pattern on the IRI to a healthy control group who had at least two timepoints of IRI data available, we included 130 neurologically and cognitively healthy older adults (age: 68.8±7.6; sex [M/F]: 56/74) from the Hillblom Network Program. The parent studies (PPG, ALLFTD, Hillblom) were conducted in accordance with IRB approval from each study institution, and all participants and their informants gave their consent to participate and to share data.
Clinical measures
To measure emotional and cognitive empathy, we used informant-ratings on the EC and PT subscales of the IRI informant questionnaire14 because they show the best psychometric characteristics among the four IRI subscales18,19 and are the most widely used informant-based measures of empathy in dementia.2,20,21 The 7-item EC subscale assesses people’s tendency to generate an other-centered prosocial response resulting from correctly inferring another’s emotional state and resulting psychological needs (emotional empathy). By contrast, the 7-item PT subscale measures people’s tendency to spontaneously imagine the thought processes and perspective of another person (cognitive empathy). Each participant had an informant who described the subject’s current level of empathy using a 5-point Likert scale, ranging from “does not describe well” to “describes very well”. Each subscale score ranged between 7 and 35, with higher scores showing higher levels of empathy. The informants were carefully identified based on the relationship and closeness to the participant, frequency of contact, and cognitive status of the informant. The majority of informants were spouses (62%), followed by adult children, siblings or other relatives (26%), friends (7%), and others (5%).
We used another informant-based measure, the CDR® Dementia Staging Instrument plus Behavior and Language domains from the NACC FTLD Module (CDR® plus NACC FTLD) as a proxy of disease severity. The measure is an extension of the standard CDR, and includes two additional domains that are predominantly affected in FTD: behavior and language.22 Each patient’s CDR® plus NACC FTLD global score was calculated based on the scoring rules by Miyagawa et al. (2020)20 (0=normal, 0.5=very mildly impaired/MCI stage, 1=mildly impaired, 2=moderately impaired, 3=severely impaired).
The Zarit Burden Interview24 is a 22-item self-report measure of stress and burden experienced by caregivers. The questionnaire was used to assess burden in different domains, including behavioral symptoms and functional status of the patient, interpersonal relationships, finances, physical health, and social life.
Neuroimaging
Acquisition and preprocessing of structural images was performed as described in the supplementary materials. We used the Desikan brain atlas25 and defined regions of interest (ROIs) in each network. The bilateral anterior insula (AI), dorsal anterior cingulate cortex (ACC), thalamus, and amygdala ROIs comprise the SN10 (Supplementary Fig. 1). The SAN ROIs included the bilateral caudate, nucleus accumbens, temporal pole (medial part), lateral orbitofrontal cortex (OFC), and the subgenual ACC.11 The ROIs of the DMN were defined in the bilateral dorsomedial prefrontal cortex, posterior cingulate cortex, hippocampus, parahippocampal gyrus, retrosplenial cortex, inferior parietal lobule, medial orbitofrontal cortex, temporal pole (lateral part), middle temporal gyrus, and supramarginal gyrus.12
Statistical analyses
Linear mixed effects (LME) models were performed in SAS Version 9.4 (Proc mixed) to examine in the full sample (n=594, 666 observations) whether the IRI scores are a significant predictor of the CDR® plus NACC FTLD score, controlling for age at first evaluation and sex. To investigate whether pathogenic variant carriers and non-carriers showed a different pattern of change in empathy over time, we added pathogenic variant status and the interaction pathogenic variant status by CDR® plus NACC FTLD to the model. To examine cross-sectional differences in empathy between asymptomatic FTD pathogenic variant carriers (C9orf72, GRN, MAPT) and asymptomatic non-carriers, we performed linear modelling (Proc GLM) and included each subscale as outcome variable, covarying for diagnostic group, age at first evaluation, and sex. In a subsample of asymptomatic individuals, as well as patients with behavioral MCI and bvFTD (n=449) who had valid cross-sectional data of both Zarit Burden Interview and IRI available, linear modelling was performed to examine whether each subscale score predicted Zarit Burden score, controlling for age at first evaluation and sex.
In the subsample of patients with two or more timepoints of IRI data available (n=91, 212 observations), we used LME models with random intercepts and slopes to examine whether the IRI scores declined over time and whether the slope of decline differed (1) between pathogenic variant carriers and non-carriers, and (2) between patients with different disease severities at baseline. To investigate whether rate of change in empathy was similar in patients with different disease severities according to the CDR® plus NACC FTLD score, we divided the patient sample into three groups: patients with very mild (CDR®=0.5; n=20), mild (CDR®=1.0; n=43), and moderate/severe (CDR®=2/3; n=26) disease stage at baseline. The models were comprised of disease duration, age at symptom onset, and sex. In the first interaction model, the variable pathogenic variant status and the interaction of disease duration by pathogenic variant status were included. The second interaction model was comprised of the variables CDR® plus NACC FTLD at baseline and the interaction with disease duration.
We also investigated whether rate of decline on the EC and PT subscales was associated with rate of atrophy progression in predefined ROIs in the SN, SAN, and DMN. In the subsample of 51 patients (124 observations) who had at least two timepoints of valid IRI and structural imaging scans of sufficient quality collected on the same 3T scanner MRI data available, separate LME models with random intercepts and slopes were fitted for each subscale as outcome, and ROI, disease duration, age at symptom onset, sex, and TIV were included as predictors in the models. Because the gray matter volumes in the three networks were highly correlated (r SN/SAN: 0.94; r SN/DMN: 0.92; r SAN/DMN: 0.89), we also included the mean gray matter volume in the two networks of no interest as covariates.
RESULTS
Demographic and clinical features
The demographic and clinical features of the full sample are described in Table 1. The longitudinal subsample consisted of symptomatic pathogenic variant carriers (n=50) and symptomatic non-carriers (n=41) with different disease stages of bvFTD. Age at symptom onset was statistically significantly (p=0.020) younger in pathogenic variant carriers (M±SD: 51.0±11.7) than in non-carriers (M±SD: 57.7±8.4). Carriers and non-carriers did not statistically significantly differ with regard to disease duration, proportion of males and females, or education, thus we did not use these variables as potential confounds in subsequent analyses.
Table 1:
Demographic and clinical characteristics of patient sample.
| Asymptomatic pathogenic variant− | Asymptomatic pathogenic variant+ | bvFTD pathogenic variant− | bvFTD pathogenic variant+ | Behavioral MCI pathogenic variant+ | p-value | |
|---|---|---|---|---|---|---|
| n | 163 | 114 | 219 | 88 | 10 | -- |
| Age at first evaluation | 47.9 (13.6) | 43.5 (14.5) | 62.7 (8.7) | 58.4 (8.5) | 53.8 (12.5) | <0.0001 |
| Pathogenic variant, C9orf72, GRN, MAPT | --- | 46, 30, 38 | --- | 45, 13, 30 | 4, 3, 3 | --- |
| Sex, M/F | 59/103 | 50/63 | 138/80 | 44/43 | 6/4 | <0.0001 |
| Education | 15.5 (2.5) | 15.6 (2.6) | 15.7 (2.9) | 15.3 (2.8) | 14.8 (2.0) | =0.512 |
| Global CDR® plus NACC FTLD | 0 | 0 | 1.6 (0.7) | 1.6 (0.7) | 0.6 (0.2) | <0.001 |
bvFTD=behavioral variant frontotemporal dementia; MCI=Mild Cognitive Impairment; C9orf72=Chromosome 9 open reading frame 72; GRN=Progranulin; MAPT=Microtubule-associated protein tau; M=Male, F=Female; CDR® plus NACC FTLD= CDR® Dementia Staging Instrument plus Behavior and Language domains from the NACC FTLD Module
Longitudinal behavior subsample: Number of patients with 2 timepoints: 34; Number of patients with 3 timepoints: 13; Number of patients with 4+ timepoints: 4; Number of healthy controls with 2 timepoints: 55; Number of healthy controls with 3 timepoints: 30; Number of healthy controls with 4+ timepoints: 45.
Cross-sectional behavioral modelling
Empathy by disease severity in the full sample:
CDR® plus NACC FTLD was a statistically significant predictor of both the EC (p<0.001) and PT (p<0.001) score (Fig. 1A/B). The results showed that the EC score significantly declined at each stage from asymptomatic to very mild (estimate=4.38, 95%CI=[2.79, 5.97], p<0.001), very mild to mild (estimate=−3.52, 95%CI=[−5.19, −1.84], p<0.001), and mild to moderate/severe (estimate=2.93, 95%CI=[1.71, 4.14], p<0.001) disease (Table 2). Similarly, the PT score showed a statistically significant drop between asymptomatic and very mild stages (estimate=5.64, 95%CI=[3.81, 7.48], p<0.001), very mild and mild stages (estimate=−3.24, 95%CI=[−4.82, −1.13], p=0.002), as well as mild and moderate/severe (estimate=1.49, 95%CI=[0.18, 2.79], p=0.023) stages. Because the interaction of CDR® plus NACC FTLD by pathogenic variant status did not reach statistical significance in either EC or PT models in our sample, further analyses of this relationship were not performed.
Fig. 1.
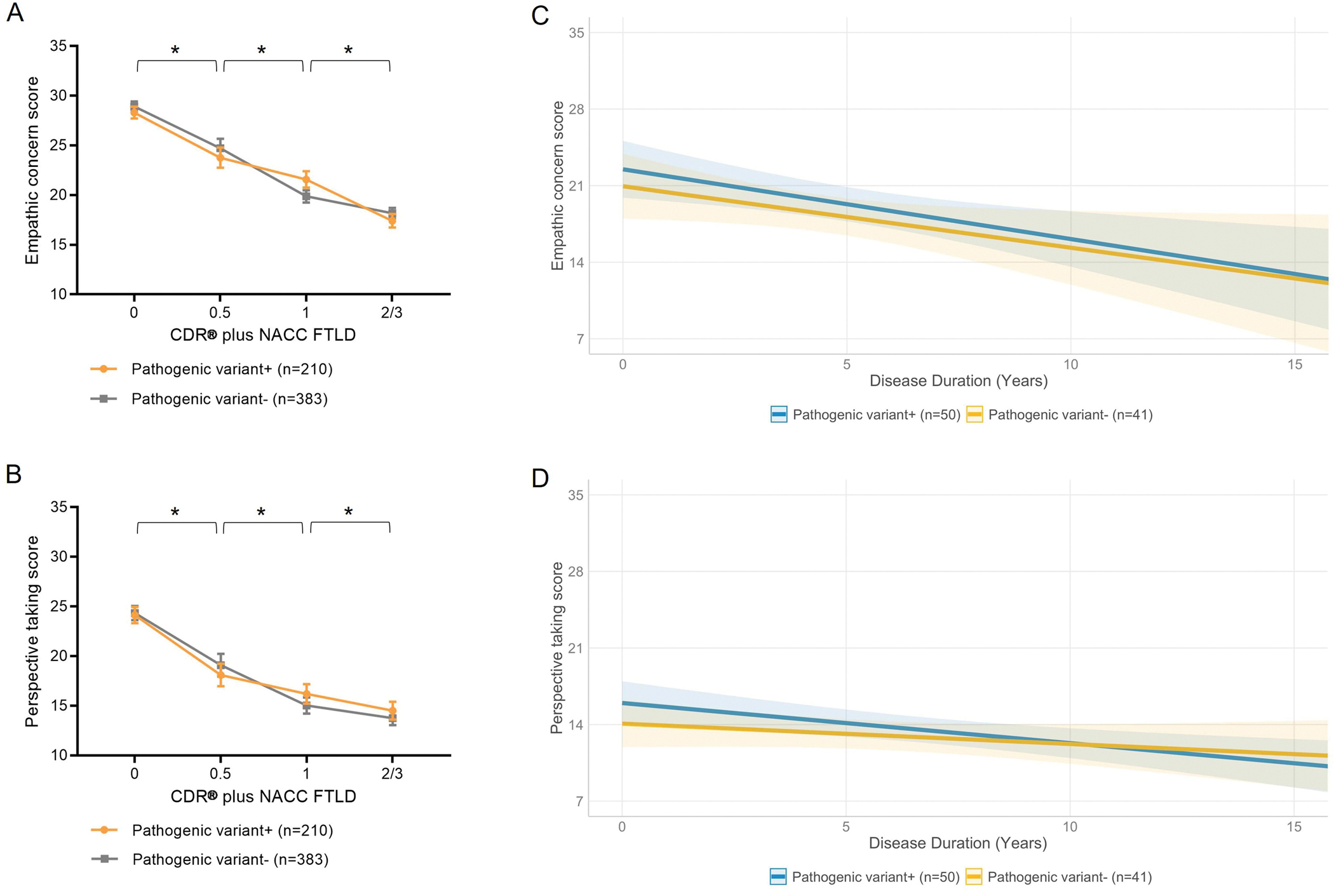
EC and PT scores reflect disease severity measured by the global CDR® plus NACC FTLD score regardless of pathogenic variant status, and worsen at a similar rate over time in pathogenic variant carriers and non-carriers.
(A) LME model analysis in the full sample (n=594) revealed a significant main effect of CDR® plus NACC FTLD (p<0.001) with regard to the EC score, showing that the score changes as a function of disease stage from asymptomatic to very mild (estimate=4.38, 95%CI=[2.79, 5.97], p<0.001), very mild to mild (estimate=−3.52, 95%CI=[−5.19, −1.84], p<0.001), and mild to moderate/severe (estimate=2.93, 95%CI=[1.71, 4.14], p<0.001) disease. The interaction between CDR® plus NACC FTLD and pathogenic variant status did not reach statistical significance. (B) Similar to the EC subscale, CDR® plus NACC FTLD significantly predicted the PT score (p<0.001), showing that the score significantly worsened between asymptomatic and very mild (estimate=5.64, 95%CI=[3.81, 7.48], p<0.001), very mild and mild (estimate=−3.24, 95%CI=[−4.82, −1.13], p=0.002), as well as between mild and moderate/severe (estimate=1.49, 95%CI=[0.18, 2.79], p=0.023) disease stage. The interaction CDR® plus NACC FTLD by disease stage at baseline did not reach statistical significance for predicting the PT score. (C) In the fully longitudinal sample (n=91), disease duration significantly predicted (estimate=−0.29, 95%CI=[−0.59, −0.00], p=0.049) the EC score, demonstrating that patients with longer disease duration had lower EC score compared to patients with shorter disease duration. However, the interaction disease duration by pathogenic variant status was not statistically significant. (D) The PT score was significantly predicted by disease duration (estimate=−0.49, 95%CI=[−0.90, −0.09], p=0.019), showing that the score significantly decreased over time with longer disease duration. The interaction between disease duration and pathogenic variant status did not reach statistical significance for predicting the PT score. Age at symptom onset and sex were included as covariates of no interest in each analysis. CDR® plus NACC FTLD=CDR® Dementia Staging Instrument plus Behavior and Language domains from the NACC FTLD Module.
Table 2:
Summary of significant results showing the relationship among empathy, disease stage, disease duration, and neuroanatomy.
| Sample | Modeled variables | b coefficient | 95% CI | p-value |
|---|---|---|---|---|
|
| ||||
| Full (n=594, 666 obs.) | Empathic Concern (EC) | |||
| -Asymptomatic (0) vs. very mild (0.5) | 4.38 | 2.79, 5.97 | <0.001 | |
| -Very mild (0.5) vs. mild (1) | −3.52 | −5.19, −1.84 | <0.001 | |
| -Mild (1) vs. moderate/severe (2/3) | 2.93 | 1.71, 4.14 | <0.001 | |
| Perspective Taking (PT) | ||||
| -Asymptomatic (0) vs. very mild (0.5) | 5.64 | 3.81, 7.48 | <0.001 | |
| -Very mild (0.5) vs. mild (1) | −3.24 | −4.82, −1.13 | =0.002 | |
| -Mild (1) vs. moderate/severe (2/3) | 1.49 | 0.18, 2.79 | =0.023 | |
|
| ||||
| Asymptomatic (n=277, 277 obs.) | Empathic Concern (EC) | |||
| -Asymptomatic (0) C9orf72 vs. asymptomatic (0) pathogenic variant negative |
η2=0.03 | 0.00, 0.07 | =0.067 | |
|
| ||||
| Longitudinal (n=91, 212 obs.) | Disease duration | |||
| -Empathic Concern (EC) | −0.58 | −0.88, −0.28 | <0.001 | |
| -Perspective Taking (PT) | −0.29 | −0.50, −0.08 | =0.010 | |
| -CDR® plus NACC FTLD/EC | −0.79 | −1.5, −0.11 | =0.003 | |
| -CDR® plus NACC FTLD/PT | −0.34 | −0.87, 0.19 | =0.050 | |
| Anatomic correlations | ||||
| -Left thalamus/EC | 0.01 | 0.00, 0.01 | <0.001 | |
| -Left caudate/EC | 0.01 | 0.00, 0.01 | =0.049 | |
| -Left inferior parietal/PT | 0.00 | 0.00, 0.01 | =0.023 | |
| -Right temporal pole/PT | −0.01 | −0.01, −0.00 | =0.036 | |
obs=observations, CDR® plus NACC FTLD= CDR® Dementia Staging Instrument plus Behavior and Language domains from the NACC FTLD Module
Relationship between empathy and caregiver burden:
Lower EC (estimate=−0.03, 95%CI=[−0.06, −0.00], p=0.033) and PT (estimate=−0.04, 95%CI=[−0.09, −0.00], p=0.043) scores in patients were significant predictors of higher Zarit Burden score in caregivers, showing that caregivers of patients with lower levels of empathy reported more burden in their day-to-day life.
Empathy in asymptomatic pathogenic variant positive and negative individuals:
Diagnostic group (C9orf72, GRN, MAPT, non-carriers) was a significant predictor of the EC score (p=0.036) in an omnibus analysis, though in post-hoc Dunnett-Hsu tests none of the individual asymptomatic pathogenic variant carrier groups showed a significant group difference from the asymptomatic non-carriers. The largest quantitative difference was between asymptomatic C9orf72 carriers (M±SD: 27.0±0.8) and asymptomatic non-carriers (28.95±0.40) was small enough to not be clinically significant and the effect size was small (Eta-square: 0.03; 95%CI=[0.00, 0.07], p=0.067). Similarly, asymptomatic GRN and asymptomatic MAPT carriers did not show a statistically significant difference from non-carriers. In contrast to the EC subscale, diagnostic group did not reach statistical significance for predicting the PT score.
Longitudinal behavioral modelling
Empathy over time in the longitudinal subsample:
Disease duration was a significant predictor of both EC (estimate=−0.58, 95%CI=[−0.88, −0.28], p<0.001) and PT (estimate=−0.29, 95%CI=[−0.50, −0.08], p=0.010) scores (Fig. 1 C/D). Disease duration also remained a significant predictor of EC (estimate=−0.65, 95%CI=[−1.02, −0.28], p=0.001) and PT (estimate=−0.36, 95%CI=[−0.63, −0.10], p=0.015) scores when pathogenic variant status and the interaction of disease duration by pathogenic variant status were added to the model, though the interaction of disease duration by pathogenic variant status did not reach statistical significance for predicting the EC or PT scores. To check whether the EC and PT scores showed a non-linear progression over time, we added the quadratic time (disease duration) term to the model. Our results showed that the quadratic term did not reach statistical significance in both the EC and PT model and therefore we proceeded with the simpler model for parsimony and interpretability reasons
Trajectory of empathy in different disease stages:
When disease severity at baseline measured by the CDR® plus NACC FTLD and its interaction with disease duration were added to the model, disease duration was a significant predictor of EC score (estimate=−0.79, 95%CI=[−1.5, −0.11], p=0.003), though the interaction was not independently significant (Fig. 2 A). The predictors disease duration (estimate=−0.34, 95%CI=[−0.87, 0.19], p=0.050) and the interaction between disease severity at baseline and disease duration did not reach statistical significance in the PT model (Fig. 2 B). As for the EC model, the quadratic time term (disease duration) was not significant, thus a linear progression of both the EC and PT scores over time was used for further analyses; however, our analysis may have been underpowered for detecting a quadratic effect. For comparison, the mean annual slope of change in EC (0.11±0.06) and PT (0.04±0.40) score in the normal control group was stable.
Fig. 2.
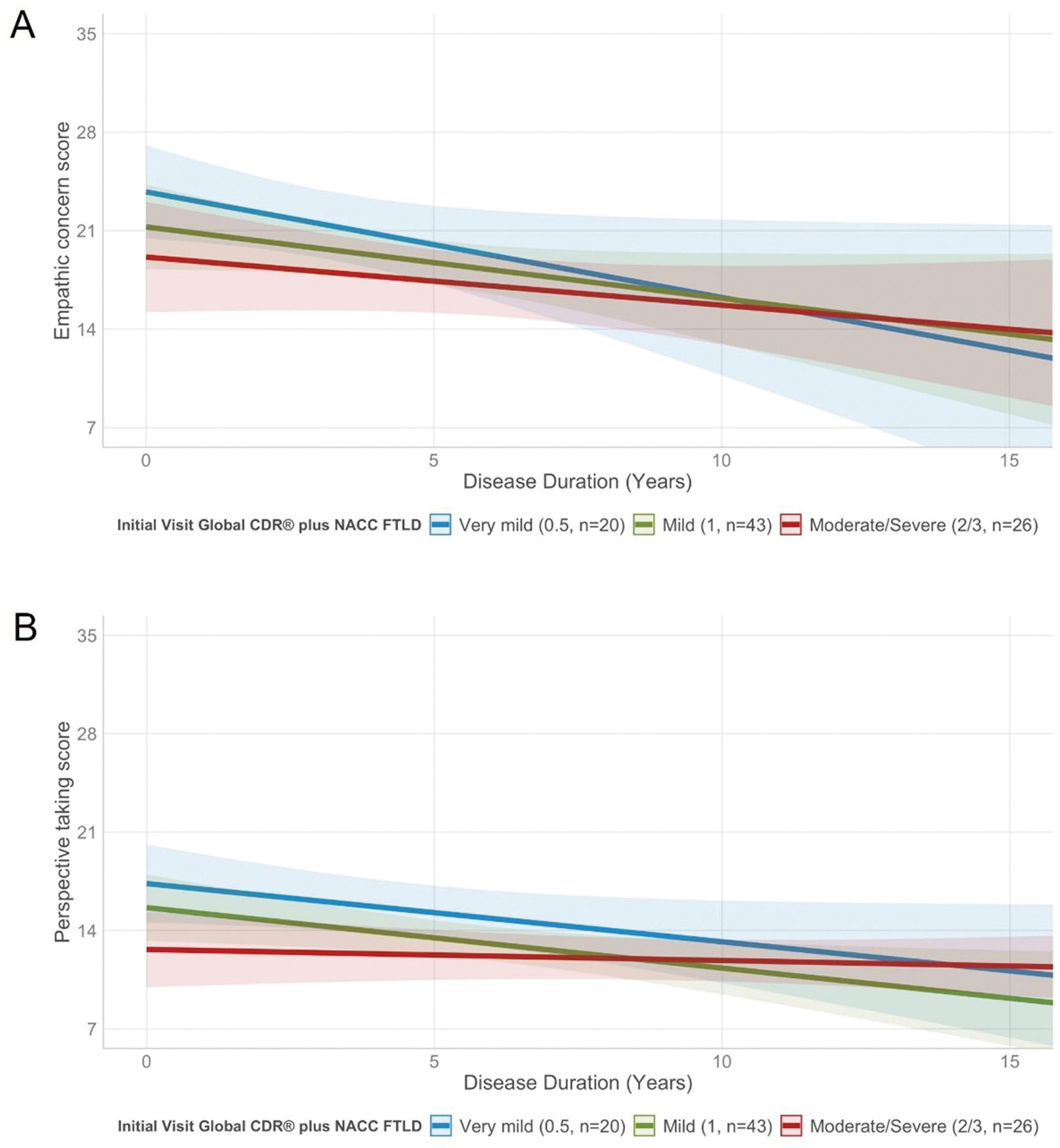
EC and PT scores of patients with early and more advanced disease stages at baseline significantly decline at a similar rate over time.
(A) The EC score was significantly predicted by disease duration (estimate=−0.42, 95%CI=[−0.98, 0.13], p=0.061), but the interaction disease duration by disease severity at baseline (very mild/mild versus moderate/severe) was not significant. This shows that patients who are in both early and more advanced disease stages show similar rates of decline on the EC subscale. (B) Disease duration significantly predicted the PT score in the main effect model (estimate=−0.21, 95%CI=[−0.57, 0.15], p=0.074). However, the interaction between disease duration and disease severity at baseline (very mild/mild versus moderate/advanced) did not reach statistical significance, demonstrating that rate of decline on the PT subscale is independent from disease severity at baseline. Age at symptom onset and sex were added to each model as covariates of no interest. CDR® plus NACC FTLD=CDR® Dementia Staging Instrument plus Behavior and Language domains from the NACC FTLD Module.
Longitudinal brain-behavior modelling
Relationship between change in empathy over time and progressive atrophy:
Faster gray matter loss in the left thalamus (estimate=0.01, 95%CI=[0.00, 0.01], p<0.001) of the SN and the left caudate (estimate=0.01, 95%CI=[0.00, 0.01], p=0.049) of the SAN was significantly associated with more rapid worsening on the EC subscale. For PT score, greater volume loss in the left inferior parietal lobule (estimate=0.00, 95%CI=[0.00, 0.01], p=0.023) and right temporal pole (estimate=−0.01, 95%CI=[−0.01, −0.00], p=0.036) of the DMN were statistically significant predictors.
DISCUSSION
This study confirms in a large sample of patients from the multi-site ALLFTD study that loss of empathy is a very early feature of bvFTD, observed in the earliest symptomatic, and in some cases even in the presymptomatic, stage of the disease, and that the changes are burdensome for caregivers. We also found evidence that empathy continues decreasing once the full phenotype is established, with lower scores in FTLD-CDR stages 2/3 than in stage 1. One of our key findings is that level of empathy is lower in very mild bvFTD than in asymptomatic individuals. In addition, the analysis we performed in the asymptomatic subgroup of carriers and non-carriers of pathogenic variants shows that individuals carrying a pathogenic C9orf72 variant have lower empathy score than either of the other two variants (GRN, MAPT) and non-carriers. Our longitudinal analyses revealed that the ability to empathize declines over time in bvFTD, and that the rate of loss of empathy corresponds to rate of volume loss in structures of the SN, SAN, and DMN. While two decades of clinical observations and smaller studies have suggested that empathy loss occurs early in bvFTD, this study is the first to provide comprehensive evidence to document this phenomenon in a well-powered sample at the very earliest disease stages.
One obstacle for early diagnosis and treatment of patients with bvFTD is that psychiatric conditions such as bipolar disorders or schizophrenia can mimic characteristic bvFTD social symptoms.26 Our findings provide novel evidence that the psychometrically validated IRI informant-based questionnaire can pick up loss of empathy in persons who are in the pre-dementia stage. The lower empathy scores in asymptomatic C9orf72 carriers compared to the other asymptomatic carrier and non-carrier groups are in line with previous studies showing that in C9orf72 carriers’ changes in behavior and in underlying key networks for social behavior predate the fully symptomatic phase of the disease, even by years.27,28 In addition, and in line with previous work,4 our study shows that loss of empathy has a negative impact on informants’ well-being, providing additional rationale for suggesting that caregivers should be incorporated early into the care planning process.
Our analyses investigating the temporal trajectory of empathy show that loss of empathy occurs at each stage in the disease progression and over time regardless of pathogenic variant status and disease severity at baseline. Consistent with cross-sectional evidence from individuals with neurodegenerative diseases,15 our longitudinal brain-behavior analyses show that loss of emotional empathy over time corresponds to progressive atrophy in subcortical regions of the SN (thalamus) and SAN (caudate) underlying basic autonomic responsiveness, emotional resonance, and awareness.29,30 In addition, we found that progressive changes in cognitive empathy are associated with loss of brain volume in lateral temporal regions of the DMN (temporal pole, inferior parietal lobule) that are involved in thinking about the emotional state of other people as well as in self-other distinction processes.31,32 The importance of both cortical and subcortical regions of these social networks for empathy is in line with previous studies from patients with neurodegenerative diseases2 and stroke33, as well as with studies on empathy for pain in healthy participants.34 Overall, our findings suggest that dementia health care providers can use the IRI to detect and monitor loss of empathy, and thus clinical and neuroanatomical progression, over the course of sporadic and genetic bvFTD. Thus, the IRI may be used as an outcome measure in ongoing clinical trials35 for patients who are in both early and more advanced stages of bvFTD.
LIMITATIONS AND CONCLUSIONS
The focus of this study was to model loss of empathy across asymptomatic and symptomatic stages of bvFTD, and we did not investigate the temporal trajectory of empathy in other neurodegenerative disease syndromes. Though previous cross-sectional studies showed the usefulness of the IRI for differential diagnosis of neurodegenerative syndromes,2,20 this work cannot answer whether the measure is able to differentiate neurodegenerative diseases as well as neurodegenerative from psychiatric conditions based on their longitudinal patterns of empathy. In our sample, pathogenic variant carriers showed earlier symptom onset than non-carriers. Though many participants did not know their own genetic status and we used informant ratings of empathy, it remains an open question whether awareness of pathogenic variant status is associated with an earlier recognition of symptoms. In addition, even though it is well known that bvFTD is a clinically, neuroanatomically, and pathologically heterogeneous syndrome, we did not examine the longitudinal patterns of empathy in different bvFTD subtypes. 36 These subtypes are associated with divergent patterns of volume loss in subcortical regions of the SN, SAN, and DMN, thus may also show different patterns of loss of empathy over time, which ought to be investigated in future research. Moreover, we were not able to investigate the relationship between empathy and other processes of social functioning (e.g., theory of mind) across different disease stages and over time because currently this data is not available for this large multisite dataset. Though previous evidence suggests that psychotropic drugs (e.g., selective serotonin inhibitors, antipsychotics) may be used for the management of behavioral symptoms in FTD,37,38 the degree to which such medications improve symptoms of empathy still needs to be investigated in future studies. Finally, future research should examine the degree to which loss of empathy in bvFTD is related to generalized loss of interest (i.e., apathy) versus direct damage to socioemotional systems including the SN, SAN, and DMN.
In conclusion, our study adds to the current literature on empathy in patients with neurodegenerative diseases by showing that the questionnaire can be broadly used for early diagnosis and to monitor clinical symptoms in patients with sporadic and genetic variants of bvFTD who are in asymptomatic and very mild symptomatic disease stages. Our findings also reiterate the need for both pharmacologic and non-pharmacologic therapeutic interventions that will target early behavioral symptoms like loss of empathy in patients with bvFTD, to mitigate the burden not only for affected patients but also for their caregivers and families.
Supplementary Material
Research in Context.
Systematic Review:
The authors reviewed studies on the neuronal correlates of empathy, and loss of empathy in behavioral variant frontotemporal dementia (bvFTD) using PubMed. Numerous studies show that empathy is a multidimensional construct that relies on regions of the salience, semantic-appraisal, and default-mode networks. While many smaller studies demonstrate that empathy is affected in bvFTD, no previous study has comprehensively investigated empathy across asymptomatic and symptomatic disease stages.
Interpretation:
Our findings show that loss of empathy occurs in very early stages of bvFTD, and further declines with disease progression. This knowledge may help clinicians identify patients with bvFTD earlier and monitor their symptom progression over time.
Future directions:
To determine the value of IRI informant-ratings for differential diagnosis of neurodegenerative diseases and distinction of bvFTD subtypes, future studies need to investigate the temporal trajectories of empathy in other neurodegenerative syndromes and within the clinically and neuroanatomically heterogeneous bvFTD syndrome.
ACKNOWLEDGEMENTS
Data collection and dissemination of the data presented in this manuscript was supported by the ALLFTD Consortium (U19: AG063911, funded by the National Institute on Aging and the National Institute of Neurological Diseases and Stroke), the former ARTFL & LEFFTDS Consortia (ARTFL: U54 NS092089, funded by the National Institute of Neurological Diseases and Stroke and National Center for Advancing Translational Sciences; LEFFTDS: U01 AG045390, funded by the National Institute on Aging and the National Institute of Neurological Diseases and Stroke), grant R01AG029577 funded by the National Institute on Aging, as well as grant P300P1_177667 from the Swiss National Science Foundation. The manuscript has been reviewed by the ALLFTD Executive Committee for scientific content. The authors acknowledge the invaluable contributions of the study participants and families as well as the assistance of the support staff at each of the participating sites.
Footnotes
POTENTIAL CONFLICTS OF INTEREST
GT: research support from the Swiss National Science Foundation.
YC: the author reports no disclosures relevant to this manuscript.
PC: the author reports no disclosures relevant to this manuscript.
BSA: research support from CDC, NIH, and Ionis.
DB: the author reports no disclosures relevant to this manuscript.
KDR: research support from NIH. She has served as an investigator for clinical trials sponsored by Avid Radiopharmaceuticals, Biogen, and Janssen Pharmaceuticals and has served as Advisory Board consultant for Biogen.
LKF: the author reports no disclosures relevant to this manuscript.
NG: research support from NIH, the Association for Frontotemporal Degeneration, Tau Consortium. She has participated in multicenter therapy studies by sponsored by Bristol Myers Squibb, Lilly, Janssen, Novartis, Pfizer, Wyeth.
JGR: receives funding from NIH and serves on editorial board for Neurology.
NRGR: receives royalties from UpToDate, has participated in multicenter therapy studies by sponsored by Biogen, TauRx, AbbVie, Novartis and Lilly. He receives research support from NIH.
MG: receives research support from NIH, Avid and Piramal. He participates in clinical trials sponsored by Biogen, TauRx, and Alector, serves as a consultant to Bracco and UCB, and serves on the Editorial Board of Neurology.
HWH: the author reports no disclosures relevant to this manuscript.
JK: the author reports no disclosures relevant to this manuscript.
WK: receives research funding from AstraZeneca, Biogen, Roche, DOD and NIH.
MIL: research support from NIH.
GL: the author reports no disclosures relevant to this manuscript.
IL: research is supported by the National Institutes of Health grants: 5P50AG005131-33, 2R01AG038791-06A, U01NS090259, U01NS100610, U01NS80818, R25NS098999, P20GM109025; U19 AG063911-1; Parkinson Study Group, Michael J Fox Foundation, Parkinson Foundation, Lewy Body Association, Roche, Abbvie, Biogen, EIP-Pharma and Biohaven Pharmaceuticals. IL was member of a Lundbeck Advisory Board and participated in a symposium organized by Sunovion. IL receives her salary from the University of California San Diego and as Chief Editor of Frontiers in Neurology.
IRM: has been a paid member of the Scientific Advisory Board for Prevail Therapeutics.
BP: the author reports no disclosures relevant to this manuscript.
EMR: the author reports no disclosures relevant to this manuscript.
KR: research support from NIH.
JCR: receives research support from NIH-NIA. He is a site PI for clinical trials sponsored by Eli Lilly.
AMS: research support from NIA-NIH and Larry L. Hillblom Foundation.
MCT: receives research funding from CIHR and NIH, and is an investigator on pharmaceutical studies with Biogen, Roche, Eli Lilly, and Boehringer.
AT: the author reports no disclosures relevant to this manuscript.
SW: receives research support from NIH.
ZKW: is partially supported by the Mayo Clinic Center for Regenerative Medicine, the gifts from The Sol Goldman Charitable Trust, and the Donald G. and Jodi P. Heeringa Family, the Haworth Family Professorship in Neurodegenerative Diseases fund, and The Albertson Parkinson’s Research Foundation. He served/s as PI or Co-PI on Abbvie, Inc. (M15-562 and M15-563), Biogen, Inc. (228PD201) grant, and Biohaven Pharmaceuticals, Inc. (BHV4157-206 and BHV3241-301). He serves as PI of the Mayo Clinic American Parkinson Disease Association (APDA) Information and Referral Center, and as Co-PI of the Mayo Clinic APDA Center for Advanced Research. He is a Co-Editor-in-Chief of the Neurologia i Neurochirurgia Polska (Polish Journal of Neurology and Neurosurgery). He is a former Co-Editor-in-Chief of Parkinsonism and Related Disorders, and former Associated Editor of the European Journal of Neurology.
BFB: research support from NIH, and research support for clinical trials sponsored by Biogen, Alector, and EIP Pharma. He serves on the Scientific Advisory Board of the Tau Consortium.
ALB: research support from NIH, the Tau Research Consortium, the Association for Frontotemporal Degeneration, Bluefield Project to Cure Frontotemporal Dementia, Corticobasal Degeneration Solutions, the Association for Frontotemporal Degeneration and the Alzheimer’s Association. He has served as a consultant for Abbvie, AGTC, Alector, Arkuda, Arvinas, Asceneuron, AZTherapeutics, Bioage, Ionis, Lundbeck, Passage BIO, Regeneron, Samumed, Transposon and UCB, and received research support from Biogen, Eisai, Eli Lilly, Genentech, Novartis, Roche and TauRx.
HJR: has received research support from Biogen Pharmaceuticals, has consulting agreements with Wave Neuroscience and Ionis Pharmaceuticals, and receives research support from NIH.
KPR: receives research funding from NIH, Quest Diagnostics, Rainwater Charitable Foundation, and Marcus Family Foundation.
REFERENCES
- 1.Dermody N, Wong S, Ahmed R, Piguet O, Hodges JR, Irish M. Uncovering the Neural Bases of Cognitive and Affective Empathy Deficits in Alzheimer’s Disease and the Behavioral-Variant of Frontotemporal Dementia. JAlzheimer’s Dis. 2016;(Preprint):1–16. [DOI] [PubMed] [Google Scholar]
- 2.Rankin KP, Gorno-Tempini M, Allison SC, et al. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129(Journal Article):2945–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shany-Ur T, Lin N, Rosen HJ, Sollberger M, Miller BL, Rankin KP. Self-awareness in neurodegenerative disease relies on neural structures mediating reward-driven attention. Brain. 2014;137(Pt 8):2368–2381. doi: 10.1093/brain/awu161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown CL, Lwi SJ, Goodkind MS, et al. Empathic Accuracy Deficits in Patients with Neurodegenerative Disease: Association with Caregiver Depression. AmJGeriatrPsychiatry. 2018;26(4):484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeda A, Sturm VE, Rankin KP, Ketelle R, Miller BL, Perry DC. Relationship Turmoil and Emotional Empathy in Frontotemporal Dementia. Alzheimer Disease & Associated Disorders. 2019;33(3):260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baez S, Morales JP, Slachevsky A, et al. Orbitofrontal and limbic signatures of empathic concern and intentional harm in the behavioral variant frontotemporal dementia. Cortex. 2016;75:20–32. doi: 10.1016/j.cortex.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 7.Cerami C, Dodich A, Canessa N, et al. Neural correlates of empathic impairment in the behavioral variant of frontotemporal dementia. Alzheimer’s & Dementia. 2014;10(6):827–834. [DOI] [PubMed] [Google Scholar]
- 8.Decety J, Lamm C. Human empathy through the lens of social neuroscience. ScientificWorldJournal. 2006;6(Journal Article):1146–1163. doi: 10.1100/tsw.2006.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaki J, Ochsner KN. The neuroscience of empathy: progress, pitfalls and promise. NatNeurosci. 2012;15(5):675–680. [DOI] [PubMed] [Google Scholar]
- 10.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. JNeurosci. 2007;27(9):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seeley WW, Zhou J, Kim EJ. Frontotemporal Dementia: What Can the Behavioral Variant Teach Us about Human Brain Organization? Neuroscientist. 2011;(Journal Article). doi: 10.1177/1073858411410354 [DOI] [PubMed] [Google Scholar]
- 12.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. ProcNatlAcadSciUSA 2004;101(13):4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis A Measuring individual differences in empathy: Evidence for a multidimensional approach. JPersSocPsychol. 1996;44(Journal Article):113–126. [Google Scholar]
- 15.Eslinger PJ, Moore P, Anderson C, Grossman M. Social cognition, executive functioning, and neuroimaging correlates of empathic deficits in frontotemporal dementia. JNeuropsychiatry ClinNeurosci. 2011;23(1):74–82. doi: 10.1176/appi.neuropsych.23.1.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–2477. doi: 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boeve B, Bove J, Brannelly P, et al. The longitudinal evaluation of familial frontotemporal dementia subjects protocol: Framework and methodology. Alzheimers Dement. 2020;16(1):22–36. doi: 10.1016/j.jalz.2019.06.4947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality & Social Psychology. 1983;44(113–126). [Google Scholar]
- 19.Cliffordson C The hierarchical structure of empathy: Dimensional organization and relations to social functioning. ScandJPsychol. 2002;43(Journal Article):49–59. [DOI] [PubMed] [Google Scholar]
- 20.Rankin KP, Kramer JH, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cognitive and Behavioral Neurology. 2005;18(1):28–36. [DOI] [PubMed] [Google Scholar]
- 21.Shdo SM, Ranasinghe KG, Gola KA, et al. Deconstructing empathy: Neuroanatomical dissociations between affect sharing and prosocial motivation using a patient lesion model. Neuropsychologia. 2017;(Journal Article). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knopman DS, Weintraub S, Pankratz VS. Language and behavior domains enhance the value of the clinical dementia rating scale. Alzheimers Dement. 2011;7(3):293–299. doi: 10.1016/j.jalz.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyagawa T, Brushaber D, Syrjanen J, et al. Utility of the global CDR® plus NACC FTLD rating and development of scoring rules: Data from the ARTFL/LEFFTDS Consortium. Alzheimer’s & Dementia. 2020;16(1):106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649–655. [DOI] [PubMed] [Google Scholar]
- 25.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 26.Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. JClinPsychiatry. 2011;72(2):126–133. doi: 10.4088/JCP.10m06382oli [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohrer JD, Nicholas JM, Cash DM, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. The Lancet Neurology. 2015;14(3):253–262. doi: 10.1016/S1474-4422(14)70324-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell LL, Greaves CV, Bocchetta M, et al. Social cognition impairment in genetic frontotemporal dementia within the GENFI cohort. Cortex. 2020;133:384–398. doi: 10.1016/j.cortex.2020.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craig AD. How do you feel -- now? The anterior insula and human awareness. Nature Reviews in Neuroscience. 2009;10(Journal Article):59–70. [DOI] [PubMed] [Google Scholar]
- 30.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. NatNeurosci. 2004;7(2):189–195. [DOI] [PubMed] [Google Scholar]
- 31.Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004;3(2):71–100. [DOI] [PubMed] [Google Scholar]
- 32.Ruby P, Decety J. What you believe versus what you think they believe: a neuroimaging study of conceptual perspective-taking. EurJNeurosci. 2003;17(11):2475–2480. [DOI] [PubMed] [Google Scholar]
- 33.Hillis AE. Inability to empathize: brain lesions that disrupt sharing and understanding another’s emotions. Brain. 2014;137(Pt 4):981–997. doi: 10.1093/brain/awt317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54(3):2492–2502. doi: 10.1016/j.neuroimage.2010.10.014 [DOI] [PubMed] [Google Scholar]
- 35.Tsai RM, Boxer AL. Therapy and clinical trials in frontotemporal dementia: past, present, and future. Journal of Neurochemistry. 2016;138(S1):211–221. doi: 10.1111/jnc.13640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranasinghe KG, Rankin KP, Pressman PS, et al. Distinct Subtypes of Behavioral Variant Frontotemporal Dementia Based on Patterns of Network Degeneration. JAMA neurology. 2016;73(9):1078–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pressman PS, Miller BL. Diagnosis and Management of Behavioral Variant Frontotemporal Dementia. Biological Psychiatry. 2014;75(574–581). Accessed August 29, 2022. https://www.sciencedirect.com/science/article/abs/pii/S0006322313009876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manoochehri M, Huey ED. Diagnosis and management of behavioral issues in frontotemporal dementia. CurrNeurolNeurosciRep. 2012;12(5):528–536. doi: 10.1007/s11910-012-0302-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


