Abstract
Parkinson’s disease (PD) which has various pathological mechanisms, recently, it is attracting attention to the mechanism via microbiome-gut-brain axis. 6-Shogaol, a representative compound of ginger, have been known for improving PD phenotypes by reducing neuroinflammatory responses. In the present study, we investigated whether 6-shogaol and ginger attenuate degeneration induced by Proteus mirabilis (P. mirabilis) on the intestine and brain, simultaneously. C57BL/6J mice received P. mirabilis for 5 days. Ginger (300 mg/kg) and 6-shogaol (10 mg/kg) were treated by gavage feeding for 22 days including the period of P. mirabilis treatment. Results showed that 6-shogaol and ginger improved motor dysfunction and dopaminergic neuronal death induced by P. mirabilis treatment. In addition, they suppressed P. mirabilis-induced intestinal barrier disruption, pro-inflammatory signals such as toll-like receptor and TNF-α, and intestinal α-synuclein aggregation. Moreover, ginger and 6-shogaol significantly inhibited neuroinflammation and α-synuclein in the brain. Taken together, 6-shogaol and ginger have the potential to ameliorate PD-like motor behavior and degeneration of dopaminergic neurons induced by P. mirabilis in mice. Here, these findings are meaningful in that they provide the first experimental evidence that 6-shogaol might attenuate PD via regulating gut-brain axis.
Keywords: Parkinson’s disease, Microbiota-Gut-Brain axis, 6-shogaol, ginger, Proteus mirabilis
INTRODUCTION
Parkinson’s disease (PD) is a multi-pathological neurodegenerative disease accompanied by motor symptoms such as bradykinesia, tremor, rigidity, and postural instability from the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc). PD has key pathological features, including nigrostriatal lewy bodies, composed primarily of accumulated α-synuclein and neuroinflammatory responses that are characterized by reactive gliosis (Raza et al., 2019). Before the onset of motor symptoms, PD patients suffer from many non-motor symptoms, including olfactory, gastrointestinal, cardiovascular, and urological problems.
Several studies have reported that changes in peripheral biological factors in PD are closely connected to the etiology and progression of the disease. Among them, the microbiota-gut-brain axis (MGB axis) is the concept that receives the most attention in explaining the crosstalk between the periphery and the brain. Numerous studies have reported changes in the microbial composition of PD patients. In addition, animal studies have demonstrated that the intestinal microbiota and its virulence factors could exacerbate PD progression, whereas well-defined metabolic alterations based on the MGB axis have not yet been elucidated.
Recently, studies of permeability and inflammation in the intestines of PD patients and PD animal models have been reported. Both intestinal penetrability and lipopolysaccharide (LPS)-binding protein in plasma were found to be increased significantly in PD patients (Forsythe and Bienenstock, 2010). Increased intestinal permeability and α-synuclein accumulation were observed in the large intestine of PD mice administered LPS systemically (Kelly et al., 2014). Gut microbial imbalance is an important factor in intestinal pathology. In fact, evidence of gut microbial changes in PD patients has been published. In a 16S ribosomal RNA (rRNA) gene pyrosequencing analysis of the fecal microbiota of PD patients, a higher abundance of the Enterobacteriaceae family was seen in non-tremor dominant PD patients, who were observed to have faster progression, worse outcomes, and more severe colonic α-synuclein pathology (Scheperjans et al., 2015). A recent experimental study revealed that gut microbiota can regulate neuroinflammation and motor dysfunction in α-synuclein overexpressing PD mice (Sampson et al., 2016). We previously reported that mice administered a specific bacterium, Proteus mirabilis (P. mirabilis) demonstrated induced motor deficits. When treating P. mirabilis, we found that dopaminergic neuronal death, neuroinflammation and α-synuclein aggregation were induced in both the brain and the colon, simultaneously (Choi et al., 2018).
Ginger, the rhizome of Zingiber officinale Roscoe, is one of the most commonly consumed food ingredients with a long history of medicinal use stretching back thousands of years. Recent studies have demonstrated that 6-shogaol (6S), a dehydrated form of 6-gingerol and a marker compound for quality check of ginger, is more stable due to the thermal liability of 6-gingerol and has more potent pharmacological effects. It is also known as the main pharmacological compound of ginger.
Its neuropharmacological actions for neurodegenerative diseases have been reported in various studies. 6S inhibits abnormal Aβ accumulation in the hippocampal and cortical regions and ameliorates memory impairment in AD transgenic mice (Na et al., 2016). This compound also attenuates Aβ oligomer-induced memory loss, neuronal damage, and neuroinflammation in mice (Moon et al., 2014). The anti-PD actions of ginger have been explored in studies of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD models, which showed that ginger extract significantly inhibits MPTP-induced motor impairment, dopaminergic neuronal damage, and striatal dopamine deletion (Angelopoulou et al., 2022). 6S inhibits motor dysfunction, dopaminergic neuronal damage, and gliosis in MPTP-induced PD mice (Park et al., 2013). It also inhibits apoptotic cell death as well as the upregulated nucleic translocation of nuclear factor (erythroid-derived 2)-like 2 against 6-hyrodxydopmaine (6-OHDA) neurotoxicity in PC12 cells (Peng et al., 2015). Recently, in addition to its effects on brain neurological disorders, a previous study investigating the effects of ginger on GI dysfunction also revealed that 6S can regulate intestinal pro-inflammation, gut barrier intensity levels, and enteric neuronal dysfunction in the MPTP-induced mouse intestine (Huh et al., 2020). These results indicate that 6S and ginger have effects on intestinal environments in a disease condition, whereas the mechanisms of the effects of 6S and ginger on intestinal and brain degeneration via the gut-brain axis have not yet been reported.
In this study, we aimed to investigate whether a treatment with ginger extract (GE) and its active compound, 6S, could attenuate P. mirabilis-induced neurodegeneration, intestinal disruption and motor deficits. Herein, to evaluate whether GE and 6S ameliorate movement impairments, we performed behavioral assessments with P. mirabilis-treated mice. To determine the effects of GE and 6S on P. mirabilis-induced neurodegeneration in the mouse brain, we examined dopaminergic neuronal cell survival, neuroinflammation and α-syn aggregation by immunostaining. In addition, we observed the effects of GE and 6S on the colonic tight junction, signals of toll-like receptor 4 (TLR4) and intestinal α-syn aggregation as induced by an oral treatment of P. mirabilis.
MATERIALS AND METHODS
Materials
Rabbit anti-tyrosine hydroxylase (TH), rat anti-dopamine transporter (DAT), immobilon-P transfer membranes, paraformaldehyde (PFA), 3,3-diaminobenzidine (DAB), dimethyl sulfoxide (DMSO), triton X-100, sodium chloride, sodium bicarbonate (NaHCO3), sucrose, slide mounting medium, proteinase K, phosphate-buffered saline (PBS) were purchased from Merck Millipore (Burlington, MA, USA). Goat anti-ionized calcium binding adaptor molecule 1 (Iba1), goat anti-TH, rabbit anti-α-synuclein aggregate antibody were purchased from Abcam (Cambridge, UK). Mouse anti-α-synuclein phosphor (Ser129) antibody was purchased from Biolegend (San Diego, CA, USA). Mouse anti-occludin and chicken anti-goat Alexa Fluor 488 were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Mouse anti-TLR4 and goat anti-tumor necrosis factor-alpha (TNF-α) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Rabbit anti-β-actin was purchased from Bethyl Laboratories (Montgomery, TX, USA). Anti-fade fluorescent medium was purchased from Dako Agilent (Santa Clara, CA, USA). Sodium dodecyl sulfate (SDS), protein assay reagent, Tween 20, ammonium persulfate, acrylamide, enzyme-linked chemiluminescence (ECL) reagent, and skimmed milk were purchased from Bio-Rad Laboratories (Hercules, CA, USA). Biotinylated goat anti-rabbit IgG, rabbit anti-rat IgG, goat anti-rabbit IgG Cy3 conjugate Alexa 594, normal goat serum, normal rabbit serum, and avidin-biotin complex (ABC) were purchased from Vector Labs (Newark, CA, USA). Horseradish peroxidase-conjugated (HRP) anti-mouse and anti-goat secondary antibody were purchased from Enzo Life Sciences (Farmingdale, NY, USA).
Preparation of GE and 6-shogaol
Ginger was purchased from the Kwangmyungdang pharmaceutical Co., Ltd. (Ulsan, Korea). GE was prepared as previously reported methods with several modification (Huh et al., 2018). In brief, fresh ginger was heated at 120°C for 2 h, and then dried for 24 h. Heated ginger was extracted with 20-fold of 70% ethanol at 70°C for 3 h by reflux extraction. The extract was filtered, lyophilized (yield (%)=9.48%), and stored at –20°C until use at the College of Pharmacy, Kyung Hee University (Seoul, Korea). 6-Shogaol was synthesized and provided from Prof. Dongyun Shin at the College of Pharmacy, Gachon University (Incheon, Korea).
Animals and administration of P. mirabilis and drugs
Seven-week-old male C57BL/6J mice used in this study were purchased from Daehan Biolink (Eumseong, Korea). After the adaptation for 7 days, mice were housed in separate cages per group (n=6 per cage) at an ambient temperature of 23 ± 1°C and relative humidity 60 ± 10% under a 12 h light/dark cycle and were allowed free access to water and food. All animal studies were performed in accordance with the “Principles of Laboratory Animal Care” (NIH publication number 80-23, revised 1996) and approved by the “Animal Care and Use Guidelines” of Kyung Hee University, Seoul, Korea (the approval number: KHUASP(SE)-17-004).
P. mirabilis treatment was described in the previous study (Choi et al., 2018). The collected P. mirabilis was orally administrated to mice for 5 days (2×108 CFU/0.2 mL sterilized PBS per mice). The mice were sacrificed at 16 days after the last administration of P. mirabilis.
Both GE and 6S were dissolved in 10% DMSO with saline. Oral administration with GE or 6S was started 1 day before P. mirabilis treatment for a total of 22 days. Mice were divided into 5 groups: (1) NOR group (saline (p.o.)-treated plus sterilized PBS (p.o.)-treated group, N=8); (2) PM group (saline (p.o.)-treated plus P. mirabilis (p.o.)-treated group, N=8); (3) GE group (GE 300 mg/kg/day (p.o.)-treated plus P. mirabilis (p.o.)-treated group, N=8); and (4) 6S group (6S 10 mg/kg/day (p.o.)-treated plus P. mirabilis (p.o.)-treated group, N=8). The scheme for experimental procedure was described in Fig. 1A.
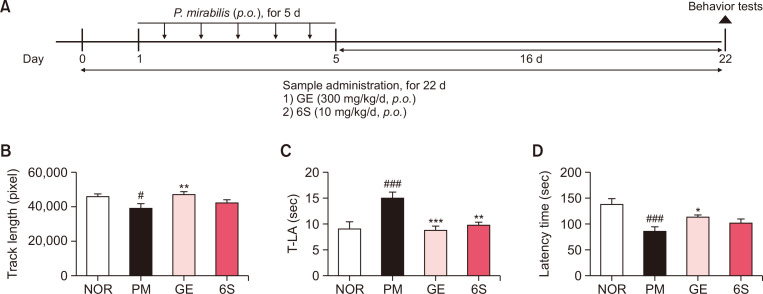
Fig. 1.
6-shogaol and ginger treatment attenuate motor impairment induced by P. mirabilis administration. (A) Experimental protocol of this study. (B) The open field test, (C) Pole test and (D) Rotarod test were performed at 16 days after the last administration of P. mirabilis. Values were expressed as means ± SEM. #p<0.05 and ###p<0.001 vs. NOR group; *p<0.05, **p<0.01 and ***p<0.001 vs. PM group. N=8 for each group.
Behavior tests
Behavior tests were conducted as previously described (Choi et al., 2018). Brief information was provided as follows.
Open field test
Open field test was performed between 9 p.m. and 2 a.m. to avoid diurnal variation. The mice were placed in the testing chamber (40×25×18 cm) with white floors, followed by a 30-min recording period using a computerized automatic analysis system (Viewer; Biobserve, Bonn, Germany). The data collected by computer included the total distance traveled by tracking the center of the animal.
Pole test
Pole test was performed on the 15 days after P. mirabilis administration. The mice were held on the top of the pole (diameter 8 mm, height 55 cm, with a rough surface). The locomotor activity time (T-LA) needed for the mice to climb down and place all four feet on the floor was recorded with a 30 s cut-off limit. Each trial had a cut off limit of 30 s.
Rotarod test
Rotarod test was performed on the 16 days after the last administration of P. mirabilis. The rotarod unit consists of a rotating spindle (7.3 cm diameter) and five individual compartments. After two or three times of training (8-10 rpm rotation speed), the rotation speed was increased to 16 rpm in a test session. The time each mouse remained on the rotating bar was recorded over three trials per mouse with a maximum length of 3 min per trial. Data are presented as the mean time on the rotating bar over the three test trials.
Biological sample preparation
For immunohistochemical studies, at 24 h after behavioral tests, mice were perfused transcardially with 0.05 M PBS, and then fixed with cold 4% PFA in a 0.1 M phosphate buffer. Brains were removed and post-fixed in a 0.1 M phosphate buffer containing 4% PFA overnight at 4°C and then immersed in a solution containing 30% sucrose in 0.05 M PBS for cryoprotection. Serial 30 µm-thick coronal sections were cut on a freezing microtome (Leica, Wetzlar, Germany) and stored in cryoprotectant (25% ethylene glycol, 25% glycerol, and 0.05 M phosphate buffer) at 4°C until use. For western blot analysis, the mice were decapitated and the brains or distal colons were isolated and stored at −80°C until use.
Immunohistochemistry
The floating brain sections of the striatum (ST) region (AP 0 to 0.5 mm) and SNpc region (AP -3.5 to -3.0 mm) were incubated overnight with a rabbit anti-TH (1:1,000) after reacting with 1% hydrogen peroxide. They were subsequently incubated with a biotinylated goat anti-rabbit IgG antibody (1:200), followed by incubation in an avidin-biotin complex solution. 3,3’-diaminobenzidine was used to develop the color of every section and the images were photographed using an optical light microscope (Olympus Microscope System BX51; Olympus, Tokyo, Japan). The optical density of TH-positive fibers was measured in the ST at ×40 magnification using Image J software (National Institutes of Health, Bethesda, MD, USA). The TH-positive cells in the SNpc were measured at ×200 magnification using Image J software. Optical density measurement and cell counts were determined by an experimenter who was blinded to the treatment conditions, and the outcome for each animal was the mean of the results from the three sections.
Immunofluorescence
To examine the activation of microglia in the ST, double immunofluorescence staining was performed. The brain sections were washed with PBS and incubated with a goat anti-Iba1 antibody (1:1,000) overnight at 4°C in the presence of 0.3% triton X-100. After rinsing in PBS, the sections were incubated with chicken anti-goat Alexa 488 (1:500) for 1 h and then 4’,6-diamidino-2-phenylindole staining was performed for 20 min. To examine the activation of microglia (TH+Iba-1) in the SN, double immunofluorescence staining was performed. The SN sections for TH+Iba-1 double staining was incubated with a rabbit anti-TH (1:1,000) for 6 h at room temperature. After washing with PBS, the sections were incubated with a goat anti-rabbit IgG Cy3 conjugate Alexa 594 (1:500) and then incubated with a goat anti-Iba-1 antibody (1:1,000) overnight at 4°C. After rinsing in PBS, the sections were incubated with chicken anti-goat Alexa 488 (1:500) for 1 h. The sections for TH+α-synuclein filament double immunostaining were incubated with a goat anti-TH (1:1,000, ab101853, Abcam) for 6 h at room temperature. After washing with PBS, the sections were incubated with chicken anti-goat Alexa 488 (1:500) for 1 h and then incubated with a rabbit anti-α-synuclein filament antibody (1:1,000, ab209538, Abcam) for 24 h at 4°C. The sections were incubated with goat anti-rabbit IgG Cy3 conjugate Alexa 594 (1:500, DI-1549, Vector Laboratories, Newark, CA, USA) for 1 h.
All immunofluorescent sections were mounted with anti-fade fluorescent medium (Wako chemical, Osaka, Japan). The images were photographed using an optical bright-field or fluorescence microscope (BX51, Olympus). The number of Iba-1 or α-syn positive cells was quantified according to stereological counting and was determined by an experimenter who was blinded to the treatment conditions, and the outcome for each animal was the mean of the results from the three sections.
Western blotting
The protein samples were transferred onto immobilon-P membranes and the membranes were blocked with 5% skim milk in tris-buffered saline-0.01% Tween 20 (TBST). Then, the membranes were incubated overnight at 4°C with primary antibodies as follows: phosphorylated α-syn (1:1,000), TLR4 (1:500), TNF-α (1:500), occludin (1:500), and β-actin (1:3,000). After that, the membranes were washed three times for 10 min with TBST, and the blots were incubated with respective HRP secondary antibodies for 1 h at room temperature. Thereafter, the membranes were washed again three times for 10 min with TBST. The intensities of the bands were normalized to the β-actin band intensity using Multi Gauge software (Fuji photo film Co, Ltd., Tokyo, Japan).
Statistical analysis
All statistical parameters were calculated using Graphpad Prism 8.0.1 software (GraphPad Software, Inc., San Diego, CA, USA). Values were expressed as the mean ± standard error of the mean (SEM). Statistical significance was determined by the one-way ANOVA followed by Tukey’s post hoc test. Differences with a p-value less than 0.05 were considered statistically significant.
RESULTS
GE and 6S ameliorate P. mirabilis-induced motor dysfunction in mice
To evaluate the effects of GE and 6S on P. mirabilis-induced movement, several motor behavior tests were performed. In open field test, the track length for 30 min in PM group was significantly decreased compared with NOR group, whereas GE treatment at 300 mg/kg/d significantly restored the activity (Fig. 1B). In addition, we found that P. mirabilis-induced motor impairment was significantly reversed by administration of GE and 6S in the pole test (Fig. 1C). Moreover, latency time in rotarod test significantly shortened by P. mirabilis treatment was significantly recovered by GE (Fig. 1D). These results show that GE and 6S treatment could attenuate P. mirabilis-induced movement impairment.
GE and 6S protect brain dopaminergic neurons against P. mirabilis
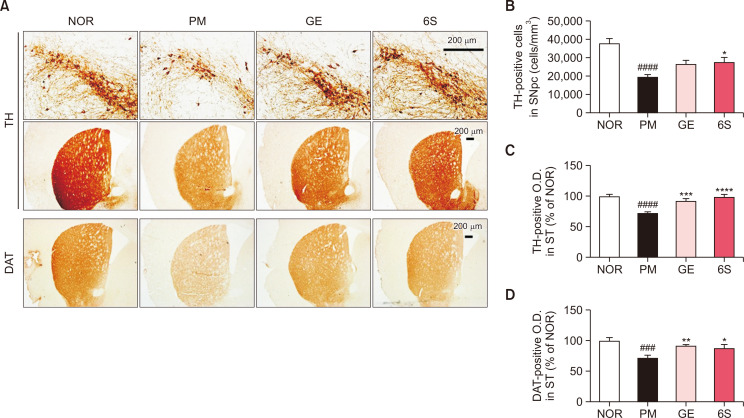
To determine the protective effects of GE and 6S on dopaminergic neuronal damage provoked by P. mirabilis, TH immunostaining for SN and ST tissue was performed (Fig. 2A). Surprisingly, treatment with GE and 6S significantly inhibited P. mirabilis-induced dopaminergic neuronal damage in both the SNpc (Fig. 2B) and the ST, respectively (Fig. 2C). These results suggest that GE and 6S treatment effectively protect brain dopaminergic neurons against P. mirabilis induction.
Fig. 2.
6-shogaol and ginger treatment inhibit dopaminergic neuronal death induced by P. mirabilis administration. (A) Representative images of TH-immunoreactivity in the ST and SNpc and DAT-immunoreactivity in the ST. (B) The number of TH-positive neurons in the SNpc was counted. The optical density of (C) TH immunoreactivity or (D) that of DAT immunoreactivity in the ST were measured. Values were expressed as means ± SEM. ###p<0.001 and ####p<0.0001 vs. NOR group; *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001 vs. PM group. N=8 for each group.
In addition, we investigated whether GE and 6S affects the alterations of striatal DAT levels like dopaminergic neurons. The optical density of DAT immunoreactivity in ST was significantly reduced in the P. mirabilis only treated group versus the normal group and GE or 6S treatment showed the significant increase of DAT immunoreactivity reduced by P. mirabilis (Fig. 2D).
GE and 6S inhibit P. mirabilis-induced neuroinflammation in mice
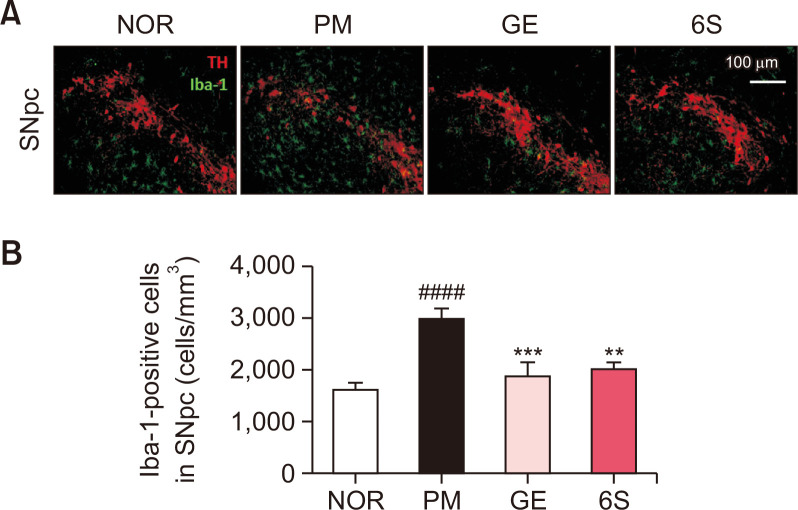
To determine the inhibitory effects of GE and 6S on neuroinflammation following the administration of P. mirabilis, double immunofluorescence staining of TH and Iba-1 for SN tissue was performed. Treatment with GE and 6S significantly inhibited P. mirabilis-induced microglial activation in the SNpc (Fig. 3). These results suggest that GE and 6S treatment protect brain dopaminergic neurons from P. mirabilis-induced neuroinflammatory responses.
Fig. 3.
6-shogaol and ginger treatment inhibits microglial activation induced by P. mirabilis administration. (A) The representative image and (B) the number of activated microglia (Iba-1-positive cells; green) was significantly increased in the SNpc of the brain (TH-positive area; red) after P. mirabilis treatment. Values were expressed as means ± SEM. ####p<0.0001 vs. NOR group; **p<0.01 and ***p<0.001 vs. PM group. N=8 for each group.
GE and 6S suppress the intestinal barrier disruption and inflammation after P. mirabilis administration
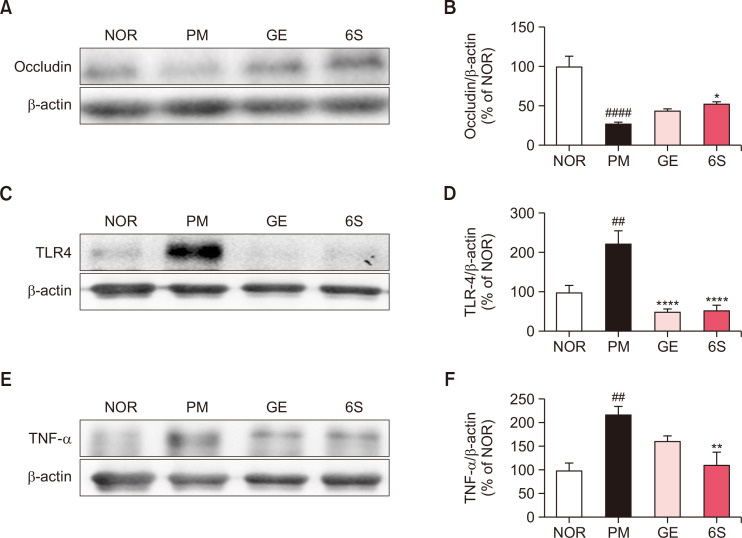
To evaluate the protective effects of GE and 6S on P. mirabilis-induced epithelial barrier disruption in colon, the levels of occludin, an epithelial barrier protein, was performed by western blotting. The band intensity of occludin in distal colon was significantly reduced in the P. mirabilis only treated group versus the normal group. GE treatment and 6S treatment exhibited the inhibitory effects against the breakdown of epithelial barriers in colon by P. mirabilis (Fig. 4A , 4B).
Fig. 4.
6-shogaol and ginger treatment inhibits P. mirabilis-induced intestinal condition. The representative immunoblotting images of (A) occludin, (C) TLR4 and (E) TNF-α. The band intensities of (B) occludin and (D) TLR4 and (F) TNF-α in the intestine were measured. Values were expressed as means ± SEM. ##p<0.01 and ####p<0.0001 vs. NOR group. *p<0.05, **p<0.01 and ****p<0.0001 vs. PM group. N=6 for each group.
To investigate the colonic inflammatory responses induced by P. mirabilis, we evaluated the bacteria-mediated proinflammation and found that GE and 6S significantly decreased P. mirabilis-induced overexpression of TLR4 in the mouse colon, respectively (Fig. 4C, 4D). In addition, GE and 6S inhibited the expression of proinflammatory cytokine TNF-α induced by P. mirabilis (Fig. 4E, 4F).
GE and 6S inhibit the α-syn aggregation in both brain and colon of P. mirabilis-treated mice
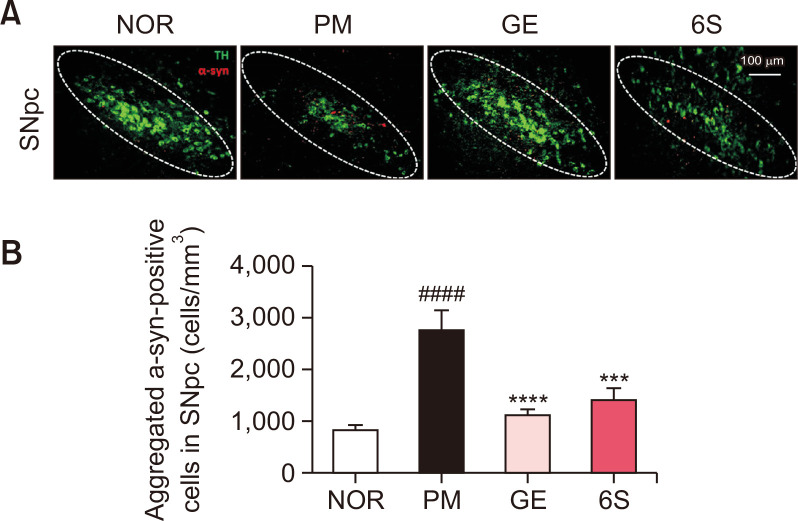
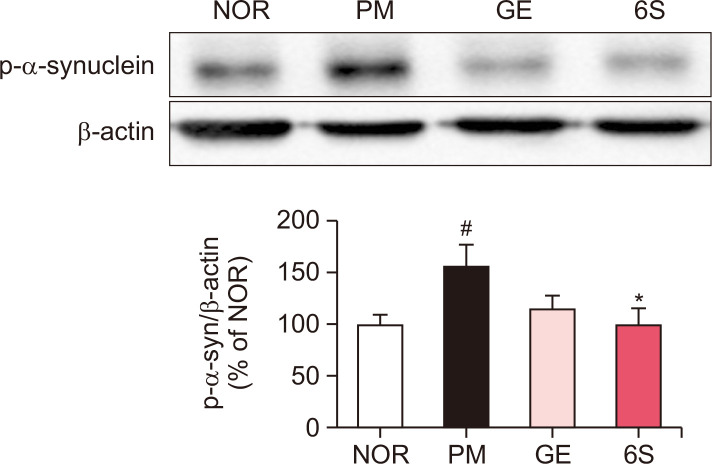
As previous report, P. mirabilis treatment stimulates the aggregation of α-synuclein in the colon and the brain. To investigate the inhibitory effects of GE and 6S on the α-synuclein aggregation in P. mirabilis-treated mouse brain, immunoreactivity of α-synuclein filaments in the SN was measured. Results showed that P. mirabilis treatment induced α-synuclein aggregation in the brain compared to the normal mouse; whereas GE and 6S significantly decreased α-synuclein filaments increased by P. mirabilis in the mouse SN (Fig. 5). We also found that P. mirabilis induced the phosphorylation of α-synuclein in the mouse colon. However, GE and 6S showed the significant decrease of phosphorylated α-synuclein in the mouse colon (Fig. 6).
Fig. 5.
6-shogaol and ginger treatment inhibits brain α-syn aggregation induced by P. mirabilis administration. The representative image and the number of aggregated α-syn (α-syn-positive signals; red) was significantly increased in the SNpc of the brain (TH-positive area; green) after P. mirabilis treatment. Values were expressed as means ± SEM. ####p<0.0001 vs. NOR group; ***p<0.001 and ****p<0.0001 vs. PM group. N=8 for each group.
Fig. 6.
6-shogaol and ginger treatment inhibits P. mirabilis-induced intestinal phospho-α-syn. The representative immunoblotting image and the band intensity of phospho-α-syn in the intestine was measured. Values were expressed as means ± SEM. #p<0.05 vs. NOR group. *p<0.05, *p<0.05 vs. PM group. N=6 for each group.
DISCUSSION
Recently, studies of mechanisms mediated by gut-brain communications and on the derivation of control materials are being conducted ultimately to treat brain degenerative diseases. Among them, we confirmed P. mirabilis-induced dopaminergic neuronal death, neuroinflammation, and α-synuclein aggregation in the brain and confirmed that P. mirabilis also affected gut-brain communication by inducing motor disorders. Based on this previous study, the present study verified whether GE and 6S show efficacy with regard to improving PD and neuroinflammation in the brain by regulating the intestinal environment.
The effects of 6S on brain degenerative diseases are widely reported. These effects, such as protecting brain neuronal cells and improving behaviors, have been found to be induced through the regulation of neuroinflammation, and additional studies are underway to determine as to whether these alterations actually act directly on the brain. Ginger and 6S have recently been reported to regulate intestinal inflammatory responses and the enteric nervous system in brain disease models. Accordingly, it was predicted that 6S could affect the brain based on the gut-brain axis, implying that 6S does not act directly on the brain. In previous studies, it was confirmed that 6S and ginger protect against MPTP-induced ENS degradation and intestinal inflammatory activation, respectively (Huh et al., 2020). These factors in turn likely affect not only the normalization of the intestinal environment, but also the brain. We used a P. mirabilis-treated mouse model in this study to predict changes in the gut and the corresponding effects on the brain, meaning that the processing of the model can have intestinal regulatory outcomes regardless of whether 6S progresses past the blood-brain barrier.
In another exploration here, we discovered certain effects of 6S and ginger on P. mirabilis-induced motor dysfunction, dopaminergic neurodegeneration, neuroinflammation, dysregulation of dopamine transmission, particulary striatal DAT loss and brain α-syn aggregation. Several previous reports provide clues about such protective effects of 6-gingerol on the intestinal barrier based on ischemic insults an on; the anti-colon cancer effects of ginger stemming from the lowering of the actions of bacterial enzymes such as β-glucuronidase and mucinase (Manju and Nalini, 2006). These result from the inhibitory actions of ginger and its phenolic components on the quorum sensing activity, which is related to the density of the bacterial population, and is affected by cell-cell communication and the sharing of chemical signal molecules (Kumar et al., 2014). Thus, the anti-biofilm activity of ginger may contribute to its ameliorating effects on P. mirabilis-induced PD phenotypes via the inhibition of P. mirabilis colonization in the gut of mice orally treated P. mirabilis. In addition, the anti-PD actions of ginger and its compounds, as explored in various PD models, support the results of the present study (Kabuto et al., 2005; Kabuto and Yamanushi, 2011; Choi et al., 2015; Peng et al., 2015); ginger protects the epithelial barriers against P. mirabilis insults. Several previous studies discuss the potential of ginger on intestinal barrier conditions. Luettig et al. (2016) demonstrated the protective effects of ginger on TNF-α-induced epithelial barrier damage via the inhibition of NF-κB cell-signaling in HT29 colon cells. Ginger also exhibited a maintenance function of the intestinal barrier function against ischemia-reperfusion injuries or inflammatory mediators such as LPS, TNF-α, and interferon-gamma through the suppression of the p38 mitogen-activated protein kinase pathway in Caco-2 colon cells (Li et al., 2017).
Meanwhile, ginger possesses biological properties that modulate alterations of gut microbiota according to findings from Samanta and coworkers. Their study revealed that pigs given feed containing antibiotics exhibited gut dysbiosis, showing the reduction in the abundance of gut bacteria with probiotic features, whereas the level increased significantly after ginger supplementation, similar to the outcome of the prebiotic inulin (Samanta et al., 2015). A recent interesting study also found that encapsulated beads consisting of ginger and a probiotic bacterium (Lactobacillus acidophilus) effectively restricted the increase of oxidative stress, the inflammatory burden, and colonic permeability in rat given dextran sulfate sodium (Deol et al., 2017). These data suggest the regulatory role of ginger on the composition of gut microbiota, also indicating that additional studies of the relationship between its effects on PD phenotypes and its ability to modulate the gut microbiota are needed.
In conclusion, this study suggests that GE and 6S have the potential to ameliorate PD-like motor behavior and the degeneration of dopaminergic neurons induced by P. mirabilis in mice. The findings of this study are meaningful in that they provide the first experimental evidence that 6S may attenuate PD via regulating microbiome-induced abnormalities in both the intestine and the brain.
Funding Statement
ACKNOWLEDGMENTS This study was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education [NRF-2018R1D1A1B07048099] and grants from the National Research Foundation of Korea funded by the Korean government [2022M3A9B6017813].
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Angelopoulou E., Paudel Y. N., Papageorgiou S. G., Piperi C. Elucidating the beneficial effects of ginger (Zingiber officinale Roscoe) in Parkinson's disease. ACS Pharmacol. Transl. Sci. 2022;5:838–848. doi: 10.1021/acsptsci.2c00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. G., Kim N., Ju I. G., Eo H., Lim S. M., Jang S. E., Kim D. H., Oh M. S. Oral administration of Proteus mirabilis damages dopaminergic neurons and motor functions in mice. Sci. Rep. 2018;8:1275. doi: 10.1038/s41598-018-19646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. S., Bae W. Y., Park C., Jeong J. W. Zingerone activates VMAT2 during MPP(+) -induced Cell Death. Phytother. Res. 2015;29:1783–1790. doi: 10.1002/ptr.5435. [DOI] [PubMed] [Google Scholar]
- Deol P. K., Khare P., Singh D. P., Soman G., Bishnoi M., Kondepudi K. K., Kaur I. P. Managing colonic inflammation associated gut derangements by systematically optimised and targeted ginger extract-Lactobacillus acidophilus loaded pharmacobiotic alginate beads. Int. J. Biol. Macromol. 2017;105:81–91. doi: 10.1016/j.ijbiomac.2017.06.117. [DOI] [PubMed] [Google Scholar]
- Forsythe P., Bienenstock J. Immunomodulation by commensal and probiotic bacteria. Immunol. Invest. 2010;39:429–448. doi: 10.3109/08820131003667978. [DOI] [PubMed] [Google Scholar]
- Huh E., Choi J. G., Noh D., Yoo H. S., Ryu J., Kim N. J., Kim H., Oh M. S. Ginger and 6-shogaol protect intestinal tight junction and enteric dopaminergic neurons against 1-methyl-4-phenyl 1,2,3,6-tetrahydropyridine in mice. Nutr. Neurosci. 2020;23:455–464. doi: 10.1080/1028415X.2018.1520477. [DOI] [PubMed] [Google Scholar]
- Huh E., Lim S., Kim H. G., Ha S. K., Park H. Y., Huh Y., Oh M. S. Ginger fermented with Schizosaccharomyces pombe alleviates memory impairment via protecting hippocampal neuronal cells in amyloid beta(1-42) plaque injected mice. Food Funct. 2018;9:171–178. doi: 10.1039/C7FO01149K. [DOI] [PubMed] [Google Scholar]
- Kabuto H., Nishizawa M., Tada M., Higashio C., Shishibori T., Kohno M. Zingerone [4-(4-hydroxy-3-methoxyphenyl)-2-butanone] prevents 6-hydroxydopamine-induced dopamine depression in mouse striatum and increases superoxide scavenging activity in serum. Neurochem. Res. 2005;30:325–332. doi: 10.1007/s11064-005-2606-3. [DOI] [PubMed] [Google Scholar]
- Kabuto H., Yamanushi T. T. Effects of zingerone [4-(4-hydroxy-3-methoxyphenyl)-2-butanone] and eugenol [2-methoxy-4-(2-propenyl)phenol] on the pathological progress in the 6-hydroxydopamine-induced Parkinson's disease mouse model. Neurochem. Res. 2011;36:2244–2249. doi: 10.1007/s11064-011-0548-5. [DOI] [PubMed] [Google Scholar]
- Kelly L. P., Carvey P. M., Keshavarzian A., Shannon K. M., Shaikh M., Bakay R. A., Kordower J. H. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson's disease. Mov. Disord. 2014;29:999–1009. doi: 10.1002/mds.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. V., Murthy P. S., Manjunatha J. R., Bettadaiah B. K. Synthesis and quorum sensing inhibitory activity of key phenolic compounds of ginger and their derivatives. Food Chem. 2014;159:451–457. doi: 10.1016/j.foodchem.2014.03.039. [DOI] [PubMed] [Google Scholar]
- Li Y., Xu B., Xu M., Chen D., Xiong Y., Lian M., Sun Y., Tang Z., Wang L., Jiang C., Lin Y. 6-Gingerol protects intestinal barrier from ischemia/reperfusion-induced damage via inhibition of p38 MAPK to NF-kappaB signalling. Pharmacol. Res. 2017;119:137–148. doi: 10.1016/j.phrs.2017.01.026. [DOI] [PubMed] [Google Scholar]
- Luettig J., Rosenthal R., Lee I. M., Krug S. M., Schulzke J. D. The ginger component 6-shogaol prevents TNF-alpha-induced barrier loss via inhibition of PI3K/Akt and NF-kappaB signaling. Mol. Nutr. Food Res. 2016;60:2576–2586. doi: 10.1002/mnfr.201600274. [DOI] [PubMed] [Google Scholar]
- Manju V., Nalini N. Effect of ginger on bacterial enzymes in 1,2-dimethylhydrazine induced experimental colon carcinogenesis. Eur. J. Cancer Prev. 2006;15:377–383. doi: 10.1097/00008469-200610000-00001. [DOI] [PubMed] [Google Scholar]
- Moon M., Kim H. G., Choi J. G., Oh H., Lee P. K., Ha S. K., Kim S. Y., Park Y., Huh Y., Oh M. S. 6-Shogaol, an active constituent of ginger, attenuates neuroinflammation and cognitive deficits in animal models of dementia. Biochem. Biophys. Res. Commun. 2014;449:8–13. doi: 10.1016/j.bbrc.2014.04.121. [DOI] [PubMed] [Google Scholar]
- Na J. Y., Song K., Lee J. W., Kim S., Kwon J. 6-Shogaol has anti-amyloidogenic activity and ameliorates Alzheimer's disease via CysLT1R-mediated inhibition of cathepsin B. Biochem. Biophys. Res. Commun. 2016;477:96–102. doi: 10.1016/j.bbrc.2016.06.026. [DOI] [PubMed] [Google Scholar]
- Park G., Kim H. G., Ju M. S., Ha S. K., Park Y., Kim S. Y., Oh M. S. 6-Shogaol, an active compound of ginger, protects dopaminergic neurons in Parkinson's disease models via anti-neuroinflammation. Acta Pharmacol. Sin. 2013;34:1131–1139. doi: 10.1038/aps.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S., Yao J., Liu Y., Duan D., Zhang X., Fang J. Activation of Nrf2 target enzymes conferring protection against oxidative stress in PC12 cells by ginger principal constituent 6-shogaol. Food Funct. 2015;6:2813–2823. doi: 10.1039/C5FO00214A. [DOI] [PubMed] [Google Scholar]
- Raza C., Anjum R., Shakeel N. U. A. Parkinson's disease: Mechanisms, translational models and management strategies. Life Sci. 2019;226:77–90. doi: 10.1016/j.lfs.2019.03.057. [DOI] [PubMed] [Google Scholar]
- Samanta A. K., Jayaram C., Jayapal N., Sondhi N., Kolte A. P., Senani S., Sridhar M., Dhali A. Assessment of fecal microflora changes in pigs supplemented with herbal residue and prebiotic. PLoS One. 2015;10:e0132961. doi: 10.1371/journal.pone.0132961.0e2aebd2309e467aa7a56c2605128db2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson T. R., Debelius J. W., Thron T., Janssen S., Shastri G. G., Ilhan Z. E., Challis C., Schretter C. E., Rocha S., Gradinaru V., Chesselet M. F., Keshavarzian A., Shannon K. M., Krajmalnik-Brown R., Wittung-Stafshede P., Knight R., Mazmanian S. K. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 2016;167:1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F., Aho V., Pereira P. A., Koskinen K., Paulin L., Pekkonen E., Haapaniemi E., Kaakkola S., Eerola-Rautio J., Pohja M., Kinnunen E., Murros K., Auvinen P. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov. Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]