Abstract
Innate immunity is a first line defence system in the body which is for sensing signals of danger such as pathogenic microbes or host-derived signals of cellular stress. Pattern recognition receptors (PRR’s), which present in the cell memebrane, are suspect the infection through pathogen-associated molecular patterns (PAMP), and activate innate immunity with response to promote inflammation via inflammatory cells such as macrophages and neutrophils, and cytokines. Inflammasome are protein complexes which are part of innate immunity in inflammation to remove pathogens and repair damaged tissues. What is the important role of inflammation in disease? In this review, we are focused on the action mechanism of NLRP3 inflammasome in inflammatory diseases such as asthma, atopic dermatitis, and sepsis.
Keywords: Asthma, Atopic dermatitis, Inflammasome, Sepsis
INTRODUCTION
Inflammation is a complex and a protective response by the immune system against physical, chemical and infective agents. However, it is frequent that inflammatory response to several stimuli leads to the damaging of normal tissues (Nathan, 2002; Rankin, 2004). Every year millions of the people are affected by chronic and acute inflammatory diseases such as asthma, atopic dermatitis and sepsis (Lambrecht and Hammad, 2003; Bel, 2013).
Atopic dermatitis (AD) is a common skin disease in children and it is multifactorial inflammatory disease (Liang et al., 2016). AD is an allergic disease which is mediated by T-helper 2. Th-2 initiates interleukin (IL)-4, IL-5, and IL-13 expression in AD, which stimulates B cells, mast cells, and epidermal cells to produce IgE and various cytokines and induce degranulation cytokines (Brandt and Sivaprasad, 2011; Kabashima, 2013; Weidinger and Novak, 2016). Innate immune functions and the regulations of adaptive immune responses there is Interleukins have important role. SNPs in the Nlrp3 gene are associated with atopic dermatitis (Macaluso et al., 2007; Bivik et al., 2013; Zhang et al., 2015). Sepsis is a harmful systemic inflammatory response to infection. IL-1family are inflammasome associated cytokines and propagates the acute inflammatory response (Luo et al., 2014).
The inflammasome is discovered by Martinon and colleagues in 2002, which is a protein complex known to promote inflammation (Martinon et al., 2002).
We are herein focused on the action mechanism of NLRP3 inflammasome in inflammatory diseases. In particular, the relationship between atopic dermatitis, asthma and sepsis and inflammasome was studied (Table 1).
Table 1.
Inflammatory diseases and their NLRP3 activators and cytokines which is released by NLRP3 activation to specific diseases respectively
| Disease | NLRP3 Activators | Cytokines | References |
|---|---|---|---|
| Asthma | Various pathogens, toxins, bacteria, RNA and Der f1 | IL-1β, IL-18 | Whelan et al., 2004; Mariathasan and Monack, 2007; Besnard et al., 2011; Tsai et al., 2018 |
| Atopic Dermatitis | Staphylococcus aureus, S. aureus linked α-toxins | IL-1β, IL-18, IL-5, IL-31 | Marples et al., 1973; Tomi et al., 2005; Arend et al., 2008; Munoz-Planillo et al., 2009; Boguniewicz and Leung, 2010; Niebuhr et al., 2014 |
| Sepsis | ROS, mtDNA,TLR4 agonist and LPS | IL-1β, IL-18, IL-33 | Martin et al., 2003; Bauernfeind et al., 2009; Willingham et al., 2009; Yin et al., 2011; Kamo et al., 2013; Luo et al., 2014 |
INFLAMMATION AND INNATE IMMUNITY
When the body effected by some infection inflammation is an immediate response from the innate immunity and it is like a barrier and prevent the infection, allows repair of damaged tissue after the elimination of the pathogens. Introduction of pathogens or mechanical injury to cells or tissue initiates the inflammation. Pattern recognition receptors (PRR’s) which are attached to the membrane of cell are toll-like receptor (TLRs) or Nod-like receptors (NLRs) for recognition of inflammatory events (dos Santos, 2012). PRRs recognize the pathogen-associated molecular patterns in an infection and when any mechanical damage is present. Damage associated molecular patterns (DAMPs) are also recognized by PRRs. Innate immunity responds with a sterile inflammatory response after the recognition of a PAMPs or DAMPs and those responses uses the innate immune cells which are macrophages, neutrophils cytokines to promote inflammation (Land, 2013).
INFLAMMASOME
Inflammasome are major complexes which are involved in innate immunity activities such as infection and changes in cellular homeostasis to initiate response to remove pathogens and repair tissue damage by activates pro-caspase-1, which then proceeds to cleave the pro-inflammatory cytokines IL-1β and IL-18 (Wilson et al., 1994; Man and Kanneganti, 2016). There are four structural subsets in inflammasome which are include nucleotide-binding oligomerization domain receptors (NLR) family, pyrin domain containing 1 (NLRP1), NLRP3, NLR family CARD domain-containing protein 4 (NLRC4) and absent in melanoma (AIM2) (van de Veerdonk et al., 2011). Pyroptosis, which is inflammatory form of cell death initiated by inflammasome, is due to an activating cleavage of gasdermin D, which forms pores in the plasma membrane (Kayagaki et al., 2015; Shi et al., 2015; Kesavardhana and Kanneganti, 2017).
NARP3 INFLAMMASOME AND INFLAMMATORY DISEASES
NLRP3 is most studied and focused inflammasome by many researchers, which is present with over 90 disease associated mutations. Leucine-rich repeats, nucleotide - binding domains (NBD), and an N-terminal pyrin domain in NLRP3 allowing recruitment of ASC activate pro caspase-1 (Hoffman et al., 2001; Kanneganti et al., 2006; Masters et al., 2009). An inflammasome complex containing ASC and caspase-1 which is formed by NLRP3 inflammasome and which complex respond to a wide range of infections and stress stimuli. There is a two-step process is required for NLRP3 activation. DAMPs and PAMPs are initiated the first step which are upregulate the pro-IL-1β, pro-IL-18 and components of inflammasome and assembly of the components into the inflammasome structure. The production of IL-1β, a pro-inflammatory cytokine, is the second step of NLRP3 activation (Bauernfeind et al., 2011a; Gross et al., 2011). Low K+ concentrations in the environment, a wide range of bacteria and viruses are triggered NLRP3 activation (Petrilli et al., 2007). In the presence of ATP microbial substances such as muramy l peptide, lipopolysaccharide and bacterial RNA are activating NLRP3 (Kanneganti et al., 2006), also bacterial toxin like nigericin and maitotoxin can activate NLRP3 (Mariathasan et al., 2004).
The activation of NLRP3 mechanism explained by several hypotheses. One of the hypotheses proposes NLRP3 activation requires low K+ concentration. Some toxins can form pores in the membrane of cells allowing for K+ efflux (Perregaux and Gabel, 1994). The extracellular ATP causes K+ efflux which induce the activation of P2X7 receptors on pannexin 1 (Colomar et al., 2003). Another model proposes DAMPs leads NLRP3 activation (Hornung et al., 2008). These also has been suggested that reactive oxygen species (ROS) cause the breakdown of thioredoxin and its interacting protein (dos Santos, 2012). The recruitment of ASC and pro-caspase 1 into the inflammasome structure is induced by NLRP3 receptor when TXNIP therein binds on it.
Asthma and NLRP3
More than 300 million people affected by asthma in worldwide, which is chronic inflammatory disease that is defined as reversible airway narrowing and is characterized by episodic symptoms of dyspnea, wheezing and coughing. Bronchial epithelial cells (BEC) are physical barrier and first line of defence against inhalants such as microorganisms, gases and allergens (Lambrecht and Hammad, 2012; Bel, 2013). In the asthma pathogenesis loss of BEC integrity is a hallmark and there is strong evidence of sloughing of found in bronchial biopsy samples from patients with mild to severe asthma (Chanez, 2005; Martinez-Giron and van Woerden, 2010). Airway structural changes is leading to remodelling of the airways because of the repetitive cycles injury and repair of BECs (Holgate, 2000; Davies, 2009; Holgate et al., 2009).
The sensitization with OVA and alum activates the NLRP3 inflammasome which leads to IL-1β maturation and humoral adaptive immune response. Allergic lung inflammation with lower eosinophil influx and impaired Th2 response causes by NLRP3 deficiency (Besnard et al., 2011).
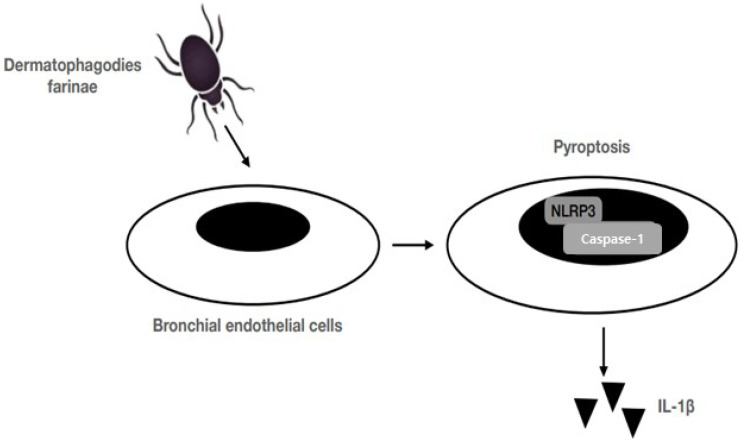
Microbial infections and non-infectious stimuli induces the host cell death which process is called pyroptosis and it is depending upon caspase-1 activation which mediates cell death and cleaves and secretes proinflammatory cytokines, such as interleukin (IL)-1β and IL-18 (Yeretssian et al., 2008; Bergsbaken et al., 2009). Cell death and pro-inflammatory signals cause tissue damage and which lead to permanent structural changes (Tanaka et al., 2001). In airway epithelia inflammatory injuries contributed by pyroptosis which act as pathogenic mechanism. Conversion of pro-IL-1β to mature IL-1β catalysed by Caspase-1 which is a key inflammatory mediator controls both local and systemic immune response (Denes et al., 2012). IL-1β plays an important role in the early phase of asthma pathogenesis and to modulate airway construction and relaxation responses directly on the airway smooth muscle (Whelan et al., 2004). Recent research demonstrated Der f 1 increased the proteolytic activation and activity caspase 1 which in turn induced the secretion of IL-1β from BECs (Tsai et al., 2018). For the recognition of exogenous microbial components or endogenous destructive cellular factors NLRs plays an important role in innate immunity. Various pathogens, toxins, bacterial RNA and uric acid triggered the activation of NLRP3 inflammasome via toll-like receptors and P2X7 receptors (Mariathasan and Monack, 2007). Here one evidence to the Der f1 increased the association of NLRP3 and caspase-1. The Der f1 induced activity of caspase-1 was shown to decrease in pHBECs and HBE-135 cells when NLRP3 was knockdown and IL-1β, pyroptosis also reduced (Tsai et al., 2018) (Fig. 1).
Fig. 1.
Proposed scheme of Der f1-induced pyroptosis in bronchial epithelial cells (Figure modified from Tsai et al., 2018). The Der f1 allergen induces BEC death through the caspase-1 pathway, referred to as pyroptosis. Following pyroptosis, BECs secrete interleukin (IL)-1β, which may perpetuate asthma pathogenesis. Der f1, Dermatophagodies farina allergen 1; NLRP3, NOD-like receptor family pyrin domain-containing protein 3.
Atopic dermatitis and NLRP3
Atopic dermatitis is a common skin disease which is mediated by Th2 through the inflammasomes. The host protects itself from pathogens by innate immune system, which initiates the repair process of injury or trauma. Almost 80-100% of AD patients are colonized with Staphylococcus aureus (S. aureus) and strong correlation between disease severity and S. aureus colonization of lesional and nonlesional skin (Marples et al., 1973; Tomi et al., 2005; Boguniewicz and Leung, 2010). Innate immunity is activated by pathogen-recognition receptors such as Toll-like receptors and nucleotide-binding oligomerization domain receptors (NLRs) (Soumelis et al., 2002; Jiao et al., 2016). The most well-known inflammasome NLRP3 links to staphylococcal α-toxin to caspase-1 activation through the formation of a multiprotein platform called the inflammasome and secrets IL-1β, and NLRP3 also recruits apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC). And then pro-caspase-1 is activated to caspase-1 when assembled of inflammasome and leads to release of IL-1β and IL-18 (Arend et al., 2008; Munoz-Planillo et al., 2009). Normal NLRP3 activation contributes host defence but excessive activation leads to inflammatory diseases.
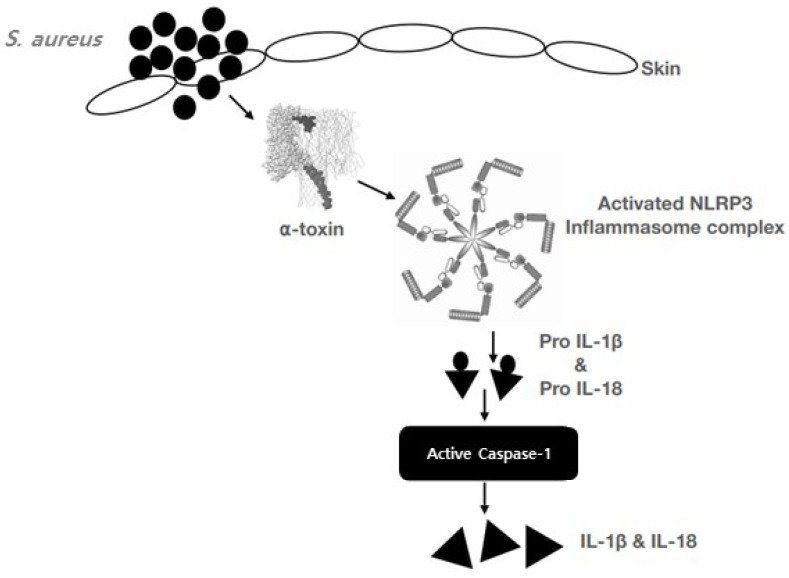
There are several mechanisms initiated by S. aureus and its toxins that result in AD. Super antigens of S. aureus have the ability to induce cutaneous lymphocyte-associated antigen (CLA) expression as a skin-homing receptor on circulating T cells. Keratinocyte-derived chemokines and thymic stromal lymphopoietin (TSLP) induce the recruitment of T cells, Th2 cell differentiation, and the induction of T cells to secrete IL-5 and IL-31. Mast cell degranulation induced by α-toxin and which is activates the nucleotide-binding oligomerization domain receptor protein 3 (NLRP3) inflammasome that eventually (Fig. 2).
Fig. 2.
The mechanism of NLRP3 activation in atopic dermatitis. S. aureus induce the super antigens TSST-1, which release the chemokines, TSLP from keratinocytes and recruit the T cells. α-toxin is link with S. aureus, activates the NLRP3 inflammasome which activates the caspase-1 and it cleavage the pro-IL-1β and pro-IL-18 to IL-1β and IL-18 (Niebuhr et al., 2014).
It is reported that Nlrp3 gene polymorphism activates the inflammasome to induce the allergic inflammatory response and increase the risk of atopic dermatitis (Macaluso et al., 2007; Zhang et al., 2015). 2,4 dinitrochlorobenzene (DNCB) and House dust mite (HDM) initiates the NLRP3 inflammasome signalling pathways and increase of NLRP3 and ASC expression, which enhanced the maturation of caspase-1 and IL-1β and induction of pyroptosis (Bivik et al., 2013). NLRP3 inflammasome activation by house dust mites are present in higher frequencies in the surroundings of patients with AD and induce caspase-1 dependent release of IL-1β and IL-18 from human keratinocytes (Jang et al., 2018).
Sepsis and NLRP3
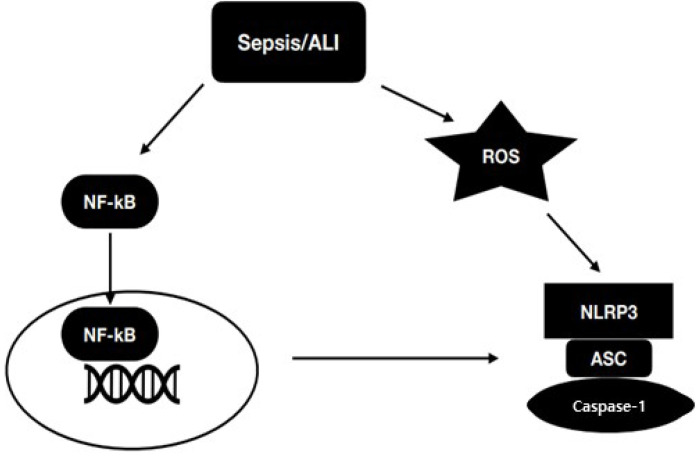
A harmful systematic inflammatory response to an infection is called sepsis (Luo et al., 2014). The patient with end-organ dysfunction lead by sepsis are particularly at risk of developing acute lung injury (ALI). Mostly 10-15% people affected by ALI are hospitalized in ICU. NLRP3 inflammasome dysregulation lead to sepsis and ALI (Martin et al., 2003). NLRP3 inflammasome activation initiate the filtration of inflammatory cells in the lung and lead to lung injury (Luo et al., 2014). IL-33 and HMGB1 which are depend on NLRP3 inflammasome plays an important role in sepsis-induced ALI (Luo et al., 2014). IL-33 belongs to IL-1 cytokine family and it is increases inflammatory response in the lung (Yin et al., 2011). In sepsis high mobility group box 1 (HMGB1) induces a late lethal systemic inflammation and NLRP3 and ASC/caspase-1/IL-1β signalling promotes HMGB1 induction and release (Willingham et al., 2009; Kamo et al., 2013). TLR4 agonist induces NLRP3, pro-caspase 1, pro-IL-1β and pro-IL-18 expressions through NF-kB pathway. Gram-negative bacteria are most common bacteria for sepsis and LPS mediated inflammatory cytokines release, which is most common agonist in polymicrobial sepsis (Bauernfeind et al., 2009). Monocytes and macrophages in the lung are activated and release inflammatory cytokines when binding the LPS with TLR4. MyD88-dependent and independent pathways are activated by LPS through NF-kB resulting in the induction of NLRP3 protein expression. NF-kB plays a vital role in NLRP3 in LPS induced NLRP3 expression and associated pro-mediators (Bauernfeind et al., 2009). Reactive oxygen species (ROS), mitochondrial DNA (mtDNA) also activates the NLRP3 and promote NLRP3 expression at the transcriptional level (Zhou et al., 2010; Bauernfeind et al., 2011b; Shimada et al., 2012). Mitochondrial damage is also involved in Sepsis and overproduction of ROS and mtDNA released from mitochondria participate in the pathogenesis of sepsis (Fig. 3).
Fig. 3.
Mechanism of NLRP3 activation in sepsis and ALI. ROS and NF-kB activates the NLRP3 inflammasome.
Drug Development of inflammatory diseases
Usually drugs are developed for diseases to target the receptors, enzymes, protein and gene etc. The process of drug development for inflammatory diseases mainly targeting on inflammasomes. Cytokine release inhibitory drugs targets the NLRP3 and AIM2 inflammasomes (Coll et al., 2011). The NLRP3 inflammasome complex is a potential target for the development of therapeutics for patients with inflammatory bowel diseases (Bauer et al., 2010). Fc11a-2 has an ability to inhibit the activation of NLRP3 inflammasome (Liu et al., 2013). Fc11a-2 mechanism of action in colitis is inhibition of NLRP3 inflammasome functions as it suppressed the activation of caspase-1 and reduced the production of IL-1β/IL-18 in macrophages. Resveratrol inhibit the secretion of IL-1β by down-regulating the protein and mRNA levels of IL-1β (Liu et al., 2013). Resveratrol mainly elevates the Siert1 and inhibit the NLRP3 inflammasome to prevent the inflammation (Fu et al., 2013). For the attenuation of Sepsis Resveratrol targets the inhibition of the NLRP3 inflammasome which prevents the over-release of pro-inflammatory cytokines and inflammatory cell infiltration and tissue impairment (Fu et al., 2013). Tranilast (TR) directly targets NLRP3 inflammasome to suppress the inflammation. TR blocks the assembly of NLRP3 inflammasome (Huang et al., 2018). TR improve the dysfunction of both glucose and lipid metabolism through inhibiting the NLRP3 inflammasome.
CONCLUSION
The inflammasomes play a prominent role in inflammatory diseases. Endogenous danger signals such as ATP or uric acid crystals released form dying cells are activated the inflammasomes. NLRP3 inflammasome is crucial to inflammatory diseases such as Asthma, Atopic dermatitis and Sepsis. NLRP3 inflammasome activation leads the inflammatory diseases through different pathways, which associate to ASC and caspase-1 and initiates release of the inflammatory cytokines such as IL-1β and IL-18 and also involves in pyroptosis. Actually infection recognized by PAMPs or DAMPs in the host then innate immunity is respond and promote the inflammation through immune cells such as macrophages, neutrophils, and release of proinflammatory cytokines. Usually bacteria, viruses and low K+ concentration environments are can activate the NLRP3. Pyroptosis is depends upon caspase-1 which is activated by NLRP3 and lead to permanent changes in airways and airway epithelial inflammatory injuries in asthma pathogenic mechanism. IL-1β also responsible for early phase of asthma which is initiated by NLRP3 inflammasome through caspase-1. Mostly Atopic dermatitis is caused by Staphylococcus aureus, which α-toxin links to NLRP3 inflammasome to activate caspase-1 and release IL-1β, IL-18. NLRP3 dependent IL-33 and HMB1 release, which plays a crucial role in Sepsis induced by TLR4 agonist and the associate with caspase-1 induces release IL-1β and IL-18 through NF-kB pathway. It is suggested that NLRP3 inflammasome is a target for drugs which will cure the inflammatory diseases such as Asthma, Atopic dermatitis and Sepsis.
Funding Statement
ACKNOWLEDGMENTS This paper was supported by the Academic Research Fund of Dr. Myung Ki (MIKE) Hong in 2021.
Footnotes
CONFLICT OF INTEREST
The authors confirm that they have no conflicts of interest.
REFERENCES
- Arend W. P., Palmer G., Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- Bauer C., Duewell P., Mayer C., Lehr H. A., Fitzgerald K. A., Dauer M., Tschopp J., Endres S., Latz E., Schnurr M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- Bauernfeind F., Ablasser A., Bartok E., Kim S., Schmid-Burgk J., Cavlar T., Hornung V. Inflammasomes: current understanding and open questions. Cell. Mol. Life Sci. 2011a;68:765–783. doi: 10.1007/s00018-010-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind F., Bartok E., Rieger A., Franchi L., Núñez G., Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 2011b;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind F. G., Horvath G., Stutz A., Alnemri E. S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B. G., Fitzgerald K. A., Hornung V., Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bel E. H. Clinical practice. Mild asthma. N. Engl. J. Med. 2013;369:549–557. doi: 10.1056/NEJMcp1214826. [DOI] [PubMed] [Google Scholar]
- Bergsbaken T., Fink S. L., Cookson B. T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard A. G., Guillou N., Tschopp J., Erard F., Couillin I., Iwakura Y., Quesniaux V., Ryffel B., Togbe D. NLRP3 inflammasome is required in murine asthma in the absence of aluminum adjuvant. Allergy. 2011;66:1047–1057. doi: 10.1111/j.1398-9995.2011.02586.x. [DOI] [PubMed] [Google Scholar]
- Bivik C., Verma D., Winge M. C., Lieden A., Bradley M., Rosdahl I., Söderkvist P. Genetic variation in the inflammasome and atopic dermatitis susceptibility. J. Invest. Dermatol. 2013;133:2486–2489. doi: 10.1038/jid.2013.168. [DOI] [PubMed] [Google Scholar]
- Boguniewicz M., Leung D. Y. Recent insights into atopic dermatitis and implications for management of infectious complications. J. Allergy Clin. Immunol. 2010;125:4–13. doi: 10.1016/j.jaci.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt E. B., Sivaprasad U. Th2 cytokines and atopic dermatitis. J. Clin. Cell. Immunol. 2011;2:110. doi: 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanez P. Severe asthma is an epithelial disease. Eur. Respir. J. 2005;25:945–946. doi: 10.1183/09031936.05.00038605. [DOI] [PubMed] [Google Scholar]
- Coll R. C., Robertson A., Butler M., Cooper M., O'Neill L. A. The cytokine release inhibitory drug CRID3 targets ASC oligomerisation in the NLRP3 and AIM2 inflammasomes. PLoS One. 2011;6:e29539. doi: 10.1371/journal.pone.0029539.ed2df02dd6da4d1aa07f6cc150804fbc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomar A., Marty V., Médina C., Combe C., Parnet P., Amédée T. Maturation and release of interleukin-1beta by lipopolysaccharide-primed mouse Schwann cells require the stimulation of P2X7 receptors. J. Biol. Chem. 2003;278:30732–30740. doi: 10.1074/jbc.M304534200. [DOI] [PubMed] [Google Scholar]
- Davies D. E. The role of the epithelium in airway remodeling in asthma. Proc. Am. Thorac. Soc. 2009;6:678–682. doi: 10.1513/pats.200907-067DP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes A., Lopez-Castejon G., Brough D. Caspase-1: is IL-1 just the tip of the ICEberg? Cell Death Dis. 2012;3:e338. doi: 10.1038/cddis.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos C. C. The role of the inflammasome in ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 2012;185:1141–1144. doi: 10.1164/rccm.201204-0649ED. [DOI] [PubMed] [Google Scholar]
- Fu Y., Wang Y., Du L., Xu C., Cao J., Fan T., Liu J., Su X., Fan S., Liu Q., Fan F. Resveratrol inhibits ionising irradiation-induced inflammation in MSCs by activating SIRT1 and limiting NLRP-3 inflammasome activation. Int. J. Mol. Sci. 2013;14:14105–14118. doi: 10.3390/ijms140714105.5211154dca064060a055145404bb90e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross O., Thomas C., Guarda G., Tschopp J. The inflammasome: an integrated view. Immunol. Rev. 2011;243:136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- Hoffman H. M., Mueller J. L., Broide D. H., Wanderer A. A., Kolodner R. D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate S. T., Roberts G., Arshad H. S., Howarth P. H., Davies D. E. The role of the airway epithelium and its interaction with environmental factors in asthma pathogenesis. Proc. Am. Thorac. Soc. 2009;6:655–659. doi: 10.1513/pats.200907-072DP. [DOI] [PubMed] [Google Scholar]
- Holgate S. T. Epithelial damage and response. Clin. Exp. Allergy. 2000;30 Suppl 1:37–41. doi: 10.1046/j.1365-2222.2000.00095.x. [DOI] [PubMed] [Google Scholar]
- Hornung V., Bauernfeind F., Halle A., Samstad E. O., Kono H., Rock K. L., Fitzgerald K. A., Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Jiang H., Chen Y., Wang X., Yang Y., Tao J., Deng X., Liang G., Zhang H., Jiang W., Zhou R. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 2018;10:e8689. doi: 10.15252/emmm.201708689.ec29081353024f058b1f7bb888706528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H. Y., Koo J. H., Lee S. M., Park B. H. Atopic dermatitis-like skin lesions are suppressed in fat-1 transgenic mice through the inhibition of inflammasomes. Exp. Mol. Med. 2018;50:1–9. doi: 10.1038/s12276-018-0104-3.0101c1dc156f473cada9b2d1b02772c4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao D., Wong C. K., Qiu H. N., Dong J., Cai Z., Chu M., Hon K. L., Tsang M. S., Lam C. W. NOD2 and TLR2 ligands trigger the activation of basophils and eosinophils by interacting with dermal fibroblasts in atopic dermatitis-like skin inflammation. Cell. Mol. Immunol. 2016;13:535–550. doi: 10.1038/cmi.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J. Dermatol. Sci. 2013;70:3–11. doi: 10.1016/j.jdermsci.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Kamo N., Ke B., Ghaffari A. A., Shen X. D., Busuttil R. W., Cheng G., Kupiec-Weglinski J. W. ASC/caspase-1/IL-1β signaling triggers inflammatory responses by promoting HMGB1 induction in liver ischemia/reperfusion injury. Hepatology. 2013;58:351–362. doi: 10.1002/hep.26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti T. D., Ozören N., Body-Malapel M., Amer A., Park J. H., Franchi L., Whitfield J., Barchet W., Colonna M., Vandenabeele P., Bertin J., Coyle A., Grant E. P., Akira S., Núñez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- Kayagaki N., Stowe I. B., Lee B. L., O'Rourke K., Anderson K., Warming S., Cuellar T., Haley B., Roose-Girma M., Phung Q. T., Liu P. S., Lill J. R., Li H., Wu J., Kummerfeld S., Zhang J., Lee W. P., Snipas S. J., Salvesen G. S., Morris L. X., Fitzgerald L., Zhang Y., Bertram E. M., Goodnow C. C., Dixit V. M. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- Kesavardhana S., Kanneganti T. D. Mechanisms governing inflammasome activation, assembly and pyroptosis induction. Int. Immunol. 2017;29:201–210. doi: 10.1093/intimm/dxx018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht B. N., Hammad H. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nat. Rev. Immunol. 2003;3:994–1003. doi: 10.1038/nri1249. [DOI] [PubMed] [Google Scholar]
- Lambrecht B. N., Hammad H. The airway epithelium in asthma. Nat. Med. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- Land W. G. Transfusion-related acute lung injury: the work of DAMPs. Transfus. Med. Hemother. 2013;40:3–13. doi: 10.1159/000345688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Chang C., Lu Q. The genetics and epigenetics of atopic dermatitis-filaggrin and other polymorphisms. Clin. Rev. Allergy Immunol. 2016;51:315–328. doi: 10.1007/s12016-015-8508-5. [DOI] [PubMed] [Google Scholar]
- Liu W., Guo W., Wu J., Luo Q., Tao F., Gu Y., Shen Y., Li J., Tan R., Xu Q., Sun Y. A novel benzo[d]imidazole derivate prevents the development of dextran sulfate sodium-induced murine experimental colitis via inhibition of NLRP3 inflammasome. Biochem. Pharmacol. 2013;85:1504–1512. doi: 10.1016/j.bcp.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Luo Y. P., Jiang L., Kang K., Fei D. S., Meng X. L., Nan C. C., Pan S. H., Zhao M. R., Zhao M. Y. Hemin inhibits NLRP3 inflammasome activation in sepsis-induced acute lung injury, involving heme oxygenase-1. Int. Immunopharmacol. 2014;20:24–32. doi: 10.1016/j.intimp.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Macaluso F., Nothnagel M., Parwez Q., Petrasch-Parwez E., Bechara F. G., Epplen J. T., Hoffjan S. Polymorphisms in NACHT-LRR (NLR) genes in atopic dermatitis. Exp. Dermatol. 2007;16:692–698. doi: 10.1111/j.1600-0625.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- Man S. M., Kanneganti T. D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat. Rev. Immunol. 2016;16:7–21. doi: 10.1038/nri.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S., Monack D. M. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- Mariathasan S., Newton K., Monack D. M., Vucic D., French, Lee W. P., Roose-Girma M., Erickson S., Dixit V. M. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- Marples R. R., Heaton C. L., Kligman A. M. Staphylococcus aureus in psoriasis. Arch. Dermatol. 1973;107:568–570. doi: 10.1001/archderm.1973.01620190044010. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Mannino D. M., Eaton S., Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Martínez-Girón R., van Woerden H. C. Disruption of airway epithelium in asthma pathogenesis: are protozoa responsible? Proc. Am. Thorac. Soc. 2010;7:161. doi: 10.1513/pats.7.2.161a. [DOI] [PubMed] [Google Scholar]
- Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Masters S. L., Simon A., Aksentijevich I., Kastner D. L. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*) Annu. Rev. Immunol. 2009;27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Planillo R., Franchi L., Miller L. S., Núñez G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J. Immunol. 2009;183:3942–3948. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Points of control in infl ammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Niebuhr M., Baumert K., Heratizadeh A., Satzer I., Werfel T. Imparied NLRP3 inflammasome expression and function in atopic dermatitis due to Th2 milieu. Allergy. 2014;69:1058–1067. doi: 10.1111/all.12428. [DOI] [PubMed] [Google Scholar]
- Perregaux D., Gabel C. A. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 1994;269:15195–15203. doi: 10.1016/S0021-9258(17)36591-2. [DOI] [PubMed] [Google Scholar]
- Pétrilli V., Papin S., Dostert C., Mayor A., Martinon F., Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- Rankin J. A. Biological mediators of acute inflammation. AACN Clin. Issues. 2004;15:3–17. doi: 10.1097/00044067-200401000-00002. [DOI] [PubMed] [Google Scholar]
- Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- Shimada K., Crother T. R., Karlin J., Dagvadorj J., Chiba N., Chen S., Ramanujan V. K., Wolf A. J., Vergnes L., Ojcius D. M., Rentsendorj A., Vargas M., Guerrero C., Wang Y., Fitzgerald K. A., Underhill D. M., Town T., Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumelis V., Reche P. A., Kanzler H., Yuan W., Edward G., Homey B., Gilliet M., Ho S., Antonenko S., Lauerma A., Smith K., Gorman D., Zurawski S., Abrams J., Menon S., McClanahan T., de Waal-Malefyt Rd R., Bazan F., Kastelein R. A., Liu Y. J. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Miyazaki N., Oashi K., Teramoto S., Shiratori M., Hashimoto M., Ohmichi M., Abe S. IL-18 might reflect disease activity in mild and moderate asthma exacerbation. J. Allergy Clin. Immunol. 2001;107:331–336. doi: 10.1067/mai.2001.112275. [DOI] [PubMed] [Google Scholar]
- Tsai T., Chiang K., Hung J., Chang W., Lin H., Shien J., Chong I., Hsu Y. Der f1 induces pyroptosis in human bronchial epithelia via the NLRP3 inflammasome. Int. J. Mol. Med. 2018;41:757–764. doi: 10.3892/ijmm.2017.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomi N. S., Kränke B., Aberer E. Staphylococcal toxins in patients with psoriasis, atopic dermatitis, and erythroderma, and in healthy control subjects. J. Am. Acad. Dermatol. 2005;53:67–72. doi: 10.1016/j.jaad.2005.02.034. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk F. L., Netea M. G., Dinarello C. A., Joosten L. A. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Weidinger S., Novak N. Atopic dermatitis. Lancet. 2016;387:1109–1122. doi: 10.1016/S0140-6736(15)00149-X. [DOI] [PubMed] [Google Scholar]
- Whelan R., Kim C., Chen M., Leiter J., Grunstein M. M., Hakonarson H. Role and regulation of interleukin-1 molecules in pro-asthmatic sensitised airway smooth muscle. Eur. Respir. J. 2004;24:559–567. doi: 10.1183/09031936.04.00133803. [DOI] [PubMed] [Google Scholar]
- Willingham S. B., Allen I. C., Bergstralh D. T., Brickey W. J., Huang M. T., Taxman D. J., Duncan J. A., Ting J. P. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J. Immunol. 2009;183:2008–2015. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. P., Black J. A., Thomson J. A., Kim E. E., Griffith J. P., Navia M. A., Murcko M. A., Chambers S. P., Aldape R. A., Raybuck S. A., Livingston D. J. Structure and mechanism of interleukin-1 beta converting enzyme. Nature. 1994;370:270–275. doi: 10.1038/370270a0. [DOI] [PubMed] [Google Scholar]
- Yeretssian G., Labbé K., Saleh M. Molecular regulation of inflammation and cell death. Cytokine. 2008;43:380–390. doi: 10.1016/j.cyto.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Yin H., Li X., Yuan B., Zhang B., Hu S., Gu H., Jin X., Zhu J. Heme oxygenase-1 ameliorates LPS-induced acute lung injury correlated with downregulation of interleukin-33. Int. Immunopharmacol. 2011;11:2112–2117. doi: 10.1016/j.intimp.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Fan H. W., Zhang J. Z., Wang Y. M., Xing H. J. NLRP3 rs35829419 polymorphism is associated with increased susceptibility to multiple diseases in humans. Genet. Mol. Res. 2015;14:13968–13980. doi: 10.4238/2015.October.29.17. [DOI] [PubMed] [Google Scholar]
- Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]