Abstract
Introduction
Prostatic stromal sarcoma is an extremely rare malignancy of the prostate with a poor prognosis.
Case presentation
A 65‐year‐old man presented with dyschezia, and computed tomography showed a large prostate mass. The diagnosis was prostate stromal sarcoma by transrectal needle biopsy. Magnetic resonance imaging suggested rectal infiltration. The patient underwent 4 courses of neoadjuvant chemotherapy with gemcitabine and docetaxel hydrate followed by total pelvic exenteration.
Conclusion
No recurrence has occurred at 5 years after the surgery. This is the first report of complete resection in prostate stromal sarcoma after neoadjuvant chemotherapy with gemcitabine and docetaxel hydrate.
Keywords: docetaxel, gemcitabine, neoadjuvant chemotherapy, prostate, stromal sarcoma
Abbreviations & Acronyms
- AC
adjuvant chemotherapy
- ACT
actinomycin
- AWD
alive with disease
- CDDP
cisplatin
- CPT‐11
irinotecan
- CT
computed tomography
- CTh
chemotherapy
- DOD
dead of disease
- DOXO
doxorubicin
- DR
distant recurrence
- DTX
docetaxel hydrate
- Dx
diagnosis
- ER
estrogen receptors
- GEM
gemcitabine
- IFO
ifosfamide
- LR
local recurrence
- MMC
mitomycin C
- MRI
magnetic resonance imaging
- n.r.
not reported
- NAC
neoadjuvant chemotherapy
- NCI CTCAE
National Cancer Institute Common Terminology Criteria for Adverse Events
- NED
no evidence of disease
- PgR
progesterone receptors
- PIRA
pirarubicin
- PR
partial response
- PSPUMP
proliferation of uncertain malignant potential
- PSS
prostate stromal sarcoma
- RCP
radical cystoprostatectomy
- RP
radical prostatectomy
- RT
radiotherapy
- SD
stable disease
- STUMP
stromal tumors of uncertain malignant potential
- VCR
vincristine
- Vp‐16
etoposide
Keynote message.
PSS is an extremely rare malignancy with a poor prognosis. Surgical resection is considered the treatment of choice as the effectiveness of NAC is unknown. Here, we report a case of PSS, in which the tumor was completely resected after NAC with GEM and DTX.
Introduction
PSS is an extremely rare malignancy of the prostate with a poor prognosis. In 1998, PSS was classified into 2 types: PSS and STUMP. Surgical resection is the treatment of choice, as the effectiveness of NAC is unknown. 1 Here, we report a case of PSS, in which the tumor was completely resected after NAC with GEM and DTX.
Case report
A 65‐year‐old man presented with dyschezia and was admitted to a local hospital in Thailand.
Rectal examination indicated a prostate mass, and contrast‐enhanced CT showed a contrast‐enhanced, 62 × 40 mm2 mass with rectal invasion, mainly in the right lobe of the prostate (Fig. 1a). The PSA value was 1.95 ng/ml (normal range at <4.0 ng/ml).
Fig. 1.
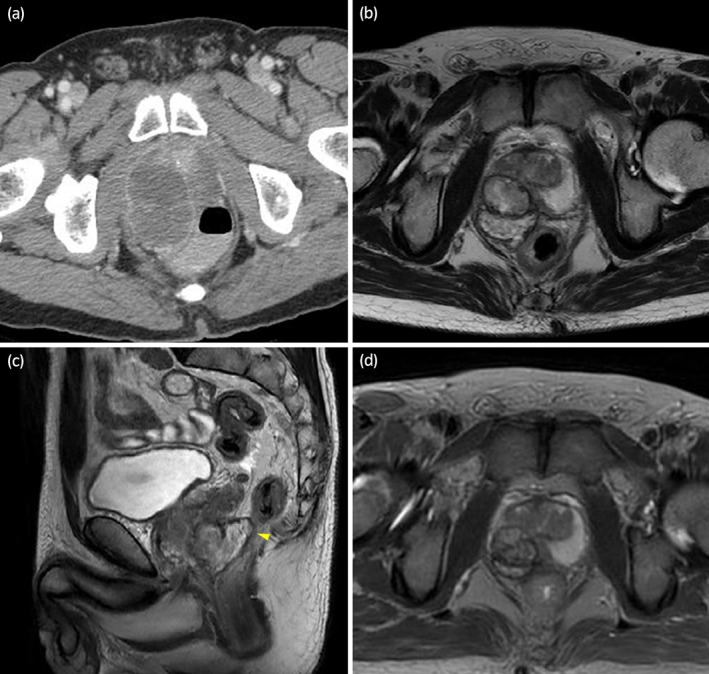
(a) Contrast‐enhanced CT shows a 62 × 40 mm2 mass in the right prostatic lobe. (b, c) MRI after two courses of CTh shows a 48% reduction in tumor size and suggests rectal invasion (yellow arrowhead). (d) MRI after four courses of CTh shows a 75% reduction in tumor size from the initial examination.
Prostate biopsy showed a high concentration of nuclei, necrosis, and small atypical cells, as well as increased nuclear fragmentation in the right lobe. Staining was positive for vimentin, negative for CD34, PSA, desmin, cytokeratin, and CD117, and highly positive for Ki‐67 (80%), indicating PSS. Whole‐body CT showed no lymph nodes or distant metastases.
The patient was diagnosed with PSS.
Chemotherapy with GEM 1 g/m2 (days 1, 8) and DTX 70 mg/m2 (day 1) was administered. After two courses of CTh, MRI showed that the volume of the mass was reduced by 48% to 36 × 36 mm, indicating a PR to the CTh (Fig. 1b,c). T2‐weighted MRI showed possible invasion of the rectal mucosa and serosa. After undergoing one more course of CTh, the patient returned to Japan for further treatment at our hospital.
Physical examination, all other routine blood tests, and urine tests were normal. Transrectal prostate biopsy was performed to evaluate the response to CTh. On pathological examination, coagulative necrosis of the tumor tissue, with surrounding fibrosis, histiocytic infiltration and hemosiderin deposition were observed, suggesting the effects of CTh. However, the histology was difficult to determine.
Based on the tumor shrinkage and the results of the prostate biopsy at our hospital, CTh with GEM and DTX was considered to be effective.
Another course was performed following the regimen for endometrial cancer in our hospital, a combination of GEM (900 mg/m2; days 1, 8) and DTX (75 mg/m2;days 1). Neutropenia [grade4 by NCI CTCAE] occurred on days 13–16 of treatment. MRI showed that the tumor had reduced by 75% from the initial examination (Fig. 1d). Total pelvic exenteration, an ileal conduit, and colostomy were planned.
After an incision in the middle lower abdomen, we observed no adhesions to surrounding organs, but the border between the tumor and rectum was indistinct. Since the length of the rectum from the pelvic floor to the tumor was found to be more than 3 cm, the anus was preserved, and a temporary small bowel stoma was created with combined resection of the rectum for a margin. Macroscopically, the right lobe of the prostate showed a 2.7 × 2.3 cm2, yellowish‐white, smooth‐surfaced ampullary area with necrosis and hemorrhage (Fig. 2). Histopathological findings showed necrotic tissue in the right lobe of the prostate with a small tumor component in the center. There was complex growth of spindle‐shaped cells with round to oval nuclei and narrow cytoplasm (Fig. 3a). Immunohistochemically, the tumor cells were negative for desmin, CD34, C‐kit, PgR, and ER, indicating sarcoma (Fig. 3b–e). The percentage of Ki‐67 positive cells was 50% (Fig. 3f). Vitrification and fibrosis extended into the surrounding fat, suggesting that there may have been extraprostatic invasion prior to treatment. Based on the histopathological findings, the final Dx was PSS.
Fig. 2.
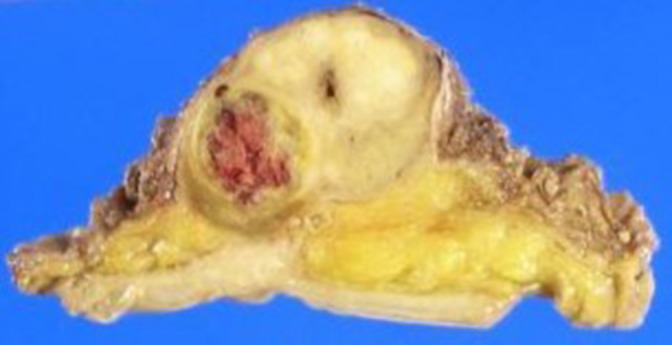
Macroscopically, the right lobe of the resected prostate shows a 2.7 × 2.3 cm3, yellowish‐white, smooth‐surfaced ampullary area with necrosis and hemorrhage.
Fig. 3.
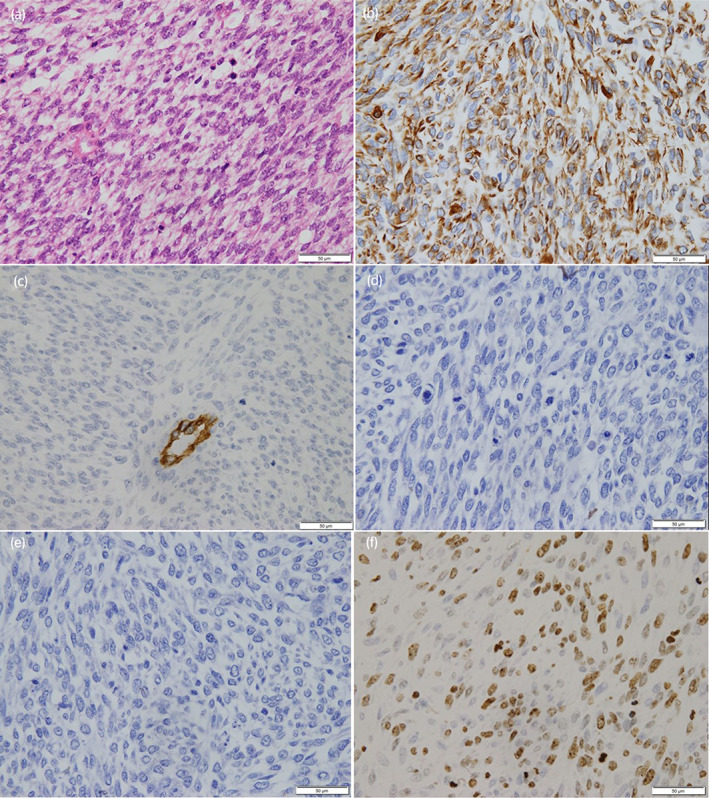
Histopathology of the prostatic stromal sarcoma. Spindle‐shaped cells are observed with an increased nucleocytoplasmic ratio. (a) Immunohistochemistry shows that the tumor is positive for vimentin (b), but negative for CD34, PgR, and ER (c–e). (f)The percentage of Ki‐67 positive cells was 50%.
The patient was discharged on postoperative day 39. The small bowel stoma was closed 126 days after total pelvic organ resection, and there was no sign of recurrence or metastasis at 5 years after surgery.
Discussion
The main histological types of prostate sarcoma in adults are leiomyosarcoma and rhabdomyosarcoma, whereas PSS is a rarer histological type. Approximately 30 cases of PSS have been reported. 1 , 2 PSS was reported previously using various names, such as phyllodes tumor; however, in 1998, cases of PSS where considered either prostatic stromal PSPUMP or PSS according to the degree of cellularity, mitotic figures, necrosis, and stromal overgrowth for the purpose of understanding biological prognosis and guiding treatment. 3 The WHO has since adopted the names STUMP and PSS. 4
Analysis of the PSS reports indicated that patients were 22 to 65 years old (median age, 38 years). Although most initial symptoms of PSS are dysuria, hematuria, and urinary retention, PSS has no specific clinical symptoms. 2 The most common metastatic site is the lung. 1
There are no specific imaging findings for PSS; however, in the present case, MRI detected rectal invasion. PSS may not increase PSA levels, which were normal in the present case. 2
Compared to STUMP, PSS shows greater cellularity, mitosis, necrosis and stromal overgrowth. 5
While immunohistochemistry can help diagnose PSS, it does not provide a definitive Dx. 2 PSS is generally vimentin, CD34, and PgR positive, and negative for ER, but CD34 and PgR were negative in this case. 1 , 2 Most cases show an increased Ki‐67 labeling index, with the present case showing an 80% increase. 1
The treatment of PSS is mainly surgical resection, with extended resection selected according to the degree of tumor growth. 1 Complete excision is recommended because PSS may rapidly develop LR or distant metastasis after incomplete excision. 5 This case achieved long‐term survival with an extended resection for tumor control.
It is not known whether radiation therapy or CTh can prolong survival. 1 Median survival is <15 months, and 10% of patients have a survival rate of >3 years. 6 Eleven reports of CTh for PSS describe the regimens and outcomes, including the present case. 1 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 The reports are summarized in Table 1. Doxorubicin and IFO were the most commonly selected regimens. 8 , 9 The regimen with GEM and DTX was reported to be useful in a Phase II study for nongastrointestinal stromal sarcomas. 16 Although the use of GEM and DTX for PSS has been reported only in the present case, it showed a clear tumor reduction effect of 75%, and surgical resection was performed at an appropriate time, thus avoiding permanent colostomy and achieving both cancer control and quality of life with a good long‐term prognosis. Results suggest that extended resection after NAC may be a treatment option for locally advanced PSS if histological Dx is obtained before surgery.
Table 1.
Treatment, response to CTh and outcomes in 11 patients with prostatic stromal sarcoma
| Ref. (year) | Age | Tumor extension | Treatment first line | Second line | Drugs (cycles) | Response to CT | Outcome | |
|---|---|---|---|---|---|---|---|---|
| 1 | 11 (2001) | 36 | Prostate extending to neck of bladder | RP | NAC + RT (70 Gy/35f) | DOXO + IFO (6) | PR | LR, DOD 20 months after Dx |
| 2 | 8 (2002) | 35 | Prostate, pelvic wall | RP + RT (60 Gy) | NAC + lobectomy | DOXO + IFO (5) | PR | DR (lung), NED 5 months after lobectomy |
| 3 | 12 (2003) | 78 | Prostate extending to bladder, abdominal walls | RP + AC | VCR + MMCO + DOXO + CDDP (6) | n.r. | DR (liver), DOD 6 months after CTh | |
| 4 | 7 (2006) | 19 | Prostate | RP | NAC + RT (49.2 Gy) | VP‐16 + IFO + CDDP (4) | PR | NED 48 months after CTh and RT |
| 5 | 13 (2007) | 52 | Prostate | RCP | NAC + metastatectomy | CDDP + PIRA + IFO (3) | n.r. | LR, AWD 12 months after metastatectomy |
| 6 | 14 (2010) | 31 | Prostate | NAC + RP | Cystectomy | DOXO + IFO (2) | SD | LR, DOD 3 months after cystectomy |
| 7 | 15 (2010) | 34 | Prostate | RP + RT (60 Gy) | Lung metastatectomy + palliative RT and CTh | DOXO + IFO (1) | PR | LR, DR (lung), DOD 25 months after Dx |
| 8 | 16 (2012) | 63 | Prostate extending to bladder | NAC + total pelvic exenteration | CDDP + CPT‐11 (1) | n.r. | NED 16 months after RCP | |
| 9 | 9 (2014) | 14 | Prostate extending to bladder, seminal vesicles, lymph nodes, bone | NAC + RT (50 Gy) + RCP | IFO + VCR + ACT + DOXO (9) | PR | DOD 15 months after Dx | |
| 10 | 2 (2020) | 40 | Prostate extending to the seminal vesicles and the rectal muscularis propria. | Total pelvic exenteration + AC | DOXO + IFO (4) | n.r. | NED 10 months after RCP | |
| 11 | Present case | 65 | Prostate extended to rectum | NAC + total pelvic exenteration | DTX + GEM (4) | PR | NED 5y after RCP |
Conclusion
In the present case of PSS, NAC using GEM and DTX and total pelvic exenteration resulted in survival without recurrence at 5 years after surgery, suggesting the effectiveness of multidisciplinary treatment for PSS.
Author contributions
Naoto Hodotsuka: Writing – original draft. Yasutomo Suzuki: Supervision. Kyota Suzuki: Writing – review and editing. Yuichiro Honda: Writing – review and editing. Shuma Endo: Writing – review and editing. Eigo Kuribayashi: Writing – review and editing. Kotaro Obayashi: Writing – review and editing. Tadaaki Minowa: Writing – review and editing. Tsutomu Hatori: Visualization. Yukihiro Kondo: Supervision.
Conflict of interest
The authors declare no conflicts of interests.
Approval of research protocol by an Institutional Reviewer Board
Not applicable.
Informed consent
The patient provided informed consent for publication of this case report.
Registry and the Registration No. of the study/trial
Not applicable.
Acknowledgments
Not applicable.
References
- 1. Ueda S, Okada K, Kanzawa M, Fukuda T, Furukawa J, Fujisawa M. A case of prostate stromal sarcoma involving the rectum. J. Surg. Case Rep. 2020; 6: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang W, Liu A, Wu J, Niu M. Prostatic stromal sarcoma: a case report and literature review. Medicine 2018; 97: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gaudin PB, Rosai J, Epstein JI. Sarcomas and related proliferative lesions of specialized prostatic stroma: a clinicopathologic study of 22 cases. Am. J. Surg. Pathol. 1998; 22: 148–62. [DOI] [PubMed] [Google Scholar]
- 4. Eble JN, Sauter G, Epstein JI, Sesterhenn IA. World Health Organization Classification of Tumors. Pathology and Genetics. Tumors of the Urinary System and Male Genital Organs IARC Press, Lyon, 2004; 209–11. [Google Scholar]
- 5. Osaki M, Osaki M, Takahashi C, Miyagawa T, Adachi H, Ito H. Prostatic stromal sarcoma: case report and review of literature. Pathol. Int. 2003; 53: 407–11. [DOI] [PubMed] [Google Scholar]
- 6. Choi SH, Kim TH, Yoon GS, Chung SK, Kim BW, Kwon TG. Two different surgical approaches for prostatic stromal sarcoma: robot‐assisted laparoscopic radical prostatectomy and open radical cysto‐prostatectomy with ileal conduit. Korean J. Urol. 2014; 55: 620–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sakura M, Tsukamoto T, Yonese J, Ishikawa Y, Aoki N, Fukui I. Successful therapy of a malignant phyllodes tumor of the prostate after postoperative local failure. Urology 2006; 67: e11–845.e13. [DOI] [PubMed] [Google Scholar]
- 8. Lam KC, Yeo W. Chemotherapy induced complete remission in malignant phyllodes tumor of the prostate metastasizing to the lung. J. Urol. 2002; 168: 1104–5. [DOI] [PubMed] [Google Scholar]
- 9. Cavaliere E, Alaggio R, Castagnetti M, Scarzello G, Bisogno G. Prostatic stromal sarcoma in an adolescent: the role of chemotherapy. Rare Tumors 2014; 6: 146–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raeve HD, Jeuris W, Wyndaele JJ, Marck EV. Cystosarcoma phyllodes of the prostate with rhabdomyoblastic differentiation. Pathol. Res. Pract. 2001; 197: 657–62. [DOI] [PubMed] [Google Scholar]
- 11. Agrawal V, Sharma D, Wadhwa N. Case report: malignant phyllodes tumor of prostate. Int. Urol. Nephrol. 2003; 35: 37–9. [DOI] [PubMed] [Google Scholar]
- 12. Morikawa T, Goto A, Tomita K et al. Recurrent prostatic stromal sarcoma with massive high‐grade prostatic intraepithelial neoplasia. J. Clin. Pathol. 2007; 60: 330–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim JY, Cho YM, Ro JY. Prostatic stromal sarcoma with rhabdoid features. Ann. Diagn. Pathol. 2010; 14: 453–6. [DOI] [PubMed] [Google Scholar]
- 14. Colombo P, Ceresoli GL, Boiocchi L et al. Prostatic stomal tumor with fatal outcome in a young man: histopathological and immunohistochemical case presentation. Rare Tumors 2010; 2: e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamazaki H, Ohyama T, Tsuboi T et al. Prostatic stromal sarcoma with neuroectodermal differentiation. Diagn. Pathol. 2012; 7: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maki RG. Gemcitabine and docetaxel in metastatic sarcoma: past, present, and future. Oncologist 2007; 12: 999–1006. [DOI] [PubMed] [Google Scholar]


