Abstract
L-Ascorbic acid (AsA) is more commonly known as vitamin C and is an indispensable compound for human health. As a major antioxidant, AsA not only maintains redox balance and resists biological and abiotic stress but also regulates plant growth, induces flowering, and delays senescence through complex signal transduction networks. However, AsA content varies greatly in horticultural crops, especially in fruit crops. The AsA content of the highest species is approximately 1,800 times higher than that of the lowest species. There have been significant advancements in the understanding of AsA accumulation in the past 20 years. The most noteworthy accomplishment was the identification of the critical rate-limiting genes for the 2 major AsA synthesis pathways (L-galactose pathway and D-galacturonic acid pathway) in fruit crops. The rate-limiting genes of the former are GMP, GME, GGP, and GPP, and the rate-limiting gene of the latter is GalUR. Moreover, APX, MDHAR, and DHAR are also regarded as key genes in degradation and regeneration pathways. Interestingly, some of these key genes are sensitive to environmental factors, such as GGP being induced by light. The efficiency of enhancing AsA content is high by editing upstream open reading frames (uORF) of the key genes and constructing multi-gene expression vectors. In summary, the AsA metabolism has been well understood in fruit crops, but the transport mechanism of AsA and the synergistic improvement of AsA and other traits is less known, which will be the focus of AsA research in fruit crops.
Introduction
L-Ascorbic acid (AsA), namely vitamin C, has important functions and antioxidant effects in organisms. It is an indispensable nutrient for human health. However, primates have lost the ability to synthesize AsA due to the mutation of the enzyme in the last step of AsA synthesis. Therefore, AsA must be part of the diet from AsA-rich vegetables and fruits. For plants, AsA not only plays important roles as an antioxidant and quenching free radical, in particular during photosynthesis and photoprotection (Smirnoff 2011), but also is involved in cell growth and division and plant hormone biosynthesis (Lisko et al. 2014). Significant progress has been made in our understanding of AsA metabolism in plants. Many of the major advances in AsA research have been achieved by studying AsA-enriched fruit crops. Although AsA can be detected in all plants, AsA levels show wide variation in fruit crops, and within the same plant there are also significant differences between tissues and organs (Davey et al. 2000). Therefore, various and multifaceted regulatory mechanisms are expected to exist in fruit crops that control AsA metabolism (Liu et al. 2022a).
Wide variation of AsA levels in fruit crops
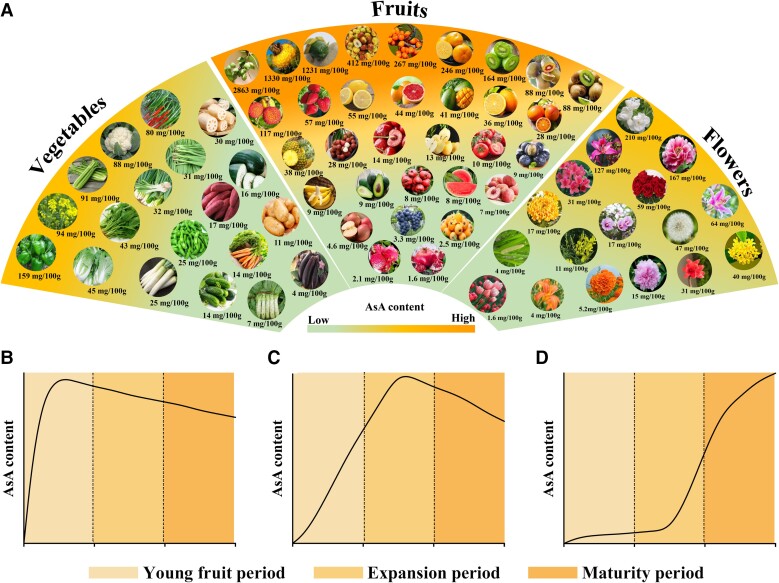
The AsA content of different species of fruit varies greatly, and the AsA content of the same fruit will also have significant differences under different growth conditions or maturities (Valente et al. 2011). The AsA content of the main horticultural crops is listed in Fig. 1A and Supplemental Table S1; all the data were collected from related studies and reports. In fruit, the highest AsA content is the Kakadu plum and the lowest is pomegranate, with the former approximately 1,800 times that of the latter (Miller et al. 1993; Valente et al. 2011). The content of AsA in kiwifruit also varies greatly, the highest being in Actinidia eriantha, which can reach 2,127 mg/100 g fresh weight (FW) (Liao et al. 2021a). In vegetables, the highest AsA content is in sweet pepper and the lowest is in eggplant, which can be 159 mg/100 and 4 mg/100 g, respectively (Ye 2011). In flowers, the highest is in jasmine with 210.63 mg/100 g, and the lowest is in tulip with 1.58 mg/100 g (Wang 2003; Xing 2004).
Figure 1.
AsA content in various horticultural crops (A) and accumulation patterns (B–D). All the data and figures were collected from related studies (Ye 2011; Huang 2013) and reports as well as the Web of Science, China National Knowledge Infrastructure, and public websites. Panels B–D represent different AsA accumulation patterns. The data used to draw schematic diagram were obtained from kiwifruit (A. eriantha), Chinese jujube (cv “Mazao”), and chestnut rose (cv “Guinong 5”) (Huang 2013; Lu et al. 2022; Liu et al. 2022b).
The AsA content also shows different accumulation patterns among fruit crops. Generally, fruit crops can be classified into 3 types according to the periods of AsA peak accumulation appears during fruit growth and development. The first type is AsA peak accumulation at the young fruit period, such as kiwifruit (Liao et al. 2021b) and apple (Li 2009) (Fig. 1B). The second type is AsA peak accumulation at the fruit expansion period, such as jujube (Chen 2015) (Fig. 1C). The third type is fruit AsA peak accumulation at the maturity period, such as chestnut rose (Huang 2013), strawberry (Luo et al. 2019), and tomato (Ioannidi et al. 2009) (Fig. 1D).
Tissue and subcellular distribution of AsA
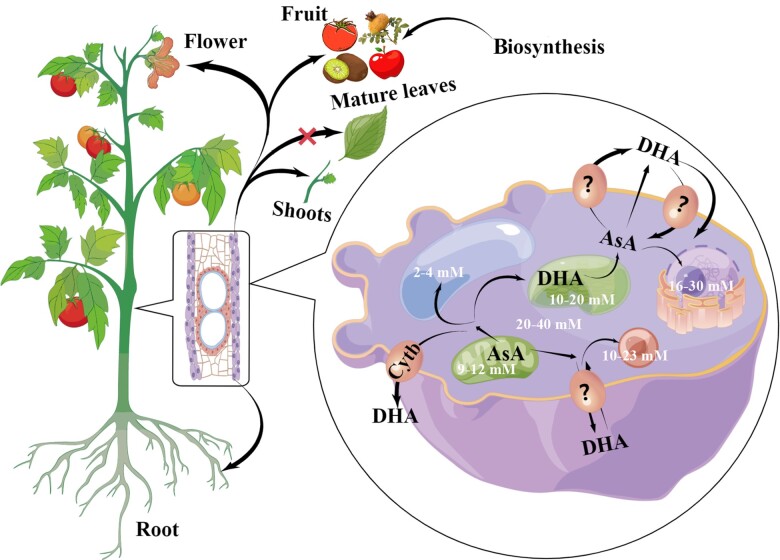
Different plant tissues have different AsA levels, with photosynthetic and storage tissues generally having higher AsA content, and younger tissue having higher AsA content than aged tissue. In Actinidia chinensis cv. “Jinyan” and apple cv. “Gala,” mature leaves were found to have higher AsA content than young leaves, and the pericarp had higher AsA content than the pulp (Li et al. 2008; Liao et al. 2022). In addition, AsA content in different tissues are cultivar specific among different cultivars of the same species. For example, in A. chinensis cv. “Hongyang,” the pulp has higher AsA content than the pericarp (Liao et al. 2022). There is a developing consensus that AsA synthesis can occur within the phloem (Hancock et al. 2003). There were reports on AsA biosynthesis in sink organs (e.g. fruit), including tomato, kiwifruit, chestnut rose, and apple, with higher AsA levels found in the vascular tissues (Li et al. 2008; Huang 2013). Studies on apple and chestnut rose fruit suggest that the accumulation of AsA in fruit may utilize AsA synthesized in other tissues, transported in the long-distance transport tissue in the form of oxidized DHA, which together with in situ synthesis leads to the accumulation of AsA (Li et al. 2008; Huang 2013) (Fig. 2). However, this research cannot fully answer the question of whether AsA accumulation in sink organs occurs as a result of biosynthesis in situ or import from the leaves. Some studies have demonstrated that in long-distance AsA transport from source to sink in model plants, AsA was transported to root tips, shoots, and floral organs but not to mature leaves (Franceschi and Tarlyn 2002; Tedone et al. 2004) (Fig. 2).
Figure 2.
Long-distance transport and metabolism of AsA in plant organelles. AsA was accumulated into phloem and transported to root tips, shoots, and floral organs, but generally not to mature leaves. At the cytological level, AsA is synthesized in mitochondria and then enters the cytoplasm for transport to various organelles. In addition, AsA and DHA can also be transported outside the cell membrane by simple diffusion or transport proteins, such as the Cytb. After AsA functions in the chloroplast, DHA is produced and transported into the cytoplasm. Elements were modified from FigDraw (https://www.figdraw.com/static/index.html).
AsA content also differs among organelles. Usually, the cytoplasm and peroxisomes contain higher levels of AsA, approximately 20 to 40 mM and 10 to 23 mM, respectively (Zechmann et al. 2011). This is followed by the nucleus and chloroplast, approximately 6 to 30 mM and 10 to 20 mM, respectively, with mitochondria having approximately 9 to 12 mM. The lowest levels are in the vacuole, with approximately 2 to 4 mM (Bartoli et al. 2000). Amyloplasts are essentially free of AsA. In addition, the AsA content in chloroplasts was significantly increased under photo-oxidative stress (Zechmann et al. 2011). Due to the last enzymatic step of the main AsA synthesis pathway being located in the mitochondria, much of the synthesized AsA is derived within the mitochondria. However, the concentration of AsA in the mitochondria is lower than that in the cytoplasm, which indicates that AsA leaves the mitochondria via transporters after being synthesized (Horemans et al. 2000) (Fig. 2). It has been found that transmembrane transport of AsA occurs in the chloroplast, vacuole, and plasma membrane, and the transport of AsA on the chloroplast and plasma membrane is an active transport process mediated by a protein carrier (Horemans et al. 2000; Szarka et al. 2004, 2007). The transmembrane transport of AsA into or out of the mitochondria and peroxisomes is unclear, with the possibility that AsA diffuses from mitochondria to the cytoplasm. It is worth mentioning that the apoplast lacks NADPH, GSH, and the corresponding reaction enzymes, resulting in a lack of AsA recycling in the apoplast (Horemans et al. 2008). Therefore, the transporters of AsA and DHA must be present between symplastically isolated cells and tissues. Except for Cytb, which was shown to indirectly cause the transfer of AsA, other putative transporters have not been validated at the molecular level (Rivas et al. 2008; Kosti et al. 2012).
Recognized pathways and genes contributing AsA accumulation in fruit crops
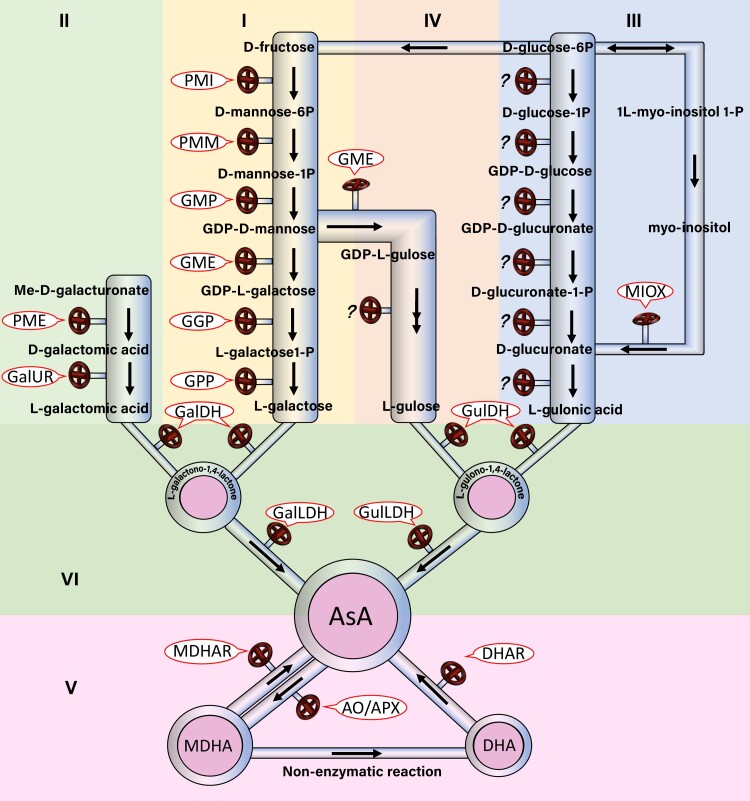
The metabolic pathway of AsA in plants is more complicated than in animals. At present, AsA metabolic pathways are mainly divided into biosynthetic, degradation, and cyclic regeneration pathways (Fig. 3), and it is recognized that there are 4 major biosynthetic pathways: L-galactose, D-galacturonic acid, inositol, and L-gulose. These pathways have been well studied in fruits, such as strawberry and kiwifruit (Foyer et al. 2020; Liao et al. 2021c; Liu et al. 2022a).
Figure 3.
Proposed pathways for AsA metabolism. L-galactose pathway (I), D-galacturonic acid pathway (II), inositol pathway (III), L-gulose pathway (IV), degradation and cyclic regeneration pathway (V) of AsA metabolism, common in the synthetic pathway (VI). PME: Methylesterase; PMI: Mannose-6-phosphate isomerase; PMM: Phosphomannose mutase; GulDH: L-gulose-1,4-lactyl dehydrogenase; GR: Glutathione reductase.
L-galactose pathway
The L-galactose pathway was the first discovered and is recognized as the main synthetic pathway in fruit crops, including apple (Li 2009), kiwifruit (Wei et al. 2021; Liao et al. 2021d), and jujube (Lu et al. 2022). The key enzymes involved in the L-galactose pathway have been largely characterized. Among these, there are 4 recognized key rate-limiting enzyme genes in this pathway: GMP, GME, GGP, and GPP.
GMP (EC 2.7.7.22, GDP-D-mannose pyrophosphorylase) is considered to be the first rate-limiting enzyme of the L-galactose pathway. There was a high correlation between GMP expression and AsA content; AsA content of the plant decreases, and the plant dies rapidly after silencing GMP (Zou et al. 2006; Badejo et al. 2008; Lin et al. 2021). The GMP gene promoter of acerola (Malpighia glabra) had higher activity than the cauliflower mosaic virus 35S and Arabidopsis GMP promoters (Badejo et al. 2008). GMP gene family members also had tissue expression specificity; for example, SlGMP3 and AeGMP2 were confirmed to be involved in AsA synthesis in leaves of tomato (Zhang et al. 2013) and kiwifruit (Liao et al. 2021a), respectively.
GME (EC 5.1.3.18, GDP-D-mannose-3′,5′-epimerase) not only catalyzes GDP-mannose to GDP-L-galactose but also to GDP-L-gulose, considered key evidence for the existence of the L-gulose pathway. In kiwifruit (Bulley et al. 2009) and blueberry (Liu et al. 2015), there was a significant correlation between GME expression level and AsA content during fruit development. A quantitative trait locus (QTL) study of tomato found that GME and AsA contents were closely related (Zou et al. 2006; Stevens et al. 2007). After inhibition of the GME genes, AsA content of the plant was significantly reduced, reactive oxygen species accumulated, and leaves were bleached (Gilbert et al. 2009). Conversely, overexpression of the GME gene can significantly increase AsA content and enhance plant resistance to stress (Imai et al. 2012; Ma et al. 2014). However, some studies have suggested that GME does not limit AsA content. For example, there was no significant correlation between the expression of the GME gene and AsA in tomato (Ioannidi et al. 2009), and overexpression of GME of kiwifruit in Arabidopsis did not significantly change the AsA level (Bulley et al. 2009).
GGP (EC 2.7.7.69, GDP-L-galactose phosphorylase) is considered to be the core gene that regulates AsA (Alegre et al. 2020; Anisimova et al. 2021). The expression of GGP during fruit development in kiwifruit with differing AsA levels was consistent with the changes in the accumulation rate of AsA (Bulley et al. 2009). Recent research on high AsA (A. eriantha) and low AsA (Actinidia rufa) of kiwifruit found that AceGGP3 was highly expressed and positively correlated with high AsA content in the fruit. Furthermore, GGP3 expression also was correlated to AsA concentration in the A. eriantha × A. rufa hybrid, and the expression of the AceGGP3 allele derived from A. eriantha was significantly higher than that of the A. rufa–derived allele AcrGGP3 (Liu et al. 2022a). When the GGP gene of kiwifruit was overexpressed in tobacco, the AsA content in tobacco leaves increased 3-fold, and the AsA content in Arabidopsis was increased by approximately 4-fold. If GGP and GME of kiwifruit were cotransformed into Arabidopsis, the AsA content can be increased by approximately 7-fold (Bulley et al. 2009). In addition, the expression of GGP was also regulated by light (Dowdle et al. 2007) and an upstream open reading frame (Laing et al. 2015; Zhang et al. 2018) to regulate AsA metabolism. Using research on kiwifruit variation in the GGP1 promoter region appears to be key to differences in GGP expression and AsA content in A. eriantha and A. rufa (Wei et al. 2021).
GPP (L-galactose-1-phosphate) was first isolated from kiwifruit, and GPP protein exists as a dimer in kiwifruit with a high AsA content (Laing et al. 2004a) but exists in monomer form in apple with low content (Guo et al., 2011). However, when the GPP gene was suppressed, the AsA content and GPP activity could be detected, indicating that there were other AsA synthesis pathways or other enzymes with GPP-like catalytic activity (Lorence et al. 2004; Conklin et al. 2006; Zhang et al. 2008). In the studies of tomato (Ioannidi et al. 2009), apple leaves (Li et al. 2009), and AsA overaccumulation mutant lines of Arabidopsis (Matteo et al. 2003), GPP was the only gene whose expression was consistent with changes in AsA content. Therefore, the transcriptional regulation of GPP plays an important role in regulating the synthesis and accumulation of AsA (Li et al. 2013a).
The D-galacturonic acid pathway
The D-galacturonic acid pathway is regarded as the main biosynthetic pathway in chestnut rose (An 2004; Huang 2013), sweet orange (Xu et al. 2013), and grape (Cruz-Rus et al. 2010) and is also used as a secondary biosynthetic pathway in kiwifruit (Li et al. 2011). Moreover, this pathway is considered to play a greater role in some tissues at certain stages of plant development. For example, AsA in chestnut rose leaves is mainly synthesized through the D-galacturonic acid pathway (An 2004).
GalUR (EC 1.1.1.19, D-galacturonic acid reductase) was first cloned from strawberry, and overexpression of strawberry GalUR caused a 10-fold to 50-fold increase in GalUR activity and a 2-fold to 3-fold increase in AsA content (Agius et al. 2003). The expression of GalUR in different tissues and development of kiwifruit has shown that GalUR was highly correlated with AsA content. However, the homology of GalUR among different kiwifruit species was low, of approximately 50% to 60% (Li et al. 2011; Wu 2015). Although GalUR gene expression was unrelated to AsA content in chestnut rose, GalUR gene expression was significantly upregulated during the rapid increases in AsA, and the L-galactose pathway was not active at this time (Huang 2013). Studies have also reported that iron deficiency stress can induce the expression of the GalUR gene in apple leaves (Tian 2007). However, it was shown that GalUR on kiwifruit and apples does not participate in the biosynthesis of AsA in leaves (Li 2009). The functional characterization of GalUR-encoded protein has not yet been well characterized, and its selectivity and specificity for D-galacturonic acid has not been determined.
The inositol pathway and L-gulose pathway
Research on the inositol pathway and the L-gulose pathway has mainly occurred in model plants, and the understanding of these pathways in fruit crops is still relatively limited. So far, the gene for the L-gulose pathway in plants has not been identified. Although key genes on the inositol pathway were identified on the chestnut rose, there was no significant correlation between their expression and AsA (Huang 2013).
MIOX (EC 1.13.99.1, Myo-inositol oxygenase) was involved in not only AsA synthesis but also in cell wall formation (Arner et al. 2002). Studies have reported that AsA content increases when MIOX was overexpressed (Lorence et al. 2004; Endres and Tenhaken 2009), but other studies show the opposite results (Kanter et al. 2005; Siddique et al. 2013). Findings in chestnut rose were consistent with the latter, suggesting no significant correlation between MIOX and AsA content (Huang 2013). In A. eriantha, MIOX was found to be involved in the accumulation of AsA during fruit development and was also closely related to A. eriantha fruit ripening (Liao et al. 2021a). MIOX has also been found to play an important role in other metabolism (Siddique et al. 2009; Eckardt 2010; Pieslinger et al. 2010), suggesting the inositol pathway in plants is secondary to AsA synthesis. In addition, SlIMP3 demonstrated high affinity with the L-galactose 1-phosphat and D-myoinositol 3-phosphate and acted as a bifunctional enzyme in the biosynthesis of AsA and myoinositol. Overexpression of SlIMP3 not only improved AsA and myoinositol content but also increased cell wall thickness, improved fruit firmness, delayed fruit softening, decreased water loss, and extended shelf-life (Zheng et al. 2022a).
Common genes that function in several synthetic pathways
Among the many enzymes involved in AsA synthesis, 2 enzymes are shared by the 4 AsA biosynthetic pathways, namely GalDH and GalLDH. These 2 enzymes are also considered to be necessary for AsA biosynthesis and have been studied in several fruit crops.
Among all AsA synthesis–related enzymes, GalDH (EC 1.1.1.117, L-galactose dehydrogenase) is the only one that participates in AsA synthesis but not in any other biochemical reactions. Although the GalDH gene has been cloned from fruit crops such as kiwifruit (Laing et al. 2004b) and apple (Xiao et al. 2007), the relationship between GalDH activity and AsA accumulation has not been clearly reported. Overexpression of GalDH can increase the activity of GalDH but not AsA content (Gatzek et al. 2002), perhaps due to the feedback inhibition of GalDH enzyme by high AsA. This feedback mechanism has been confirmed in spinach, where 1 mM AsA can reduce GalDH enzyme activity by 41% (Pallanca and Smirnoff 2000; Mieda et al. 2004). In addition, due to the very high conversion efficiency of GalDH enzyme toward L-galactose, exogenous L-galactose can be converted into AsA (Smirnoff and Wheeler 2000), resulting in a low L-galactose content.
GalLDH (EC 1.1.1.117, L-galactose-1,4-lactone dehydrogenase) is the last enzyme in the synthesis of AsA. The GalLDH gene has been identified in many plants, such as kiwifruit (Wu 2015) and chestnut rose (An et al. 2005a). GalLDH was highly specific for L-galactose-1,4-lactone, and the enzymatic activity was inhibited by high concentrations of L-galactose-1,4-lactone. A large number of studies have confirmed that the GalLDH gene and AsA content were highly correlated (Pateraki et al. 2004; Liao et al. 2021a). Antisense suppression of GalLDH mRNA led to a significant decline in the GalLDH activity (Tabata et al. 2001). However, attempts to increase AsA via GalLDH failed, mainly because this protein was located in the inner mitochondria membrane (Alhagdow et al. 2007). It has been reported that this gene may be induced by light to regulate AsA content (Smirnoff and Wheeler 2000; Dowdle et al. 2007; Liao et al. 2019), perhaps via light induction of expression (Tamaoki et al. 2003) or photorespiration-dependent changes directly affecting GalLDH enzyme activity (Millar et al. 2003; Bartoli et al. 2006).
Degradation and cyclic regeneration pathway
In plants, the degradation and cyclic regeneration pathways are coupled together to maintain the balance of AsA. Due to AsA's role in removing reactive oxygen species, these pathways are integral to plant resistance to biotic and abiotic stresses (Chen et al. 2003; Singh et al. 2014). Plants mainly degrade AsA through AO (EC 1.10.3.3, ascorbate oxidase) and APX (EC:1.11.1.11, ascorbate peroxidase) and regenerate AsA through MDHAR (EC 1.6.5.4, monodehydroascorbate reductase) and DHAR (EC 1.8.5.1, dehydroascorbate reductase).
AO and APX both would use AsA as an electron donor, and it reduces H2O2 to H2O. The inhibition of AO gene expression can increase the AsA content by 40% and improve the salt tolerance of the plant (Sanmartin et al. 2003; Yamamoto et al. 2005; Zhang et al. 2011a). Silencing of AO results in inhibition growth and altered AsA levels and ripening patterns in melon fruit (Chatzopoulou et al. 2020). Unlike AO, many studies have shown that the activity of APX enzyme has a strong correlation with AsA content (Singh et al. 2014; Chiang et al. 2015). It appears that fruit crops have more members of the APX gene family than herbaceous crops. Research has focused on the ability of APX to improve resistance to external stress (Wang et al. 2005) and not the role of APX gene in regulating AsA content. The APX gene that plays a key role in fruit development has been identified in A. eriantha (Liao et al. 2020), which also responds to light stress.
In plants, MDHAR is widely distributed and found in chloroplast, cytoplasm, mitochondria, and peroxisome (Lunde et al. 2006; Li et al. 2010). Studies on persimmon show that MDHAR was closely correlated to the metabolism of AsA content in the leaves and fruit (Pu 2008). Overexpression of the MDHAR gene not only increased the AsA content but also enhanced the resistance of transgenic lines to ozone, salt damage, and drought stress (Eltayeb et al. 2007; Stevens et al. 2008). However, overexpressed MDHAR gene from fruit crops showed that the AsA content of transgenic plants was significantly downregulated (Haroldsen et al. 2011; Gest et al. 2013). The results with the latter were also obtained in kiwifruit (A. eriantha); this study speculated that increased MDHAR enzyme activity promotes APX enzyme activity, which leads to the decrease of AsA content (He 2022).
DHAR combines with GSH to catalyze DHA and reduce it into AsA. DHAR genes have been cloned from spinach, chestnut rose, kiwifruit, and other fruit crops (Chen et al. 2003; Niu et al. 2007) and have shown that DHAR had a significant positive regulatory effect on AsA content. However, DHAR was not been considered as the key gene for the accumulation of high AsA levels in chestnut rose (An et al. 2005b). Overexpression of DHAR in model plants has shown that the AsA content increased by 2 to 4 times, and the content of glutathione also significantly increased (Chen et al. 2003; Chen and Gallie 2005). In contrast, inhibition of DHAR gene expression led to inhibition of plant growth (Chen and Gallie 2006), and resistance to O3 decreased (Chen and Gallie 2005), resulting in obvious photoinhibition (Chen and Gallie 2008).
Transcriptional regulators of AsA levels and response to environmental factors
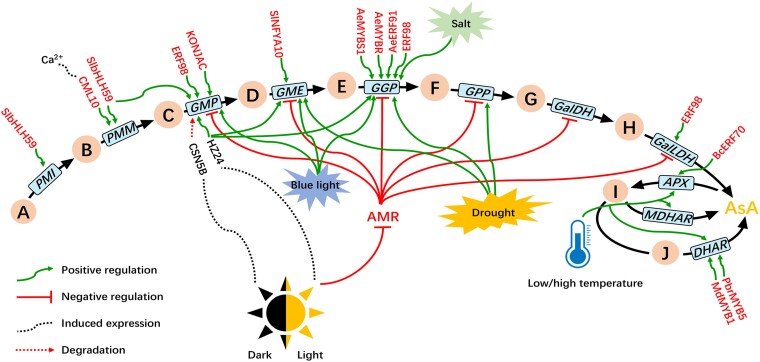
Although many studies on the functional genes related to AsA metabolism have been reported, the abundant variation in AsA content among different species in plants suggests there was complex interaction between genes and transcription factors (TFs). There were several reports on the regulation of AsA content by TFs in fruit crops (Zheng et al. 2022a) (Fig. 4). For example, MdERF98, an apple ethylene response factor, can directly bind to the promoter of MdGMP1 to activate transcription (Zhang et al. 2012; Ma et al. 2022). Studies in pears have shown that PbrMYB5 can bind to the promoter of PbrDHAR2 to regulate AsA content as well as affect the cold tolerance of plants (Xing et al. 2018). In cabbage, BcERF70 acts on the DRE (dehydration responsive element) motif in 7 target gene promoters to regulate AsA content (Yuan et al. 2020). In addition, a recent study showed that AcERF91 (Chen et al. 2021), AceMYBS1 (Liu et al. 2022b), and AcMYBR (Liu et al. 2021) could affect AsA content in kiwifruit fruit by regulating the expression of AcGGP3.
Figure 4.
Reported TFs and environmental regulatory networks for AsA accumulation. Green arrows indicate promotion of expression or AsA accumulation, red arrows indicate inhibition of expression or AsA accumulation. A) D-fructose-6P, (B) D-mannose-6P, (C) D-mannose-1P, (D) GDP-D-mannose, (E) GDP-L-galactose, (F) L-galactose1-P, (G) L-galactose, (H) L-galactono-1,4-lactone, (I) monodehydroascorbate, (J) dehydroascorbate. AMR: AsA mannose pathway regulator.
The biosynthesis of AsA is extremely sensitive to light. Studies have shown that AsA content significantly decreases after bagging treatments in many fruits, such as kiwifruit (Liao et al. 2019), pear (Xing et al. 2018), and apple (Ma et al. 2022). The transcript abundance of genes encoding enzymes involved in AsA biosynthesis shows diurnal fluctuations influenced by light. This presumably reflects a need for antioxidants to detoxify reactive oxygen species produced during photosynthesis. Studies in apples show that the F-box protein MdAMR1L1 interacts with MdGMP1 and promotes its degradation through the ubiquitination pathway, thereby inhibiting AsA synthesis (Ma et al. 2022). Light negatively regulates AMR gene expression, which then regulates expression of other key genes, affecting AsA levels (Zhang et al. 2009). CSN5B, part of the COP9 signalosome complex, promotes GMP expression (Wang et al. 2013). Similar studies have reported that AtAMR1 can negatively regulate the genes in the L-galactose pathway, including GMP, GME, GGP, GPP, GalDH, and GalLDH (Zhang et al. 2009) (Fig. 4). At the same time, the GGP gene is induced not only by blue light but also by drought and salt stress (Wang et al. 2022). Also light can directly affect the expression of other AsA metabolism-related genes, including GalLDH, MDHAR (Liao et al. 2019), and PMI (Majed and Karim 2017).
The regulation of AsA content by transcriptional activation or repression is also affected by variations in the promoter regions of functional genes. One study of different kiwifruit species showed that there was a 183-bp deletion in the GGP promoter of A. eriantha, resulting in different GGP expression and AsA content in A. eriantha (high AsA content) and A. rufa (low AsA content) (Wei et al. 2021). Sequence analysis of the deleted fragment found that there were some negatively regulated cis-acting elements in the GGP promoter of A. rufa, which reduced the transcription level of GGP (Li et al. 2014). Cis-acting elements such as G-Box and ABRE motifs in the promoter of the GGP gene of kiwifruit can regulate GGP expression under different light conditions (Li et al. 2013a, 2013b).
Challenges and promising ways to enhance AsA content in fruit crops
Enhancement of AsA content in fruit crops attracts considerable attention, not only to strengthen its nutritional value but also to improve stress tolerance. At present, studies on AsA in fruit crops have turned to elaborate regulatory networks, and functional characterization of the key structural genes and TFs has been ongoing. The next primary focus is to use these genes and TFs for genetic improvement in crops. In molecular breeding, genetic engineering is one of the preferred technologies for scientists and breeders. Among the studies of improving the AsA content by using genetic engineering technology, GGP, GME, GMP, and DHAR have a large number of research reports, which can respectively increase the AsA content of tomato fruit by 6.2 times, 1.6 times, 1.5 times, and 1.6 times, respectively (Bulley et al. 2011; Haroldsen et al. 2011; Zhang et al. 2011b; Cronje et al. 2012). However, overexpression of these genes was also accompanied by some morphological fruit alterations, such as seedlessness. It is highly likely that the dynamic balance of reactive oxygen species in plants was disrupted. Using multiple expression vectors to simultaneously overexpress the genes related to AsA synthesis, degradation and regeneration will be an alternative strategy to enhance the AsA content in fruit crops. The disadvantage of this method is the need for the acquisition of numerous transgenic lines to seek the best overexpression combination. In addition, the key regulatory TFs that regulate AsA through population genetic mapping can also be used to construct multiple expression vectors. It must be mentioned that editing the uORF of key genes of AsA provides a generalizable, efficient method for manipulating translation of mRNA that could be applied to enhance crop vitamin C, especially for GGP (Laing et al. 2015; Li et al. 2018; Zhang et al. 2018).
Using physical or chemical methods to induce the accumulation of AsA content is another simple strategy in fruit crops. This needs to be based on the studies of effects of treatments on key genes related to AsA metabolism. At present, we know that AsA content would be affected by some abiotic factors, including light, abscisic acid (ABA), and methyl jasmonate (Liao et al. 2019; Liu et al. 2022a). This knowledge will be useful for the applications of AsA enhancements in fruit crops.
Conclusions
The content of AsA in plants is affected by the biosynthesis, degradation, regeneration, and transport of AsA. There has been intensive study of these pathways with the key genes in the L-galactose pathway being identified, with a large number of studies confirming the function of various key genes. The genes related to AsA degradation and regeneration are less studied. The degradation and regeneration of AsA play important roles in both biotic and abiotic stresses. Genes that encode proteins that transport AsA or transcriptionally regulate AsA metabolism have recently been identified. The study of the regulation of AsA levels is becoming clear, especially in response to changes in the environment.
Competing interests
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
The authors thank all the scientists who have contributed to the research of L-ascorbic acid.
Contributor Information
Guanglian Liao, National Key Laboratory for Germplasm Innovation & Utilization of Horticultural Crops, Huazhong Agricultural University, Wuhan, Hubei 430070, PR China; Kiwifruit Institute, Jiangxi Agricultural University, Nanchang, Jiangxi 330045, PR China.
Qiang Xu, National Key Laboratory for Germplasm Innovation & Utilization of Horticultural Crops, Huazhong Agricultural University, Wuhan, Hubei 430070, PR China.
Andrew C Allan, The New Zealand Institute for Plant and Food Research Limited (Plant & Food Research) Mt Albert, Private Bag 92169, Auckland Mail Centre, Auckland 1142, New Zealand; School of Biological Sciences, University of Auckland, Private Bag 92019, Auckland, New Zealand.
Xiaobiao Xu, Kiwifruit Institute, Jiangxi Agricultural University, Nanchang, Jiangxi 330045, PR China.
Author contributions
GLL: Writing original draft. QX and ACA: Reviewing and editing. XBX: Supervision, conceptualization. All the authors read and approved the final manuscript.
Data availability
The datasets supporting the conclusions of this article are included.
Funding
This study was supported by the National Natural Science Foundation of China (32160692 to X.B.X and 31925034 to Q.X). The funding bodies played no role in the design of the study; collection, analysis, and interpretation of the data, and in writing the manuscript.
References
- Agius F, González-Lamothe R, Caballero JL, Muñoz-Blanco J, Botella MA, Valpuesta V. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat Biotechnol. 2003:21(2):177–181. 10.1038/nbt777 [DOI] [PubMed] [Google Scholar]
- Alegre M, Steelheart C, Baldet P, Rothan C, Just D, Okabe Y, Ezura H, Smirnoff N, Gustavo E, Grozeff G, et al. Deficiency of GDP-L-galactose phosphorylase, an enzyme required for ascorbic acid synthesis, reduces tomato fruit yield. Planta. 2020:251(2):54. 10.1007/s00425-020-03345-x [DOI] [PubMed] [Google Scholar]
- Alhagdow M, Mounet F, Gilbert L, Nunes-Nesi A, Garcia V, Just D, Petit J, Beauvoit B, Fernie AR, Rothan C, et al. Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol. 2007:145(4):1408–1422. 10.1104/pp.107.106500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An HM. Physiological mechanism of accumulating high level L-ascorbic acid, and molecular cloning and expression of its key biosynthetic enzyme in Rosa roxburghii tratt. PhD dissertation. Hangzhou, Zhejiang, China: Zhejiang University; 2004. [Google Scholar]
- An HM, Chen LG, Fan WG. Cloning of cDNA fragment of L-galactono-1,4-lactone dehydrogenase and its expression in different organs of Rosa roxburghii tratt. Scientia Agricultura Sinica. 2005a:38(3):571–575. [Google Scholar]
- An HM, Chen LG, Fan WG, Liu QL. Relationship between ascorbic acid accumulation and related enzyme activities in fruit of Rosa roxburghii Tratt. J Plant Physiology Mol Biol. 2005b:31(4):431–436. [PubMed] [Google Scholar]
- Anisimova OK, Seredin TM, Shchennikova AV, Kochieva EZ, Filyushin MA. Estimation of the vitamin C content and GDP-L-galactose phosphorylase gene (VTC2) expression level in leek (Allium porrum L.) cultivars. Russ J Plant Physiol. 2021:68(1):85–93. 10.1134/S1021443720060023 [DOI] [Google Scholar]
- Arner RJ, Prabhu KS, Thompson JT, Hildenbrandt GR, Liken AD, Reddy CC. myo-Inositol oxygenase: molecular cloning and expression of a unique enzyme that oxidizes myo-inositol and D-chiro-inositol. Biochem J. 2002:360(Pt2):213–220. 10.1042/0264-6021:3600313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badejo AA, Tanaka N, Esaka M. Analysis of GDP-D-mannose pyrophosphorylase gene promoter from acerola (Malpighia glabra) and increase in ascorbate content of transgenic tobacco expressing the acerola gene. Plant Cell Physiol. 2008:49(1):126–132. 10.1093/pcp/pcm164 [DOI] [PubMed] [Google Scholar]
- Bartoli CG, Pastori GM, Foyer CH. Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol. 2000:123(1):335–344. 10.1104/pp.123.1.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli CG, Yu JP, Gómez F, Fernández L, McIntosh L, Foyer CH. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J Exp Bot. 2006:57(8):1621–1631. 10.1093/jxb/erl005 [DOI] [PubMed] [Google Scholar]
- Bulley S, Wright M, Rommens C, Yan H, Rassam M, Lin-Wang K, Andre C, Brewster D, Karunairetnam S, Allan AC, et al. Enhancing ascorbate in fruits and tubers through over-expression of the L-galactose pathway gene GDP-L-galactose phosphorylase. Plant Biotechnol J. 2011:10(4):390–397. 10.1111/j.1467-7652.2011.00668.x [DOI] [PubMed] [Google Scholar]
- Bulley SM, Rassam M, Hoser D, Otto W, Schünemann N, Wright M, MacRae E, Gleave A, Laing W. Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-L-galactose guanyltransferase is a major control point of vitamin C biosynthesis. J Exp Bot. 2009:60(3):765–778. 10.1093/jxb/ern327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzopoulou F, Sanmartin M, Mellidou I, Pateraki I, Koukounaras A, Tanou G, Kalamaki MS, Veljović-Jovanović S, Antić TC, Kostas S, et al. Silencing of ascorbate oxidase results in reduced growth, altered ascorbic acid levels and ripening pattern in melon fruit. Plant Physiol Biochem. 2020:156:291–303. 10.1016/j.plaphy.2020.08.040 [DOI] [PubMed] [Google Scholar]
- Chen Y, Shu P, Wang RC, Du XF, Xie Y, Du K, Deng H, Li MZ, Zhang Y, Grierson D, et al. Ethylene response factor AcERF91 affects ascorbate metabolism via regulation of GDP-galactose phosphorylase encoding gene (AcGGP3) in kiwifruit. Plant Sci. 2021:313:111063. 10.1016/j.plantsci.2021.111063 [DOI] [PubMed] [Google Scholar]
- Chen YY. Study on the mechanism of ascorbic acid metabolism in Ziziphus jujube and Ziziphus jujube mill. Var. spinosa. Master’s thesis. Baoding, Hebei, China: Hebei Agricultural University; 2015. [Google Scholar]
- Chen Z, Gallie DR. Increasing tolerance to ozone by elevating foliar ascorbic acid confers greater protection against ozone than increasing avoidance. Plant Physiol. 2005:138(3):1673–1689. 10.1104/pp.105.062000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gallie DR. Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol. 2006:142(2):775–787. 10.1104/pp.106.085506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gallie DR. Dehydroascorbate reductase affects non-photochemical quenching and photosynthetic performance. J Biol Chem. 2008:283(31):21347–21361. 10.1074/jbc.M802601200 [DOI] [PubMed] [Google Scholar]
- Chen Z, Young TE, Ling J, Chang S, Gallie DR. Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA. 2003:100(6):3525–3530. 10.1073/pnas.0635176100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CM, Chen LFO, Shih SW, Lin KH. Expression of eggplant ascorbate peroxidase increases the tolerance of transgenic rice plants to flooding stress. J Plant Biochem Biotechnol. 2015:24(3):257–267. 10.1007/s13562-014-0265-7 [DOI] [Google Scholar]
- Conklin PL, Gatzek S, Wheeler GL, Dowdle J, Raymond MJ, Rolinski S, Isupov M, Littlechild JA, Smirnoff N. Arabidopsis thaliana VTC4 encodes L-galactose-1-P phosphatase, a plant ascorbic acid biosynthetic enzyme. J Biol Chem. 2006:28(23):15662–15670. 10.1074/jbc.M601409200 [DOI] [PubMed] [Google Scholar]
- Cronje C, George GM, Fernie AR, Bekker J, Kossmann J, Bauer R. Manipulation of l-ascorbic acid biosynthesis pathways in Solanum lycopersicum: elevated GDP-mannose pyrophosphorylase activity enhances l-ascorbate levels in red fruit. Planta. 2012:235(3):553–564. 10.1007/s00425-011-1525-6 [DOI] [PubMed] [Google Scholar]
- Cruz-Rus E, Botella MA, Valpuesta V, Gomez-Jimenez MC. Analysis of genes involved in L-ascorbic acid biosynthesis during growth and ripening of grape berries. J Plant Physiol. 2010:167(9):739–748. 10.1016/j.jplph.2009.12.017 [DOI] [PubMed] [Google Scholar]
- Davey MW, Montagu MV, Inzé D, Sanmartin M, Kanellis A, Smirnoff N, Benzie IJJ, Strain JJ, Favell D, Fletcher J. Plant L-ascorbic acid: chemistry, function, metabolism, biovailability and effects of processing. J Sci Food Agric 2000:80(7):825–860. [DOI] [Google Scholar]
- Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007:52(4):673–689. 10.1111/j.1365-313X.2007.03266.x [DOI] [PubMed] [Google Scholar]
- Eckardt NA. Myo-inositol biosynthesis genes in Arabidopsis: differential patterns of gene expression and role in cell death. Plant Cell. 2010:22(3):537. 10.1105/tpc.110.220310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Shibahara T, Inanaga S, Tanaka K. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta. 2007:225(5):1255–1264. 10.1007/s00425-006-0417-7 [DOI] [PubMed] [Google Scholar]
- Endres S, Tenhaken R. Myoinositol oxygenase controls the level of myoinositol in Arabidopsis, but does not increase ascorbic acid. Plant Physiol. 2009:149(2):1042–1049. 10.1104/pp.108.130948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Kyndt T, Hancock RD. Vitamin C in plants: novel concepts, new perspectives, and outstanding issues. Antioxid Redox Signal. 2020:32(7):463–485. 10.1089/ars.2019.7819 [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Tarlyn NM. L-ascorbic acid is accumulated in source leaf phloem and transported to sink tissues in plants. Plant Physiol. 2002:130(2):649–656. 10.1104/pp.007062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzek S, Wheeler GL, Smirnoff N. Antisense suppression of L-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated L-galactose synthesis. Plant J. 2002:30(5):541–553. 10.1046/j.1365-313X.2002.01315.x [DOI] [PubMed] [Google Scholar]
- Gest N, Garchery C, Gautier H, Nez AJ, Stevens R. Light-dependent regulation of ascorbate in tomato by a monodehydroascorbate reductase localized in peroxisomes and the cytosol. Plant Biotechnol J. 2013:11(3):344–354. 10.1111/pbi.12020 [DOI] [PubMed] [Google Scholar]
- Gilbert L, Alhagdow M, Nunes-Nesi A, Quemener B, Guillon F, Bouchet B, Faurobert M, Gouble B, Page D, Garcia V, et al. GDP-D-mannose 3,5-epimerase (GME) plays a key role at the intersection of ascorbate and non-cellulosic cell-wall biosynthesis in tomato. Plant J. 2009:60(3):499–508. 10.1111/j.1365-313X.2009.03972.x [DOI] [PubMed] [Google Scholar]
- Guo CM, Li MJ, Ma FW, Liang D, Zhang M. cDNA cloning and expression analysis of L-galactose-1-phosphate phosphatase in apple. Sci Agric Sin. 2011:44(4):762–770. 10.3864/j.issn.0578-1752.2011.04.014 [DOI] [Google Scholar]
- Hancock RD, McRae D, Haupt S, Viola R. Synthesis of L-ascorbic acid in the phloem. BMC Plant Biol. 2003:3(1):1–13. 10.1186/1471-2229-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroldsen IM, Chi-Ham CL, Kulkarni S, Lorence A, Bennett AB. Constitutively expressed DHAR and MDHAR influence fruit, but not foliar ascorbate levels in tomato. Plant Physiol Biochem. 2011:49(10):1244–1249. 10.1016/j.plaphy.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YQ. Identification of MDHAR family genes and the function analysis of AeMDHAR3 in Actinidia eriantha. Master’s thesis. Nanchang, Jiangxi, China: Jiangxi Agricultural University; 2022. [Google Scholar]
- Horemans N, Foyer CH, Han A. Transport and action of ascorbate at the plant plasma membrane. Trends Plant Sci. 2000:5(6):263–267. 10.1016/S1360-1385(00)01649-6 [DOI] [PubMed] [Google Scholar]
- Horemans N, Szarka A, Bock MD, Raeymaekers T, Potters G, Levine M, Banhégyi G, Guisez Y. Dehydroascorbate and glucose are taken up into Arabidopsis thaliana cell cultures by two distinct mechanisms. FEBS Lett. 2008:582(18):2714–2718. 10.1016/j.febslet.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. Molecular mechanism for the accumulation of high content of L-ascorbic acid in chestnut ross. PhD dissertation. Wuhan, Hubei, China: Huazhong Agricultural University; 2013. [Google Scholar]
- Imai T, Ban Y, Yamamoto T, Moriguchi T. Ectopic overexpression of peach GDP-D-mannose pyrophosphorylase and GDP-D-mannose-3′,5′-epimerase in transgenic tobacco. Plant Cell Tissue Organ Cult. 2012:111(1):1–13. 10.1007/s11240-012-0165-2 [DOI] [Google Scholar]
- Ioannidi E, Kalamaki MS, Engineer C, Pateraki I, Alexandrou D, Mellidou I, Giovannonni J, Kanellis AK. Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions. J Exp Bot. 2009:60(2):663–678. 10.1093/jxb/ern322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter U, Usadel B, Guerineau F, Li Y, Pauly M, Tenhaken R. The inositol oxygenase gene family of Arabidopsis is involved in the biosynthesis of nucleotide sugar precursors for cell-wall matrix polysaccharides. Planta. 2005:221(2):243–254. 10.1007/s00425-004-1441-0 [DOI] [PubMed] [Google Scholar]
- Kosti V, Lambrinidis G, Myrianthopoulos V, Diallinas G, Mikros E. Identification of the substrate recognition and transport pathway in a eukaryotic member of the nucleobase-ascorbate transporter (NAT) family. PLoS One. 2012:7(7):e41939. 10.1371/journal.pone.0041939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing WA, Bulley S, Wright M, Cooney J, Jensen D, Barraclough D, MacRae E. A highly specific L-galactose-1-phosphate phosphatase on the path to ascorbate biosynthesis. Proc Natl Acad Sci USA. 2004a:101(48):16976–16981. 10.1073/pnas.0407453101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing WA, Frearson N, Bulley S, MacRae E. Kiwifruit L-galactose dehydrogenase: molecular, biochemical and physiological aspects of the enzyme. Funct Plant Biol. 2004b:31(10):1015–1025. 10.1071/FP04090 [DOI] [PubMed] [Google Scholar]
- Laing WA, Martínez-Sánchez M, Wright MA, Bulley SM, Brewster D, Dare AP, Rassam M, Wang D, Storey R, Macknight RC, et al. An upstream open reading frame is essential for feedback regulation of ascorbate biosynthesis in Arabidopsis. Plant Cell. 2015:27(3):772–786. 10.1105/tpc.114.133777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wu QY, Sun YL, Wang LY, Yang XH, Meng QW. Overexpression of chloroplastic monodehydroascorbate reductase enhanced tolerance to temperature and methyl viologen-mediated oxidative stresses. Physiol Plant. 2010:139:421–434. 10.1111/j.1399-3054.2010.01369.x [DOI] [PubMed] [Google Scholar]
- Li J, Li MJ, Liang D, Cui M, Ma FW. Expression patterns and promoter characteristics of the gene encoding Actinidia deliciosa L-galactose-1-phosphate phosphatase involved in the response to light and abiotic stresses. Mol Biol Rep. 2013a:40(2):1473–1485. 10.1007/s11033-012-2190-y [DOI] [PubMed] [Google Scholar]
- Li J, Li MJ, Liang D, Ma FW, Lei YS. Comparison of expression pattern, genomic structure, and promoter analysis of the gene encoding GDP-l-galactose phosphorylase from two Actinidia species. Sci Hortic. 2014:169:206–213. 10.1016/j.scienta.2014.02.024 [DOI] [Google Scholar]
- Li J, Liang D, Li MJ, Ma FW. Light and abiotic stresses regulate the expression of GDP-L-galactose phosphorylase and levels of ascorbic acid in two kiwifruit genotypes via light-responsive and stress-inducible cis-elements in their promoters. Planta. 2013b:238(3):535–547. 10.1007/s00425-013-1915-z [DOI] [PubMed] [Google Scholar]
- Li MJ. Physiological and molecular mechanism of ascorbate formation and accumulation in apple and kiwifruit. PhD dissertation. Yangling, Shaanxi, China: Northwest A&F University; 2009. [Google Scholar]
- Li MJ, Liu J, Liang D, Guo CM, Ma FW. The relationship between GalUR expression and ascorbate accumulation in kiwifruit. Acta Horticulturae Sinica. 2011:38(09):1641–1649. 10.16420/j.issn.0513-353x.2011.09.004 [DOI] [Google Scholar]
- Li MJ, Ma FW, Guo CM, Liu J. Ascorbic acid formation and profiling of genes expressed in its synthesis and recycling in apple leaves of different ages. Plant Physiology Biochemical. 2009:48(4):216–224. 10.1016/j.plaphy.2010.01.015 [DOI] [PubMed] [Google Scholar]
- Li MJ, Ma FW, Zhang M, Pu F. Distribution and metabolism of ascorbic acid in apple fruits (Malus domestica borkh cv. Gala). Plant Sci. 2008:174(6):606–612. 10.1016/j.plantsci.2008.03.008 [DOI] [Google Scholar]
- Li TD, Yang XP, Yu Y, Si XM, Zhai XW, Zhang HW, Dong WX, Gao CC, Xu C. Domestication of wild tomato is accelerated by genome editing. Nat Biotechnol. 2018:36(12):1160–1163. 10.1038/nbt.4273 [DOI] [PubMed] [Google Scholar]
- Liao GL, Chen L, He YQ, Li XS, Lv ZX, Yi SY, Zhong M, Huang CH, Jia DF, Qu XY, et al. Three metabolic pathways are responsible for the accumulation and maintenance of high AsA content in kiwifruit (Actinidia eriantha). BMC Genom. 2021a:22(1):13. 10.1186/s12864-020-07311-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao GL, He YQ, Li XS, Zhong M, Huang CH, Yi SY, Liu Q, Xu XB. Effects of bagging on fruit flavor quality and related gene expression of AsA synthesis in Actinidia eriantha. Sci Hortic. 2019:256:108511. 10.1016/j.scienta.2019.05.038 [DOI] [Google Scholar]
- Liao GL, He YQ, Liu Q, Huang CH, Jia DF, Qu XY, Xu XB. Difference analysis of AsA content in different tissues of different genotypes kiwifruit (Actinidia spp.). Acta Agric Univ Jiangxi. 2022:44(02):351–357. 10.13836/j.jjau.2022036 [DOI] [Google Scholar]
- Liao GL, Liu Q, Li YQ, Zhong M, Huang CH, Jia DF, Xu XB. Identification and expression profiling analysis of ascorbate peroxidase gene family in Actinidia chinensis (Hongyang). J Plant Res. 2020:133(5):715–726. 10.1007/s10265-020-01206-y [DOI] [PubMed] [Google Scholar]
- Liao GL, Liu Q, Xu XB, He YQ, Li YQ, Wang HL, Ye B, Huang CH, Zhong M, Jia DF. Metabolome and transcriptome reveal novel formation mechanism of early mature trait in kiwifruit (Actinidia eriantha). Front Plant Sci. 2021b:12:760496. 10.3389/fpls.2021.760496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao GL, Xu XB, Huang CH, Zhong M, Jia DF. Resource evaluation and novel germplasm mining of Actinidia eriantha. Sci Hortic. 2021c:282:110037. 10.1016/j.scienta.2021.110037 [DOI] [Google Scholar]
- Liao GL, Zhong M, Jiang ZQ, Tao JJ, Jia DF, Qu XY, Huang CH, Liu Q, Xu XB. Genome-wide association studies provide insights into the genetic determination of flower and leaf traits of Actinidia eriantha. Front Plant Sci. 2021d:12:730890. 10.3389/fpls.2021.730890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YX, Zhang JH, Wu LT, Zhang YT, Chen Q, Li MY, Zhang Y, Luo Y, Wang Y, Wang XR, et al. Genome-wide identification of GMP genes in Rosaceae and functional characterization of FaGMP4 in strawberry (Fragaria × ananassa). Genes Genom. 2021:43(6):587–599. 10.1007/s13258-021-01062-7 [DOI] [PubMed] [Google Scholar]
- Lisko K, Aboobucker S, Torres R, Lorence A. Engineering elevated vitamin c in plants to improve their nutritional content, growth, and tolerance to abiotic stress. Phytochem Biosynth Funct Appl. 2014:44:109–128. 10.1007/978-3-319-04045-5_6 [DOI] [Google Scholar]
- Liu FH, Wang L, Gu L, Zhao W, Su HY, Cheng XH. Higher transcription levels in ascorbic acid biosynthetic and recycling genes were associated with higher ascorbic acid accumulation in blueberry. Food Chem. 2015:188:399–405. 10.1016/j.foodchem.2015.05.036 [DOI] [PubMed] [Google Scholar]
- Liu HB, Wei LZ, Ni Y, Chang LL, Dong J, Zhong CF, Sun R, Li ST, Xiong R, Wang GX, et al. Genome-wide analysis of ascorbic acid metabolism related genes in Fragaria × ananassa and its expression pattern analysis in strawberry fruits. Front Plant Sci. 2022a:13:954505. 10.3389/fpls.2022.954505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Wu RM, Bulley SM, Zhong CH, Li DW. Kiwifruit MYBS1-like and GBF3 transcription factors influence L-ascorbic acid biosynthesis by activating transcription of GDP-L-galactose phosphorylase 3. New Phytol. 2022b:234(5):1782–1800. 10.1111/nph.18097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Xie XD, Zhong CH, Li DW. Comparative transcriptome analysis revealed the key genes regulating ascorbic acid synthesis in Actinidia. Int J Mol Sci. 2021:22(23):12894. 10.3390/ijms222312894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorence A, Chevone BI, Mendes P, Nessler CL. myo-Inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol. 2004:134(3):1200–1205. 10.1104/pp.103.033936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DY, Wu Y, Pan QH, Zhang YP, Qi YY, Bao WH. Identification of key genes controlling L-ascorbic acid during jujube (Ziziphus jujuba Mill.) fruit development by integrating transcriptome and metabolome analysis. Front Plant Sci. 2022:13:950103. 10.3389/fpls.2022.950103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde C, Baumann U, Shirley N, Drew DP, Finche GB. Gene structure and expression pattern analysis of three monodehydroascorbate reductase (MDHAR) genes in Physcomitrella patens: implications for the evolution of the MDHAR family in plants. Plant Mol Biol. 2006:60(2):259–275. 10.1007/s11103-005-3881-8 [DOI] [PubMed] [Google Scholar]
- Luo Y, Lin YX, Mo F, Ge C, Jiang LY, Zhang Y, Chen Q, Sun B, Wang Y, Wang XR, et al. Sucrose promotes strawberry fruit ripening and affects ripening-related processes. Int J Genomics. 2019:2019:9203057. 10.1155/2019/9203057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SY, Li HX, Wang L, Li BY, Wang ZY, Ma BQ, Ma FW, Li MJ. F-box protein MdAMR1L1 regulates ascorbate biosynthesis in apple by modulating GDP-mannose pyrophosphorylase. Plant Physiol. 2022:188(1):653–669. 10.1093/plphys/kiab427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LC, Wang YR, Liu WX, Liu ZP. Overexpression of an alfalfa GDP-mannose 3, 5-epimerase gene enhances acid, drought and salt tolerance in transgenic Arabidopsis by increasing ascorbate accumulation. Biotechnol Lett. 2014:36(11):2331–2341. 10.1007/s10529-014-1598-y [DOI] [PubMed] [Google Scholar]
- Majed SO, Karim AY. Phylogenetic relationship among phosphomannose isomerase (PMI), phosphomannose mutase (PMM) and GDP-D-mannose pyrophosphorylase (GMP) in photosynthetic eukaryotes. ZANCO J Pure Appl Sci. 2017:28:s27–s38. [Google Scholar]
- Matteo AD, Hancock RD, Ross HA, Frusciante L, Viola R. Characterisation of Chlorella pyrenoidosa L-ascorbic acid accumulating mutants: identification of an enhanced biosynthetic enzyme activity and cloning of the putative gene from Arabidopsis thaliana. Comp Biochem Physiol—Part A Mol Integr Physiol. 2003:134:155. [Google Scholar]
- Mieda T, Yabuta Y, Rapolu M, Motoki T, Takeda T, Yoshimura K, Ishikawa T, Shigeoka S. Feedback inhibition of spinach L-galactose dehydrogenase by L-ascorbate. Plant Cell Physiol. 2004:45(9):1271–1279. 10.1093/pcp/pch152 [DOI] [PubMed] [Google Scholar]
- Millar AH, Mittova V, Kiddle G, Heazlewood JL, Bartoli CG, Theodoulou FL, Foyer CH. Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiol. 2003:133(2):443–447. 10.1104/pp.103.028399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JB, James KW, Maggiore PMA. Tables of composition of Australian aboriginal foods. Australia Aboriginal Studies Press; Canberra, Australia; 1993. [Google Scholar]
- Niu XY, Lei YS, Liang D, Ma FW, Wang XR. Cloning and sequence analysis of cDNA fragments of L-galactono-1,4-lactone dehydrogenase and dehydroascorbate reductase from fruit of Actinidia deliciosa. J Northwest A & F Univ. 2007:35(12):57–62. 10.13207/j.cnki.jnwafu.2007.12.009 [DOI] [Google Scholar]
- Pallanca JE, Smirnoff N. The control of ascorbic acid synthesis and turnover in pea seedlings. J Exp Bot. 2000:51(345):669–674. 10.1093/jexbot/51.345.669 [DOI] [PubMed] [Google Scholar]
- Pateraki I, Sanmartin M, Kalamaki MS, Gerasopoulos D, Kanellis AK. Molecular characterization and expression studies during melon fruit development and ripening of L-galactono-1,4-lactone dehydrogenase. J Exp Bot. 2004:55(403):1623–1633. 10.1093/jxb/erh186 [DOI] [PubMed] [Google Scholar]
- Pieslinger AM, Hoepflinger MC, Tenhaken R. Cloning of glucuronokinase from Arabidopsis thaliana, the last missing enzyme of the myo-inositol oxygenase pathway to nucleotide sugars. J Biol Chem. 2010:285(5):2902–2910. 10.1074/jbc.M109.069369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu F. Studies on ascorbic acid diversity and metabolism in fruits of persimmon germplasm. Master’s thesis. Yangling, China: Northwest A & F University; 2008. [Google Scholar]
- Rivas CI, Zúiga FA, Salas-Burgos A, Mardones L, Ormazabal V, Vera JC. Vitamin C transporters. J Physiol Biochem. 2008:64(4):357–375. 10.1007/BF03174092 [DOI] [PubMed] [Google Scholar]
- Sanmartin M, Drogoudi PD, Lyons T, Pateraki I, Barnes J, Kanellis AK. Over-expression of ascorbate oxidase in the apoplast of transgenic tobacco results in altered ascorbate and glutathione redox states and increased sensitivity to ozone. Planta. 2003:216(6):918–928. 10.1007/s00425-002-0944-9 [DOI] [PubMed] [Google Scholar]
- Siddique S, Endres S, Atkins JM, Szakasits D, Wieczorek K, Hofmann J, Blaukopf C, Urwin PE, Tenhaken R, Grundler FMW. Myo-inositol oxygenase genes are involved in the development of syncytia induced by Heterodera schachtii in Arabidopsis roots. New Phytol. 2009:184(2):457–472. 10.1111/j.1469-8137.2009.02981.x [DOI] [PubMed] [Google Scholar]
- Siddique S, Endres S, Sobczak M, Radakovic ZS, Fragner L, Grundler FMW, Weckwerth W, Tenhaken R, Bohlmann H. Myo-inositol oxygenase is important for the removal of excess myo-inositol from syncytia induced by Heterodera schachtii in Arabidopsis roots. New Phytol. 2013:201(2):476–485. 10.1111/nph.12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Mishra A, Jha B. Over-expression of the peroxisomal ascorbate peroxidase (SbpAPX) gene cloned from halophyte Salicornia brachiata confers salt and drought stress tolerance in transgenic tobacco. Mar Biotechnol (New York). 2014:16(3):321–332. 10.1007/s10126-013-9548-6 [DOI] [PubMed] [Google Scholar]
- Smirnoff N. Vitamin C: the metabolism and functions of ascorbic acid in plants. Adv Bot Res. 2011:59:107–177. 10.1016/B978-0-12-385853-5.00003-9 [DOI] [Google Scholar]
- Smirnoff N, Wheeler GL. Ascorbic acid in plants: biosynthesis and function. CRC Crit Rev Plant Sci. 2000:19(4):267–290. 10.1080/07352680091139231 [DOI] [PubMed] [Google Scholar]
- Stevens R, Buret M, Duffé P, Garchery C, Baldet P, Rothan C, Causse M. Candidate genes and quantitative trait loci affecting fruit ascorbic acid content in three tomato populations. Plant Physiol. 2007:143(4):1943–1953. 10.1104/pp.106.091413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R, Page D, Gouble B, Garchery C, Zamir D, Causse M. Tomato fruit ascorbic acid content is linked with monodehydroascorbate reductase activity and tolerance to chilling stress. Plant Cell Environ. 2008:31(8):1086–1096. 10.1111/j.1365-3040.2008.01824.x [DOI] [PubMed] [Google Scholar]
- Szarka A, Horemans N, Bánhegyi G, Han A. Facilitated glucose and dehydroascorbate transport in plant mitochondria. Arch Biochem Biophys. 2004:428(1):73–80. 10.1016/j.abb.2004.05.011 [DOI] [PubMed] [Google Scholar]
- Szarka A, Horemans N, Kovács Z, Gróf P, Mayer M, Bánhegyi G. Dehydroascorbate reduction in plant mitochondria is coupled to the respiratory electron transfer chain. Physiol Plant. 2007:129(1):225–232. 10.1111/j.1399-3054.2006.00810.x [DOI] [Google Scholar]
- Tabata K, Ôba K, Suzuki K, Esaka M. Generation and properties of ascorbic acid-deficient transgenic tobacco cells expressing antisense RNA for L-galactono-1,4-lactone dehydrogenase. Plant J. 2001:27(2):139–148. 10.1046/j.1365-313x.2001.01074.x [DOI] [PubMed] [Google Scholar]
- Tamaoki M, Mukai F, Asai N, Nakajima N, Kubo A, Aono M, Saji H. Light-controlled expression of a gene encoding l-galactono-γ-lactone dehydrogenase which affects ascorbate pool size in Arabidopsis thaliana. Plant Sci. 2003:164(6):1111–1117. 10.1016/S0168-9452(03)00122-5 [DOI] [Google Scholar]
- Tedone L, Hancock RD, Alberino S, Haupt S, Viola R. Long-distance transport of L-ascorbic acid in potato. BMC Plant Biol. 2004:4(1):16. 10.1186/1471-2229-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian RJ. The transfurmation and expression of gene GalUR of apple and transfurmation gene HS into alfalfa and salt-resistant research of different variety alfalfa. Master’s thesis. Chongqing, China: Southwest University; 2007. [Google Scholar]
- Valente A, Albuquerque TG, Sanches-Silva A, Costa HS. Ascorbic acid content in exotic fruits: a contribution to produce quality data for food composition database. Food Res Int. 2011:44(7):2237–2242. 10.1016/j.foodres.2011.02.012 [DOI] [Google Scholar]
- Wang FZ. Study on chemical composition of jasmine flower residue and its utilization. Master’s thesis. Hefei, Anhui Province, China: Anhui Agricultural University; 2003. [Google Scholar]
- Wang J, Yu YW, Zhang ZJ, Quan RD, Zhang HW, Ma LG, Deng XW, Huang RF. Arabidopsis CSN5B interacts with VTC1 and modulates ascorbic acid synthesis. Plant Cell. 2013:25(2):625–636. 10.1105/tpc.112.106880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WJ, Liu XY, Cheng C, Xie XD, Li LL, Bai J, Liu P, Zhong CH, Li DW. Effects of salt and drought stresses and light quality on vitamin C content and expression of synthetic genes in kiwifruit leaves. J Fruit Sci. 2022:39(02):203–210. 10.13925/j.cnki.gsxb.20210392 [DOI] [Google Scholar]
- Wang YJ, Wisniewski ME, Meilan R, Webb R, Fuchigami L, Boyer C. Overexpression of cytosolic ascorbate peroxidase in tomato (Lycopersicon esculentum) confers tolerance to chilling and salt stress. J Am Soc Horticul Sci. 2005:130(2):167–173. 10.21273/JASHS.130.2.167 [DOI] [Google Scholar]
- Wei H, Li F, Song ZJ, Yuan YL, Mao K, Li MJ. Study on the relationship between the promoter region of GGP1 and ascorbic acid content in kiwifruit. Plant Physiol J. 2021:57(12):2357–2365 [Google Scholar]
- Wu H. Cloning and quantitative expression analysis of ascorbate synthase-related genes in Actinidia eriantha. Master’s thesis. Nanchang, Jiangxi Province, China: Jiangxi Agricultural University; 2015. [Google Scholar]
- Xiao NN, Ma FW, Zhang JK, Liang D. Cloning and sequence analysis of full-length cDNA encoding L-galactose dehydrogenase from apple. J Northwest A & F Uni (Natural Science Edition). 2007:35(04): 175–178. 10.13207/j.cnki.jnwafu.2007.04.038 [DOI] [Google Scholar]
- Xing HW. Analysis of nutritious components in tulip petals and azalea petals. J Chongqing Technol Bus Univ (Natural Science Edition). 2004:22(01):53–55 [Google Scholar]
- Xing CH, Liu Y, Zhao LY, Zhang SL, Huang XS. A novel MYB transcription factor regulates ascorbic acid synthesis and affects cold tolerance. Plant Cell Environ. 2018:42(3):832–845. 10.1111/pce.13387 [DOI] [PubMed] [Google Scholar]
- Xu Q, Chen LL, Ruan X, Chen D, Zhu A, Chen C, Bertrand D, Jiao WB, Hao BH, Lyon MP, et al. The draft genome of sweet orange (Citrus sinensis). Nat Genet. 2013:45(1):59–66. 10.1038/ng.2472 [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Bhuiyan MNH, Waditee R, Tanaka Y, Esaka M, Oba K, Jagendorf AT, Takabe T. Suppressed expression of the apoplastic ascorbate oxidase gene increases salt tolerance in tobacco and Arabidopsis plants. J Exp Bot. 2005:56(417):1785–7196. 10.1093/jxb/eri167 [DOI] [PubMed] [Google Scholar]
- Ye ZB. Quality analysis of horticultural products. China Agricultural Press; Beijing, China; 2011. [Google Scholar]
- Yuan JP, Yu ZH, Lin TT, Wang L, Chen X, Liu TK, Wang JJ, Hou XL, Li Y. BcERF070, a novel ERF (ethylene-response factor) transcription factor from non-heading Chinese cabbage, affects the accumulation of ascorbic acid by regulating ascorbic acid-related genes. Mol Breed. 2020:40(1):2. 10.1007/s11032-019-1065-5 [DOI] [Google Scholar]
- Zechmann B, Liou L, Koffler BE, Horvat L, Tomašić A, Fulgosi H, Zhang Z. Subcellular distribution of glutathione and its dynamic changes under oxidative stress in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2011:11(8):631–642. 10.1111/j.1567-1364.2011.00753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Liu J, Zhang Y, Cai X, Gong P, Zhang J, Wang T, Li H, Ye Z. Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Cell Rep. 2011a:30(3):389–398. 10.1007/s00299-010-0939-0 [DOI] [PubMed] [Google Scholar]
- Zhang WY, Lorence A, Gruszewski HA, Chevone BI, Nessler CL. AMR1, An Arabidopsis gene that coordinately and negatively regulates the mannose/L-galactose ascorbic acid biosynthetic pathway. Plant Physiol. 2009:150(2):942–950. 10.1104/pp.109.138453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WY, Gruszewski HA, Chevone BI, Nessler CL. An Arabidopsis purple acid phosphatase with phytase activity increases foliar ascorbate. Plant Physiol. 2008:146(2):431–440. 10.1104/pp.107.109934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YY, Li H, Shu WB, Zhang CJ, Zhang W, Ye ZB. Suppressed expression of ascorbate oxidase gene promotes ascorbic acid accumulation in tomato fruit. Plant Molecular Biology Rep. 2011b:29(3):638–645. 10.1007/s11105-010-0271-4 [DOI] [Google Scholar]
- Zhang CJ, Ouyang B, Yang CX, Zhang XH, Liu H, Zhang YY, Zhang JH, Li HX, Ye ZB. Reducing AsA leads to leaf lesion and defence response in knock-down of the AsA biosynthetic enzyme GDP-D-mannose pyrophosphorylase gene in tomato plant. PLOS One. 2013:8:e1987. 10.1371/journal.pone.0061987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HW, Si XM, Ji X, Fan R, Liu JX, Chen KL, Wang DW, Gao CX. Genome editing of upstream open reading frames enables translational control in plants. Nature Biotechnol. 2018:36(9):894–898. 10.1038/nbt.4202 [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Wang J, Zhang RX, Huang RF. The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J. 2012:71(2):273–287. 10.1111/j.1365-313X.2012.04996.x [DOI] [PubMed] [Google Scholar]
- Zheng XZ, Gong M, Zhang QD, Tan HQ, Li LP, Tang YW, Li ZG, Peng MC, Deng W. Metabolism and regulation of ascorbic acid in fruits. Plants. 2022a:11(12):1602. 10.3390/plants11121602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XZ, Yuan YJ, Huang BW, Hu XW, Tang YW, Xu X, Wu MB, Gong ZH, Luo YQ, Gong M, et al. Control of fruit softening and ascorbic acid accumulation by manipulation of SlIMP3 in tomato. Plant Biotechnol J. 2022b:20(6):1213–1225. 10.1111/pbi.13804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou LP, Li HX, Ouyang B, Zhang JH, Ye ZB. Cloning, expression, and mapping of GDP-D-mannose pyrophosphorylase cDNA from tomato (Lycopersicon esculentum). Acta Genetica Sinica. 2006:33(8):757–764. 10.1016/S0379-4172(06)60108-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are included.