Abstract
Background
Sleeve gastrectomy (SG) is effective in the treatment of cardiometabolic complications of obesity but is associated with bone loss.
Purpose
To determine the long-term effects of SG on vertebral bone strength, density, and bone marrow adipose tissue (BMAT) in adolescents and young adults with obesity.
Materials and Methods
This 2-year prospective nonrandomized longitudinal study enrolled adolescents and young adults with obesity who underwent either SG (SG group) or dietary and exercise counseling without surgery (control group) at an academic medical center from 2015 to 2020. Participants underwent quantitative CT of the lumbar spine (L1 and L2 levels) to assess bone density and strength, proton MR spectroscopy to assess BMAT (L1 and L2 levels), and MRI of the abdomen and thigh to assess body composition. Student t and Wilcoxon signed-rank tests were used to compare 24-month changes between and within groups. Regression analysis was performed to evaluate associations between body composition, vertebral bone density, strength, and BMAT.
Results
A total of 25 participants underwent SG (mean age, 18 years ± 2 [SD], 20 female), and 29 underwent dietary and exercise counseling without surgery (mean age, 18 years ± 3, 21 female). Body mass index (BMI) decreased by a mean of 11.9 kg/m2 ± 5.21 [SD] after 24 months in the SG group (P < .001), while it increased in the control group (mean increase, 1.49 kg/m2 ± 3.10; P = .02). Mean bone strength of the lumbar spine decreased after surgery compared with that in control subjects (mean decrease, −728 N ± 691 vs −7.24 N ± 775; P < .001). BMAT of the lumbar spine increased after SG (mean lipid-to-water ratio increase, 0.10 ± 0.13; P = .001). Changes in vertebral density and strength correlated positively with changes in BMI and body composition (R = 0.34 to R = 0.65, P = .02 to P < .001) and inversely with vertebral BMAT (R = −0.33 to R = −0.47, P = .03 to P = .001).
Conclusion
SG in adolescents and young adults reduced vertebral bone strength and density and increased BMAT compared with those in control participants.
Clinical trial registration no. NCT02557438
© RSNA, 2023
See also the editorial by Link and Schafer in this issue.
Summary
Adolescents and young adults with obesity had lower bone strength as assessed with quantitative CT and higher bone marrow adipose tissue as assessed with MR spectroscopy 24 months after sleeve gastrectomy compared with control participants who did not undergo surgery.
Key Results
■ In a 2-year prospective longitudinal study of adolescents and young adults with obesity who underwent sleeve gastrectomy (SG) (n = 25) and those who did not undergo surgery (control group) (n = 29), mean bone strength of the lumbar spine as assessed with quantitative CT decreased after surgery compared with that in control subjects (mean decrease, −728 N ± 691 [SD] vs −7.24 N ± 775; P < .001).
■ Mean lumbar bone marrow adipose tissue as assessed with MR spectroscopy increased after SG (baseline lipid-to-water ratio, 0.38 ± 0.15; 24-month lipid-to-water ratio, 0.48 ± 0.19; P = .001).
Introduction
Obesity in children and adolescents has increased in the recent past (1). Childhood obesity places a large economic burden on the health care system and is associated with a wide spectrum of cardiometabolic diseases, such as type 2 diabetes mellitus and arterial hypertension (1–3). Children and adolescents who are obese are more likely to be obese as adults (4). Furthermore, most metabolic conditions associated with obesity are reversible if obesity is treated in the short term, but they cause irreversible impairment of organ function when obesity is present over a longer period (5). To prevent these long-term consequences, metabolic and bariatric surgery (MBS) is the most effective treatment for long-lasting weight loss and reduction of obesity-related comorbidities in adults (6). For adolescents, the Teen–Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study showed similar effects as MBS in adolescents who underwent sleeve gastrectomy (SG) and were observed for up to 5 years after surgery. The success of SG in this cohort was comparable to that in adults, as was a reversal of obesity-induced comorbidities (7). Thus, SG and other types of MBS are increasingly performed in adolescents and young adults.
While MBS has beneficial effects on cardiometabolic health, it may be detrimental to the skeleton. Sleeve gastrectomy reduces gastric volume, thereby affecting hormone secretion, which in turn can influence bone health (8,9), with an increased risk and prevalence of fractures in adults (10,11). Some studies have investigated the effects of SG and other types of MBS on bone health in adolescents and young adults (12–14). In general, MBS causes reductions in bone mineral density (BMD), measured with dual-energy x-ray absorptiometry (12), and impairment in bone microarchitecture of the appendicular skeleton (14).
Advances in CT, such as biomechanical CT, use noninvasive techniques like finite element analysis to estimate bone strength from quantitative CT scans. This type of analysis enables prediction of fracture risk in older adults or adults with chronic diseases (15–17). Also, a fat depot that has attracted active investigation over the past decade is bone marrow adipose tissue (BMAT), a biomarker for skeletal integrity and metabolic risk (18,19). Studies have shown that BMAT increases 1 year after MBS (20,21). BMAT can be assessed noninvasively using proton MR spectroscopy (18), which allows accurate longitudinal investigations. However, to our knowledge, at present, no longer-term studies investigating the effect of MBS on bone strength with biomechanical CT or on BMAT with proton MR spectroscopy have been performed in adolescents or young adults with obesity.
The hypothesis was that SG would lead to a decrease in vertebral bone density and strength and an increase in BMAT compared with control participants. Thus, the purpose of this study was to investigate the 2-year effects of SG on vertebral bone strength and BMAT in adolescents and young adults.
Materials and Methods
This prospective longitudinal study was performed at an academic medical center. The study was approved by the institutional review board and conducted in compliance with Health Insurance Portability and Accountability regulations. Written informed consent or assent was obtained from all study participants or their legal guardians. In a prior study, baseline vertebral BMD and BMAT were reported for 52 of the 54 participants (21). In the current study, biomechanical CT analysis and 24-month BMAT data are reported. The study was registered on ClinicalTrials.gov as NCT02557438.
Participants
Adolescents and adults (age range, 13–24 years) with moderate to severe obesity (body mass index [BMI] ≥ 35 kg/m2 for adults; BMI ≥ 120% of the age- and sex-specific 95th percentile for adolescents) were enrolled from 2015 to 2020. Two nonrandomized groups were defined. The surgical group included participants who intended to undergo sleeve gastrectomy (SG), with at least one obesity-related comorbidity or a BMI of 40 kg/m2 or higher. The second group comprised participants who did not plan to undergo SG but who received dietary and exercise counseling. For this analysis, the presence of a quantitative CT study at baseline and again at 24 months was required. Exclusion criteria for both groups were pregnancy, history of medical disorders or use of medications affecting bone metabolism, history of smoking (>10 cigarettes per day), history of substance abuse per the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V), and body weight of more than 200 kg due to weight limitations of our equipment.
All study participants underwent physical examination, blood tests, and imaging at baseline (before surgery in the SG group) and at 24 months. Self-reported race and ethnicity were documented. All participants were advised to take supplemental vitamin D throughout the study. In addition, the following recommendations were made based on 25-hydroxyvitamin D levels (21–30 ng/mL [52.4–74.9 nmol/L], 4000 IU per day; 12–20 ng/mL [29.9–49.9 nmol/L], 50 000 IU per week for 2 months; <12 ng/mL [<29.9 nmol/L], 50 000 IU per week for 3 months followed by a maintenance dose).
Physical Examination and Blood Panel
Physical examination included assessment of Tanner stage at baseline and assessment of BMI and fasting 25-hydroxyvitamin D, calcium, phosphorus, and parathyroid hormone levels at both visits.
Biomechanical CT
Helical noncontrast CT of the L1 and L2 vertebrae was performed with a clinical scanner (Lightspeed Pro; GE Healthcare), with participants in the supine position and with the following scan parameters: tube voltage of 120kV, tube current of 100mA, section thickness of 2.5 mm, section interval of 2.5 mm, field of view of 500 mm, table height of 144 mm, CT dose index volume of 6.91 mGy, and dose-length product of 87.6 mGy · cm. All scans were performed with a calibration phantom (Mindways Software).
Vertebral bone density and biomechanical properties were assessed with validated Food and Drug Administration–approved proprietary software (VirtuOst; O.N. Diagnostics) (16). The following variables were collected as averages from the level of the L1 and L2 vertebrae: (a) vertebral compressive strength (in Newtons), determined as the force required to virtually fracture the participant’s lumbar spine under compressive loading conditions; (b) vertebral bending stiffness (in kilonewton meters per radian), determined by subjecting the vertebra to an anterior-posterior bending moment, with greater stiffness representing greater resistance to bending forces (22); and (c) volumetric BMD (in milligrams per cubic centimeter) of the entire vertebral body (integral BMD), the trabecular bone in the vertebral center, and the cortical bone compartment (defined as the outer 2 mm of bone in the vertebral body).
Proton MR Spectroscopy
Proton MR spectroscopy of the lumbar spine was performed after an overnight fast with a 3-T scanner (Siemens Trio; Siemens Healthineers). A point-resolved spatially localized spectroscopy pulse sequence was used with and without water suppression (repetition time msec/echo time msec, 3000/30; eight acquisitions; 1024 data points; receiver bandwidth, 1000 Hz) with fixed voxel size. Voxels were placed in anterior L1 and L2 vertebral bodies, avoiding cortical bone and the posterior venous plexus. Automated optimization of gradient shimming was performed for each voxel placement (23). Acquisition time was 2 minutes 24 seconds. Measurements are reported as averages of measurements of the L1 and L2 vertebral bodies.
Fitting of proton MR spectroscopy data was performed using LCModel (version 6.3–0 K; Stephen Provencher). Metabolites were quantified using eddy current correction and water scaling. A customized fitting algorithm for bone marrow analysis provided estimates for all lipid signals combined (0.9, 1.3, 1.6, 2.3, 5.2, and 5.3 ppm) scaled to unsuppressed water at 4.7 ppm and expressed as lipid-to-water ratios
All image acquisitions and analyses were performed blinded to the group assignment and under the supervision of a musculoskeletal radiologist with 17 years of experience (M.A.B.).
MRI
Participants underwent single-section imaging (T1-weighted fast spin-echo pulse sequence; repetition time msec/echo time msec, 300/12; section thickness, 10 mm; echo train length, four; matrix, 512 × 512) of the abdomen at the level of the L4 vertebra and of the midthigh for body composition assessment. Acquisition time was 42 seconds. Cross-sectional areas of abdominal visceral and subcutaneous adipose tissue and of the muscles at the midthigh level were determined with commercial software (VITRAK; Merge/eFilm).
Statistical Analyses
Statistical analyses were performed using JMP Statistical Software (version 15; SAS Institute) (F.H., M.A.B.). All variables were tested for normality of distribution using the Wilk-Shapiro test. The SG and control groups were compared at baseline and 24 months using the Student t test or Wilcoxon rank-sum test for continuous variables (depending on data distribution) and the χ2 test for categorical variables. With 54 participants, the study was powered at 90% to detect a 2.6% difference between groups for 24-month changes in bone parameters at an α level of .05 based on an estimated SD of change of 2.85%. The 24-month changes within groups were assessed using the paired Wilcoxon signed-rank test. As BMI was different between groups at baseline, multivariable analysis was used to assess differences between groups after controlling for baseline BMI. In addition, potential confounders of the study groups were controlled for by using a multivariate fit model controlled for sex, race, and age. To determine weight loss–independent effects of SG on bone, analyses were also controlled for 24-month changes in BMI. Correlations between biomechanical CT parameters and BMAT were determined using nonparametric Spearman correlation. Data for continuous variables are presented as means ± SDs, and data for categorical variables are presented as frequencies and percentages. P < .05 was considered indicative of a significant difference.
Results
Baseline Participant Characteristics
A total of 54 participants completed quantitative CT at baseline and 24 months (SG group: n = 25; mean age, 18 years ± 2; 20 female; control group: n = 29; mean age, 18 years ± 3; 21 female) and comprised the study group (Fig 1). Age, sex, race distribution, serum bone parameters, body composition, quantitative CT parameters, and BMAT at baseline were comparable between groups (Table 1).
Figure 1:
Study flow diagram.
Table 1:
Participant Characteristics at Baseline
Body Composition and Serum Bone Parameters
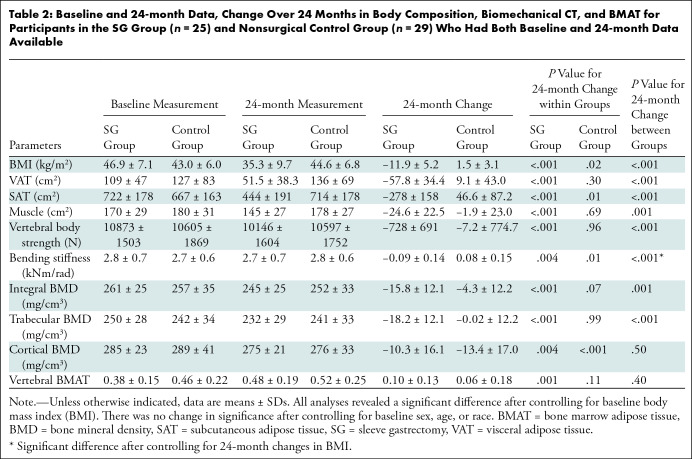
On average, participants in the SG group reduced their BMI by 11.9 kg/m2 ± 5.21 in the 24 months after surgery (P < .001), whereas there was an average increase in BMI in the control group of 1.49 kg/m2 ± 3.10 (P = .02) (between-group difference, P < .001). Accordingly, abdominal visceral adipose tissue decreased in the SG group (mean decrease, −57.83 cm2 ± 34.40) but increased in the control group (mean increase, 9.07 cm2 ± 43.00; P < .001), and subcutaneous adipose tissue decreased in the SG group (mean decrease, −278.17 cm2 ± 158.26, P < .001) but increased in control group (mean increase, 46.59 cm2 ± 87.24; P = .01). Thigh muscle decreased within the SG group (mean decrease, −24.60 cm2 ± 22.49; P < .001) and compared with controls (P < .001). All between-group 24-month changes remained significant after controlling for baseline BMI but lost significance after controlling for 24-month change in BMI (Table 2).
Table 2:
Baseline and 24-month Data, Change Over 24 Months in Body Composition, Biomechanical CT, and BMAT for Participants in the SG Group (n = 25) and Nonsurgical Control Group (n = 29) Who Had Both Baseline and 24-month Data Available
Phosphorus levels increased in the SG group after 24 months (mean increase, 0.27 mg/dL ± 0.43 [0.09 mmol/L ± 0.14]; P = .01). No 24-month changes in calcium (SG group, 0.05 mg/dL ± 0.46 [0.01 mmol/L ± 0.12]; control group, −0.03 mg/dL ± 0.40 [−0.01 mmol/L ± 0.1]; P = .50), vitamin D (SG group, 4.82 ng/mL ± 9.66 [12.03 nmol/L ± 24.11]; control group, −0.19 ng/dL ± 10.25 [−0.47 nmol/L ± 25.58]; P = .08), and parathyroid hormone (SG group, 2.64 ng/L ± 13.08; control group, 3.96 ng/L ± 10.98; P = .72) levels were observed between groups.
Biomechanical CT Parameters
Forty-six participants had matching L1 and L2 levels at baseline and 24 months. In four patients and one patient, the T12 and L1 levels and the L2 and L3 levels, respectively, were scanned at baseline and 24 months due to transitional anatomy. In three participants, only the L2 vertebra was investigated due to a mismatch between lumbar levels imaged at baseline and at the 24-month visit.
Bone strength decreased by an average of −728 N ± 691 within the SG group (P < .001) (Fig 2), without evidence of a change among control participants (mean decrease, −7.24 N ± 775, P = .96). Trabecular BMD decreased in the SG group (mean decrease, −18.2 mg/cm3 ± 12.1; P < .001) without evidence of a change in the control group (mean decrease, −0.02 mg/cm3 ± 12.2; P = .99). Moreover, bending stiffness, integral BMD, and cortical BMD decreased from the baseline to 24-month visit in both groups (Table 2). The between-group 24-month changes remained significant after controlling for baseline BMI; however, they lost significance after controlling for 24-month change in BMI, except for bending stiffness (P = .03), suggesting that the reductions in biomechanical parameters are primarily driven by weight loss.
Figure 2:
Biomechanical noncontrast CT analysis of the L1 vertebra in an 18-year-old woman with severe obesity (body mass index [BMI], 48.4 kg/m2) prior to sleeve gastrectomy (SG) and 24 months after surgery (BMI, 26.6 kg/m2). L1 vertebra was loaded to 9820 N at both visits for comparison purposes. Breaking strength was (A) 11 920 N at baseline prior to SG and (B) 9820 N at 24 months after surgery. Cutout views of the finite element models under compressive load depict the distribution of bone mineral density (black and white areas) and bone failure (colored areas). Red indicates tissue that failed earlier during the compressive load (weaker bone). Shades of gray indicate different bone densities, with white being dense bone and black being little or no bone mineral.
Bone Marrow Adipose Tissue
All proton MR spectroscopy examinations were of sufficient quality for analysis. Total BMAT of the lumbar spine increased within the SG group (mean lipid-to-water ratio, 0.10 ± 0.13; P = .001) (Fig 3) without evidence of a difference between groups (P = .40) (Table 2).
Figure 3:
Proton MR spectroscopy of the L1 vertebra to assess bone marrow adipose tissue (BMAT) in an 18-year-old woman with severe obesity (body mass index [BMI], 42.1 kg/m2) (A) prior to sleeve gastrectomy and (B) 24 months after surgery (BMI, 28.5 kg/m2) show an increase in BMAT content (0.86 lipid-to-water ratio before surgery vs 1.40 lipid-to-water ratio after surgery).
Correlation between Biomechanical CT and BMAT Changes
Changes in vertebral strength, bending stiffness, and integral and trabecular BMD correlated positively with changes in BMI, visceral and abdominal subcutaneous adipose tissue, and muscle (R = 0.34 to R = 0.65, P = .02 to P < .001) and inversely with vertebral BMAT (R = −0.33 to R = −0.47, P = .001 to P = .03) (Table 3). These findings indicate that the greatest decreases in bone biomechanical properties occurred in participants with the largest reduction in BMI and in those who lost the most abdominal fat and muscle (Fig 4). There was no evidence of associations between 24-month changes in body composition and 24-month changes in cortical BMD (Table 3).
Table 3:
Correlations between 24-month Changes in Vertebral Density and Strength Parameters and Body Composition and BMAT
Figure 4:
Regression analyses between 24-month changes in vertebral bone strength and body mass index (BMI) and vertebral bone marrow adipose tissue (BMAT). Analyses show (A) a positive correlation between change in bone strength and change in BMI and (B) an inverse correlation between change in bone strength and change in BMAT.
Discussion
This study aimed to investigate the long-term effects of sleeve gastrectomy on bone strength in adolescents and young adults with obesity, as measured with biomechanical CT and proton MR spectroscopy, and to compare them with nonsurgical control participants.
Our findings were as follow: (a) vertebral strength was reduced 24 months after SG as compared with that in control participants (SG group, −728 N ± 691; control group, −7.24 N ± 775; P < .001), (b) SG led to an increase in BMAT (mean lipid-to-water ratio, 0.10 ± 0.13; P = .001), and (c) 24-month changes in vertebral BMAT showed an inverse association with 24-month changes in bone biomechanical properties (R = −0.33 to R = −0.47, P = .001 to P = .03), enabling us to confirm negative effects of BMAT on the skeleton.
While biomechanical parameters decreased in the SG group, there was no change in the control group (P ≤ .001). We did not observe an increase in trabecular and cortical bone parameters or strength estimates in the control group, which would be expected in adolescence, which is a critical time for bone accrual (9). The lack of change in the control group implies that obesity itself has negative effects on bone in adolescents, preventing the expected bone accrual in this adolescent population. Participants with the greatest weight, fat, and muscle loss were those with the greatest declines in vertebral bone density and biomechanical properties.
The impact of mechanic loading on vertebral bone is well known, and muscle especially has positive effects on BMD and bone strength (24). Of note, the reductions in biomechanical CT parameters lost significance after controlling for the 24-month change in BMI, suggesting that mechanical unloading in the SG group was the main mechanism for reduction in bone biomechanical properties. However, the significant reduction in bending stiffness after SG remained significant after controlling for change in BMI, suggesting that this parameter is influenced by factors other than mechanical unloading.
SG was effective in causing sustained weight loss, with participants experiencing an average decrease in BMI of 12 kg/m2, while participants in the nonsurgical control group did not benefit from diet and exercise counseling with respect to weight loss and demonstrated a BMI increase of about 1.5 kg/m2 over the study duration (P < .001).
Childhood obesity negatively affects quality of life and leads to reduced life expectancy (1,2,25,26). In children with severe obesity, MBS is the most effective means to achieve weight loss, with SG being the most common procedure in this age group (6). However, our study also highlights the known negative effects of MBS on bone health (10,13). While most studies examining bone health after MBS in adolescents have assessed changes in dual-energy x-ray absorptiometry measures of areal bone density or used high-resolution peripheral quantitative CT to assess changes in the peripheral skeleton (radius and tibia) over a year (less frequently, 2 years), we assessed effects of SG on the axial skeleton (spine) using quantitative CT 2 years after surgery and compared these changes with those in a group of nonsurgical control participants (ie, adolescents with moderate to severe obesity of similar age, who were followed without surgery). The longer-term assessment and inclusion of a nonsurgical control group are relevant, given the importance of the pubertal years in accruing bone for the attainment of peak bone mass (9).
We performed detailed CT-based analysis of biomechanical properties of the lumbar vertebrae, which revealed significant reductions of all relevant surrogates of biomechanical properties, including vertebral strength and bending stiffness. Biomechanical parameters and BMD, as well as the microenvironment, affect skeletal integrity. In that regard, BMAT has received attention as an imaging biomarker of skeletal integrity and metabolic risk. High BMAT has been shown to be a risk factor for fracture and a negative predictor of bone strength (19). In our study, BMAT of the lumbar spine increased 24 months after SG and was inversely associated with strength parameters, enabling us to confirm the negative impact of BMAT on skeletal integrity.
As bariatric surgery becomes more frequent in adolescents, its effect on bone health needs to be emphasized, especially to the physicians who will continue to provide routine medical care to these patients. Raising awareness about the importance of bone health will allow for monitoring and management of low bone mass, optimal dietary supplementation with vitamin D and calcium, and initiation of appropriate therapy, if necessary. Moreover, the observed effects of MBS on BMAT might represent a target for therapies. Most important, our results suggest that adolescents are susceptible to bone loss after SG in a similar way as adults, who are known to experience deterioration of bone quality after a period of weight loss (27).
Our study had limitations. First, we did not randomly assign participants to the SG or control group. Thus, study participants in the SG group had a higher BMI at baseline. Thus, we controlled all analyses for baseline BMI. Second, our study focused on parameters impacting long-term to lifetime fracture risk; however, our follow-up period was only 24 months.
In conclusion, our 24-month prospective longitudinal study showed that sleeve gastrectomy in adolescents and young adults impairs bone health, with a reduction in biomechanical bone properties and an increase in lumbar bone marrow adipose tissue (BMAT) when compared with control participants who did not undergo surgery. These changes were largely explained by reductions in body mass index. A longer-term study based on clinical outcomes, such as fractures, would be required to further investigate the impact of reduction in biomechanical bone parameters and increase in BMAT on fracture risk.
Acknowledgments
Acknowledgments
The authors thank David Lee, PhD, and Tony M. Keaveny, PhD, for their help with the analysis of biomechanical CT scans.
Supported by the National Institutes of Health (grants K23DK110419 and P30DK057521 [V.S.], K24HD071843 [M.M.], R01 DK103946 [M.M., M.A.B.], and K24DK109940 [M.A.B.]).
Data sharing: Data generated or analyzed during the study are available from the corresponding author by request.
Disclosures of conflicts of interest: F.A.H. No relevant relationships. V.S. No relevant relationships. S.T. No relevant relationships. I.B. No relevant relationships. A.P.L.L. No relevant relationships. M.L.B. President of the American Society for Bone and Mineral Research. M.M. Royalties from UpToDate; consulting fees from Sanofi and Abbvie; honorarium from International Consortium of Eating Disorders; support for travel, lodging, and food at the 2023 PESTOLA meeting and the 2023 International Meeting of Pediatric Endocrinology from the respective societies; President and board member of the Pediatric Endocrine Society; editor-in-chief for the American Board of Pediatrics. M.A.B. No relevant relationships.
Abbreviations:
- BMAT
- bone marrow adipose tissue
- BMD
- bone mineral density
- BMI
- body mass index
- MBS
- metabolic and bariatric surgery
- SG
- sleeve gastrectomy
References
- 1. Skinner AC , Ravanbakht SN , Skelton JA , Perrin EM , Armstrong SC . Prevalence of Obesity and Severe Obesity in US Children, 1999-2016 . Pediatrics 2018. ; 141 ( 3 ): e20173459 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Cesare M , Sorić M , Bovet P , et al . The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action . BMC Med 2019. ; 17 ( 1 ): 212 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finkelstein EA , Graham WC , Malhotra R . Lifetime direct medical costs of childhood obesity . Pediatrics 2014. ; 133 ( 5 ): 854 – 862 . [DOI] [PubMed] [Google Scholar]
- 4. Abdullah A , Peeters A , de Courten M , Stoelwinder J . The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies . Diabetes Res Clin Pract 2010. ; 89 ( 3 ): 309 – 319 . [DOI] [PubMed] [Google Scholar]
- 5. Stanford FC , Mushannen T , Cortez P , et al . Comparison of Short and Long-Term Outcomes of Metabolic and Bariatric Surgery in Adolescents and Adults . Front Endocrinol (Lausanne) 2020. ; 11 : 157 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Griggs CL , Perez NP Jr , Goldstone RN , et al . National Trends in the Use of Metabolic and Bariatric Surgery Among Pediatric Patients With Severe Obesity . JAMA Pediatr 2018. ; 172 ( 12 ): 1191 – 1192 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inge TH , Courcoulas AP , Jenkins TM , et al . Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents . N Engl J Med 2016. ; 374 ( 2 ): 113 – 123 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Angrisani L , Santonicola A , Iovino P , et al . Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014 . Obes Surg 2017. ; 27 ( 9 ): 2279 – 2289 . [Published correction appears in Obes Surg 2017;27(9):2290-2292.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gordon CM , Zemel BS , Wren TA , et al . The Determinants of Peak Bone Mass . J Pediatr 2017. ; 180 : 261 – 269 . [DOI] [PubMed] [Google Scholar]
- 10. Saad RK , Ghezzawi M , Habli D , Alami RS , Chakhtoura M . Fracture risk following bariatric surgery: a systematic review and meta-analysis . Osteoporos Int 2022. ; 33 ( 3 ): 511 – 526 . [DOI] [PubMed] [Google Scholar]
- 11. Schwartz AV , Sigurdsson S , Hue TF , et al . Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults . J Clin Endocrinol Metab 2013. ; 98 ( 6 ): 2294 – 2300 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beamish AJ , Gronowitz E , Olbers T , Flodmark CE , Marcus C , Dahlgren J . Body composition and bone health in adolescents after Roux-en-Y gastric bypass for severe obesity . Pediatr Obes 2017. ; 12 ( 3 ): 239 – 246 . [DOI] [PubMed] [Google Scholar]
- 13. Kaulfers AM , Bean JA , Inge TH , Dolan LM , Kalkwarf HJ . Bone loss in adolescents after bariatric surgery . Pediatrics 2011. ; 127 ( 4 ): e956 – e961 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Misra M , Singhal V , Carmine B , et al . Bone outcomes following sleeve gastrectomy in adolescents and young adults with obesity versus non-surgical controls . Bone 2020. ; 134 : 115290 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allaire BT , Lu D , Johannesdottir F , et al . Prediction of incident vertebral fracture using CT-based finite element analysis . Osteoporos Int 2019. ; 30 ( 2 ): 323 – 331 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keaveny TM , Clarke BL , Cosman F , et al . Biomechanical Computed Tomography analysis (BCT) for clinical assessment of osteoporosis . Osteoporos Int 2020. ; 31 ( 6 ): 1025 – 1048 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang X , Sanyal A , Cawthon PM , et al . Prediction of new clinical vertebral fractures in elderly men using finite element analysis of CT scans . J Bone Miner Res 2012. ; 27 ( 4 ): 808 – 816 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jarraya M , Bredella MA . Clinical imaging of marrow adiposity . Best Pract Res Clin Endocrinol Metab 2021. ; 35 ( 4 ): 101511 . [DOI] [PubMed] [Google Scholar]
- 19. Pachón-Peña G , Bredella MA . Bone marrow adipose tissue in metabolic health . Trends Endocrinol Metab 2022. ; 33 ( 6 ): 401 – 408 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bredella MA , Greenblatt LB , Eajazi A , Torriani M , Yu EW . Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue . Bone 2017. ; 95 : 85 – 90 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bredella MA , Singhal V , Hazhir Karzar N , et al . Effects of Sleeve Gastrectomy on Bone Marrow Adipose Tissue in Adolescents and Young Adults with Obesity . J Clin Endocrinol Metab 2020. ; 105 ( 11 ): e3961 – e3970 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crawford RP , Keaveny TM . Relationship between axial and bending behaviors of the human thoracolumbar vertebra . Spine 2004. ; 29 ( 20 ): 2248 – 2255 . [DOI] [PubMed] [Google Scholar]
- 23. Bredella MA , Gill CM , Gerweck AV , et al . Ectopic and serum lipid levels are positively associated with bone marrow fat in obesity . Radiology 2013. ; 269 ( 2 ): 534 – 541 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu EW . Bone metabolism after bariatric surgery . J Bone Miner Res 2014. ; 29 ( 7 ): 1507 – 1518 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caprio S , Santoro N , Weiss R . Childhood obesity and the associated rise in cardiometabolic complications . Nat Metab 2020. ; 2 ( 3 ): 223 – 232 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gibson-Smith D , Halldorsson TI , Bot M , et al . Childhood overweight and obesity and the risk of depression across the lifespan . BMC Pediatr 2020. ; 20 ( 1 ): 25 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lindeman KG , Rushin CC , Cheney MC , Bouxsein ML , Hutter MM , Yu EW . Bone Density and Trabecular Morphology at Least 10 Years After Gastric Bypass and Gastric Banding . J Bone Miner Res 2020. ; 35 ( 11 ): 2132 – 2142 . [DOI] [PubMed] [Google Scholar]






![Biomechanical noncontrast CT analysis of the L1 vertebra in an 18-year-old woman with severe obesity (body mass index [BMI], 48.4 kg/m2) prior to sleeve gastrectomy (SG) and 24 months after surgery (BMI, 26.6 kg/m2). L1 vertebra was loaded to 9820 N at both visits for comparison purposes. Breaking strength was (A) 11 920 N at baseline prior to SG and (B) 9820 N at 24 months after surgery. Cutout views of the finite element models under compressive load depict the distribution of bone mineral density (black and white areas) and bone failure (colored areas). Red indicates tissue that failed earlier during the compressive load (weaker bone). Shades of gray indicate different bone densities, with white being dense bone and black being little or no bone mineral.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6bb3/10315522/d27988e57425/radiol.223256.fig2.jpg)
![Proton MR spectroscopy of the L1 vertebra to assess bone marrow adipose tissue (BMAT) in an 18-year-old woman with severe obesity (body mass index [BMI], 42.1 kg/m2) (A) prior to sleeve gastrectomy and (B) 24 months after surgery (BMI, 28.5 kg/m2) show an increase in BMAT content (0.86 lipid-to-water ratio before surgery vs 1.40 lipid-to-water ratio after surgery).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6bb3/10315522/e68ab888d55b/radiol.223256.fig3.jpg)

