Abstract
The role of the composition of the gut microbiota on human health is not well understood. However, during the past decade, an increased emphasis has been placed on the influence of the impact of nutrition on the composition of gut microbiota and how the gut microbiota affects human health. The current review focuses on the role of some of the most studied phytochemicals on the composition of the gut microbiota. First, the review highlights the state of the research evidence regarding dietary phytochemical consumption and gut microbiota composition, including the influence of phytochemicals such as polyphenols, glucosinolates, flavonoids, and sterols that are present in vegetables, nuts, beans, and other foods. Second, the review identifies changes in health outcomes with altered gut microbiota composition, in both animal and human model studies. Third, the review highlights research that includes both associations between dietary phytochemical consumption and gut microbiota composition, and associations between the gut microbiota composition and health outcomes, in order to elucidate the role of the gut microbiota in the relationship between dietary phytochemical consumption and health outcomes in humans and animals. The current review indicated that phytochemicals can beneficially alter gut microbiota composition and decrease the risk for some diseases, such as cancers, and improve some cardiovascular and metabolic risk biomarkers. There is an urgent demand for high-quality studies that determine the relationships between the consumption of phytochemicals and health outcomes, examining gut microbiota as a moderator or mediator.
Keywords: gut microbiota, phytochemicals, phenolics, 16S rRNA, bacteria
INTRODUCTION
The associations between health-promoting human dietary patterns, a healthy gut, and chronic disease prevention are well documented [1]. One such health-promoting dietary pattern is a plant-based diet, which has recently gained popularity [2]. Plants, including fruits and vegetables, provide primary and secondary metabolites. Primary metabolites are amino acids, enzymes, coenzymes, and nucleosides [3]. Secondary metabolites, also known as phytochemicals, are the result of several chemical pathways such as the Shikimik Pathway, also known as the phenylpropanoid pathway, and are derived from the transformation of primary metabolites. This transformation process occurs under environmental stresses such as ultraviolet (UV) radiation, microbial infections, mechanical wounding, and chemical stressors such as herbicides [4]. Phytochemicals are essential in defensive roles for plants, such as resistance to diseases (viruses, fungi, bacteria, and insects), herbivores (insects and mammals), plant competitors, and abiotic stress (e.g. UV radiation, heat, tissue damage, etc.) [5].
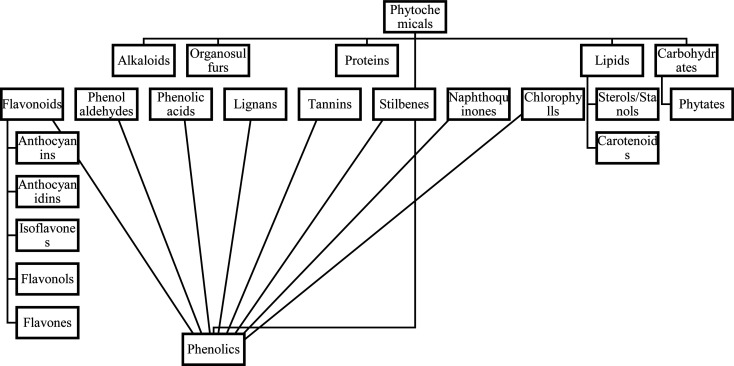
Phytochemicals are comprised of six main groups which are shown in Fig. 1. The largest group of phytochemicals, phenolics, consists of six subgroups which are flavonoids (comprised of eight subgroups), phenolic acids (for example curcumin), stilbenes (for example resveratrol), catechins, and lignans [6]. Phytochemicals are used for medicinal, nutritive, and cosmetic purposes, and humans can benefit either directly or indirectly from the consumption of phytochemicals found in fruits and vegetables. Examples of indirect uses of phytochemicals include food additives, flavors, medicines, and industrially important medications containing phytochemicals [7].
Fig. 1.
Organization of phytochemical classifications [91].
Since phytochemicals are metabolized by the body as xenobiotics, their bioavailability tends to be low [8]. Following consumption, only 5–10% of phytochemicals are absorbed into the bloodstream through the small intestine and may be further metabolized in the liver. The remaining 90–95% of these compounds reach the colon, where the colonic microbiota modify the structural composition into absorbable metabolites via hydrolysis, reduction, dihydroxylation, demethylation, decarboxylation, and ring fission [9]. For instance, polyphenols that are not absorbed in the small intestine make it to the colon, where they are significantly structurally altered. The intestinal bacteria actively hydrolyze glycosides into aglycones, and degrade them to simple phenolic acids [10]. Since the intestinal microbiota produces active metabolites, this activity is central to the biological action of polyphenols. As an example, daidzein is transformed in the large intestine to produce the active metabolite equol [11, 12].
Associations between the gut microbiota, dietary intake, and health outcomes need to be elucidated. Gut microbiota composition analysis categorizes diversity into two types, alpha and beta diversity. The alpha type is a measurement of microbiome diversity in a single sample, and the beta type is a measurement of diversity between two communities [13]. Eukaryotic organisms, viruses, bacteria, and archaea make up the human microbiome [14]. The microbiota is a term for the group of bacteria; genes, along with the microbiota, comprise the microbiome [15]. The gut microbiome varies greatly between people, as genetics, diet, physical activity, medical interventions, and other factors affect the diversity and quantity of the microbiome. Some gut bacterial genome diversity has been determined via fecal sampling methods, and in fact, the most used method for analyzing the microbiome is stool sampling, though this method does not fully capture α and β diversity of gut microbiomes, especially in the small intestine [16]. Other than stool sampling, the primary method of analysis is polymerase chain reaction (PCR) [17]. In PCR, 16S rRNA is used to amplify the variants of bacteria in the gut microbiome [18].
In healthy humans, the gut microbiota is likely to be dominated by members of the phyla Bacteroidetes and Firmicutes; in contrast, in those with poor health, the gut microbiota may be dominated by bacterial phyla such as Proteobacteria (which contains Escherichia coli), Verrucomicrobia, Actinobacteria, or Fusobacteria. However, the interpretation of a healthy or unhealthy gut is complex, as the composition of bacteria varies within and between individuals [19]. For example, one previous study concluded that the rapid effects of vegan and carnivore diets on gut microbiome composition were evident in both alpha and beta diversity [20].
Continuous imbalance of the microbial composition of the gut is called dysbiosis, which is correlated with obesity and diabetes [15], inflammatory bowel disease [21], alcoholic and nonalcoholic fatty liver disease [22], and hepatocellular carcinoma [23]. Physical and health status can also influence gut microbiota composition, and in addition, the immune system is thought to affect gut microbiota composition. It has become obvious that gut microbiota dysbiosis is linked to a wide range of human diseases and ailments [24]. The complex association between the gut microbiota and the human mucosal immune system helps to attain and maintain immunological homeostasis. This reciprocal system makes determining causality quite challenging. Studies of the relationship between diet and microbiome composition date back more than a century ago [25]. Through technological advances, researchers have observed that diet can affect intestinal gas production and bacterial metabolism [26]. The gut microbiota can break down some dietary indigestible fibers, producing short-chain fatty acids (SFCAs) as a potential energy source, and these SCFAs are associated with decreased risk of some diseases such as colon cancer [27]. All of the associations mentioned thus far have been primarily observed in non-human animals, mainly rodents; however, there is ongoing research in humans [28]. Recent studies have shown that SCFAs produced by gut, might be correlated with diet–gut–brain–behavior interactions, which could prevent or mitigate cravings for unhealthful foods [29]. Moreover, another study showed an association between SCFA produced by intestinal gut microbiota, and blood pressure regulation [30]. With this background in mind, the primary goal of this review was to examine the potential importance of consuming active phytochemicals for beneficially altering gut microbiota composition and to better understand what the state of the current literature regarding whether the gut microbiota serves as an independent health outcome/therapeutic target, or as a mediator or moderator in the relationship between phytochemical consumption and subsequent health outcomes. This review explores the observational and clinical studies which have discussed the roles of phytochemical consumption after reaching the large intestine, on gut microbiota composition, and plausible resultant health outcomes. Therefore, this review comprises five parts based on the included categories of phytochemicals. In each category, the associations between phytochemical consumption, changes in gut microbiota composition, and associated health outcomes, both in human and animal studies are included when both are available.
LITERATURE SEARCH
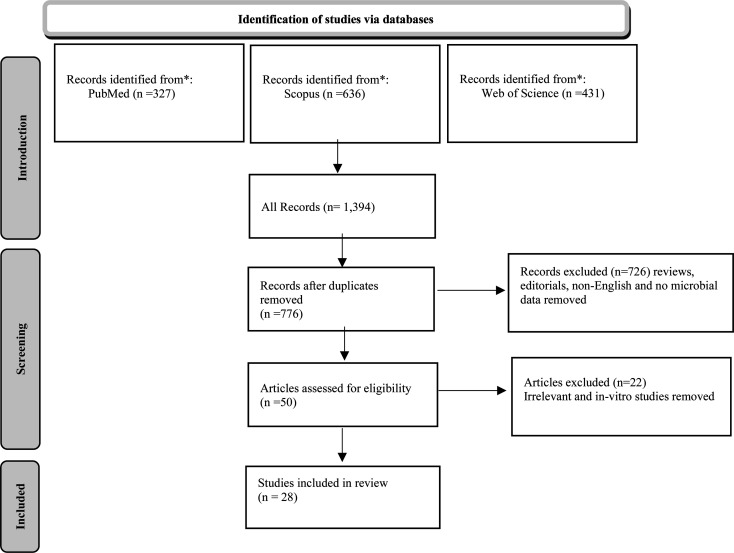
In order to examine the peer-reviewed research available on the interactions between phytochemicals and gut microbiota composition, a comprehensive literature search was conducted in PubMed and SCOPUS until March 2022. The following keywords were used: Gastrointestinal Microbiomes, Microbiome, Gut Microbiotas, Gastrointestinal Flora, Gastrointestinal Microbiota, Gastrointestinal Microbial Community, Gastric Microbiome, Intestinal Microbiome, Intestinal Microbiota, Intestinal Microflora, Enteric Bacteria, Dietary Phytochemical, Plant Bioactive Compound, Plant-Derived Chemical, and Phytonutrient. Boolean Operators “AND”, “OR” were used to combine keywords. All observational and experimental studies of human and animal participants that included phytochemical consumption, gut microbiota composition, and measured health-related outcomes, were included. Reviews, editorials, letters, and articles not written in English, were excluded. A PRISMA flow diagram of the study selection process is shown in Fig. 2.
Fig. 2.
PRISMA flow diagram of study selection process.
ASSOCIATIONS BETWEEN CONSUMPTION OF FLAVONOIDS, GUT MICROBIOTA COMPOSITION, AND HEALTH OUTCOMES
Flavonoids in general
Human studies
One of the subgroups of polyphenols, flavonoids, which consists of 5 other subgroups, was used to determine associations with gut microbiota changes. Li and Somerset studied adults with cystic fibrosis (CF) using a flavonoid-specific food frequency questionnaire (FFQ) to estimate participants’ flavonoid intake. This study showed that flavonoid intake was correlated with an increase in Actinomyces, and a reduction in class Coriobacteriia [31]. Since the gut microbiota composition of patients with CF comprises more Actinobacteria as compared with patients without CF, it is possible that flavonoids, especially gallocatechin in black tea, might decrease colorectal cancer (CRC) and lung cancer in CF patients by modifying gut microbiota composition (decreased Coriobacteriia) [32]. However, considering the small sample size, the study design, and data collection methods, these effects are only speculative and require more investigation in future studies.
Isoflavones
Human studies
Legumes, soybeans, green beans, and mung beans are abundant in isoflavones including daidzin, the glycoside form of daidzein. Equol, produced by the metabolic conversion of daidzein by the gut bacteria, is an isoflavandiol nonsteroidal estrogen [33]. About one third to one half of humans (depending on the community) can generate equol, as compared with non-human animal species which are all capable of this conversion [34]. Equol production has been reported as 40–70% among Asian populations versus 20–30% Western populations [35]. Phytoestrogens are known to have hormonal effects on animals and cell culture systems. In premenopausal women, where estrogen levels are high, phytoestrogen consumption may reduce endogenous estrogen levels and activity. In postmenopausal women, when estrogen levels are low, phytoestrogens may operate as estrogen agonists. Given the numerous health benefits associated with higher estrogen levels generally, hormonal impacts may account for positive epidemiological associations in populations consuming soy, where a lower risk of chronic diseases and menopausal symptoms have been shown [36,37,38]. However, whether these health benefits are modulated by the gut microbiome, is unknown. Recently, with the advancement of new research techniques, more studies have been conducted in an attempt to determine the role of the gut microbiome following isoflavonoid consumption. For example, a cross-sectional study demonstrated associations between isoflavonoids (genistein and equol) and changes in lipid profiles as well as gut microbiome composition changes. Results indicated a negative association for Haemophilus parainfluenzae and a positive association for Klebsiella pneumoniae and Lachnospiraceae bacterium-8_1_57FAA with total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and apolipoprotein B (ApoB). Further, a higher relative abundance of H. parainfluenzae, and lower levels of K. pneumoniae and Lachnospiraceae bacterium-8_1_57FAA were shown in participants who were able to produce equol as compared to non-producers. These results suggest that microbial equol synthesis may play a role in the variations in blood lipid levels following consumption of isoflavonoids [39]. In addition, a clinical trial in menopausal women showed a strong positive association between consumption of soy and prevalence of the genus Bifidobacterium and Rothia in equol producers [40]. However, a 6-month trial in menopausal women showed no associations between equol levels and lipid profiles both in producers and non-producers, despite alterations in gut microbiome composition [41]. Also, a randomized clinical trial has shown that soy isoflavone group had significantly better scores on cognitive tests compared to the placebo group, indicating that soy isoflavones may have a positive effect on cognitive function in patients with Alzheimer’s disease [42]. Moreover, a cross-sectional study showed that equol producers had significantly better scores on the Mini-Mental State Examination (MMSE) compared to non-producers, indicating a positive association between equol production and cognitive function in older adults [43].
Genistein
Animal studies
Genistein is the primary isoflavonoid found in soy, which was included in three separate studies. First, it was studied to assess the effects on gut microbiota composition, metabolic endotoxemia, and cognitive performance in mice given a high-fat diet. Genistein altered gut microbiota composition by increasing the genus Prevotella and Akkermansia, which were associated with lower lipopolysaccharides (LPS), and decreased metabolic endotoxemia. A reduction in LPS and endotoxemia correlated with a decrease in neuroinflammation and improvements in cognitive function [44]. Genistein can stimulate the oxidation of fat in subcutaneous adipose tissue and decrease fat formation through unidentified mechanisms [45]. Moreover, these findings are consistent with earlier research, which showed that a rise in Akkermansia muciniphila strengthened the gut barrier by thickening the mucous layer and reducing the influx of LPS. Together, by reducing inflammation, these changes may lessen metabolic abnormalities in lipid and carbohydrate metabolism [46]. Second, genistein was studied to determine effects on gut microbiota alterations and adverse effects of chemotherapy, by transplanting feces to mice from human participants with breast cancer, who were receiving chemotherapy. Patients had their feces collected before and after chemotherapy in order to transplant to mice. Each patient’s feces were transplanted to four mice, and the mice were then given a corn oil diet without genistein (control), or a corn oil diet with genistein (intervention). Feces were collected each month and sequencing was performed using 16S rRNA. By transplanting breast cancer patients’ feces, researchers were able to successfully humanize germ-free mice. Genistein decreased members of the genus Paraprevotella, Anaerostipes, and Bacteroides, and increased members of genus Lactococcus, Akkermansia, and Eubacterium before tumor induction. These alterations, in turn, increased SCFA production. However, after tumor induction, gut bacteria diversity was reduced [47]. Third, genistein was found to exacerbate type 1 diabetes, solely in female offspring. It was hypothesized that in female offspring, genistein could down-regulate some pro-inflammatory genes such as IL-10 and IgM, which are assumed to contribute to gut dysbiosis and increases in Enterobacteriales. The rise in Enterobacteriales were associated with increased inflammation and worsened type 1 diabetes symptoms in female mice. However, in male offspring, genistein had the opposite effect by decreasing inflammation through a reduction in T cells [48].
Flavanols
The most commonly studied flavanols include quercetin and kaempferol.
Quercetins
Human studies
Quercetin is consumed through foods or supplements and has been shown to have beneficial effects on health outcomes. A randomized clinical trial in polycystic ovary syndrome (PCOS) patients showed that quercetin supplementation increased adiponectin while decreasing homeostasis model assessment-estimated insulin resistance (HOMA-IR), testosterone, luteinizing hormone (LH), fasting blood sugar (FBS), and insulin. Therefore, quercetin may be a useful supplement for PCOS, as indicated by these reported effects on adiponectin levels [49]. In healthy males, previous research has indicated that after quercetin supplementation, plasma concentrations of uric acid decreased, without inducing changes in body mass index (BMI) [50]. However, the underlying mechanisms for these beneficial health effects in humans require further research, and the role of the gut microbiome needs to be clarified.
Animal studies
Quercetin is transformed to metabolites such as 3,4-dihydroxyphenylacetic acid by Bacteroides fragilis, Clostridium perfringens, Eubacterium ramulus, Streptococcus S-2, Lactobacillus L-2, Bifidobacterium B 9, and Bacteroides JY-6 after digestion in the gut [51,52,53]. These metabolites are shown to prevent cholesterol-induced mitochondrial dysfunction and cholesterol-impaired insulin secretion in pancreatic β-cells [54]. Studies have shown that quercetins alter gut microbiome composition by increasing α diversity, which is an indication of a healthy gut and stable intestinal ecosystem [55]. There was a reduction in the abundance of Verrocomicrobia and an increase in microbiome diversity and the abundances of Actinobacteria, Cyanobacteria, and Firmicutes. Some modifications in gut microbiota composition were associated with health biomarkers. For example, Actinobacteria correlated negatively with intestinal cholesterol and positively with coprostanol. Also, Firmicutes correlated negatively with cholesterol. Together, these findings showed that quercetin affects the pro-inflammatory cytokines production and atherogenic lipid metabolites, which is correlated to a rise in the number of Actinobacteria while a reduction in the number of Firmicutes. Therefore, quercetin might have beneficial effects on atherosclerotic diseases and oxidative stress, which are likely to be due to the modulation of indicators of inflammation like IL-6 and malondialdehyde (MDA) [56]. Quercetin was also studied to determine its effects on nonalcoholic fatty liver disease (NAFLD) development through gut microbiota modulation. Mice were grouped into two different sets of donors and receivers of fecal microbiota transplantation. Gut microbiota donors were selected based on obesity status and NAFLD-related biomarkers, after consuming a high-fat diet and quercetin. Fecal transplantation recipients were selected to show metabolic and variations in phenotype caused by interactions between quercetin, diets, and transplanted microbiota. The details of the within-group categories are listed in Table 1. The findings showed that in the high-fat diet fed receiver groups, the Akkermansia genus was found to be positively associated with butyrate production (p-value=0.01) and negatively associated with body weight (p-value=0.001), HOMA-IR (p-value=0.01), NAFLD Activity Score (p-value=0.001), and inflammasome activation (p-value=0.05). There was a notable increase in the Akkermansia genus when transferring from the donor to the receiver mice in the high-fat diet fed. The Akkermensia genus potentially plays a crucial part in developing protective metabolic phenotypes against NAFLD [57]. Moreover, the associations between gut microbiota composition, trans-resveratrol, and quercetin in high-fat, sucrose diet-fed rats, were assessed with the aim of controlling gut microbiota dysbiosis and enhancing gut health. Mainly quercetin was found to alter gut microbiota composition, and these changes are reported in detail in Tables 2, 3. For instance, quercetin increased Bacteroides vulgatus and A. muciniphila, which are inversely associated with obesity. Quercetin also reduced Eubacterium cylindroides and Bilophila wadsworthia which are correlated with obesity caused by diet. Quercetin alone or in combination with resveratrol decreased insulin resistance and HOMA-IR. The processes through which these decreases occur are not clear [58]. Quercetin elevated gut microbial diversity when vitamin D levels were low (including Facklamia and Aerococcus) and decreased Aβ plaques, tau phosphorylation, neuroinflammation, and miR-26a and miR-125b, in the hippocampus and cortex. These alterations may play a role in improving cognition in rats; however, the mechanisms require more research [59].
Table 1. Summary of included studies.
| Phytochemicals | Study | Animal or human | Changes in gut | Health outcome | Determination of associations between gut |
|---|---|---|---|---|---|
| participants | microbiota composition | assessment | microbiota changes and health outcomes | ||
| Phytochemicals | [80] | Human | ✓ | ✗ | ✗ |
| Lipid phytochemicals | [83] | Human | ✓ | ✓ | ✗ |
| (Phytosterols) | [85] | Human | ✗ | ✓ | ✗ |
| [86] | Human | ✓ | ✓ | ✗ | |
| [87] | Human | ✓ | ✓ | ✗ | |
| Protein phytochemical | [82] | Human | ✓ | ✓ | ✓ |
| (Glucosinolate) | |||||
| Polyphenols | [71] | Human | ✓ | ✓ | ✓ |
| Polyphenols (Lignan) | [78] | Human | ✓ | ✗ | ✗ |
| Polyphenols (Flavonoids) | [32] | Human | ✓ | ✓ | ✓ |
| Polyphenols | [49] | Human | ✓ | ✓ | ✗ |
| [Flavonoids (Quercetins)] | [50] | Human | ✓ | ✓ | ✗ |
| Polyphenols (Isoflavonoids) | [39] | Human | ✓ | ✓ | ✓ |
| [41] | Human | ✓ | ✗ | ✗ | |
| [40] | Human | ✓ | ✗ | ✗ | |
| Polyphenols + Alkaloids | [79] | Human | ✓ | ✗ | ✗ |
| [81] | Animal | ✓ | ✓ | ✓ | |
| Polyphenols | [56] | Animal | ✓ | ✓ | ✓ |
| [Flavonoids (Quercetin)] | [57] | Animal | ✓ | ✓ | ✓ |
| [59] | Animal | ✓ | ✓ | ✗ | |
| Polyphenols [Flavonoids | [58] | Animal | ✓ | ✓ | ✓ |
| (Quercetin + trans-resveratrol)] | |||||
| Polyphenols | [60] | Animal | ✓ | ✓ | ✓ |
| [Flavonoids (Kaempferol)] | |||||
| Polyphenols | [65] | Animal | ✓ | ✓ | ✓ |
| [Flavonoids (Baicalein)] | [67] | Animal | ✓ | ✓ | ✓ |
| Polyphenols | [68] | Animal | ✓ | ✓ | ✗ |
| [Flavonoids (Chrysin)] | |||||
| Polyphenols | [46] | Animal | ✓ | ✓ | ✓ |
| [Flavonoids (Genistein)] | [47] | Animal | ✓ | ✓ | ✓ |
| [48] | Animal | ✓ | ✓ | ✓ | |
| Polyphenols | [70] | Animal | ✓ | ✓ | ✓ |
| [Flavonoids (Anthocyanin)] |
Table 2. Summary of included studies: human studies.
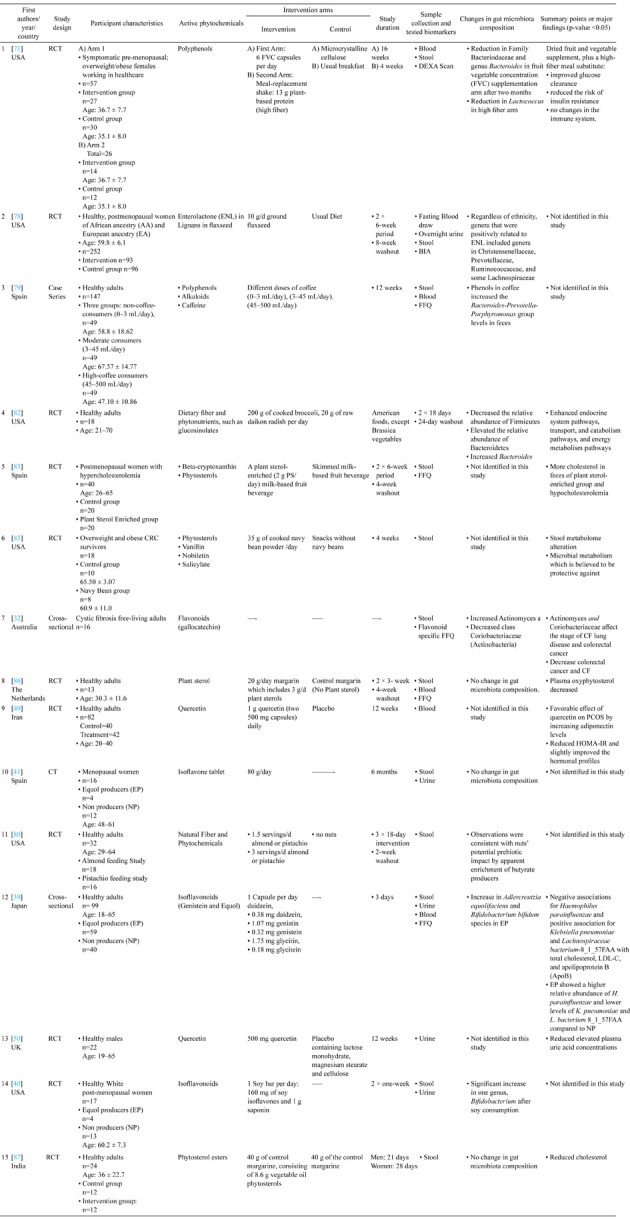
The Table was organized by date of publication from the most recent to the oldest. All the papers used the 16s rRNA method to amplify the variants of bacteria in the gut microbiome. RCT: Randomized Clinical Trial; CRC: Colorectal Cancer; OGTT: Oral Glucose Tolerance Test; BIA: Bioimpedance Analysis; ENL: enterolactone; PS: Plant Sterol; HFD: High Fat Diet; CF: Cystic Fibrosis; FVC: Fruit and Vegetable Concentrate; MDA: malondialdehyde; TNF: tumor necrosis factor; MCP: monocyte chemoattractant protein; IL-6: interleukin; HFS: High Fat Sucrose; SCFA: Short Chain Fatty Acid.
Table 3. Summary of included studies: animal studies.
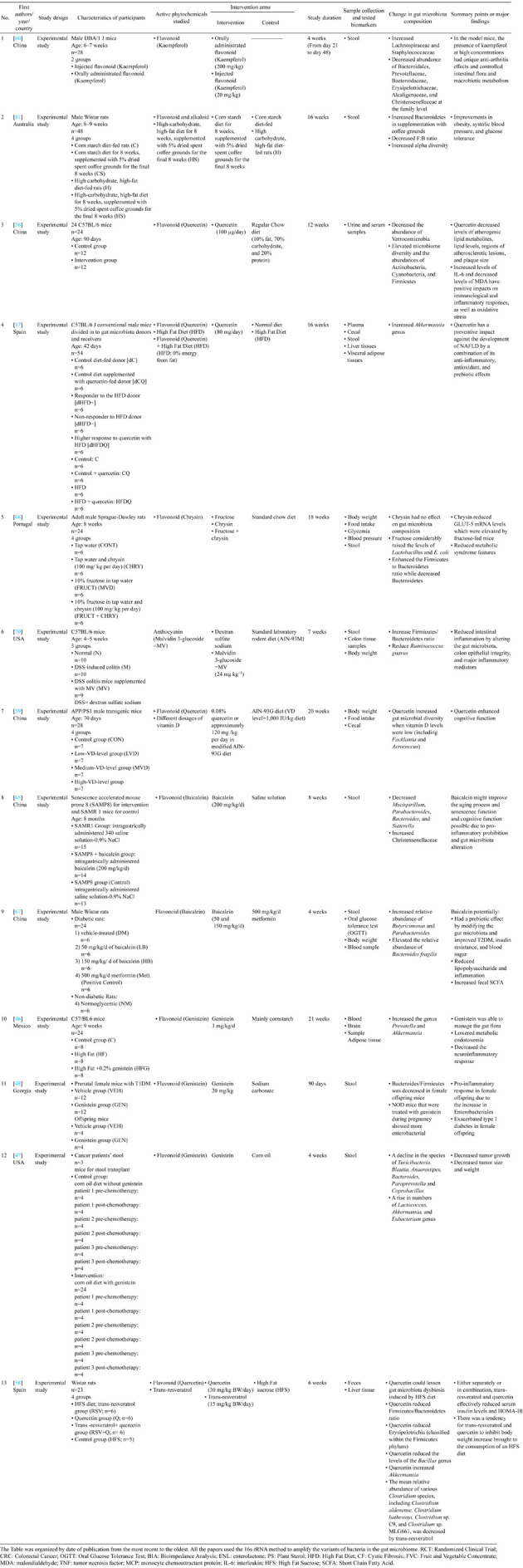
The Table was organized by date of publication from the most recent to the oldest. All the papers used the 16s rRNA method to amplify the variants of bacteria in the gut microbiome. RCT: Randomized Clinical Trial; CRC: Colorectal Cancer; OGTT: Oral Glucose Tolerance Test; BIA: Bioimpedance Analysis; ENL: enterolactone; PS: Plant Sterol; HFD: High Fat Diet; CF: Cystic Fibrosis; FVC: Fruit and Vegetable Concentrate; MDA: malondialdehyde; TNF: tumor necrosis factor; MCP: monocyte chemoattractant protein; IL-6: interleukin; HFS: High Fat Sucrose; SCFA: Short Chain Fatty Acid.
Kaempferol
Animal studies
Another flavonoid that is common in vegetables and fruit is kaempferol. Kaempferol has been correlated to anti-arthritis effects, which may be due to modulation in gut flora, which was shown to modulate energy production, tryptophan, fatty acid, and secondary bile metabolism [60].
Flavones
Baicalein
Animal studies
Baicalein is another type of flavons, found in Scutellariae baicalensis Georgi. It is thought to have antioxidant [61], anti-inflammatory [62], and anti-aging effects [63]. Baicalein may be linked to some factors that reduce oxidative stress, including elevated catalase activity (CAT), elevated glutathione (GSH) levels, and reduced oxidized glutathione (GSSG) levels [64]. In a study, the associations between baicalein, gut microbiota composition, and cognitive function in mice were assessed. Baicalein changed the abundance of five genera in the mice, decreasing Mucispirillum, Parabacteroides, Bacteroides, and Sutterella. In contrast, there was an increase in Christensenellaceae. According to correlational analysis, the abundances of Mucispirillum, Bacteroides, and Sutterella were adversely associated with cognitive function, but Christensenellaceae was positively associated with cognition. Additionally, Christensenellaceae abundance was negatively associated with IL-6 and TNF-α. Mucispirillum and Bacteroides were also correlated with IL-6 in the brain cortex. Gut microbiota modification and suppression of cerebral pro-inflammatory cytokines may be associated with improvements in cognitive function in mice [65]. The impact of baicalein on gut microbiota composition and glycemic and lipid markers were assessed in a study of diabetic rats [66]. Results indicated that baicalein changed the gut microbiota composition in rats with diabetes by elevating α diversity, a relative abundance of Butyricimonas and Parabacteroides, and a relative abundance of B. fragilis. With these changes, the gut microbiome composition of diabetic rats resembled healthy rats. Increased ratios of Firmicutes to Bacteroidetes were shown to be positively associated with inflammation, insulin resistance, and increased blood glucose [66]. Since baicalein decreased this ratio, it may improve type 2 diabetes mellitus (T2DM) abnormalities, insulin resistance, and inflammation. Furthermore, baicalein increased SCFA-producing bacteria such as A. muciniphila, which increased the fecal SCFA and the thickness of the mucus layer and and beneficially affected gut barrier function, possibly preventing T2DM in diabetic rats [67].
Chrysin
Animal studies
Another flavonoid, chrysin, was studied to determine its effects on gut microbiota composition and metabolic syndrome in rats. Chrysin alone did not influence gut microbiota composition. Therefore, it does not prevent metabolic syndrome through gut microbiota modulation. However, chrysin could reduce GLUT-5 (glucose transporter) mRNA gene expression, which was elevated in fructose-fed mice, and possibly contribute to reductions in metabolic syndrome risk factors [68].
Anthocyanins
Human studies
A study conducted using a mouse model, showed that anthocyanin malvidin 3-glucoside (MV), predominantly occurring in blueberries, modulated gut microbiota composition by decreasing the abundance of Firmicutes, while increasing the abundance of Bacteroidetes so that the Firmicutes:Bacteroidetes (F:B) ratio was reduced. Reductions in the F:B ratio is assumed to have pro-inflammatory effects. Moreover, MV reduced the abundance of Ruminococcus gnavus, which is thought to be associated with elevated disease activity in inflammatory bowel disease (IBD) patients. In addition, MV inhibited bacterial outer membrane lipoproteins, oxidative phosphorylation, and LPS production pathways. The development and severity of IBD are both influenced by decreased fecal butyrate content [69]. The genus Clostridium, which is known to contain species that produce butyrate, as well as the class Clostridia, were both much more prevalent after MV supplementation [70].
ASSOCIATIONS BETWEEN CONSUMPTION OF OTHER POLYPHENOLS, GUT MICROBIOTA COMPOSITION, AND HEALTH OUTCOMES
Polyphenols
Human studies
Polyphenols consists of 8 subgroups which is shown in Fig. 1. Numerous studies have identified the associations between polyphenols, including their subgroups, with modifications in gut microbiota composition, and health outcomes. However, most human studies have not been conclusive regarding how gut microbiota alterations could affect health status. A recent study of obese/overweight women showed that supplementation with fruit and vegetable concentrates (FVC) and high fiber plant-based protein shakes with polyphenols, changed gut microbiota composition and significantly reduced Bacteriodaceae and genus Bacteroides and genus Lactococcus. At the same time, there were improvements in glucose clearance, and the authors speculated that there was a reduction in insulin resistance, which would then be associated with a decreased risk of T2DM. However, the same study indicated no effects on immune markers [71]. This result is in line with findings from earlier research that the Bacteroides genus is capable of complex carbohydrate metabolism and may be protective against T2DM [72].
Lignans
Human studies
Lignan has an estrogen like structure, acting as a phytoestrogen in humans [73]. Lignan is found in seeds, vegetables, cereals, legumes, nuts, fruits, and to a lesser extent in beverages like tea and coffee [74]. Dietary lignan is converted to enterodiol and enterolactone by the gut microbiota, and these metabolites have anti-inflammatory, anti-oxidant, estrogenic/anti-estrogenic, and anti-cancer effects which are thought to be health promoting [75, 76]. Several studies have shown that increased dietary lignan consumption is associated with decreased cardiovascular disease (CVD) risk [77]. However, the associations between gut microbiome composition and subsequent health outcomes following lignan consumption, have not been clearly determined. Flaxseed, which is abundant in enterolactone, a type of lignan, was used in a study of postmenopausal women with African American (AA) and European American (EA) ancestry. Results of the study showed that women with different ancestries had different gut microbiota compositions, both at the baseline and after enterolactone intervention. For example, there were significant differences in the abundance of 18 genera among AA participants and 17 genera among EA participants; 12 of these were shared by the two groups. However, following the flaxseed intervention, only 9 genera were shared by participants in the AA group (21 genera) and the EA group (14 genera). There were no associations between lignan, α and β diversity of microbiome composition and the onset of any chronic diseases [78].
PHYTOCHEMICAL COMBINATIONS, GUT MICROBIOTA COMPOSITION, AND HEALTH OUTCOMES
Flavonoids and alkaloids
Human studies
There are not many studies investigating the associations between combinations of phytochemicals and gut microbiota composition. One such clinical study that included 147 participants, assessed the associations between some phenols such as flavonoids and alkaloids combined as the active phytochemicals in coffee, and alterations in gut microbiota composition. Participants were divided into tertiles based on the daily coffee consumption. Flavonoids and alkaloids in the highest tertile of coffee consumption showed a significant elevation in the prevalence of Bacteroides-Prevotella-Porphyromonas in feces. However, this study did not aim to determine how gut microbiota changes might affect health outcomes [79]. Likewise, in another clinical trial, the associations between the phytonutrients in pistachios and almonds, and gut microbiota composition, were assessed without determination of associations with health outcomes. Nut consumption increased butyrate and bifidobacterial. Pistachio consumption had a larger effect on butyrate composition than almonds and was connected to a reduction in the lactic acid bacteria. Therefore, nut consumption modifies gut microbiota composition in ways that align with health promotion, but no direct health outcomes have been determined in the studies conducted thus far [80].
Animal studies
The effects of ground coffee consumption, abundant in alkaloids and flavonoids, was studied during a high-carbohydrate, high-fat diet that induced metabolic syndrome and cardiovascular remodeling in male Wistar rats [81]. This 16-week controlled study showed that ground coffee consumption increased Bacteroidetes (p-value=0.05), decreased the F:B ratio (p-value=0.0027), and increased α diversity (p-value=0.0005). In terms of health outcomes, coffee grounds decreased body weight, abdominal fat, total body fat mass, systolic blood pressure, and plasma triglycerides, and non-esterified fatty acid concentrations. Coffee ground consumption also improved glucose tolerance and the structure and function of the heart and liver. The changes in the gut microbiota composition were also significantly associated with improvements in glucose tolerance as well as in adiposity and systolic blood pressure [81].
PROTEIN PHYTOCHEMICALS, GUT MICROBIOTA COMPOSITION, AND HEALTH OUTCOMES
Glucosinolate
Human studies
Broccoli and radish consumption are abundant in glucosinolate. Broccoli increased Bacteroides, the relative abundance of Bacteroidetes, and reduced the relative abundance of Firmicutes [82]. Therefore, it altered β diversity. In participants with BMIs <26 kg/m2, broccoli consumption increased glucosinolate metabolites, which supports the idea that variations in the presence of health-promoting glucosinolate metabolites may relate to alterations in the microbiota. Since the greatest peak in plasma metabolites was positively associated with the difference in Bacteroidetes from baseline to post-intervention with broccoli consumption (r=0.69, p-value=0.04). Firmicutes variation from baseline to post-intervention was likely to be negatively associated (r=0.66, p-value=0.05), but variation in the Bacteroidetes to Firmicutes ratio was likely to be positively associated (r=0.58, p-value=0.1). Following the trial, Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUST), a predictive software package to infer the metabolic activities of the microbiota by comparing compositions, was used to assess the function of the gut microbiota. Overall, consumption of broccoli increased the number of pathways involved in energy metabolism (p-value=0.01), transport and catabolism (p=0.04), and endocrine system functions (p-value=0.05), while decreasing the number of pathways related to membrane transport (p-value=0.03). Additionally, the protein families involved in transcription control and cellular transportation, were among those anticipated to be elevated in the broccoli group (e.g., RNA polymerase sigma-70 factor) [82].
LIPID PHYTOCHEMICALS, GUT MICROBIOTA COMPOSITION, AND HEALTH OUTCOMES
Phytosterols
Human studies
Plant phytosterol consumption was studied in a trial where two milk-based fruit beverages were fortified with plant sterols or a placebo without enrichment. Plant phytosterols consumption in the intervention group was shown to decrease cholesterol absorption. As the structure of plant sterols is the same as cholesterol, the amount of cholesterol was measured in feces. Since more cholesterol was in the feces in the intervention group, plant phytosterol consumption was associated with hypocholesterolemia in participants. However, the gut microbiota composition was not studied in this trial. The authors assumed that gut microbiota alterations could be related to the amount of cholesterol in the feces [83]. Another study showed that when colorectal cancer survivors consumed navy beans, which are abundant in sterols, the stool metabolome and microbial metabolism of amino acids, fatty acids, and phytochemicals were altered. For example, there was an increase in anacardic acid, 4-hydroxyphenylacetate, cadaverine, diacetylchitobiose, and 5,6 dihydrothymine. Five metabolites increased and were recognized as navy bean metabolites: ophthalmate, piperidine, adenosine-2′, 3′-cyclic monophosphate, and N-methylpipecolate. Therefore, navy bean consumption may modulate the metabolism of nutrients and metabolic pathways which have been shown previously to be protective against colorectal cancer [84]. However, gut microbiota alterations were not directly studied, and it was only hypothesized that changes in metabolites and feces composition might be correlated with changes in gut microbiota composition [85]. Moreover, another study assessed the associations between plant stanols, and saturated plant sterols, on gut microbiota changes. With 20 g margarine consumption per day, which contains 3 g of plant stanol, plasma oxyphytosterol decreased. However, there were no alterations in gut microbiota composition [86]. These findings are in line with another study that assessed the associations between daily consumption of 40 g margarine in females, sex hormones, and gut microbiota changes. Results indicated no significant effects on gut microbiota composition or sex hormone levels. However, margarine, enriched with phytosterol esters, decreased serum low-density lipoprotein (LDL) cholesterol and total cholesterol. The improvements in health outcomes occurred despite lack of alterations in gut microbiota composition [87]. The primary purpose of the current review was to identify the current state of the published research regarding the consumption of phytochemicals, associations with gut microbiota composition, and related health outcomes in human and non-human animals. Most of the previous gut microbiota research has been conducted in non-human animal models, and clinical studies in humans identifying the roles that gut microbiota plays in disease pathogenesis are still lacking. Based on previous human and non-human animal studies, the phytochemical associations with gut microbiota composition and health outcomes fall into two broad categories; 1) Regular consumption of some active phytochemicals can alter gut microbiota composition in ways that have been shown to be correlated with positive health outcomes. This is particularly true in non-human animals, for example, different types of flavonoids altered gut microbiota composition which was correlated with improved glycemic markers, inflammation, cognitive function, and oxidative stress; 2) Some phytochemicals have been shown to improve markers of disease risk without significant changes in the gut microbiota, for example, phytosterol esters present in navy beans have positive impact on colon health in cancer survivors without changing gut microbiota composition.
DISCUSSION
There is little evidence in the current published research literature that connects phytochemical consumption, gut microbiota alterations, and health outcomes. While many of the published studies offer feasible mechanistic rationale; however, future research that includes all three of these components are needed. For example, antioxidants are beneficial for decreasing the risk for cancer, however, a well-known outcome of this type of research is supplementation with some antioxidants (vitamin C, A, E). Unfortunately, supplementation with vitamin E in smokers, who would be at increased risk for developing cancer, has been shown to increase the risk [88].
There is an urgent need to determine whether microbiome alterations serve as moderators or mediators between phytochemical consumption and non-communicable health outcomes, or whether the gut microbiota is an independent therapeutic target. The limitations of the current published research leave important gaps that make interpretation difficult. This is particularly true given the transient nature and changeability of the gut microbiota composition with acute lifestyle (diet and physical activity) interventions as well as the influence of aging. Previous research suggests distinct differences in the composition of gut microbiota with aging, potentially associated with dietary intake and specific nutrients [89]. These changes include increases in proteolytic bacteria and decreases in saccharolytic bacteria associated with sarcopenia and decreased longevity. Additionally, some research suggests that prebiotics and probiotics may help to mitigate these age-related changes [90]. Future research should investigate the effects of age-related changes on the gut microbiota and the efficacy of consumption of some phytochemicals as potential therapeutic approaches. Most of the initial research on the gut microbiota has been conducted in animal models, based on these studies, there has been a lot of interest generated for research in humans, and substantial new grant funding designated for this type of research. However, little has been published that connects phytochemical consumption, alterations in gut microbiota composition, and non-communicable health outcomes.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST
None.
REFERENCES
- 1.Neuhouser ML. 2019. The importance of healthy dietary patterns in chronic disease prevention. Nutr Res 70: 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuso PJ, Ismail MH, Ha BP, Bartolotto C. 2013. Nutritional update for physicians: plant-based diets. Perm J 17: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeshi K, Crayn D, Ritmejerytė E, Wangchuk P. 2022. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 27: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caretto S, Linsalata V, Colella G, Mita G, Lattanzio V. 2015. Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int J Mol Sci 16: 26378–26394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagare S, Bhatia M, Tripathi N, Pagare S, Bansal YK. 2015. Secondary metabolites of plants and their role: overview. Curr Trends Biotechnol Pharm 9: 293–304. [Google Scholar]
- 6.Rao SR, Ravishankar GA. 2002. Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20: 101–153. [DOI] [PubMed] [Google Scholar]
- 7.Ravishankar GA, Venkataraman LV. 1990. Food applications of plant cell cultures. Curr Sci 59: 914–920. [Google Scholar]
- 8.Holst B, Williamson G. 2008. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr Opin Biotechnol 19: 73–82. [DOI] [PubMed] [Google Scholar]
- 9.D’Archivio M, Filesi C, Varì R, Scazzocchio B, Masella R. 2010. Bioavailability of the polyphenols: status and controversies. Int J Mol Sci 11: 1321–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aura AM, Martin-Lopez P, O’Leary KA, Williamson G, Oksman-Caldentey KM, Poutanen K, Santos-Buelga C. 2005. In vitro metabolism of anthocyanins by human gut microflora. Eur J Nutr 44: 133–142. [DOI] [PubMed] [Google Scholar]
- 11.Muñoz Y, Garrido A, Valladares L. 2009. Equol is more active than soy isoflavone itself to compete for binding to thromboxane A(2) receptor in human platelets. Thromb Res 123: 740–744. [DOI] [PubMed] [Google Scholar]
- 12.Setchell KD, Brown NM, Lydeking-Olsen E. 2002. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr 132: 3577–3584. [DOI] [PubMed] [Google Scholar]
- 13.Lozupone CA, Knight R. 2008. Species divergence and the measurement of microbial diversity. FEMS Microbiol Rev 32: 557–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitman WB, Coleman DC, Wiebe WJ. 1998. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA 95: 6578–6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. 2007. The human microbiome project. Nature 449: 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguirre de Cárcer D, Cuív PO, Wang T, Kang S, Worthley D, Whitehall V, Gordon I, McSweeney C, Leggett B, Morrison M. 2011. Numerical ecology validates a biogeographical distribution and gender-based effect on mucosa-associated bacteria along the human colon. ISME J 5: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juraschek SP, Appel LJ, Anderson CAM, Miller ER, 3rd. 2013. Effect of a high-protein diet on kidney function in healthy adults: results from the OmniHeart trial. Am J Kidney Dis 61: 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinclair L, Osman OA, Bertilsson S, Eiler A. 2015. Microbial community composition and diversity via 16S rRNA gene amplicons: evaluating the illumina platform. PLoS One 10: e0116955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, Creasy HH, Earl AM, FitzGerald MG, Fulton RS, Giglio MG, Hallsworth-Pepin K, Lobos EA, Madupu R, Magrini V, Martin JC, Mitreva M, Muzny DM, Sodergren EJ, Versalovic J, Wollam AM, Worley KC, Wortman JR, Young SK, Zeng Q, Aagaard KM, Abolude OO, Allen-Vercoe E, Alm EJ, Alvarado L, Andersen GL, Anderson S, Appelbaum E, Arachchi HM, Armitage G, Arze CA, Ayvaz T, Baker CC, Begg L, Belachew T, Bhonagiri V, Bihan M, Blaser MJ, Bloom T, Bonazzi V, Paul Brooks J, Buck GA, Buhay CJ, Busam DA, Campbell JL, Canon SR, Cantarel BL, Chain PSG, Chen IMA, Chen L, Chhibba S, Chu K, Ciulla DM, Clemente JC, Clifton SW, Conlan S, Crabtree J, Cutting MA, Davidovics NJ, Davis CC, DeSantis TZ, Deal C, Delehaunty KD, Dewhirst FE, Deych E, Ding Y, Dooling DJ, Dugan SP, Michael Dunne W, Scott Durkin A, Edgar RC, Erlich RL, Farmer CN, Farrell RM, Faust K, Feldgarden M, Felix VM, Fisher S, Fodor AA, Forney LJ, Foster L, Di Francesco V, Friedman J, Friedrich DC, Fronick CC, Fulton LL, Gao H, Garcia N, Giannoukos G, Giblin C, Giovanni MY, Goldberg JM, Goll J, Gonzalez A, Griggs A, Gujja S, Kinder Haake S, Haas BJ, Hamilton HA, Harris EL, Hepburn TA, Herter B, Hoffmann DE, Holder ME, Howarth C, Huang KH, Huse SM, Izard J, Jansson JK, Jiang H, Jordan C, Joshi V, Katancik JA, Keitel WA, Kelley ST, Kells C, King NB, Knights D, Kong HH, Koren O, Koren S, Kota KC, Kovar CL, Kyrpides NC, La Rosa PS, Lee SL, Lemon KP, Lennon N, Lewis CM, Lewis L, Ley RE, Li K, Liolios K, Liu B, Liu Y, Lo CC, Lozupone CA, Dwayne Lunsford R, Madden T, Mahurkar AA, Mannon PJ, Mardis ER, Markowitz VM, Mavromatis K, McCorrison JM, McDonald D, McEwen J, McGuire AL, McInnes P, Mehta T, Mihindukulasuriya KA, Miller JR, Minx PJ, Newsham I, Nusbaum C, O’Laughlin M, Orvis J, Pagani I, Palaniappan K, Patel SM, Pearson M, Peterson J, Podar M, Pohl C, Pollard KS, Pop M, Priest ME, Proctor LM, Qin X, Raes J, Ravel J, Reid JG, Rho M, Rhodes R, Riehle KP, Rivera MC, Rodriguez-Mueller B, Rogers YH, Ross MC, Russ C, Sanka RK, Sankar P, Fah Sathirapongsasuti J, Schloss JA, Schloss PD, Schmidt TM, Scholz M, Schriml L, Schubert AM, Segata N, Segre JA, Shannon WD, Sharp RR, Sharpton TJ, Shenoy N, Sheth NU, Simone GA, Singh I, Smillie CS, Sobel JD, Sommer DD, Spicer P, Sutton GG, Sykes SM, Tabbaa DG, Thiagarajan M, Tomlinson CM, Torralba M, Treangen TJ, Truty RM, Vishnivetskaya TA, Walker J, Wang L, Wang Z, Ward DV, Warren W, Watson MA, Wellington C, Wetterstrand KA, White JR, Wilczek-Boney K, Wu Y, Wylie KM, Wylie T, Yandava C, Ye L, Ye Y, Yooseph S, Youmans BP, Zhang L, Zhou Y, Zhu Y, Zoloth L, Zucker JD, Birren BW, Gibbs RA, Highlander SK, Methé BA, Nelson KE, Petrosino JF, Weinstock GM, Wilson RK, White O, Human Microbiome Project Consortium.2012. Structure, function and diversity of the healthy human microbiome. Nature 486: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. 2007. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104: 13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. 2012. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J, Zhao F, Lin B, Feng J, Wu X, Liu Y, Zhao L, Zhu B, Wei Y. 2020. Gut microbiota participates in antithyroid drug induced liver injury through the lipopolysaccharide related signaling pathway. Front Pharmacol 11: 598170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson CT, Sharma V, Elmén L, Peterson SN. 2015. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin Exp Immunol 179: 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asatoor AM. 1964. Studies on metabolism by intestinal bacteria. Proceedings of the Association of Clinical Biochemists 3: 82–87. [Google Scholar]
- 26.Le Nevé B, Derrien M, Tap J, Brazeilles R, Cools Portier S, Guyonnet D, Ohman L, Störsrud S, Törnblom H, Simrén M. 2019. Fasting breath H2 and gut microbiota metabolic potential are associated with the response to a fermented milk product in irritable bowel syndrome. PLoS One 14: e0214273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carretta MD, Quiroga J, López R, Hidalgo MA, Burgos RA. 2021. Participation of short-chain fatty acids and their receptors in gut inflammation and colon cancer. Front Physiol 12: 662739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, Goedert JJ, Hayes RB, Yang L. 2013. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst 105: 1907–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medawar E, Haange SB, Rolle-Kampczyk U, Engelmann B, Dietrich A, Thieleking R, Wiegank C, Fries C, Horstmann A, Villringer A, von Bergen M, Fenske W, Veronica Witte A. 2021. Gut microbiota link dietary fiber intake and short-chain fatty acid metabolism with eating behavior. Transl Psychiatry 11: 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. 2015. Gut dysbiosis is linked to hypertension. Hypertension 65: 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Somerset S. 2018. Associations between flavonoid intakes and gut microbiota in a group of adults with cystic fibrosis. Nutrients 10: 1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tunney MM, Field TR, Moriarty TF, Patrick S, Doering G, Muhlebach MS, Wolfgang MC, Boucher R, Gilpin DF, McDowell A, Elborn JS. 2008. Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am J Respir Crit Care Med 177: 995–1001. [DOI] [PubMed] [Google Scholar]
- 33.Mayo B, Vázquez L, Flórez AB. 2019. Equol: a bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients 11: 2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekikawa A, Ihara M, Lopez O, Kakuta C, Lopresti B, Higashiyama A, Aizenstein H, Chang YF, Mathis C, Miyamoto Y, Kuller L, Cui C. 2019. Effect of S-equol and soy isoflavones on heart and brain. Curr Cardiol Rev 15: 114–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Setchell KD, Clerici C. 2010. Equol: pharmacokinetics and biological actions. J Nutr 140: 1363S–1368S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou T, Meng C, He P. 2019. Soy isoflavones and their effects on xenobiotic metabolism. Curr Drug Metab 20: 46–53. [DOI] [PubMed] [Google Scholar]
- 37.Luca SV, Macovei I, Bujor A, Miron A, Skalicka-Woźniak K, Aprotosoaie AC, Trifan A. 2020. Bioactivity of dietary polyphenols: the role of metabolites. Crit Rev Food Sci Nutr 60: 626–659. [DOI] [PubMed] [Google Scholar]
- 38.Hu WS, Lin YM, Kuo WW, Pan LF, Yeh YL, Li YH, Kuo CH, Chen RJ, Padma VV, Chen TS, Huang CY. 2016. Suppression of isoproterenol-induced apoptosis in H9c2 cardiomyoblast cells by daidzein through activation of Akt. Chin J Physiol 59: 323–330. [DOI] [PubMed] [Google Scholar]
- 39.Zheng W, Ma Y, Zhao A, He T, Lyu N, Pan Z, Mao G, Liu Y, Li J, Wang P, Wang J, Zhu B, Zhang Y. 2019. Compositional and functional differences in human gut microbiome with respect to equol production and its association with blood lipid level: a cross-sectional study. Gut Pathog 11: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakatsu CH, Armstrong A, Clavijo AP, Martin BR, Barnes S, Weaver CM. 2014. Fecal bacterial community changes associated with isoflavone metabolites in postmenopausal women after soy bar consumption. PLoS One 9: e108924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guadamuro L, Delgado S, Redruello B, Flórez AB, Suárez A, Martínez-Camblor P, Mayo B. 2015. Equol status and changes in fecal microbiota in menopausal women receiving long-term treatment for menopause symptoms with a soy-isoflavone concentrate. Front Microbiol 6: 777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gleason CE, Fischer BL, Dowling NM, Setchell KD, Atwood CS, Carlsson CM, Asthana S. 2015. Cognitive effects of soy isoflavones in patients with Alzheimer’s disease. J Alzheimers Dis 47: 1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Igase M, Igase K, Tabara Y, Ohyagi Y, Kohara K. 2017. Cross-sectional study of equol producer status and cognitive impairment in older adults. Geriatr Gerontol Int 17: 2103–2108. [DOI] [PubMed] [Google Scholar]
- 44.Zhao J, Bi W, Xiao S, Lan X, Cheng X, Zhang J, Lu D, Wei W, Wang Y, Li H, Fu Y, Zhu L. 2019. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep 9: 5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y, Wang P, Sang S. 2019. Dietary genistein inhibits methylglyoxal-induced advanced glycation end product formation in mice fed a high-fat diet. J Nutr 149: 776–787. [DOI] [PubMed] [Google Scholar]
- 46.López P, Sánchez M, Perez-Cruz C, Velázquez-Villegas LA, Syeda T, Aguilar-López M, Rocha-Viggiano AK, Del Carmen Silva-Lucero M, Torre-Villalvazo I, Noriega LG, Torres N, Tovar AR. 2018. Long-term genistein consumption modifies gut microbiota, improving glucose metabolism, metabolic endotoxemia, and cognitive function in mice fed a high-fat diet. Mol Nutr Food Res 62: e1800313. [DOI] [PubMed] [Google Scholar]
- 47.Paul B, Royston KJ, Li Y, Stoll ML, Skibola CF, Wilson LS, Barnes S, Morrow CD, Tollefsbol TO. 2017. Impact of genistein on the gut microbiome of humanized mice and its role in breast tumor inhibition. PLoS One 12: e0189756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang G, Xu J, Cai D, Chen SY, Nagy T, Guo TL. 2018. Exacerbation of type 1 diabetes in perinatally genistein exposed female Non-Obese Diabetic (NOD) mouse is associated with alterations of gut microbiota and immune homeostasis. Toxicol Sci 165: 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rezvan N, Moini A, Janani L, Mohammad K, Saedisomeolia A, Nourbakhsh M, Gorgani-Firuzjaee S, Mazaherioun M, Hosseinzadeh-Attar MJ. 2017. Effects of quercetin on adiponectin-mediated insulin sensitivity in polycystic ovary syndrome: a randomized placebo-controlled double-blind clinical trial. Horm Metab Res 49: 115–121. [DOI] [PubMed] [Google Scholar]
- 50.Shi Y, Williamson G. 2016. Quercetin lowers plasma uric acid in pre-hyperuricaemic males: a randomised, double-blinded, placebo-controlled, cross-over trial. Br J Nutr 115: 800–806. [DOI] [PubMed] [Google Scholar]
- 51.Peng X, Zhang Z, Zhang N, Liu L, Li S, Wei H. 2014. In vitro catabolism of quercetin by human fecal bacteria and the antioxidant capacity of its catabolites. Food Nutr Res 58: 23406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z, Peng X, Li S, Zhang N, Wang Y, Wei H. 2014. Isolation and identification of quercetin degrading bacteria from human fecal microbes. PLoS One 9: e90531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blaut M, Schoefer L, Braune A. 2003. Transformation of flavonoids by intestinal microorganisms. Int J Vitam Nutr Res 73: 79–87. [DOI] [PubMed] [Google Scholar]
- 54.Carrasco-Pozo C, Gotteland M, Castillo RL, Chen C. 2015. 3,4-Dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, protects against pancreatic β-cells dysfunction induced by high cholesterol. Exp Cell Res 334: 270–282. [DOI] [PubMed] [Google Scholar]
- 55.Iszatt N, Janssen S, Lenters V, Dahl C, Stigum H, Knight R, Mandal S, Peddada S, González A, Midtvedt T, Eggesbø M. 2019. Environmental toxicants in breast milk of Norwegian mothers and gut bacteria composition and metabolites in their infants at 1 month. Microbiome 7: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nie J, Zhang L, Zhao G, Du X. 2019. Quercetin reduces atherosclerotic lesions by altering the gut microbiota and reducing atherogenic lipid metabolites. J Appl Microbiol 127: 1824–1834. [DOI] [PubMed] [Google Scholar]
- 57.Porras D, Nistal E, Martínez-Flórez S, Olcoz JL, Jover R, Jorquera F, González-Gallego J, García-Mediavilla MV, Sánchez-Campos S. 2019. Functional interactions between gut microbiota transplantation, quercetin, and high-fat diet determine non-alcoholic fatty liver disease development in germ-free mice. Mol Nutr Food Res 63: e1800930. [DOI] [PubMed] [Google Scholar]
- 58.Etxeberria U, Arias N, Boqué N, Macarulla MT, Portillo MP, Martínez JA, Milagro FI. 2015. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J Nutr Biochem 26: 651–660. [DOI] [PubMed] [Google Scholar]
- 59.Lv M, Yang S, Cai L, Qin LQ, Li BY, Wan Z. 2018. Effects of quercetin intervention on cognition function in APP/PS1 mice was affected by vitamin D status. Mol Nutr Food Res 62: e1800621. [DOI] [PubMed] [Google Scholar]
- 60.Aa LX, Fei F, Qi Q, Sun RB, Gu SH, Di ZZ, Aa JY, Wang GJ, Liu CX. 2020. Rebalancing of the gut flora and microbial metabolism is responsible for the anti-arthritis effect of kaempferol. Acta Pharmacol Sin 41: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shieh DE, Liu LT, Lin CC. 2000. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res 205A: 2861–2865. [PubMed] [Google Scholar]
- 62.Lee W, Ku SK, Bae JS. 2015. Anti-inflammatory effects of Baicalin, Baicalein, and Wogonin in vitro and in vivo. Inflammation 38: 110–125. [DOI] [PubMed] [Google Scholar]
- 63.Duan DD, Gao L, Wang KX, Qin XM, Zhou YZ, Du GH. 2016. [Baicalein prolongs the lifespan of Drosophila melanogaster through antioxidation activity]. Yao Xue Xue Bao 51: 1401–1406 (in Chinese). [PubMed] [Google Scholar]
- 64.Gao L, Duan DD, Zhang JQ, Zhou YZ, Qin XM, Du GH. 2016. A bioinformatic approach for the discovery of antiaging effects of baicalein from scutellaria baicalensis georgi. Rejuvenation Res 19: 414–422. [DOI] [PubMed] [Google Scholar]
- 65.Gao L, Li J, Zhou Y, Huang X, Qin X, Du G. 2018. Effects of baicalein on cortical proinflammatory cytokines and the intestinal microbiome in senescence accelerated mouse prone 8. ACS Chem Neurosci 9: 1714–1724. [DOI] [PubMed] [Google Scholar]
- 66.Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y, Levy E, Marette A. 2015. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64: 872–883. [DOI] [PubMed] [Google Scholar]
- 67.Zhang B, Sun W, Yu N, Sun J, Yu X, Li X, Xing Y, Yan D, Ding Q, Xiu Z, Ma B, Yu L, Dong Y. 2018. Anti-diabetic effect of baicalein is associated with the modulation of gut microbiota in streptozotocin and high-fat-diet induced diabetic rats. J Funct Foods 46: 256–267. [Google Scholar]
- 68.Andrade N, Marques C, Andrade S, Silva C, Rodrigues I, Guardão L, Guimarães JT, Keating E, Calhau C, Martel F. 2019. Effect of chrysin on changes in intestinal environment and microbiome induced by fructose-feeding in rats. Food Funct 10: 4566–4576. [DOI] [PubMed] [Google Scholar]
- 69.Rechner AR, Smith MA, Kuhnle G, Gibson GR, Debnam ES, Srai SK, Moore KP, Rice-Evans CA. 2004. Colonic metabolism of dietary polyphenols: influence of structure on microbial fermentation products. Free Radic Biol Med 36: 212–225. [DOI] [PubMed] [Google Scholar]
- 70.Liu F, Wang TTY, Tang Q, Xue C, Li RW, Wu VCH. 2019. Malvidin 3-glucoside modulated gut microbial dysbiosis and global metabolome disrupted in a murine colitis model induced by dextran sulfate sodium. Mol Nutr Food Res 63: e1900455. [DOI] [PubMed] [Google Scholar]
- 71.van der Merwe M, Moore D, Hill JL, Keating FH, Buddington RK, Bloomer RJ, Wang A, Bowman DD. 2021. The impact of a dried fruit and vegetable supplement and fiber rich shake on gut and health parameters in female healthcare workers: a placebo‐controlled, double‐blind, randomized clinical trial. Microorganisms 9: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sedighi M, Razavi S, Navab-Moghadam F, Khamseh ME, Alaei-Shahmiri F, Mehrtash A, Amirmozafari N. 2017. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb Pathog 111: 362–369. [DOI] [PubMed] [Google Scholar]
- 73.Abd El-Mawla AM, Beerhues L. 2002. Benzoic acid biosynthesis in cell cultures of Hypericum androsaemum. Planta 214: 727–733. [DOI] [PubMed] [Google Scholar]
- 74.Rothwell JA, Perez-Jimenez J, Neveu V, Medina-Remón A, M’hiri N, García-Lobato P, Manach C, Knox C, Eisner R, Wishart DS, Scalbert A. 2013. Phenol-Explorer 3.0: a major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database (Oxford) 2013: bat070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Plaha NS, Awasthi S, Sharma A, Kaushik N. 2022. Distribution, biosynthesis and therapeutic potential of lignans. 3 Biotech 12: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grosso G, Godos J, Lamuela-Raventos R, Ray S, Micek A, Pajak A, Sciacca S, D’Orazio N, Del Rio D, Galvano F. 2017. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: level of evidence and limitations. Mol Nutr Food Res 61: 61. [DOI] [PubMed] [Google Scholar]
- 77.Tresserra-Rimbau A, Rimm EB, Medina-Remón A, Martínez-González MA, de la Torre R, Corella D, Salas-Salvadó J, Gómez-Gracia E, Lapetra J, Arós F, Fiol M, Ros E, Serra-Majem L, Pintó X, Saez GT, Basora J, Sorlí JV, Martínez JA, Vinyoles E, Ruiz-Gutiérrez V, Estruch R, Lamuela-Raventós RM, PREDIMED Study Investigators.2014. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr Metab Cardiovasc Dis 24: 639–647. [DOI] [PubMed] [Google Scholar]
- 78.McCann SE, Hullar MAJ, Tritchler DL, Cortes-Gomez E, Yao S, Davis W, O’Connor T, Erwin D, Thompson LU, Yan L, Lampe JW. 2021. Enterolignan production in a flaxseed intervention study in postmenopausal us women of african ancestry and european ancestry. Nutrients 13: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.González S, Salazar N, Ruiz-Saavedra S, Gómez-Martín M, de Los Reyes-Gavilán CG, Gueimonde M. 2020. Long-term coffee consumption is associated with fecal microbial composition in humans. Nutrients 12: 1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ukhanova M, Wang X, Baer DJ, Novotny JA, Fredborg M, Mai V. 2014. Effects of almond and pistachio consumption on gut microbiota composition in a randomised cross-over human feeding study. Br J Nutr 111: 2146–2152. [DOI] [PubMed] [Google Scholar]
- 81.Bhandarkar NS, Mouatt P, Goncalves P, Thomas T, Brown L, Panchal SK. 2020. Modulation of gut microbiota by spent coffee grounds attenuates diet-induced metabolic syndrome in rats. FASEB J 34: 4783–4797. [DOI] [PubMed] [Google Scholar]
- 82.Kaczmarek JL, Liu X, Charron CS, Novotny JA, Jeffery EH, Seifried HE, Ross SA, Miller MJ, Swanson KS, Holscher HD. 2019. Broccoli consumption affects the human gastrointestinal microbiota. J Nutr Biochem 63: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cuevas-Tena M, Bermúdez JD, Silvestre RLÁ, Alegría A, Lagarda MJ. 2019. Impact of colonic fermentation on sterols after the intake of a plant sterol-enriched beverage: a randomized, double-blind crossover trial. Clin Nutr 38: 1549–1560. [DOI] [PubMed] [Google Scholar]
- 84.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6: 1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baxter BA, Oppel RC, Ryan EP. 2018. Navy beans impact the stool metabolome and metabolic pathways for colon health in cancer survivors. Nutrients 11: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baumgartner S, Mensink RP, Smet E, Konings M, Fuentes S, de Vos WM, Plat J. 2017. Effects of plant stanol ester consumption on fasting plasma oxy(phyto)sterol concentrations as related to fecal microbiota characteristics. J Steroid Biochem Mol Biol 169: 46–53. [DOI] [PubMed] [Google Scholar]
- 87.Ayesh R, Weststrate JA, Drewitt PN, Hepburn PA. 1999. Safety evaluation of phytosterol esters. Part 5. Faecal short-chain fatty acid and microflora content, faecal bacterial enzyme activity and serum female sex hormones in healthy normolipidaemic volunteers consuming a controlled diet either with or without a phytosterol ester-enriched margarine. Food Chem Toxicol 37: 1127–1138. [DOI] [PubMed] [Google Scholar]
- 88.Wu QJ, Xiang YB, Yang G, Li HL, Lan Q, Gao YT, Zheng W, Shu XO, Fowke JH. 2015. Vitamin E intake and the lung cancer risk among female nonsmokers: a report from the Shanghai Women’s Health Study. Int J Cancer 136: 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, Abe F, Osawa R. 2016. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol 16: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bischoff SC. 2016. Microbiota and aging. Curr Opin Clin Nutr Metab Care 19: 26–30. [DOI] [PubMed] [Google Scholar]
- 91.Bolling BW, Chen CYO, McKay DL, Blumberg JB. 2011. Tree nut phytochemicals: composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr Res Rev 24: 244–275. [DOI] [PubMed] [Google Scholar]