Cheng William Hong
Cheng William Hong, MD, MS
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,✉,
Victoria Chernyak
Victoria Chernyak, MD, MS
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Jin-Young Choi
Jin-Young Choi, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Sonia Lee
Sonia Lee, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Chetan Potu
Chetan Potu, BS
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Timoteo Delgado
Timoteo Delgado, BS
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Tanya Wolfson
Tanya Wolfson, MA
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Anthony Gamst
Anthony Gamst, PhD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Jason Birnbaum
Jason Birnbaum, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Rony Kampalath
Rony Kampalath, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Chandana Lall
Chandana Lall, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
James T Lee
James T Lee, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Joseph W Owen
Joseph W Owen, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Diego A Aguirre
Diego A Aguirre, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Mishal Mendiratta-Lala
Mishal Mendiratta-Lala, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Matthew S Davenport
Matthew S Davenport, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
William Masch
William Masch, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Alexandra Roudenko
Alexandra Roudenko, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Sara C Lewis
Sara C Lewis, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Andrea Siobhan Kierans
Andrea Siobhan Kierans, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Elizabeth M Hecht
Elizabeth M Hecht, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Mustafa R Bashir
Mustafa R Bashir, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Giuseppe Brancatelli
Giuseppe Brancatelli, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Michael L Douek
Michael L Douek, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Michael A Ohliger
Michael A Ohliger, MD PhD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
An Tang
An Tang, MD MSc
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Milena Cerny
Milena Cerny, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Alice Fung
Alice Fung, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Eduardo A Costa
Eduardo A Costa, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Michael T Corwin
Michael T Corwin, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
John P McGahan
John P McGahan, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Bobby Kalb
Bobby Kalb, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Khaled M Elsayes
Khaled M Elsayes, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Venkateswar R Surabhi
Venkateswar R Surabhi, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Katherine Blair
Katherine Blair, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Robert M Marks
Robert M Marks, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Natally Horvat
Natally Horvat, MD, PhD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Shaun Best
Shaun Best, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Ryan Ash
Ryan Ash, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Karthik Ganesan
Karthik Ganesan, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Christopher R Kagay
Christopher R Kagay, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Avinash Kambadakone
Avinash Kambadakone, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Jin Wang
Jin Wang, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Irene Cruite
Irene Cruite, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Bijan Bijan
Bijan Bijan, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Mark Goodwin
Mark Goodwin, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Guilherme Moura Cunha
Guilherme Moura Cunha, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Dorathy Tamayo-Murillo
Dorathy Tamayo-Murillo, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Kathryn J Fowler
Kathryn J Fowler, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1,
Claude B Sirlin
Claude B Sirlin, MD
1From the Department of Radiology and Biomedical Imaging, University of California San Francisco, 513 Parnassus Ave, S255, Box 0628, San Francisco, CA 94143 (C.W.H., M.A.O.); Liver Imaging Group, Department of Radiology, University of California, San Diego, San Diego, Calif (C.W.H., C.P., T.D., D.T.M., K.J.F., C.B.S.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (V.C., N.H.); Department of Radiology, Yonsei University, Seoul, South Korea (J.Y.C.); Department of Radiology, University of California Irvine, Orange, Calif (S.L., R.K.); Computational and Applied Statistics Laboratory, University of California San Diego, San Diego, Calif (T.W., A.G.); Department of Radiology, New York University, New York, NY (J.B.); Department of Radiology, University of Florida, Jacksonville, Fla (C.L.); Department of Radiology, University of Kentucky, Lexington, Ky (J.T.L., J.W.O.); Department of Radiology, Fundación Santa Fe de Bogotá, Bogotá, Colombia (D.A.A.); Department of Radiology, University of Michigan, Ann Arbor, Mich (M.M.L., M.S.D., W.M.); Department of Radiology, Allegheny Health Network, Pittsburgh, Pa (A.R.); Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY (S.C.L.); Department of Radiology, New York-Presbyterian/Weill Cornell Medical Center, New York, NY (A.S.K., E.M.H.); Departments of Radiology and Medicine, Duke University Medical Center, New York, NY (M.R.B.); Section of Radiology, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), University Hospital Paolo Giaccone, Palermo, Italy (G.B.); Department of Radiology, University of California Los Angeles, Los Angeles, Calif (M.L.D.); Department of Radiology, Radiation Oncology and Nuclear Medicine, Université de Montréal, Montréal, Canada (A.T., M.C.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.F.); CEDRUL-Centro de Diagnóstico por Imagem, João Pessoa, Brazil (E.A.C.); Department of Radiology, University of California Davis, Sacramento, Calif (M.T.C., J.P.M.); Radiology Limited, Tucson, Ariz (B.K.); Department of Abdominal Imaging, University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E., V.R.S., K.B.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); University of São Paulo/Hospital Sírio-Libanês, São Paulo, Brazil (N.H.); Department of Radiology, University of Kansas, Kansas City, Kan (S.B., R.A.); Sir H. N. Reliance Foundation Hospital and Research Centre, Mumbai, India (K.G.); Department of Radiology, California Pacific Medical Center, San Francisco, Calif (C.R.K.); Department of Radiology, Massachusetts General Hospital, Boston, Mass (A.K.); The 3rd Affiliated Hospital, Sun Yat-sen University, Guangzhou, China (J.W.); Inland Imaging, Spokane, Wash (I.C.); Sutter Medical Group, Sacramento, Calif (B.B.); Austin Health, Melbourne, Australia (M.G.); Department of Radiology, University of Washington, Seattle, Wash (G.M.C.).
1
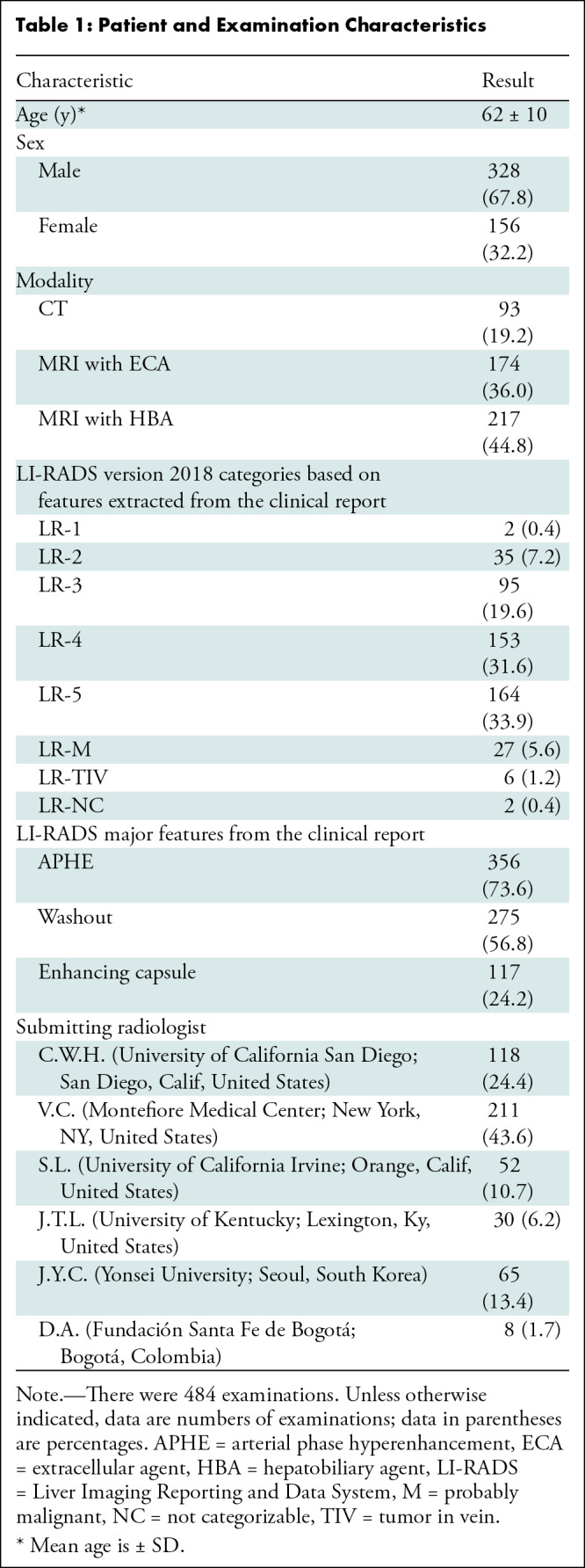
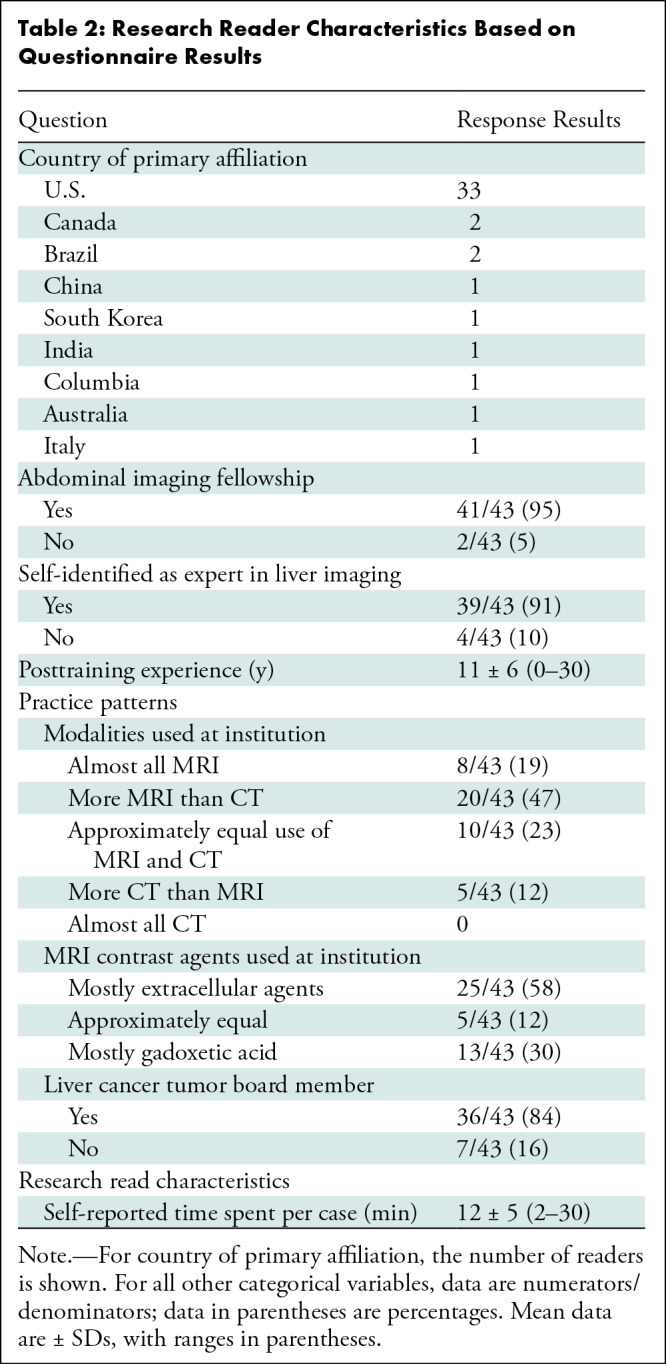
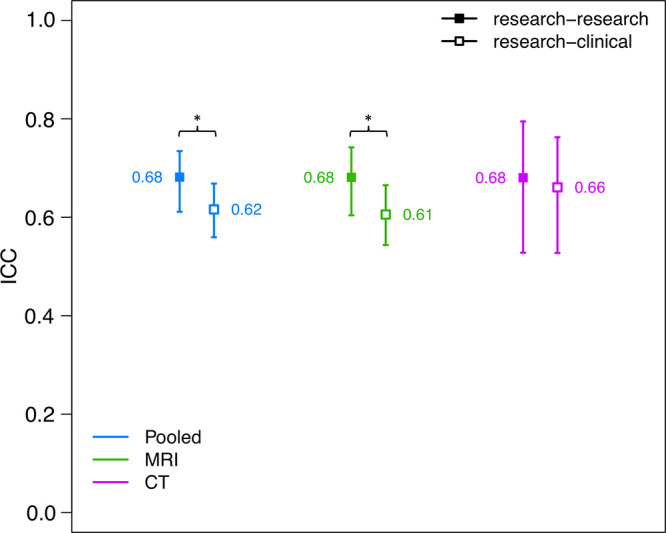
![Plot shows intraclass correlation coefficient (ICC) reader agreement for dichotomized classification of Liver Imaging Reporting and Data System (LI-RADS) version 2018 for the following dichotomized categories: probably or definitely malignant versus other, LR-5 (definitely hepatocellular carcinoma [HCC]) versus other, and LR-M (probably or definitely malignant, not specific for HCC) versus other. Agreement among research reads only (research-research; ■) and between research and clinical reads (research-clinical; □) are shown. Tails represent 95% CIs. * P < .05 by nonparametric bootstrap with per-case resampling. Research-research agreement for malignant categories was better than research-clinical agreement.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/70dc/10315518/9e221d726a19/radiol.222855.fig4.jpg)
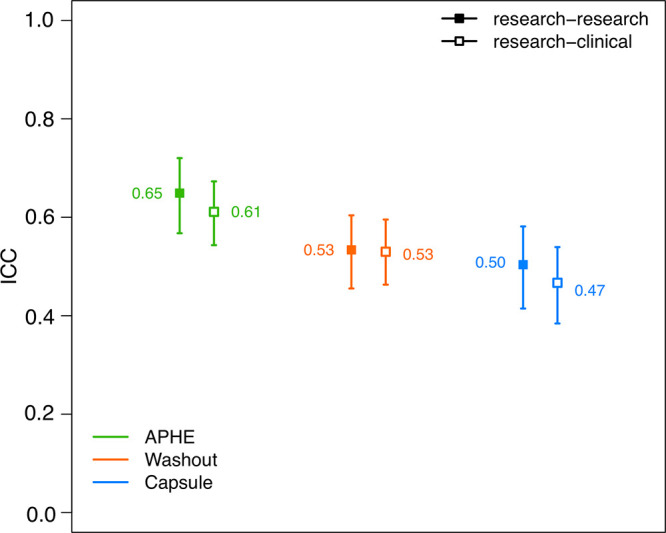
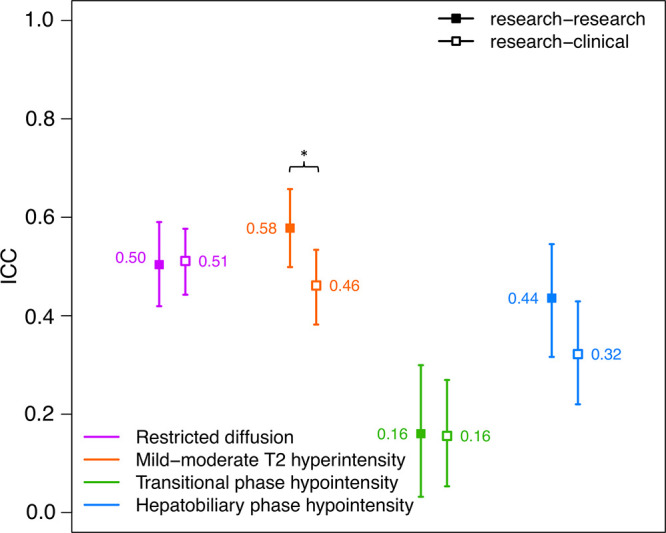



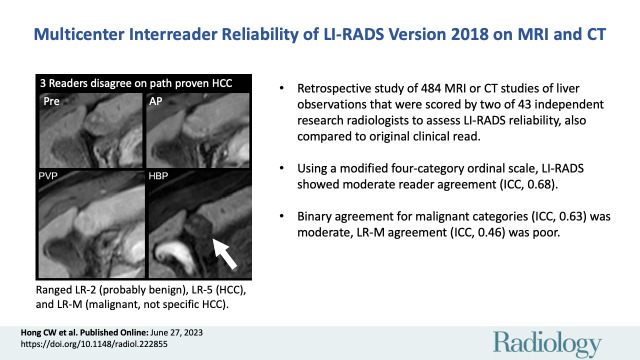
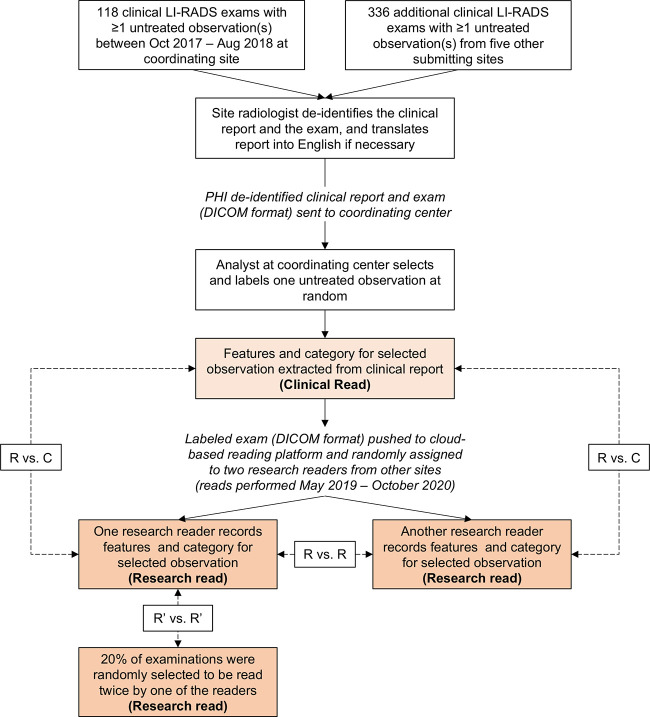
![MRI scans show (A) reader disagreement and (B) reader agreement. (A) Gadoxetic acid–enhanced MRI scans in a 56-year-old male patient with cirrhosis secondary to hepatitis C. From left to right: contrast-unenhanced (Pre), arterial phase (AP), portal venous phase (PVP), and hepatobiliary phase (HBP) images. This 21-mm hepatobiliary phase hypointense observation (arrow) was characterized on the clinical read as having nonrim arterial phase hyperenhancement and washout appearance and was categorized as Liver Imaging Reporting and Data System (LI-RADS) category LR-5 (definitely hepatocellular carcinoma [HCC]). The first research reader characterized it as having a targetoid appearance and categorized it as LR-M (probably or definitely malignant, not specific for HCC). The second research reader characterized it as having no major features and paralleling the blood pool and categorized it as LR-2 (probably benign). It was subsequently resected and found to be a well-differentiated HCC. (B) Extracellular contrast–enhanced MRI scans in a 61-year-old female patient with cirrhosis secondary to hepatitis C. From left to right: contrast-unenhanced, arterial phase, portal venous phase, and delayed-phase (DP) images. This 31-mm observation (arrow) in the caudate lobe was characterized on the clinical read as having arterial phase hyperenhancement, washout appearance, and capsule appearance, and was categorized as LI-RADS category LR-5 (definitely HCC). Both research readers also categorized this observation as LR-5. The patient died of intracranial hemorrhage a few months later.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/70dc/10315518/9d8029290ef8/radiol.222855.fig3.jpg)