Abstract
Purpose
Coronary artery disease (CAD) is the primary cause of mortality in developing countries. Off-pump coronary artery bypass grafting (OPCAB) offers more upside in revascularization by preventing cardiopulmonary bypass trauma and minimizing aortic manipulation. Even though cardiopulmonary bypass is not involved, OPCAB still causes a significant systemic inflammatory response. This study determines the prognostic values of the systemic immune-inflammation index (SII) towards perioperative outcomes in patients who underwent OPCAB surgery.
Patients and methods
This was a single-center retrospective study at the National Cardiovascular Center Harapan Kita, Jakarta, using secondary data from electronic medical records and medical record archives of all patients who underwent OPCAB from January 2019 through December 2021. A total of 418 medical records were obtained, and 47 patients were excluded based on the exclusion criteria. The values of SII were calculated from preoperative laboratory data of segmental neutrophil count, lymphocyte count, and platelet count. Patients were divided into two groups based on the SII cutoff value of 878.056 x 103/mm3.
Results
The baseline SII values of 371 patients were calculated, among which 63 (17%) patients had preoperative SII values of ≥878.057 x 103/mm3. High SII values were a significant predictor of prolonged ventilation (RR 1.141, 95% CI 1.001–1.301) and prolonged ICU stay (RR 1.218, 95% CI 1.021–1.452) after OPCAB surgery. A positive correlation was observed between SII and hospital length of stay after OPCAB surgery. From the receiver operating characteristic curve analysis, SII predicted prolonged ventilation duration, with an area under the curve of 0.658 (95% CI 0.575–0.741, p = 0.001).
Conclusion
High preoperative SII values are capable of predicting prolonged mechanical ventilation and intensive care unit stay after OPCAB surgery.
Keywords: systemic immune-inflammation index, off-pump coronary artery bypass graft, systemic inflammation, mechanical ventilation, intensive care unit stay
Introduction
Coronary artery disease (CAD) is one of the major causes of disability and mortality in developed countries and is expected to increase in developing countries.1 Treatment of CAD by myocardial revascularization aims to reduce chest pain symptoms and improve patient outcomes.2 Off-pump coronary artery bypass grafting (OPCAB) is a revascularization method without the involvement of cardiopulmonary bypass. The OPCAB procedure is increasingly becoming a choice due to its advantages in reducing cardiopulmonary bypass trauma, while minimizing aortic manipulation.3 However, OPCAB offers less complete revascularization and is associated with higher mortality at ten years, reaching 33.4% compared to 29.6% in on-pump coronary artery bypass grafting (CABG).4
Atherosclerosis is a low-grade inflammatory condition that affects coronary artery endothelium.5 Systemic inflammatory responses are an integral factor in determining postoperative morbidity and mortality.6 Neutrophil activation in OPCAB causes a spike in neutrophil count due to sequestration. Monocyte and neutrophil activation prompts platelet activations, which release cytokines as a systemic inflammatory response.7 The systemic immune-inflammation index (SII) is a novel index proposed by Hu et al in 2014, involving neutrophil, lymphocyte, and platelet counts and capable of reflecting a patient’s inflammatory and immune status.8 Dividing the neutrophyl count by the lymphocyte count will produce the neutrophil-to-lymphocyte ratio (NLR). Multiplying NLR by the platelet count results in SII. In cardiac surgery, the decrease in platelet counts, together with the increase in NLR, has been shown as an effective indicator on early mortality and renal failure.9 However, previous studies of SII in OPCAB patients are still limited.
Dey et al observed that SII independently predicted poor postoperative outcomes. Furthermore, a significant correlation was found between SII values and the duration of mechanical ventilation and intensive care unit (ICU) length of stay.10 Yang et al concluded that a higher SII value is independently associated with a higher future risk of developing cardiac death in CAD patients after coronary intervention. Improvements in the risk prediction of major cardiovascular events were observed in SII compared to traditional risk factors.11
Patients undergoing OPCAB are still vulnerable to inflammatory responses. Generally, inflammatory indices will increase in the earlier phase of on-pump CABG. However, a delayed response due to prothrombotic conditions was observed in OPCAB.6 Higher SII values were previously associated with poor outcomes.12,13 The current study aims to assess the prognostic values of SII for morbidity and mortality outcomes in OPCAB patients.
Methods
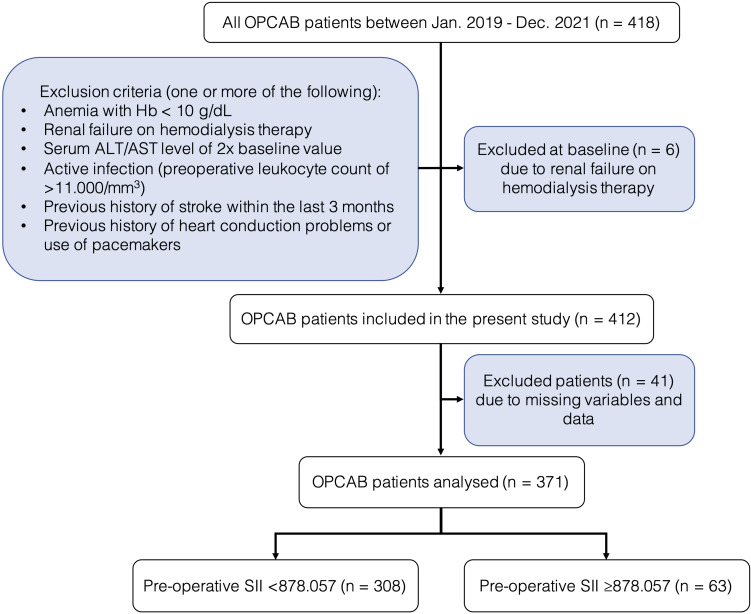
This was a single-center, retrospective study approved by the Institutional Review Board of the National Cardiovascular Center Harapan Kita (LB.02.01/VII/008/KEP008/2023). This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was waived due to the retrospective nature of our study, and all patients’ data were anonymized and de-identified before analysis. This study included consecutive cases of patients who underwent OPCAB surgery at the National Cardiovascular Center Harapan Kita from January 2019 through December 2021. Patients over the age of 18 who underwent a non-emergency OPCAB procedure are eligible. Patients with the presence of one or more of the following are excluded: patients with missing variables and data, anemia with hemoglobin (Hb) < 10 g/dL, renal failure on hemodialysis therapy, a serum ALT/AST level of twice the baseline value, active infection (preoperative total leukocyte count of > 11.000/mm3), previous history of stroke within the last three months, and heart conduction problems or use of pacemakers. Dropout criteria included conversion to on-pump CABG. The flow chart for the present study is illustrated in Figure 1.
Figure 1.
Study flow diagram.
Preoperative characteristics data of the patients such as age, sex, body mass index (BMI), history of hypertension, diabetes mellitus, hyperlipidemia, chronic obstructive pulmonary disorder, smoking history, active smoker, family history of CAD, left ventricular ejection fraction (LVEF), presence of left main (LM) disease and EuroSCORE II were all recorded. Preoperative laboratory data recorded are segmental neutrophil count, lymphocyte count, and platelet count, which are then calculated for the NLR and SII. Patients were divided into two groups based on the SII value cutoff: <878.056 x 103/mm3 and ≥878.057 x 103/mm3. The value of NLR was calculated by dividing the neutrophil count by the lymphocyte count. The value of SII was calculated by multiplying the neutrophil count by the platelet count and dividing by the lymphocyte count.
Data regarding the procedure, including the duration of the surgery, number of grafts, the requirement of an intra-aortic balloon pump (IABP), and blood transfusion, were recorded. Perioperative outcomes such as the vasoactive-inotropic score (VIS), duration of mechanical ventilation (DO-MV), length of intensive care unit stay (LOS-ICU), length of hospital stay (LOS-H), the incidence of postoperative atrial fibrillation (PoAF), stroke, acute myocardial infarct (MI), and mortality were also recorded. VIS was obtained by adding dopamine (μg/kg/min) + dobutamine (μg/kg/min) + 10 × milrinone (μg/kg/min) + 100 × epinephrine (μg/kg/min) + 100 × norepinephrine (μg/kg/min) + 10,000 × vasopressin (μg/kg/min).
Standard anesthesia procedures and techniques were followed in this study. All anesthesia procedures performed, including induction, monitoring, and maintenance protocols, were in accordance with the standard institutional protocol. Experienced cardiac surgeons performed all the surgeries. A median sternotomy was performed, followed by the harvesting of the left internal mammary artery and a venous graft. Following the procedure, all patients are transported to the ICU.
Patients were evaluated hourly for eligibility for extubation in the ICU, where hemodynamically stable patients (without the need for high-dose vasoactive inotropic support with urine output of >0.5 mL/kg/hours) are weaned off the ventilator and subsequently extubated. Prolonged mechanical ventilation was accepted as >24 hours since all operations were performed by avoiding unnecessary use of anesthetic agents. Prolonged ICU stay was accepted as length of stay of >48 hours in the ICU. Stable patients are then transported to the surgery recovery ward. Prolonged hospital stay was accepted as length of hospital stay >7 days. Mortality rates will be recorded until the thirtieth postoperative day.
Statistical Analysis
Data analysis was performed using SPSS for Mac version 23.0 (SPSS Inc., Chicago, IL, USA). The significance level was 0.05 (two-tailed) with a predetermined power of 90%. The Kolmogorov‑Smirnov test was used for performing the normality test. Normally distributed continuous variables were expressed in mean ± standard deviation (SD) and analyzed using the unpaired t‑test, while non-normally distributed data were expressed in median (minimum‑maximum) and analyzed using the Mann‑Whitney U-test. Categorical variables were expressed in number and frequency and subsequently analyzed using the Chi‑square test. A p-value of < 0.05 was considered statistically significant. A receiver operating curve analysis was done on significant outcomes to determine a new SII cutoff using the largest Youden Index. The correlation between significant variables and SII was tested with Pearson’s or Spearman correlation. Variables with a p value of < 0.05 are considered significant.
Results
Among the 371 patients in the present study, 63 (17%) patients had preoperative SII values of ≥878.057 x 103/mm3. The demographic characteristics are presented in Table 1, followed by laboratory parameters in Table 2 and perioperative data in Table 3. EuroSCORE II, neutrophil count, lymphocyte count, platelet count, NLR, and SII were significantly different (p <0.05) between the low and high SII groups.
Table 1.
Patients Characteristics
| Variables | SII <878.057 (n = 308) | SII ≥878.057 (n = 63) | p-value |
|---|---|---|---|
| Age, years (Mean ± SD) | 58.94 ± 7.87 | 60.63 ± 6.64 | 0.111 |
| Sex | |||
|
259 (84.1%) | 51 (81%) | 0.670 |
|
49 (15.9%) | 12 (19%) | |
| Body Mass Index, kg/m2 (Median [min-max]) | 25.59 (13.71–40.27) | 24.77 (20.00–35.00) | 0.396 |
| Hypertension (%) | 197 (64%) | 43 (68.3%) | 0.565 |
| Diabetes Mellitus (%) | 104 (33.8%) | 27 (42.9%) | 0.193 |
| Dyslipidemia (%) | 90 (29.2%) | 18 (28.6%) | 1.000 |
| Chronic Obstructive Pulmonary Disorder (%) | 4 (1.3%) | 3 (4.8%) | 0.183 |
| Smoking History (%) | 165 (53.6%) | 39 (61.9%) | 0.267 |
|
9 (2.9%) | 5 (7.9%) | 0.123 |
| Recent MI (%) | 109 (35.4%) | 16 (25.4%) | 0.145 |
| Family History of CAD (%) | 50 (16.2%) | 9 (14.3%) | 0.844 |
| Preoperative Ejection Fraction, % (Median [min-max]) | 57 (15–86) | 55 (21–80) | 0.363 |
| LM Disease (%) | 92 (29.9%) | 25 (39.7%) | 0.138 |
| EuroSCORE II (Median [min-max]) | 1.02 (0.50–9.79) | 1.19 (0.55–5.95) | 0.030* |
Note: *Statistical significance (p < 0.05) is marked with.
Abbreviations: CAD, coronary artery disease; LM, left main; MI, myocardial infarct; SD, standard deviation; SII, systemic immune-inflammation index.
Table 2.
Laboratory Parameters
| Variables | SII <878.057 (n = 308) | SII ≥878.057 (n = 63) | p-value |
|---|---|---|---|
| Neutrophil Count (Median [min-max]) | 57.25 (30.00–76.80) | 69.7 (31.20–86.10) | <0.001* |
| Lymphocyte Count (Median [min-max]) | 29.75 (8.80–57.10) | 18.00 (2.40–29.30) | <0.001* |
| Platelet Count (Mean ± SD) | 242.16 ± 56.75 | 318.54 ± 90.04 | <0.001* |
| NLR (Median [min-max]) | 1.92 (0.53–5.09) | 3.85 (1.79–34.04) | <0.001* |
| SII (Median [min-max]) | 466.059 (119.790–877.272) | 1131.330 (878.718–8788.138) | <0.001* |
Note: *Statistical significance (p < 0.05) is marked with.
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; SD, standard deviation; SII, systemic immune-inflammation index.
Table 3.
Procedural Data
| Variables | SII <878.057 (n = 308) | SII ≥878.057 (n = 63) | p-value |
|---|---|---|---|
| Duration of surgery, minutes (Median [min-max]) | 270 (97–646) | 270 (90–450) | 0.306 |
| Number of grafts (%) | |||
|
7 (2.3%) | 0 (0%) | 0.062 |
|
89 (28.9%) | 11 (17.5%) | |
|
205 (66.6%) | 52 (82.5%) | |
|
7 (2.3%) | 0 (0%) | |
| IABP (%) | 26 (8.4%) | 8 (12.7%) | 0.408 |
| Blood Transfusion (%) | 38 (12.3%) | 9 (14.3%) | 0.829 |
|
33 (10.7%) | 8 (12.7%) | 0.813 |
|
9 (2.9%) | 2 (3.2%) | 1.000 |
|
5 (1.6%) | 1 (1.6%) | 1.000 |
Abbreviations: FFP, fresh frozen plasma; IABP, intra-aortic balloon pump; PRC, packed red cell; TC, thrombocyte concentrate.
The primary outcomes noted in the present study are presented in Table 4. Perioperative data such as DO-MV, LOS-ICU, and LOS-H are significantly different (p < 0.05) between the low and high SII groups. Significantly longer DO-MV, LOS-ICU, and LOS-H durations were observed in the high SII group, as depicted in Table 4.
Table 4.
Perioperative Outcomes
| Variables | SII <878.057 (n = 308) | SII ≥878.057 (n = 63) | p-value |
|---|---|---|---|
| VIS (Median [min-max]) | 2.5 (0.0–508.0) | 3.0 (0.0–508.0) | 0.407 |
| PoAF (%) | 19 (6.2%) | 3 (4.8%) | 0.890 |
| Stroke (%) | 2 (0.6%) | 1 (1.6%) | 1.000 |
| MI (%) | 8 (2.6%) | 2 (3.2%) | 1.000 |
| DO-MV, hours (Median [min-max]) | 10 (3–229) | 12 (4–240) | 0.047* |
| LOS-ICU, hours (Median [min-max]) | 22 (2–326) | 23 (8–312) | 0.049* |
| LOS-H, days (Median [min-max]) | 7 (1–55) | 7 (1–36) | 0.035* |
| Mortality (%) | 12 (3.9%) | 3 (4.8%) | 1.000 |
Note: *Statistical significance (p < 0.05) is marked with.
Abbreviations: DO-MV, duration of mechanical ventilation; LOS-H, length of hospital stay; LOS-ICU, length of intensive care unit stay; MI, myocardial infarct; PoAF, postoperative atrial fibrillation; VIS, vasoactive-inotropic score.
Significant numerical variables were converted into categorical variables: DO-MV >24 hours were converted into prolonged ventilation; LOS-ICU >48 hours were converted into prolonged ICU stay; and LOS-H >7 days were converted into prolonged hospital stay. On bivariate analysis, high SII values were found to be a significant predictor of prolonged ventilation (RR 1.141, 95% CI 1.001–1.301) and prolonged ICU stay (RR 1.218, 95% CI 1.021–1.452) after OPCAB surgery (Table 5).
Table 5.
Analysis of High SII Values in Predicting Poor Perioperative Outcomes
| Variables | RR | 95% CI | p-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Prolonged Mechanical Ventilation (DO-MV >24 hours) | 1.141 | 1.001 | 1.301 | 0.019* |
| Prolonged ICU Stay (LOS-ICU > 48 hours) | 1.218 | 1.021 | 1.452 | 0.011* |
| Prolonged Hospital Stay (LOS-H > 7 days) | 1.173 | 0.920 | 1.496 | 0.212 |
Note: *Statistical significance (p < 0.05) is marked with.
Abbreviations: CI, confidence interval; DO-MV, duration of mechanical ventilation; ICU, intensive care unit; LOS-H, length of hospital stay; LOS-ICU, length of intensive care unit stay; RR, relative risk.
No correlation was observed between SII and DO-MV or LOS-ICU. However, a very weak, positive correlation was observed (p = 0.010, R = 0.133) between SII and LOS-H after OPCAB surgery (Table 6). From the receiver operating characteristic curve analysis, SII predicted prolonged ventilation duration, with an area under the curve of 0.658 (95% CI 0.575–0.741, p = 0.001). The present study obtained a new cutoff value of 600.897 x 103/mm3 (64.3% sensitivity, 63.8% specificity) for SII (Figure 2).
Table 6.
Correlation Between SII and Perioperative Outcomes
| Variables | SII | |
|---|---|---|
| p-value | R-value | |
| VIS | 0.069 | 0.095 |
| DO-MV, hours | 0.402 | 0.044 |
| LOS-ICU, hours | 0.200 | 0.067 |
| LOS-H, days | 0.010* | 0.133* |
Notes: *Statistical significance (p < 0.05) is marked with.
Abbreviations: DO-MV, duration of mechanical ventilation; LOS-H, length of hospital stay; LOS-ICU, length of intensive care unit stay; SII, systemic immune-inflammation index; VIS, vasoactive-inotropic score.
Figure 2.
Receiver operating characteristic curve (ROC) for prediction of mechanical ventilation duration based on SII. AUC, area under curve; SII, systemic immune-inflammation index. The highest area under the curve of 0.658 (p = 0.001) for systemic immune-inflammation index, with its respective cutoff value of 600.897 x 103/mm3.
Discussion
In our current study, SII predicts prolonged ventilation and prolonged ICU stay after OPCAB surgery. High SII values were found to be a predictor of prolonged ventilation (DO-MV >24 hours) and prolonged ICU stay (LOS-ICU >48 hours). Our findings suggest that high SII values serve as a predictor of perioperative outcomes in patients undergoing OPCAB. Several factors have been shown to contribute to inflammation in prolonged ventilation after OPCAB. The fundamental factors identified are the stress of the surgery and procedures such as sternotomy, aortic manipulation, pericardial suction, and harvesting of the internal mammary artery, all of which provoke a systemic inflammatory response. High tidal volume ventilation in the immediate postoperative period of cardiac surgery has been associated with prolonged mechanical ventilation, prolonged ICU stay, and an increased risk of organ dysfunction.14 The use of inotropic agents affects the systemic and pulmonary vasculature, causing hemodynamic changes strongly associated with prolonged mechanical ventilation.15 Furthermore, inflammation of the lungs due to injury perpetrated by mechanical ventilation may be considered another factor contributing to prolonged mechanical ventilation after the procedure. This mechanism directly affects prolonged ICU stay in patients after OPCAB.14 Prolonged ICU stay in patients undergoing OPCAB has yet to be specifically studied further. However, the use of intra-aortic balloon pumps, more than two inotropes, and perioperative myocardial infarction are independent risk factors in patients undergoing open heart surgery.16
OPCAB provides complete revascularization while minimizing significant systemic inflammation. Endothelial cells express neutrophil and monocyte chemotactic factors, P-selectins and E-selectins, in response to stress and pressure on arteries and microvasculature. Then followed by leukocyte sequestration, reduction of inducible nitric oxide (iNOS), and platelet and clotting factor activation.6 Endothelial dysfunction causes an upregulation of prothrombotic factors. Contact with prothrombotic factors will activate platelets which release cytokines, resulting in leukocyte activation and leukocyte-platelet complex sequestration in the myocardium and other organs.6 Tissue trauma and heparin-protamine complexes activate complement via a classic pathway, which begins the production of the cytotoxic complex C5b-9 and major anaphylotoxins C3a and C5a. Simultaneously, in OPCAB, surgical trauma causes an early drop in T (CD3+) and B (CD19+) lymphocytes after surgery, indicating an active response in preventing excessive immune responses. Changes in lymphocyte activity showed that surgical trauma results in an extremely fast lymphocyte activation with specific immunological purposes of apoptosis and anergy.17 Apoptosis will further accelerate lymphocyte turnover and consequently cause a postoperative lymphocyte decrease.
The recent development of SII as a novel index reflecting each patient’s immune and inflammatory status has proved to be widely associated with poor outcomes in cancer patients.8,18 Previous studies have established NLR as an important prognostic marker. Gurbuz et al showed NLR elevation as a risk factor of major adverse cardiac and cerebrovascular events (MACCE) in patients who underwent CABG operation.19 The platelet count in SII serves as an important inflammatory parameters that mediate the release of cytokines and chemokines, which have important effects on vascular wall inflammation. Study of SII on CABG by Abanoz et al determined that SII value is an independent predictor for postoperative major adverse cardiac and cerebrovascular events.20
Baseline patient characteristics in the present study observed significantly different EuroSCORE II values, neutrophil, lymphocyte, and platelet counts between both groups. In Table 1, we observed that more than 80% of the patients in the study are male. Jabagi et al observed that women with CAD are less likely than men to undergo multiple arterial revascularizations, which is likely a result of women being diagnosed with CAD at a later stage than men. Jabagi et al also found higher American Society of Anesthesiologists scores which lead to the hypothesis that women experience more systemic disease and represent a riskier surgical candidate, accounting for why surgeons are reluctant to perform longer, riskier multiple arterial revascularization procedures in women.21 Moreover, a significant correlation was observed between SII and LOS-H after OPCAB surgery.
In this study, SII predicts poor perioperative outcomes after OPCAB surgery, as observed by the higher percentages of stroke (1.6% vs 0.6%), MI (3.2% vs 2.6%), and mortality (4.8% vs 3.9%), in the high SII group compared to the low SII group. This result is consistent with a previous retrospective study on CAD patients by Yang et al, which similarly observed higher percentages of cardiac death (6.3% vs 2.2%), non-fatal MI (6.8% vs 4.6%), and non-fatal stroke (2.7% vs 1.9%) in the high SII group with a cutoff of 694.3 x 103/mm3.11
Higher median VIS scores (3.0 vs 2.5), longer median durations of DO-MV (12 hours vs 10 hours), and LOS-ICU (23 hours vs 22 hours) were also observed between the high SII and low SII groups. Similar findings were reported by Dey et al, who observed significantly higher durations of DO-MV and LOS-ICU in the poor outcome group.10
Higher SII values had previously been associated with poor outcomes.12,13 In this study, we observed that higher SII values were independent predictors for prolonged mechanical ventilation and ICU stay, contributing to poor outcomes. We also observed a weak positive correlation (p = 0.010, R = 0.133) between SII and LOS-H after OPCAB surgery, which shows that as the SII value increases, the duration of hospital stay will follow. Contrary to our findings, previous findings by Dey et al observed a positive correlation between SII and DO-MV and LOS-ICU but no correlation between SII and LOS-H.10
The ROC analysis for SII (AUC = 0.658, p = 0.001) yielded a cutoff value of 600.897 x 103/mm3 with a sensitivity of 64.3% and specificity of 63.8%. Our findings were similar to a previous study by Urbanowicz et al, which focused on novel inflammatory markers for long-term mortality prediction after OPCAB and observed SII (AUC = 0.669, p = 0.001) with a sensitivity of 60.0% and specificity of 70.11% with a cutoff value of 953 x 103/mm3.22 We hypothesized that differences in SII cutoff values are due to the patient population. In this case, our cutoff value is in accordance with the Indonesian population.
Limitations and Implications
Limitations in this study include the lack of complete medical records available to be accessed, hence only including the last three years’ worth of digitalized medical records. Another limitation is that the population of this study is limited to a single-center Indonesian population. Further multi-center studies involving different populations in Asia will be needed to better reflect the immune-inflammatory status of the Asian population. The clinical implications of the present study should be noted. Using a simple and cheap morbidity and prognostic predictor in SII should at least be considered when assessing preoperative feasibility and prognoses. When encountering elective patients who wish to undergo OPCAB with an SII value on the higher side of the scale, anesthesiologists should consider the probability of morbidities such as prolonged mechanical ventilation and prolonged ICU stay.
Conclusion
After OPCAB surgery, patients with high preoperative SII values have significantly longer ventilation duration and ICU stay. High preoperative SII values reflect poor immune and inflammatory status and are associated with poor outcomes. In clinical practice, SII could be used as an easy, cheap, and practical prognostic tool to identify patients with a high risk of morbidity and poor perioperative outcomes after OPCAB surgery.
Data Sharing Statement
Individual deidentified participant data from this study will be made available upon request after publication and will be available for 36 months following article publication. Researchers need to state their aims of analysis and provide a methodologically sound proposal directed towards the corresponding author.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4(13):256–268. doi: 10.21037/atm.2016.06.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen RV, Hjortbak MV, Bøtker HE. Ischemic heart disease: an update. Semin Nucl Med. 2020;50(3):195–207. doi: 10.1053/j.semnuclmed.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 3.Gaudino M, Angelini GD, Antoniades C, et al. Off‐pump coronary artery bypass grafting: 30 years of debate. JAHA. 2018;7(16):e009934. doi: 10.1161/JAHA.118.009934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chikwe J, Lee T, Itagaki S, Adams DH, Egorova NN. Long-term outcomes after off-pump versus on-pump coronary artery bypass grafting by experienced surgeons. J Am Coll Cardiol. 2018;72(13):1478–1486. doi: 10.1016/j.jacc.2018.07.029 [DOI] [PubMed] [Google Scholar]
- 5.Shrivastava AK, Singh HV, Raizada A, Singh SK. C-reactive protein, inflammation and coronary heart disease. Egyptian Heart J. 2015;67(2):89–97. doi: 10.1016/j.ehj.2014.11.005 [DOI] [Google Scholar]
- 6.Sondekoppam RV, Arellano R, Ganapathy S, Cheng D. Pain and inflammatory response following off-pump coronary artery bypass grafting. Curr Opin Anaesthesiol. 2014;27(1):106–115. doi: 10.1097/ACO.0000000000000036 [DOI] [PubMed] [Google Scholar]
- 7.Raja SG, Berg GA. Impact of off-pump coronary artery bypass surgery on systemic inflammation: current best available evidence. J Cardiac Surgery. 2007;22(5):445–455. doi: 10.1111/j.1540-8191.2007.00447.x [DOI] [PubMed] [Google Scholar]
- 8.Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442 [DOI] [PubMed] [Google Scholar]
- 9.Engin M. Are pre and postoperative platelet to lymphocyte ratio and neutrophil to lymphocyte ratio associated with early postoperative AKI Following CABG? Braz J Cardiovasc Surg. 2020;35(2). doi: 10.21470/1678-9741-2019-0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dey S, Kashav R, Kohli JK, et al. Systemic immune-inflammation index predicts poor outcome after elective off-pump CABG: a retrospective, single-center study. J Cardiothorac Vasc Anesth. 2021;35(8):2397–2404. doi: 10.1053/j.jvca.2020.09.092 [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Wu C, Hsu P, et al. Systemic immune‐inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50(5). doi: 10.1111/eci.13230 [DOI] [PubMed] [Google Scholar]
- 12.Urbanowicz T, Michalak M, Al-Imam A, et al. The significance of systemic immune-inflammatory index for mortality prediction in diabetic patients treated with off-pump coronary artery bypass surgery. Diagnostics. 2022;12(3):634. doi: 10.3390/diagnostics12030634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tosu AR, Kalyoncuoglu M, Biter Hİ, et al. Prognostic value of systemic immune-inflammation index for major adverse cardiac events and mortality in severe aortic stenosis patients after TAVI. Medicina. 2021;57(6):588. doi: 10.3390/medicina57060588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badenes R, Lozano A, Belda FJ. Postoperative pulmonary dysfunction and mechanical ventilation in cardiac surgery. Crit Care Res Pract. 2015;2015:1–8. doi: 10.1155/2015/420513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daza-Arana JE, Lozada-Ramos H, Ávila-Hernández DF, Ordoñez - Mora LT, Sánchez DP. Prolonged mechanical ventilation following coronary artery bypass graft in Santiago De Cali, Colombia. VHRM. 2022;18:767–781. doi: 10.2147/VHRM.S367108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tunc M, Sahutoglu C. Risk factors for prolonged intensive care unit stay after open heart surgery in adults. Turk J Anaesth Reanim. 2018;46(4):283–291. doi: 10.5152/TJAR.2018.92244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blacher C, Neumann J, Jung LA, Lucchese FA, Ribeiro JP. Off-pump coronary artery bypass grafting does not reduce lymphocyte activation. Int J Cardiol. 2005;101(3):473–479. doi: 10.1016/j.ijcard.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 18.Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of Systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9(18):3295–3302. doi: 10.7150/jca.25691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurbuz O, Kumtepe G, Ozkan H, et al. Predictive value of neutrophil-lymphocyte ratio for long-term cardiovascular event following coronary artery bypass grafting. Braz J Cardiovasc Surg. 2020;35(3). doi: 10.21470/1678-9741-2018-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abanoz M, Engin M. Investigation of systemic immune inflammatory index and prognostic nutritional index in prediction of major adverse cardiovascular and cerebral events occurring after coronary artery bypass operations. Acta Medica Alanya. 2021;5(3):263–269. doi: 10.30565/medalanya.929006 [DOI] [Google Scholar]
- 21.Jabagi H, Tran DT, Hessian R, Glineur D, Rubens FD. Impact of gender on arterial revascularization strategies for coronary artery bypass grafting. Ann Thorac Surg. 2018;105(1):62–68. doi: 10.1016/j.athoracsur.2017.06.054 [DOI] [PubMed] [Google Scholar]
- 22.Urbanowicz T, Michalak M, Olasińska-Wiśniewska A, et al. Neutrophil counts, neutrophil-to-lymphocyte ratio, and systemic inflammatory response index (SIRI) Predict mortality after off-pump coronary artery bypass surgery. Cells. 2022;11(7):1124. doi: 10.3390/cells11071124 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual deidentified participant data from this study will be made available upon request after publication and will be available for 36 months following article publication. Researchers need to state their aims of analysis and provide a methodologically sound proposal directed towards the corresponding author.