Abstract
Long-term administration of levodopa (L-DOPA) to patients with Parkinson’s disease (PD) commonly results in involuntary dyskinetic movements, as is known for L-DOPA-induced dyskinesia (LID). 5-Hydroxytryptophan (5-HTP) has recently been shown to alleviate LID; however, no biochemical alterations to aberrant excitatory conditions have been revealed yet. In the present study, we aimed to confirm its anti-dyskinetic effect and to discover the unknown molecular mechanisms of action of 5-HTP in LID. We made an LID-induced mouse model through chronic L-DOPA treatment to 6-hydroxydopamine-induced hemi-parkinsonian mice and then administered 5-HTP 60 mg/kg for 15 days orally to LID-induced mice. In addition, we performed behavioral tests and analyzed the histological alterations in the lesioned part of the striatum (ST). Our results showed that 5-HTP significantly suppressed all types of dyskinetic movements (axial, limb, orolingual and locomotive) and its effects were similar to those of amantadine, the only approved drug by Food and Drug Administration. Moreover, 5-HTP did not affect the efficacy of L-DOPA on PD motor manifestations. From a molecular perspective, 5-HTP treatment significantly decreased phosphorylated CREB and ΔFosB expression, commonly known as downstream factors, increased in LID conditions. Furthermore, we found that the effects of 5-HTP were not mediated by dopamine1 receptor (D1)/DARPP32/ERK signaling, but regulated by AKT/mTOR/S6K signaling, which showed different mechanisms with amantadine in the denervated ST. Taken together, 5-HTP alleviates LID by regulating the hyperactivated striatal AKT/mTOR/S6K and CREB/ΔFosB signaling.
Keywords: Parkinson’s disease, Levodopa, Levodopa-induced dyskinesia, 5-Hydroxytryptophan, Serotonin
INTRODUCTION
Levodopa (L-DOPA) is the gold standard for treating patients with Parkinson’s disease (PD), the second most common age-related neurodegenerative disease (Reeve et al., 2014; Tambasco et al., 2018). However, approximately 70% of patients who receive L-DOPA experience involuntary movements within 5 years, called L-DOPA-induced dyskinesia (LID) (Pandey and Srivanitchapoom, 2017). It is accompanied by abnormal movements, including uncontrolled body writhing and twisting. (Thanvi et al., 2007).
In the normal state, several neuronal systems maintain appropriate dopamine concentrations by its expulsion and reuptake. Since both dopaminergic and serotonergic (5-HT) neurons possess aromatic L-amino acid decarboxylase that converts L-DOPA to dopamine, these neuronal systems modulate dopamine release from the intrasynaptic cleft in spiny projection neurons (SPNs). As PD progresses with the denervation of the dopaminergic neurons in the brain, L-DOPA is merely converted into dopamine by 5-HT neurons. Unlike dopaminergic neurons, 5-HT neurons do not have dopamine uptake functions, so that excessive dopamine levels in the synaptic cleft are induced. Consequently, a surge in dopamine concentration generates hyperactivated dopamine 1 receptor (D1)-downstream molecular signals and other aberrant biochemical signals, which lead to involuntary movement disorders (Calabresi et al., 2008; Cenci, 2014). Amantadine is the only medication approved by Food and Drug Administration (FDA) for LID therapy (Sharma et al., 2018). It normalizes excitatory signals by maintaining the closed status of glutamate N-methyl-D-aspartate (NMDA) receptor channels and reducing calcium influx into the postsynaptic neurons (Blanpied et al., 2005; Inzelberg et al., 2006; Junho and de Oliveira, 2019). However, its narrow therapeutic window along with severe side effects, including constipation, vomiting and decreased appetite, makes it difficult for the patients to take the medication (Pandey and Srivanitchapoom, 2017).
To overcome these limitations, several targets have been presented for LID research. Among them, regulating the 5-HT system seems to be crucial, since dopamine and 5-HT, translocated by vesicular monoamine transporter 2, release competitively in the synaptic cleft of the 5-HT neurons (Carta et al., 2008). It was discovered that the 5-HT1 receptor agonist reduces L-DOPA-derived dopamine release and subsequent LID development by activating the 5-HT autoreceptor, which plays a role in regulating the release of neurotransmitters (Carta et al., 2007). In addition, chronic administration of the 5-HT1 receptor agonist, eltoprazine, alleviated dyskinesia by normalizing D1/extracellular signal-regulated kinase (ERK) and mechanistic target of rapamycin (mTOR) signal and restoring synaptic plasticity in an LID-induced rat model (Ghiglieri et al., 2016). On the other hand, targeting the 5-HT autoreceptor might have the disadvantage of extensively decreasing dopamine concentration and 5-HT release in the synaptic cleft, which lead to mood disorders, such as depression (Albert et al., 1996; Kannari et al., 2001; Nautiyal and Hen, 2017).
5-Hydroxytryptophan (5-HTP), a precursor of 5-HT, has also been explored in LID research due to its advantages. In contrast to 5-HT, 5-HTP can easily cross the blood-brain barrier (Nakatani et al., 2008). Moreover, it dampens excessive dopamine levels but does not decline 5-HT levels, unlike the 5-HT autoreceptor. In previous studies, Tronci et al. (2013) found that treatment with 5-HTP had an anti-dyskinetic effect in an LID-induced rat model without interfering with L-DOPA efficacy. In addition, they discovered that seven patients with LID taking 50 mg 5-HTP with L-DOPA for 4 weeks, showed significant improvement in dyskinesia compared to the placebo group, including five patients, using a unified dyskinesia rating scale and unified Parkinson’s disease rating scale IV (Meloni et al., 2020). These studies showed the potential effects of 5-HTP in treating LID; however, the biochemical modulations of 5-HTP in response to various excitatory signals occurring in LID have not yet been elucidated.
The objective of this study was to confirm whether 5-HTP produces an anti-dyskinetic effect in LID-induced mice and to explore its unknown molecular mechanisms. First, we chronically administered L-DOPA to hemi-parkinsonian mice to induce LID manifestations. To confirm the anti-dyskinetic effect of 5-HTP, we performed an abnormal involuntary movements (AIMs) test after 5-HTP treatment for 15 days. In addition, we checked whether the drug may alter the motor improvement of L-DOPA by performing the cylinder test. Finally, to discover the 5-HTP effect on abnormally hyperactivated molecular signals, pathological factors related to LID were analyzed by Western blotting in the striatum (ST) of mice.
MATERIALS AND METHODS
Animals and drug administration
6-weeks-old ICR mice (30-34 g) were used in this study. The mice were purchased from Daehan Biolink Co. Ltd. (Eumseong, Korea). All mice were housed in cages (40×25×18 cm) under controlled humidity (60 ± 10%), temperature (23 ± 1°C) and a 12-h light/dark cycle with a free access to water and food. The mice were housed for 7 days before the experiments. All animal experiments in this study were conducted in accordance with the Animal Care and Use guidelines of Kyung Hee University, Seoul, Korea (approval number; KHUASP (SE)-20-316).
To make a hemi-parkinsonian mouse model, 6-hydroxydopamine (6-OHDA; Sigma-Aldrich, MO, USA) surgeries were performed under anesthesia with tribromoethanol (312.5 mg/kg, intraperitoneal [i.p.]). The surgical procedures were performed as previously described (Eo et al., 2019). Briefly, at a rate of 0.5 μL/min, the animals in the SHAM group received 2 μL vehicle (saline with 0.1% ascorbic acid) stereotaxically and the remaining animals were injected with 6-OHDA (8 μg/μL) to the right ST (coordinates with respect to bregma in mm: AP 0.5, ML 2.0, DV−3.0) using a Hamilton syringe (Hamilton, NV, USA). After a recovery period of 14 days, we performed an apomorphine-induced rotation test, and the mice that did not perform 125 turns in 25 min were excluded from the present study.
To prepare LID-induced mice, we followed a previously described procedure (Huh et al., 2018). Except for the animals in the SHAM (vehicle-lesioned, n=7) and PD (6-OHDA-lesioned, n=6) groups, which received the vehicle (saline, peroral [p.o.]), the remaining animals were treated with L-DOPA with benserazide (80 mg/kg with 20 mg/kg, p.o.) for 28 days from 21th day after surgery and then tested for LID modeling through the AIMs test.
L-DOPA-treated mice were divided into three groups: (1) LID group (oral cotreatment with L-DOPA and vehicle as saline, n=6), (2) 5-HTP group (oral cotreatment with L-DOPA and 5-HTP 60 mg/kg, n=6) and (3) AMAN group (oral cotreatment of L-DOPA and amantadine 40 mg/kg, n=5). From the 49th day after surgery, 5-HTP and amantadine were administered 1 h before L-DOPA administration for 15 days. Drugs were dissolved in saline, and L-DOPA was specifically used in suspensions under shading conditions. L-DOPA, benserazide, 5-HTP and amantadine were purchased from Sigma-Aldrich.
Behavior tests
The AIMs test was used to evaluate the severity of LID in animals (Azkona et al., 2014; Paolone et al., 2015; Hamadjida et al., 2018; Calabrese et al., 2020). To check the anti-dyskinetic effects of 5-HTP, the test was assessed by scoring the severity of the four subtypes individually. Each subtype was scored on a scale from to 0-4 (0=absent, 1=occasionally (<50% observation time), 2=frequent (>50% observation time), 3=continuous and disrupted by threatening stimulation, 4=present all time, severe, not disrupted). The total AIMs score was calculated by summing the scores of the four subtypes or those of each session. As soon as L-DOPA was administered, each mouse was placed in a cylinder (diameter 135 mm; height 225 mm) and observed for 1 min every 20 min for 180 min by two individually trained researchers.
To investigate the effect of drugs on L-DOPA efficacy, we performed a cylinder test on the 13th day of drug treatment. Each mouse was placed in a cylinder (diameter 110 mm; height 150 mm) 1 h after L-DOPA administration. The left or right movements of the forepaws touching the wall were recorded for 5 min for each mouse. The contralateral paw use was calculated as follows:
(The number of times the right forepaw touches the wall) / (The number of times the total forepaw touches the wall)×100 (%).
Tissue preparation and Western blot analysis
1h after L-DOPA administration, the animals were sacrificed by cervical dislocation on the 16th day of drug administration. The 6-OHDA-injected right ST regions were dissected and stored at –80°C until histological analysis. To investigate the effect of 5-HTP on pathological factors related to LID, ST was lysed using RIPA buffer containing protease and phosphatase following the manufacturer’s instructions (ThermoFisher, Waltham, MA, USA).
To determine the biochemical mechanism of 5-HTP action in LID, we analyzed the mice of ST by Western blotting. Proteins were equalized at the same concentration using the Bradford assay (Bio-Rad, Hercules, CA, USA). After proteins were separated by size using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, they were transferred to the polyvinylidene difluoride (PVDF) membranes (Millipore, Burlington, MA, USA). The membranes were incubated with 5% skim milk or 5% BSA (Bio-Rad) in 25 mM Tris-Cl, 150 mM NaCl, and 0.005 % Tween-20 for 1 h and then incubated with primary antibodies in 1% skim milk or 5% BSA overnight at 4°C. Primary antibodies were delta FBJ murine osteosarcoma viral oncogene homolog B (ΔFosB), dopamine and cyclic adenosine monophosphate regulated phosphorylation of 32 kDa (DARPP32), phospho-Thr202/Thr204-ERK1/2 (P-ERK1/2) a serine/threonine-specific protein kinase (AKT), phospho-Ser437-AKT (P-AKTS473), mTOR, phospho-Ser2481-mTOR (P-mTORS2481), ribosomal S6 kinase (S6K), phospho-Thr389-S6K (P-S6KT389), GAPDH; (cell signaling, MA, USA); ERK, cyclic adenosine monophosphate response element-binding protein (CREB), Phospho-Ser133-CREB (P-CREBS133); (Santa Cruz, CA, USA), and phospho-Thr34-DARPP32 (P-DARPP32T34); (Phosphosolutions, CO, USA). After washing for 15 min with Tris-buffered saline containing 1% Tween 20, the membranes were incubated with secondary antibodies for 1 h. Immunoactive bands were developed using an ECL reagent and visualized using the ChemiDocTM XRS+ system (Bio-Rad).
Statistical analysis
All statistical parameters were calculated using GraphPad Prism 8.0.1 software (GraphPad Software, San Diego, CA, USA). Values are expressed as mean ± standard error of the mean (SEM). The results were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test or two-way ANOVA followed by Dunnett’s multiple comparison test. Additionally, the correlation analysis was analyzed by Pearson’s correlation coefficient. Differences with a p-value less than 0.05 were considered statistically significant.
RESULTS
5-HTP reduced dyskinetic movements in LID-induced mice
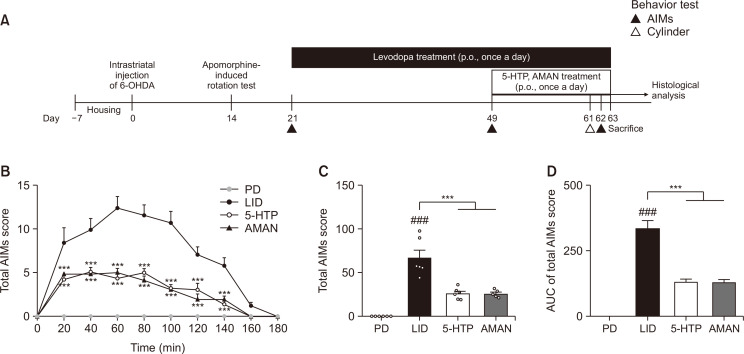
To confirm whether treatment with 5-HTP decreased dyskinetic movements in LID-induced mice, we conducted an AIMs test for 180 min on the 14th day of drug treatment (Fig. 1A). From 20 min to 140 min after L-DOPA administration, mice in the 5-HTP group showed significantly suppressed abnormal dyskinetic movements compared to those in the LID group, which was similar to that observed in the AMAN group. In particular, at 60 min after L-DOPA treatment, mice in the LID group showed the highest AIMs score during the test sessions; however, the 5-HTP-treated group significantly reduced score compared with the LID group (Fig. 1B). In addition, the total AIMs score of the 5-HTP group, which is the sum of the four AIMs subtype scores for 180 min, decreased compared with that of the LID group (Fig. 1C). In an aspect of the area under curve (AUC) of graph for changing AIMs score over time, we also found that increased AUC in the LID group was significantly attenuated by treatment of 5-HTP (Fig. 1D). Additionally, the 5-HTP group showed similar effects as those of the AMAN group.
Fig. 1.
Anti-dyskinetic effect of 5-HTP on LID. Experimental schedule (A), AIMs score during 180 min as soon as the start of L-DOPA administration (B). Sum of the 4 AIMs subtypes scores (axial, limb, orolingual and locomotive) during 180min (C). AUC of total AIMs score graph over time (D). Data were analyzed by two-way ANOVA, followed by Dunnett’s post hoc test (B) or one-way ANOVA, followed by Dunnett’s post hoc test (C, D). ###p<0.001 compared with the PD group; ***p<0.001 compared with the LID group. Values were presented as means ± of SEM. PD: 6-OHDA-lesioned mouse (n=6), LID: 6-OHDA-lesioned mouse treated with vehicle+L-DOPA (n=6), 5-HTP: 6-OHDA-lesioned mouse treated with 5-HTP+L-DOPA (n=6), AMAN: 6-OHDA-lesioned mouse treated with AMAN+L-DOPA (n=5).
5-HTP reduced AIMs per each subtype
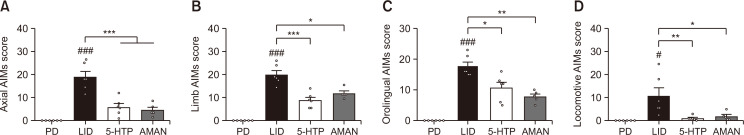
To determine the subtypes of the anti-dyskinetic effect of 5-HTP, the AIMs scores for the four subtypes were compared. The four subtypes of AIMs scores were highly increased in LID-induced mice. As shown in Fig. 2A, the increased axial AIMs score in the LID group was significantly diminished by treatment with 5-HTP 60 mg/kg. Additionally, other subtype results showed that mice of the 5-HTP group had significantly decreased dyskinetic movements compared to those of chronically L-DOPA treated mice (Fig. 2B-2D). The inhibitory effect showed similar impact on the AMAN group.
Fig. 2.
Effect of 5-HTP on AIMs of each subtype (axial; A, limb; B, orolingual; C and locomotive; D). Data were analyzed by one-way ANOVA, followed by Dunnett’s post hoc test. ###p<0.001 and #p<0.05 compared with the PD group; ***p<0.001, **p<0.01 and *p<0.05 compared with the LID group. Values were presented as means ± of SEM. PD: 6-OHDA-lesioned mouse (n=6), LID: 6-OHDA-lesioned mouse treated with vehicle+L-DOPA (n=6), 5-HTP: 6-OHDA-lesioned mouse treated with 5-HTP+L-DOPA (n=6), AMAN: 6-OHDA-lesioned mouse treated with AMAN+L-DOPA (n=5).
5-HTP did not interfere with L-DOPA efficacy on parkinsonian motor behavior
For LID therapies, maintaining the efficacy of L-DOPA is as important as suppressing dyskinesia. Therefore, we performed a cylinder test to evaluate its intervention on the L-DOPA effect. The forelimb usage on the contralateral side of the lesioned part in the PD group (Fig. 3) was significantly lower than that of the SHAM group. However, L-DOPA administration resulted in a significant recovery. In addition to the LID group, the 5-HTP and AMAN groups did not show a disturbance on the L-DOPA effect, indicating that 5-HTP does not interfere with L-DOPA efficacy.
Fig. 3.
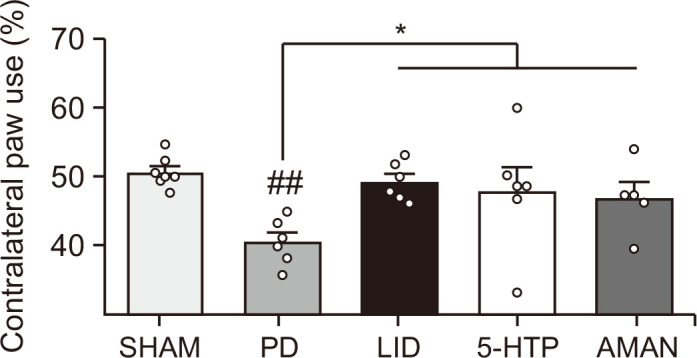
Effect of 5-HTP on the L-DOPA motor efficacy. The 5-HTP administration was done 1 h before L-DOPA treatment. After 1 h of L-DOPA treatment, the test was performed. Data were analyzed by one-way ANOVA, followed by Dunnett’s post hoc test. ##p<0.01 compared with the SHAM group and *p<0.05 compared with the PD group. Values were presented as means ± of SEM. SHAM: vehicle-lesioned mouse (n=7), PD: 6-OHDA-lesioned mouse (n=6), LID: 6-OHDA-lesioned mouse treated with vehicle+L-DOPA (n=6), 5-HTP: 6-OHDA-lesioned mouse treated with 5-HTP+L-DOPA (n=6), AMAN: 6-OHDA-lesioned mouse treated with AMAN+L-DOPA (n=5).
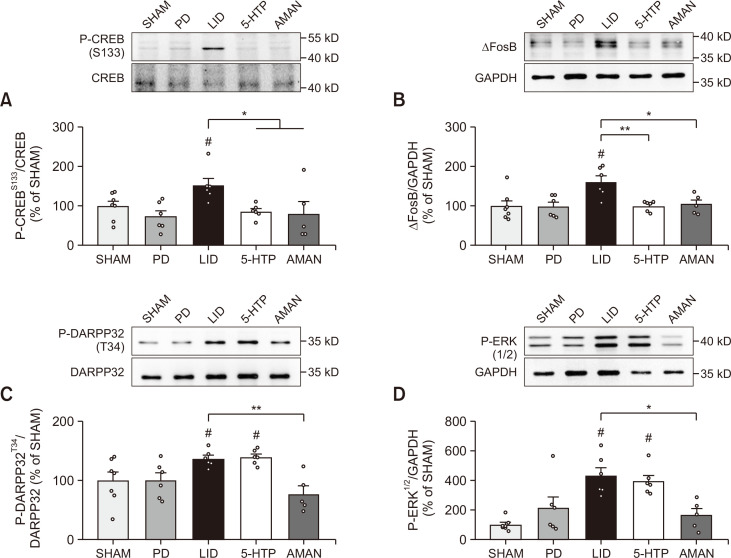
5-HTP normalized aberrant CREB and ΔFosB signaling, but did not affect D1/DARPP32/ERK signaling in LID-induced mouse ST
We next investigated whether 5-HTP altered several possible factors related to abnormal signals caused by excessive dopamine in the ST of LID mice. First, we evaluated the expression of phosphorylated CREB and ΔFosB, which are commonly known downstream factors in LID conditions. As shown in Fig. 4A and 4B, chronic treatment of L-DOPA increased phosphorylation of CREB and ΔFosB expression compared with the PD group, but mice treated with 5-HTP 60 mg/kg orally showed normalization of both overexpressed protein levels of phosphorylated CREB and ΔFosB similar to those of amantadine treated mice. Next, we assessed the commonly altered D1/DARPP32/ERK pathway to elucidate how these downstream scales were regulated. Phosphorylation of DARPP32 and ERK levels was excessively increased in the LID group (Fig. 4C, 4D). Interestingly, treatment of amantadine in LID-induced mice inhibited its phosphorylation, but oral administration of 60 mg/kg 5-HTP did not affect its expression. Taken together, we found that 5-HTP regulated ΔFosB and CREB signaling, but not by D1/DARPP32/ERK signaling, unlike amantadine.
Fig. 4.
Molecular mechanisms of 5-HTP on the phosphorylated CREB, ΔFosB and D1/DARPP32/ERK signaling in the ST. Expressions of P-CREBS133/CREB (A), ΔFosB/GAPDH (B), P-DARPP32T34/DARPP32 (C) and P-ERK1/2/GADPH (D) in the lesioned ST were quantified as compared to the SHAM group. Data were analyzed by one-way ANOVA, followed by Dunnett’s post hoc test. #p<0.05 compared with the PD group; **p<0.01 and *p<0.05 compared with the LID group. Values were presented as means ± of SEM. SHAM: vehicle-lesioned mouse (n=7), PD: 6-OHDA-lesioned mouse (n=6), LID: 6-OHDA-lesioned mouse treated with vehicle+L-DOPA (n=6), 5-HTP: 6-OHDA-lesioned mouse treated with 5-HTP+L-DOPA (n=6), AMAN: 6-OHDA-lesioned mouse treated with AMAN+L-DOPA (n=5).
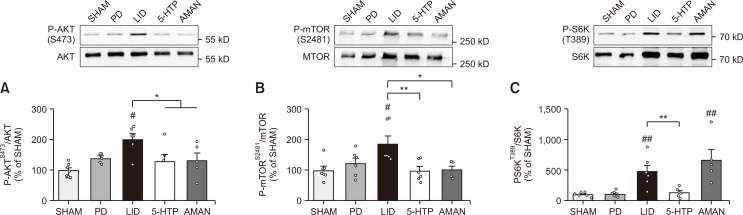
5-HTP normalized AKT/mTOR/S6K pathway in LID-induced mouse ST
In order to determine an additional pathway for regulating phosphorylated CREB and ΔFosB, we analyzed another pathway, AKT/mTOR, which could also be activated by the 5-HT receptor (Meffre et al., 2012). As shown in Fig. 5A, phosphorylation of AKT was highly elevated in the ST of LID mice compared to that of PD mice. However, its levels were stabilized in the 5-HTP group. In addition, the AKT downstream factor, mTOR, was significantly phosphorylated by chronic L-DOPA treatment. Consistent with the change in AKT expression, the 5-HTP group showed an inhibitory effect on the phosphorylation of mTOR compared with the LID group (Fig. 5B). We also found that 5-HTP mediated the AKT/mTOR signal similar to that of the AMAN group. By confirming that 5-HTP mediated AKT/mTOR, we evaluated whether 5-HTP could specifically regulate mTOR downstream factors, such as S6K. Phosphorylation of S6K in the LID group was significantly increased compared to that in the PD group. Elevated phosphorylation of S6K was significantly decreased in the 5-HTP group, which was not observed in the AMAN group (Fig. 5C).
Fig. 5.
Molecular mechanisms of 5-HTP on the AKT/mTOR/S6K signaling in the ST. Expressions of P-AKTS473/AKT (A), P-mTORS2481/mTOR (B) and P-S6KT389/AKT (C) in the lesioned ST were quantified as compared to the SHAM group. Data were analyzed by one-way ANOVA, followed by Dunnett’s post hoc test. ##p<0.01 and #p<0.05 compared with the PD group; **p<0.01 and *p<0.05 compared with the LID group. Values were presented as means ± of SEM. SHAM: vehicle-lesioned mouse (n=7), PD: 6-OHDA-lesioned mouse (n=6), LID: 6-OHDA-lesioned mouse treated with vehicle+L-DOPA (n=6), 5-HTP: 6-OHDA-lesioned mouse treated with 5-HTP+L-DOPA (n=6), AMAN: 6-OHDA-lesioned mouse treated with AMAN+L-DOPA (n=5).
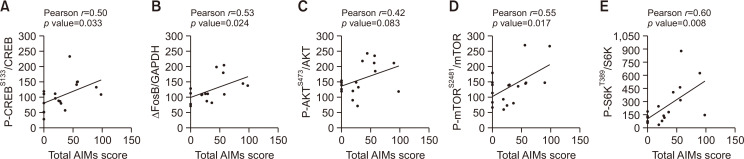
Striatal biomolecules altered by 5-HTP correlated with dyskinesia induction
The relationship between the total AIMs score and expression of proteins modified by 5-HTP 60 mg/kg treatment such as ΔFosB and phosphorylated CREB, AKT, mTOR and S6K was analyzed by Pearson’s correlation. Altered factors by 5-HTP were positively correlated with development of LID (Fig. 6). Particularly, expression of ΔFosB and phosphorylated CREB, mTOR and S6K showed statistically significant correlation with AIMs score. Collectively, 5-HTP reduced LID by modulating AKT/mTOR/S6K and CREB/ΔFosB signals.
Fig. 6.
Scatter plots of correlation between biomolecular expression by 5-HTP and severity of dyskinesia. The P-CREB/CREB (A), ΔFosB/GAPDH (B), P-AKT/AKT (C), P-mTOR/mTOR (D) and P-S6K/S6K (E) expressions were used for correlation analysis. Data were analyzed by Pearson’s correlation coefficient.
DISCUSSION
In this study, we aimed to demonstrate whether 5-HTP would have similar anti-dyskinetic effects as those of amantadine as a positive control, while maintaining the movement improvement in PD. In addition, we analyzed the unknown pathological molecular pathways in the LID condition. Our study revealed that treatment with 5-HTP reduced LID and sustained L-DOPA efficacy in parkinsonian motor disorders as much as amantadine in dyskinetic mice. In addition, for the first time, we found that 5-HTP specifically modulated the AKT/mTOR and CREB/ΔFosB pathways, independent of D1/DARPP32/ERK signaling.
5-HTP is attracting attention in LID research because of its non-reducing ability of 5-HT levels as opposed to the 5-HT autoreceptor agonist, which is well established in this area (Tronci et al., 2013; Paolone et al., 2015; Meloni et al., 2020). It is interpreted that 5-HTP maintains 5-HT mediated signals, and furthermore, selectively modulates biochemical cascades related to dyskinetic movements. Our data indicated that 5-HTP treatment showed similar effects as those of amantadine in terms of the behavior aspects (Fig. 1-3), but differed in molecular mechanisms. Specifically, the expression of the hyper-activated D1/DARPP32/ERK pathway was decreased by amantadine, but not by 5-HTP (Fig. 4). Amantadine, a previously approved drug for LID, acts not only as an NMDA receptor antagonist but also as a dopamine receptor agonist; therefore, its use for long-term treatment of LID remains controversial (Pettorruso et al., 2012; Marxreiter et al., 2017). In addition, D1-mediated signaling plays a primary role in motor circuit and LID occurrence in the ST (Aubert et al., 2005; Nishi et al., 2011). Therefore, various targets involved in D1 mediated cAMP regulation have been proposed; however, it raises concerns that direct regulation of D1 signaling could suppress locomotive activity as seen in PD. Therefore, alternative therapies that regulate aberrant biochemical signals have more advantages than the direct regulation of specific receptor activity for LID (Urs et al., 2015). Therefore, 5-HTP overcomes the disadvantages of specific receptor target therapies and at the same time, controls LID by showing a similar ability as that of amantadine.
In excitatory biochemical signals, the D1-downstreatm molecular pathway called D1/DARPP32/ERK signaling is triggered when the surge of dopamine activates D1 at the post-synapse in SPNs of dyskinetic mice by increasing cyclic adenosine monophosphate (cAMP) production (Feyder et al., 2011). We first hypothesized that 5-HTP would reduce elevated activation of D1/DARPP32/ERK through downregulation of cAMP levels by activating 5-HT1A/B receptor (Shimizu and Ohno, 2013), as in a previous study, it was discovered that the effect of 5-HTP on LID was partially agonistic towards 5-HT1A/B receptor (Tronci et al., 2013). In our study, we confirmed that 5-HTP did not affect the D1/DARPP32/ERK pathway. Several studies have suggested alternative pathways that regulate phosphorylated CREB, mTOR and truncation of FosB, but not by the D1/DARPP32/ERK pathway. Ryu et al. (2021) reported that coadministration of β-lapachone with L-DOPA attenuated LID by regulating the glycogen synthase kinase 3β pathway, but did not significantly impact the D1/ERK pathway in denervated ST. In addition, Eshraghi et al. (2020) observed that the mTOR pathway could be mediated independent of ERK signaling. Taken together, these results could indicate that the inhibitory effect of dyskinesia is not necessarily mediated through D1-mediated signaling.
Our results also suggested that 5-HTP specifically acted on the mTOR pathway, unlike amantadine that did not alter the phosphorylated S6K expression (Fig. 5). Amantadine reduced the phosphorylation of mTOR in hepatitis A-infected Huh7 cells (Sasaki-Tanaka et al., 2022). However, its effect on S6K phosphorylation was not elucidated yet. S6K activity is mainly mediated by phosphorylation on T389 or T229 site, which independently regulate its activity. Unlike T389 site that we analyzed, the phosphorylation on T229 requires PDK1 (phosphoinositide-dependent protein kinase 1) activation. (Magnuson et al., 2012). Amantadine is an anti-virus agent against virus A that is affected by PDK1/AKT/mTOR pathway (Murray et al., 2012). Collectively, amantadine might induce the phosphorylation of T229, not T389. However, further study about molecular mechanism of amantadine should be needed.
The mTOR pathway is a promising target for LID. Regarding the behavioral aspect, acute and chronic administration of rapamycin, an mTOR complex1 inhibitor, diminished dyskinesia with sustaining L-DOPA efficacy on motor symptoms in hemiparkisonian rodent model (Brugnoli et al., 2016; Calabrese et al., 2020). Phosphorylated S6K, the mTOR downstream cascade, also robustly increased in the dyskinetic mice, which has a strong positive correlation with the relevance of the total AIMs score (Eshraghi et al., 2020). Furthermore, the regulation of mTOR signaling is closely involved in synaptic plasticity, which impacts dyskinesia. In the LID condition, loss of striatal bidirectional synaptic plasticity occurred (Picconi et al., 2018); however, pre-administration of rapamycin restored the loss of synaptic plasticity in LID-induced rats (Calabrese et al., 2020). Besides, S6K1 knockout mice showed deficits in the early phase of LTP related to memory acquisition in the hippocampus (Antion et al., 2008). Also, striatal mTOR/S6K signaling altered by 5-HTP showed strongly high correlation with dyskinesia (Fig. 6). Based on these above mentions, 5-HTP actions by the regulation of mTOR signaling could play a significant role in the treatment of LID.
In addition, the AKT/mTOR pathway is also associated with 5-HT receptors, especially, 5-HT1, 2 and 6 receptors in several disease models. For example, the activation of 5-HT1A receptor stimulated AKT/mTOR signaling and showed an antidepressant effect by ketamine in the rodent medial prefrontal cortex (Chaki and Fukumoto, 2019). In addition, cannabis promoted the AKT/mTOR pathway through the 5-HT2 receptor in the mouse cortex (Ibarra-Lecue et al., 2018). Also, Meffre et al. (2012) discovered that the 5-HT6 receptor induced AKT/mTOR activation in the prefrontal cortex of schizophrenia rodent models. Thus, considering several previous studies comprehensively, 5-HTP administration would specifically affect the AKT/mTOR pathway by activating 5-HT receptors.
By taking L-DOPA gradually, increased phosphorylation of CREB has been also found and then produces ΔFosB, which is also associated with LID development and synaptic plasticity (Nestler et al., 2001; Alberini, 2009; Sharma et al., 2015; Beck et al., 2019). Hence, normalization of increased CREB phosphorylation and ΔFosB levels is important in the LID system. AKT has been reported to mediate CREB and mTOR through 5-HT receptors (Chilmonczyk et al., 2017). In previous studies, 5-HT stimulated AKT, CREB and 5-HT7 receptor- mediated AKT/CREB signaling is involved in liver regeneration in rat hepatocytes (Svejda et al., 2013; Ballou et al., 2018). In our findings, 5-HTP modulated not only the AKT/mTOR pathway but also CREB phosphorylation and ΔFosB (Fig. 4, 5). Therefore, normalization of abnormal molecular ratio might be beneficial for the regulation of impaired synaptic plasticity. However, further studies are needed to assess the potential contribution to the loss of depotentiation in SPNs.
Considering that 5-HTP acts as a 5-HT1A/B receptor agonist and its regulation system of mTOR, it might regulate glutamate levels. In fact, the 5-HT1 receptor agonist, eltoprazine, attenuated LID by decreasing the elevated glutamate level (Paolone et al., 2015). Moreover, Brugnoli et al. (2016) discovered that elevated striatal glutamate levels were diminished by treatment with rapamycin in 6-OHDA-lesioned L-DOPA-treated mice. It is well established that glutamate levels in the ST increase under LID conditions. Thus, further studies on the regulation of glutamate levels and related pathways by 5-HTP need to be conducted.
Taken together, our present study demonstrated that the administration of 5-HTP specifically modulated AKT/mTOR/S6K and CREB/ΔFosB pathway-related synaptic plasticity in LID-induced mouse ST. Moreover, these effects evoked similar anti-dyskinetic effects compared to amantadine without worsening L-DOPA efficacy. In view of the above-mentioned results, 5-HTP might prevent L-DOPA-induced dyskinetic movements by specifically regulating phosphorylation of AKT and simultaneously normalizing mTOR/S6K and CREB/ΔFosB signaling in PD.
Footnotes
CONFLICT OF INTEREST
Author M.G.P is employed by MetaCen therapeutics Incorporation of Korea. All authors declare no other competing interests.
REFERENCES
- Alberini C. M. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert P. R., Lembo P., Storring J. M., Charest A., Saucier C. The 5-HT1A receptor: signaling, desensitization, and gene transcription. Neuropsychopharmacology. 1996;14:19–25. doi: 10.1016/S0893-133X(96)80055-8. [DOI] [PubMed] [Google Scholar]
- Antion M. D., Merhav M., Hoeffer C. A., Reis G., Kozma S. C., Thomas G., Schuman E. M., Rosenblum K., Klann E. Removal of S6K1 and S6K2 leads to divergent alterations in learning, memory, and synaptic plasticity. Learn. Mem. 2008;15:29–38. doi: 10.1101/lm.661908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert I., Guigoni C., Hakansson K., Li Q., Dovero S., Barthe N., Bioulac B. H., Gross C. E., Fisone G., Bloch B., Bezard E. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann. Neurol. 2005;57:17–26. doi: 10.1002/ana.20296. [DOI] [PubMed] [Google Scholar]
- Azkona G., Sagarduy A., Aristieta A., Vazquez N., Zubillaga V., Ruiz-Ortega J. A., Perez-Navarro E., Ugedo L., Sanchez-Pernaute R. Buspirone anti-dyskinetic effect is correlated with temporal normalization of dysregulated striatal DRD1 signalling in L-DOPA-treated rats. Neuropharmacology. 2014;79:726–737. doi: 10.1016/j.neuropharm.2013.11.024. [DOI] [PubMed] [Google Scholar]
- Ballou Y., Rivas A., Belmont A., Patel L., Amaya C. N., Lipson S., Khayou T., Dickerson E. B., Nahleh Z., Bryan B. A. 5-HT serotonin receptors modulate mitogenic signaling and impact tumor cell viability. Mol. Clin. Oncol. 2018;9:243–254. doi: 10.3892/mco.2018.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck G., Singh A., Zhang J., Potts L. F., Woo J. M., Park E. S., Mochizuki H., Mouradian M. M., Papa S. M. Role of striatal deltaFosB in l-Dopa-induced dyskinesias of parkinsonian nonhuman primates. Proc. Natl. Acad. Sci. U. S. A. 2019;116:18664–18672. doi: 10.1073/pnas.1907810116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpied T. A., Clarke R. J., Johnson J. W. Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J. Neurosci. 2005;25:3312–3322. doi: 10.1523/JNEUROSCI.4262-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnoli A., Napolitano F., Usiello A., Morari M. Genetic deletion of Rhes or pharmacological blockade of mTORC1 prevent striato-nigral neurons activation in levodopa-induced dyskinesia. Neurobiol. Dis. 2016;85:155–163. doi: 10.1016/j.nbd.2015.10.020. [DOI] [PubMed] [Google Scholar]
- Calabrese V., Di Maio A., Marino G., Cardinale A., Natale G., De Rosa A., Campanelli F., Mancini M., Napolitano F., Avallone L., Calabresi P., Usiello A., Ghiglieri V., Picconi B. Rapamycin, by inhibiting mTORC1 signaling, prevents the loss of striatal bidirectional synaptic plasticity in a rat model of L-DOPA-induced dyskinesia. Front. Aging Neurosci. 2020;12:230. doi: 10.3389/fnagi.2020.00230.a7602896de9f424fb37861e0572fc994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P., Di Filippo M., Ghiglieri V., Picconi B. Molecular mechanisms underlying levodopa-induced dyskinesia. Mov. Disord. 2008;23 Suppl 3:S570–S579. doi: 10.1002/mds.22019. [DOI] [PubMed] [Google Scholar]
- Carta M., Carlsson T., Kirik D., Bjorklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- Carta M., Carlsson T., Munoz A., Kirik D., Bjorklund A. Serotonin-dopamine interaction in the induction and maintenance of L-DOPA-induced dyskinesias. Prog. Brain Res. 2008;172:465–478. doi: 10.1016/S0079-6123(08)00922-9. [DOI] [PubMed] [Google Scholar]
- Cenci M. A. Presynaptic mechanisms of l-DOPA-induced dyskinesia: the findings, the debate, and the therapeutic implications. Front. Neurol. 2014;5:242. doi: 10.3389/fneur.2014.00242.7d0bef78780a498b8e0e87f475c30bdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S., Fukumoto K. Role of serotonergic system in the antidepressant actions of mGlu2/3 receptor antagonists: similarity to ketamine. Int. J. Mol. Sci. 2019;20:1270. doi: 10.3390/ijms20061270.df17ea8ba81849bbbc278fed7299c0e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilmonczyk Z., Bojarski A. J., Pilc A., Sylte I. Serotonin transporter and receptor ligands with antidepressant activity as neuroprotective and proapoptotic agents. Pharmacol. Rep. 2017;69:469–478. doi: 10.1016/j.pharep.2017.01.011. [DOI] [PubMed] [Google Scholar]
- Eo H., Kwon Y., Huh E., Sim Y., Choi J. G., Jeong J. S., Du X. F., Soh H. Y., Hong S. P., Kim Pak Y., Oh M. S. Protective effects of DA-9805 on dopaminergic neurons against 6-hydroxydopamine-induced neurotoxicity in the models of Parkinson's disease. Biomed. Pharmacother. 2019;117:109184. doi: 10.1016/j.biopha.2019.109184. [DOI] [PubMed] [Google Scholar]
- Eshraghi M., Ramirez-Jarquin U. N., Shahani N., Nuzzo T., De Rosa A., Swarnkar S., Galli N., Rivera O., Tsaprailis G., Scharager-Tapia C., Crynen G., Li Q., Thiolat M. L., Bezard E., Usiello A., Subramaniam S. RasGRP1 is a causal factor in the development of l-DOPA-induced dyskinesia in Parkinson's disease. Sci. Adv. 2020;6:eaaz7001. doi: 10.1126/sciadv.aaz7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyder M., Bonito-Oliva A., Fisone G. L-DOPA-induced dyskinesia and abnormal signaling in striatal medium spiny neurons: focus on dopamine D1 receptor-mediated transmission. Front. Behav. Neurosci. 2011;5:71. doi: 10.3389/fnbeh.2011.00071.8a77899003f640cd923c5ea34b135bea [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiglieri V., Mineo D., Vannelli A., Cacace F., Mancini M., Pendolino V., Napolitano F., di Maio A., Mellone M., Stanic J., Tronci E., Fidalgo C., Stancampiano R., Carta M., Calabresi P., Gardoni F., Usiello A., Picconi B. Modulation of serotonergic transmission by eltoprazine in L-DOPA-induced dyskinesia: Behavioral, molecular, and synaptic mechanisms. Neurobiol. Dis. 2016;86:140–153. doi: 10.1016/j.nbd.2015.11.022. [DOI] [PubMed] [Google Scholar]
- Hamadjida A., Nuara S. G., Bedard D., Gaudette F., Beaudry F., Gourdon J. C., Huot P. The highly selective 5-HT2A antagonist EMD-281,014 reduces dyskinesia and psychosis in the l-DOPA-treated parkinsonian marmoset. Neuropharmacology. 2018;139:61–67. doi: 10.1016/j.neuropharm.2018.06.038. [DOI] [PubMed] [Google Scholar]
- Huh E., Choi J. G., Sim Y., Oh M. S. An integrative approach to treat Parkinson's disease: ukgansan complements L-dopa by ameliorating dopaminergic neuronal damage and L-dopa-induced dyskinesia in mice. Front. Aging Neurosci. 2018;10:431. doi: 10.3389/fnagi.2018.00431.969871ec87be4014a2b58786bf3327fc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra-Lecue I., Mollinedo-Gajate I., Meana J. J., Callado L. F., Diez-Alarcia R., Uriguen L. Chronic cannabis promotes pro-hallucinogenic signaling of 5-HT2A receptors through Akt/mTOR pathway. Neuropsychopharmacology. 2018;43:2028–2035. doi: 10.1038/s41386-018-0076-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzelberg R., Bonuccelli U., Schechtman E., Miniowich A., Strugatsky R., Ceravolo R., Logi C., Rossi C., Klein C., Rabey J. M. Association between amantadine and the onset of dementia in Parkinson's disease. Mov. Disord. 2006;21:1375–1379. doi: 10.1002/mds.20968. [DOI] [PubMed] [Google Scholar]
- Junho B. T., de Oliveira V. F. The role of NMDA receptor antagonists, amantadine and memantine, in schizophrenia treatment: a systematic review. Arch. Psychiatry Psychother. 2019;46:165–168. doi: 10.1590/0101-60830000000218. [DOI] [Google Scholar]
- Kannari K., Yamato H., Shen H., Tomiyama M., Suda T., Matsunaga M. Activation of 5-HT(1A) but not 5-HT(1B) receptors attenuates an increase in extracellular dopamine derived from exogenously administered L-DOPA in the striatum with nigrostriatal denervation. J. Neurochem. 2001;76:1346–1353. doi: 10.1046/j.1471-4159.2001.00184.x. [DOI] [PubMed] [Google Scholar]
- Magnuson B., Ekim B., Fingar D. C. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- Marxreiter F., Winkler J., Uhl M., Madzar D. A case report of severe delirium after amantadine withdrawal. Case Rep. Neurol. 2017;9:44–48. doi: 10.1159/000460814.395eafe4422049a09e83b2a904a29b0f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffre J., Chaumont-Dubel S., Mannoury, la Cour C., Loiseau F., Watson D. J., Dekeyne A., Seveno M., Rivet J. M., Gaven F., Deleris P., Herve D., Fone K. C., Bockaert J., Millan M. J., Marin P. 5-HT(6) receptor recruitment of mTOR as a mechanism for perturbed cognition in schizophrenia. EMBO Mol. Med. 2012;4:1043–1056. doi: 10.1002/emmm.201201410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni M., Puligheddu M., Sanna F., Cannas A., Farris R., Tronci E., Figorilli M., Defazio G., Carta M. Efficacy and safety of 5-Hydroxytryptophan on levodopa-induced motor complications in Parkinson's disease: a preliminary finding. J. Neurol. Sci. 2020;415:116869. doi: 10.1016/j.jns.2020.116869. [DOI] [PubMed] [Google Scholar]
- Murray J. L., McDonald N. J., Sheng J., Shaw M. W., Hodge T. W., Rubin D. H., O'Brien W. A., Smee D. F. Inhibition of influenza A virus replication by antagonism of a PI3K-AKT-mTOR pathway member identified by gene-trap insertional mutagenesis. Antivir. Chem. Chemother. 2012;22:205–215. doi: 10.3851/IMP2080. [DOI] [PubMed] [Google Scholar]
- Nakatani Y., Sato-Suzuki I., Tsujino N., Nakasato A., Seki Y., Fumoto M., Arita H. Augmented brain 5-HT crosses the blood-brain barrier through the 5-HT transporter in rat. Eur. J. Neurosci. 2008;27:2466–2472. doi: 10.1111/j.1460-9568.2008.06201.x. [DOI] [PubMed] [Google Scholar]
- Nautiyal K. M., Hen R. Serotonin receptors in depression: from A to B. F1000Res. 2017;6:123. doi: 10.12688/f1000research.9736.1.afe733ffdbb74be7b673966ea76c638f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E. J., Barrot M., Self D. W. DeltaFosB: a sustained molecular switch for addiction. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A., Kuroiwa M., Shuto T. Mechanisms for the modulation of dopamine d(1) receptor signaling in striatal neurons. Front. Neuroanat. 2011;5:43. doi: 10.3389/fnana.2011.00043.8adffa607f484fabb167d57c0a598d0f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S., Srivanitchapoom P. Levodopa-induced dyskinesia: clinical features, pathophysiology, and medical management. Ann. Indian Acad. Neurol. 2017;20:190–198. doi: 10.4103/aian.AIAN_239_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G., Brugnoli A., Arcuri L., Mercatelli D., Morari M. Eltoprazine prevents levodopa-induced dyskinesias by reducing striatal glutamate and direct pathway activity. Mov. Disord. 2015;30:1728–1738. doi: 10.1002/mds.26326. [DOI] [PubMed] [Google Scholar]
- Pettorruso M., Martinotti G., Di Nicola M., Onofrj M., Di Giannantonio M., Conte G., Janiri L. Amantadine in the treatment of pathological gambling: a case report. Front. Psychiatry. 2012;3:102. doi: 10.3389/fpsyt.2012.00102.28b3f46f376e476084e0b4e5d78c258c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picconi B., De Leonibus E., Calabresi P. Synaptic plasticity and levodopa-induced dyskinesia: electrophysiological and structural abnormalities. J. Neural. Transm. (Vienna) 2018;125:1263–1271. doi: 10.1007/s00702-018-1864-6. [DOI] [PubMed] [Google Scholar]
- Reeve A., Simcox E., Turnbull D. Ageing and Parkinson's disease: why is advancing age the biggest risk factor? Ageing Res. Rev. 2014;14:19–30. doi: 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Y. K., Park H. Y., Go J., Lee I. B., Choi Y. K., Lee C. H., Kim K. S. beta-Lapachone ameliorates L-DOPA-induced dyskinesia in a 6-OHDA-induced mouse model of Parkinson's disease. Mol. Med. Rep. 2021;23:217. doi: 10.3892/mmr.2021.11856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki-Tanaka R., Shibata T., Moriyama M., Okamoto H., Kogure H., Kanda T. Amantadine and rimantadine inhibit hepatitis A virus replication through the induction of autophagy. J. Virol. 2022;96:e0064622. doi: 10.1128/jvi.00646-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Singh S., Sharma V., Singh V. P., Deshmukh R. Neurobiology of l-DOPA induced dyskinesia and the novel therapeutic strategies. Biomed. Pharmacother. 2015;70:283–293. doi: 10.1016/j.biopha.2015.01.029. [DOI] [PubMed] [Google Scholar]
- Sharma V. D., Lyons K. E., Pahwa R. Amantadine extended-release capsules for levodopa-induced dyskinesia in patients with Parkinson's disease. Ther. Clin. Risk Manag. 2018;14:665–673. doi: 10.2147/TCRM.S144481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Ohno Y. Improving the treatment of Parkinson's disease: a novel approach by modulating 5-HT(1A) receptors. Aging Dis. 2013;4:1–13. [PMC free article] [PubMed] [Google Scholar]
- Svejda B., Kidd M., Timberlake A., Harry K., Kazberouk A., Schimmack S., Lawrence B., Pfragner R., Modlin I. M. Serotonin and the 5-HT7 receptor: the link between hepatocytes, IGF-1 and small intestinal neuroendocrine tumors. Cancer Sci. 2013;104:844–855. doi: 10.1111/cas.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambasco N., Romoli M., Calabresi P. Levodopa in Parkinson's disease: current status and future developments. Curr. Neuropharmacol. 2018;16:1239–1252. doi: 10.2174/1570159X15666170510143821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanvi B., Lo N., Robinson T. Levodopa-induced dyskinesia in Parkinson's disease: clinical features, pathogenesis, prevention and treatment. Postgrad. Med. J. 2007;83:384–388. doi: 10.1136/pgmj.2006.054759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronci E., Lisci C., Stancampiano R., Fidalgo C., Collu M., Devoto P., Carta M. 5-Hydroxy-tryptophan for the treatment of L-DOPA-induced dyskinesia in the rat Parkinson's disease model. Neurobiol. Dis. 2013;60:108–114. doi: 10.1016/j.nbd.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Urs N. M., Bido S., Peterson S. M., Daigle T. L., Bass C. E., Gainetdinov R. R., Bezard E., Caron M. G. Targeting beta-arrestin2 in the treatment of L-DOPA-induced dyskinesia in Parkinson's disease. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E2517–E2526. doi: 10.1073/pnas.1502740112. [DOI] [PMC free article] [PubMed] [Google Scholar]