Abstract
Gardner syndrome has head and neck manifestations that may be recognized during dental visits. Features such as multiple gnathic osteomas, impacted supernumerary teeth, and multiple foci of idiopathic osteosclerosis can be easily identified on dental radiographs, prompting the clinician to refer the patient for further investigation. A dental examination and routine radiographs play a vital role in revealing the extracolonic presentation of Gardner syndrome, which facilitates timely screening and detection of colorectal cancer and other malignancies associated with this condition. This report discusses the case of a 50-year-old Caucasian man who presented with a hard swelling of the left angle of the mandible and was diagnosed with Gardner syndrome based on abnormal findings from an oral examination, dental imaging, and medical and family history.
Keywords: Gardner Syndrome, Cone-Beam Computed Tomography, Osteoma, Osteosclerosis
Familial adenomatoid polyposis (FAP) is an autosomal dominant syndrome characterized by the presence of multiple colorectal polyps with a marked tendency toward malignancy.1 FAP with osteomas and benign tumors of the soft tissue is referred to as Gardner syndrome in the literature.2 Symptoms such as diarrhea, constipation, rectal bleeding, anemia, and abdominal pain may be present at the time of diagnosis. People affected by Gardner syndrome have a high risk of developing colorectal cancer at an early age. The prevalence of cancer in patients with symptomatic FAP ranges from 47% to 67%, and 59% of patients diagnosed with Gardner syndrome die from colorectal cancer due to extensive metastasis.3 Almost 100% of colorectal polyps will undergo malignant transformation if left untreated.4
Patients with Gardner syndrome can exhibit various abnormalities of the bone, teeth, and soft tissues. Osteomas usually appear around puberty and become evident before intestinal polyps.5 They may occur at the angle of the mandible and induce marked facial deformity and limited mouth opening. The triad of dental abnormalities comprises odontomas, supernumerary teeth, and impacted teeth.6 The common soft tissue manifestations are epidermoid or sebaceous cysts and desmoid tumors.7
As a multisystemic disease, the diagnosis and treatment of Gardner syndrome may be delayed if a healthcare professional focuses only on their area of expertise instead of taking a systematic approach. This report presents the case of a 50-year-old Caucasian man who had been treated for rectal and liver cancers, but was not diagnosed with Gardner syndrome until being seen in the authors’ dental clinic.
Case Report
A 50-year-old Caucasian man was referred to the Department of Oral and Maxillofacial Surgery at East Carolina University School of Dental Medicine for the evaluation of multiple radiopaque lesions on a panoramic radiograph. The patient’s medical history included stage 4 rectal and liver cancer with a surgical history of ostomy bag placement. The cancer was subsequently treated with chemotherapy, which was completed about 1 year prior to his appointment at the authors’ clinic. The patient also had undergone the surgical excision of multiple epidermal cysts.
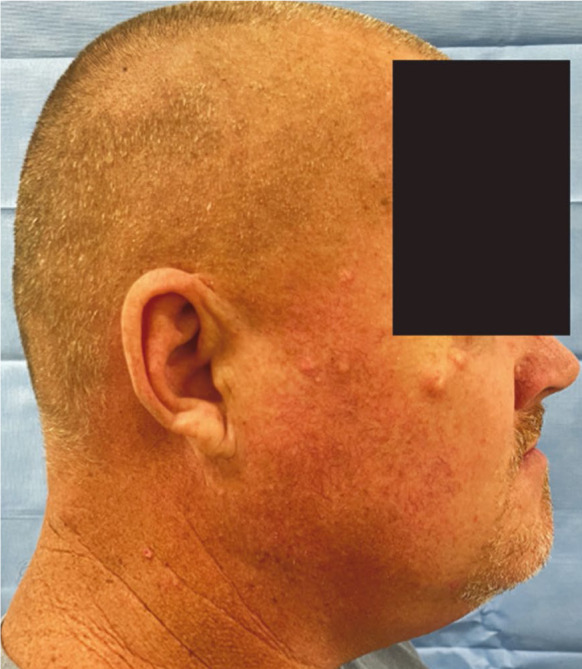
A comprehensive head and neck examination revealed facial asymmetry on the left side of the mandible, which was hard on palpation (Fig. 1A). The patient denied any symptoms and reported that the facial swelling had been present for over a decade. An extraoral examination also revealed multiple, smooth, non-ulcerated, epidermal cysts with yellow-white discoloration that were soft on palpation in the right malar-temporal area (Fig. 1B).
Fig. 1A. A frontal view of the patient’s face shows an extraoral asymmetry on the lower left side.
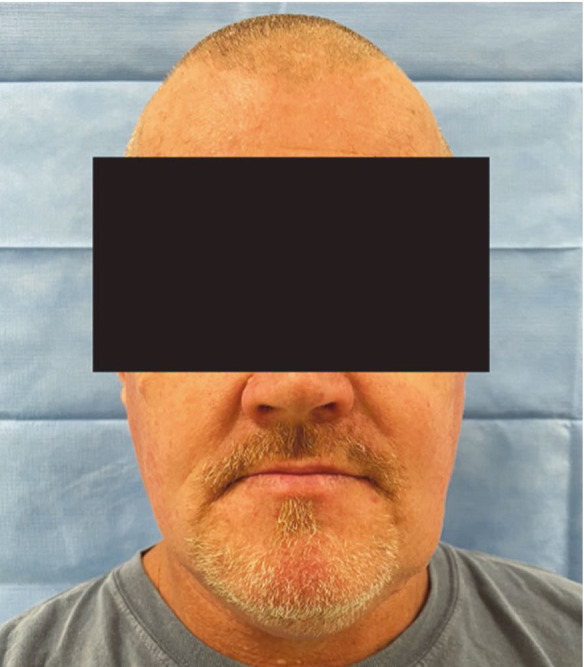
Fig. 1B. A lateral view of the patient’s face illustrates multiple epidermal cysts in the malar and temporal regions.
Intraorally, the patient had multiple missing and mobile teeth, and poor oral hygiene characterized by calculus and debris accumulation. The left mandibular second premolar was identified as partially erupted, and a few deciduous teeth, including the right maxillary second primary molar, and right mandibular first and second primary molars, were also retained and mobile.
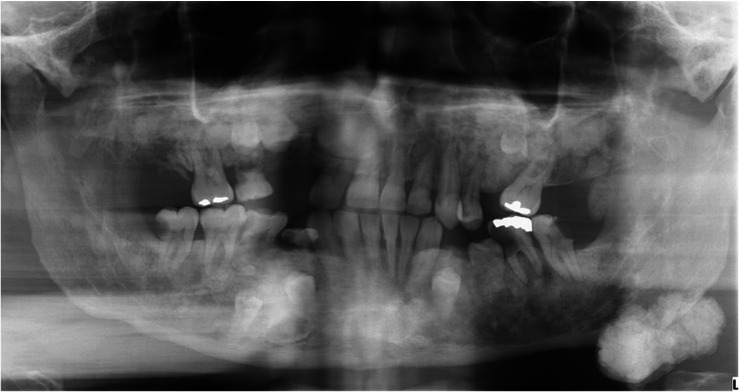
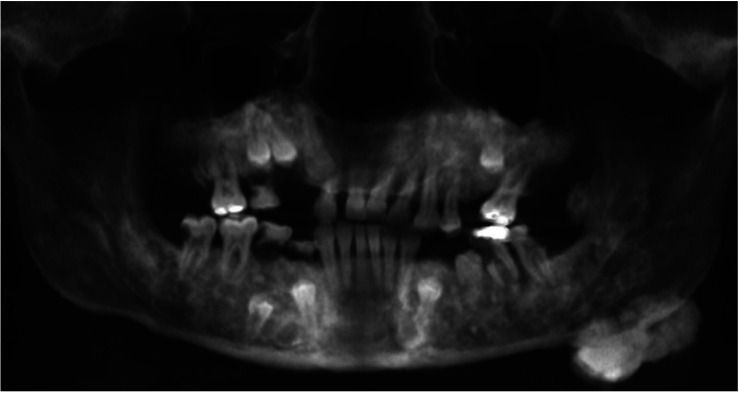
The panoramic radiograph revealed multiple ill-defined radiopaque areas throughout the maxilla and mandible (Fig. 2). Large lobulated radiopaque lesions abutting the inferior border of the left mandibular angle and anterior surface of the left ascending ramus were also observed. The right maxillary permanent canine, first and second premolars, left maxillary permanent second premolar, left mandibular permanent first premolar, and right mandibular permanent first and second premolars were recognized as impacted (Fig. 2).
Fig. 2. A panoramic radiograph demonstrates multiple impacted teeth, idiopathic osteosclerosis, and osteomas at the inferior border and the anterior ascending ramus of the left mandible.
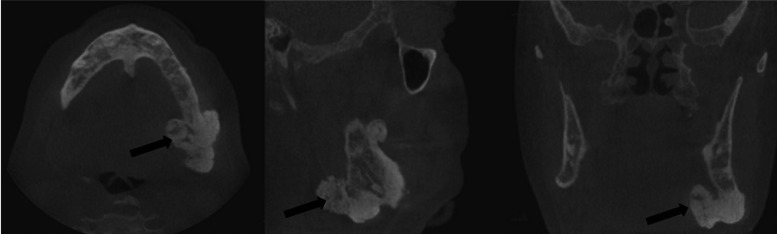
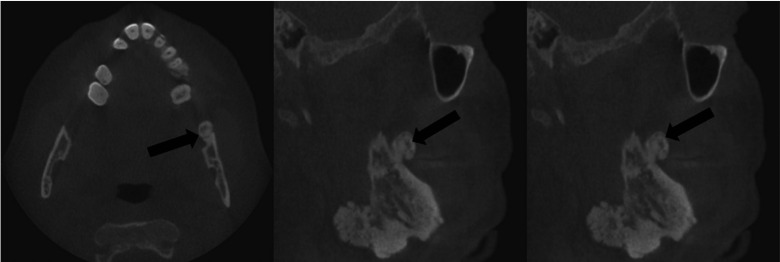
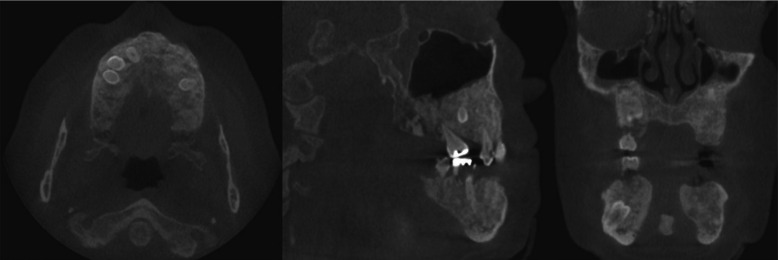
Cone-beam computed tomography was performed for a further assessment (Fig. 3A). Multiple osteomas, including 1 attached at the left mandibular angle (Fig. 3B) and 1 abutting the anterior border of the left ascending ramus (Fig. 3C), were noted. Multiple impacted teeth and widespread idiopathic osteosclerosis causing alveolar expansion were also observed in the maxilla and mandible on reconstructed orthogonal and panoramic projections (Figs. 3D and 4).
Fig. 3A. Three-dimensional cone-beam computed tomographic reconstruction with bone window demonstrates multiple tooth impaction, alveolar expansion, and osteomas at the inferior border and anterior ascending ramus of the left mandible.
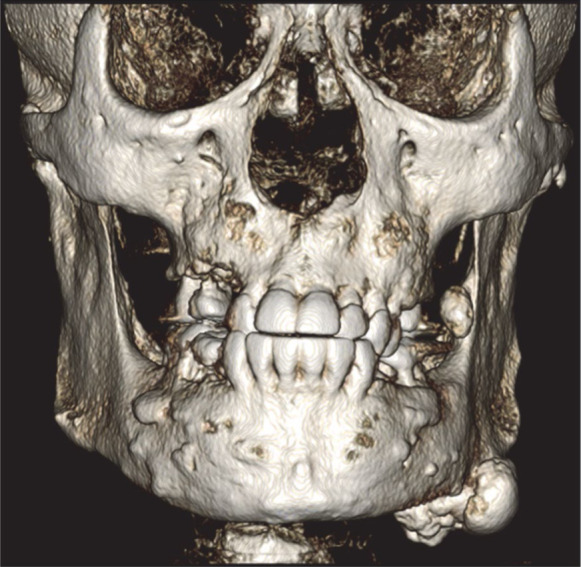
Fig. 3B. An osteoma is noted at the left mandibular angle on the orthogonal reconstructions of a cone-beam computed tomographic scan.
Fig. 3C. Another osteoma is noted abutting the anterior border of the left ascending ramus in orthogonal reconstructions of a cone-beam computed tomographic scan.
Fig. 3D. Multiple impacted teeth and widespread idiopathic osteosclerosis causing alveolar expansion are observed in the maxilla and mandible on orthogonal reconstructions of a cone-beam computed tomographic scan.
Fig. 4. Panoramic reconstruction of a cone-beam computed tomographic scan demonstrates multiple impacted teeth and widespread idiopathic osteosclerosis of both arches, as well as osteoma attached at the inferior border and anterior ascending ramus of the left mandible.
A further discussion with the patient about his condition revealed that his 2 nieces had been diagnosed with Garner syndrome. Based on the patient’s head and neck examination, panoramic radiograph, cone-beam computed tomographic (CBCT) scan, and medical and family history, he was informed that he had Gardner syndrome as well. Restorative work, periodontal cleaning, and extraction were planned for the patient. Removal of the osteomas was not proposed at this time since they did not present cosmetic or functional concerns. Informed consent was obtained from the patient for publishing the case with minimal disclosure of confidential information.
Discussion
FAP is an inherited condition that arises from mutations in the adenomatoid polyposis coli (APC) gene, which produce premalignant polyps in the colon and rectum. These polyps usually occur during adolescence, with a malignant transformation around the fourth decade of life.8 Frameshift and nonsense mutations account for more than 90% of cases. It is hypothesized that the mutated gene initiates a neoplastic process by disrupting several stages of cell development. The APC gene is considered a tumor suppressor gene. When deleted or mutated, as occurs in most FAP-associated lesions, it triggers tumorigenesis in the gastrointestinal (GI) tract.9 The patient presented herein reported a history of liver and rectal cancer, which was treated with surgical resection and consequent placement of an ostomy bag. This concurs with the GI symptoms of most FAP patients.
Although GI malignancy is the hallmark of FAP, a constellation of extraintestinal lesions is considered a critical component of the diagnostic criteria and prognostic indicators for Gardner syndrome. These include osteomas, dental abnormalities, and skin pathology.
Osteomas are a significant component of Gardner syndrome. The frontal bones are the most frequently involved, followed by the maxilla and mandible.10,11 Although most of these lesions can be palpated during a physical examination, they are also easily detected during routine radiographic examination. Lobulated peripheral osteomas are most frequently found at the mandibular angle arising from the surface of the bone.4,11 This patient’s panoramic radiograph and CBCT scan showed lobulated osteomas on the left angle and ascending ramus of the mandible, as well as widespread heterogeneous idiopathic osteosclerosis involving the maxilla and mandible.
Dental abnormalities are found in 30% to 75% of affected individuals, including tooth ankylosis, multiple unerupted/impacted teeth, supernumerary teeth, anodontia, hypercementosis, and compound odontomas.4 Impacted permanent teeth and retained primary teeth were observed in our patient upon clinical and radiographic examinations. Interestingly, a specific mutation in the APC gene was recently described to be closely associated with dental anomalies, especially supernumerary teeth and odontomas.12
Besides hard tissue abnormalities, the commonest skin manifestations of Gardner syndrome include epidermoid or sebaceous cysts (66%). They are found on the face, scalp, and extremities, and are usually asymptomatic. Some patients may develop mild pruritus or signs of inflammation. This patient demonstrated multiple epidermal cysts in the malar and temporal region, which is a common skin indication in this group of patients.
As a multisystemic condition, patients with FAP may present other extraintestinal lesions such as nasopharyngeal angiofibromas, desmoid fibromatosis, adrenal adenomas, and malignant neoplasms of the liver, gallbladder, biliary tract, thyroid, and central nervous system.13,14,15 A rare manifestation of FAP is the development of hepatoblastoma, which in non-FAP circumstances, is the most common pediatric liver malignancy.16 Although uncommon, patients with an APC germline mutation have a high risk of developing hepatoblastomas.17 The patient presented herein disclosed a history of hepatoblastoma, which had been treated with surgical removal and chemotherapy.
APC gene testing helps in the pre-symptomatic diagnosis for patients at risk or with a familial history of FAP. Screening for FAP-associated tumors should be done for patients with a known APC mutation and known family history.18 Detailed investigations in patients with extraintestinal manifestations suggestive of FAP should not be overlooked. Currently, testing is recommended for patients with close relatives known to have FAP, 10 to 20 intestinal polyps, and patients with colorectal adenomas in combination with the extraintestinal manifestations of FAP, such as gnathic osteomas and dental abnormalities.19 In this case, the presence of a variety of gnathic, dental, and skin manifestations, a past medical history of liver and colorectal malignancies, and a familial history of FAP prompted the clinical diagnosis of Gardner syndrome. Genetic testing was recommended for the patient.
Surprisingly, the patient was not aware of the diagnosis until being seen in our clinic. Since dental-gnathic manifestations normally develop prior to the malignant transformation of colorectal polyps in individuals with Gardner syndrome, the dental profession is at the front line to screen and initiate proper referrals and work-ups for these patients to preserve lives and reduce medical costs. This case also reiterates the importance of a holistic mindset and interprofessional approach for the assessment and management of patients with Gardner syndrome.
In conclusion, patients with Gardner syndrome experience a high mortality rate if they are not promptly diagnosed and managed properly. Dental professionals are well-positioned to identify classic extraintestinal manifestations of Gardner syndrome, such as osteomas, odontomas, impacted/supernumerary teeth and hypercementosis during head and neck examinations and through routine dental radiography. A thorough patient interview with attention to the family history is also essential for screening and appropriate referral. If known, the family history should include first- and second-degree relatives with a history of cancer or gastrointestinal disease, the age of diagnosis, and any relevant genetic testing. Regardless of the type of practice or specialty, dental practitioners should become familiar with the oral manifestations of systemic diseases and promptly refer suspected patients for further evaluations and testing.
Footnotes
Conflicts of Interest: None
References
- 1.Baldino ME, Koth VS, Silva DN, Figueiredo MA, Salum FG, Cherubini K. Gardner syndrome with maxillofacial manifestation: a case report. Spec Care Dentist. 2019;39:65–71. doi: 10.1111/scd.12339. [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi T, Takenoshita Y, Kubo K, Iida M. Natural course of jaw lesions in patients with familial adenomatosis coli (Gardner’s syndrome) Int J Oral Maxillofac Surg. 1993;22:226–230. doi: 10.1016/s0901-5027(05)80641-1. [DOI] [PubMed] [Google Scholar]
- 3.Chimenos-Küstner E, Pascual M, Blanco I, Finestres F. Hereditary familial polyposis and Gardner’s syndrome: contribution of the odonto-stomatology examination in its diagnosis and a case description. Med Oral Patol Oral Cir Bucal. 2005;10:402–409. [PubMed] [Google Scholar]
- 4.Cankaya AB, Erdem MA, Isler SC, Cifter M, Olgac V, Kasapoglu C, et al. Oral and maxillofacial considerations in Gardner’s syndrome. Int J Med Sci. 2012;9:137–141. doi: 10.7150/ijms.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomaidis V, Seretis K, Tsoucalas G, Razos K, Vasilopoulos A, Efenti GM, et al. A case of early FAP diagnosis with extraintestinal manifestations on the face. Acta Med Acad. 2019;48:217–224. doi: 10.5644/ama2006-124.260. [DOI] [PubMed] [Google Scholar]
- 6.Panjwani S, Bagewadi A, Keluskar V, Arora S. Gardner’s syndrome. J Clin Imaging Sci. 2011;1:65. doi: 10.4103/2156-7514.92187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fotiadis C, Tsekouras DK, Antonakis P, Sfiniadakis J, Genetzakis M, Zografos GC. Gardner’s syndrome: a case report and review of the literature. World J Gastroenterol. 2005;11:5408–5411. doi: 10.3748/wjg.v11.i34.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma H, Brosens LA, Offerhaus GJ, Giardiello FM, de Leng WW, Montgomery EA. Pathology and genetics of hereditary colorectal cancer. Pathology. 2018;50:49–59. doi: 10.1016/j.pathol.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 10.Dawlatly ED, Al-Qurain AA, Salih-Mahmud M. Gardner’s syndrome presenting with a giant osteoma. Ann Saudi Med. 1997;17:542–544. doi: 10.5144/0256-4947.1997.542. [DOI] [PubMed] [Google Scholar]
- 11.Madani M, Madani F. Gardner’s syndrome presenting with dental complaints. Arch Iran Med. 2007;10:535–539. [PubMed] [Google Scholar]
- 12.Yu F, Cai W, Jiang B, Xu L, Liu S, Zhao S. A novel mutation of adenomatous polyposis coli (APC) gene results in the formation of supernumerary teeth. J Cell Mol Med. 2018;22:152–162. doi: 10.1111/jcmm.13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valanzano R, Curia MC, Aceto G, Veschi S, De Lellis L, Catalano T, et al. Genetic evidence that juvenile nasopharyngeal angiofibroma is an integral FAP tumour. Gut. 2005;54:1046–1047. doi: 10.1136/gut.2005.065508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coffin CM, Hornick JL, Zhou H, Fletcher CD. Gardner fibroma: a clinicopathologic and immunohistochemical analysis of 45 patients with 57 fibromas. Am J Surg Pathol. 2007;31:410–416. doi: 10.1097/01.pas.0000213348.65014.0a. [DOI] [PubMed] [Google Scholar]
- 15.Attard TM, Giglio P, Koppula S, Snyder C, Lynch HT. Brain tumors in individuals with familial adenomatous polyposis: a cancer registry experience and pooled case report analysis. Cancer. 2007;109:761–766. doi: 10.1002/cncr.22475. [DOI] [PubMed] [Google Scholar]
- 16.Trobaugh-Lotrario AD, López-Terrada D, Li P, Feusner JH. Hepatoblastoma in patients with molecularly proven familial adenomatous polyposis: clinical characteristics and rationale for surveillance screening. Pediatr Blood Cancer. 2018;65:e27103. doi: 10.1002/pbc.27103. [DOI] [PubMed] [Google Scholar]
- 17.Yang A, Sisson R, Gupta A, Tiao G, Geller JI. Germline APC mutations in hepatoblastoma. Pediatr Blood Cancer. 2018;65:e26892. doi: 10.1002/pbc.26892. [DOI] [PubMed] [Google Scholar]
- 18.Giardiello FM, Brensinger JD, Petersen GM. AGA technical review on hereditary colorectal cancer and genetic testing. Gastroenterology. 2001;121:198–213. doi: 10.1053/gast.2001.25581. [DOI] [PubMed] [Google Scholar]
- 19.Provenzale D, Gupta S, Ahnen DJ, Bray T, Cannon JA, Cooper G, et al. Genetic/familial high-risk assessment: colorectal version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:1010–1030. doi: 10.6004/jnccn.2016.0108. [DOI] [PubMed] [Google Scholar]