Key Points
The combinatorial dual-epitope DC vaccine is safe.
This vaccine regimen induced an immune response in a minority of patients.
One patient had an exceptional response and long-term survival.
Abstract
Previous work from our group and others has shown that patients with breast cancer can generate a T cell response against specific human epidermal growth factor 2 (HER2) epitopes. In addition, preclinical work has shown that this T cell response can be augmented by Ag-directed mAb therapy. This study evaluated the activity and safety of a combination of dendritic cell (DC) vaccination given with mAb and cytotoxic therapy. We performed a phase I/II study using autologous DCs pulsed with two different HER2 peptides given with trastuzumab and vinorelbine to a study cohort of patients with HER2-overexpressing and a second with HER2 nonoverexpressing metastatic breast cancer. Seventeen patients with HER2-overexpressing and seven with nonoverexpressing disease were treated. Treatment was well tolerated, with one patient removed from therapy because of toxicity and no deaths. Forty-six percent of patients had stable disease after therapy, with 4% achieving a partial response and no complete responses. Immune responses were generated in the majority of patients but did not correlate with clinical response. However, in one patient, who has survived >14 y since treatment in the trial, a robust immune response was demonstrated, with 25% of her T cells specific to one of the peptides in the vaccine at the peak of her response. These data suggest that autologous DC vaccination when given with anti-HER2–directed mAb therapy and vinorelbine is safe and can induce immune responses, including significant T cell clonal expansion, in a subset of patients.
Introduction
Metastatic breast cancer is a leading cause of cancer-related mortality in women. Significant advances have been made in the treatment of human epidermal growth factor 2 (HER2)-overexpressing breast cancer through the development of novel HER2-targeted therapies; however, metastatic disease remains incurable with modern therapeutics. Although the use of trastuzumab, pertuzumab, and trastuzumab emtansine (T-DM1) in localized breast cancer has markedly improved early breast cancer outcomes, continued innovation is essential to improve outcomes for patients who develop metastatic disease despite initial HER2-targeted treatment (1, 2).
Despite modest efficacy of the immune checkpoint inhibitor pembrolizumab in programmed death-ligand 1–positive metastatic hormone receptor–negative, HER2-negative breast cancer, anti–programmed death-ligand 1 and anti–CTLA-4 agents have not been shown to improve outcomes in HER2-positive breast cancer (3). Instead, the current treatment for HER2-positive metastatic breast cancer involves anti-HER2 mAbs such as trastuzumab and pertuzumab given with taxane chemotherapy, Ab–drug conjugates such as ado-T-DM1 and trastuzumab deruxtecan, and small molecule inhibitors of HER2 such as tucatinib and lapatinib given with trastuzumab and capecitabine chemotherapy. More recently, clinical data have suggested that patients with HER2-expressing, but not HER2-overexpressing, tumors, termed HER2-low tumors, are also likely to benefit from HER2-targeted agents (4).
Peptides derived from HER2 are immunogenic in a large proportion of patients, and peptide vaccines have shown immunological activity in the adjuvant setting, although the clinical benefit of this approach is unclear (5–10). DNA vaccination targeting the HER2 intracellular domain has been shown to be safe and to generate Ag-specific T cell responses as well (11). In the advanced or metastatic setting, vaccine-primed T cells have been expanded ex vivo and given in adoptive transfer (12). Dendritic cell (DC) vaccines offer an alternative immunotherapy approach that uses peptides derived from intracellular proteins such as HER2 to generate HER2-specific CD8+ CTLs capable of lysing autologous breast cancer cells (13–20). This provides another strategy to target and destroy HER2-expressing cancer cells that is hypothesized to complement existing therapies. Compared with mRNA and peptide vaccines, DC vaccines are more stable and do not rely on the vaccine being taken up by APCs. Previous studies have shown that DCs can be produced from CD34+ hematopoietic progenitor cells and subsequently act as APCs capable of initiating a T cell response in the absence of in vivo priming (21–31). Studies of peptide-pulsed DCs have been performed in other advanced cancer types with immune responses detected in >60% of treated patients (32). Objective responses and improved median overall survival (OS) rates have been reported in several of these studies, although durable clinical responses have been infrequent with DC-based vaccines to date (33–42). In ductal carcinoma in situ, a breast cancer precursor lesion, the magnitude of anti-HER2 CD4+ T cell responses measured in the sentinel lymph node after neoadjuvant DC vaccination was associated with pathological complete response at the time of surgery (43).
CD34+-derived DCs have been favored over monocyte-derived DCs based on their ability to elicit more potent T cell immune responses against cancer (44–46). To enhance the activity of the DC vaccine generated for this study, we targeted two epitopes in the HER2 protein: E75 and E90. The rationale for dual targeting of these peptides comes from preclinical work suggesting E90-specific responses enhanced the E75 antitumor response. In addition to identification of robust tumor Ags, the activity of DC vaccines relies on cell-intrinsic factors. Our group and others have found that Abs, through engaging Fc receptors on DCs, can enhance the maturity and activity of those cells, possibly lessening the propensity toward an immunosuppressive DC phenotype (47, 48). In addition, immunological cell death, which can be mediated by specific chemotherapy drugs, may be critical to the activation of innate immune cells such as DCs and to alteration of the immunosuppressive tumor microenvironment (49). Thus, to enhance the function of the DC vaccine in this study, we administered it with the anti-HER2 mAb trastuzumab and the vinca alkaloid vinorelbine.
In this article, we report the results of two sequential phase I/II clinical trials investigating the efficacy of a multiepitope DC vaccine given with cytotoxic chemotherapy and HER2-targeted therapy in women with HER2-expressing and then HER2-overexpressing metastatic breast cancer (32, 50–55). The primary endpoint of the initial study was safety and tolerability, whereas that of the subsequent study was response rate. The secondary endpoint focused on evaluating Ag-specific immune responses before and after therapy. With a >14-y follow-up of the longest surviving patient, we report on the outcome and immune activity of this combined therapy approach.
Materials and Methods
Patient eligibility
All patients had histologically proven metastatic breast cancer. HLA-A0201 positivity by DNA genotyping was required because the two peptides used bind to HLA-A0201. Breast cancer receptor status was assigned according to American Society of Oncology/College of American Pathologists guidelines at the time the study was conducted. The first trial included patients with HER2-expressing, but not HER2-overexpressing, tumors, defined as 1+ staining by immunohistochemistry (IHC) staining or 2+ IHC staining and not amplified by fluorescence in situ hybridization (FISH) (single-probe average HER2 copy number < 6 signals/nuclei and/or a dual-probe HER2/CEP17 ratio < 2.2) (56, 57). The subsequent trial required HER2 overexpression, defined as 3+ staining by IHC staining or 2+ staining and amplified by FISH. Patients could have either hormone receptor–positive or –negative tumors, with hormone receptor–positive disease defined by at least 1% of tumor cells staining positive for the estrogen and/or progesterone receptor. Patients were required to be ≥18 y old with Eastern Cooperative Oncology Group performance status 0–2. Cardiac ejection fraction was required to be >45%, and required laboratory parameters included serum creatinine <2.0 mg/dl, ALT/AST ≤3 times the upper limit of normal (ULN) (≤5 times if liver metastases present), bilirubin ≤2 times ULN, absolute neutrophil count >1500/mm3, hemoglobin ≥10, and platelet count >100,000/mm3. Patients with stable CNS metastases were permitted to enroll. Prior trastuzumab was allowed; however, patients who had received prior vinorelbine were excluded. No hormonal therapy or cytotoxic chemotherapy was allowed within 14 d of initial apheresis. No systemic steroids were allowed on trial. Concomitant bisphosphonate therapy was allowed for patients with osseous metastases.
Study design
The first study in patients with HER2-expressing, but not HER2-overexpressing, metastatic breast cancer (more recently termed HER2-low, but not designated as such at the time of the study design) was a phase I study to determine safety. The subsequent phase II study in patients with tumors with HER2-positive tumors was designed with a primary objective to test the hypothesis that the addition of a multiepitope DC vaccine to trastuzumab and vinorelbine would increase the response rate among women with metastatic breast cancer. Based on previously reported response rates of 25%–30% for single agent vinorelbine in the metastatic setting, this study was designed to include 26 patients to provide a power of 89% to detect a difference in response rate from 35% to 60% with an α error ≤0.10 (58–60). The secondary objective was to evaluate the ability of peptide-pulsed DCs plus trastuzumab to induce functional Ag-specific T cells. Each patient was planned to receive six biweekly vaccines unless removed from the study for safety reasons or disease progression.
Vinorelbine and trastuzumab were selected based on preclinical data regarding the activation of DCs by Abs independent of the expression of the Ag bound to the Ab (61). A biweekly dosing schedule was chosen given similar efficacy with reduced myelosuppression compared with weekly dosing (62).
The studies were reviewed and approved by the University of North Carolina Institutional Review Boards (ethics approval was provided by University of North Carolina Office of Human Research Ethics Institutional Review Board approval numbers 03-1571 and 05-2860) and conducted in accordance with ethical principles per the Declaration of Helsinki. Both trials were registered on ClinicalTrials.gov (NCT0088985 and NCT00266110). All participants provided written informed consent. The study was monitored per institutional protocol by the Institutional Data Safety and Monitoring Committee at the University of North Carolina.
We assessed the in vitro response to therapy using tetramer analysis and intracellular cytokine assays (Supplemental Figs. 1–3). T cell activity was measured after the administration of the vaccine. An immune response was defined as a 3-fold increase in the numbers of tetramer-positive cells comparing prevaccine with peak postvaccine values, if the postvaccine value was >0.5% or a 2-fold increase if the prevaccine value was >1%. Similarly, we considered a 3-fold increase in the percent of cells generating IFN-γ or IL-4 using an irrelevant peptide compared with the peptides in the vaccine by ELISPOT, or by intracellular cytokine or CD107 degranulation assay assessment, or a 2-fold increase in prevaccine to postvaccine values if the prevaccine value for intracellular cytokine staining is >1% to indicate a response to this therapy.
Procedures and treatments
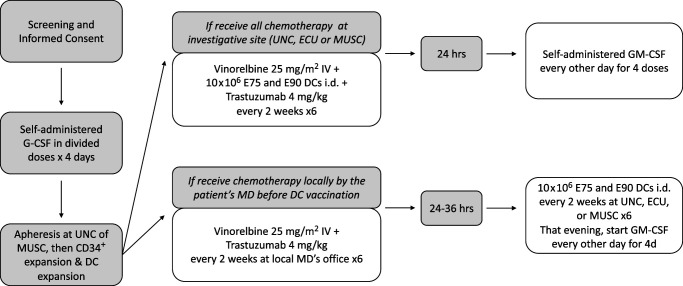
Following patient informed consent and trial enrollment, patients received G-CSF and then underwent apheresis followed by CD34+ cell selection and expansion (Fig. 1). Patients then received vinorelbine 25 mg/m2 i.v. plus trastuzumab 4 mg/kg plus peptide-pulsed DCs every 2 wk. For the phase I trial, patients received a dose escalation of peptide-pulsed DCs at either 2 × 106 or 10 × 106. For the phase II trial, 10 × 106 peptide-pulsed DCs were administered, with sufficient cells generated to allow patients to receive six injections. For the phase II trial, GM-CSF was administered s.c. every other day for four doses starting 24 h after treatment.
FIGURE 1.
Study schema.
CD34+ peripheral blood stem cells were mobilized after the administration of G-CSF 10 µg/kg s.c. each day in divided doses for 4 d before a 15-l apheresis collection. PBSCs were expanded and differentiated using GM-CSF, FLT3-ligand, stem cell factor, and IL-4. They were then matured ex vivo with GM-CSF, IL-4, TNF, IFN-α, and IL-6. The mature, differentiated DCs were then pulsed with HER2 peptides and activated with CD40L. This method produces cells with the surface characteristics and functional activity of DCs. These cells were then cryopreserved for future injection. Infused peptide-pulsed DCs exceeded 80% viability as per the lot release criteria, and at least 75% of injected cells had 2 log expression of HLA-DR over control. All injected DC vaccines demonstrated sterility and effective DC potency. Immune monitoring results were performed on PBLs of patients before and after DC vaccines.
Generation of autologous peptide-pulsed DCs
Autologous DCs were differentiated from the CD34+ progenitor cells isolated from a 15-l leukapheresis by CliniMACS CD34 selection (Miltenyi Biotech, Gladbach, Germany). CD34+ selected cells were incubated at 37°C, 5% CO2 in AIM V media (Life Technologies) with 10% human AB serum (GeminiBio, West Sacramento CA) and GM-CSF 800 U/ml (Sanofi, Paris, France), Flt3 ligand 100 ng/ml (PeproTech, Cranberry, NJ), and stem cell factor 50 ng/ml (PeproTech) in Costar Ultra-low Attachment plates at an initial concentration of 0.3 × 106/ml in 3 ml of six-well cluster plates for the first 5 d and then GM-CSF, SCF, Flt3L, and IL-4 (PeproTech) until day 8. After day 8, the expanding cells are cultured in GM-CSF and IL-4 only and subdivided in half when the concentration approached 0.8 × 106/ml to sustain maximum proliferation. Cells were expanded by this method up to 14 d with replenished cytokines every 48 h when the cultures were either cryopreserved or used to differentiate into mature DCs.
Cryovials of in vitro–expanded CD34+-derived cells were thawed and differentiated for an additional 5 d in AIM V with 10% human AB serum, GM-CSF, IL-4, and TNF 20 ng/ml (PeproTech). The TNF was replenished daily. IFN-α (Schering, Kenilworth, NJ) 1000 U/ml and IL-6 (PeproTech) 1000 U/ml were added to the differentiation media the last 48 h of culture before testing for cell-surface marker expression by flow cytometry and DC potency in an allogeneic MLR.
DCs were incubated overnight with 50 mg/ml of either HER2/neu GMP peptide E75 (KIFGSLAFL) or E90 (CLTSTVQLV) (Multiple Peptide Systems, San Diego, CA) and with CD40L (PeproTech) 1 mg/ml for an hour before washing with saline with 2% human serum albumin (Talecris Biotherapeutics) before viability and sterility testing. After thawing, E75 and E90 peptide-pulsed DCs were immediately used for injection.
Clinical assessments
Clinical response was defined according to RECIST 1.0 criteria. A partial response was defined as at least a 30% decrease in the longest diameters of target lesions, taking as reference the baseline longest diameter. Progressive disease was defined as at least a 20% increase in the longest diameters of target lesions, taking as a reference the smallest longest diameter recorded since the treatment started or the appearance of one or more new lesions. Stable disease was defined as neither sufficient shrinkage nor increase to qualify as a partial response or progressive disease. Restaging studies inclusive of CT chest, abdomen, pelvis, plain radiography, and/or bone scan as clinically necessary were performed after the third cycle of therapy and at least 15 d after the completion of therapy. Restaging was repeated every 3 mo in patients who stayed on therapy beyond six cycles.
Toxicity was evaluated each cycle using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3. Dose-limiting toxicities (DLTs) included grade III neurologic, pulmonary, cardiac, hepatic, or renal toxicity and any grade IV toxicity except hematologic toxicity, which was considered a DLT only if related to life-threatening bleeding, febrile neutropenia, or causes a ≥2-wk treatment delay. Patients who experienced a DLT were required to come off the study. Serious adverse events (SAEs) included life-threatening or life-limiting events and events that required hospitalization.
Correlative assessments
To evaluate the ability of peptide-pulsed DCs plus trastuzumab to induce functional Ag-specific T cells, we measured ex vivo Ag-specific T cell activity against peptide-pulsed and tumor targets by tetramer staining and intracellular cytokine assays using PBMCs isolated from the bloodstream 7 ± 1 d posttherapy. PBMCs were stained with CD8–Pacific Orange (MHCD0830; Life Technologies), along with PE–HLA-A*02:01 tetramers generated with E75 peptide, E90 peptide, or a negative control peptide, all from Beckman Coulter. For single-cell sorting, sort gates were determined by setting the CD8+Tetramer+ gate so that it included no cells in the negative tetramer sample. Tetramer+CD8+ T cells were sorted by an iCyt Reflection high-speed sorter at 1 cell/well into a 96-well PCR plate with each well containing 4 μl buffer (0.5× PBS, 10 mM DTT, and 8 U RNaseOUT; Invitrogen). Plates were kept frozen at −80°C before RT-PCR analysis (63).
HLA typing
All patients underwent initial screening informed consent before HLA testing. Patients who consented for the study then underwent an evaluation for the expression of HLA-A0201 using DNA-based techniques.
Intracellular cytokine staining and upregulation of CD107 expression
PBMCs were cocultured at a 1:5 ratio with unpulsed autologous DCs, E75 peptide-pulsed DCs, or E90 peptide-pulsed DCs in AIM V 10% human AB serum and unlabeled anti-CD28/49 (BD Biosciences, San Jose, CA) and anti–CD107-PE (Pharmingen, San Jose, CA) for an hour. Brefeldin A and GolgiStop (BD Biosciences) 5 mg/ml each were added, and the staining and stimulation were continued for an additional 5 h. After FACS lyse and FACS (Sigma) permeabilization steps, the PBMC:DC-stimulated cells were blocked with 200 ng/ml murine IgG before staining with anti-CD8 PerCP and anti–IFN-γ FITC (BD Biosciences) and fixing in 1% formalin (Polysciences, Warrington, PA) in PBS before acquisition on FACSCalibur flow cytometer (BD Biosciences).
Tetramer staining
PBMCs were resuspended in 0.5% human serum albumin in PBS and 200 ng/ml purified mouse IgG (Sigma) before incubation with CD8-FITC (Beckman-Coulter) and either PE E75, E90, or negative tetramer (iTAg MHC Class I Human Tetramer-SA-PE; MBL International Corporation, Woburn, MA). Stained cells were washed and fixed in 1% formalin (Polysciences) in PBS an hour before acquisition on FACSCalibur flow cytometer (BD Biosciences).
In vitro stimulation of E75- or E90-specific CD8 effector T cells
Peptide-pulsed DCs were cocultured with autologous PBMCs in AIM V 10% human AB serum and IL-2 (20 U/ml) and IL-7 (10 ng/ml) (PeproTech) at a ratio of 20 PBMCs:1 DC for the initial stimulation for 7 d and at a ratio of 100 PBMCs:1 peptide-pulsed DC for restimulation at 14 d. The effector T cells assays for tetramer and the upregulation of CD107 and IFN-γ were performed after 7 d poststimulation.
Single-cell PCR and sequencing
RT-PCR amplification and sequencing of TCRβ clonotypes was performed using multiplex primers and nested PCR covering all human TCRβ V region genes and the β-chain C region (64). PCR products were treated with Exonuclease I and shrimp alkaline phosphatase and sequenced by the UNC Genome Analysis Facility. TCRβ sequence identifications were made using the International ImMunoGeneTics/HighV-QUEST software tool (65).
TCR sequencing and repertoire profiling
Sequence data were processed using Python and R scripts developed in the laboratory. TCR sequences were analyzed for the presence of the conserved invariant cysteine and FGXG motifs that define the CDR3 in one reading frame. Sequences that did not exhibit this motif or had stop codons in the motif reading frame were excluded. TRBV and TRBJ gene identifications were made by either exact alignment or the highest scoring Smith–Waterman alignment to the germline reference gene sequences annotated in ImMunoGeneTics (http://www.imgt.org/IMGTrepertoire) (64, 66).
Data analysis
Data were analyzed in R v4.0.3 (67), additionally using packages survminer (68), ggplot2 (69), viridis (70), and igraph (71). The OS curve was plotted to include the 95% confidence intervals (CIs) around the Kaplan–Meier estimator. Pairwise comparisons of prevaccine and postvaccine T cell population features were done using paired Welch t tests. Associations of T cell population features with OS were evaluated using Cox proportional hazards modeling with individual features as the predictor variables and OS as the response variable. The manuscript was generated using the CONSORT reporting guidelines (72). Regarding availability of data and materials, correlative study data will be provided to investigators at the publication of these data.
Results
This was a single-site phase I/II study in which 52 patients consented to HLA testing for LCCC 0310, and 99 consented for HLA testing for LCCC 0418. Of these, 12 patients were eligible for therapy on LCCC 0310, with 7 female patients enrolled at the University of North Carolina Hospitals. Thirty patients were eligible for LCCC 0418 with 19 female patients enrolled on the phase II trial, LCCC 0418, for tumors that overexpressed HER2 (Fig. 1). Patient decision was the primary reason eligible patients did not enroll. Enrollment was from April 16, 2004, to December 2, 2011. Two patients died before receiving treatment on the trial but are included in the intent-to-treat group for OS. Baseline patient characteristics are listed in Table I. The majority of patients had hormone receptor–positive breast cancer (83%). Nearly 80% of patients had visceral metastases at the time of trial participation. One-third of patients had no prior lines of chemotherapy-containing treatment in the metastatic setting, whereas 46% had one prior line and 21% had two or more prior lines. No patients on LCCC 0310 (Her2-expressing) had received prior trastuzumab. The majority of patients on LCCC 0418 had received trastuzumab before trial participation (82%). Five patients had prior HER2-directed therapy with lapatinib, and one patient had prior T-DM1. Because toxicities and efficacy were similar, clinical outcomes were combined while we evaluated the immune correlatives separately.
Table I. Baseline patient characteristics.
| Patient Characteristics (n = 24) | Total number with (range) for age and (percentages) for others |
|---|---|
| Age, y (range) | 56 (39–69) |
| Sex, n (%) | |
| Female | 24 (100) |
| Race, n (%) | |
| Black | 2 (8.3) |
| White | 22 (91.7) |
| Hormone receptor status, n (%) | |
| Positive | 20 (83.3) |
| Negative | 4 (16.7) |
| HER2 receptor status, n (%) | |
| 1+ IHC | 2 (8.3) |
| 2+ IHC | 8 (33.3) |
| Not amplified by FISH | 4 |
| FISH not performed | 2 |
| FISH amplified | 2 |
| 3+ IHC | 14 (58.3) |
| Visceral metastases, n (%) | |
| Yes | 19 (79.2) |
| No | 5 (20.8) |
| CNS metastases, n (%) | |
| Yes | 2 (8.3) |
| No | 22 (91.7) |
| Prior lines of chemotherapy in the advanced setting, n (%) | |
| 0 | 8 (33.3) |
| 1 | 11 (45.8) |
| 2 | 4 (16.7) |
| 3+ | 1 (4.2) |
| Prior trastuzumab | |
| Yes | 14 (58.3) |
| No | 10 (41.7) |
Assessments included hormone receptor positivity, presence of visceral metastases, prior lines of chemotherapy, use of trastuzumab, and/or previous HER2-directed therapies. Data exclude the two patients who died before receiving treatment on trial.
Characteristics of the infused DCs expanded from CD34 progenitors are provided in Supplemental Fig. 1. The infused DCs expressed high levels of CD40, CD86, CD11c, and HLA-DR with moderate expression of CD83 and very minimal expression of CD14, consistent with the infusion of activated and mature DCs.
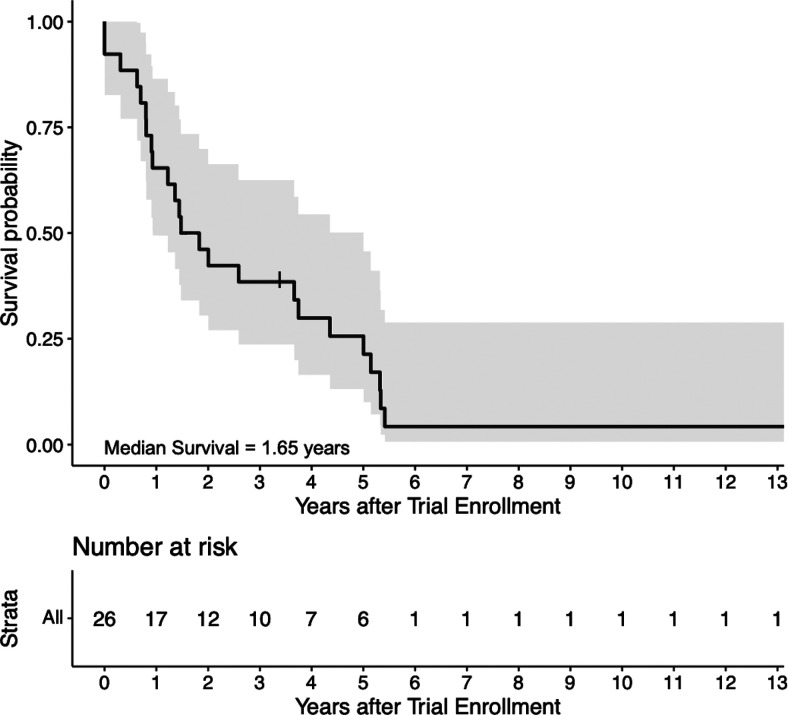
Of the 24 patients treated in both trials, one patient (4%) had a partial response. Eleven patients (46%) achieved stable disease as best response on treatment. There were no complete responses. Fifteen patients (63%) received six or more DC vaccines, with the maximum number of vaccines received by a single patient being nine. The progression-free survival was 3.3 mo, with a range from 1.1 to 9.5 mo. Median OS was 1.65 y (Fig. 2).
FIGURE 2.
Overall survival in patients treated with dual-epitope HER2 DC vaccination. The Kaplan–Meier estimate of OS in the intention-to-treat population is shown with its 95% CI. Two patients were enrolled but died of disease progression before the first vaccination. One patient had prolonged survival >10 y and continues to be alive at the time of her last clinic follow-up on December 3, 2020.
The only grade 3/4 treatment-related adverse events (TRAEs) were hematologic (one patient with grade 4 neutropenia; one with grade 3 leukopenia, not otherwise specified; and one with grade 3 anemia) (Table II). The most common TRAEs were anemia, neutropenia, and fatigue. Hypocalcemia was the next most common adverse event. Five patients experienced fevers and/or chills. Two patients experienced grade 2 allergic reactions to GM-CSF, one of which qualified as an SAE for which the patient discontinued the study. One patient experienced a grade 1 injection reaction to the DC vaccine and was removed from the study. This was considered a DLT. The only additional DLT was observed with neutropenia, requiring a dose reduction of vinorelbine in three patients. Two additional SAEs occurred as a result of hospitalizations for symptoms unrelated to treatment, with one leading to a survival event while the patient was on study.
Table II. TRAEs, SAEs, and DLTs.
| Adverse Event | Any Grade | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Any | 20 | 17 | 13 | 3 | 2 |
| Abdominal pain | 2 | 1 | 1a | ||
| Allergic reaction | 1 | 1a | |||
| Anemia | 8 | 7 | 1 | ||
| Constipation | 2 | 2 | |||
| Cough | 1 | 1 | |||
| Diarrhea | 2 | 2 | |||
| Fatigue | 7 | 6 | 1 | ||
| Fever/Chills | 5 | 5b | |||
| Hyperglycemia | 1 | 1 | |||
| Hypoalbuminemia | 2 | 1 | 1 | ||
| Hypocalcemia | 6 | 3 | 3 | ||
| Hypokalemia | 3 | 3 | |||
| Hyponatremia | 4 | 4 | |||
| Hypomagnesemia | 2 | 2 | |||
| Injection-site reaction | 2 | 1c | 1 | ||
| Leukopenia, not specified | 5 | 3 | 1 | 1 | |
| Lymphopenia | 4 | 1 | 3 | ||
| Nausea | 3 | 3 | |||
| Neutropenia | 7 | 3 | 4c | 1c | |
| Musculoskeletal pain | 3 | 2 | 1 | ||
| Shortness of breath | 2 | 1 | 1a | ||
| Strep throat | 1 | 1 | |||
| Thrombocytopenia | 2 | 1 | 1 | ||
| Upper respiratory infection | 1 | 1 |
Additional grade 1 events were observed in one patient: alanine aminotransferase increase, aspartate aminotransferase increase, creatinine increase, headache, hypermagnesemia, hypotension, rhinitis, sensory neuropathy, shortness of breath, urinary tract infection, and weight loss.
SAEs: hospitalization for abdominal pain (n = 1), unrelated to treatment; hospitalization for shortness of breath (n = 1), unrelated to treatment; and allergic reaction to leukocyte growth factor (n = 1), patient removed from study.
Regimen was interrupted (fever [n = 1]).
DLTs: neutropenia (n = 3), dose reduced; injection-site reaction (n = 1), therapy discontinued.
Reasons for trial discontinuation included completion of therapy (either six vaccine treatments or more than six, which was discontinued when vaccine doses were completely used) (n = 10), progressive disease (n = 12), and leukine injection reaction (n = 2). The five patients who received more than six vaccines had a longer survival compared with those who did not complete vaccination, with all surviving >3.5 y beyond their first treatment on trial, with one of these patients remaining alive >14 y after vaccination. Details regarding her clinical course are available in Supplemental Fig. 4.
Evaluation of E75- and E90-specific CD8+ T cell responses to therapeutic vaccination
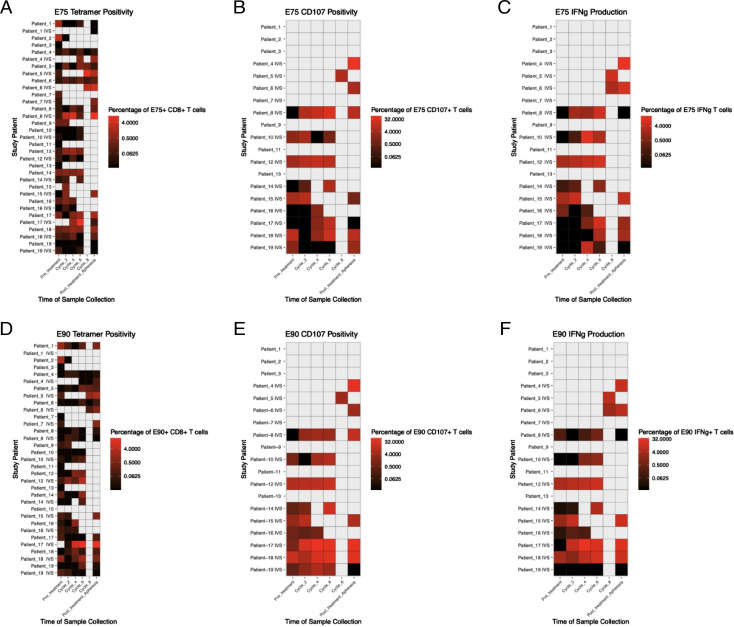
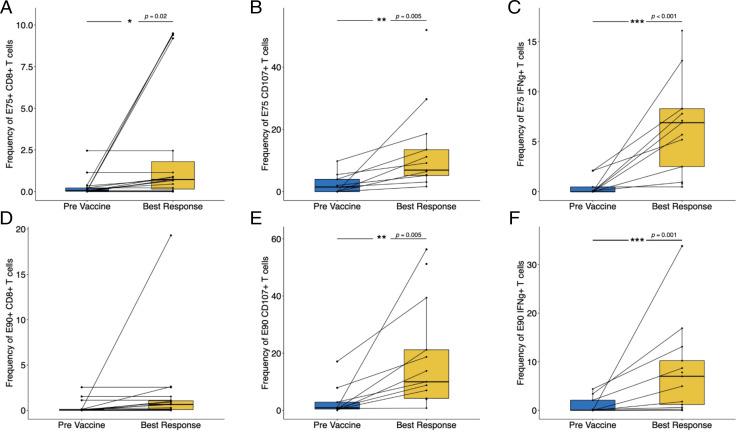
For the LCCC 0418 study cohort, we used flow cytometry assays to quantify E75 and E90 Ag-specific CD8+ T cells, measure IFN-γ secretion, and evaluate cytotoxic activity via staining for cell-surface CD107a on peripheral blood T cell populations. Representative examples of tetramer, ELISPOT, and CD107+ T cells, respectively, are shown in Supplemental Fig. 2. Immune monitoring results for this study cohort over the study time points are summarized in Fig. 3. Only E75- and E90-specific CD8+ T cell numbers were measured in the LCCC 0310 study cohort (Supplemental Fig. 3). Although there was variability in time to best immunological response, epitope-specific T cell number, IFN-γ production, and cytotoxicity were all increased postvaccination relative to prevaccination levels (Fig. 4). Immunological vaccine responses were heterogeneous, with patients having different overall magnitudes of response and different responses across the variables tested (number of epitope-specific CD8+ T cells, IFN-γ secretion, cell-surface CD107a expression) (Supplemental Fig. 3). None of the variables tested were significantly associated with OS postvaccination (Table III).
FIGURE 3.
Immune monitoring results in the LCCC 0418 study cohort. Leukapheresis samples were analyzed before first vaccination and at the end of the study. Peripheral blood samples were analyzed on the days of cycles 2, 4, 6, and 8 in vaccinated patients. Heatmaps show (A) frequency of E75-specific CD8+ T cells, (B) frequency of CD8+ T cells positive for CD107 surface staining after stimulation with E75 peptide, (C) frequency of CD8+ T cells expressing IFN-γ after stimulation with E75 peptide, (D) frequency of E90-specific CD8+ T cells, (E) frequency of CD8+ T cells positive for CD107a surface staining after stimulation with E90 peptide, and (F) frequency of CD8+ T cells expressing IFN-γ after stimulation with E90 peptide. Gray boxes represent time points when samples were not collected.
FIGURE 4.
Increase of E75- and E90-specific T cell responses in treated patients. Pretreatment immune monitoring measurements were compared with the time point of best response on treatment: (A) frequency of E75-specific CD8+ T cells, (B) frequency of CD8+ T cells positive for CD107 surface staining after stimulation with E75 peptide, (C) frequency of CD8+ T cells expressing IFN-γ after stimulation with E75 peptide, (D) frequency of E90-specific CD8+ T cells, (E) frequency of CD8+ T cells positive for CD107 surface staining after stimulation with E90 peptide, and (F) frequency of CD8+ T cells expressing IFN-γ after stimulation with E90 peptide.
Table III. Variables tested to probe heterogeneity of immunological vaccine responses.
| Variable | Hazard Ratio | 95% CI | p Value |
|---|---|---|---|
| E75 Tetramer | 0.93 | 0.81–1.07 | 0.32 |
| E75 CD107 | 1.01 | 0.97–1.05 | 0.55 |
| E75 IFNg | 1.00 | 0.87–1.16 | 0.99 |
| E90 tetramer | 1.03 | 0.92–1.15 | 0.59 |
| E90 CD107 | 1.02 | 0.99–1.05 | 0.25 |
| E90 IFNg | 1.02 | 0.95–1.10 | 0.61 |
Assessment of vaccine responses was based on number of epitope-specific CD8+ T cells, IFN-γ secretion, cell-surface CD107a expression as determined by flow cytometric analysis. None of the variables tested were significantly associated with OS postvaccination.
TCR repertoire profiling of E75-specific CD8+ T cells
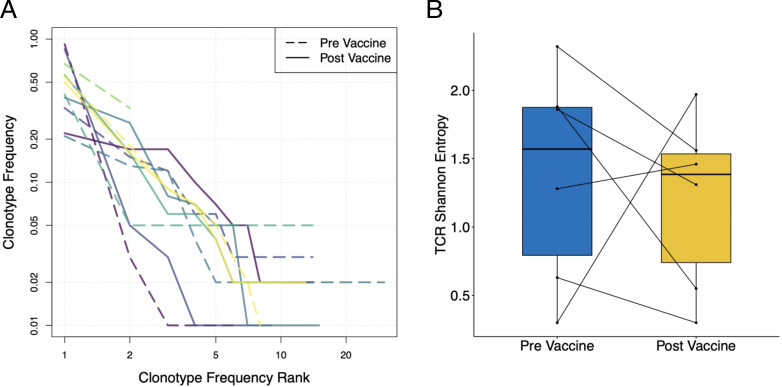
We performed single-cell sorting of E75-specific CD8+ T cells followed by TCRβ sequencing to evaluate diversity of the Ag-specific TCR repertoire in the context of vaccine therapy. Repertoire diversity was not significantly different in prevaccination versus postvaccination samples (Fig. 5).
FIGURE 5.
TCRβ repertoire profiling in treated patients. (A) Rank-frequency plot showing frequency distributions of prevaccine (dashed lines) and postvaccine (solid lines) E75-specific CD8+ T cell populations. Distinct colors represent individual patients. (B) TCR repertoire diversity measurements of E75-specific CD8+ T cell populations before and after vaccine therapy.
Robust E75-specific CD8+ T cell response in an exceptional responder
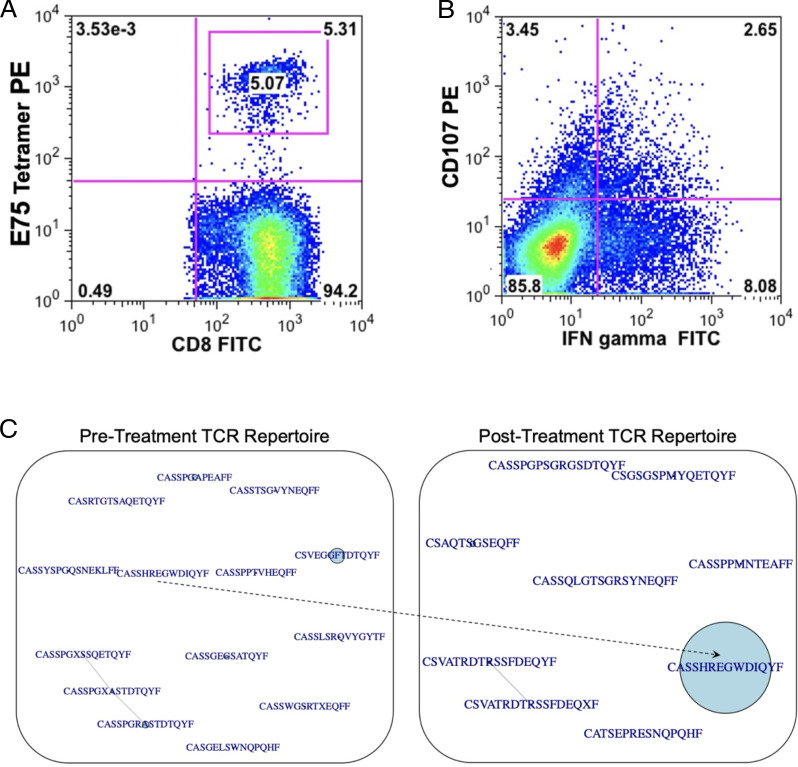
Given the remarkable survival at 14 y for one patient treated with this multiepitope DC vaccination, we performed an in-depth characterization of the immune response in this patient posttherapy. This patient had an increased frequency of E75-specific CD8+ T cells, CD8+ T cell production of IFN-γ, and cell-surface expression of CD107a after vaccination (Fig. 6A, 6B). Using next-generation sequencing, we also found decreased TCR repertoire diversity (increased clonality) in the E75-specific CD8+ T cell population after therapeutic vaccination. Before vaccine therapy, we identified 14 different clonotypes as demonstrated by different CDR3s from T cells in the bloodstream from this patient specific for the E75 or E90 epitopes. Interestingly, after vaccination, we identified a dominant single TCRβ clonotype with the CDR amino acid sequence CASSHREGWDIQYF, specific for the E75 epitope, which was present at low frequency in the pretreatment TCR repertoire but became the dominant clonotype posttreatment (Fig. 6C) with >25% of the repertoire composed of this single clone.
FIGURE 6.
Robust E75-specific CD8+ T cell responses in an exceptional responder. (A) Frequency of E75-specific CD8+ T cells. (B) Frequency of CD8+ T cells positive for CD107 surface staining after stimulation with E75 peptide (y-axis) and frequency of CD8+ T cells expressing IFN-γ after stimulation with E75 peptide (x-axis). (C) Sequence similarity networks showing TCR repertoires of the sorted E75-specific CD8+ T cells. TCR clonotypes were considered identical if they expressed the same V gene, J gene, and CDR3 gene segment. CDR3 amino acid sequences are shown, with central circles corresponding to relative abundance in the TCR repertoire. The sequence CASSHREGWDIQYF was present in a low frequency in the pretreatment repertoire and expanded to become a dominant clonotype in the posttreatment TCR repertoire.
Discussion
In this article, we provide evidence that peptide-pulsed DCs can be effectively generated and given in combination with chemotherapy and HER2-targeted therapy, with few grade ≥2 adverse events and all but one patient able to complete at least three vaccinations. The combination of autologous peptide-pulsed DCs with trastuzumab and vinorelbine stable disease led to stable disease in 46% and partial response in 4% with a median survival of 1.65 y. Quite surprisingly, one patient, who had the most robust immunological response postvaccination, continues to experience long-term survival with continued bone-only disease >14 y after trial participation.
We did not find that the level of expression of HER2 was a determinant of response to this vaccine because patients with HER2 expression, but not overexpression (treated in the phase I study), responded similarly to those with HER2 overexpression in the phase II study. Our rationale for studying this patient population was preclinical data suggesting that a very low level of expression of immunodominant peptide/MHC complexes could induce a CD8+ T cell response. Our findings are particularly interesting given recent data demonstrating clinically significant therapeutic activity of the HER2-targeted Ab drug conjugate trastuzumab deruxtecan in patients with low levels of HER2 expression (73). Given that patients with metastatic hormone receptor–positive and triple-negative breast cancer have few, if any, options besides chemotherapy in the later line setting, which rarely provides durable responses, these data suggest additional development of targeted and immune-based therapeutics specific to HER2 that take advantage of even low levels of HER2 expression.
DC vaccination induced an immune response as demonstrated by increased Ag-specific T cell responses, including increased E75+CD8+ T cells by tetramer analysis, increased frequency of IFN-γ–secreting T cells with E75 and E90 stimulation, and increased frequency of CD107+ T cells with E75 and E90 stimulation. Four of six patients with available data had increased TCR repertoire clonality after vaccination. However, these responses were not correlated with clinical efficacy, which is similar to published data (74). There are a number of reasons for this that include a lack of understanding of what level of Ag-specific T cell number or functional activity in the bloodstream correlates with a clinical response postvaccination, the absence of data regarding T cell activity at the tumor site, possible local immunosuppression that may limit the efficacy of activated T cells, the potential development of T cell exhaustion, which had not been well characterized when this study was underway, and limitations on the effector activity being measured. Although we found that the vaccine elicited greater number of T cells after treatments, these values did not approach those found in individuals after viral infection.
We found one patient who had an extremely robust immune response to this therapy (see Supplemental Fig. 4 for treatment schema). Before vaccination, she had evidence of an oligoclonal response to HER2 with a very small population of T cells that expressed the immunodominant TCR found postvaccination. After vaccination, she had a robust T cell response to the E75 epitope with an increased percentage of T cells specific for E75 that generated IFN-γ and lytic activity by in vitro stimulation after the completion of vaccine therapy. In addition, she had E75-specific tetramer-positive cells present postvaccination. Finally, close to 25% of her T cell repertoire postvaccination was represented by one clonal CDR3 that was present at a much lower frequency before vaccination, strongly suggesting that the vaccine was capable of expanding an endogenous immune response. Quite interestingly, this patient is alive 14 y after the diagnosis of metastatic breast cancer (postvaccine treatment is summarized in Supplemental Fig. 4).
The limitations of this study include the absence of a control group that received vinorelbine and trastuzumab without vaccination (although most patients in the phase II trial had received prior chemotherapy and 82% had received trastuzumab) to allow the vaccine contribution to be assessed alone, the absence of immune assessment at the tumor site, and the modest sample size. Immune monitoring was performed on the peripheral blood; however, in the context of neoadjuvant HER2 DC vaccination for Ductal Carcinoma in Situ, clinical response was associated with T cell responses measured in the sentinel lymph node, but not in blood (43). In addition, this study was performed before the development of newer therapies for HER2-expressing tumors, which limits its applicability to current standard of care.
In conclusion, multiepitope DC vaccines can be successfully generated and safely given to patients with metastatic breast cancer. Vaccine-specific T cell responses were seen in the context of concurrent chemotherapy plus HER-2–targeted therapy, even in patients who had multiple lines of therapy before vaccination. Immunodominant Ag responses varied between individuals, although both Ags tested were presented by HLA-A*02:01 (and all enrolled patients expressed this HLA allele). Although much vaccine work in HER2+ breast cancer has focused on the E75, AE37, and GP2 peptides, as well as peptide mixtures, in this study, we saw T cell responses elicited to the E90 peptide as well. There was only one objective response in this study cohort despite more frequent generation of vaccine epitope-specific T cell responses; however, a significant number of patients treated had stable disease, and nearly 30% of patients survived >5 y from their initial treatment in the trial, which is longer than historical survival for patients with metastatic breast cancer. T cell responses after vaccination were heterogeneous and not statistically associated with improved survival. One patient, with the most robust expansion of a T cell clone postvaccination, is still alive 14 y after treatment with a small frequency clone prevaccination greatly expanded by this therapy. These findings provide an impetus for a larger phase clinical trial to test the efficacy of this combination in patients with metastatic breast cancer, as well as to include the E90 peptide in future multiepitope HER2 vaccine formulations.
Supplementary Material
Footnotes
This work was supported by the National Institutes of Health, National Cancer Institute (R21 CA089961 and P50CA058223 to J.S.S.) and the Susan G. Komen for the Cure Foundation (to J.S.S.).
The online version of this article contains supplemental material.
- CI
- confidence interval
- DLT
- dose-limiting toxicity
- FISH
- fluorescence in situ hybridization
- HER2
- human epidermal growth factor 2
- IHC
- immunohistochemistry
- OS
- overall survival
- SAE
- serious adverse event
- T-DM1
- trastuzumab emtansine
- TRAE
- treatment-related adverse event
Disclosures
B.G.V. has received research funding from Merck Inc., Genecentric, and Cancer Research Institute. C.K.A. has received research funding from PUMA, Lilly, Merck, Seattle Genetics, Nektar, Tesaro, G1-Therapeutics, ZION, Novartis, Pfizer, Astra Zeneca, and Elucida. C.K.A. has a compensated consultant role for Genentech, Eisai, IPSEN, Seattle Genetics, Astra Zeneca, Novartis, Immunomedics, Elucida, and Athenex. C.K.A. has received honoraria from Genentech, Eisai, IPSEN, Seattle Genetics, Astra Zeneca, Novartis, Immunomedics, Elucida, and Athenex. C.K.A. receives royalties from UpToDate and Jones and Bartlet. F.A.C. has received research funding from Bristol Myers Squibb, Replimune, and Amgen. J.S.S. has received research funding from Merck Inc., Carisma Therapeutics, and GlaxoSmithKline. J.S.S. is a compensated consultant for PIQUE Therapeutics. J.S.S. owns intellectual property with Tessa Therapeutics for the use of lymphodepletion and CD30.CAR T cells for patients with Hodgkin’s lymphoma and has filed for intellectual property protection for the use of STING agonists to enhance CAR T cell therapy for the treatment of patients with solid tumors. The other authors have no financial conflicts of interest.
References
- 1. von Minckwitz, G., Procter M., de Azambuja E., Zardavas D., Benyunes M., Viale G., Suter T., Arahmani A., Rouchet N., Clark E., et al. APHINITY Steering Committee and Investigators . 2017. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 377: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. von Minckwitz, G., Huang C. S., Mano M. S., Loibl S., Mamounas E. P., Untch M., Wolmark N., Rastogi P., Schneeweiss A., Redondo A., et al. KATHERINE Investigators . 2019. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380: 617–628. [DOI] [PubMed] [Google Scholar]
- 3. Cortes, J., Cescon D. W., Rugo H. S., Nowecki Z., Im S.-A., Yusof M. M., Gallardo C., Lipatov O., Barrios C. H., Holgado E., et al. KEYNOTE-355 Investigators . 2020. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396: 1817–1828. [DOI] [PubMed] [Google Scholar]
- 4. Diéras, V., Deluche E., Lusque A.. 2022. Abstract PD8-02: trastuzumab deruxtecan (T-DXd) for advanced breast cancer patients (ABC), regardless HER2 status: a phase II study with biomarkers analysis (DAISY). Cancer Res. 82(Suppl. 4): PD8-02. [Google Scholar]
- 5. Benavides, L. C., Gates J. D., Carmichael M. G., Patil R., Holmes J. P., Hueman M. T., Mittendorf E. A., Craig D., Stojadinovic A., Ponniah S., Peoples G. E.. 2009. The impact of HER2/neu expression level on response to the E75 vaccine: from U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. [Published erratum appears in 2009 Clin. Cancer Res. 15: 5601.] Clin. Cancer Res. 15: 2895–2904. [DOI] [PubMed] [Google Scholar]
- 6. Mittendorf, E. A., Clifton G. T., Holmes J. P., Clive K. S., Patil R., Benavides L. C., Gates J. D., Sears A. K., Stojadinovic A., Ponniah S., Peoples G. E.. 2012. Clinical trial results of the HER-2/neu (E75) vaccine to prevent breast cancer recurrence in high-risk patients: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer 118: 2594–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mittendorf, E. A., Ardavanis A., Litton J. K., Shumway N. M., Hale D. F., Murray J. L., Perez S. A., Ponniah S., Baxevanis C. N., Papamichail M., Peoples G. E.. 2016. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget 7: 66192–66201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clifton, G. T., Litton J. K., Arrington K., Ponniah S., Ibrahim N. K., Gall V., Alatrash G., Peoples G. E., Mittendorf E. A.. 2017. Results of a phase Ib trial of combination immunotherapy with a CD8+ T cell eliciting vaccine and trastuzumab in breast cancer patients. Ann. Surg. Oncol. 24: 2161–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mittendorf, E. A., Lu B., Melisko M., Price Hiller J., Bondarenko I., Brunt A. M., Sergii G., Petrakova K., Peoples G. E.. 2019. Efficacy and safety analysis of nelipepimut-S vaccine to prevent breast cancer recurrence: a randomized, multicenter, phase iii clinical trial. Clin. Cancer Res. 25: 4248–4254. [DOI] [PubMed] [Google Scholar]
- 10. Brown, T. A.II, Mittendorf E. A., Hale D. F., Myers J. W. III, Peace K. M., Jackson D. O., Greene J. M., Vreeland T. J., Clifton G. T., Ardavanis A., et al. 2020. Prospective, randomized, single-blinded, multi-center phase II trial of two HER2 peptide vaccines, GP2 and AE37, in breast cancer patients to prevent recurrence. Breast Cancer Res. Treat. 181: 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Disis, M. L. N., Guthrie K. A., Liu Y., Coveler A. L., Higgins D. M., Childs J. S., Dang Y., Salazar L. G.. 2023. Safety and outcomes of a plasmid DNA vaccine encoding the ERBB2 intracellular domain in patients with advanced-stage ERBB2-positive breast cancer: a phase 1 nonrandomized clinical trial. JAMA Oncol. 9: 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Disis, M. L., Dang Y., Coveler A. L., Marzbani E., Kou Z. C., Childs J. S., Fintak P., Higgins D. M., Reichow J., Waisman J., Salazar L. G.. 2014. HER-2/neu vaccine-primed autologous T-cell infusions for the treatment of advanced stage HER-2/neu expressing cancers. Cancer Immunol. Immunother. 63: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Disis, M. L., Pupa S. M., Gralow J. R., Dittadi R., Menard S., Cheever M. A.. 1997. High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J. Clin. Oncol. 15: 3363–3367. [DOI] [PubMed] [Google Scholar]
- 14. Disis, M. L., Gooley T. A., Rinn K., Davis D., Piepkorn M., Cheever M. A., Knutson K. L., Schiffman K.. 2002. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J. Clin. Oncol. 20: 2624–2632. [DOI] [PubMed] [Google Scholar]
- 15. Fisk, B., Blevins T. L., Wharton J. T., Ioannides C. G.. 1995. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J. Exp. Med. 181: 2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuhns, J. J., Batalia M. A., Yan S., Collins E. J.. 1999. Poor binding of a HER-2/neu epitope (GP2) to HLA-A2.1 is due to a lack of interactions with the center of the peptide. J. Biol. Chem. 274: 36422–36427. [DOI] [PubMed] [Google Scholar]
- 17. Lustgarten, J., Theobald M., Labadie C., LaFace D., Peterson P., Disis M. L., Cheever M. A., Sherman L. A.. 1997. Identification of Her-2/Neu CTL epitopes using double transgenic mice expressing HLA-A2.1 and human CD.8. Hum. Immunol. 52: 109–118. [DOI] [PubMed] [Google Scholar]
- 18. Navabi, H., Jasani B., Adams M., Evans A. S., Mason M., Crosby T., Borysiewicz L.. 1997. Generation of in vitro autologous human cytotoxic T-cell response to E7 and HER-2/neu oncogene products using ex-vivo peptide loaded dendritic cells. Adv. Exp. Med. Biol. 417: 583–589. [DOI] [PubMed] [Google Scholar]
- 19. Reilly, R. T., Gottlieb M. B., Ercolini A. M., Machiels J. P., Kane C. E., Okoye F. I., Muller W. J., Dixon K. H., Jaffee E. M.. 2000. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 60: 3569–3576. [PubMed] [Google Scholar]
- 20. Zaks, T. Z., Rosenberg S. A.. 1998. Immunization with a peptide epitope (p369-377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res. 58: 4902–4908. [PubMed] [Google Scholar]
- 21. Bell, D., Young J. W., Banchereau J.. 1999. Dendritic cells. Adv. Immunol. 72: 255–324. [DOI] [PubMed] [Google Scholar]
- 22. Hart, D. N. 1997. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 90: 3245–3287. [PubMed] [Google Scholar]
- 23. Lotze, M. T., Shurin M., Davis I., Amoscato A., Storkus W. J.. 1997. Dendritic cell based therapy of cancer. Adv. Exp. Med. Biol. 417: 551–569. [DOI] [PubMed] [Google Scholar]
- 24. Nestle, F. O., Banchereau J., Hart D.. 2001. Dendritic cells: on the move from bench to bedside. Nat. Med. 7: 761–765. [DOI] [PubMed] [Google Scholar]
- 25. Ni, K., O’Neill H. C.. 1997. The role of dendritic cells in T cell activation. Immunol. Cell Biol. 75: 223–230. [DOI] [PubMed] [Google Scholar]
- 26. Schuler, G., Steinman R. M.. 1997. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J. Exp. Med. 186: 1183–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mackensen, A., Herbst B., Köhler G., Wolff-Vorbeck G., Rosenthal F. M., Veelken H., Kulmburg P., Schaefer H. E., Mertelsmann R., Lindemann A.. 1995. Delineation of the dendritic cell lineage by generating large numbers of Birbeck granule-positive Langerhans cells from human peripheral blood progenitor cells in vitro. Blood 86: 2699–2707. [PubMed] [Google Scholar]
- 28. Herbst, B., Köhler G., Mackensen A., Veelken H., Kulmburg P., Rosenthal F. M., Schaefer H. E., Mertelsmann R., Fisch P., Lindemann A.. 1996. In vitro differentiation of CD34+ hematopoietic progenitor cells toward distinct dendritic cell subsets of the birbeck granule and MIIC-positive Langerhans cell and the interdigitating dendritic cell type. Blood 88: 2541–2548. [PubMed] [Google Scholar]
- 29. Herbst, B., Köhler G., Mackensen A., Veelken H., Mertelsmann R., Lindemann A.. 1997. CD34+ peripheral blood progenitor cell and monocyte derived dendritic cells: a comparative analysis. Br. J. Haematol. 99: 490–499. [DOI] [PubMed] [Google Scholar]
- 30. Caux, C., Dezutter-Dambuyant C., Schmitt D., Banchereau J.. 1992. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature 360: 258–261. [DOI] [PubMed] [Google Scholar]
- 31. Caux, C., Massacrier C., Vanbervliet B., Dubois B., Durand I., Cella M., Lanzavecchia A., Banchereau J.. 1997. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood 90: 1458–1470. [PubMed] [Google Scholar]
- 32. Draube, A., Klein-González N., Mattheus S., Brillant C., Hellmich M., Engert A., von Bergwelt-Baildon M.. 2011. Dendritic cell based tumor vaccination in prostate and renal cell cancer: a systematic review and meta-analysis. PLoS One 6: e18801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anguille, S., Smits E. L., Lion E., van Tendeloo V. F., Berneman Z. N.. 2014. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 15: e257–e267. [DOI] [PubMed] [Google Scholar]
- 34. Tjoa, B. A., Erickson S. J., Bowes V. A., Ragde H., Kenny G. M., Cobb O. E., Ireton R. C., Troychak M. J., Boynton A. L., Murphy G. P.. 1997. Follow-up evaluation of prostate cancer patients infused with autologous dendritic cells pulsed with PSMA peptides. Prostate 32: 272–278. [DOI] [PubMed] [Google Scholar]
- 35. Murphy, G. P., Tjoa B. A., Simmons S. J., Jarisch J., Bowes V. A., Ragde H., Rogers M., Elgamal A., Kenny G. M., Cobb O. E., et al. 1999. Infusion of dendritic cells pulsed with HLA-A2-specific prostate-specific membrane antigen peptides: a phase II prostate cancer vaccine trial involving patients with hormone-refractory metastatic disease. Prostate 38: 73–78. [DOI] [PubMed] [Google Scholar]
- 36. Nestle, F. O., Alijagic S., Gilliet M., Sun Y., Grabbe S., Dummer R., Burg G., Schadendorf D.. 1998. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 4: 328–332. [DOI] [PubMed] [Google Scholar]
- 37. Brossart, P., Wirths S., Stuhler G., Reichardt V. L., Kanz L., Brugger W.. 2000. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood 96: 3102–3108. [PubMed] [Google Scholar]
- 38. Thurner, B., Haendle I., Röder C., Dieckmann D., Keikavoussi P., Jonuleit H., Bender A., Maczek C., Schreiner D., von den Driesch P., et al. 1999. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J. Exp. Med. 190: 1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dillman, R., Selvan S., Schiltz P., Peterson C., Allen K., Depriest C., McClay E., Barth N., Sheehy P., de Leon C., Beutel L.. 2004. Phase I/II trial of melanoma patient-specific vaccine of proliferating autologous tumor cells, dendritic cells, and GM-CSF: planned interim analysis. Cancer Biother. Radiopharm. 19: 658–665. [DOI] [PubMed] [Google Scholar]
- 40. Dillman, R. O., Selvan S. R., Schiltz P. M., McClay E. F., Barth N. M., DePriest C., de Leon C., Mayorga C., Cornforth A. N., Allen K.. 2009. Phase II trial of dendritic cells loaded with antigens from self-renewing, proliferating autologous tumor cells as patient-specific antitumor vaccines in patients with metastatic melanoma: final report. Cancer Biother. Radiopharm. 24: 311–319. [DOI] [PubMed] [Google Scholar]
- 41. Dillman, R. O., Cornforth A. N., Depriest C., McClay E. F., Amatruda T. T., de Leon C., Ellis R. E., Mayorga C., Carbonell D., Cubellis J. M.. 2012. Tumor stem cell antigens as consolidative active specific immunotherapy: a randomized phase II trial of dendritic cells versus tumor cells in patients with metastatic melanoma. J. Immunother. 35: 641–649. [DOI] [PubMed] [Google Scholar]
- 42. Sheikh, N. A., Petrylak D., Kantoff P. W., Dela Rosa C., Stewart F. P., Kuan L. Y., Whitmore J. B., Trager J. B., Poehlein C. H., Frohlich M. W., Urdal D. L.. 2013. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol. Immunother. 62: 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lowenfeld, L., Mick R., Datta J., Xu S., Fitzpatrick E., Fisher C. S., Fox K. R., DeMichele A., Zhang P. J., Weinstein S. P., et al. 2017. Dendritic cell vaccination enhances immune responses and induces regression of HER2pos DCIS independent of route: results of randomized selection design trial. Clin. Cancer Res. 23: 2961–2971. [DOI] [PubMed] [Google Scholar]
- 44. Shinde, P., Melinkeri S., Santra M. K., Kale V., Limaye L.. 2019. Autologous hematopoietic stem cells are a preferred source to generate dendritic cells for immunotherapy in multiple myeloma patients. Front. Immunol. 10: 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bernhard, H., Disis M. L., Heimfeld S., Hand S., Gralow J. R., Cheever M. A.. 1995. Generation of immunostimulatory dendritic cells from human CD34+ hematopoietic progenitor cells of the bone marrow and peripheral blood. Cancer Res. 55: 1099–1104. [PubMed] [Google Scholar]
- 46. Gu, Y. Z., Zhao X., Song X. R.. 2020. Ex vivo pulsed dendritic cell vaccination against cancer. Acta Pharmacol. Sin. 41: 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Merck, E., de Saint-Vis B., Scuiller M., Gaillard C., Caux C., Trinchieri G., Bates E. E.. 2005. Fc receptor gamma-chain activation via hOSCAR induces survival and maturation of dendritic cells and modulates Toll-like receptor responses. Blood 105: 3623–3632. [DOI] [PubMed] [Google Scholar]
- 48. Akiyama, K., Ebihara S., Yada A., Matsumura K., Aiba S., Nukiwa T., Takai T.. 2003. Targeting apoptotic tumor cells to Fc gamma R provides efficient and versatile vaccination against tumors by dendritic cells. J. Immunol. 170: 1641–1648. [DOI] [PubMed] [Google Scholar]
- 49. Vanmeerbeek, I., Sprooten J., De Ruysscher D., Tejpar S., Vandenberghe P., Fucikova J., Spisek R., Zitvogel L., Kroemer G., Galluzzi L., Garg A. D.. 2020. Trial watch: chemotherapy-induced immunogenic cell death in immuno-oncology. OncoImmunology 9: 1703449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zitvogel, L., Apetoh L., Ghiringhelli F., André F., Tesniere A., Kroemer G.. 2008. The anticancer immune response: indispensable for therapeutic success? J. Clin. Invest. 118: 1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schlom, J. 2012. Therapeutic cancer vaccines: current status and moving forward. J. Natl. Cancer Inst. 104: 599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen, G., Emens L. A.. 2013. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol. Immunother. 62: 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gabrilovich, D. I. 2007. Combination of chemotherapy and immunotherapy for cancer: a paradigm revisited. Lancet Oncol. 8: 2–3. [DOI] [PubMed] [Google Scholar]
- 54. Wheeler, C. J., Das A., Liu G., Yu J. S., Black K. L.. 2004. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin. Cancer Res. 10: 5316–5326. [DOI] [PubMed] [Google Scholar]
- 55. Bracci, L., Schiavoni G., Sistigu A., Belardelli F.. 2014. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 21: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hammond, M. E., Hayes D. F., Dowsett M., Allred D. C., Hagerty K. L., Badve S., Fitzgibbons P. L., Francis G., Goldstein N. S., Hayes M., et al. American Society of Clinical Oncology; College of American Pathologists . 2010. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch. Pathol. Lab. Med. 134: e48–e72. [DOI] [PubMed] [Google Scholar]
- 57. Wolff, A. C., Hammond M. E., Schwartz J. N., Hagerty K. L., Allred D. C., Cote R. J., Dowsett M., Fitzgibbons P. L., Hanna W. M., Langer A., et al. American Society of Clinical Oncology/College of American Pathologists . 2007. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 131: 18–43. [DOI] [PubMed] [Google Scholar]
- 58. Gasparini, G., Caffo O., Barni S., Frontini L., Testolin A., Guglielmi R. B., Ambrosini G.. 1994. Vinorelbine is an active antiproliferative agent in pretreated advanced breast cancer patients: a phase II study. J. Clin. Oncol. 12: 2094–2101. [DOI] [PubMed] [Google Scholar]
- 59. Degardin, M., Bonneterre J., Hecquet B., Pion J. M., Adenis A., Horner D., Demaille A.. 1994. Vinorelbine (navelbine) as a salvage treatment for advanced breast cancer. Ann. Oncol. 5: 423–426. [DOI] [PubMed] [Google Scholar]
- 60. Weber, B. L., Vogel C., Jones S., Harvey H., Hutchins L., Bigley J., Hohneker J.. 1995. Intravenous vinorelbine as first-line and second-line therapy in advanced breast cancer. J. Clin. Oncol. 13: 2722–2730. [DOI] [PubMed] [Google Scholar]
- 61. Burstein, H. J., Harris L. N., Marcom P. K., Lambert-Falls R., Havlin K., Overmoyer B., Friedlander R. J. Jr., Gargiulo J., Strenger R., Vogel C. L., et al. 2003. Trastuzumab and vinorelbine as first-line therapy for HER2-overexpressing metastatic breast cancer: multicenter phase II trial with clinical outcomes, analysis of serum tumor markers as predictive factors, and cardiac surveillance algorithm. J. Clin. Oncol. 21: 2889–2895. [DOI] [PubMed] [Google Scholar]
- 62. Stathopoulos, G. P., Rigatos S. K., Pergantas N., Tsavdarides D., Athanasiadis I., Malamos N. A., Stathopoulos J. G.. 2002. Phase II trial of biweekly administration of vinorelbine and gemcitabine in pretreated advanced breast cancer. J. Clin. Oncol. 20: 37–41. [DOI] [PubMed] [Google Scholar]
- 63. Vincent, B. G., Young E. F., Buntzman A. S., Stevens R., Kepler T. B., Tisch R. M., Frelinger J. A., Hess P. R.. 2010. Toxin-coupled MHC class I tetramers can specifically ablate autoreactive CD8+ T cells and delay diabetes in nonobese diabetic mice. J. Immunol. 184: 4196–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hunsucker, S. A., McGary C. S., Vincent B. G., Enyenihi A. A., Waugh J. P., McKinnon K. P., Bixby L. M., Ropp P. A., Coghill J. M., Wood W. A., et al. 2015. Peptide/MHC tetramer-based sorting of CD8+ T cells to a leukemia antigen yields clonotypes drawn nonspecifically from an underlying restricted repertoire. Cancer Immunol. Res. 3: 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li, S., Lefranc M. P., Miles J. J., Alamyar E., Giudicelli V., Duroux P., Freeman J. D., Corbin V. D., Scheerlinck J. P., Frohman M. A., et al. 2013. IMGT/HighV QUEST paradigm for T cell receptor IMGT clonotype diversity and next generation repertoire immunoprofiling. Nat. Commun. 4: 2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Manso, T., Folch G., Giudicelli V., Jabado-Michaloud J., Kushwaha A., Nguefack Ngoune V., Georga M., Papadaki A., Debbagh C., Pégorier P., et al. 2022. IMGT® databases, related tools and web resources through three main axes of research and development. Nucleic Acids Res. 50(D1): D1262–D1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. R Core Team . 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 68. Kassambara, A., Kosinski M., Biecek P.. 2021. survminer: drawing survival curves using ‘ggplot2’. R package version 0.4.9. R Foundation for Statistical Computing, Vienna, Austria. https://cran.r-project.org/web/packages/survminer/survminer.pdf. [Google Scholar]
- 69. Wickham, H. 2016. ggplot2: elegant graphics for data analysis. Springer-Verlag New York, New York. [Google Scholar]
- 70. Garnier, S., Ross N., Rudis R., Camargo A. P., Sciaini M., Scherer C.. 2021. Rvision: colorblind-friendly color maps for R. R package version 0.6.1. R Foundation for Statistical Computing, Vienna, Austria. https://rdrr.io/cran/viridis/. [Google Scholar]
- 71. Csardi, G., Nepusz T.. 2006. The igraph software package for complex network research. InterJournal. Complex Syst. 1695: 1–9. Available at: http://igraph.org. [Google Scholar]
- 72. Schulz, K. F., Altman D. G., Moher D.. 2010. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J. Pharmacol. Pharmacother. 1: 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Modi, S., Jacot W., Yamashita T., Sohn J., Vidal M., Tokunaga E., Tsurutani J., Ueno N. T., Prat A., Chae Y. S., et al. 2022. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387: 9–20. https://doi.org/10.1056/NEJMoa2203690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dees, E. C., McKinnon K. P., Kuhns J. J., Chwastiak K. A., Sparks S., Myers M., Collins E. J., Frelinger J. A., Van Deventer H., Collichio F., et al. 2004. Dendritic cells can be rapidly expanded ex vivo and safely administered in patients with metastatic breast cancer. Cancer Immunol. Immunother. 53: 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.