Abstract
Background
MYCN-amplified RB1 wild-type (MYCNARB1+/+) retinoblastoma is a rare but clinically important subtype of retinoblastoma due to its aggressive character and relative resistance to typical therapeutic approaches. Because biopsy is not indicated in retinoblastoma, specific MRI features might be valuable to identify children with this genetic subtype.
Purpose
To define the MRI phenotype of MYCNARB1+/+ retinoblastoma and evaluate the ability of qualitative MRI features to help identify this specific genetic subtype.
Materials and Methods
In this retrospective, multicenter, case-control study, MRI scans in children with MYCNARB1+/+ retinoblastoma and age-matched children with RB1−/− subtype retinoblastoma were included (case-control ratio, 1:4; scans acquired from June 2001 to February 2021; scans collected from May 2018 to October 2021). Patients with histopathologically confirmed unilateral retinoblastoma, genetic testing (RB1/MYCN status), and MRI scans were included. Associations between radiologist-scored imaging features and diagnosis were assessed with the Fisher exact test or Fisher-Freeman-Halton test, and Bonferroni-corrected P values were calculated.
Results
A total of 110 patients from 10 retinoblastoma referral centers were included: 22 children with MYCNARB1+/+ retinoblastoma and 88 control children with RB1−/− retinoblastoma. Children in the MYCNARB1+/+ group had a median age of 7.0 months (IQR, 5.0–9.0 months) (13 boys), while children in the RB1−/− group had a median age of 9.0 months (IQR, 4.6–13.4 months) (46 boys). MYCNARB1+/+ retinoblastomas were typically peripherally located (in 10 of 17 children; specificity, 97%; P < .001) and exhibited plaque or pleomorphic shape (in 20 of 22 children; specificity, 51%; P = .011) with irregular margins (in 16 of 22 children; specificity, 70%; P = .008) and extensive retina folding with vitreous enclosure (specificity, 94%; P < .001). MYCNARB1+/+ retinoblastomas showed peritumoral hemorrhage (in 17 of 21 children; specificity, 88%; P < .001), subretinal hemorrhage with a fluid-fluid level (in eight of 22 children; specificity, 95%; P = .005), and strong anterior chamber enhancement (in 13 of 21 children; specificity, 80%; P = .008).
Conclusion
MYCNARB1+/+ retinoblastomas show distinct MRI features that could enable early identification of these tumors. This may improve patient selection for tailored treatment in the future.
© RSNA, 2023
Supplemental material is available for this article.
See also the editorial by Rollins in this issue.
Summary
MYCN-amplified RB1 wild-type retinoblastoma has distinct features compared with RB1 pathogenic variant–driven retinoblastoma at MRI, including tumors in a peripheral (anterior) location with plaque or pleomorphic shape, irregular margins, tumor-retinal folding, and peritumoral blood.
Key Results
■ In this retrospective study of 110 patients, unique MRI features enabled differentiation of patients with MYCN-amplified RB1 wild-type retinoblastoma (MYCNARB1+/+) (n = 22) from patients with RB1 pathogenic variant–driven retinoblastoma (n = 88).
■ MRI features that showed high specificity for MYCNARB1+/+ identification included peripheral location (anteriorly to the equator) (97%; P < .001), peritumoral hemorrhage (88%; P < .001), subretinal hemorrhage with a fluid-fluid level (95%; P = .005), and tumorretinal folding with vitreous enclosure (94%; P < .001).
Introduction
Retinoblastoma is the most common malignant eye tumor in children (incidence of one in 17 000 live births), usually diagnosed before the child reaches 5 years of age (1). Tumorigenesis is predominantly initiated by two genetic events involving both alleles of the tumor suppressor gene RB1 (ie, RB1−/− retinoblastoma). A new molecular subtype of retinoblastoma without pathogenic variants in the RB1 gene (RB1+/+) has been discovered. Instead, a high level of MYCN amplification was found to be the driving force in the initiation of retinal tumors (MYCN-driven or MYCN-amplified RB1 wild-type [MYCNARB1+/+] retinoblastoma) in approximately 1%–2% of patients with retinoblastoma (2–5). Although MYCN amplifications also exist in RB1−/− tumors, the clinical appearance suggests a distinct phenotype for RB1+/+ tumors driven by MYCN amplification (6,7). This MYCNARB1+/+ subtype was found to be a unilateral subtype and manifests with larger tumors in children at a very young age; it showed strikingly distinct histopathologic features with poorly differentiated tumors consisting of neuroblastic cells and few rosettes (2). Additionally, a relative resistance to typical therapeutic approaches was found (8). While eye-preserving treatment is increasingly attempted in the children with MYCNARB1+/+ retinoblastoma, salvaging an eye in this aggressive subtype of retinoblastoma may result in a higher risk of uncontrolled disease. Therefore, early identification of patients with MYCNARB1+/+ retinoblastoma is clinically important and may enable a distinct treatment approach.
Detecting genetic traits of retinoblastoma is increasingly challenging in clinical practice due to the lack of availability of histopathologic material for molecular testing. Tumor biopsy is contraindicated because of the associated risk of local tumor seeding and metastasis. Furthermore, enucleated specimens have become less available due to the increasing use of eye-saving treatment options, even in advanced stages (9,10). Development of noninvasive stratification methods is therefore important for subtype recognition and implementation of novel targeted therapies. The recently emerging minimally invasive technique of obtaining cell-free tumor DNA from the aqueous humor may become advantageous (11). MRI similarly has great potential for molecular subtype differentiation with the advantages of being noninvasive and widely used in children with retinoblastoma for supporting the diagnosis, examining disease extent, and screening for intracranial disease (12–15). The imaging phenotype of MYCNARB1+/+ retinoblastoma, however, remains unclear. The purpose of this study was to assess MRI features of MYCNARB1+/+ retinoblastoma and to evaluate whether this genetic subtype can be differentiated from RB1−/− retinoblastoma using qualitative MRI features.
Materials and Methods
Patients
The institutional review board of Vrije Universiteit University Medical Center (institutional review board no. 00002991) in Amsterdam, the Netherlands, approved this multicenter, retrospective, case-control study; the requirement to obtain informed consent was waived. This study was performed in accordance with the Standards for Reporting of Diagnostic Accuracy Studies, or STARD, statement guidelines.
MRI scans from patients with retinoblastoma were retrospectively collected. MRI scans were acquired between June 2001 and February 2021 and collected between May 2018 and October 2021 from retinoblastoma referral centers. Case identification was performed by addressing both published authors who studied patients with MYCNARB1+/+ retinoblastoma and retinoblastoma referral centers. Six patients (three from Germany and three from the Netherlands) were previously reported in the first report, to our knowledge, on the MYCNARB1+/+ subtype retinoblastoma (2) and one patient (from India) was previously reported in a case report (8). For each identified patient with the MYCNARB1+/+ retinoblastoma subtype, matched controls with RB1−/− retinoblastoma were included at a ratio of 1:4. Inclusion criteria were (a) histopathologically confirmed diagnosis of retinoblastoma, (b) unilateral disease, (c) MRI examination performed after 1995 and including at least noncontrast T1-weighted and gadolinium-based contrast agent–enhanced T1-weighted sequences, and (d) genetic analysis of tumor material: for MYCNARB1+/+ retinoblastomas, this included MYCN amplification and RB1 wild-type expression, and for RB1−/− retinoblastomas, this included biallelic RB1 pathogenic variants. Genetic information was collected retrospectively; no genetic tests were performed for the current study. Case-control matching was performed based on age, date of MRI examination, and referral site. In instances where control MRI examinations from the same institution were unavailable, control MRI examinations from a different institution were used.
MRI Feature Selection and Assessment
MRI features were adopted from a previously validated imaging atlas of retinoblastoma (Table S1) (16). These included tumor morphologic features (location, growth pattern, shape) and tumor composition (homogeneity, calcifications, enhancement, necrosis). Three imaging features were added: (a) peritumoral hemorrhage (focal hypointense signal intensity on T2-weighted images specifically covering the surface of the tumor); (b) tumor-retinal folds with vitreous enclosure; and (c) a detached hyaloid membrane. The three added features were defined after unblinded review by independent readers not involved in further feature scoring (C.M.d.B. and R.W.J., with 4 and 5 years of experience, respectively, in ocular MRI). Subsequently, two radiologists with expertise in retinoblastoma imaging (P.d.G. and M.C.d.J., with 10 and 17 years of experience, respectively, in ocular MRI) blinded to patient details (including age and genetic information) individually assessed all MRI scans for all features. Disagreements were resolved by consensus in a separate meeting. Imaging features that were marked as indeterminate after consensus were excluded from analysis (eg, the feature “subretinal seeding” is indeterminate in cases without retinal detachment). No central pathology review was performed, but histopathologic examinations were reviewed in six patients from the Netherlands to be able to demonstrate radiopathologic correlations.
Statistical Analysis
Interobserver MRI reader variability was assessed by calculating Fleiss κ. Data distribution was assessed using the Shapiro-Wilk test. The two-tailed Fisher exact test and Fisher-Freeman-Halton test were used for the statistical analyses. Bonferroni-corrected P < .05 was considered indicative of statistically significant difference. Statistical calculations were performed using SPSS software (version 26, IBM).
Results
Patients
Of the 110 patients with retinoblastoma included in the study, 22 had the MYCNARB1+/+ molecular subtype and 88 had the RB1−/− molecular subtype. Data for patients with MYCNARB1+/+ retinoblastoma were included from 10 retinoblastoma referral centers in Amsterdam, the Netherlands (n = 6); Paris, France (n = 4); Essen, Germany (n = 3); Phoenix, Arizona, U.S. (n = 2); Portland, Oregon, U.S. (n = 2); Lausanne, Switzerland (n = 1); Atlanta, Georgia, U.S. (n = 1); Iowa City, Iowa, U.S. (n = 1); Memphis, Tennessee, U.S. (n = 1); and Kolkata, India (n = 1). Figure 1 demonstrates the identification and inclusion process for patients in this study. No patients were excluded. Patient demographic characteristics are available in Table 1; the case-control study design resulted in similar groups with no significant differences in patient age or the year of the MRI examination.
Figure 1:
Flowchart diagram shows inclusion of patients in this case-control study with a ratio between cases and controls of 1:4. Cases are patients with MYCN-amplified RB1 wild-type (MYCNARB1+/+) retinoblastoma, and controls are patients with biallelic pathogenic variation of RB1–driven (ie, RB1−/−) retinoblastoma.
Table 1:
Patient Demographic Characteristics
Tumor Characteristics of MYCNARB1+/+ Retinoblastoma
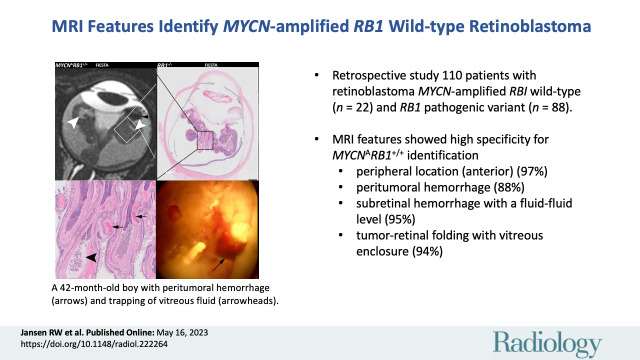
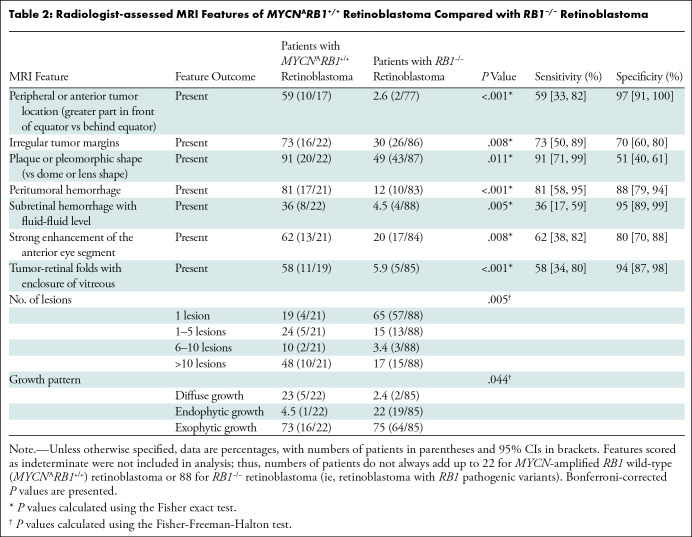
Identified associations between imaging features and the MYCNARB1+/+ subtype of retinoblastoma are listed in Table 2; features scored as indeterminate resulted in patient numbers not always adding up to the total of 22 patients with MYCNARB1+/+ retinoblastoma and 88 patients with RB1−/− retinoblastoma. A peripheral tumor location (anteriorly to the equator) showed a 97% specificity for MYCNARB1+/+ retinoblastoma and was found in 59% of children with MYCNARB1+/+ retinoblastoma (10 of 17) compared with 2.6% of children with RB1−/− retinoblastoma (two of 77) (P < .001) (Fig 1). Extensive retinal folds were found in the MYCNARB1+/+ subtype, in which the tumor-affected retina was seen to enclose parts of the vitreous (Figs 1–3). This MRI feature showed a specificity of 94% for the MYCNARB1+/+ subtype and was present in 58% of children with MYCNARB1+/+ retinoblastoma (11 of 19) versus in 5.9% of children with RB1−/− (five of 85) (P < .001). Another MRI feature associated with the MYCNARB1+/+ subtype was irregular tumor margins, which occurred in 73% of patients with MYCNARB1+/+ (16 of 22) versus 30% of patients with RB1−/− retinoblastoma (26 of 86) (specificity, 70%; P = .008) (Fig 3). MYCNARB1+/+ retinoblastomas more frequently had plaque or pleomorphic shape (in 20 of 22 children [91%]) compared with RB1−/− retinoblastomas (in 43 of 87 children [49%]), with the latter showing relatively more dome- or lens-shaped tumors (P = .011) (Fig 3). Additionally, a larger number of tumor foci (P = .005) and a diffuse infiltrative growth pattern (P = .044) were found to be associated with MYCNARB1+/+ retinoblastoma. Table S1 lists the frequency and interobserver agreement of all assessed imaging features. The frequency of individual imaging features showed a wide range (0%–91%), as did the interobserver agreement (κ = 0.03–0.81).
Table 2:
Radiologist-assessed MRI Features of MYCNARB1+/+ Retinoblastoma Compared with RB1−/− Retinoblastoma
Figure 3:
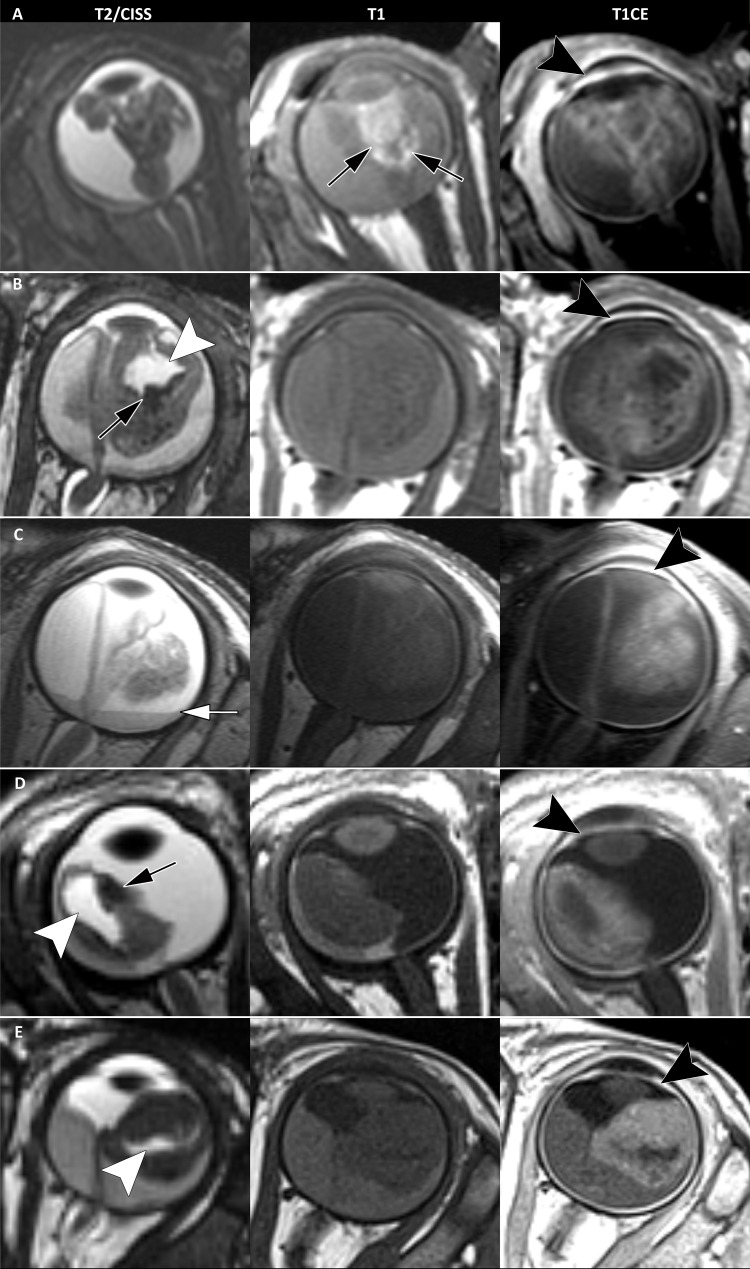
Example T2-weighted constructive interference in steady state (CISS), T1-weighted noncontrast, and T1-weighted contrast-enhanced (CE) images in five children with MYCN-amplified RB1 wild-type (MYCNARB1+/+) retinoblastoma show typical MRI phenotypes in an (A) 11-month-old boy, (B) 4-month-old girl, (C) 6-month-old boy, (D) 3-month-old girl, and (E) 4-month-old girl. Typical MRI features include anterior tumor location, irregular tumor margins, plaque or pleomorphic shape, a diffuse growth pattern, and numerous tumor lesions. Peritumoral hemorrhage is shown (black arrows in A, B, and D). T1 hyperintensity suggests the presence of peritumoral blood in A. Subretinal hemorrhagic fluid-fluid level is shown (white arrow in C). Tumor-retina folding with enclosure of the vitreous is denoted (white arrowheads in B, D, and E). Strong enhancement of the anterior eye segment is present in all five patients (black arrowheads on T1-weighted sequences with gadolinium-based contrast agent).
Figure 2:
MRI phenotype MYCN-amplified RB1 wild-type (MYCNARB1+/+) retinoblastoma versus retinoblastoma driven by RB1 pathogenic variation (RB1−/−). (A) Representative MRI scans in a 42-month-old boy with MYCNARB1+/+ retinoblastoma show relatively anterior tumor location, irregular margins, a pleomorphic shape, tumor-retinal folding with retina-retina contact (arrowheads), and peritumoral blood (arrows). Axial T1-weighted images with and without gadolinium-based contrast agent, T2-weighted image, and subtraction image are included. (B) MRI scan in a 10-month-old girl with RB1−/− retinoblastoma shows a posterior location, smooth margins, and a lens shape without vitreous inclusion or peritumoral blood. Axial T1-weighted images with and without gadolinium-based contrast agent, T2-weighted image, and subtraction image are included. CE = contrast enhanced, FIESTA = fast imaging employing steady-state acquisition.
Peritumoral Intraocular Findings in MYCNARB1+/+ Retinoblastoma
Peritumoral hemorrhage was more often observed at MRI of MYCNARB1+/+ retinoblastoma (in 17 of 21 children [81%]) compared with MRI of RB1−/− retinoblastoma (in 10 of 83 children [12%]) and was also apparent at histopathologic examination in a patient with both MRI and histopathologic data available (Fig 4). Additionally, children with MYCNARB1+/+ retinoblastoma showed more subretinal blood to the extent that there was a fluid-fluid level present (MYCNARB1+/+, eight of 22 children [36%]; RB1−/−, four of 88 children [4.5%]), a finding that showed 95% specificity for MYCN-amplified tumors. Moreover, the MYCN-amplified tumors showed a more intense enhancement within the anterior eye segment (MYCNARB1+/+, in 13 of 21 children [62%]; RB1−/−, in 17 of 84 children [20%]; P = .008) (Fig 3).
Figure 4:
Axial MRI scans (left column), histopathologic images (middle columns), and fundoscopic images (right column) in two children with MYCN-amplified RB1 wild-type (ie, MYCNARB1+/+) retinoblastoma, (A) a 42-month-old boy and (B) 3-month-old girl. MRI scans show peritumoral hemorrhage (black arrows) and retinal detachment with multiple retinal folds with vitreous enclosure (black and white arrowheads). Scale bars of 25 mm are shown in the bottom right corner. Fundoscopic images show extensive peritumoral blood (black arrows, without spatial correlation).
Discussion
Because retinoblastomas driven by MYCN amplification (ie, MYCNARB1+/+) have an aggressive nature and relative resistance to typical chemotherapy approaches, identification of patients with this retinoblastoma subtype is clinically important. As tissue biopsy is not safe in retinoblastoma, we sought to determine whether MRI could be used to identify this rare retinoblastoma subtype. Compared with retinoblastoma driven by biallelic pathogenic variation of RB1 (ie, RB1−/−), MYCNARB1+/+ tumors were recognizable at pretreatment MRI by their peripheral location anteriorly to the equator (specificity, 97%; P < .001), irregular margins (specificity, 70%; P = .008), diffuse growth pattern (P = .044) with many tumor foci (P = .005), peritumoral hemorrhage (specificity, 88%; P < .001), and extensive folding of the affected retina enclosing parts of the vitreous (specificity, 94%; P < .001). These imaging findings could supplement clinical findings, such as unilaterality and young age, for pretreatment recognition of MYCNARB1+/+ retinoblastoma.
The diffuse growth pattern, irregular margins, and multiple tumor foci found at MRI in MYCNARB1+/+ retinoblastoma may suggest rapid tumor growth at a young age. Aggressive tumor growth and young age were also described in clinical evaluations (2). Children with MYCNARB1+/+ retinoblastoma in our study had a median age of 7 months, considerably younger than patient population with unilateral RB1−/− retinoblastoma (approximately 24 months [17]). The finding of a peripheral location anteriorly to the equator at MRI was surprising in these young children, considering retinal development proceeds from center (posterior) to periphery (anterior). Most of the retina was affected in these patients, suggesting either fast tumor growth from posterior to anterior or extensive multifocality. A peripheral location by itself is, unlike the other described features for MYCNARB1+/+ tumors, not a sign of advanced-stage disease in the population with general (RB1−/−) retinoblastoma. The peripheral location may, however, lead to a diagnosis at a relatively advanced stage in the absence of leukocoria as an early symptom, as seen in central tumors. In children with RB1−/− retinoblastoma, tumors originating from the peripheral retina are seen in patients of older age, mostly with an unaffected central (posterior) retina (18). Fast tumor growth is also a possible explanation for other imaging features of MYCNARB1+/+ tumors. For instance, the folding of a tumor-filled retina with vitreous enclosure is probably caused by rapid growth in a convoluting manner, in which one fold of the retina attaches to an opposite fold. In our study, this was confirmed at histopathologic evaluation. These vitreous invaginations were large and often unifocal compared with multifocal smaller cavitations seen in cavitary retinoblastoma (19). Peritumoral hemorrhage and subretinal hemorrhage with fluid-fluid levels could also be related to fast tumor growth. These MRI features are likely due to the tumor outgrowing its blood supply, resulting in hypoxia. Hypoxia induces upregulation of proangiogenic factors, including the vascular endothelial growth factor gene, resulting in the formation of fragile blood vessels that may hemorrhage more easily (20). This may also explain the frequent finding of anterior eye segment enhancement in MYCNARB1+/+ retinoblastoma: poorly developed regions of the blood-ocular barrier possibly result in extravascular leakage of contrast material into the anterior eye chamber (21). We suggest that the majority of imaging findings in patients with MYCNARB1+/+ retinoblastoma reflect the early onset and fast growth of this subtype, which is congruent with clinical, histopathologic, and molecular findings from previously described patients with this retinoblastoma subtype (2,8,22–24).
In addition to supporting the previously established difference in molecular makeup and histopathologic appearance, our study also showed that MYCNARB1+/+ tumors have a unique phenotypic appearance at MRI (2–4,8). This provides further evidence that MYCNARB1+/+ is a distinct retinoblastoma subtype with different behavior from RB1−/−, potentially benefiting from new targeted treatments. In a recent study, a novel therapy (MLN4924 [Pevonedistat, Takeda], a neddylation inhibitor) was tested in human retinoblastoma xenografts in vivo, and chemosensitivity was observed in both MYCNARB1+/+ and RB1−/− tumors (25). Such compounds may be specifically valuable in treatment of MYCNARB1+/+ retinoblastoma, as these tumors have been described to respond poorly to traditional chemotherapeutic regimens (8). Another described approach includes the use of anti-GD2 monoclonal antibodies, since chemorefractory MYCNARB1+/+ retinoblastoma has been shown to express ganglioside GD2 (26). Multiple compounds for MYC inhibition have entered clinical-phase research, although more research is needed to bring therapies from bench to bedside (27). In both research and clinical settings, MRI features can aid in patient selection for targeted treatments against the MYCNARB1+/+ subtype (26).
Our study had several limitations. First, the subtype MYCNARB1+/+ is rare, and available MRI data are limited. We had to acquire data internationally from multiple centers, and MRI protocols and quality varied. Generally, MRI requirements for retinoblastoma include a field strength above 1.5 T, a section thickness of less than 2 mm, a T2-weighted sequence, and T1-weighted sequences before and after intravenous administration of a gadolinium-based contrast agent (13). Second, interreader agreement of imaging features showed a wide range, possibly explained in part by insufficient MRI quality for features requiring detailed evaluation. Third, the low prevalence of the MYCNARB1+/+ subtype resulted in large CIs for prediction parameters for morphologic features.
In conclusion, MYCN-amplified RB1 wild-type retinoblastomas show distinct MRI features that could enable early identification of this more aggressive retinoblastoma subtype.
Initiation and coordination of this study was funded by Stichting Kinderen Kankervrij (KIKA) (grant no. 342) and the Hanarth Foundation (grant for project titled MRI-based Deep Learning Segmentation and Quantitative Radiomics in Retinoblastoma: A Next Step Toward Personalized Interventions). Departmental funding was received by Casey Eye Institute, Oregon Health & Science University (A.H.S., A.K.M., and O.E.U.) from the National Institutes of Health (grant P30 EY010572) in addition to unrestricted departmental funding from Research to Prevent Blindness. Departmental funding was received by Emory Eye Center, Ocular Oncology Service (O.E.U., G.B.H., and H.G.) via National Eye Institute core grant P30 EY006360.
Data sharing: Data generated or analyzed during the study are available from the corresponding author by request.
Disclosures of conflicts of interest: R.W.J. No relevant relationships. C.M.d.B. No relevant relationships. L.C. No relevant relationships. S.G. No relevant relationships. S.v.E. No relevant relationships. J.L.J. No relevant relationships. A.R. No relevant relationships. A.H.S. Consulting fees from Castle Biosciences; payment for lecture at Immunocore educational symposium; support for travel from Immunocore. A.K.M. No relevant relationships. P.M. No relevant relationships. O.E.U. No relevant relationships. G.B.H. No relevant relationships. H.G. No relevant relationships. H.C.B. No relevant relationships. K.E.N. No relevant relationships. R.C.B. Consultant for Aileron Therapeutics. S. Sen No relevant relationships. S. Sirin No relevant relationships. H.J.B. No relevant relationships. P.G. No relevant relationships. C.J.D. No relevant relationships. J.A.C. No relevant relationships. P.v.d.V. No relevant relationships. R.B. No relevant relationships. J.D. No relevant relationships. A.C.M. No relevant relationships. M.C.d.J. No relevant relationships. P.d.G. No relevant relationships.
Abbreviation:
- MYCN A RB1 +/+
- MYCN-amplified RB1 wild-type
References
- 1. Moll AC , Kuik DJ , Bouter LM , et al . Incidence and survival of retinoblastoma in the Netherlands: a register based study 1862-1995 . Br J Ophthalmol 1997. ; 81 ( 7 ): 559 – 562 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rushlow DE , Mol BM , Kennett JY , et al . Characterisation of retinoblastomas without RB1 mutations: genomic, gene expression, and clinical studies . Lancet Oncol 2013. ; 14 ( 4 ): 327 – 334 . [DOI] [PubMed] [Google Scholar]
- 3. Ewens KG , Bhatti TR , Moran KA , et al . Phosphorylation of pRb: mechanism for RB pathway inactivation in MYCN-amplified retinoblastoma . Cancer Med 2017. ; 6 ( 3 ): 619 – 630 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh HP , Shayler DWH , Fernandez GE , et al . An immature, dedifferentiated, and lineage-deconstrained cone precursor origin of N-Myc-initiated retinoblastoma . Proc Natl Acad Sci U S A 2022. ; 119 ( 28 ): e2200721119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blixt MKE , Hellsand M , Konjusha D , et al . MYCN induces cell-specific tumorigenic growth in RB1-proficient human retinal organoid and chicken retina models of retinoblastoma . Oncogenesis 2022. ; 11 ( 1 ): 34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Price EA , Patel R , Scheimberg I , et al . MYCN amplification levels in primary retinoblastoma tumors analyzed by Multiple Ligation-dependent Probe Amplification . Ophthalmic Genet 2021. ; 42 ( 5 ): 604 – 611 . [DOI] [PubMed] [Google Scholar]
- 7. Lillington DM , Goff LK , Kingston JE , et al . High level amplification of N-MYC is not associated with adverse histology or outcome in primary retinoblastoma tumours . Br J Cancer 2002. ; 87 ( 7 ): 779 – 782 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zugbi S , Ganiewich D , Bhattacharyya A , et al . Clinical, genomic, and pharmacological study of MYCN-amplified RB1 wild-type metastatic retinoblastoma . Cancers (Basel) 2020. ; 12 ( 9 ): 2714 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abramson DH , Gobin YP , Francis JH . Orbital retinoblastoma treated with intra-arterial chemotherapy . Ophthalmology 2021. ; 128 ( 10 ): 1437 . [DOI] [PubMed] [Google Scholar]
- 10. Zhou C , Wen X , Ding Y , et al . Eye-preserving therapies for advanced retinoblastoma: a multicenter cohort of 1678 patients in China . Ophthalmology 2022. ; 129 ( 2 ): 209 – 219 . [DOI] [PubMed] [Google Scholar]
- 11. Berry JL , Xu L , Kooi I , et al . Genomic cfDNA analysis of aqueous humor in retinoblastoma predicts eye salvage: the surrogate tumor biopsy for retinoblastoma . Mol Cancer Res 2018. ; 16 ( 11 ): 1701 – 1712 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Jong MC , van der Meer FJ , Göricke SL , et al . Diagnostic accuracy of intraocular tumor size measured with MR imaging in the prediction of postlaminar optic nerve invasion and massive choroidal invasion of retinoblastoma . Radiology 2016. ; 279 ( 3 ): 817 – 826 . [DOI] [PubMed] [Google Scholar]
- 13. de Graaf P , Göricke S , Rodjan F , et al . Guidelines for imaging retinoblastoma: imaging principles and MRI standardization . Pediatr Radiol 2012. ; 42 ( 1 ): 2 – 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Jong MC , Kors WA , de Graaf P , Castelijns JA , Kivelä T , Moll AC . Trilateral retinoblastoma: a systematic review and meta-analysis . Lancet Oncol 2014. ; 15 ( 10 ): 1157 – 1167 . [DOI] [PubMed] [Google Scholar]
- 15. Jansen RW , de Bloeme CM , Brisse HJ , et al . MR imaging features to differentiate retinoblastoma from Coats’ disease and persistent fetal vasculature . Cancers (Basel) 2020. ; 12 ( 12 ): 3592 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jansen RW , de Jong MC , Kooi IE , et al . MR imaging features of retinoblastoma: association with gene expression profiles . Radiology 2018. ; 288 ( 2 ): 506 – 515 . [DOI] [PubMed] [Google Scholar]
- 17. Broaddus E , Topham A , Singh AD . Incidence of retinoblastoma in the USA: 1975-2004 . Br J Ophthalmol 2009. ; 93 ( 1 ): 21 – 23 . [DOI] [PubMed] [Google Scholar]
- 18. King BA , Parra C , Li Y , et al . Spatiotemporal patterns of tumor occurrence in children with intraocular retinoblastoma . PLoS One 2015. ; 10 ( 7 ): e0132932 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raval V , Kaliki S . Cavitary retinoblastoma: a review of literature . Surv Ophthalmol 2022. ; 67 ( 3 ): 723 – 728 . [DOI] [PubMed] [Google Scholar]
- 20. Stitt AW , Simpson DA , Boocock C , Gardiner TA , Murphy GM , Archer DB . Expression of vascular endothelial growth factor (VEGF) and its receptors is regulated in eyes with intra-ocular tumours . J Pathol 1998. ; 186 ( 3 ): 306 – 312 . [DOI] [PubMed] [Google Scholar]
- 21. de Graaf P , van der Valk P , Moll AC , et al . Contrast-enhancement of the anterior eye segment in patients with retinoblastoma: correlation between clinical, MR imaging, and histopathologic findings . AJNR Am J Neuroradiol 2010. ; 31 ( 2 ): 237 – 245 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moulin AP , Stathopoulos C , Marcelli F , Schoumans Pouw J , Beck-Popovic M , Munier FL . Secondary enucleated retinoblastoma with MYCN amplification . Ophthalmic Genet 2021. ; 42 ( 3 ): 354 – 359 . [DOI] [PubMed] [Google Scholar]
- 23. Afshar AR , Pekmezci M , Bloomer MM , et al . Next-generation sequencing of retinoblastoma identifies pathogenic alterations beyond RB1 inactivation that correlate with aggressive histopathologic features . Ophthalmology 2020. ; 127 ( 6 ): 804 – 813 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roohollahi K , de Jong Y , van Mil SE , Fabius AWM , Moll AC , Dorsman JC . High-level MYCN-amplified RB1-proficient retinoblastoma tumors retain distinct molecular signatures . Ophthalmol Sci 2022. ; 2 ( 3 ): 100188 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aubry A , Yu T , Bremner R . Preclinical studies reveal MLN4924 is a promising new retinoblastoma therapy . Cell Death Discov 2020. ; 6 ( 1 ): 2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schaiquevich P , Francis JH , Cancela MB , Carcaboso AM , Chantada GL , Abramson DH . Treatment of retinoblastoma: what is the latest and what is the future . Front Oncol 2022. ; 12 : 822330 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Llombart V , Mansour MR . Therapeutic targeting of “undruggable” MYC . EBioMedicine 2022. ; 75 : 103756 . [DOI] [PMC free article] [PubMed] [Google Scholar]