Abstract
The vascular lab is an essential tool in diagnosing intracranial and extracranial disease including vasospasm from subarachnoid hemorrhage and carotid artery stenosis in the setting of stroke or transient ischemic attack. This article discusses the indications, protocol, and diagnostic criteria for transcranial doppler (TCD) and carotid artery duplex ultrasound.
Intracranial and extracranial arterial testing by way of transcranial doppler (TCD) and carotid imaging carries enormous implications and can provide life or death information. The learning curve for these techniques is steep but can be mastered with repetition and precise technique.
TCD is performed in the setting of subarachnoid hemorrhage (SAH) associated with ruptured aneurysms, traumatic injuries, and sequelae of stroke, and carries a high rate of morbidity and mortality, where degenerated blood products in the subarachnoid space can lead to vasoactive reactions, in particular, vasospasm.1,2 This occurs in up to 30% of patients with SAH and may lead to ischemic stroke in the distribution of the affected vascular segment.3 TCD is a noninvasive method of assessing intracranial cerebral blood flow. It was first developed to detect vasospasm and is used widely in this regard to assist clinical management and decision making to determine the need for invasive neurointerventional procedures to treat vasospasm.
On the other hand, carotid artery imaging is used most often in the setting of cardiovascular disease – cardiovascular disease being the leading cause of death and morbidity in the United States. Myocardial infarction and cerebrovascular accident (CVA) or stroke are the two main contributing disease processes. Stroke is a leading cause of serious long-term disability and mortality. In the United States, someone has a stroke every 40 seconds, and someone dies from a stroke every 3.5 minutes.4 Carotid artery stenosis and thromboembolic disease related to unstable plaque is a major cause of CVA. First developed in the 1970s to evaluate for carotid artery stenosis, carotid duplex ultrasound (CDUS), provides a safe, accurate, reproduceable, and cost effect method of diagnosing and follow-up for extracranial cerebrovascular disease.4
Indications
TCD
The primary use of TCD is for evaluation of vasospasm (Figure 1). When a patient presents with SAH, TCD is obtained as early as possible in the hospital course to establish a baseline for future comparison. Cerebral artery vasospasm typically occurs 4 to 14 days after SAH. At our institution, TCD is obtained daily for 14 to 21 days after presentation with SAH.3 Daily studies are compared to assess for changes in velocity to determine the presence of vasospasm.
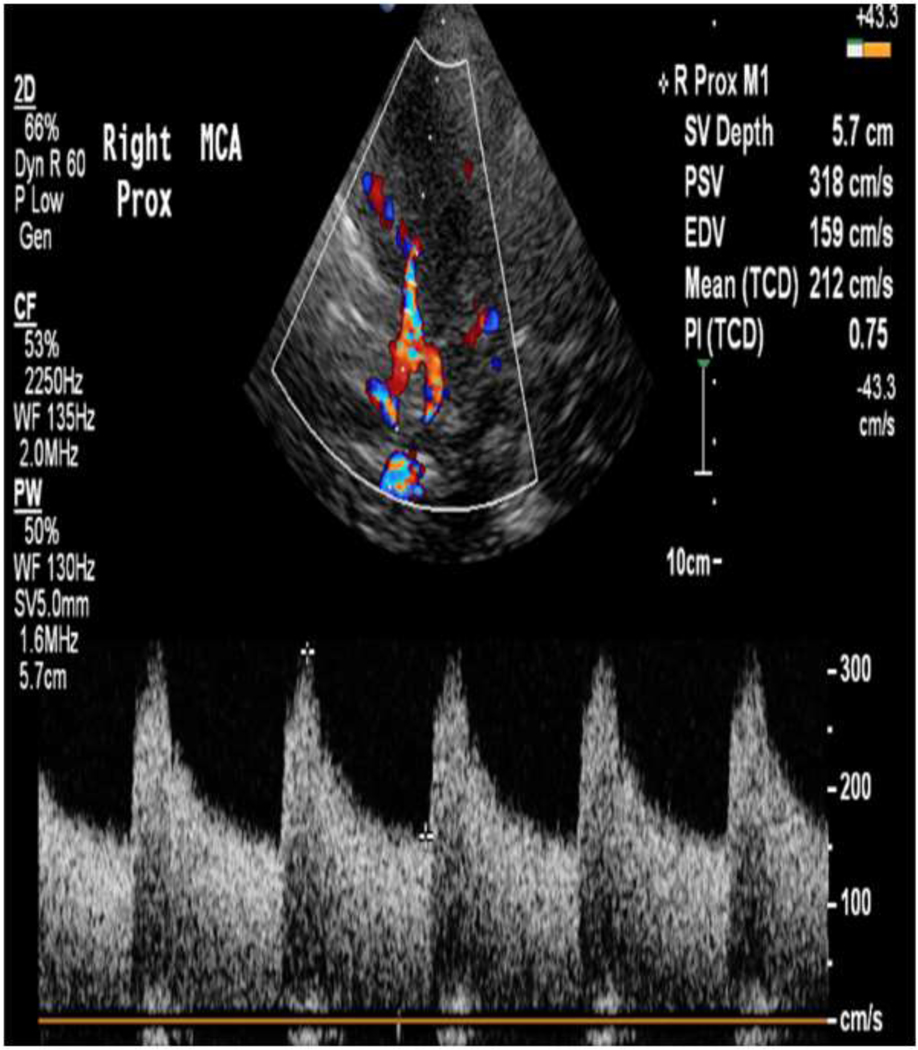
Figure 1:
59 year old female with a ruptured aneurysm leading to subarachnoid hemorrhage. TCD of the right MCA shows elevated TAMAX of 212 cm/s consistent with severe vasospasm. This was confirmed with a calculated Lindegaard ratio of >6 (not shown).
In addition to vasospasm, TCD can be employed as a diagnostic tool for several other disease states. First, it can be used as a minimally invasive technique to assess for right-to-left cardiac or intrapulmonary shunts (Figure 2). In the case of cryptogenic stroke, right-to-left shunts are a likely etiology, in the absence of carotid artery disease, intracardiac thrombus, or vegetative valvular disease. While transesophageal echocardiogram (TEE) with agitated saline bubble solution can be used, TCD bubble studies do not require sedatives, are less invasive, and have been shown to have increased sensitivity over TEE.5 Second, TCD can be used in risk stratification for patients with sickle cell disease (SCD) (Figure 3). Patients with SCD are at increased risk of stroke. In the Stroke Prevention Trial for Sickle Cell Anemia (STOP trial), elevated time averaged peak flow velocities (>200cm/s) were associated with 40% increased stroke risk in the next 3 years.6 At-risk patients should be screened every 6 months to assess for changes in flow velocities. This can be used to determine the need for transfusion for stroke prevention. TCD has also been used for determination of brain death (Figure 4). This is particularly important in those patients being considered for organ donation. It is used as a confirmatory test when clinical criteria cannot be applied, for example hypothermia, drug, or metabolic confounders, etc. When used appropriately, abnormal waveforms on TCD in brain death has a sensitivity of 91–99% and specificity of 100%.7 Lesser utilized indications include assessing for intracranial arterial stenosis, acute embolic stroke, and for elevated intracranial pressures.
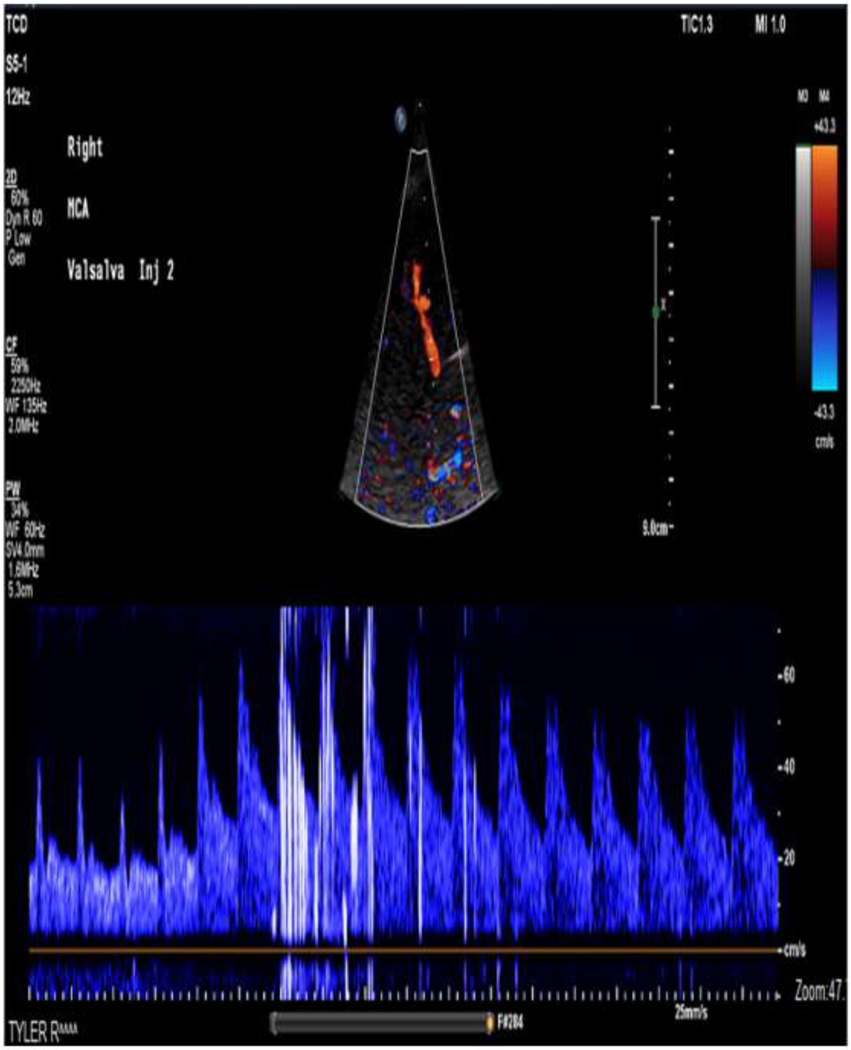
Figure 2:
53 year old female presents with cryptogenic stroke. TCD of the right MCA was performed with injection of agitated saline. Imaging was performed during release of Valsalva maneuver. 10–20 High-Intensity Transient Signals (HITS) were noted. This is consistent with a moderate right-to-left intracardiac shunt. This patient underwent closure of a patent foramen ovale (PFO).
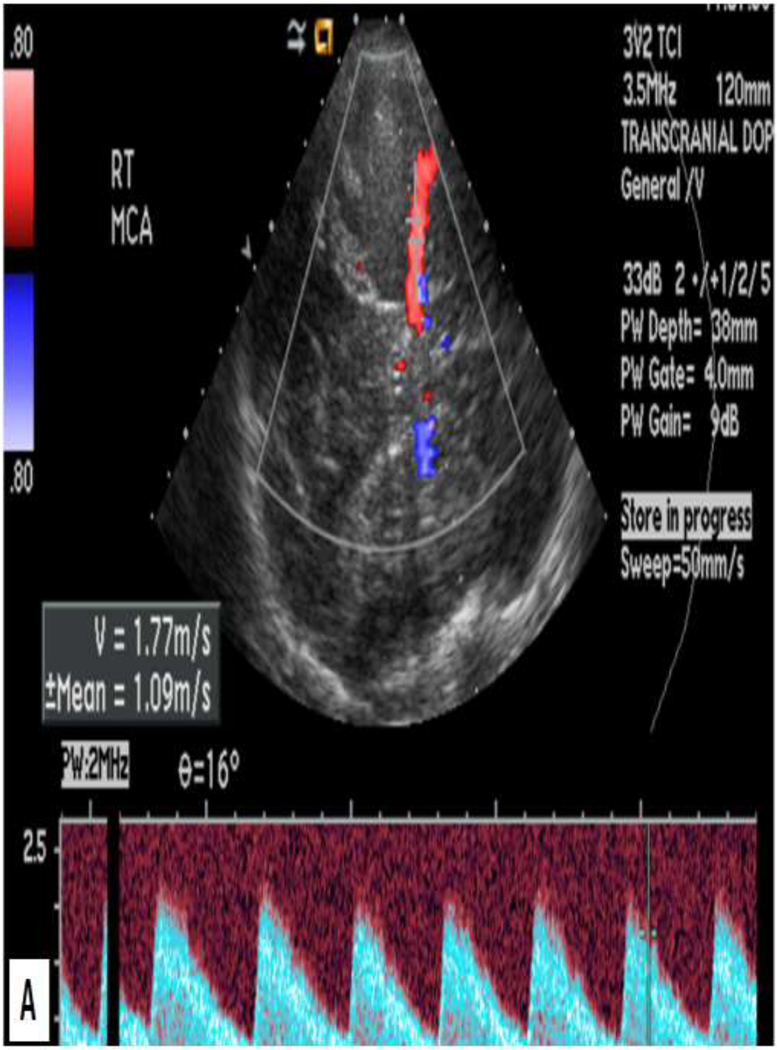
Figure 3:
19 year old female presenting in sickle cell crisis. TCD of the right (A) and left (B) middle cerebral arteries shows abnormally elevated mean flow velocities of 177 cm/s and 189 cm/s, respectively. The patient was treated with blood transfusion to decrease stroke risk.
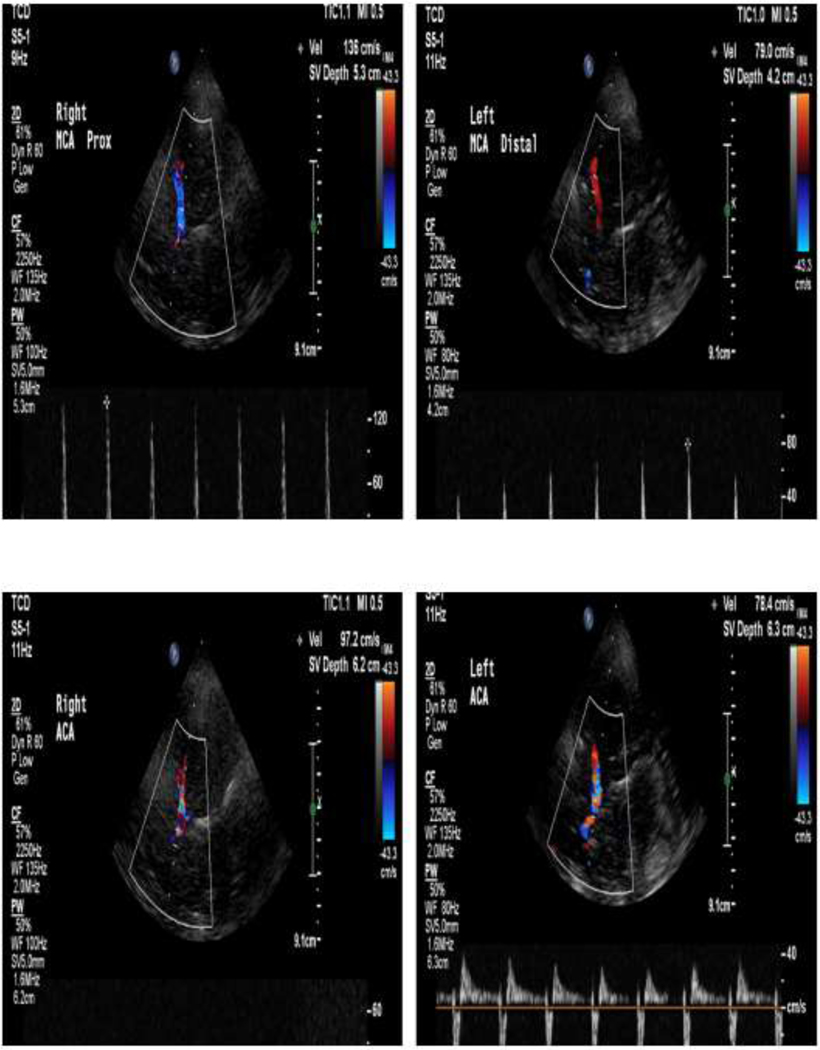
Figure 4:
42 year old patient with anoxic brain injury. TCD of right and left MCA and ACA showing high-resistant, reverberating flow suggesting brain death. However, the posterior circulation is not shown. For conformation of brain death on TCD, both the anterior and posterior circulation must be shown.
Per cms.gov, the following indications constitute a medical necessity for TCD:8
Detection and evaluation of the downstream hemodynamic effects of severe stenosis (≥ 60% diameter reduction) and major basal intracranial arteries (≥50% diameter reduction) or occlusion of the extracranial or major basal intracranial arteries
Detection and serial evaluation of cerebral vasospasm in the setting of subarachnoid hemorrhage
Evaluation of intracranial hemodynamic abnormalities in the setting of suspected brain death
Intraoperative and perioperative monitoring of intracranial flow velocity and hemodynamic patterns during carotid endarterectomy. The professional component is only reimbursed if it is provided during the operative procedure by a physician that is not part of the operating team
Evaluation in the setting of cerebral embolization
Assessment of hemodynamic effects, patterns, and extent of collateral circulation in patients with severe stenosis or occlusion, when used to guide patient care
Assessment of stroke risk in children aged two to sixteen years with homozygous sickle cell disease
In the detection of residual right to left shunting after repair/closure of an intracardiac or intrapulmonary shunt
Multiple cerebrovascular procedures may be allowed during the same patient visit if the physician/provider documents medical necessity in the patient’s medical record.
Carotid duplex ultrasound
The safe and rapid evaluation of patients with extracranial cerebrovascular disease makes CDUS particularly appealing for a wide range of patients and presentations. As a screening tool, CDUS is recommended in asymptomatic patients with known risk factors of cardiovascular disease by multiple societies, including the Society of Interventional Radiology and American Stroke Association.9 If significant carotid disease is present with stenosis of > 20%, follow up screening is recommended (Table 1). Not only is the presence of significant carotid artery stenosis important in screening but also the intima-media thickness (IMT). In recent years, IMT has been described as an important marker for cardiovascular disease and often can be used to reclassify patients in standard risk models. However, its routine use to assess cardiovascular disease is generally not recommended.10
Table 1.
Assessment for vasospasm using TAMAX and Lindegaard ratio:13
| TAMAX (cm/s) | Lindegaard ratio | Interpretation of findings |
|---|---|---|
| <120 | <3 | Normal |
| 120–200 | 3–6 | Mild vasospasm |
| >200 | >6 | Severe vasospasm |
CDUS is also used in the evaluation of symptomatic patients. Patients with a documented CVA or those with a suspected transient ischemic attack (TIA) should undergo CDUS to assess for carotid stenosis.11 This will determine the need for medical therapy, surgery, or endovascular therapy to treat the disease to prevent further events. From a Medicare reimbursement standpoint in the symptomatic patient, strict indications are required (Table 2).
Table 2.
Assessment for vasospasm using the mean flow velocity of the MCA and the Lindegaard ratio.17
| Mean flow velocity (cm/s) | Lindegaard ratio | Interpretation of findings |
|---|---|---|
| <120 | ≤3 | Hyperemia only |
| >80 | 3–4 | Hyperemia plus possible mild |
| spasm | ||
| ≥120 | 3–4 | Hyperemia plus mild spasm |
| ≥120 | 4–5 | Hyperemia plus moderate spasm |
| ≥120 | 5–6 | Moderate spasm |
| ≥180 | 6 | Moderate to severe spasm |
| ≥200 | ≥6 | Severe spasm |
| ≥200 | 4–6 | Hyperemia plus moderate spasm |
| ≥200 | 3–4 | Hyperemia plus mild or residual spasm |
| ≥200 | <3 | Hyperemia only |
In those patients who undergo carotid intervention with carotid endarterectomy (CEA) or carotid artery stenting (CAS) (including transfemoral and transcarotid artery revascularization), routine surveillance is indicated to assess for recurrent stenosis. Following CAS in our practice, follow up CDUS is typically performed at 1 month, 6 months, and yearly thereafter. A similar scheduled is used for following up after CEA.
In addition to carotid stenosis, CDUS is indicated for evaluation of other disease processes. Giant cell arteritis or temporal arteritis is an inflammatory process that affects large and medium-sized vessels in middle-aged and elderly women. CDUS is uniquely positioned to evaluate the temporal arteries and its branches to screen for this pathology.12 This can direct the need for further evaluation, including temporal artery biopsy, or treatment. Although the following are not direct indications for obtaining an exam, incidental findings are often discovered, including, carotid dissection, thrombus, cardiac arrythmias, fibromuscular dysplasia among others. Solid masses in the neck including, lymphadenopathy, salivary and thyroid nodules, glomus tumors, and more may be discovered at the time of CDUS.
Per cms.gov, the following indications constitute a medical necessity for CDUS:
Asymptomatic or symptomatic neck bruits
Amaurosis fugax
- Localized cerebral or ocular transient ischemic attacks (such as):
- sensory loss
- weakness of one side of the face
- slurred speech
- weakness of an extremity
Medical history strongly suggests that syncope is due to vertebrobasilar or bilateral carotid artery disease
Recent neurologic or cerebrovascular event
Prior to major cardiac or vascular surgery when a bruit is noted on physical exam or there is a history of prior neurologic or cerebrovascular event
After carotid endarterectomy or follow-up of previously documented stenoses
Pulsating neck mass
Evaluation of blunt or penetrating neck trauma, including iatrogenic
Ocular microembolism
The following imaging schedule is recommended by cms.gov for reimbursement:8
Stenosis of 20–49% - annual study
Stenosis of 50–79% - study every six months
Stenosis of 80–99% - study every 6 months if surgery is not performed
Post carotid endarterectomy - at 6 weeks, 6 months, and one year. During the first year, follow-up studies should be on the treated side unless signs and symptoms, or previously identified disease is present in the contralateral carotid artery are indications for a bilateral procedure.
Of note if a patient becomes symptomatic repeat duplex scans are allowed outside the above schedule.
Protocol
TCD13
Patient is in position to access acoustic windows.
2-MHz transducer used
Transtemporal window (Figure 5): Evaluate the Circle of Willis with color and spectral Doppler (no angle correction), recording the highest time-averaged maximum velocity (TAMAX = PSV+(EDVx2)/3) and pulsatility index (PI = PSV-EDV)/TAMAX) for each artery: MCA, ACA, terminal ICA, and PCA P1 segment.
Suboccipital window (Figure 6): Evaluate the following with color and spectral Doppler (no angle correction), recording the highest TAMAX and PI for each artery: right vertebral artery, left vertebral artery, and basilar artery.
Transorbital window (Figure 7): Evaluate the following with color and spectral Doppler (no angle correction), recording the highest TAMAX and PI for the ophthalmic artery.
Submandibular window: Evaluate the following with color and spectral Doppler (no angle correction), recording the highest TAMAX and PI for each artery: distal portion of the right carotid artery and distal portion of the left carotid arteries.
Calculate and record the Lindegaard ratio (MCA TAMAX/Distal ICA TAMAX) for both sides.
Calculate and record the Sviri ratio (Sviri ratio = TAMAX of the basilar artery / TAMAX of the extracranial vertebral artery) for both sides.
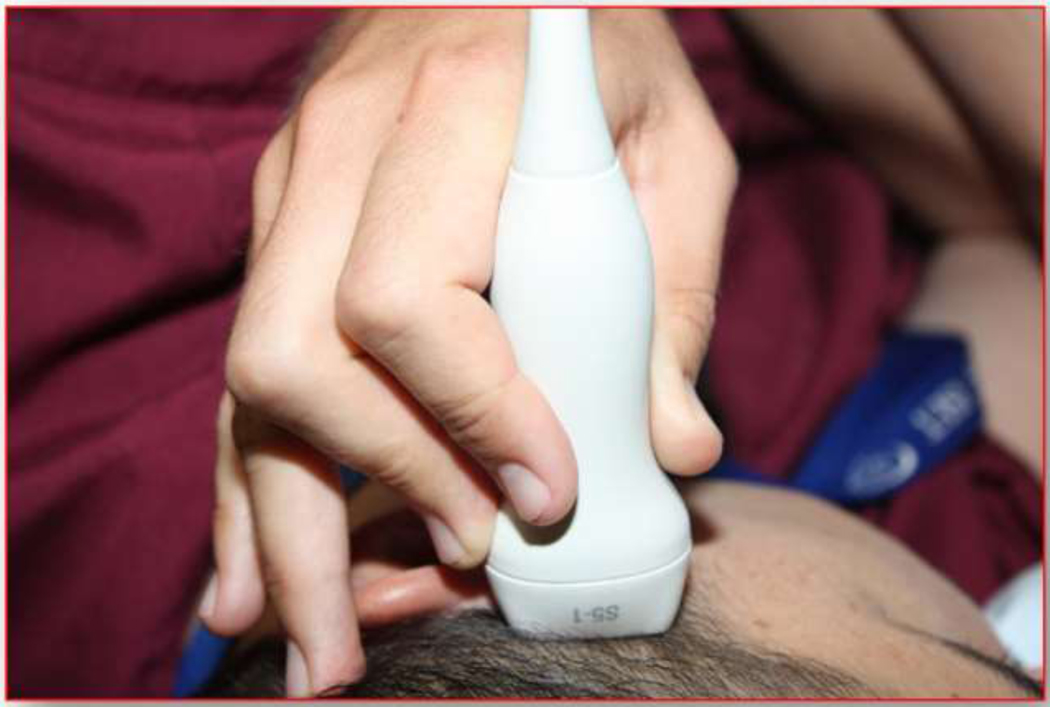
Figure 5.
Photograph of placement of the probe for a transtemporal window.
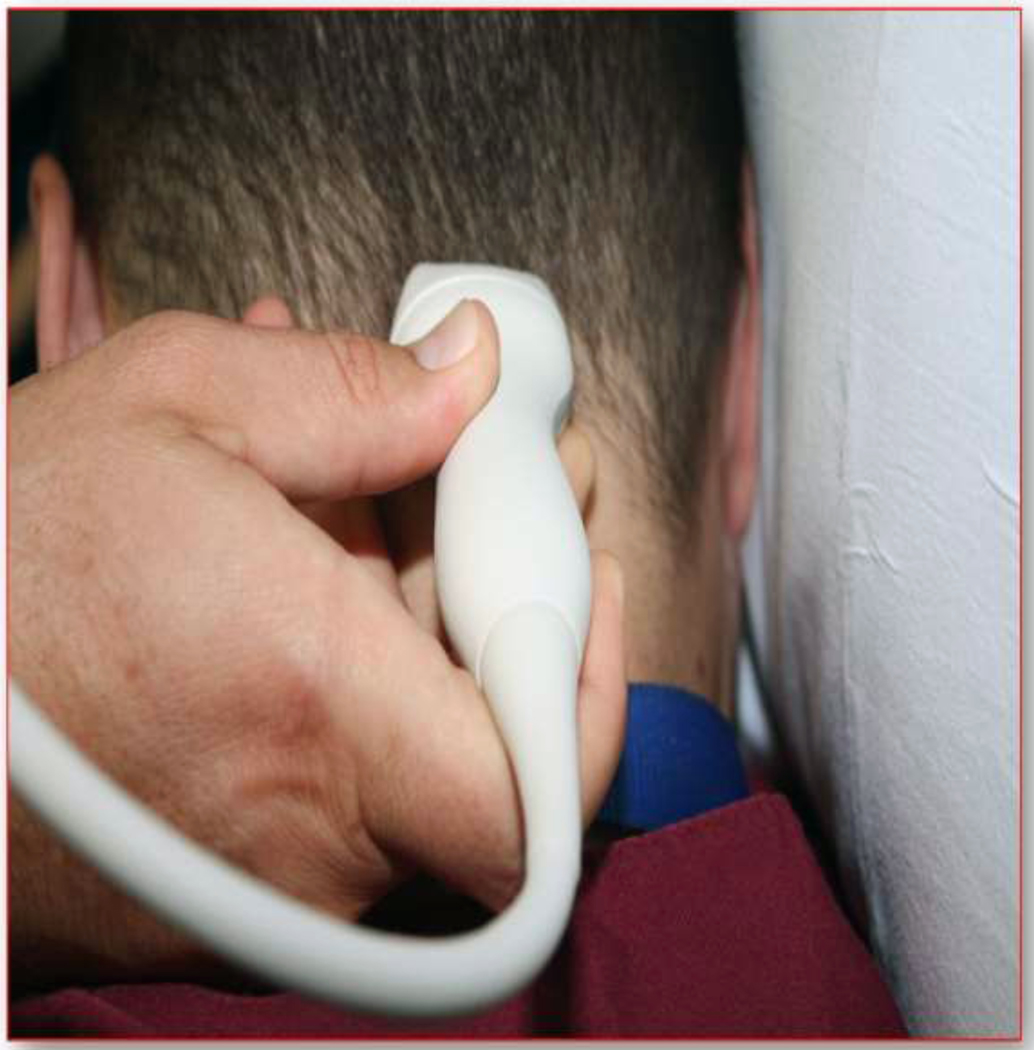
Figure 6.
Photograph of placement of the probe for a suboccipital window.
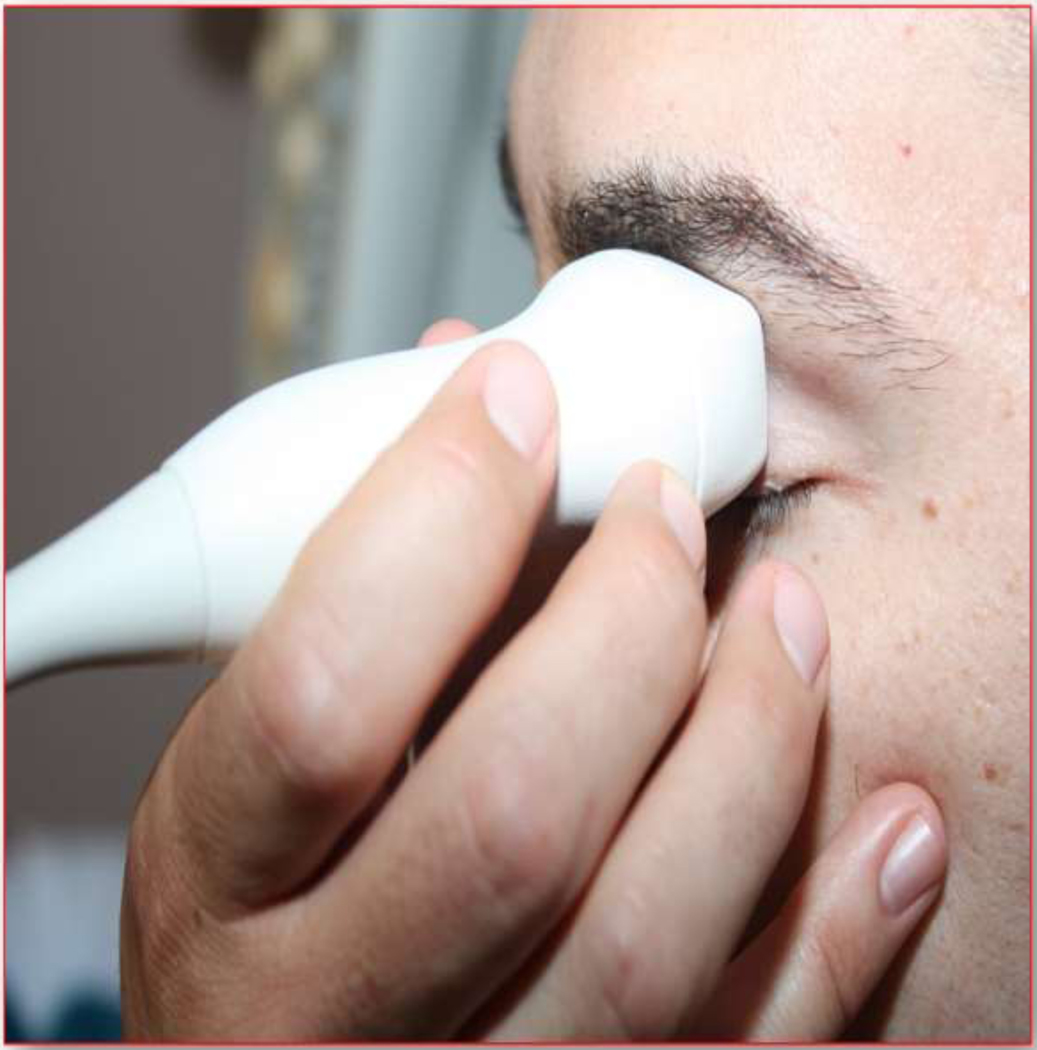
Figure 7.
Photograph of placement of the probe for transorbital window.
Imaging pears and parameters to keep in mind for certain key arteries are the following:14
For the ophthalmic artery:
Acoustic window: Transorbital
Depth: 40–50 mm
Transducer orientation: Slightly medial
Flow direction: Towards the probe
Mean flow velocity: 16–26 cm/s
For the MCA:
Acoustic window: Transtemporal
Depth: 60–75 mm
Transducer orientation: En face
Flow direction: Towards the probe
Mean flow velocity: 46–86 cm/s
For the ACA:
Acoustic window: Transtemporal
Depth: 60–75 mm
Transducer orientation: Anterior
Flow direction: Away from the probe
Mean flow velocity: 41–76 cm/s
For the PCA:
Acoustic window: Transtemporal
Depth: 60–75 mm
Transducer orientation: Posterior
Flow direction: Towards the probe
Mean flow velocity: 33–64 cm/s
For the vertebral artery:
Acoustic window: Suboccipital
Depth: 45–75 mm
Transducer orientation: Superior and oblique
Flow direction: Away from the probe
Mean flow velocity: 27–55 cm/s
For the basilar artery:
Acoustic window: Transtemporal
Depth: 70–120 mm
Transducer orientation: Superior
Flow direction: Away from the probe
Mean flow velocity: 30–57 cm/s
Carotid duplex ultrasound
Patient is supine, with patient head slightly hyper extended and rotated away from the side being examined
9 MHz Linear transducer is used. Use lowest possible power settings.
- Common carotid artery:
- Grayscale: note the presence and characteristics of plaques – smooth, homogeneous, heterogeneous, calcification. Anterior, posterior, and lateral view of the carotid should be imaged to improve visualization in the shadow of a calcified plaque
- Color: note any disturbance of flow.
- Spectral waveform: 60 degree or less Doppler angle correction parallel to the flow jet or the vessel wall
- Areas of suspected stenosis are evaluated in the region proximal to the stenosis, at the point of maximum stenosis and distal to the stenosis. Peak systole and end diastole velocity is measured in the internal carotid artery using a 60 degree or less Doppler angle correction parallel to the flow jet or the vessel wall.
- Record representative measurement of the PSV and EDV in the proximal, middle, and distal potions of the common carotid artery
Internal carotid artery: Repeat above for the proximal, mid, and distal potions of the internal carotid artery.
External carotid artery: Repeat above for the external carotid artery. Temporal tapping of the ipsilateral superficial temporal artery may be used for vessel identification
Measure the Intima-media thickness of the ICA
Calculate the ICA/CCA PSV ratio.
Vertebral artery: Color and spectral waveform are used to evaluate the vertebral artery – to assess the presence or absence of flow, the direction of flow direction as well as spectral Doppler waveforms morphology. Subclavian artery evaluation is performed if there is reversal of flow in the vertebral artery.
Diagnostic criteria
TCD
Patient is imaged every day, at the same time every day. Day 2–5 after subarachnoid hemorrhage (SAH), one is looking to detect the development of vasospasm before it is clinically evident to escalate hemodynamic management as needed. Day 5–12 after SAH patient is imaged to detect progression of vasospasm and to plan interventions such as angioplasty or intra-arterial drug therapy. Day 12 till the end of the ICU course, patient is imaged for resolution of vasospasm after treatment or intervention, sustainability of patency of arteries, and for late or rebound vasospasm.15
When the patient is scanned, mean flow velocities are obtained. Mean flow velocity of <120 cm/s in the MCA has a high negative predictive value for vasospasm and ≥200 cm/s in the MCA has a high specificity for vasospasm.16 The acquired mean flow velocity and calculated TAMAX, pulsatility index, Lindegaard ratio, and Sviri ratio are used to assess for vasospasm. Calculations are performed as follows:
The time-averaged maximum velocity (TAMAX) also known as the time-averaged peak (TAP) flow velocities is calculated using peak systolic velocity (PSV) and end diastolic velocity (EDV) is calculated as follows: TAP = PSV+(EDVx2)/3
The pulsatility index (PI) is calculated as follows: PI = (PSV-EDV) / TAMAX.
The Lindegaard ratio is calculated by dividing the MCA TAMAX flow velocity (TAMAXMCA) by the extracranial (most distal portion of the submandibular) internal carotid artery (TAMAXEC ICA) TAP flow velocity as follows: Lindegaard ratio = TAMAXMCA/TAMAXEC ICA
The Sviri ratio is calculated by dividing the basilar TAMAX flow velocity (TAMAXBA) by the extracranial vertebral artery TAMAX flow velocity (TAMAXEC VA) as follows: Sviri ratio = TAMAXBA / TAMAXEC VA
Table 1, 2, and 3 describe how mean flow velocity, TAMAX, Lindegaard ratio, and Sviri ratio are used in vasospasm assessment. Increased PI has been associated with elevated intracranial pressure and can be followed with each TCD study to alert the clinician of any possible changes of intracranial pressure.15
Table 3.
Assessment for vasospasm using the mean flow velocity in the basilar artery and the Sviri ratio.17
| Mean flow velocity (cm/s) | Sviri ratio | Interpretation of findings |
|---|---|---|
| >70 | > 2 | Vasospasm |
| >85 | >2.5 | Moderate or severe vasospasm |
| >85 | >3 | Severe vasospasm |
Carotid ultrasound
There has been considerable variation in criteria used to interpret the degree of ICA stenosis in carotid duplex ultrasound. To reduce the variability, the Intersocietal Accreditation Commission (IAC) recommended IAC-accredited labs to use the guidelines set by the Society of Radiologists in Ultrasound (SRU), and after a period, compared results of the CDUS using the SRU to that of contrast angiography. They found that the SRU guideline PSV threshold of 125cm/sec for 50% stenosis was overly sensitive and had inadequate specific and accuracy, supporting the adoption of increasing the PSV threshold of 180 cm/s.18 Therefore, the IAC now recommends the modified criteria to diagnosis ICA stenosis seen in Table 4.19
Table 4.
Modified SRU guidelines for the assessment of carotid artery stenosis.
| Degree of stenosis, % | ICA PSV, cm/s | Plaque estimate, % | ICA/CCA PSV ratio |
ICA EDV, cm/s |
|---|---|---|---|---|
| Normal | <180 | None | <2 | <40 |
| <50 | <180 | <50 | <2 | <40 |
| 50–69 | 180–230 | >50 | 2–4 | 40–100 |
| >70 | >230 | >50 | >4 | >100 |
| Near occlusion | High, low, or undetectable | Visible | Variable | Variable |
| Total occlusion | Undetectable | Visible, no detectable lumen | Not applicable | Not applicable |
Diagnostic criteria at a glance
TCD
| Degree of MCA vasospasm | Mean flow velocity (cm/s) | Lindegaard ratio |
|---|---|---|
| Mild | 120–149 | 3–6 |
| Moderate | 150–199 | 3–6 |
| Severe | >200 | >6 |
| Degree of basilar artery vasospasm | Mean flow velocity (cm/s) | Sviri ratio |
| Vasospasm present | >70 | >2 |
| Moderate or severe | >85 | >2.5 |
| Severe | >85 | >3 |
Carotids
| Degree of stenosis, % | ICA PSV, cm/s | Plaque estimate, % | ICA/CCA PSV ratio |
ICA EDV, cm/s |
|---|---|---|---|---|
| Normal | <180 | None | <2 | <40 |
| <50 | <180 | <50 | <2 | <40 |
| 50–69 | 180–230 | >50 | 2–4 | 40–100 |
| >70 | >230 | >50 | >4 | >100 |
| Near occlusion | High, low, or undetectable | Visible | Variable | Variable |
| Total occlusion | Undetectable | Visible, no detectable lumen | Not applicable | Not applicable |
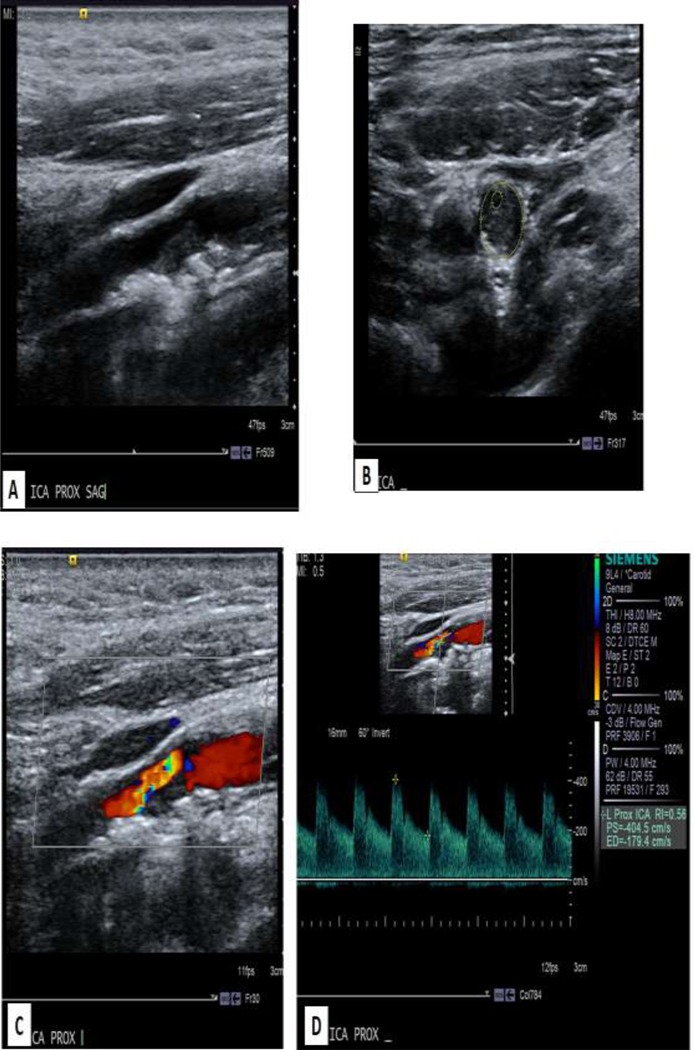
Figure 8:
62 year old male with history of head and neck cancer status post radiation with surveillance ultrasound. A demonstrates longitudinal gray scale with narrowing due to atherosclerotic plaque. B demonstrates transverse view with measurement of the small lumen. C. Color image demonstrates narrowing with aliasing. D. Spectral waveform demonstrates a PSV of 404.5 cm/s. In comparison, the common carotid artery PSV was 101.1 cm/s (not shown). Therefore, the ICA stenosis is significant.
Footnotes
Brian Schiro
Disclosures:
Medtronic – Speaker
Cook - Speaker
Penumbra – Speaker
Philips - Consultant
Muhammad Hasan
No Disclosures
Yolanda Bryce
Disclosures:
Hologic – Consultant
Pfizer – Speaker
Boston Scientific – Speaker
Costantino Peña
Disclosures:
Philips Healthcare – Consultant
Shockwave Medical – Speaker
Penumbra – Speaker
BD Bard – Speaker
Cordis – Speaker
Cook Medical - Speaker
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brian Schiro, Miami Cardiac and Vascular Institute, 8950 N Kendall Dr Suite 607W Miami, FL 33176.
Muhammad Hasan, Miami Cardiac and Vascular Institute, 8950 N Kendall Dr Suite 607W, Miami, FL 33176.
Yolanda Bryce, Memorial Sloan Kettering Cancer Center, 1275 York Avenue New York, NY 10065.
Costantino Peña, Miami Cardiac and Vascular Institute, 8950 N Kendall Dr Suite 607W, Miami, FL 33176.
References
- 1.Macdonald RL, Hunsche E, Schuler R, Wlodarczyk J, Mayer SA. Quality of life and healthcare resource use associated with angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Stroke 2012;43(4):1082–8. DOI: 10.1161/STROKEAHA.111.634071. [DOI] [PubMed] [Google Scholar]
- 2.Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke 2007;38(8):2315–21. DOI: 10.1161/STROKEAHA.107.484360. [DOI] [PubMed] [Google Scholar]
- 3.Suarez JI. Diagnosis and Management of Subarachnoid Hemorrhage. Continuum (Minneap Minn) 2015;21(5 Neurocritical Care):1263–87. DOI: 10.1212/CON.0000000000000217 [DOI] [PubMed] [Google Scholar]
- 4.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022;145(8):e153–e639. DOI: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 5.Mahmoud AN, Elgendy IY, Agarwal N, Tobis JM, Mojadidi MK. Identification and Quantification of Patent Foramen Ovale-Mediated Shunts: Echocardiography and Transcranial Doppler. Interv Cardiol Clin 2017;6(4):495–504. DOI: 10.1016/j.iccl.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 6.Lee MT, Piomelli S, Granger S, et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood 2006;108(3):847–52. DOI: 10.1182/blood-2005-10-009506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wijdicks EFM. The Diagnosis of Brain Death. New England Journal of Medicine 2001;344(16):1215–1221. DOI: 10.1056/nejm200104193441606. [DOI] [PubMed] [Google Scholar]
- 8.https://www.cms.gov/medicare-coverage-database/, accessed July 27, 2022
- 9.Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation 2011;124(4):489–532. DOI: 10.1161/CIR.0b013e31820d8d78 [DOI] [PubMed] [Google Scholar]
- 10.Willeit P, Tschiderer L, Allara E, et al. Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk: Meta-Analysis of 119 Clinical Trials Involving 100 667 Patients. Circulation 2020;142(7):621–642. DOI: 10.1161/CIRCULATIONAHA.120.046361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009;40(6):2276–93. DOI: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 12.Jianu DC, Jianu SN, Dan TF, et al. Ultrasound Technologies and the Diagnosis of Giant Cell Arteritis. Biomedicines 2021;9(12). DOI: 10.3390/biomedicines9121801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. https://www.gundersenhealth.org/app/files/public/3ae770e1-a5a5-42b4-84ce-0ac23a194e77/TCD-for-Vasospasm-with-worksheet.pdf .
- 14.Kirsch JD, Mathur M, Johnson MH, Gowthaman G, Scoutt LM. Advances in transcranial Doppler US: imaging ahead. Radiographics 2013;33(1):E1–E14. DOI: 10.1148/rg.331125071. [DOI] [PubMed] [Google Scholar]
- 15.Samagh N, Bhagat H, Jangra K. Monitoring cerebral vasospasm: How much can we rely on transcranial Doppler. J Anaesthesiol Clin Pharmacol. 2019. Jan-Mar;35(1):12–18. doi: 10.4103/joacp.JOACP_192_17. PMID: 31057233; PMCID: PMC6495622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vora YY, Suarez-Almazor M, Steinke DE, Martin ML, Findlay JM. Role of transcranial Doppler monitoring in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1999;44:1237–47 [PubMed] [Google Scholar]
- 17.Kumar G, Alexandrov AV. Vasospasm Surveillance With Transcranial Doppler Sonography in Subarachnoid Hemorrhage. J Ultrasound Med 2015;34(8):1345–50. DOI: 10.7863/ultra.34.8.1345. [DOI] [PubMed] [Google Scholar]
- 18.Gornik HL, Rundek T. Gardener H, et al. Optimization of duplex velocity criteria for diagnosis of internal carotid artery (ICA) stenosis: A report of the Intersocietal Accreditation Commission (IAC) Vascular Testing Division Carotid Diagnostic Criteria Committee. Vascular Medicine 2021;e-pubilshed ahead of print. Available for free download at: https://journals.sagepub.com/doi/full/10.1177/1358863X211011253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leers SA. Improving Quality in Noninvasive Testing by Certification and Accreditation. InNoninvasive Vascular Diagnosis: A Practical Textbook for Clinicians 2022. Feb 24 (pp. 3–16). Cham: Springer International Publishing [Google Scholar]