Abstract
A frequent eating pattern may alter glycemic control and augment postprandial insulin concentrations in some individuals due to the subsequent meal truncating the previous postprandial period. This study examined glucose, insulin, c-peptide and glucose-dependent insulinotropic peptide (GIP) responses in obese individuals when meals were consumed in a high frequency pattern (every 2-h, 6M) or in a low frequency pattern (every 4-h, 3M) over 12-h. We also examined these postprandial responses to high protein, frequent meals (6MHP). Thirteen obese subjects completed three 12-h study days during which they consumed 1500 kcal: 1) 3M – 15% protein, 65% carbohydrate; 2) 6M –15% protein, 65% carbohydrate, and 3) 6MHP – 45% protein, 35% carbohydrate. Blood samples were taken every 10 min and analyzed for glucose, insulin, c-peptide, and GIP. Insulin total area under the curve (tAUC) and peak insulin concentrations (P<0.05) were greater with 3M than with 6M, but the glucose tAUC was not different between conditions. 6MHP (glucose: 64.3±1.5 g/dl*min, insulin: 22.7±2.1 μIU/dL for 12-h) lowered glucose and insulin excursions more so over 12-h than either the 3M (glucose: 70.5±1.4 g/dL*min, insulin: 31.6±2.1 μIU/dL*min for 12-h) or 6M condition (glucose: 70.3±1.5 g/dL*min, insulin: 26.8±2.5 μIU/dL*min for 12-h, P<0.01). Insulin secretion, GIP concentrations, and the glucose/insulin ratio were unaltered by meal frequency or composition. In obese subjects, consuming meals in a low frequency pattern does not alter glucose tAUC, but increases postprandial insulin responses. The substitution of protein for carbohydrate in a frequent meal pattern results in tighter glycemic control and reduced postprandial insulin responses.
Keywords: meal test, GIP, obesity, frequent feeding, insulin secretion
Introduction
A high fasting insulin concentration commonly exists with obesity, impaired glucose tolerance, and type 2 diabetes mellitus (T2D) (1-3). There is evidence in Zucker rats that the onset of hyperinsulinemia precedes the development of obesity and muscle insulin resistance; and the magnitude of hyperinsulinemia is proportional to the duration of obesity (4). Additionally, obese rats treated with diazoxide (inhibits insulin release) demonstrated reduced weight-gain and improved glucose tolerance (5). Subsequent research (6) in obese humans with hyperinsulinemia demonstrated that administration of diazoxide over 8 weeks led to attenuation of hyperinsulinemia, greater weight loss, greater decrease in body fat, and reduced the acute insulin response to glucose, without inducing glucose intolerance. Based on these findings, it has been hypothesized that hyperinsulinemia may play a more prominent role in the development of obesity, insulin resistance, and T2D than previously thought, and that therapies alleviating hyperinsulinemia should be targeted for the prevention of these chronic conditions (7-9).
While numerous conditions and factors are known to increase mean insulin concentration, the most apparent and modifiable factor is diet - in particular, the macronutrient composition of the diet, and the pattern (frequency of meals per day) of caloric intake. A more frequent eating pattern was shown to result in prolonged periods of hyperglycemia and/or hyperinsulinemia in healthy individuals (10), which is suggested to be a result of truncating the postprandial period with a subsequent meal; thereby maintaining consistently high insulin concentrations throughout the day. Previously, we also observed elevated glucose AUC throughout the day with 6 meals compared to 3 meals over 12-h (P<0.05), while insulin AUC was reduced (11). However, Solomon et al (12) found no difference in the insulin or glucose tAUC over an 8 h period when comparing low feeding frequency (2 meals/day) to a high feeding frequency (12 meals/day) in lean individuals. One limitation of these studies has been the recruitment of non-obese, healthy individuals only. Jenkins et al (13) reported that spreading the nutrient load (3 meals and one snack vs 13 snacks) resulted in reduced glucose, insulin and c-peptide concentrations in older (age range: 37-81 y), overweight (106-129% overweight) patients with T2D. Taken together these studies suggests a divergent response to increased meal frequency in lean individuals versus overweight individuals with T2D, thus reexamination of this question would be prudent in a more homogenous population to identify the influence of obesity alone.
Given the paucity of research examining the impact of meal frequency on glucose and insulin concentrations in obese individuals (12, 13), the purpose of this study was to examine the postprandial glucose and insulin response when meals are separated by 2-h vs. 4-h in obese individuals over a 12-h period. More specifically, we were interested in identifying insulin concentrations in this population in response to altered meal frequency regimes. We speculated that shorter intervals between meals would result in prolonged hyperglycemia with concomitant lower insulin concentrations due to altered insulin secretion in obese individuals.
Substituting protein for carbohydrate also decreases postprandial glucose and insulin responses in both the short-term (14) and longer-term (15), thus the meal composition must also be considered. Wolever and colleagues (16) reported that a high protein meal (30 g) increased the insulin response but had no effect on insulin secretion rate. Therefore, the second purpose of this study was to establish the postprandial insulin response to frequent high protein meals over 12-h in obese individuals. We hypothesized substituting protein for carbohydrate during a high frequency meal pattern would reduce glucose concentrations, resulting in decreased insulin AUC and secretion rates.
Experimental Methods
Subjects:
Thirteen obese men and women (age: 29-50 y) with a fasting blood glucose concentration (< 100 mg/dL) were recruited for this study. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the University of Missouri Institutional Review Board. Written informed consent was obtained from all subjects/patients. The inclusion criteria were: obese individuals with a BMI 30-45 kg/m2, waist circumference > 88 cm, body fat percentage > 35%, non-smokers, sedentary lifestyle (< 60 min of physical activity per week), not pregnant, and no prior history of heart, lung, kidney, endocrine, or liver disease (17). No subject was taking any prescription medications known to alter glucose or lipid metabolism and were weight stable over the last 6 months. Baseline information about dietary intake, physical activity, and medical history were obtained via a self-reported questionnaire.
Study Design:
Parts of the experimental design have been published previously (17). All subjects completed three 12-h study days during which they consumed 1,500 kcals (6,276 KJ). There was a low frequency meal regimen (3 meals (3M): 500 kcal/meal (2,092 KJ: 18.8 g protein, 81.2 g carbohydrate, 10 g fat) consumed every 4-h, high frequency meal regimen (6 meals (6M): 250 kcal/meal (1,046 KJ: 9.4 g protein, 40.6 g carbohydrate, 5 g fat) consumed every 2-h, HF high protein meal (6MHP): 250 kcal/meal every 2-h; 28 g protein, 21.9 g carbohydrate, 5 g fat. Approximately one month elapsed between each study day, and the three study days were completed in a counterbalanced order. The study days were energy-matched. The meals utilized a liquid shake (Nutritional Shake, Walgreens, Deerfield, IL). For the 6MHP condition, protein was added which consisted of Pro Complex whey protein (Pro Complex, Optimum Nutrition Inc., Aurora, IL, USA) (11) and branched-chain amino acids which also contained a small amount of fat (~2 g) sufficient to balance the fat component of the dietary conditions at 20%. We used liquid meals for this protocol, as previous work has shown that alterations in the macronutrient composition of liquids does not affect gastric emptying rate (18) and so that the rate of eating could be more precisely controlled, thereby eliminating the effect of eating speed on glucose and insulin responses.
Study day:
Following a 12-h overnight fast, a venous catheter was placed into the antecubital vein and kept patent with a saline drip. Blood samples were drawn from a stopcock at baseline and every 10 min for 12-h. On the 3M day, meals were at 0700 h (following baseline samples), 1100 and 1500 h and on the 6M regimens were 0700, 0900, 1100, 1300, 1500 and 1700 h. Subjects were sedentary throughout the day with only minor activity. Blood samples were analyzed for glucose, insulin, c-peptide and glucose-dependent insulinotropic peptide (GIP).
Anthropometric Measures:
As part of the screening, all subjects had their height and weight measured using a digital scale and stadiometer. Waist and hip circumference were taken with a measuring tape. Body fat percentage was calculated using the BOD POD® according to the manufacturer’s guidelines (COSMED Corp, Little Elm, TX USA). Lung volume was measured and body fat was estimated using the Siri equation.
Dietary Assessment:
Since prior dietary patterns can influence the postprandial responses, subjects were required to keep a dietary record during the three days prior to each 12-h visit. The dietary record from the first study day was given to each subject on their subsequent visit and subjects were asked to repeat the same dietary pattern prior to the subsequent visit (17). Total energy and macro/micronutrient content were determined from the records using Food Processor SQL, version 10.8 (ESHA Research, Salem, OR, USA).
Blood Handling and Analysis:
Blood samples were placed in serum separator tubes, centrifuged at 3000 RPM for 15 min and stored at −80°C until analysis. Blood glucose concentrations were determined using colorimetric assays (Fischer Scientific, Inc., Houston, TX, USA) (coefficient of variation (CV)-1.8%). Serum insulin concentrations were determined using an Immulite 1000 Immunoassay System (Siemens Healthcare Diagnostics, Inc., Deerfield, IL, USA). C-peptide and GIP concentrations were assayed with kits from Millipore (Billerica, MA, USA) using Luminex xMap Technology (Linco Research, St. Charles, MO, USA) on a Luminex 100/200 platform (Luminex Corporation, Austin, TX, USA). Inter-assay coefficients of variation (CV) for insulin, c-peptide and GIP were 8.3%, 7.9% and 9%, and intra-assay CV for insulin, c-peptide and GIP were 6.9%, 8.2% and 7.9%, respectively. All samples for a given subject were run in the same assay.
Data Analysis and Statistical Analysis:
The homeostatic model assessment of insulin resistance (HOMA-IR) (19) and quantitative insulin sensitivity check index (QUICKI) (20) were used to calculate insulin resistance and sensitivity, respectively, prior to each condition. Total area under the curve (tAUC) for the postprandial responses over 12-h was calculated using the trapezoidal method (21). The postprandial glycemic and insulin excursions were derived from the absolute difference in nadir (baseline insulin concentration prior to each meal) to peak concentration.
Insulin secretion was determined using the c-peptide data. A pulse profile was analyzed using a multi-parameter deconvolution technique (AutoDecon, Pulse_XP software; University of Virginia) which derives quantitative estimates of attributes of c-peptide secretory pulses and half-life from measured c-peptide concentrations (22, 23). A Gaussian distribution of secretory rate was assumed using the serum c-peptide concentrations and it was assumed to match insulin at a 1:1 ratio. Basal secretion was estimated for each condition. C-peptide pulses were considered significant if they were able to be distinguished from zero with a 95% statistical significance as previously reported (22, 24).
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) statistical software, version 19.0 (IBM Inc, Chicago, IL, USA). For the primary analysis, a mixed-model ANOVA with repeated measures was used to compare the tAUC values between conditions for all variables, and the baseline value on each study day for each respective hormone was used as a covariate. Since baseline values can influence the hormonal response, we corrected for the baseline value of each subject to control for day to day variation. The variables for the deconvolution analysis were also analyzed with an AVOVA with repeated measures. A Pearson product correlation was run on the hormone variables. Statistical significance was set at P ≤ 0.05 and values are reported as mean ± standard error of the mean.
For the bootstrapping analysis, we subtracted each subject’s hormone concentration at each time point from one study day with the comparison study day at the same time point (e.g. 3M-6M) (25). The change in hormone concentrations was plotted over time with simultaneous 95% confidence bands. The 95% confidence regions were derived using bootstrapping technique. This 95% confidence region avoids the problem of multiple point-wise comparisons. A significant response was defined as occurring when the lower 95% confidence limit for the curve was more than zero, and significant suppression of hormone release was defined as occurring when the upper 95% confidence limit for the regression curve was less than zero (25). This analysis was done for each study day for glucose and insulin concentrations.
Results
Eleven women and 3 men completed the study. We found no significant differences in fasting glucose, insulin, C-peptide, or GIP concentrations between the study days. The mean fasting insulin concentration across the three study days was 9.9±1.0 μIU/ml, while mean fasting glucose concentration was 80.8±2.2 mg/dL. The participants remained weight stable while participating in this study and weighed 100.0±3.7 kg (weight gain/loss < 2 kg) and had a BMI of 35.5±1.0 kg/m2. Subjects had a mean percent body fat of 45.9±1.8%. Their HOMA-IR was 2.1±0.2, and insulin sensitivity (QUICKI) was 0.32±0.05. There were no dietary differences (energy consumed or composition) during the 3 d prior to each condition. Subjects consumed ~ 2,500 kcal each of the 3 days prior to the study day (48% carbohydrate, 15% protein and 37% fat) (17).
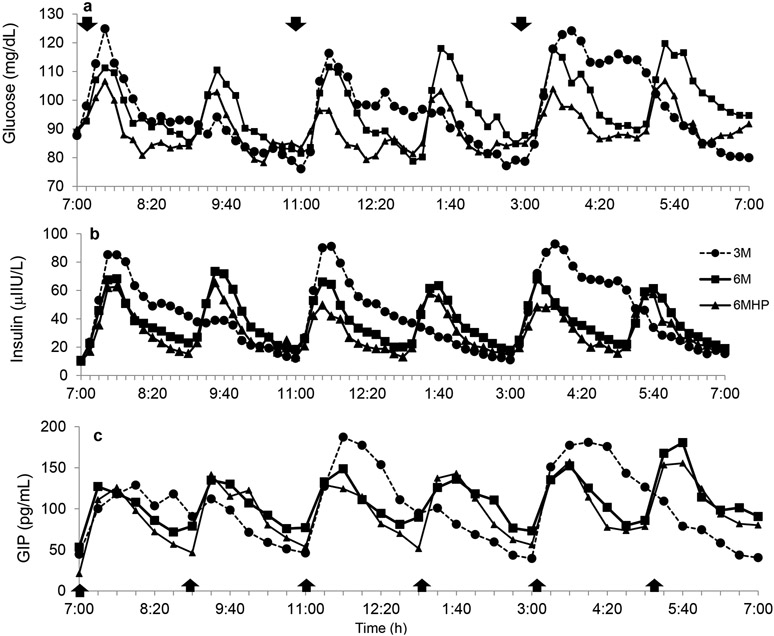
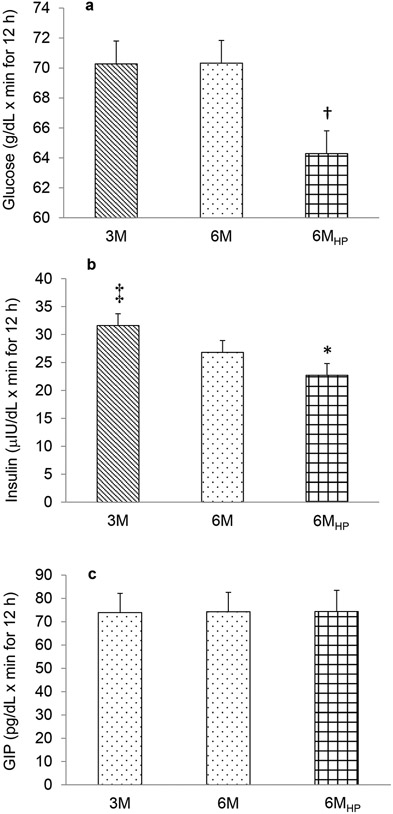
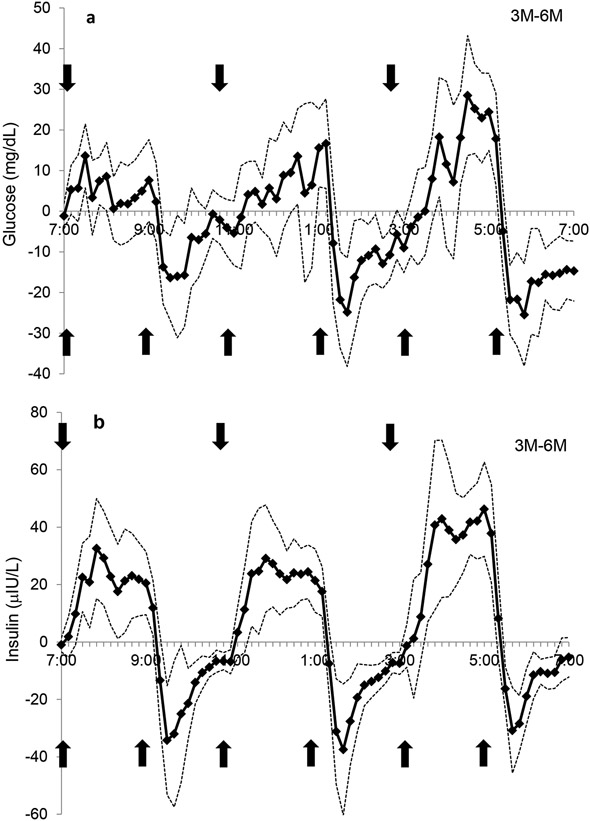
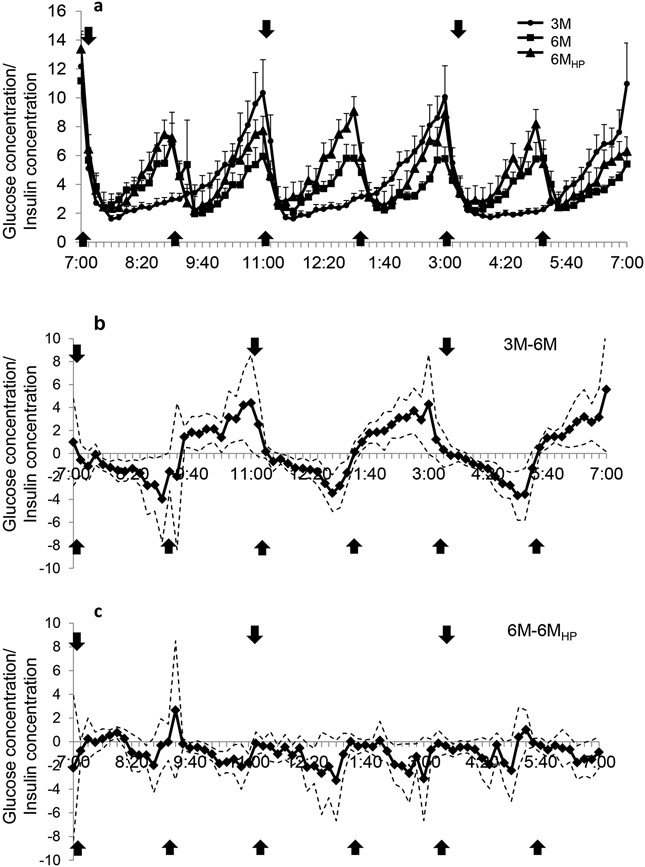
The pattern of the postprandial glucose and insulin responses over the 12-h day are illustrated in Figures 1a and b, respectively. Total glucose AUC adjusted for fasting glucose levels was not different between 3M vs. 6M regimens (P=0.98, Figure 2a), but the postprandial changes in peak glucose levels were greater with the 3M condition at the first meal (39.2±4.8 mg/dL) than with both the 6M and 6MHP condition (27.5±3.7 and 15.6±2.9 mg/dL, respectively, P<0.0001) (Figure 1a). The postprandial peak glucose changes were also significantly higher for 6M than 6MHP (P<0.001, Figure 1a). Within each study day, there was no significant difference in the change to peak with each meal, thus the glucose response at subsequent meals did not differ from the first meal which followed 12-h of fasting. Comparison of postprandial glucose on the 3M day with the 6M day revealed periods of higher glucose concentrations on the 3M day (min: 7:30-7:40, 7:50-8:00, 12:20-12:40, 1:00-1:20, 3:50-4:00, 4:20-5:20; total 130 min/day) (Figure 3a) and lower glucose concentrations (the lines passing below the x axis) at the 2nd, 4th and 5th meal (min: 9:20-9:40, 1:30-3:10, 5:30-7:00; total 210 min/day), indicating overall glucose concentrations were lower for a greater amount of time during the day while on the 3M pattern.
Figure 1.
The pattern of glucose response over 12-h for each study day for glucose (a), insulin (b), and glucose-dependent insulinotropic peptide (GIP) (c). 3M- low frequency meals, 6M – high frequency meals, 6MHP- high frequency, high protein meal. Mean±SE. n=13 (2 men/11 women).  indicates a meal.
indicates a meal.
Figure 2.
Total area under the curve adjusted for fasting levels for glucose (a), insulin (b), and glucose-dependent insulinotropic peptide (GIP) (c). 3M- low frequency meals, 6M – high frequency meals, 6MHP- high frequency, high protein meal. †P<0.01 6MHP vs. 3M and 6M; ‡P<0.05 3M vs. 6M; *P<0.001 6MHP vs. 3M and 6M. Mean±SE. n=13 (2 men/11 women)
Figure 3.
The comparison of the 3M-6M day for A) glucose and B) insulin concentrations over 12-h. The solid line represents the difference between study days and simultaneous 95% confidence limits (dotted line) are shown. Significant stimulation of glucose or insulin release by the meal occurred when the lower 95% confidence limit was more than zero. Significant suppression of glucose or insulin release occurred when the upper 95% confidence limit was less than zero. Arrows indicate meals.  indicates a meal.
indicates a meal.
Insulin tAUC adjusted for fasting insulin was greater on the 3M compared to the 6M regimen (P<0.05; Figure 2b). The postprandial insulin excursions on the 3M day (mean: 91.8±13.5 μIU/L) were significantly greater (P<0.001) at all meals than on the 6M days (mean: 55.3±10.8 μIU/L). Insulin concentrations closely followed meal ingestion which demonstrated higher insulin concentrations on the 3M day than on the 6M day at the times which corresponded with meal ingestion (min: 7:20-9:10, 11:30-1:20, 3:40-5:20; total 320 min/day) (Figure 3b.), while on the 6M day higher insulin levels were observed in those intervals only when the additional meals were consumed (min: 9:20-11:10, 1:30-3:10, 5:30-6:50; total 290 min/day), indicating insulin concentrations were higher for a slightly greater amount of time while on the 3M.
Examining the tAUC for each meal revealed that on the 3M and 6M day, there is no significant difference across meals in the glucose or insulin tAUC at each respective meals, although there was a trend for slightly lower glucose tAUC for the meals earlier in the day than was observed later in the day. Insulin tAUC showed a similar trend in the 3M condition but not the 6M condition.
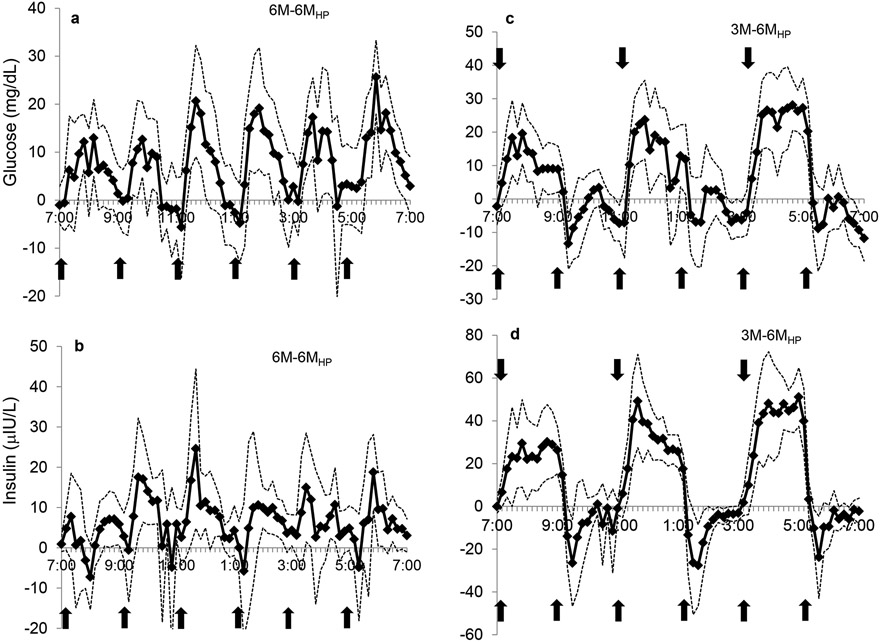
Frequent HP meals resulted in a significantly lower glucose tAUC compared to both the 3M and 6M conditions (P<0.01 and P<0.01, respectively) (Figure 2a). Peak glucose levels on the 6MHP day were significantly lower (P<0.01) than the respective peaks on the 3M day, and approached significance on the 6M day (P<0.06). Insulin tAUC was greater on both the 6M and 3M day than the 6MHP day (P<0.0001) (Figure 2b). Peak insulin responses were greater on the 3M day than 6MHP at each corresponding meal of the 3M day (P<0.01) but peak insulin responses on the 6M day were only significantly greater at the 3rd and 5th meal than on the 6MHP day (P<0.01) (Figure 1b). The contrast in glycemic and insulin excursions during the 6MHP regimen compared to the 6M and 3M regimens are illustrated in Figure 4. Compared to the 6M condition, the glucose concentrations with the 6MHP condition were usually lower for about 50 min post meal (Figure 4a), while the insulin concentrations were lower at meals 2, 3, 4, and for very short periods of time following meals 5 and 6 (Figure 4b). In comparison to the 3M condition, with the 6MHP condition glucose levels were lower for ~80 min post meal after meal 1, 3, and 5 (Figure 4c). The insulin concentrations followed a very similar trend of being much higher on the 3M condition for ~2-h post meal (Figure 4d). With the 6MHP condition, glucose and insulin tAUC was not different across meals on that study day.
Figure 4.
The comparison of the 6M-6MHP and 3M-6MHP days for glucose (a,c) and insulin (b,d) concentrations, respectively, over 12-h. Refer to Fig 3 for more detail.  indicates a meal.
indicates a meal.
The highest glucose/insulin ratio occurred at fasting and immediately prior to each meal (Figure 5a). Following consumption of the meals, this ratio dropped rapidly as the magnitude of increase in insulin concentration was greater than the magnitude of increase in glucose concentration. Approximately 40 min post-meal the ratio began to rise under all conditions. The slope of this rise between 40-120 min post-meal demonstrated a condition effect, such that a significantly lower slope for 3M was observed compared to 6MHP (p<0.01) and for 6M (P<0.05). There was no difference in the slope between 6M and 6MHP condition. Further, there was no difference in this effect between meals across the day.
Figure 5.
A) Pattern of the ratio of glucose/insulin concentrations over 12-h for each study day, B) comparison of the 3M-6M ratio of glucose/insulin concentrations and C) comparison of the 6M-6MHP ratio of glucose/insulin concentrations. Refer to Fig 3 for more detail.  indicates a meal.
indicates a meal.
C-peptide and GIP tAUC were not different between study days for meal frequency or meal composition. Figure 1c shows the GIP pattern of response, and as can be seen from the tAUC the response is very similar across all study days (Figure 2c). Deconvolution of the c-peptide data provided insight into insulin secretion characteristics for the 12-h period. There were no differences in the insulin secretion characteristics between the study days (Table 1). Fasting GIP and c-peptide levels were significantly correlated with insulin tAUC (r=0.449, p<0.01 and r=0.751, p<0.000 respectively).
Table 1:
Deconvolution Parameters for C-Peptide
| LFM | HFM | HFHP | |
|---|---|---|---|
| Secretory Pulses (pulses/12 h) | 5.4 (0.5) | 6.7 (0.4) | 6.1 (0.5) |
| Half Width (nmol/L) | 20.9 (4.0) | 12.2 (1.5) | 16.3 (3.4) |
| Half Life (min) | 71.9 (8.6) | 67.2 (8.4) | 69.4 (7.3) |
| Pulse Mass (nmol/L/pulse) | 4929 (945) | 4189 (686) | 5319 (972) |
| Peak Amplitude (nmol/L/pulse) | 405 (94) | 417 (96) | 573 (210) |
| Pulse interval (min) | 140.5 (16.1) | 111.7 (10.2) | 112.4 (6.3) |
| Production Rate (mass/pulse) | 23,859 (3,793) | 26,718 (4,465) | 28,717 (7,135) |
All values reported as means and standard error of the mean. LFM-low frequency meals, HFM-high frequency meals, HP-high protein.
p=0.07 vs HFM
P=0.08 vs HFM; n=13; mean (SE)
Discussion
The eating regimen of frequent meals has become common practice but the impact of such an eating pattern on glycemic and insulinemic excursions over the course of a 12-h day has not been well studied, particularly in obese individuals who often demonstrate hyperinsulinemia while being normoglycemic. The findings from the present study show, for the first time, that in obese individuals: 1) the larger less frequent meals (3M) result in greater insulin responses (peak insulin concentration and 12-h AUC) than what is observed with 6M; 2) while peak glucose concentrations were higher in the 3M regimens, the glucose concentrations remained largely unaffected by the pattern (3M vs. 6M) of caloric intake during the study day, 3) replacing carbohydrates with protein reduced glucose and insulin concentrations substantially throughout the day, and 4) insulin secretion and insulin clearance were not altered by acute changes in meal frequency or meal composition.
While glucose tAUC was not different between the 3M and 6M regimens, the postprandial response to meals were very different between the conditions. The 3M contained a larger energy and carbohydrate load/meal thus resulting in higher peak glucose levels following meal consumption and for a number of short intervals throughout the day. Peak glucose values were 30% higher following the initial meal ingestion on the 3M day compared to the 6M, but no differences occurred with subsequent meals. This observation contrasts our early finding in healthy young individuals that 6M meals resulted in higher glucose levels across the day (11). However the findings parallel with those of Solomon et al (12) who compared the glucose/insulin responses between meals consumed at low frequency (every 4-h) or high frequency (every 40 min) for 8 h. Likewise, Munsters and Saris (10) demonstrated greater insulin and glucose fluctuations but lower glucose AUC in healthy young men, following a low frequency diet (3 meals/day) or high frequency diet (14 meals/day).
Despite equivalent glucose concentrations, overall insulin levels tended to be ~10% higher on the 3M day, and this finding is in line with previous data from our lab (17). The large insulin excursions closely tracked when the meals were consumed (Figure 3) on each of the respective days, but insulin responses were greater on the 3M day for about 390 min across the day while they were greater on the 6M day for 290 min. Thus, if the hypothesis regarding chronically elevated insulin levels as being deleterious (8, 26) hold true, then meals which are high in carbohydrate consumed in a 6M pattern may be beneficial for obese individuals particularly if they have elevated insulin responses to meals. Future long term studies manipulating meal frequency need to be conducted to establish the long term effects this may have on insulin secretion and/or insulin resistance in a variety of populations.
A number of studies have reported diurnal variations in glucose tolerance in lean individuals (27, 28); which may be a circadian variation in the responsiveness of the α-cell to glucose, resulting in an attenuation in insulin sensitivity later in the day (28). However in obese subjects, this circadian variation is believed to be absent (29), which is supported by our data. While we did not include direct measures of insulin sensitivity, there were only slight differences in the postprandial insulin or glucose concentrations or the glucose/insulin ratio in response to meals ingested at subsequent times throughout the day in any of the dietary conditions studied. Slight divergences between glucose and insulin responses throughout the day are expected and these divergences may be more apparent in obese individuals as it is often not uncommon to see normal glucose responses in this population but higher insulin levels to achieve these levels.
We also tested the hypothesis that substitution of carbohydrate with protein would lower the postprandial blood glucose and insulin response. As expected, there was a significant decrease in the absolute concentration of blood glucose and insulin in response to the 6MHP condition in the present study, which is most likely due to the reduced total carbohydrate load. When compared to the 6M day, the 6MHP condition resulted in lower glucose levels for ~310 min (~43% of the day) over the 12-h, while the insulin levels were lower about 260 min (~36%) throughout the day. The insulinotropic effects of protein are well characterized in the literature. Frid et al (30) demonstrated that addition of whey protein to meals containing rapidly digested and absorbed carbohydrates resulted in an insulinotropic effect; thereby reducing glucose concentration. Of note, however, Lan-Pridhainy et al. (16) demonstrated postprandial hyperinsulinemia only when 30 g of protein are consumed. In the present study, participants ingested ~28 g of whey protein in the 6MHP condition. When assessing the deconvolution parameters for c-peptide, there appears to be no change in any of the secretory or half-life parameters studied in the present investigation. Likewise, neither the insulinogenic ratio (tAUC insulin/tAUC glucose) nor the glucose/insulin ratio provides support for an insulinotropic effect in the present investigation during the 6MHP meal pattern. Indeed there is a trend for the glucose/insulin ratio to be higher during the 6MHP condition (Figure 5a) which translates into extended periods throughout the day (~120 min in the morning; ~80 min in the afternoon; Figure 5c) where the glucose/insulin ratio is elevated in the 6MHP condition (insulinotropic effect would result in a reduction in this ratio).
Other mechanisms associated with the hypoglycemic effect of protein are suggested to be due to differences in gastric emptying (8) or enhanced insulin secretion through augmented GIP and GLP-2 secretion (9). Frid et al. (30) observed that postprandial GIP responses were higher after whey ingestion than when compared to a reference meal containing no whey (alternate source of protein). While we observed no differences in GIP levels between study days (6M vs. 6MHP), it is important to note that the present investigation substituted protein for carbohydrate as opposed to adding it to the meal. Thus, the lack of significant difference between the 6MHP and 6M meals is surprising. Finally, it is of interest to note the appearance of a possible diurnal trend in the GIP responses to meals in the present study, with a trend for an increasing peak GIP response in subsequent meals (Figure 1c). While previous research has observed a more rapid early response in GIP following ingestion of a standardized meal in the morning compared to the afternoon (AUC in first 30 min following meal ingestion) in healthy subjects, there were no differences in the concentration of GIP in the remaining 270 min (31) nor in the peak GIP concentration. Further investigation into the GIP responses over the course of the day in both healthy and clinical populations would be of interest.
Surprisingly, there are a limited number of studies which have examined postprandial glycemic control in response to different meal frequency patterns, and these have utilized discernible study protocols. This study’s strength is that we assessed this response over a 12-h study period and have captured possible diurnal variations in glucose tolerance (28). Further, our frequent sampling techniques allow for us to more carefully analyze the pattern of response than some of the previous studies which have sampled hourly (10, 13). Additionally, many of the previous studies have recruited only lean healthy individuals (10, 12), and the study which did recruit individuals with T2D, recruited a heterogenous group based on age, sex and body-weight status (13). One limitation of the current study is that we did not include a 3MHP trial. We did not include this group since this study in obese subjects was modelled after our study in lean subjects (11). In hindsight, this arm would have allowed us to establish the effect of meal frequency on the HP condition. In addition it could be argued that we should have included more energy in the meals, as larger meals may more closely reflect what these subjects habitually consume; however, we selected an energy intake that would keep these subjects in energy balance during the study day in light of the fact that they were physically inactive all day. Additionally the results presented here represent the short term response to meal frequency and meal composition and provide insight into the glycemic responses. However, this study does not identify if the beneficial responses seen with 6M would still be manifested in a long term study. Future research needs to address whether long term changes in meal frequency alter insulin sensitivity.
The present study demonstrated in an obese population that ingestion of more frequent meals (6-meal eating pattern vs. 3-meal eating pattern) (i) results in no change in tAUC for glucose despite significantly lower tAUC for insulin and; (ii) reduces postprandial insulin excursions without altering c-peptide secretory parameters or tAUC for GIP. Further research is needed in individuals with type 2 diabetes to establish the effects of long term ramifications of adopting frequent meals on insulin sensitivity, clearance and secretion. Further, the substitution of protein for carbohydrates reduces tAUC for insulin and glucose and does not alter the glucose/insulin ratio, secretory parameters for c-peptide or GIP responses. Finally, no significant diurnal patterns emerged across any of the parameters investigated in the current study.
Acknowledgements:
Sincere thanks to our subjects who donated a tremendous amount of time to this study, and to the nurses on the Clinical Research Unit at the University of Missouri.
Financial support:
This project was partially supported by NIH R21DK084467. NIH had no role in the design, analysis or writing of this article."
Footnotes
Conflict of Interest: We have no conflicts of interest to declare.
References
- 1.Pories WJ, MacDonald KG Jr., Morgan EJ, Sinha MK, Dohm GL, Swanson MS, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr. 1992;55(2 Suppl):582S–5S. [DOI] [PubMed] [Google Scholar]
- 2.Reed MA, Pories WJ, Chapman W, Pender J, Bowden R, Barakat H, et al. Roux-en-Y gastric bypass corrects hyperinsulinemia implications for the remission of type 2 diabetes. J Clin Endocrinol Metab. 2011;96(8):2525–31. [DOI] [PubMed] [Google Scholar]
- 3.Schulze MB, Thorand B, Fritsche A, Haring HU, Schick F, Zierer A, et al. Body adiposity index, body fat content and incidence of type 2 diabetes. Diabetologia. 2012;55(6):1660–7. [DOI] [PubMed] [Google Scholar]
- 4.Zarjevski N, Doyle P, Jeanrenaud B. Muscle insulin resistance may not be a primary etiological factor in the genetically obese fa/fa rat. Endocrinology. 1992;130(3):1564–70. [DOI] [PubMed] [Google Scholar]
- 5.Alemzadeh R, Jacobs W, Pitukcheewanont P. Antiobesity effect of diazoxide in obese Zucker rats. Metabolism. 1996;45(3):334–41. [DOI] [PubMed] [Google Scholar]
- 6.Alemzadeh R, Langley G, Upchurch L, Smith P, Slonim AE. Beneficial effect of diazoxide in obese hyperinsulinemic adults. J Clin Endocrinol Metab. 1998;83(6):1911–5. [DOI] [PubMed] [Google Scholar]
- 7.Corkey BE. Banting lecture 2011: hyperinsulinemia: cause or consequence? Diabetes. 2012;61(1):4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pories WJ, Dohm GL. Diabetes: have we got it all wrong? Hyperinsulinism as the culprit: surgery provides the evidence. Diabetes Care. 2012;35(12):2438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizza RA, Mandarino LJ, Genest J, Baker BA, Gerich JE. Production of insulin resistance by hyperinsulinaemia in man. Diabetologia. 1985;28(2):70–5. [DOI] [PubMed] [Google Scholar]
- 10.Munsters MJ, Saris WH. Effects of meal frequency on metabolic profiles and substrate partitioning in lean healthy males. PLoS One. 2012;7(6):e38632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmstrup M, Owens C, Fairchild T, Kanaley J. Effect of meal frequency on glucose and insulin excursions over the course of a day. The European e-Journal of Clinical Nutrition and Metabolism. 2010;5(6):e277–e80. [Google Scholar]
- 12.Solomon TP, Chambers ES, Jeukendrup AE, Toogood AA, Blannin AK. The effect of feeding frequency on insulin and ghrelin responses in human subjects. Br J Nutr. 2008:1–10. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins DJ, Ocana A, Jenkins AL, Wolever TM, Vuksan V, Katzman L, et al. Metabolic advantages of spreading the nutrient load: effects of increased meal frequency in non-insulin-dependent diabetes. Am J Clin Nutr. 1992;55(2):461–7. [DOI] [PubMed] [Google Scholar]
- 14.Kalogeropoulou D, Lafave L, Schweim K, Gannon MC, Nuttall FQ. Leucine, when ingested with glucose, synergistically stimulates insulin secretion and lowers blood glucose. Metabolism. 2008;57(12):1747–52. [DOI] [PubMed] [Google Scholar]
- 15.Gannon MC, Nuttall FQ. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes. 2004;53(9):2375–82. [DOI] [PubMed] [Google Scholar]
- 16.Lan-Pidhainy X, Wolever TM. The hypoglycemic effect of fat and protein is not attenuated by insulin resistance. Am J Clin Nutr. 2010;91(1):98–105. [DOI] [PubMed] [Google Scholar]
- 17.Heden TD, Liu Y, Sims LJ, Whaley-Connell AT, Chockalingam A, Dellsperger KC, et al. Meal Frequency Differentially Alters Postprandial Triacylglycerol and Insulin Concentrations in Obese Women. Obesity (Silver Spring). 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetze O, Steingoetter A, Menne D, van der Voort IR, Kwiatek MA, Boesiger P, et al. The effect of macronutrients on gastric volume responses and gastric emptying in humans: A magnetic resonance imaging study. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G11–7. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. [DOI] [PubMed] [Google Scholar]
- 20.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–10. [DOI] [PubMed] [Google Scholar]
- 21.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–31. [DOI] [PubMed] [Google Scholar]
- 22.Engdahl JH, Veldhuis JD, Farrell PA. Altered pulsatile insulin secretion associated with endurance training. J Appl Physiol. 1995;79(6):1977–85. [DOI] [PubMed] [Google Scholar]
- 23.Johnson ML, Veldhuis PP, Grimmichova T, Farhy LS, Evans WS. Validation of a deconvolution procedure (AutoDecon) for identification and characterization of fasting insulin secretory bursts. J Diabetes Sci Technol. 2010;4(5):1205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pritzlaff CJ, Wideman L, Weltman JY, Abbott RD, Gutgesell ME, Hartman ML, et al. Impact of acute exercise intensity on pulsatile growth hormone release in men. J Appl Physiol. 1999;87:498–504. [DOI] [PubMed] [Google Scholar]
- 25.Kanaley JA, Weltman JY, Pieper KS, Weltman A, Hartman ML. Cortisol and growth hormone responses to exercise at different times of day. J Clin Endocrinol Metab. 2001;86:2881–9. [DOI] [PubMed] [Google Scholar]
- 26.Corkey BE. Diabetes: have we got it all wrong? Insulin hypersecretion and food additives: cause of obesity and diabetes? Diabetes Care. 2012;35(12):2432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saad A, Dalla Man C, Nandy DK, Levine JA, Bharucha AE, Rizza RA, et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes. 2012;61(11):2691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol. 1992;262(4 Pt 1):E467–75. [DOI] [PubMed] [Google Scholar]
- 29.Lee A, Ader M, Bray GA, Bergman RN. Diurnal variation in glucose tolerance. Cyclic suppression of insulin action and insulin secretion in normal-weight, but not obese, subjects. Diabetes. 1992;41(6):750–9. [DOI] [PubMed] [Google Scholar]
- 30.Frid AH, Nilsson M, Holst JJ, Bjorck IM. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr. 2005;82(1):69–75. [DOI] [PubMed] [Google Scholar]
- 31.Lindgren O, Mari A, Deacon CF, Carr RD, Winzell MS, Vikman J, et al. Differential islet and incretin hormone responses in morning versus afternoon after standardized meal in healthy men. J Clin Endocrinol Metab. 2009;94(8):2887–92. [DOI] [PubMed] [Google Scholar]