Abstract
BACKGROUND
Radical gastrectomy (RG) is commonly used in the treatment of patients with gastric cancer (GC), but this procedure may lead to stress responses, postoperative cognitive dysfunction, and blood coagulation abnormalities in patients.
AIM
To investigate the influences of dexmedetomidine (DEX) on stress responses and postoperative cognitive and coagulation functions in patients undergoing RG under general anesthesia (GA).
METHODS
One hundred and two patients undergoing RG for GC under GA from February 2020 to February 2022 were retrospectively reviewed. Of these, 50 patients had received conventional anesthesia intervention [control group (CG)] and 52 patients had received DEX in addition to routine anesthesia intervention [observation group (OG)]. Inflammatory factor (IFs; tumor necrosis factor-α, TNF-α; interleukin-6, IL-6), stress responses (cortisol, Cor; adrenocorticotropic hormone, ACTH), cognitive function (CF; Mini-Mental State Examination, MMSE), neurological function (neuron-specific enolase, NSE; S100 calcium-binding protein B, S100B), and coagulation function (prothrombin time, PT; thromboxane B2, TXB2; fibrinogen, FIB) were compared between the two groups before surgery (T0), as well as at 6 h (T1) and 24 h (T2) after surgery.
RESULTS
Compared with T0, TNF-α, IL-6, Cor, ACTH, NSE, S100B, PT, TXB2, and FIB showed a significant increase in both groups at T1 and T2, but with even lower levels in OG vs CG. Both groups showed a significant reduction in the MMSE score at T1 and T2 compared with T0, but the MMSE score was notably higher in OG compared with CG.
CONCLUSION
In addition to a potent inhibitory effect on postoperative IFs and stress responses in GC patients undergoing RG under GA, DEX may also alleviate the coagulation dysfunction and improve the postoperative CF of these patients.
Keywords: Dexmedetomidine, Radical gastrectomy, General anesthesia, Inflammatory factors, Stress responses
Core Tip: Radical gastrectomy (RG), a minimally invasive procedure, is reported to be the optimal cure for gastric cancer (GC) with the advantages of lesser pain and faster recovery. Dexmedetomidine (DEX) is used in a wide range of clinical scenarios. Available evidence suggests that DEX can reduce perioperative inflammation and stress and exert a certain protective effect on cognitive function (CF) in elderly patients after laparoscopic cholecystectomy. In this study, we aimed to assess the influence of DEX on stress responses, CF, and coagulation function of GC patients undergoing RG under general anesthesia, with a view to contributing to the improvement of prognosis in these patients.
INTRODUCTION
Despite the advances in the diagnosis and treatment of gastric cancer (GC), the postoperative prognosis of patients remains unsatisfactory[1]. Radical gastrectomy (RG), a minimally invasive procedure, is reported to be the optimal cure for GC with the advantages of lesser pain and faster recovery[2,3]. However, this procedure may induce physiological abnormalities such as excessive release of inflammatory factors (IFs), stress responses, and blood hypercoagulability[4]. The excessive release of IFs is known to adversely affect the central nervous system, resulting in neurological impairment and increased risk of postoperative cognitive dysfunction[5,6]. Studies have shown that cognitive dysfunction is a common adverse event after cardiac surgery, with approximately one-third of patients suffering from cognitive decline at 6 wk after surgery[7]. Thus, it is incumbent on researchers to search for effective measures to improve the postoperative cognitive function (CF) of GC patients undergoing RG under general anesthesia (GA) from the perspectives of IFs, stress responses, CF, and coagulation function.
Optimization of anesthesia strategy can help reduce postoperative adverse events in patients undergoing RG for GC, with a certain protective effect on vital organ functions and postoperative CF[8,9]. Dexmedetomidine (DEX) is a multipotent central α-2 adrenergic agonist with sedative, analgesic, and anti-sympathetic functions, which is often used as an anesthetic adjuvant[10]. It is used in a wide range of clinical scenarios. Besides RG, it can also be used in colorectal cancer surgery, joint replacement, cardiac surgery, and other clinical scenarios, helping to reduce the risk of delirium in elderly patients[11]. Available evidence suggests that DEX can reduce perioperative inflammation and stress and exert a certain protective effect on CF in elderly patients after laparoscopic cholecystectomy[12,13].
In this study, we aimed to assess the influence of DEX on stress responses, CF, and coagulation function of GC patients undergoing RG under GA, with a view to contributing to the improvement of prognosis in these patients.
MATERIALS AND METHODS
General data
This was a retrospective study approved by the Ethics Committee of The Second Affiliated Hospital of Guangxi Medical University. The study population comprised of 102 patients with GC who underwent RG under GA at our hospital between February 2020 and February 2022. Patients who received routine anesthesia intervention were included in the control group (CG; n = 50) while those who received DEX in combination with conventional anesthesia intervention were included in the observation group (OG; n = 52). The two groups were comparable with respect to baseline clinical characteristics (P > 0.05).
Criteria for patient enrollment and exclusion
All the included patients met the surgical indications for GC and underwent GA, with the America Society of Anesthesiologist (ASA) classification II or III[14], intact case data, no mental illness or mental disorders, and active cooperation with the research.
The exclusion criteria for this study were as follows: Severe arrhythmia as confirmed by electrocardiograph (ECG); diseases such as severe malnutrition, anemia and abnormal liver function; diabetes, hypertension or coronary heart disease; infectious diseases.
Intervention methods
CG group received routine anesthesia intervention. OG group received was DEX in addition to routine anesthesia intervention.
For all the patients, blood pressure, ECG, and pulse oxygen saturation were routinely monitored after entering the operating room, and venous access was established. DEX infusion was initiated before conventional induction and discontinued before the heart resumed beating. In OG, DEX was injected intravenously at a loading dose of 0.5 μg/kg followed by a continuous infusion at a rate of 0.2–0.6 μg/kg/h; patients in the CG were administered normal saline at the same dose. After the above procedure, both groups of patients underwent routine anesthesia induction in the same manner, namely, administration of intravenous midazolam, fentanyl, atracurium, and propofol. Endotracheal intubation and mechanical ventilation were then performed with a tidal volume of 8–10 mL/kg and a ventilation frequency of 12–20 times/min; the PETCO2 was maintained at 35–40 mmHg. Propofol, remifentanil, and atracurium were injected intravenously for anesthesia maintenance.
Evaluation indices
After anesthesia, five milliliters of peripheral elbow venous blood was collected before surgery (T0), as well as at 6 h (T1) and 24 h (T2) after surgery. Serum was separated via centrifugation after 2 h of standing, and refrigerated at -20℃ for later use.
IFs: Serum levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were determined by enzyme-linked immunosorbent assay (ELISA).
Stress responses: ELISA was performed to quantify blood cortisol (Cor) and adrenocorticotropic hormone (ACTH) levels.
CF: According to the Mini-Mental State Examination (MMSE), the CF of patients at T0, T1, and T2 was evaluated from seven aspects: Time orientation, place orientation, registration, attention and calculation, recall, language, and copying. The lower the score, the more significant the cognitive dysfunction.
Neurological function: ELISA was employed to measure neuron-specific enolase (NSE) and S100 calcium-binding protein B (S100B) levels.
Coagulation function: An automatic hemagglutination analyzer was used to quantify coagulation function indicators prothrombin time (PT), thromboxane B2 (TXB2), and fibrinogen (FIB).
Statistical analysis
Continuous variables were presented as mean ± SD and between-group differences were assessed using the independent sample t test. Multi-group and within-group differences were assessed using one-way ANOVA. Categorical variables were presented as frequency (percentage) and between-group differences were assessed using the χ2 test. Statistical analysis was performed using SPSS 19.0. P values < 0.05 were considered indicative of statistical significance.
RESULTS
Comparison of baseline data between the two groups
There was no significant difference between the two groups with respect to sex, age, disease course, body weight, tumor staging, ASA grade, or history of hypertension and diabetes (P > 0.05) (Table 1).
Table 1.
Comparison of baseline data of two groups of gastric cancer patients undergoing radical gastrectomy under general anesthesia
|
Classification
|
Control group (n = 50)
|
Observation group (n = 52)
|
χ
2 value
|
P value
|
| Gender (male/female) | 32/18 | 29/23 | 0.718 | 0.397 |
| Age (yr) | 50.82 ± 6.65 | 49.85 ± 7.63 | 0.683 | 0.496 |
| Course of disease (yr) | 2.32 ± 0.55 | 2.25 ± 0.56 | 0.637 | 0.526 |
| Weight (kg) | 63.76 ± 8.02 | 64.38 ± 8.43 | 0.380 | 0.705 |
| Tumor staging (I/II) | 28/22 | 27/25 | 0.171 | 0.680 |
| ASA classification (II/III) | 26/24 | 30/22 | 0.334 | 0.564 |
| History of hypertension (yes/no) | 10/40 | 15/37 | 1.078 | 0.299 |
| Medical history of diabetes (yes/no) | 7/43 | 12/40 | 1.386 | 0.239 |
ASA: America Society of Anesthesiologist.
Influence of DEX on IFs
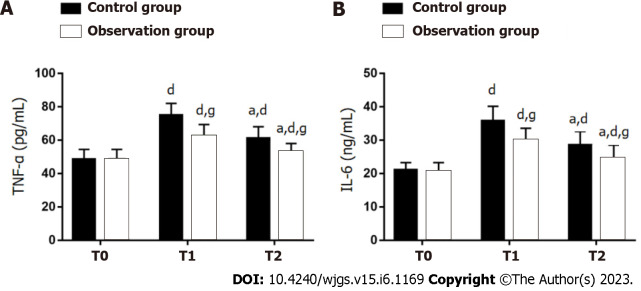
Serum levels of TNF-α and IL-6 were not significantly different between the two groups at T0 (P > 0.05). The levels showed a marked increase in both groups at T1 and T2 (P < 0.05), with significantly lower levels in OG as compared to CG (P < 0.05) (Figure 1).
Figure 1.
Influence of dexmedetomidine on inflammatory factors in gastric cancer patients undergoing radical gastrectomy under general anesthesia. A: Tumor necrosis factor-α at different time points in two groups of gastric cancer (GC) patients undergoing radical gastrectomy (RG) under general anesthesia (GA); B: Interleukin-6 at different time points in two groups of GC patients undergoing RG under GA. aP < 0.05 vs T1; dP < 0.05 vs T0; gP < 0.05 vs control group. T0: Before surgery; T1: 6 h after surgery; T2: 24 h after surgery; TNF-α: Tumor necrosis factor-α; IL-6: Interleukin-6.
Influence of DEX on stress responses
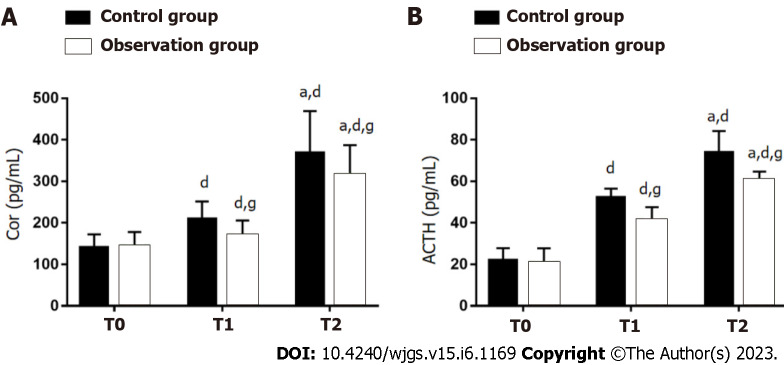
The stress responses of both groups were evaluated by measuring Cor and ACTH (Figure 2). There were no significant between-group differences with respect to Cor and ACTH at T0 (P > 0.05). Compared with T0, Cor and ACTH in both groups showed a significant increase at T1 and T2 (P < 0.05), especially in OG (P < 0.05).
Figure 2.
Influence of dexmedetomidine on stress responses of gastric cancer patients undergoing radical gastrectomy under general anesthesia. A: Cortisol at different time points in two groups of gastric cancer (GC) patients undergoing radical gastrectomy (RG) under general anesthesia (GA); B: Adrenocorticotropic hormone at different time points in two groups of GC patients undergoing RG under GA. aP < 0.05 vs T1; dP < 0.05 vs T0; gP < 0.05 vs control group. T0: Before surgery; T1: 6 h after surgery; T2: 24 h after surgery; Cor: Cortisol; ACTH: Adrenocorticotropic hormone.
Impact of DEX on CF
There was no significant between-group difference in the MMSE score at T0 (P > 0.05). MMSE scores at T1 and T2 were significantly lower than that at T0 in both groups (P < 0.05), but the scores of OG were still higher than those of CG (P < 0.05) (Figure 3).
Figure 3.
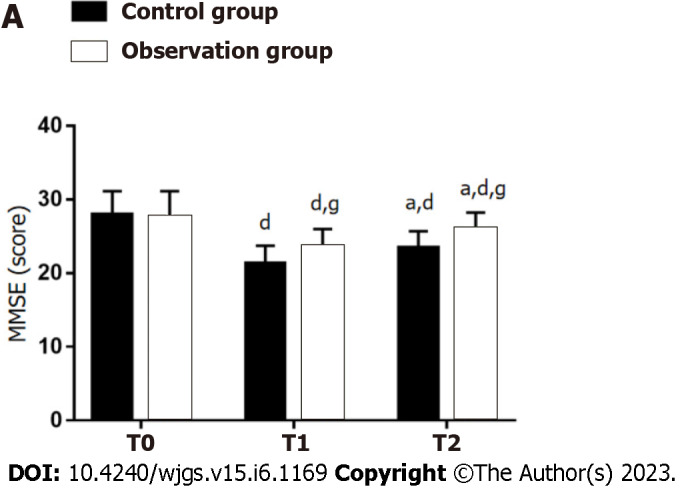
Effect of dexmedetomidine on cognitive function (Mini-Mental State Examination) of gastric cancer patients undergoing radical gastrectomy under general anesthesia. aP < 0.05 vs T1; dP < 0.05 vs T0; gP < 0.05 vs control group. T0: Before surgery; T1: 6 h after surgery; T2: 24 h after surgery; MMSE: Mini-Mental State Examination.
Effect of DEX on neurological function
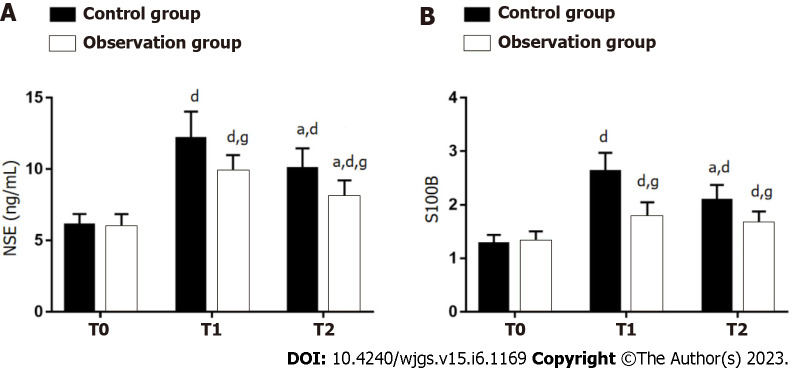
The effects of two anesthesia methods on neurological function were evaluated by detecting NSE and S100B (Figure 4). There were no significant between-group differences with respect to NSE and S100B at T0 (P > 0.05). Significant increase in NSE and S100B was observed in both groups at T1 and T2 (P < 0.05), with lower levels in OG as compared to CG (P < 0.05).
Figure 4.
Effect of dexmedetomidine on neurological function of gastric cancer patients undergoing radical gastrectomy under general anesthesia. A: Neuron-specific enolase at different time points in two groups of gastric cancer (GC) patients undergoing radical gastrectomy (RG) under general anesthesia (GA); B: S100 calcium-binding protein B at different time points in two groups of GC patients undergoing RG under GA. aP < 0.05 vs T1; dP < 0.05 vs T0; gP < 0.05 vs control group. T0: Before surgery; T1: 6 h after surgery; T2: 24 h after surgery; NSE: Neuron-specific enolase; S100B: S100 calcium-binding protein B.
Influence of DEX on coagulation function
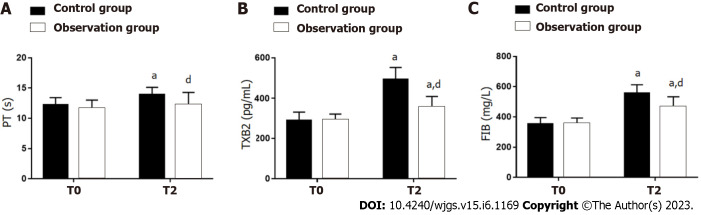
There were no significant between-group differences with respect to PT, XB2, or FIB at T0 (P > 0.05). At T1 and T2, both groups showed a significant increase in PT, TXB2 and FIB compared with the respective levels at T0 (P < 0.05), with lower levels in OG vs CG (P < 0.05) (Figure 5).
Figure 5.
Influence of dexmedetomidine on neurological function of gastric cancer patients undergoing radical gastrectomy under general anesthesia. A: Prothrombin time at different time points in two groups of gastric cancer (GC) patients undergoing radical gastrectomy (RG) under general anesthesia (GA); B: Thromboxane B2 at different time points in two groups of GC patients undergoing RG under GA; C: Fibrinogen at different time points in two groups of GC patients undergoing RG under GA. aP < 0.05 vs T0; dP < 0.05 vs control group. T0: Before surgery; T1: 6 h after surgery; T2: 24 h after surgery; PT: Prothrombin time; TXB2: Thromboxane B2; FIB: Fibrinogen.
DISCUSSION
RG is the main treatment modality for GC, but the inflammation, stress responses, and neurological dysfunction induced by surgical trauma have a negative impact on patient postoperative recovery and survival[2]. The influence of DEX on postoperative stress responses, CF, and coagulation function of GC patients undergoing RG under GA remains poorly elucidated in the contemporary literature.
Several studies have investigated the application value of DEX in RG for GC. In the study by Guo et al[15], DEX outperformed epidural anesthesia in terms of sedative and analgesic effects in elderly adults undergoing RG for GC and accelerated their recovery. Liu et al[16] focused on the influence of DEX combined with propofol on postoperative analgesia and cellular immune function during RG. The combination of the two was found to suppress postoperative stress responses, improve analgesia effects, enhance immune function, and reduce the occurrence of postoperative adverse events. In the present study, we investigated the clinical effects of DEX in GC patients undergoing RG under GA from five aspects: inflammation, stress, CF, neurological function, and coagulation function. In terms of inflammation, postoperative TNF-α and IL-6 levels were significantly lower in OG, suggesting the anti-inflammatory effect of DEX in these patients. TNF-α and IL-6 are known inflammatory indices of RG, both of which mediate the inflammatory process and participate in organ involvement and can be inhibited to some extent postoperatively under the intervention of DEX, consistent with our observations[17,18]. The anti-inflammatory mechanism of DEX may be related to the activation of cholinergic anti-inflammatory pathway to suppress systemic inflammatory responses[19]. In the stress response evaluation, Cor and ACTH in OG were found to be significantly elevated after surgery but were still lower than those in CG, suggesting that DEX used in RG has a more prominent inhibitory effect on stress responses. Consistently, Yang et al[20] also reported that DEX can alleviate stress responses in patients undergoing laparoscopic cholecystectomy, which was reflected in significant reductions in Cor and ACTH levels. Further, CF evaluation results showed that although the postoperative MMSE score of OG reduced notably just like CG, it was still significantly higher than CG, indicating a significant protective effect of DEX on the CF of patients undergoing RG under GA, which is in line with the findings of Yang et al[21]. When evaluating neurological function, NSE and S100B in OG were also found to be significantly increased as those in CG, but were still markedly lower in OG vs CG, indicating that DEX intervention can inhibit NSE and S100B in patients. NSE and S100B are known to be neurological function indices related to brain injury; the former can reflect neuronal abnormalities, while the latter is a marker of glial cell damage[22]. Zhao et al[23] also reported a neuroprotective effect of DEX in patients with hypertensive cerebral hemorrhage in the perioperative period by inhibiting NSE and S100B levels, which is consistent with our results. Finally, we verified the effect of DEX on coagulation function, and found that PT, TXB2, and FIB in OG after the intervention of DEX were significantly increased but significantly lower than those in CG, indicating that DEX can significantly improve coagulation function in patients undergoing RG under GA. Chen et al[24] also found that the application of DEX in patients undergoing RG under GA inhibited postoperative blood hypercoagulability by weakening the activation of coagulation function, which is related to the direct or indirect regulation of platelet function by DEX.
Some limitations of our study should be considered. This was a single-center retrospective study with a relatively small sample size, which may have introduced an element of bias. A larger multi-center study is required to obtain more definitive evidence.
CONCLUSION
In this study, the use of DEX demonstrated a significant clinical benefit in patients undergoing RG under GA. DEX was found to inhibit inflammation and stress reactions, as well as improve the postoperative cognitive, neurological, and coagulation functions in these patients. Our findings may provide a new reference for anesthesia management optimization and prognosis improvement of such patients.
ARTICLE HIGHLIGHTS
Research background
Radical gastrectomy (RG) is often used to treat patients with gastric cancer (GC), but it may cause stress responses, postoperative cognitive dysfunction and abnormal coagulation function.
Research motivation
The effects of dexmedetomidine (DEX) on stress responses, postoperative cognitive function and coagulation function of GC patients undergoing RG under general anesthesia were analyzed retrospectively.
Research objectives
This study aimed to optimize anesthesia strategy to help reduce the perioperative risk of GC patients receiving RG.
Research methods
One hundred and two patients undergoing RG for GC under general anesthesia were included. Of them, 50 cases receiving routine anesthesia were set as a control group (CG) and 52 cases receiving routine anesthesia plus DEX were set as an observation group (OG). Then inflammatory factors, stress responses, cognitive function, neurological function, and coagulation function of the two groups were comparatively analyzed at various time points [before (T0), and 6 h (T1) and 24 h (T2) after surgery].
Research results
Compared with T0, tumor necrosis factor-α, interleukin-6, cortisol, adrenocorticotropic hormone, neuron-specific enolase, S100 calcium-binding protein B, prothrombin time, thromboxane B2, and fibrinogen were markedly elevated at T1 and T2 in both groups, with even lower levels of these parameters in OG compared with CG. In addition, a marked reduction in the Mini-Mental State Examination (MMSE) score was observed at T1 and T2 compared with T0 in both groups, with a significantly higher MMSE score in OG vs CG at each postoperative time point.
Research conclusions
In addition to effective inhibition of inflammatory factors and stress responses in GC patients undergoing RG under general anesthesia, DEX can also alleviate coagulation dysfunction and improve postoperative cognitive function in these patients.
Research perspectives
Our findings may provide a novel reference for optimizing anesthesia management and improving outcomes in patients undergoing RG for GC.
Footnotes
Institutional review board statement: The study was reviewed and approved by The Second Affiliated Hospital of Guangxi Medical University Institutional Review Board [Approval No. 2020(KY-0141)].
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare no competing interests.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 6, 2023
First decision: March 14, 2023
Article in press: April 19, 2023
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koestler T, Switzerland; Zingg U, Switzerland S-Editor: Wang JL L-Editor: A P-Editor: Guo X
Contributor Information
Xiang-Fei Ma, Department of Anesthesiology, The Second Affiliated Hospital of Guangxi Medical University, Nanning 530007, Guangxi Zhuang Autonomous Region, China.
Shi-Jia Lv, Department of Anesthesiology, The Second Affiliated Hospital of Guangxi Medical University, Nanning 530007, Guangxi Zhuang Autonomous Region, China.
Shen-Qiao Wei, Department of Anesthesiology, The Second Affiliated Hospital of Guangxi Medical University, Nanning 530007, Guangxi Zhuang Autonomous Region, China.
Bing-Rong Mao, Department of Anesthesiology, The Second Affiliated Hospital of Guangxi Medical University, Nanning 530007, Guangxi Zhuang Autonomous Region, China.
Xiu-Xia Zhao, Department of Anesthesiology, The Second Affiliated Hospital of Guangxi Medical University, Nanning 530007, Guangxi Zhuang Autonomous Region, China.
Xiao-Qing Jiang, Department of Anesthesiology, The Second Affiliated Hospital of Guangxi Medical University, Nanning 530007, Guangxi Zhuang Autonomous Region, China.
Fei Zeng, Department of Anesthesiology, The Second Affiliated Hospital of Guangxi Medical University, Nanning 530007, Guangxi Zhuang Autonomous Region, China.
Xue-Ke Du, Department of Anesthesiology, The Second Affiliated Hospital of Guangxi Medical University, Nanning 530007, Guangxi Zhuang Autonomous Region, China. mxf17031861@126.com.
Data sharing statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Wang Q, Zhu D. The prognostic value of systemic immune-inflammation index (SII) in patients after radical operation for carcinoma of stomach in gastric cancer. J Gastrointest Oncol. 2019;10:965–978. doi: 10.21037/jgo.2019.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiao R, Peng S, Wang L, Feng M, Li Y, Sun J, Liu D, Fu J, Feng C. Ultrasound-Guided Quadratus Lumborum Block Combined with General Anaesthesia or General Anaesthesia Alone for Laparoscopic Radical Gastrectomy for Gastric Adenocarcinoma: A Monocentric Retrospective Study. Int J Gen Med. 2022;15:7739–7750. doi: 10.2147/IJGM.S382757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan W, Yang L, Li J, Dong B. Ultrasound Image-Guided Nerve Block Combined with General Anesthesia under an Artificial Intelligence Algorithm on Patients Undergoing Radical Gastrectomy for Gastric Cancer during and after Operation. Comput Math Methods Med. 2022;2022:6914157. doi: 10.1155/2022/6914157. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Robich M, Ryzhov S, Kacer D, Palmeri M, Peterson SM, Quinn RD, Carter D, Sheppard F, Hayes T, Sawyer DB, Rappold J, Prudovsky I, Kramer RS. Prolonged Cardiopulmonary Bypass is Associated With Endothelial Glycocalyx Degradation. J Surg Res. 2020;251:287–295. doi: 10.1016/j.jss.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen Y, Liu X, Shi J, Wu X. Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int J Biol Macromol. 2019;125:496–502. doi: 10.1016/j.ijbiomac.2018.11.190. [DOI] [PubMed] [Google Scholar]
- 6.Urcun YS, Altun Y, Pala AA. Early and late predictors of postoperative neurocognitive dysfunction in cardiac surgery. Ideggyogy Sz. 2022;75:231–240. doi: 10.18071/isz.75.0231. [DOI] [PubMed] [Google Scholar]
- 7.Mathew JP, White WD, Schinderle DB, Podgoreanu MV, Berger M, Milano CA, Laskowitz DT, Stafford-Smith M, Blumenthal JA, Newman MF Neurologic Outcome Research Group (NORG) of The Duke Heart Center. Intraoperative magnesium administration does not improve neurocognitive function after cardiac surgery. Stroke. 2013;44:3407–3413. doi: 10.1161/STROKEAHA.113.002703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Ren X, Ma Y, Ge L, Hu Z, Yan W. [Research progress of the role of postoperative pain in the development of postoperative cognitive dysfunction in geriatric patients] Nan Fang Yi Ke Da Xue Xue Bao. 2019;39:1122–1126. doi: 10.12122/j.issn.1673-4254.2019.09.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pajares MA, Margarit JA, García-Camacho C, García-Suarez J, Mateo E, Castaño M, López Forte C, López Menéndez J, Gómez M, Soto MJ, Veiras S, Martín E, Castaño B, López Palanca S, Gabaldón T, Acosta J, Fernández Cruz J, Fernández López AR, García M, Hernández Acuña C, Moreno J, Osseyran F, Vives M, Pradas C, Aguilar EM, Bel Mínguez AM, Bustamante-Munguira J, Gutiérrez E, Llorens R, Galán J, Blanco J, Vicente R. Guidelines for enhanced recovery after cardiac surgery. Consensus document of Spanish Societies of Anesthesia (SEDAR), Cardiovascular Surgery (SECCE) and Perfusionists (AEP) Rev Esp Anestesiol Reanim (Engl Ed) 2021;68:183–231. doi: 10.1016/j.redar.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Thomas A, Miller JL, Couloures K, Johnson PN. Non-Intravenous Sedatives and Analgesics for Procedural Sedation for Imaging Procedures in Pediatric Patients. J Pediatr Pharmacol Ther. 2015;20:418–430. doi: 10.5863/1551-6776-20.6.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sui X, Duan Q, Liu K, Li C. Postoperative delirium after long-term general anesthesia in elderly patients, how to reduce it?: Protocol of a double-blinded, randomized, placebo-controlled trial. Medicine (Baltimore) 2021;100:e25885. doi: 10.1097/MD.0000000000025885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K, Wu M, Xu J, Wu C, Zhang B, Wang G, Ma D. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: systematic review and meta-analysis. Br J Anaesth. 2019;123:777–794. doi: 10.1016/j.bja.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Yan J, Han X. Dexmedetomidine may benefit cognitive function after laparoscopic cholecystectomy in elderly patients. Exp Ther Med. 2013;5:489–494. doi: 10.3892/etm.2012.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan S, Shillcutt S, Oakes D, Muehlschegel JD, Shore-Lesserson L, Rong LQ. Gender of Abstract Presenters at the Annual Meetings of the Society of Cardiovascular Anesthesiologists and American Society of Anesthesiologists: 2016 to 2020. J Cardiothorac Vasc Anesth. 2022;36:1867–1872. doi: 10.1053/j.jvca.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo L, Liu Y, Wang M. Effect of Perioperative Dexmedetomidine Anesthesia on Prognosis of Elderly Patients with Gastrointestinal Tumor Surgery. Comput Math Methods Med. 2022;2022:7889372. doi: 10.1155/2022/7889372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu R, Suo S, Wang Y, Wang M. Effects of Dexmedetomidine and Propofol on Postoperative Analgesia and the Cellular Immune Function of Patients Undergoing Radical Gastrectomy for Gastric Cancer. Contrast Media Mol Imaging. 2022;2022:7440015. doi: 10.1155/2022/7440015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Vosoughian M, Dahi M, Dabir S, Moshari M, Tabashi S, Mosavi Z. Effects of General Anesthesia Versus Spinal Anesthesia on Serum Cytokine Release After Cesarean Section: A Randomized Clinical Trial. Anesth Pain Med. 2021;11:e111272. doi: 10.5812/aapm.111272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui J, Gao M, Huang H, Huang X, Zeng Q. Dexmedetomidine Improves Lung Function by Promoting Inflammation Resolution in Patients Undergoing Totally Thoracoscopic Cardiac Surgery. Oxid Med Cell Longev. 2020;2020:8638301. doi: 10.1155/2020/8638301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma J, Chen Q, Li J, Zhao H, Mi E, Chen Y, Yi B, Ning J, Ma D, Lu K, Gu J. Dexmedetomidine-Mediated Prevention of Renal Ischemia-Reperfusion Injury Depends in Part on Cholinergic Anti-Inflammatory Mechanisms. Anesth Analg. 2020;130:1054–1062. doi: 10.1213/ANE.0000000000003820. [DOI] [PubMed] [Google Scholar]
- 20.Yang A, Gao F. Effect of dexmedetomidine combined with propofol on stress response, hemodynamics, and postoperative complications in patients undergoing laparoscopic cholecystectomy. Am J Transl Res. 2021;13:11824–11832. [PMC free article] [PubMed] [Google Scholar]
- 21.Yang W, Kong LS, Zhu XX, Wang RX, Liu Y, Chen LR. Effect of dexmedetomidine on postoperative cognitive dysfunction and inflammation in patients after general anaesthesia: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e15383. doi: 10.1097/MD.0000000000015383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Yu ZX, Ji MS, Yan J, Cai Y, Liu J, Yang HF, Jin ZC. A Pilot Study of the Use of Dexmedetomidine for the Control of Delirium by Reducing the Serum Concentrations of Brain-Derived Neurotrophic Factor, Neuron-Specific Enolase, and S100B in Polytrauma Patients. J Intensive Care Med. 2019;34:674–681. doi: 10.1177/0885066617710643. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J, Zhou C. The protective and hemodynamic effects of dexmedetomidine on hypertensive cerebral hemorrhage patients in the perioperative period. Exp Ther Med. 2016;12:2903–2908. doi: 10.3892/etm.2016.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Shao DH, Mao ZM, Shi LL, Ma XD, Zhang DP. Effect of dexmedetomidine on blood coagulation in patients undergoing radical gastrectomy under general anesthesia: A prospective, randomized controlled clinical trial. Medicine (Baltimore) 2018;97:e11444. doi: 10.1097/MD.0000000000011444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.