Abstract
Rapidly growing interest in the study of extracellular vesicles (EVs) has led to an accumulation of evidence on their critical roles in various pathologies, as well as opportunities to design novel therapeutic EV-based applications. Efficiently exploiting the constantly expanding knowledge of the biology and function of EVs requires a deep understanding of the various possible strategies of using EVs for therapeutic purposes. Accordingly, in the present work, we have narrowed the broad therapeutic potential of EVs and consider the similarities and differences of various strategies as we articulate three major aspects (i.e., a triad) of their therapeutic uses: (i) EVs as drug targets, whereby we discuss therapeutic targeting of disease-promoting EVs; (ii) EVs as drugs, whereby we consider the natural medicinal properties of EVs and the available options for their optimization; and (iii) EVs as drug carriers, whereby we highlight the advantages of EVs as vehicles for efficacious drug delivery of natural compounds. Finally, after conducting a comprehensive review of the latest literature on each of these aspects, we outline opportunities, limitations, and potential solutions.
Keywords: Drug delivery, Microvesicle, Exosome, Natural compound, Biomimetic
Graphical Abstract
1. Introduction
Extracellular vesicles (EVs) are a group of nano-sized, membrane-embedded structures released by a variety of cell types, including both eukaryotic and prokaryotic cells (1). Depending on their origins, biogenesis pathways, biological functions, and physical characteristics (e.g., morphology and size), EVs are classified into several categories, including endosome-originated exosomes and various types of plasma membrane-derived microvesicles (2). Regardless of their nomenclature, which is still in its infancy due to the absence of any consensus definitions between categories, EVs play critical roles as transporters of biological messages between cells (3). Given the importance of intercellular communication as a hallmark of multicellular organisms (4), EVs have remained evolutionarily conserved to maintain a wide range of physiological roles. Such roles are primarily achieved via relaying information from EV-producing cell(s) to nearby or distant recipient cells through highly regulated packaging of a variety of biomolecules, such as RNA species, proteins, lipids, and sugars (5). Sequestration of disease-causing amyloid-β proteins by cerebrospinal fluid exosomes in the brain (6), immunomodulation by milk exosomes as an important regulatory interface between a mother and an infant (7–9), hemostasis regulation via coagulation enhancement (10), stimulation and/or tolerance induction in immune cells (11, 12), effects on cell polarity and tissue patterning during developmental stages, and communication at the fetomaternal interface during pregnancy are only a few among many previously described physiological roles of human EVs. Moreover, dysregulation of EV-mediated signaling has been shown in various human pathologies, giving rise to identification of certain disease-promoting EVs (13). For instance, tumor-derived EVs act in favor of tumor progression via promoting metastasis, angiogenesis, and drug resistance (14); synovial EVs in rheumatoid arthritis mediate the accumulation of autoantigens such as citrullinated proteins (15); and EVs isolated from various biological fluids demonstrate differential payloads that contribute to disease pathogenesis (16). Being revolutionary conserved, EVS are also produced by, protozoans, helminths, and bacteria that are especially important in pathogenic organisms, as they have been shown to mediate host–pathogen interactions (17, 18).
Uncovering additional aspects of the widespread roles of EVs in human physiology and various pathological conditions, as well as the responsible mechanisms, has provided a foundation for designing a variety of novel EV-based therapeutic approaches. The role of EVs in mediating disease pathogenesis, specifically in cancer (19) and infectious diseases (20), has prompted many researchers to target disease-promoting EV-mediated pathways to give rise to therapeutic outcomes. Given the advantageous structure of EVs that makes these nanoparticles favorable options for drug delivery (21), a great proportion of reviews have focused solely on the application of EVs as therapeutic tools (22–24). However, the scope of the therapeutic use of EVs extends far beyond their use as vehicles/carriers for drug delivery. Indeed, a substantial body of evidence has focused on the intrinsic properties of EVs to elicit beneficial effects without any artificial manipulation or extrinsic drug incorporation (25). Accordingly, in the present work, we have reviewed the therapeutic uses of EVs from three different perspectives—referred to as “the triad” of drug targets, drugs, and drug carriers—and comprehensively present each aspect via reviewing the most influential and recent literature in each category. Considering the numerous limitations to the efficient delivery of natural compounds, we included only studies on natural compounds in the latter section. Moreover, we present the advantages of various strategies, as well as their limitations and potential solutions to cope with each limitation. Considering the importance of exosomes as the most widely-studied population of EVs, the main focus of the current review is on exosomes, unless otherwise stated.
2. Minimal requirements for the identification of EVs
A substantial number of papers in the past decade have reported various functional properties of EVs, especially exosomes. Obvious discrepancies can be frequently seen among the results of these studies, which cannot be explained without considering the probability of variations in the nature and type of the isolated exosomes. To eliminate such ambiguities and to ensure more reliable results, the International Society for Extracellular Vesicles provided a recent update (MISEV2018) indicating the minimum specific reporting information required to validate the identity of an EV, prior to ascribing any specific functional properties to it (26). A brief but inclusive summary of the minimal information required to rigorously confirm the identity of isolated exosomes for various applications, including drug loading purposes, is as follows: 1) a quantitative description of both the exosomes and their sources via reporting the total protein contents and particle numbers for the exosomes and the culture conditions for their sources; 2) the presence of a minimum of three positive markers, such as transmembrane or cytosolic proteins, as well as the absence of one negative marker; and 3) single-particle characterization using a combination of electron microscopy and other single-analyzer techniques.
3. The importance of source selection for EVs
Theoretically, most types of human cells have the potential to secrete EVs (27); therefore, it is not groundless to postulate that any kind of primary cell or established cell line can be used as a biological factory to produce bulk amounts of EVs. In addition, EVs recovered from bodily fluids (e.g., serum, plasma, urine) are another source of EV isolation (5, 28); however, this approach is mostly used for limited isolation for diagnostic and research purposes and has yet to be regarded as a high-yield source of EVs for drug delivery applications. When selecting a source from which to isolate EVs for drug delivery, it is important to note that the source can affect membrane composition and consequently change the homing capacity and biological function of EVs. This is a particularly important consideration because EVs are not as versatile as synthetic nanoparticles in terms of their flexibility for engineering and manipulation (29). On the other hand, if EVs are selected for their natural therapeutic properties without the need for any major manipulation, source selection is also paramount because the expected biological effects of EVs directly relate to their source of purification (30). As an example, senescence of mesenchymal stem cells (MSCs) due to a high number of passages impairs the regenerative capacity of EVs produced by these cells (31), which can greatly reduce their efficacy when used in certain conditions such as spinal cord injury (32). Another finding supporting the importance of source selection for EVs is the observation of dual effects of exosomes isolated from the same cell type (i.e., MSCs) but from two different sources (i.e., bone marrow and umbilical cord), which differentially affect the function of natural killer cells (33). Dendritic cell (DC)-derived exosomes are also largely affected by the activation and maturation status of their cells of origin. These exosomes bear most of the major surface functional proteins of their parent DCs (34), and are likely optimal for directly targeting specific targets such as antigen-recognizing T cells and concomitantly loading them with molecules of interest to boost the expected response. Regardless of the effects that source variation can have on the quality of isolated EVs, MSCs and cancer cell lines have been introduced as rich sources of EVs (32, 35). Their high rates of EV production as well as their accessibility and culture-friendly properties make them good options to recover relatively copious amounts of EVs for use in drug delivery (36). MSCs are among the most studied EV producer cells, whereas the potential use of cancer cells as sources of EVs comes with a lot of safety concerns that remain to be addressed (37). Finally, new sources of EVs that are more accessible and reproducible in terms of mass production for therapeutic use, such as red blood cells (RBCs) and plant cells (38, 39), are among other available options that will be also discussed in detail in the following sections.
4. EVs for therapeutic purposes
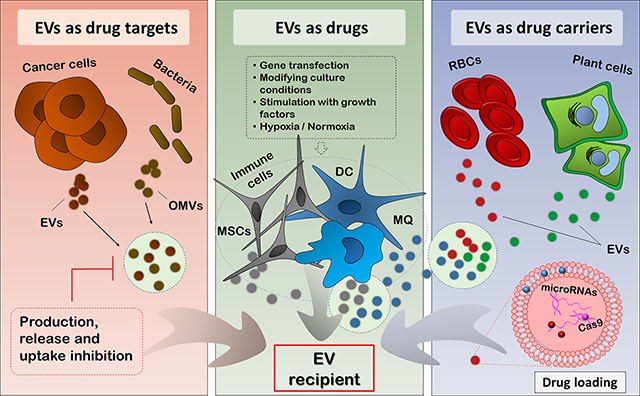
The phenomenal properties of EVs as subcellular particles give them widespread potential, and have made them exciting options to be exploited in designing novel therapies (40). The roles of EVs in various intercellular processes involved in the pathology of many diseases and their natural origin and rich content of bioactive molecules within EVs both influence their therapeutic potential (41). As design of EV-based therapies has evolved, various researchers have taken advantage of distinct aspects of EVs based on their research and therapeutic goals. The current review will discuss the therapeutic use of EVs from three different points of view (Figure 1)—as drug targets, as drugs, and as drug carriers, the latter two of which encompass the delivery of natural molecules in various forms—and discuss the latest advances in each category. To maintain clarity, we use the term “drug” as a common term to describe any agent used for its therapeutic effects.
Figure 1:
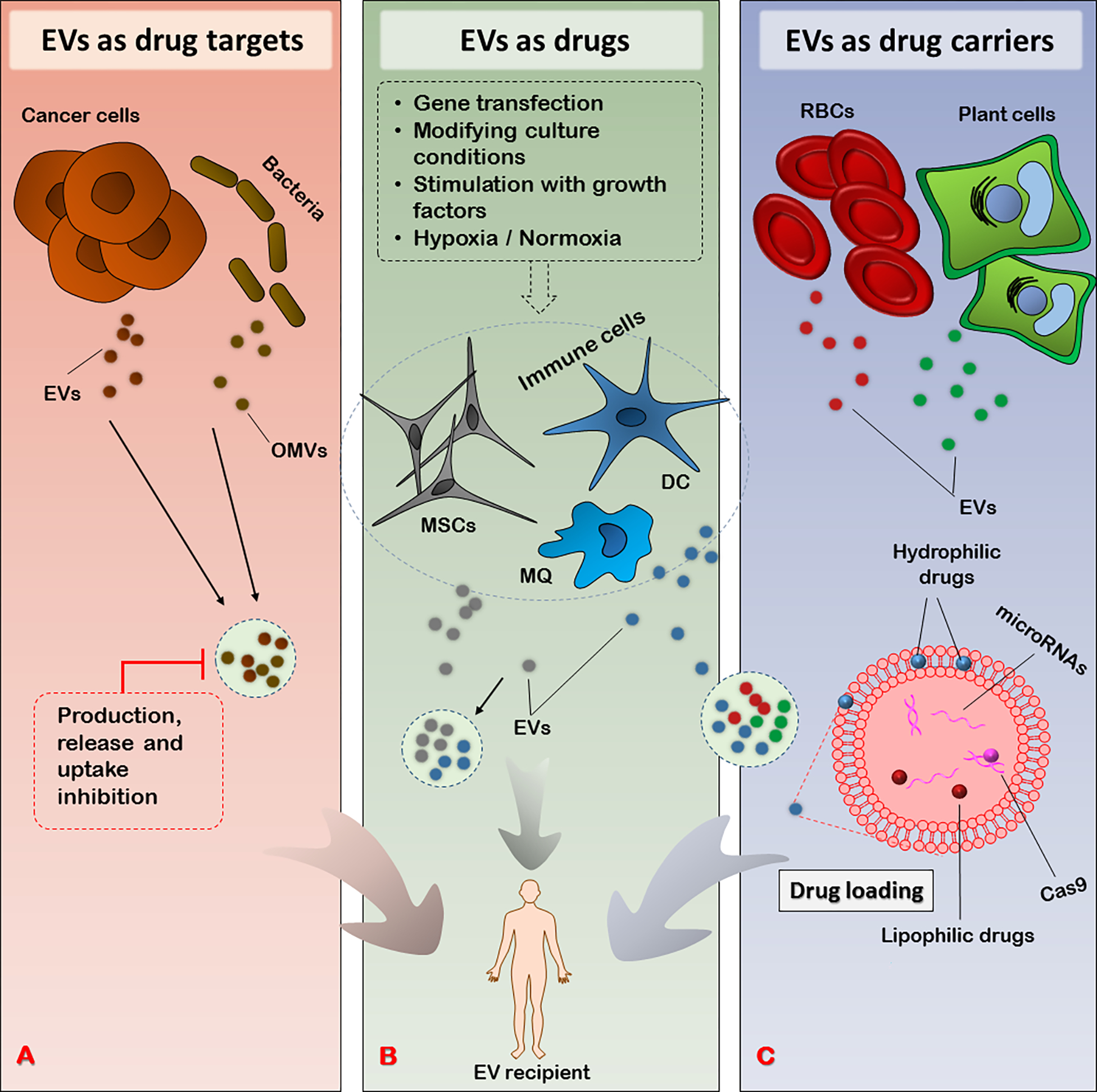
The therapeutic triad of EVs based on their application. A: EVs as drug targets. EVs released by cancer cells take part in disease promotion; OMVs released by bacteria contribute to their pathogenesis, interaction with host cells, and exotoxin release into the bloodstream. Intercepting these deleterious EV-mediated processes at three stages (production, release, and uptake) can offer therapeutic advantages. B: EVs as drugs. EVs released from MSCs, immune cells (DCs and macrophages), and other cell types, as well as EVs secreted in milk, may offer natural intrinsic medicinal effects that can be exploited for therapeutic purposes. In certain cases, the cellular sources of EVs can be manipulated to educate them toward producing more specialized and efficacious EVs richer in specific payloads of interest. C: EVs as drug carriers. EVs released from plant cells, RBCs, MSCs, and other cell types can be used for loading various types of therapeutics, including genetic material and drugs. The incorporated agents can be either hydrophobic or hydrophilic in nature. EV, Extracellular vesicle; OMV, Outer membrane vesicle; MSC, Mesenchymal stem cell; DC, Dendritic cell; MQ, Macrophage; RBC, Red blood cell
4.1. EVs as drug targets
As conveyors of intercellular communication, EVs play physiological roles, and may also take part in the initiation, exacerbation, and treatment resistance of various pathologies (42); in particular, the roles of EVs in cancer have been widely studied. For instance, tumor-derived EVs export a variety of molecules such as non-coding RNAs and enzymes required to established a favorable microenvironment, the so called “metastatic niche”, to future metastasis sites (43). The suppression of a variety of immune cells involved in cancer immune surveillance by the same EVs support tumor progression and worsen the condition (44). Finally, numerous reports describe the roles of EVs in conferring resistance of cancer cells to various chemotherapy drugs, leaving a considerable proportion of treatment regimens unsuccessful (45, 46). Thus, interfering with the pathological roles of EVs may improve treatment outcomes. In this context, effectively targeting EVs using pharmacological or natural agents is of therapeutic importance (Table 1). To target unfavorable EVs, they can be intercepted at three major points of their biogenesis and functioning: 1) their production, 2) their release from parent cells, and 3) their uptake by recipient cells (47).
Table 1:
Studies in which disease-promoting EVs were targeted using various agents (drugs) for therapeutic purposes (EVs as drug targets).
| Study type | EV type | EV source | EV-driven pathology | Targeted biogenesis stage | Targeting agent/method | Outcomes after targeting | Mechanism of action | Ref. |
|---|---|---|---|---|---|---|---|---|
| In vitro | Exosome | Myeloma cells (OPM2 and L363) | NK cell cytotoxicity suppression | Biogenesis (source modification) | EPA and DHA | ↓Suppressive effects of the myeloma exosomes on NK cells | NS | (48) |
| In vitro | Exosome | Breast cancer cells (MCF7 and MDA-MB-231) | Angiogenesis [in cancer] | Biogenesis (source modification) | DHA | ↓ Angiogenic effects of the exosomes | Change in the miRNA composition of EVs | (49) |
| In vitro | Exosome | Prostate cancer cells | Cancer promotion | Release | Manumycin A | ↓ Exosome release from cancer cells | Inhibition of the Ras/Raf/ERK1/2 and hnRNP H1 pathways | (51) |
| In vitro, in vivo | Exosome | Lung cancer cells | • Immunosuppression • Chemo resistance |
Release | GW-4869 | ↓ Exosome release from cancer cells | nSMase inhibition | (53) |
| In vitro | OMV | Bacteroides fragilis (Intestinal pathogen) | Exosome-mediated: • secretion of enterotoxin • interaction of the enterotoxin with host cells |
Potentially: Biogenesis and Uptake | Not carried out yet | Potential inhibition of pathogenesis | NA | (54) |
EVs, Extracellular vesicles; NS, not specified; NA, not applicable; NK, Natural killer cell; EPA, Eicosapentaenoic acid; DHA, Docosahexaenoic acid; miRNA, MicroRNA; OMV, Outer membrane vesicle; nSMase, neutral sphingomyelinase; Ref., References
To hinder EV production, our group modified the undesired effects of exosomes from multiple myeloma (MM) cell lines at the biogenesis level using eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), two long-chain polyunsaturated omega three fatty acids. We first demonstrated that exosomes released by MM cells suppress the cytotoxic activity of natural killer cells, the major cells of the innate immune system involved in the fight against hematological malignancies. We then showed that pre-treatment of the producer MM cell lines with either EPA or DHA can largely reverse the natural killer-suppressing effects of exosomes from MM cells, suggesting a novel method to therapeutically target MM-derived exosomes even before their release (48). In a similar effort, Hannafon et al. (2015) used DHA to reverse the pro-antigenic effects of exosomes isolated from breast cancer cell lines. They showed that changes in the microRNA (miRNA) contents of the resealed exosomes following treatment with DHA was the mechanism behind the observed effects, suggesting that such a treatment can alter exosome cargo sorting in favor of anti-tumor effects (49).
The second approach to preventing undesired EV-mediated effects is to hinder their release from the original cells; reviewed in (50). For example, Datta et al. (2017) who used bioinformatics methods to perform virtual screening of a vast number of approved drugs to identify those with possible exosome release-inhibiting effects. They identified the natural antibiotic Manumycin A as an inhibitor of exosome secretion from prostate cancer cells via inhibiting the Ras/Raf/ERK1/2 and hnRNP H1 pathways (51). These results provided the basis for the repositioning of currently existing drugs to target EV release. A review describing exosome-mediated shedding of natural killer cell inhibitory ligands (i.e., MICA and MICB) as an important escape mechanism for cancer cells suggested that targeting exosome release from these cells has therapeutic potential (52). A similar study showed that inhibition of Kras-derived exosomes of lung tumor cells can attenuate the immunosuppression and chemoresistance caused by these EVs (53). The importance of targeting EV biogenesis is not limited to their production by host cells. Prokaryotic cells (e.g., extrinsic pathogens) also produce outer membrane vesicles (OMVs) of high physiological and pathological importance. In this regard, Zakharzhevskaya et al. (2017) showed that OMVs released by the intestinal pathogen Bacteroides fragilis mediate secretion of its major enterotoxin as well as its interaction with host intestinal cells, despite the enterotoxin not being released by B. fragilis in its free form (54). These findings indicate that identifying EV-targeting methods that specifically target EV production by non-eukaryotic cells of interest may aid in developing novel therapies for some infectious diseases.
Hampering uptake of disease-promoting EVs by their target cells is the third level at which EVs can be therapeutically targeted (47). Although research findings on this strategy are still scarce, a few critical molecules (e.g., the family of Tim4 transmembrane proteins and the apoptosis-related surface phosphatidylserine) have been found to be involved in exosome uptake, making them potential targets for interventions (55).
4.2. EVs as drugs
EVs can often be applied as drugs the way they are secreted by their parent cells, taking advantage of their natural beneficial properties. A variety of cell types have EVs that can potentially be used without any specific manipulation of their contents and/or associated molecules. In this context, MSCs, immune cells such as DCs and macrophages, and even cancer cells are among the most important examples (5), which we will discuss. In certain cases, educating the parent EV-producing cells via genetic manipulation and pharmacological treatments can provide advantages over their non-manipulated/-engineered counterparts, because the produced EVs can carry a payload of interest to align with therapeutic goals. The following sections will focus on both strategies.
4.2.1. EVs from MSCs
Upon their identification more than five decades ago, MSCs were initially appreciated for their multipotent differentiation potential; however, continual uncovering of their regenerative and modulatory properties soon broadened their use as therapeutic tools in various contexts (56). Due to their abundance in various tissues and the wide range of their physiological roles, MSCs are the source of myriad active molecules, making them useful candidates to be applied in various pathologies (33). Caplan and Correa (2011) ascribed the term “drugstore” to MSCs to highlight their rich content and importance as sources of bioactive molecules (56). Despite such great potential, safety concerns regarding the uncontrolled fate of MSCs after their administration, as well as the technical difficulties of cell-based therapies, has challenged the applicability of MSCs for use in humans (32). A substantial number of studies on the mechanisms through which MSCs exert their beneficial effects have proposed that MSCs act mainly via two distinct mechanisms: direct cell-to-cell contact with their target cells and release of soluble agents (including EVs) as a means of remote modulation of their targets.
Supporting remote modulation by MSCs, it is now evident that EVs are a major component of MSC soluble mediators and can, to a large extent, recapitulate the biological effects of MSCs even in their complete absence (57–59). Such findings have opened a new window toward using MSC-derived EVs as solitary therapeutic agents and solely based on their natural contents of bioactive molecules for various purposes (Table 2). Such a strategy may not only circumvent safety concerns regarding cell-based therapies, but also enable the design of new methods to take advantage of the rich cargos of MSC-EVs. The anti-apoptotic, pro-angiogenic, anti-inflammatory, proliferative, and trophic nature of MSC-EVs gives them potent intrinsic regenerative properties, which have been exploited in multiple studies to promote tissue regeneration (e.g., in bone, skin, cartilage, heart muscle) in a variety of conditions (e.g., lung and liver fibrosis, cardiovascular disease) (60–62). Models of myocardial infarction (MI) have also revealed the vascular regeneration properties of MSC-EVs in ischemic heart injury (63). For instance, Chung et al. (2019) used EVs from endothelial progenitor cells (isolated from bone marrow of mice) to improve the hemodynamic status of mice inflicted with experimental MI. They performed intramyocardial injections at MI borders and observed significant and rapid proangiogenic effects (64). In another study, exosomes derived from MSCs of various sources (bone marrow, adipose tissue, and umbilical cord) inhibited cardiomyocyte apoptosis and promoted angiogenesis, thereby improving cardiac function and protecting the myocardium (65). In an ischemia/reperfusion (IR)-inflicted rat model, natural exosomes isolated from umbilical cord MSCs alleviated the induced injury via their rich content of the miRNA miR-20a. Binding of miR-20a to the 3ʹ UTRs of two upregulated genes, Beclin-I and FAS, and the consequent apoptosis inhibition, was suggested as the responsible mechanism (66). Ma et al. (2019) used a rat model of retinal detachment (RD) to investigate the therapeutic effects of local injection of MSC-derived exosomes (i.e., in the eyes) on RD-induced ischemic and apoptotic events responsible for vision decline. The injected exosomes significantly reduced the levels of two major inflammatory cytokines, TNF-α and IL-1β, decreased the cleavage of Atg5 (a major protein involved in autophagy), and inhibited photoreceptor cell apoptosis, which altogether resulted in maintaining a normal retinal structure. Proteomic analysis of the exosomes revealed the presence of a wide variety of anti-inflammatory, anti-apoptotic, and neuroprotective molecules (67). In a parallel study, the authors took advantage of MSC-derived exosomes to study their beneficial effects in diabetic mice with peripheral neuropathy. Treatment with MSC exosomes suppressed inflammation via decreasing the expression of inflammatory cytokines and regulating the proportion of M1 and M2 macrophages toward an anti-inflammatory phenotype, yielding a significant improvement in neurovascular structure. Bioinformatics analysis revealed the presence of TLR4/NF-κB-targeting miRNAs (interrupters of inflammation) in their isolated exosomes, which may have mediated the observed beneficial effects (68).
Table 2:
Studies in which EVs have been used for their intrinsic medicinal effects (EVs as drugs).
| Study type | EV type | EV source | Parent cell modification | Responsible natural mechanisms / contents | Context/model | Outcomes | Ref. |
|---|---|---|---|---|---|---|---|
| In vitro, in vivo | EVs (NS) | Endothelial progenitor cells | NA | • ↑ Regeneration • ↑ Angiogenesis |
Mouse experimental MI; HUVECs | → Improved ventricular contractility → Preserved ventricular geometry → Cardiovascular recovery post-MI |
(64) |
| In vitro, in vivo | Exosomes | MSCs | NA | • ↓ Cardiomyocyte apoptosis • ↑ Angiogenesis via altering VEGF, bFGF, and HGF |
Rat MI model; NRCMs | → Improved cardiac function → Myocardium protection → Cardiovascular recovery post-MI |
(65) |
| In vivo | Exosomes | MSCs | NA | • ↓ TNF-α and IL-1β • ↓ Apoptosis • ↓ Atg5 cleavage Contents: A wide range of anti-inflammatory, anti-apoptotic and neuroprotective molecules |
Rat RD model | → Maintaining normal retinal structure | (67) |
| In vivo | Exosomes | MSCs | NA | • ↓ Inflammatory cytokines • ↓ M1 and ↑ M2 macrophages; Contents: (TLR4/NF-κB)-targeting miRNAs |
Diabetic mice | → Promoted functional recovery in mice with neuropathy → Alleviated neurovascular function |
(68) |
| In vitro, in vivo | Exosomes | MSCs | miR-20a reinforcement using its mimics | • Beclin-I and FAS inhibition > apoptosis inhibition • Improved cardiac function; Contents: miR-20a |
Rat IR-induced injury | → Improved IR-induced injury Outcomes post-modification: • Almost full alleviation of the injury |
(66) |
| In vitro, in vivo | Exosomes | MSCs | miR-20b-3p overexpression (transfection) | • ↓ Oxalate-induced autophagy • ↓ Inflammation (ATG7 and TLR4 inhibition) Contents: miR-20b-3p |
CaOx-induced rat kidney stone model | → Protection against kidney stones | (70) |
| In vitro | EV fraction of total secretome | MSCs | Stimulation via culture condition modification including starvation, IL-1β addition and dexamethasone addition | • Encompassing natural contents of antimicrobial secretome including AAT | To combat lung pathogens | → Demonstrated antimicrobial efficacy against Gram-negative bacteria → Elastase inhibition → Demonstrated lung regeneration properties Outcomes post-modification: • Increased protein content and AAT production • Increased AAT gene expression |
(57) |
| In vitro, in vivo | Exosomes | Cancer cells | NA | • Presence of immune activating molecules (HSP70, etc.) • Presence of native tumor antigens • Overexpressed receptor and molecules involved in antigen sampling by DCs |
Mice and cancer cell lines | → Efficient cross-presentation of shared tumor antigens → Tumor rejection |
(80) |
| In vitro, in vivo | EVs (NS) | Cancer cells | Transfection with anti-miR-21 | • Surface tumor-targeting properties of the T-EVs • Capability of co-delivery Anti-miR-21 |
Mice and cancer cell lines: 4T1, HepG2, and SKBR3 | → Efficient targeted delivery to tumor sites | (81) |
| In vitro, in vivo | Exosomes | MQs & monocytes | NA | • Circumventing elimination of the therapeutic EVs owing to the natural origin of EVs (the host immune system) | Mouse model of PD | → Efficient accumulation in brains of PD mouse models → Efficient delivery of the loaded agent (catalase) |
(85) |
| In vitro, in vivo | Exosomes | DCs | NA | • Exploiting the natural antigen presentation-related molecules of DC-EVs combined with antigen loading • Cell-free EV-based tumor vaccine |
Mouse tumor model | → Successful CTL priming → Successful tumor eradication |
(34) |
| In vitro; In vivo | EVs (NS) | IC21 macrophages | NA | • Exploiting the rich LFA-1 surface content of MQ-EVs (Abundance of LFA-1 on EVs results in their affinity to inflamed sites with ICAM-1 overexpression) | Batten disease models: LINCL mice and TPP1 enzyme-deficient cells (CLN2) | → Targeted delivery of the therapeutic EVs to inflamed brain | (87) |
| In vivo | EVs (NS) | Bacteria (S. aureus) | NA | • Vaccine design exploiting antigenicity and adjuvant properties of microbial EVs | S. aureus lung infection | → Activation of the Th1 response → Protection against S. aureus-induced lethal pneumonia |
(93) |
| In vitro | Exosomes | Porcine milk | NA | • ↓ Expression of p53, p21, caspase 3 and 9 • Regulation of β-catenin and cycline D1 • ↑ Cell viability • ↓ mRNA levels of p21, fas and Tp53 • ↑ mRNA levels of ZO-1, OCLN and CLDN1 |
Deoxynivalenol-induced damage/IPEC-J2 | → Decreasing DON-induced damage by promoting cell proliferation and reducing apoptosis | (140) |
| In vitro | EVs | Human Breast Milk | NA | • ↑ Cellular proliferation after H/R • ↓ Apoptosis after H/R |
Necrotizing Enterocolitis/IEC-6 | → Decreased histological damage → Decreased incidence of NEC |
(97) |
| In vivo | P35K EVs P100K EVs |
Commercial cow’s milk | NA | • After P35K EVs feeding: ↑ G-CSF, GM-CSF, IL-7, CCL3, CCL4, IFN-ɣ and IFN-α ↓ IL-12-p40, IL-23, IL-4 • After P100K EVs feeding: ↓ IL-3, IL-6, IL-10, IL-12-p40, IL-17 and TNFα ↑ G-CSF, GM-CSF ↑ M-CSF, GM-CSF, IL-5 and IL-4 ↑ Expression of anti-inflammatory A20 Normalized levels of COX-2 and ZO-1 ↓ Colitis-associated microRNAs: miR-21, miR-29b and miR-125b |
Murine colitis | → Improve colitis via decreasing inflammation | (141) |
| In vitro | Exosomes | Cow Milk | NA | • ↑ Macrophage proliferation • ↑ β-catenin expression • ↑ p21 and p53 expression • ↓ Cyclin D1 expression |
Cisplatin-Induced Cytotoxicity RAW 264.7 | → Protective effect against cisplatin cytotoxicity through boosting immune system and increasing proliferation markers | (142) |
|
In vivo
In vitro |
EVs | Cow milk | NA | • In vivo: ↓Serum levels of MCP-1 and IL-6 • In vitro: ↓ TNF-α and MCP-1 ↑ Expression of GATA-3 (Th2), IL-17 (Th17), and Foxp3 |
Murine arthritis Splenocytes | → Ameliorating arthritis via cartilage pathology and bone marrow inflammation | (100) |
| In vitro | Exosomes | Camel Milk | NA | • Significant anti-proliferative activity • Suppression of migration • ↑ DNA damage • ↑ Caspase-3 activity • Bax upregulation and Bcl2 downregulation • ↓ MDA levels and iNOS mRNA • ↑ SOD, GPX, and CAT activities • ↓ Expression levels of IL1β, NF-κB, VEGF, MMP9 |
Breast cancer: MCF7 | → Anticancer effect through induction of apoptosis and inhibition of oxidative stress, inflammation, angiogenesis, and metastasis | (101) |
| In vitro | Exosomes | Porcine Milk | NA | • ↓ Expression of IL-1β, IL-6, and TNF-α • ↑ Cell viability • ↓ mRNA levels of Tp53, Fas, and Caspase-3 • ↓ Phosphorylation of IκBα and NF-κB |
LPS-Induced Apoptosis IPEC-J2 | → Decreasing LPS-induced injury by inhibiting inflammation and apoptosis | (143) |
EV, Extracellular vesicle; NS, Not specified; NA, Not applicable; MI, Myocardial infarction; HUVECs, Human umbilical vein endothelial cells, MOA, Mechanism of action; AAT, Alpha-1-antitrypsin; NRCMs, Neonatal rat cardiomyocytes; VEGF, Vascular endothelial growth factor; bFGF, Basic fibroblast growth factor, HGF, Hepatocyte growth factor; IR, Ischemia-reperfusion; TNF-α, Tumor necrosis factor alpha; IL-1β, Interleukin-1 beta; TLR4, Toll-like receptor 4; NF-κB, Nuclear factor κappa B; CaOx, Calcium oxalate; HEK293, Human embryonic kidney cell line; HSP70, Heat Shock Protein 70; DCs, Dendritic cells; PD, Parkinson’s disease; MQ, Macrophages; DCs, Dendritic cells; S. aureus, Staphylococcus aureus; Th1, Helper T lymphocyte type 1; IPEC-J2, intestinal porcine enterocytes isolated from the jejunum; IEC-6, intestinal epithelium cell; H/R, hypoxia/reoxygenation; A20, anti-inflammatory protein TNFAIP3; IL, Interleukin; RAW 264.7, Murine macrophage cell line; MCF7, Human breast cancer cells; LPS, Lipopolysaccharide; Ref., References
In addition to leveraging the intrinsic properties of MSC-derived EVs, other groups have attempted to maximize their effects by educating the EV-producing cells toward producing specialized EVs with stronger effects (69). Several strategies can be employed in this approach, including stimulating producer cells with various agents (e.g., drugs, cytokines, growth factors), changing cell culture conditions (e.g., hypoxic vs. normoxic, 3D vs. 2D culture), and engineering the cells using genetic constructs (e.g., plasmids) (60). The goals of such strategies may be to increase the concentration of a certain molecule(s) in the EVs, alter the sorting of small RNAs, or even add/delete a certain gene in the final composition of the released EVs. For instance, in an effort to exploit the natural regenerative effects of human adipose tissue derived-MSCs in the lungs, the MSC secretome was revealed to include a rich treasury of proteins and lipids necessary to maintain protease/anti-protease balance and anti-microbial activity. Interestingly, stimulation of MSCs with dexamethasone and IL-1β, combined with starvation, resulted in an increased concentration of Alpha-1-antitrypsin (AAT), the major elastase-inhibiting enzyme in the lung (57). In the previously mentioned study in which the therapeutic effect of MSC exosomes in IR-induced injury was attributed mainly to miR-20a content, reinforcement of the exosomes with mimics of this miRNA resulted in full alleviation of the ischemic injury, whereas only partial effects were elicited from the exosomes of non-manipulated MSCs (66). In another study, adipose-derived MSCs transfected with a miR-20b-3p mimic reduced calcium oxalate accumulation in rat kidneys, suggesting the use of exosomes has therapeutic potential as a safe and similarly effective alternative to MSCs to protect against kidney stones. The authors suggested downregulation of oxalate-induced autophagy and inflammation were responsible for the observed effects (70).
4.2.2. EVs from tumor cells
EVs released by tumor cells are rich sources of tumor antigens, and offer great advantages compared to other sources of tumor antigens (e.g., total cell lysates, irradiated cell extracts) (71). To elicit a potent anti-tumor immune response, several essential elements must be addressed. These include the presence of a rich source of immunogenic tumor antigens, efficient uptake of these antigens by professional antigen presenting cells (APCs), activation and maturation of the APCs, and finally presentation of the processed antigens to both helper and cytotoxic T lymphocytes (Th and CTL, respectively) (72–74). The latter can only be achieved via a specific antigen presentation pathway called “cross-presentation,” whereby an endocytosed antigen is presented in complex with the type 1 major histocompatibility complex (MHC I) (75). Identifying potential tumor antigens capable of inducing an efficient anti-tumor response specific to tumor cells has remained a constant challenge in designing novel vaccines and therapies for cancer. Tumor-specific antigens (TSA) and tumor-associated antigens (TAA) are two major groups of tumor antigens, each of which has certain advantages and limitations. TSAs are typically a specific representation of the unique antigens (“neoantigens”) produced as a result of the mutational events within the tumor cell. In contrast, TAAs can be also found on non-malignant cells, but with alterations in their expression (i.e., extent of and developmental stage of expression) due to the malignant transformation (76, 77). Given the technical difficulties of identifying and applying TSAa, TAAs have prevailed as candidate anti-tumor therapies and vaccines. Some examples of TAAs are carcinoembryonic antigen (CEA), the transmembrane glycoprotein Mucin 1 (MUC1), and melanoma-associated antigen (MAGE), each of which is either overexpressed or aberrantly expressed in certain types of cancers. However, the limited number of these antigens and their poor immunogenicity largely limit their use (78, 79). Moreover, although there is some degree of antigen overlap (shared antigens) among different tumors, the inability of the shared antigens to be efficiently cross-presented by APCs has turned the possibility of designing a single vaccine against several cancer types into a myth.
In an interesting study, the authors showed for the first time that tumor-derived exosomes contain shared tumor antigens that can be efficiently cross-presented by DCs and lead to tumor rejection (80). The presence of a discrete range of proteins (e.g., heat shock proteins [HSPs]) crucial for the activation of a potent anti-tumor response as well as native tumor antigens and the overexpression of certain molecules and receptors involved in antigen sampling by APCs were suggested as the main reasons behind this finding (80). Accordingly, using tumor-derived EVs (T-EVs) as natural samples of tumor antigens may be a desirable option for designing effective antigen-based immunotherapies. One strategy is to use these EVs for the ex vivo maturation and induction of DCs capable of inducing strong CTL responses. Given the diverse ingredients of T-EVs, such a strategy could circumvent the need for additional activation stimulators of DCs and may be an alternative to methods combining various peptides from different tumors to increase the coverage of the designed therapies/vaccines (78). Another strategy is to introduce T-EVs directly to the host to elicit the effects of interest, such as targeted delivery of certain molecules. Similar to MSCs, EV-producing tumor cells can also be primed to produce EVs of interest with improved therapeutic effects. A major drawback of this strategy is the diverse and complex composition of T-EVs, which may unexpectedly lead to undesired effects. A solution to enable exploitation of favorable surface properties of T-EVs, without undesirable effects, is to use T-EVs to produce biomimetics in which the central drug-carrying core is coated with the outer shell of the T-EV (29, 37). For example, Bose et al. (2018) used EVs isolated from two cancer cell lines (HepG2 and SKBR3) transfected with a therapeutic anti-miRNA (anti-miR-21) to functionalize synthetic gold-iron oxide nanoparticles and take advantage of their tumor-targeting capabilities (81). They suggested that EV targeting behavior is dependent upon the type of tumor cell from which it was isolated. Finally, given that cancer cells produce greater amounts of EVs than their non-malignant counterparts (50) and that their targeting behaviors can be predicted based on their original producer cells, cancer cell-derived EVs may be outstanding candidates for use as innovative targeted therapies (37).
4.2.3. EVs from immune cells
Immune cells, depending on the cell type, possess a variety of features that are reflected in their EVs. Professional APCs, including DCs, B lymphocytes, and macrophages, release EVs bearing the major components required for antigen presentation such as MHC I and II as well as the necessary co-stimulatory molecules (34, 82). Selective enrichment of distinct adhesion molecules such as CD11b, CD9, and lactadherin, which guide the EVs toward certain effector cells, has also been reported for EVs released by these immune cells (83, 84). In addition, being part of a host’s innate immune system, EVs produced by macrophages and monocytes are not entrapped by the phagocytic system, making them superior to other microparticles used for drug delivery that are frequently trapped and eliminated by phagocytes (85). These findings have tempted researchers to use immune cell-derived EVs as effective non-cellular tools to elicit beneficial immune responses. An example of such efforts is the pioneering study of Zitvogel et al. (1998), in which the natural antigen presenting features of EVs released by DCs were successfully combined with a tumor antigen loading strategy (exosome pulsing with tumor peptides) to design EV-based cell-free vaccines capable of priming CTLs in vivo. The EVs used in the study naturally harbored all the required molecules to efficiently present the loaded tumor antigens to the effector cells, which ended in eradication of the tumors in the animal models used (34). Despite the effectiveness of DC-based tumor vaccines, several drawbacks limit their applicability, such as difficulties in long-term storage and challenges regarding their targeted delivery (86). However, the findings of Zitvogel et al. and similar studies underscore the potential of cell-free EV-based tumor vaccines to overcome the limitations of conventional DC-based therapies. In addition to antigen presenting capabilities, EVs from macrophages possess other features that could be potentially exploited in designing innovative therapies. EVs from macrophages expressing adhesion molecules (e.g., LFA-1) are attracted to certain inflammatory sites where other complementary adhesion molecules (e.g., ICAM-1) are overexpressed, facilitating targeted EV interaction. Given that inflammation is an inseparable component of many conditions such as neuroinflammatory disorders, this natural feature of macrophage-derived EVs can be therapeutically exploited for targeted drug delivery to inflamed sites (87). Together, these findings support continued exploration of the natural properties of EVs released by various types of immune cells. Indeed, further research with EVs produced by less studied cells of the immune system such as natural killer cells could open new windows for designing novel therapeutics.
4.2.4. EVs from the human microbiome
Human resident microbiota play a major role in homeostasis and maintenance of physiological integrity and health (88). In addition to eukaryotic cells, bacteria are also capable of producing EVs of various types, which are classified into three categories depending on their source and biogenesis pathway: apoptotic bodies; membrane vesicles (MVs), which are mainly produced by Gram-positive bacteria and originate from bacterial inner membranes; and OMVs, which are the major EVs released by Gram-negative bacteria (89, 90). Similar to host EVs, microbial EVs contribute to numerous processes that form the complex interactions between microbiota and host cells. Some of these EVs serve as nutrient sources for host cells, whereas others may take part in metabolic processes, horizontal gene transfer, and even specific delivery of certain ingredients (e.g., nucleic acids) to recipient cells (88). MVs released by a Lactobacillus species in the gut contain known natural bacteriocins capable of killing opportunistic pathogens and other resident competitors (91). Within prokaryotic EVs, the presence of pathogen-associated molecular patterns (PAMPs), which trigger immune recognition of common microbial patterns by interaction with their corresponding pattern recognition receptors (PRRs), together with natural adjuvant properties, can trigger initiation of an anti-microbial immune response similar to that of the pathogens itself (92, 93). Accordingly, in an effort to harness this feature, EVs from Staphylococcus aureus were successfully used as a vaccine candidate to trigger an effective immune response via a pathway involving toll-like receptor 2 (TLR2) (93). Microbial EVs also mediate immunomodulation of their host cells, mainly via lipopolysaccharide (LPS). However, further study of therapeutic opportunities based on the immunomodulatory components of microbial EVs and their possible associated risks is needed.
4.2.5. EVs from milk
Breast milk contains a rich content of EVs released from a diversity of cells, including those from distal sites in the body (94). These EVs contain high levels of immune-related miRNAs that are capable of being transferred to the infant and exerting immunomodulatory effects (7). In recent years, various studies have sought to elicit direct pharmacological effects using milk-derived EVs; the majority of the studies observed significant anti-inflammatory, tolerogenic, and anti-apoptotic effects in various contexts. In a recent study, porcine milk exosomes decreased deoxynivalenol-induced injury via the up-regulation of miR-181a, miR-30c, miR-365-5p, and miR-769-3p and downregulation of their target genes in the p53 pathway and, as a result, promoted cell proliferation and inhibited cell apoptosis (95). Porcine milk also contains miRNAs that reduce LPS-induced injury via inhibiting inflammation and reducing apoptosis. mRNA expression of p53, Fas, and caspase-3 and expression of the inflammatory cytokines IL-1β, IL-6, and TNF-α are reduced following treatment of enterocytes with milk exosomes (96). In addition, human breast milk-derived EVs protected premature infants against necrotizing enterocolitis, mainly via increasing cellular proliferation and reducing apoptosis, which leads to decreased histological damage (97). Cow milk EVs were also effective in attenuating a murine model of colitis through regulating a set of inflammatory cytokines and chemokines. Upregulation of A20, a critical inflammatory protein in the NF-κB pathway, and downregulation of colitis-associated miRNAs (e.g., miR-21, miR-29b, and miR-125b) were also observed following treatment with these EVs (98). Cow milk exosomes also protect against cisplatin-induced toxicity via increasing macrophage proliferation and expression of β-catenin, p21, and p53 (99). Cow milk EVs diminished murine arthritis via improving cartilage pathology and bone marrow inflammation; expression of the regulatory T cell-promoting cytokines GATA-3, IL-17 and Foxp3 was increased, whereas the expression of the inflammatory cytokines TNF-α and MCP-1 was mitigated (100). Milk exosomes have also proven effective against breast cancer via promoting apoptosis and reducing oxidative stress and inflammation markers (101). These findings altogether indicate that milk EVs, regardless of their source, could have potent anti-inflammatory, immunomodulatory, and anti-apoptotic effects, which could potentially be used for the treatment of various inflammatory disorders.
4.3. EVs as drug carriers
In addition to delivering drugs within the EV core or membrane, the membranous EV structure can also be used as a carrier for specific ligands via their decoration with various functional molecules to elicit desired outcomes. Although a variety of bioactive molecules including small RNAs, proteins, genetic material such as CRISPR sequences, and other common drugs and molecules have been incorporated into exosomes (87), we focus mainly on the delivery of naturally-occurring molecules using EVs, in line with the goals of the present review (Table 3). Despite the widespread use and market availability of natural plant-based compounds, including total plant-based extracts and purified herbal constituents, their therapeutic efficacy is limited due to low bioavailability and solubility, hepatic deposition, and poor targeting due to their rapid unspecific uptake by other tissues (102, 103). Considering the superiority of EVs as natural drug loading tools that offer various advantages (e.g., both hydrophobic and hydrophilic loading platforms), harnessing their beneficial properties to overcome the limitations of natural plant-based compounds is an ambitious undertaking that has been carried out many researchers. Here, we review the most recent literature on the use of EVs for the delivery of natural agents.
Table 3:
Studies in which EVs have been used as carriers of natural compounds (EVs as drug carriers).
| Study type | EV type | EV source | Isolation method | Incorporated natural agent | Context/condition | Expected EV advantages | Outcomes | Ref. |
|---|---|---|---|---|---|---|---|---|
| In vitro | Membrane vesicles (1 nm–3 μm) | Broccoli root (Brassica oleracea) | Two-phase aqueous polymer technique |
Dyes: Basic fuchsin Bromophenol Fluorescents: Fluorescein Diacetate (FDA) |
Transdermal delivery | • Hydrophobic properties • Availability • Stability • Permeability • Enhanced delivery |
• Successful interaction with skin keratinocytes and delivery of loaded agents | (109) |
| In vitro | Exosomes | Cancer cell lines (MCF7, HepG2, Caco2 and PC3) | Commercial isolation kit | Bioactive saponins and flavonoids from black bean extract | Treatment of cancer cells | Enhanced uptake and delivery of the loaded compounds | • Better anti-proliferative and cytotoxic effects when loaded into exosomes | (104) |
| In vitro, In vivo | Exosomes | Naïve macrophages | Gradient ultra-centrifugation | Endogenous agent: Brain-derived neurotropic factor (BDNF) |
Presence / absence of brain inflammation | • Efficient homing • BBB crossing • Enhanced delivery of the protein cargo |
• Receptor-mediated endocytosis • Efficient BBB passing through LFA-1/ICAM-1 interactions • Better uptake in the presence of inflammation |
(108) |
| In vivo (C57BL/6j) | Exosomes | Mouse lymphoma cell line (EL-4) | Differential centrifugation + sucrose gradient isolation | Anti-inflammatory agents: Curcumin / Stat3 inhibitor (JSI124) |
Three models of brain inflammation: -LPS-induced -EAE -GL-26 brain tumor |
• Selective uptake by microglial cells • Increased solubility, stability and bioavailability |
• Exosome-encapsulated curcumin was efficiently delivered and ameliorated all three inflammation models | (107) |
| In vitro, In vivo | Exosomes | EL-4 and murine macrophage cells (RAW 264.7) | Differential centrifugation + sucrose gradient isolation | Curcumin | LPS-induced septic shock | • Inflammatory cell targeting • Reduced off-target delivery / toxicity • Enhanced stability in vitro and bioavailability in vivo |
• Septic shock amelioration • Reduced population of CD11b+Gr-1+ cells • Successful delivery to circulating myeloid cells and apoptosis induction • Enhanced anti-inflammatory activity compared to curcumin alone |
(106) |
| In vitro, In vivo | MVs | RBCs | RBC osmotic hemolysis and subsequent extrusion through 0.4μm polycarbonate membranes | Hydrophobic natural alkaloid: Camptothecin | BALB/c tumor models and lung carcinoma cells (A549) | • Reduced off-target toxicity • Overcoming low bioavailability due to hydrophobic nature • Overcoming immune-mediated clearance as opposed to synthetic carriers • Stability and retention (slow release) |
• Efficient apoptotic and cytotoxic effects in tumor cells • Promising delivery to the tumor site • Optimal for theranostic applications |
(105) |
| In vitro, In vivo | EVs (NS) | IC21 macrophages | Gradient ultra-centrifugation | TPP1 enzyme | Batten disease models: LINCL mice and TPP1 enzyme-deficient cells (CLN2) |
• Brain homing • Immune inertness • Efficacious delivery • Protection against proteolytic degradation |
• Therapeutic efficacy and increased lifespan of the affected mice | (87) |
| In vitro | MVs | Lactobacillus acidophilus | Gradient ultra-centrifugation | Bacteriocins | Opportunistic pathogen: Lactobacillus acidophilus |
• Natural bacteriocins within the composition of the MVs | • Effective adherence to the target pathogen and compromising of its growth and membrane integrity | (91) |
EV, Extracellular vesicle; NS, Not specified; BBB, Blood-brain barrier; LFA-1, Lymphocyte function-associated antigen 1, ICAM-1, Intercellular Adhesion Molecule 1; LPS, Lipopolysaccharide; EAE, Experimental autoimmune encephalomyelitis; RBCs, Red blood cells; TPP1, tripeptidyl peptidase-1; Ref., References
In two similar studies by Donoso-Quezada et al. (2019 and 2020), total black bean extract containing bioactive saponins and flavonoids, previously shown to have anti-proliferative effects on cancer cells, was loaded into exosomes isolated from a variety of breast, colon, and hepatic cancer cell lines. The exosomal form of the extract was more efficacious than its free form or even liposomal forms in hindering the proliferation of cancer cells (104). Donoso-Quezada et al. suggested that large-scale preparation of cancer cell-derived exosomes is an obstacle to their use as delivery vehicles. Circumventing this obstacle, Malhotra et al. (2019) used a less frequently used source of EVs to enhance delivery of the natural hydrophobic agent Camptothecin in a mouse model of lung carcinoma (105). They used EVs isolated from RBCs to take advantage of their immune inertness and avoid their elimination by phagocytic and undesired immune stimulation. Advantages of RBC-derived EVs include a hydrophobic core for Camptothecin loading, as well as strong retention and slow-release capacities, which make these structures promising candidates for personalized targeted drug delivery. For certain conditions in which overactivation of inflammatory cells, mainly macrophages, contributes to disease pathology, specific targeting of anti-inflammatory agents to these hostile populations becomes a major challenge. This is especially important when the therapeutic agents are natural molecules for which low stability and high levels of off-target delivery have been reported. In this regard, Sun et al. (2010) used exosomes isolated from mouse lymphoma cells and a macrophage cell line to overcome the poor stability and off-target effects of curcumin (the anti-inflammatory natural constituent of turmeric) in alleviating several inflammatory conditions including LPS-induced septic shock and lung inflammation. They showed that incorporation of curcumin into exosomes increased its stability in the circulation and reduced off-target uptake. Because the exosomes were mainly taken up by CD11b+Gr-1+ cells, a major phenotype of cells responsible for inflammatory pathologies, a consequent decrease in the number of these cells resulted in significant suppression of inflammation and inflammatory cytokine production and yielded a survival advantage in models of septic shock (106). A year later, the same team used exosomes to increase brain delivery of curcumin via the intranasal root. They hypothesized that the intranasal route and strong inclination of brain macrophages (microglial cells) to take up exosomes could improve bioavailability of systematically administered drugs in the brain. They inflicted three types of inflammation (LPS-induced septic shock, experimental autoimmune encephalomyelitis, and a GL26 tumor model), loaded their agents of interest into exosomes, and measured bioavailability and selective uptake of the drugs by the target microglia cells responsible for the inflammation. They observed promising results in all three models and concluded that intranasal delivery of exosomal curcumin increases its solubility, stability, and bioavailability in neuroinflammation (107). In another study by Haney et al. (2019), macrophage-derived EVs protected against proteolytic cleavage and immune inertness and showed efficacious targeted delivery of TPP1, an insufficiently produced enzyme, to the inflamed brain in a mouse model of Batten disease (87). In many neuroinflammatory disorders, the inflammatory nature of the brain contributes to the high affinity of macrophage-derived exosomes for the brain and aids in the targeted delivery of medicinal agents to sites of interest. Macrophage exosome-associated LFA-1 is thought to play a critical role in guiding exosomes toward inflammatory sites to interact with the naturally overexpressed ICAM-1 (108). In addition to EVs isolated from mammalian cells, other less-studied sources of EVs have been recently introduced, and may offer further advantages such as large-scale accessibility. In this regard, Yepet-Molina et al. (2020) used membrane-derived vesicles from broccoli root to facilitate transdermal delivery of dyes and fluorescent agents. Broccoli EVs successfully interacted with skin keratinocytes and enhanced the permeability, stability, availability, and delivery of the loaded agents to the skin. These findings suggest that plant-derived EVs can potentially be used as novel and widely available drug carriers, especially for transdermal delivery purposes (109). Dean et al. (2020) used MVs from Lactobacillus acidophilus as natural carriers to transfer antimicrobial bacteriocins to L. delbrueckii, an opportunistic pathogen of the same genus. They treated the MV-producing bacteria with lactacin B-inducing peptide to enrich the bacteriocins of interest within the produced. These MVs successfully transferred the antimicrobial bacteriocins to the target pathogen (L. delbrueckii) and compromised its growth and membrane integrity (91).
5. Advantages of EVs as carriers of natural molecules
5.1. Biocompatibility and immune stealth
A major limitation of synthetic nanoparticles is that they are recognized and eliminated from the bloodstream by the phagocytes of the immune system, hampering their efficacy to a great extent (110). Therefore, there is an urgent need for novel alternatives that not only possess the favorable properties of synthetic nanoparticles, but also are recognized as safe by the host`s immune system (111). In this regard, the protein corona (PrC) on the surface of EVs is a major determinant of their pharmacokinetics and bio distribution within body fluids and is mainly affected by their surrounding environment and intrinsic features (112). It has been shown that generating EV-like structures with certain PrC properties could potentially circumvent the limitations associated with the systemic delivery of EVs to the therapeutic targets (112). Due to the natural origin of EVs, they harbor the marker CD47 that is identified by the host immune system as “self.” Therefore, EVs are weak immune stimulators, if not completely inert, ensuring biocompatibility and immune stealth (113–115) (Figure 2). Moreover, therapeutically administered EVs can undergo biodegradation and be safely removed by natural pre-existing physiological metabolic pathways with minimal toxicity. Thus, EVs are desirable for use as intrinsic bioactive molecules as well as safe and effective delivery vehicles for exogenous drugs without any adverse immune reaction.
Figure 2:
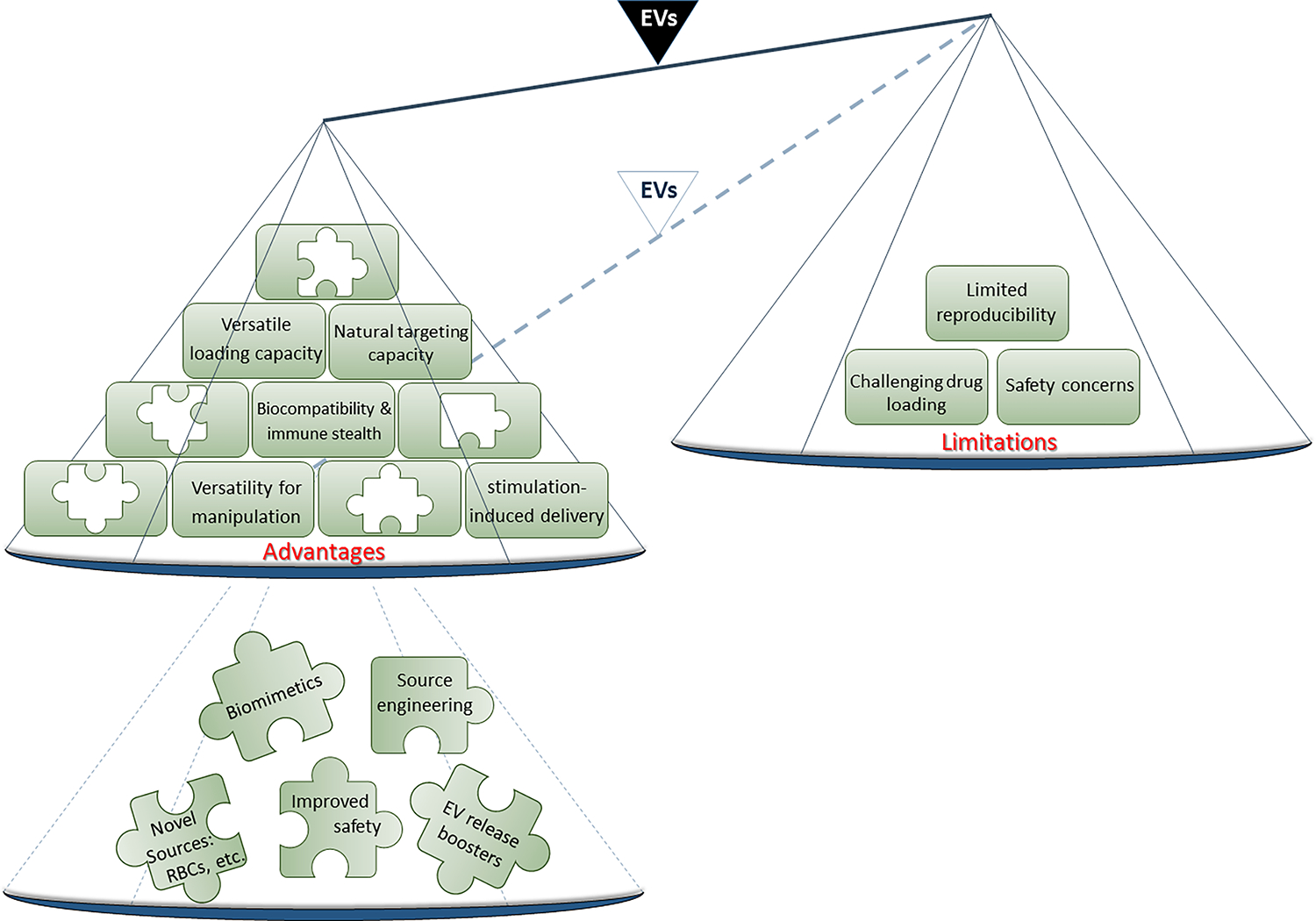
The advantages vs. limitations of EVs as carriers of natural molecules. Despite the outweighing advantages (left plate), the therapeutic use of EVs comes with limitations that need to be addressed using novel strategies (lower left plate). EV, Extracellular vesicle; RBC, Red blood cell
5.2. Versatile loading capacity
The loading of any therapeutic agent into EVs can be achieved either via physical entrapment within the EV layers or via chemical interaction of the agent with certain EV compartments (e.g., transmembrane tetraspanins) (116). For instance, in a recently introduced method, proteins of interest (CRISPR associated protein 9) were readily loaded onto breast cancer cell (MDA-MB-231)-derived EVs through their binding to cationic lipids and a subsequent passive incubation step with EVs (117). The dual hydrophilic and hydrophobic nature of EVs (i.e., the aqueous core and the space within the phospholipid shell, respectively) provides a versatile platform for both lipophilic and hydrophilic moieties (105). This is especially important for the optimal delivery of drugs with serious limitations such as low solubility and inability to cross barriers. For example, more than 98% of small and 100% of large molecules have extremely poor bioavailability in blood-brain barrier-protected sites (118, 119). This characteristic of EVs also makes them desirable for use in cosmetic formulations whose delivery to the skin is a serious obstacle (120). The membranous shell of EVs serves as protection for a variety of molecules, including protecting exogenous genetic material such as CRISPR sequences, miRNAs, and viral vectors from nucleases and protecting various enzyme cargos against proteases, ensuring safe delivery to sites of interest (121–124). EVs also offer the possibility of incorporating a range of molecules, such as total herbal extracts (125) or drugs with synergistic effects (126, 127). Additional benefits of using EVs as delivery tools include the ability to decorate EVs with specific targeting molecules (e.g., antibodies against a target antigen) and/or to simultaneously load a drug as a combinatorial strategy (128).
5.3. Natural targeting capacity
Regardless of their bioactive content, EVs released by distinct cell types appear to have affinities for certain tissues and be adapted to the microenvironment of that particular tissue (88) (Figure 3). For instance, EVs released by inflammatory cell populations such as macrophages and monocytes demonstrate molecular patterns (i.e., high LFA-1 expression) that endow them with a natural preference for inflamed sites (i.e., where LFA-1 can interact with upregulated ICAM-1) (108). A pioneering work published in Nature in 2015 showed for the first time that cancer cells that exhibit metastatic tropism for certain organs release EVs (specifically exosomes) with the same organotropic affinities (129). The integrin signature of the released EVs is responsible for such differential affinities. This interesting finding established proof-of-concept for the ambitious idea of leveraging the natural site-specific affinity of cancer-derived EVs for targeted drug delivery (123). Another advantage of using EVs as carriers is their capacity to reduce off-target delivery of natural agents whose non-specific absorption hinders their bioavailability in the target organ (107), as well as the adverse effects associated with non-specific delivery of small chemotherapeutic molecules such as doxorubicin and paclitaxel (113, 130). Moreover, the lipid membrane of EVs enables them to successfully deliver enzymes and other proteins to subcellular compartments such as lysosomes (87) and the endocytic compartment (124), a characteristic with great therapeutic potential for the targeted treatment of lysosomal disorders (87).
Figure 3:
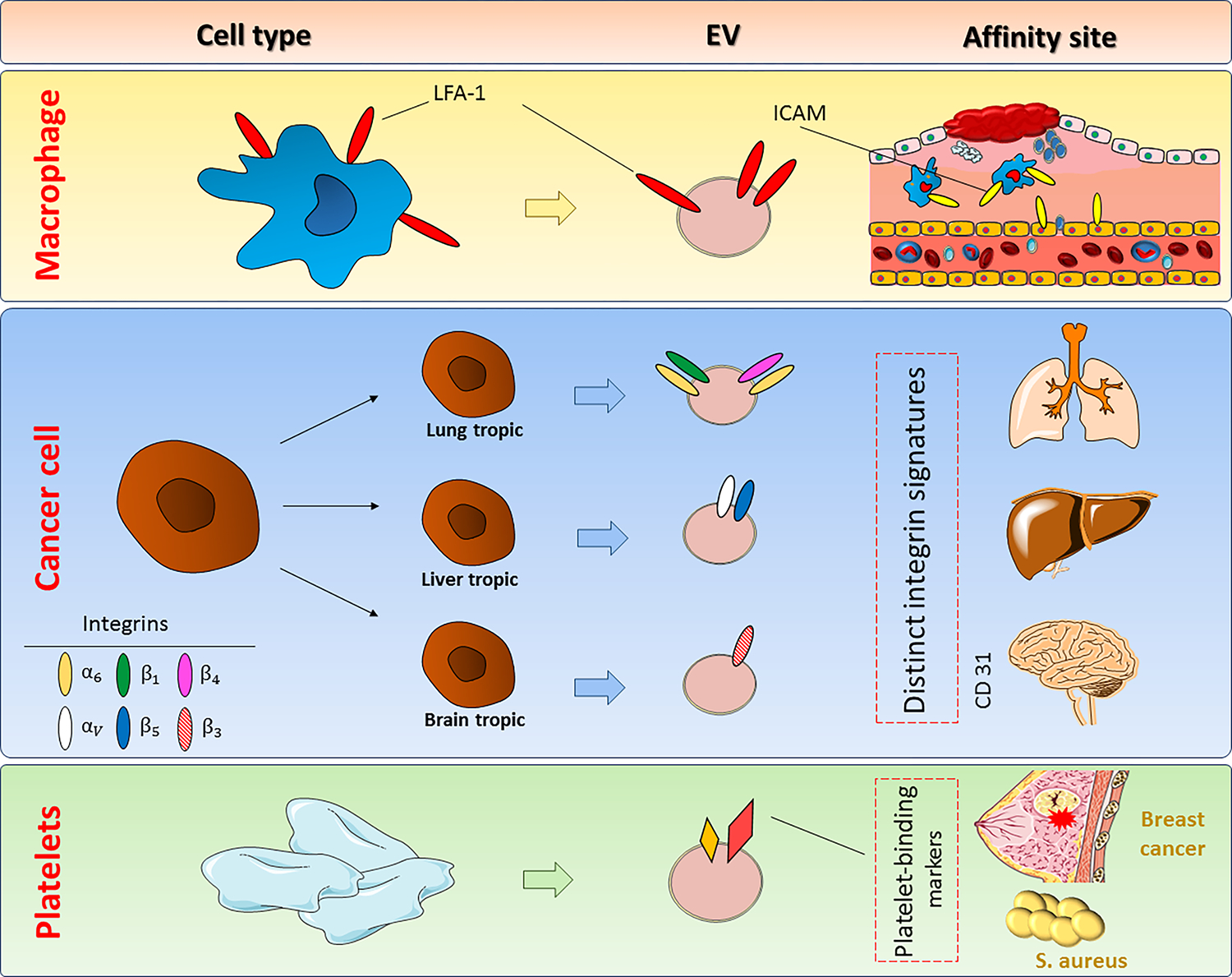
EVs released from various cells and their natural target-specific affinity. EVs from macrophages, cancer cells, and platelets possess intrinsic molecular signatures that endow them with affinities for certain targets. Identifying these natural affinities may provide novel opportunities to use these EVs in targeted drug delivery. EV, Extracellular vesicle; LFA, Lymphocyte function-associated antigen 1; ICAM-1, Intercellular Adhesion Molecule 1
5.4. Versatility for manipulation
Although synthetic nanoparticles are often highly engineerable platforms that offer a variety of options in terms of particle size and surface structure (29), EVs provide other options that enable specialized effects. This is mainly because, in addition to manipulating EVs by decorating them with specific targeting molecules (128, 131, 132), they can also be engineered indirectly through their parent cells to reflect gene products of interest or be enriched with certain molecules. This provides a great opportunity to obtain specialized EVs via strategies such as changing parent cell culture conditions or transfecting parent cells with the genes of interest.
5.5. Stimuli-responsive delivery
One advantage of EVs in terms of increasing drug delivery specificity is the potential to functionalize EVs with certain moieties sensitive to specific stimuli. Such a strategy allows for conditional and controlled release, activation, or delivery of the intended drug, provided that the stimuli, which could be either an intrinsic characteristic of a certain tissue/cell or an external trigger, is present. For example, to exploit intrinsic changes in the host as a stimuli, Gao et al. (2017) constructed glucose-responsive, insulin-containing EVs to limit insulin release to the glucose peaks that occur in patients with diabetes (133). This possibility of engineering EVs as a stimuli-responsive vehicle, coupled with their previously described advantages, give EVs great potential to overcome the current limitations of a vast variety of therapeutic strategies.
6. Limitations and recommendations
Despite the wide range of advantages that EVs offer as therapeutic tools, their efficient use is challenged by several limitations (Figure 2). Among the therapeutic triad of EVs (as drug targets, as drugs, and as drug carriers), strategies in which EVs are used for their intrinsic medicinal properties or as vehicles for drug delivery require substantial amounts of EVs to achieve therapeutic goals. Therefore, the yield and reproducibility of the sources from which EVs are obtained become paramount (134). For instance, in vitro purification of EVs from cells requires extensive culturing of an adequate number of cells, which is labor- and time-intensive (28). In addition, due to their limited expansion capabilities, cells such as MSCs undergo senescence after prolonged maintenance in culture, a phenomenon that impairs the natural regenerative effects of MSCs compared to young cells (31). Therefore, new cost-effective methods are warranted for the rapid and large-scale production of therapeutic-grade EVs (125). The only solution to this problem seems to be to foster greater EV production and release by such cells. In this regard, N-methyldopamine and norepinephrine have been used in combination to successfully boost exosome production by MSCs without any significant change in their angiogenic and immunomodulatory properties (135). Similarly, calcium phosphate particles were used to increase the reproducibility of EVs from myeloid cells. An increase in intracellular calcium concentrations following treatment with calcium phosphate was suggested as the underlying mechanism for the observed increase in EV production (136).
A second major obstacle in the therapeutic application of EVs is safety concerns associated with EVs isolated from cellular sources. The complex composition of EVs makes complete control of outcomes challenging; for example, undesired events such as horizontal gene transfer may be driven by unidentified components of the EVs (137). Using EVs from RBCs obtained from patients with the “O” blood group antigen may overcome this complication. Such a strategy would not only provide a universally available, ample source of EVs, but also eliminates to a large extent the risk of immunogenicity and horizontal delivery of unwanted genetic molecules (137). An alternative approach increasingly used in recent years is the development of biomimetics via combining the versatility of synthetic cores with the outer shell of EVs for their functional properties to produce therapeutic microparticles devoid of any unwanted content (138) (Figure 2).
A lack of optimal techniques for drug loading of EVs is another hurdle that needs to be addressed (139). EVs have low loading capacity compared to synthetic microparticles, especially for the loading of macromolecules (49). Although novel methods such as anchoring the molecules of interest to the transmembrane cholesterol molecules of EVs have been suggested to deal with this problem, this limitation still remains a major drawback. Finally, the possibility of engineering the cellular sources from which EVs are obtained is the option with the most potential to overcome the difficulties of drug loading into EVs.
Finally, to overcome the limitations of using EVs as therapeutic tools and leveraging the existing opportunities, the future research agenda in the field is as follows:
Prioritize discovery of novel sources of EVs for large-scale yield
Uncover engineering opportunities to educate cellular EV sources toward producing highly specialized EVs at high yields
Identify and develop new isolation and drug-loading techniques
7. Conclusion
Rapidly advancing research on the myriad benefits of EVs as therapeutic tools has led to a breakthrough in developing novel EV-based therapies for a variety of conditions. By therapeutically targeting disease-promoting EVs and exploiting the natural medicinal properties of EVs, we have broadened their applications to more innovative therapies. Furthermore, these natural biological vehicles have enabled improved delivery of drugs, whose instability, off-target delivery, high toxicity, and low bioavailability largely limited their therapeutic efficacy. Despite the unique advantages of EVs, the possibility of their clinical use comes with several limitations, namely low reproducibility, safety concerns due to their complex composition, and challenging drug loading. In addition to the constantly growing efforts to discover new areas of EV applicability, future research will mainly focus on overcoming these limitations in hopes of translating EVs into effective clinical treatments.
Funding:
The present work was supported by the CA72851, CA187956, CA202797 and CA214254 grants from the National Cancer Institute, National Institute of Health.
Non-standard abbreviations:
- EV
Extracellular Vesicle
- MSC
Mesenchymal Stem Cell
- DC
Dendritic Cell
- MM
Multiple Myeloma
- EPA
Eicosapentaenoic Acid
- DHA
Docosahexaenoic Acid
- OMV
Outer Membrane Vesicle
- RD
Retinal Detachment
- AAT
Alpha-1-antitrypsin
- APC
Antigen Presenting Cell
- MHC I
Major Histocompatibility Complex Type I
- TSA
Tumor-specific Antigens
- TAA
Tumor-associated Antigens
- CEA
Carcinoembryonic Antigen
- MUC1
Mucin 1
- MAGE
Melanoma-associated Antigen
- LFA1
Lymphocyte Function-associated Antigen 1
- ICAM1
Intercellular Adhesion Molecule 1
- PAMP
Pathogen-associated Molecular Patterns
- PRR
Pattern Recognition Receptors
- TLR2
Toll-like Receptor 2
- LPS
Lipopolysaccharide
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Cited literature
- 1.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual review of cell and developmental biology. 2014;30:255–89. [DOI] [PubMed] [Google Scholar]
- 2.Andaloussi SE, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nature reviews Drug discovery. 2013;12(5):347–57. [DOI] [PubMed] [Google Scholar]
- 3.Van Niel G, d’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nature reviews Molecular cell biology. 2018;19(4):213. [DOI] [PubMed] [Google Scholar]
- 4.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. Journal of Cell Biology. 2013;200(4):373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yáñez-Mó M, Siljander PR-M, Andreu Z, Bedina Zavec A, Borràs FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4(1):27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An K, Klyubin I, Kim Y, Jung JH, Mably AJ, T O’Dowd S, et al. Exosomes neutralize synaptic-plasticity-disrupting activity of Aβ assemblies in vivo. Molecular brain. 2013;6(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q, et al. Immune-related microRNAs are abundant in breast milk exosomes. International journal of biological sciences. 2012;8(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Admyre C, Johansson SM, Qazi KR, Filén J-J, Lahesmaa R, Norman M, et al. Exosomes with immune modulatory features are present in human breast milk. The Journal of immunology. 2007;179(3):1969–78. [DOI] [PubMed] [Google Scholar]
- 9.Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. The Journal of Immunology. 2006;176(3):1534–42. [DOI] [PubMed] [Google Scholar]
- 10.Dvorak H, Quay S, Orenstein N, Dvorak A, Hahn P, Bitzer A, et al. Tumor shedding and coagulation. Science. 1981;212(4497):923–4. [DOI] [PubMed] [Google Scholar]
- 11.Wang J-G, Williams JC, Davis BK, Jacobson K, Doerschuk CM, Ting JP-Y, et al. Monocytic microparticles activate endothelial cells in an IL-1β–dependent manner. Blood, The Journal of the American Society of Hematology. 2011;118(8):2366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasser O, Schifferli JrA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104(8):2543–8. [DOI] [PubMed] [Google Scholar]
- 13.Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nature cell biology. 2019;21(1):9–17. [DOI] [PubMed] [Google Scholar]
- 14.Maacha S, Bhat AA, Jimenez L, Raza A, Haris M, Uddin S, et al. Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Molecular cancer. 2019;18(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skriner K, Adolph K, Jungblut PR, Burmester GR. Association of citrullinated proteins with synovial exosomes. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 2006;54(12):3809–14. [DOI] [PubMed] [Google Scholar]
- 16.Moloudizargari M, Abdollahi M, Asghari MH, Zimta AA, Neagoe IB, Nabavi SM. The emerging role of exosomes in multiple myeloma. Blood Reviews. 2019;38:100595. [DOI] [PubMed] [Google Scholar]
- 17.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature reviews immunology. 2009;9(8):581–93. [DOI] [PubMed] [Google Scholar]
- 18.Senft AW, Philpott DE, Pelofsky AH. Electron microscope observations of the integument, flame cells, and gut of Schistosoma mansoni. The Journal of parasitology. 1961;47(2):217–29. [PubMed] [Google Scholar]
- 19.Han L, Lam EW-F, Sun Y. Extracellular vesicles in the tumor microenvironment: old stories, but new tales. Molecular cancer. 2019;18(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nature Reviews Microbiology. 2015;13(10):620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maas SL, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends in cell biology. 2017;27(3):172–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–56. [DOI] [PubMed] [Google Scholar]
- 23.Liao W, Du Y, Zhang C, Pan F, Yao Y, Zhang T, et al. Exosomes: the next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. 2019;86:1–14. [DOI] [PubMed] [Google Scholar]
- 24.Kibria G, Ramos EK, Wan Y, Gius DR, Liu H. Exosomes as a drug delivery system in cancer therapy: potential and challenges. Mol Pharm. 2018;15(9):3625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pirisinu M, Pham TC, Zhang DX, Hong TN, Nguyen LT, Le MT, editors. Extracellular vesicles as natural therapeutic agents and innate drug delivery systems for cancer treatment: Recent advances, current obstacles, and challenges for clinical translation. Semin Cancer Biol; 2020: Elsevier. [DOI] [PubMed] [Google Scholar]
- 26.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7):727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iannotta D, Yang M, Celia C, Di Marzio L, Wolfram J. Extracellular vesicle therapeutics from plasma and adipose tissue. Nano Today. 2021;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F, et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun. 2019;10(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng W, He C, Hao Y, Wang L, Li L, Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27(1):585–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G, et al. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell death & disease. 2013;4(11):e911–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23(5):812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moloudizargari M, Govahi A, Fallah M, Rezvanfar MA, Asghari MH, Abdollahi M. The mechanisms of cellular crosstalk between mesenchymal stem cells and natural killer cells: Therapeutic implications. Journal of Cellular Physiology. 2020. [DOI] [PubMed] [Google Scholar]
- 34.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nature medicine. 1998;4(5):594–600. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Ma J, Tang K, Huang B. Therapeutic use of tumor cell-derived extracellular vesicles. Extracellular Vesicles: Springer; 2017. p. 433–40. [DOI] [PubMed] [Google Scholar]
- 36.Yeo RWY, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65(3):336–41. [DOI] [PubMed] [Google Scholar]
- 37.Vázquez-Ríos AJ, Molina-Crespo Á, Bouzo BL, López-López R, Moreno-Bueno G, de la Fuente M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. J Nanobiotechnology. 2019;17(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.del Carmen Martínez-Ballesta M, Pérez-Sánchez H, Moreno DA, Carvajal M. Plant plasma membrane aquaporins in natural vesicles as potential stabilizers and carriers of glucosinolates. Colloids Surf B Biointerfaces. 2016;143:318–26. [DOI] [PubMed] [Google Scholar]
- 39.Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013;21(7):1345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villa F, Quarto R, Tasso R. Extracellular vesicles as natural, safe and efficient drug delivery systems. Pharmaceutics. 2019;11(11):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reiner AT, Witwer KW, Van Balkom BW, De Beer J, Brodie C, Corteling RL, et al. Concise review: Developing best-practice models for the therapeutic use of extracellular vesicles. Stem Cells Translational Medicine. 2017;6(8):1730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Affabris E, Mangino G, Romeo G. Introduction to the special issue: Extracellular vesicles and biological signal delivery in the inflammatory microenvironment. Cytokine Growth Factor Rev. 2020;51:10–1. [DOI] [PubMed] [Google Scholar]
- 43.Qin X, Yu S, Zhou L, Shi M, Hu Y, Xu X, et al. Cisplatin-resistant lung cancer cell–derived exosomes increase cisplatin resistance of recipient cells in exosomal miR-100-5p-dependent manner. Int J Nanomed. 2017;12:3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan FM, Saleh E, Alawadhi H, Harati R, Zimmermann WH, El-Awady R. Inhibition of exosome release by ketotifen enhances sensitivity of cancer cells to doxorubicin. Cancer Biol Ther. 2018;19(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel H, Nilendu P, Jahagirdar D, Pal JK, Sharma NK. Modulating secreted components of tumor microenvironment: A masterstroke in tumor therapeutics. Cancer Biol Ther. 2018;19(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giallombardo M, Taverna S, Alessandro R, Hong D, Rolfo C. Exosome-mediated drug resistance in cancer: the near future is here. Ther Adv Med Oncol. 2016;8(5):320–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kosaka N, Yoshioka Y, Tominaga N, Hagiwara K, Katsuda T, Ochiya T. Dark side of the exosome: the role of the exosome in cancer metastasis and targeting the exosome as a strategy for cancer therapy. Future Oncology. 2014;10(4):671–81. [DOI] [PubMed] [Google Scholar]
- 48.Moloudizargari M, Redegeld F, Asghari MH, Mosaffa N, Mortaz E. Long-chain polyunsaturated omega-3 fatty acids reduce multiple myeloma exosome-mediated suppression of NK cell cytotoxicity. Daru. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hannafon BN, Carpenter KJ, Berry WL, Janknecht R, Dooley WC, Ding W-Q. Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA). Molecular cancer. 2015;14(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moloudizargari M, Asghari MH, Abdollahi M. Modifying exosome release in cancer therapy: How can it help? Pharmacol Res. 2018;134:246–56. [DOI] [PubMed] [Google Scholar]
- 51.Datta A, Kim H, Lal M, McGee L, Johnson A, Moustafa AA, et al. Manumycin A suppresses exosome biogenesis and secretion via targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1 in castration-resistant prostate cancer cells. Cancer Lett. 2017;408:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moloudizargari M, Asghari MH, Mortaz E. Inhibiting exosomal MIC-A and MIC-B shedding of cancer cells to overcome immune escape: new insight of approved drugs. Daru. 2019;27(2):879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petanidis S, Domvri K, Porpodis K, Anestakis D, Freitag L, Hohenforst-Schmidt W, et al. Inhibition of kras-derived exosomes downregulates immunosuppressive BACH2/GATA-3 expression via RIP-3 dependent necroptosis and miR-146/miR-210 modulation. Biomed Pharmacother. 2020;122:109461. [DOI] [PubMed] [Google Scholar]
- 54.Zakharzhevskaya NB, Tsvetkov VB, Vanyushkina AA, Varizhuk AM, Rakitina DV, Podgorsky VV, et al. Interaction of Bacteroides fragilis toxin with outer membrane vesicles reveals new mechanism of its secretion and delivery. Front Cell Infect Microbiol. 2017;7(JAN). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450(7168):435–9. [DOI] [PubMed] [Google Scholar]
- 56.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bari E, Ferrarotti I, Di Silvestre D, Grisoli P, Barzon V, Balderacchi A, et al. Adipose Mesenchymal Extracellular Vesicles as Alpha-1-Antitrypsin Physiological Delivery Systems for Lung Regeneration. Cells. 2019;8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamichhane TN, Sokic S, Schardt JS, Raiker RS, Lin JW, Jay SM. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2015;21(1):45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kholia S, Ranghino A, Garnieri P, Lopatina T, Deregibus MC, Rispoli P, et al. Extracellular vesicles as new players in angiogenesis. Vascul Pharmacol. 2016;86:64–70. [DOI] [PubMed] [Google Scholar]
- 60.Tsiapalis D, O’Driscoll L. Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells. 2020;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N, et al. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589(11):1257–65. [DOI] [PubMed] [Google Scholar]
- 62.Ferguson SW, Wang J, Lee CJ, Liu M, Neelamegham S, Canty JM, et al. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep. 2018;8(1):1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, et al. Methodological Guidelines to Study Extracellular Vesicles. Circ Res. 2017;120(10):1632–48. [DOI] [PubMed] [Google Scholar]
- 64.Chung JJ, Han J, Wang LL, Arisi MF, Zaman S, Gordon J, et al. Delayed delivery of endothelial progenitor cell-derived extracellular vesicles via shear thinning gel improves postinfarct hemodynamics. J Thorac Cardiovasc Surg. 2020;159(5):1825–35.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu H, Wang Z, Liu L, Zhang B, Li B. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J Cell Biochem. 2020;121(3):2089–102. [DOI] [PubMed] [Google Scholar]
- 66.Zhang L, Song Y, Chen L, Li D, Feng H, Lu Z, et al. MiR-20a-containing exosomes from umbilical cord mesenchymal stem cells alleviates liver ischemia/reperfusion injury. J Cell Physiol. 2020;235(4):3698–710. [DOI] [PubMed] [Google Scholar]
- 67.Ma M, Li B, Zhang M, Zhou L, Yang F, Ma F, et al. Therapeutic effects of mesenchymal stem cell-derived exosomes on retinal detachment. Exp Eye Res. 2020;191:107899. [DOI] [PubMed] [Google Scholar]
- 68.Fan B, Li C, Szalad A, Wang L, Pan W, Zhang R, et al. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia. 2020;63(2):431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maqsood M, Kang M, Wu X, Chen J, Teng L, Qiu L. Adult mesenchymal stem cells and their exosomes: Sources, characteristics, and application in regenerative medicine. Life Sci. 2020;256:118002. [DOI] [PubMed] [Google Scholar]
- 70.Shi J, Duan J, Gong H, Pang Y, Wang L, Yan Y. Exosomes from miR-20b-3p-overexpressing stromal cells ameliorate calcium oxalate deposition in rat kidney. J Cell Mol Med. 2019;23(11):7268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horrevorts SK, Stolk DA, van de Ven R, Hulst M, van Het Hof B, Duinkerken S, et al. Glycan-Modified Apoptotic Melanoma-Derived Extracellular Vesicles as Antigen Source for Anti-Tumor Vaccination. Cancers. 2019;11(9):1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zou W Regulatory T cells, tumour immunity and immunotherapy. Nature Reviews Immunology. 2006;6(4):295–307. [DOI] [PubMed] [Google Scholar]
- 73.Knutson KL, Disis M. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunology, Immunotherapy. 2005;54(8):721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang R-F. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends in immunology. 2001;22(5):269–76. [DOI] [PubMed] [Google Scholar]
- 75.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nature Reviews Immunology. 2012;12(8):557–69. [DOI] [PubMed] [Google Scholar]
- 76.Parmiani G, Castelli C, Dalerba P, Mortarini R, Rivoltini L, Marincola FM, et al. Cancer immunotherapy with peptide-based vaccines: what have we achieved? Where are we going? Journal of the National Cancer Institute. 2002;94(11):805–18. [DOI] [PubMed] [Google Scholar]
- 77.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nature medicine. 2004;10(9):909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jalali SA, Parmiani G. Pre-clinical and clinical aspects of peptide-based vaccine against human solid tumors. Recent Pat Biotechnol. 2011;5(2):108–17. [DOI] [PubMed] [Google Scholar]
- 79.Kuldkepp A, Reinsalu O, Karakai M, Toomsoo E, Kurg R. Cancer-testis antigen MAGEA protein expression in extracellular vesicles released by cells. Biopolymers and Cell. 2019;35(3):192-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7(3):297–303. [DOI] [PubMed] [Google Scholar]
- 81.Bose RJC, Uday Kumar S, Zeng Y, Afjei R, Robinson E, Lau K, et al. Tumor Cell-Derived Extracellular Vesicle-Coated Nanocarriers: An Efficient Theranostic Platform for the Cancer-Specific Delivery of Anti-miR-21 and Imaging Agents. ACS Nano. 2018;12(11):10817–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Théry C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, et al. Molecular characterization of dendritic cell-derived exosomes: selective accumulation of the heat shock protein hsc73. The Journal of cell biology. 1999;147(3):599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J BIOL CHEM. 1998;273(32):20121–7. [DOI] [PubMed] [Google Scholar]
- 85.Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Markov O, Oshchepkova A, Mironova N. Immunotherapy based on dendritic cell-targeted/-derived extracellular vesicles — A novel strategy for enhancement of the anti-tumor immune response. Front Pharmacol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haney MJ, Klyachko NL, Harrison EB, Zhao Y, Kabanov AV, Batrakova EV. TPP1 Delivery to Lysosomes with Extracellular Vesicles and their Enhanced Brain Distribution in the Animal Model of Batten Disease. Adv Healthc Mater. 2019;8(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang J, Kim EK, McDowell A, Kim YK. Microbe-derived extracellular vesicles as a smart drug delivery system. Transl Clin Pharmacol. 2018;26(3):103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infection and immunity. 2012;80(6):1948–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rivera J, Cordero RJ, Nakouzi AS, Frases S, Nicola A, Casadevall A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proceedings of the National Academy of Sciences. 2010;107(44):19002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dean SN, Rimmer MA, Turner KB, Phillips DA, Caruana JC, Hervey WJI, et al. Lactobacillus acidophilus Membrane Vesicles as a Vehicle of Bacteriocin Delivery. Front Microbiol. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsatsaronis JA, Franch-Arroyo S, Resch U, Charpentier E. Extracellular vesicle RNA: a universal mediator of microbial communication? Trends in microbiology. 2018;26(5):401–10. [DOI] [PubMed] [Google Scholar]
- 93.Choi SJ, Kim M-H, Jeon J, Kim OY, Choi Y, Seo J, et al. Active immunization with extracellular vesicles derived from Staphylococcus aureus effectively protects against staphylococcal lung infections, mainly via Th1 cell-mediated immunity. PLoS ONE. 2015;10(9):e0136021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lässer C, Alikhani VS, Ekström K, Eldh M, Paredes PT, Bossios A, et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. Journal of translational medicine. 2011;9(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xie M-Y, Chen T, Xi Q-Y, Hou L-J, Luo J-Y, Zeng B, et al. Porcine milk exosome miRNAs protect intestinal epithelial cells against deoxynivalenol-induced damage. Biochemical Pharmacology. 2020:113898. [DOI] [PubMed] [Google Scholar]
- 96.Xie M-Y, Hou L-J, Sun J-J, Zeng B, Xi Q-Y, Luo J-Y, et al. Porcine milk exosome MiRNAs attenuate LPS-induced apoptosis through inhibiting TLR4/NF-κB and p53 pathways in intestinal epithelial cells. J Agric Food Chem. 2019;67(34):9477–91. [DOI] [PubMed] [Google Scholar]
- 97.Pisano C, Galley J, Elbahrawy M, Wang Y, Farrell A, Brigstock D, et al. Human Breast Milk-Derived Extracellular Vesicles in the Protection Against Experimental Necrotizing Enterocolitis. Journal of Pediatric Surgery. 2020;55(1):54–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Benmoussa A, Diallo I, Salem M, Michel S, Gilbert C, Sévigny J, et al. Concentrates of two subsets of extracellular vesicles from cow’s milk modulate symptoms and inflammation in experimental colitis. Sci Rep. 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matic S, D’Souza DH, Wu T, Pangloli P, Dia VP. Bovine Milk Exosomes Affect Proliferation and Protect Macrophages against Cisplatin-Induced Cytotoxicity. Immunological Investigations. 2020:1–15. [DOI] [PubMed] [Google Scholar]
- 100.Arntz OJ, Pieters BC, Oliveira MC, Broeren MG, Bennink MB, de Vries M, et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Molecular nutrition & food research. 2015;59(9):1701–12. [DOI] [PubMed] [Google Scholar]
- 101.Badawy AA, El-Magd MA, AlSadrah SA. Therapeutic Effect of Camel Milk and Its Exosomes on MCF7 Cells In Vitro and In Vivo. Integrative Cancer Therapies. 2018;17(4):1235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bilia AR, Bergonzi MC, Guccione C, Manconi M, Fadda AM, Sinico C. Vesicles and micelles: Two versatile vectors for the delivery of natural products. J Drug Deliv Sci Technol. 2016;32:241–55. [Google Scholar]
- 103.Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. Journal of pharmacological and toxicological methods. 2000;44(1):235–49. [DOI] [PubMed] [Google Scholar]
- 104.Donoso-Quezada J, Guajardo-Flores D, González-Valdéz J. Exosomes as nanocarriers for the delivery of bioactive compounds from black bean extract with antiproliferative activity in cancer cell lines. Materials Today: Proceedings. 2019;13:362–9. [Google Scholar]
- 105.Malhotra S, Dumoga S, Sirohi P, Singh N. Red Blood Cells-Derived Vesicles for Delivery of Lipophilic Drug Camptothecin. ACS Appl Mater Interfaces. 2019. [DOI] [PubMed] [Google Scholar]
- 106.Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19(10):1769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yuan D, Zhao Y, Banks WA, Bullock KM, Haney M, Batrakova E, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yepes-Molina L, Martínez-Ballesta MC, Carvajal M. Plant plasma membrane vesicles interaction with keratinocytes reveals their potential as carriers. J Adv Res. 2020;23:101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peng Q, Zhang S, Yang Q, Zhang T, Wei X-Q, Jiang L, et al. Preformed albumin corona, a protective coating for nanoparticles based drug delivery system. Biomaterials. 2013;34(33):8521–30. [DOI] [PubMed] [Google Scholar]
- 111.Desnick RJ, Schuchman EH. Enzyme replacement and enhancement therapies: lessons from lysosomal disorders. Nature Reviews Genetics. 2002;3(12):954–66. [DOI] [PubMed] [Google Scholar]
- 112.Imperlini E, Celia C, Cevenini A, Mandola A, Raia M, Fresta M, et al. Nano-bio interface between human plasma and niosomes with different formulations indicates protein corona patterns for nanoparticle cell targeting and uptake. Nanoscale. 2021;13(10):5251–69. [DOI] [PubMed] [Google Scholar]
- 113.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–90. [DOI] [PubMed] [Google Scholar]
- 114.Lai RC, Yeo RWY, Tan KH, Lim SK. Exosomes for drug delivery—a novel application for the mesenchymal stem cell. Biotechnol Adv. 2013;31(5):543–51. [DOI] [PubMed] [Google Scholar]
- 115.Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M. A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2014;1846(1):75–87. [DOI] [PubMed] [Google Scholar]
- 116.Choi HS, Ipe BI, Misra P, Lee JH, Bawendi MG, Frangioni JV. Tissue-and organ-selective biodistribution of NIR fluorescent quantum dots. Nano Lett. 2009;9(6):2354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Busatto S, Iannotta D, Walker SA, Di Marzio L, Wolfram J. A Simple and Quick Method for Loading Proteins in Extracellular Vesicles. Pharmaceuticals (Basel). 2021;14(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pardridge WM. Drug transport across the blood–brain barrier. Journal of cerebral blood flow & metabolism. 2012;32(11):1959–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tarhini M, Greige-Gerges H, Elaissari A. Protein-based nanoparticles: From preparation to encapsulation of active molecules. Int J Pharm. 2017;522(1–2):172–97. [DOI] [PubMed] [Google Scholar]
- 121.Pan Q, Ramakrishnaiah V, Henry S, Fouraschen S, de Ruiter PE, Kwekkeboom J, et al. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi). Gut. 2012;61(9):1330–9. [DOI] [PubMed] [Google Scholar]
- 122.Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335(1):201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu Q, Zhang Z, Zhao L, Qin Y, Cai H, Geng Z, et al. Tropism-facilitated delivery of CRISPR/Cas9 system with chimeric antigen receptor-extracellular vesicles against B-cell malignancies. J Control Release. 2020;326:455–67. [DOI] [PubMed] [Google Scholar]
- 124.Do MA, Levy D, Brown A, Marriott G, Lu B. Targeted delivery of lysosomal enzymes to the endocytic compartment in human cells using engineered extracellular vesicles. Sci Rep. 2019;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Donoso-Quezada J, Guajardo-Flores D, González-Valdez J. Enhanced exosome-mediated delivery of black bean phytochemicals (Phaseolus vulgaris L.) for cancer treatment applications. Biomed Pharmacother. 2020;131. [DOI] [PubMed] [Google Scholar]
- 126.Zhang L, Xiao H, Li J, Cheng D, Shuai X. Co-delivery of doxorubicin and arsenite with reduction and pH dual-sensitive vesicle for synergistic cancer therapy. Nanoscale. 2016;8(25):12608–17. [DOI] [PubMed] [Google Scholar]
- 127.Gong C, Tian J, Wang Z, Gao Y, Wu X, Ding X, et al. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J Nanobiotechnology. 2019;17(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li Y, Gao Y, Gong C, Wang Z, Xia Q, Gu F, et al. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine: Nanotechnology, Biology and Medicine. 2018;14(7):1973–85. [DOI] [PubMed] [Google Scholar]
- 129.Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Mark MT, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jang SC, Kim OY, Yoon CM, Choi D-S, Roh T-Y, Park J, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698–710. [DOI] [PubMed] [Google Scholar]
- 131.Li D, Yao S, Zhou Z, Shi J, Huang Z, Wu Z. Hyaluronan decoration of milk exosomes directs tumor-specific delivery of doxorubicin. Carbohydrate Research. 2020:108032. [DOI] [PubMed] [Google Scholar]
- 132.Yu M, Gai C, Li Z, Ding D, Zheng J, Zhang W, et al. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci. 2019;110(10):3173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gao L, Wang T, Jia K, Wu X, Yao C, Shao W, et al. Glucose-Responsive Supramolecular Vesicles Based on Water-Soluble Pillar[5]arene and Pyridylboronic Acid Derivatives for Controlled Insulin Delivery. Chem Eur J. 2017;23(27):6605–14. [DOI] [PubMed] [Google Scholar]
- 134.Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, et al. Applying extracellular vesicles based therapeutics in clinical trials–an ISEV position paper. J Extracell Vesicles. 2015;4(1):30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang J, Bonacquisti EE, Brown AD, Nguyen J. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells. 2020;9(3):660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shyong YJ, Chang KC, Lin FH. Calcium phosphate particles stimulate exosome secretion from phagocytes for the enhancement of drug delivery. Colloids Surf B Biointerfaces. 2018;171:391–7. [DOI] [PubMed] [Google Scholar]
- 137.Usman WM, Pham TC, Kwok YY, Vu LT, Ma V, Peng B, et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat Commun. 2018;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li Z, Zhou X, Wei M, Gao X, Zhao L, Shi R, et al. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2018;19(1):19–28. [DOI] [PubMed] [Google Scholar]
- 139.Lu M, Zhao X, Xing H, Xun Z, Zhu S, Lang L, et al. Comparison of exosome-mimicking liposomes with conventional liposomes for intracellular delivery of siRNA. Int J Pharm. 2018;550(1–2):100–13. [DOI] [PubMed] [Google Scholar]
- 140.Xie MY, Chen T, Xi QY, Hou LJ, Luo JY, Zeng B, et al. Porcine milk exosome miRNAs protect intestinal epithelial cells against deoxynivalenol-induced damage. Biochemical Pharmacology. 2020;175. [DOI] [PubMed] [Google Scholar]
- 141.Benmoussa A, Diallo I, Salem M, Michel S, Gilbert C, Sévigny J, et al. Concentrates of two subsets of extracellular vesicles from cow’s milk modulate symptoms and inflammation in experimental colitis. Sci Rep. 2019;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Matic S, D’Souza DH, Wu T, Pangloli P, Dia VP. Bovine Milk Exosomes Affect Proliferation and Protect Macrophages against Cisplatin-Induced Cytotoxicity. Immunological Investigations. 2020;49(7):711–25. [DOI] [PubMed] [Google Scholar]
- 143.Xie MY, Hou LJ, Sun JJ, Zeng B, Xi QY, Luo JY, et al. Porcine Milk Exosome MiRNAs Attenuate LPS-Induced Apoptosis through Inhibiting TLR4/NF-κB and p53 Pathways in Intestinal Epithelial Cells. J Agric Food Chem. 2019;67(34):9477–91. [DOI] [PubMed] [Google Scholar]


