Abstract
Antibiotic resistance (AR) is a silent pandemic that kills millions worldwide. Although the development of new therapeutic agents against antibiotic resistance is in urgent demand, this has presented a great challenge, especially for Gram-negative bacteria that have inherent drug-resistance mediated by impermeable outer membranes and multidrug efflux pumps that actively extrude various drugs from the bacteria. For the last two decades, multidrug efflux pumps, including AcrAB−TolC, the most clinically important efflux pump in Gram-negative bacteria, have drawn great attention as strategic targets for re-sensitizing bacteria to the existing antibiotics. This article aims to provide a concise overview of the AcrAB−TolC operational mechanism, reviewing its architecture and substrate specificity, as well as the recent development of AcrAB−TolC inhibitors.
Keywords: AcrAB−TolC, Antibiotic resistance, Gram negative bacteria, Multidrug efflux pumps, RND family
INTRODUCTION
Antibiotic resistance (AR) is a silent pandemic that kills millions worldwide. A comprehensive computational modeling based on globally collected data revealed that in 2019, almost 5 million deaths were associated with antibiotic resistance, including 1.27 million deaths directly caused by antibiotic resistant bacterial infections (1). Among these deaths, over 50% were attributable to drug-resistant Gram-negative bacteria, such as Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, which the World Health Organization has designated as priority pathogens for the urgent development of novel therapies (2).
Antibiotic resistance in Gram-negative bacteria is a combination of acquired resistance developed upon exposure to antibiotics, and intrinsic resistance resulting from the bacterial physiological characteristics, such as impermeable cell envelop, efficient drug efflux pumps, and the formation of biofilms that prevent antibiotics from reaching the bacteria. Acquired resistance, including the alteration of drug targets, inactivation, or degradation of antibiotics, often renders the bacteria with high resistance. Conversely, intrinsic resistance facilitates the initial development of resistance in Gram-negative bacteria via mainly maintaining drug concentrations at a low level, buying the time to acquire a high degree of resistance (3-5). Among the resistance mechanisms, efflux pumps play pivotal roles for both intrinsic and acquired resistance in Gram-negative bacteria (6, 7).
Efflux pumps are membrane-bound proteins that serve as a primary defense mechanism via actively exporting toxic substances, such as antibiotics, detergents, and heavy metals, whenever bacteria encounter them. Combining reduced drug penetration via the outer membrane barrier, efflux pumps make Gram-negative bacteria inherently resistant to many antibiotics. There are five main families of efflux pumps in Gram-negative bacteria: the major facilitator superfamily (MFS), the small multidrug resistance (SMR) family, the multidrug and toxin extrusion (MATE) family, the ATP-binding cassette (ABC) superfamily, the resistance-nodulation-division (RND) superfamily, and the proteobacterial antimicrobial compound efflux (PACE) family (8). The tripartite RND pumps are uniquely found in Gram negative bacteria. Although it has been reported that Staphylococcus aureus, a Gram positive bacterium, possesses FarE initially described as a RND type of efflux pump, it does not have a full architecture characterized for the tripartite RND pumps (9, 10). The tripartite RND pumps are composed of an inner membrane protein (IMP), a membrane fusion protein (MFP), and an outer membrane factor (OMF), as a tripartite complex that enables toxic compounds to be expelled, passing through the three layers of the cell envelop in Gram-negative bacteria (inner membrane-peptidoglycan-outer membrane) (Fig. 1A) (11-13). Several tripartite RND pumps have been identified in Gram-negative bacteria, such as AcrAB−TolC in Enterobacteriaceae, MexAB−OprM, MexCD−OprJ, and MexXY−OprM in Pseudomonas aeruginosa, AdeABC in Acinetobacter baumannii, CmeABC in Campylobacter jejuni, and MtrCDE in Neisseria gonorrhoeae (14-19). AcrAB−TolC is the most studied tripartite RND efflux pump. AcrAB−TolC has a notorious substrate polyspecificity that can extrude a wide range of structurally diverse toxic compounds out of the bacterial cell, including many antibiotics; thus it is often associated with multidrug resistance (20). In addition to its role in antibiotic resistance, AcrAB− TolC has been implicated in other cellular processes, such as bacterial biofilm formation, pathogenicity during infection, and adaptation to environmental stresses (21-25). For these reasons, AcrAB−TolC is a significant factor for treatment outcomes in clinics, which make the pump an attractive target for the development of therapeutic agents against Gram-negative bacteria (26, 27). This article aims to provide a concise summary of the AcrAB−TolC operational mechanism, with a glimpse of the architecture, substrates, and inhibitors of the pump.
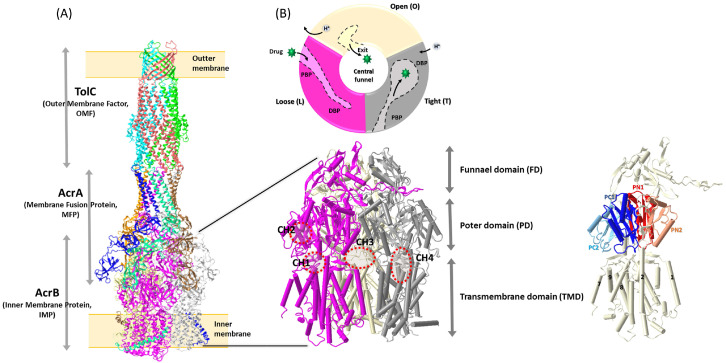
Fig. 1.
The architecture of AcrAB−TolC. (A) The cryoEM structures of a fully assembled AcrAB−TolC complex with open form of TolC (PDI ID: 5NG5) and a AcrB trimer (PDI ID: 6BAJ) with several important transmembrane helixes and subdomains in an AcrB protomer (31, 34), and (B) the conformation cycle of the three distinct protomers within trimeric AcrB, which each protomer goes through the rotation of three states termed loose (L), tight (T), and open (O) (48-50, 92).
ARCHITECTURE OF AcrAB−TolC
The structure of the whole complex of AcrAB−TolC has been resolved using various techniques that include X-ray crystallography and cryo-electron microscopy, providing insights into its structure and operational mechanism (28-31). Like other members of the tripartite RND family, AcrAB−TolC is a tripartite complex consisting of two trimeric proteins, AcrB (IMP) and TolC (OMF), bridged by hexameric AcrA (MFP) (Fig. 1A).
AcrB is a transmembrane protein with the size of 113.6 kDa (1,049 aa). In the AcrAB−TolC complex, AcrB exists as a homotrimer, and the three protomers are arranged in a triangular shape, forming a funnel-like structure that can cope with a wide range of molecules for their translocation out of the bacterial cell. Each protomer is composed of three domains: the transmembrane domain (TMD), the porter domain (PD), and the funnel domain (FD) (Fig. 1). The TMD of AcrB protomer is comprised of 12 transmembrane helices (TM1−TM12), which contain critical residues for proton translocation to conduct the energy transduction through electrochemical proton gradient across the membrane (32). A total of 36 TMs of TMDs in trimeric AcrB form the central cavity in the inner membrane, and provide a path for drug entrance (33). It has been shown that the conformational changes of the TMDs in each AcrB protomer are interconnected, affecting the function of the whole complex (34). The PD in AcrB consists of two N-terminal and two C-terminal subdomains (PN1, PN2, PC1, and PC2), and it is the major domain of AcrB for substrate binding, which during the drug efflux process, undergoes large conformational changes. Each PD of the protomer contains two substrate-binding pockets, the proximal binding pocket (PBP) between PC1 and PC2, and the distal binding pocket (DBP) between PC1 and PN2 (PN1), which are separated by 11 amino acids termed “a switch loop” (35, 36). The PD is a key structural component of AcrB that is essential for the recognition, binding, and translocation of various molecules (37). The FD, the third domain of AcrB, is a region located at the tip of AcrB toward the periplasmic end of the protein. A long loop made with two N-terminal β-sheets (Nβ 8 and 9) in the FD of each protomer stretches and penetrates the FD of another protomer, interlocking with each other and maintaining the architecture of the AcrB trimer, while the TMDs and PDs of protomers undergo conformational changes (Fig. 1) (38). A sequential peptide affinity tag experiment revealed that AcrZ, a 49 amino acid long (5.3 kDa) small protein, interacts with AcrB in one to one ratio (39). As a single helix, AcrZ interacts with MTD of AcrB in the inner membrane (28, 31, 40). A recent cryo-EM study observed that bending of AcrZ around position 16 (P16) is important for the physical interaction of AcrZ with AcrB (41). The same study also revealed that AcrZ binding to AcrB modulates the structural conformation and activity of AcrB through allosteric changes that is enhanced in a lipid environment where cardiolipin and palmityloleoyl phosphatidylglycerol are present (41).
AcrA is the 42.2 kDa (397 aa) periplasmic protein comprising MFP in the AcrAB−TolC complex, which bridges the inner membrane protein AcrB and the outer membrane protein TolC (Fig. 1). AcrA exists in two conformationally different types of protomers (protomer I and II) in a hexameric assembly of the AcrAB−TolC complex. Each AcrA protomer is subdivided into four subdomains: the membrane-proximal domain (MPD), β-barrel domain, lipoyl domain, and α-helical domain (13, 42). A recent cellular electron cryo-tomography study revealed that there are two distinct conformations of MPDs in hexameric AcrA, in which one interacts with both AcrB and the inner membrane (protomer I), while the other interacts with only AcrB (protomer II) (42). Along with the MPD, the β-barrel domains flexibly linked to the MPDs are also involved in interaction with AcrB at its FDs. The lipoyl domains and the α-helical hairpins are arranged toward TolC in a cylindrical shape, and play roles in the stabilization of the AcrA hexameric assembly, interacting with each other, and the intermeshing cogwheel-like interaction with TolC providing the TolC interface, respectively (40).
TolC is the outer membrane protein of the complex, and forms a channel across the outer membrane of the bacterial cell. TolC-like proteins are ubiquitous in Gram-negative bacteria, which are involved in various extrusion processes of diverse molecules that are either produced by bacteria (metabolites, virulence factors, and toxins), or imported into them (antibiotics, biocides, bile salts and organic solvents) (43, 44). Unlike other Gram-negative bacteria that have several TolC-like proteins, entrobacteriaceae, such as Escherichia coli, express only one TolC that needs to be recruited to an AcrAB subcomplex to complete the complex assembly (45). TolC in the AcrAB− TolC complex exists as a homotrimer with each 53.7 kDa (493 aa) protomer containing periplasmic α-barrels and outer membrane embedded β-barrels. Twelve β-barrels in three TolC protomers form an outer membrane pore, while α-barrels create a 100 Å long channel in the periplasm, which together undergo open and closed conformational changes in an iris-like manner that is powered by reorientation of the AcrA interacting with TolC at its α-helical hairpins during the substance efflux process (43-45).
GENERAL OPERATION MECHANISM OF AcrAB−TolC
During the efflux process through the AcrAB−TolC drug efflux pump, substrates move from the AcrB binding pocket through the TolC channel, and into the extracellular space. The movements of substrates through this complex have been extensively studied using a variety of techniques, which include X-ray crystallography, cryo-electron microscopy, and molecular dynamics simulations (28-31, 46). The efflux process is initiated when a substrate of AcrAB−TolC binds to AcrB, and triggers a series of conformational changes in the complex (11, 47, 48). During the drug translocation process, conformational changes of AcrB occur in a cyclic manner, in which each protomer goes through the rotation of three states termed access, binding, and extrusion, or alternatively, loose (L), tight (T), and open (O), which are adopted from F0F1−ATP synthase (Fig. 1B) (48-50). The L state, the apo form of AcrB, is presumably the initial state of the conformation cycle. In the L state of a protomer, the PBP between PC1 and PC2 subdomains is open, while the DBP between PC1 and PN2 subdomains is closed in the PD (31, 51, 52). Upon binding of substrates, the L state shifts to the T state where the PBP becomes smaller, while the DBP is open, allowing the substrates to be translocated toward the FD. At the T state stabilized by substrate binding in the DBP, the TMD undergoes the conformational change that allows protons to access the acidic residues (D407 and D408) in the TMD, which provide the proton translocation driven T to O transition of AcrB (53). The exit gate between PN1 and PN2 subdomains is open at the O state, while both the PBP and DBP are closed. As a result, substrates move out through the FD toward the funnel of AcrA−TolC complex (31, 53). Finally, the release of protons from the TMD in the O state to the cytoplasm brings the AcrB to the initial L state, closing the exit gate, and opening the PBP (54). Of note, a recent study proposed an alternative explanation for the cyclic conformational rotation of AcrB, with the hypothesis that the conformational changes in AcrB trimer are initially triggered by proton binding, instead of substrate binding. However, this hypothesis has not yet been verified by others (55).
Prior to substrate binding, the AcrAB−TolC complex is presumably at the resting state, where the AcrB trimer exists as a symmetrical structure, with all three protomers in the L state. In the resting state pump, the symmetrical (LLL) AcrB trimer is tied to a loosely packed AcrA hexamer that binds to the closed form of TolC (38). Upon substrate binding, the cyclic conformational change of AcrB occurs, converting its structure from symmetrical to asymmetrical form. Various asymmetrical AcrB trimers comprised of combinations of the different states (L− T−O, T−T−L, L−T−L) have been found through X-ray and cryo-EM analyses (31, 49). Since full AcrAB−TolC complexes with asymmetric AcrB trimers are supposed to be in the active process for substrate extrusion, the conformations of AcrA and TolC are adopted to complete the process, providing an open path for substrates to go through, out of the outer membrane. At the active stage of an AcrA hexamer, the β-barrels and MP domains associated with the AcrB trimer are reoriented, and the α-helical hairpins are tightly packed, sealing the enclosed cavity that is connected to the TolC (30, 46). The conformational changes of AcrA lead to relocation of the helices at the lower end of the TolC α-barrel in an iris-like opening, eventually forming an entire open path from AcrB to TolC toward the outer membrane (31). Several reviews have provided detailed explanations of the elaborate conformational changes in AcrAB−TolC complex during the substrate extrusion (11, 13).
Four different substrate access channels (CH1−4) have been suggested based on computational structure analyses of AcrB, of which entry sites for substrates toward the PBP and the DBP in AcrB are found to be either from the periplasm, or the outer leaflet of the inner membrane. Channel 1 (CH1) is located in the interface between PC2/TMD (TM8) that allows substrates to enter AcrB through the outer leaflet of the inner membrane (56). It has been observed that CH1 is open in the L and T states, but closed in the O state by the conformational shift of TM8 and PC2 (33, 56). The periplasmic Channel 2 (CH2) was observed in the cleft between PC1 and PC2, which leads the substrate to the PBP of the L and T states (33, 48, 56). The presence of the third channel (CH3) was observed at the central cavity, a large cavity at the bottom of the periplasmic domain and at the top of the TMS domain, formed by the residues of all three AcrB protomers in the inner membrane (57-59). It has been suggested that the CH3 can guide substrates directly to the DBP by passing the PBP, as well as the switch loop (33). Recent molecular modeling studies, followed by functional and structural analyses, have suggested a fourth channel (CH4) that begins at the TM1/TM2 groove, and extends to an interface pathway between PN2 and PC1 toward the DBP (60, 61). These different substrate access channels in distinct locations at the AcrB trimer contribute to substrate poly-specificity of AcrB, providing various routes for the extrusion of structurally unrelated diverse molecules out of the bacteria.
SUBSTRATE POLY-SPECIFICITY OF AcrAB−TolC
A wide range of substrates that include antibiotics, detergents, dyes, and other toxic compounds can be recognized and transported through AcrAB−TolC (Supplementary Table 1). The poly-specificity of AcrAB−TolC allows structurally diverse molecules to be transported by the pump, and numerous functional, structural, and mutagenesis studies have shown that the substrate specificity is initially determined by the access channels at the PD, which have certain substrate preferences (33, 49, 57, 62). The CH1 in the TMD/PC2 interface at the TM 7/8/9 groove near the vestibule guides substrates from the outer leaflet of the inner membrane to the PBP and DBP transition point (57). Phenicols, linezolid, and fusidic acid, as well as dodecyl β-D-maltoside (DDM) that has been co-crystalized with AcrB, are found to be preferred substrates for CH1 (36, 56, 60, 62). A mutagenesis study revealed that CH1 can be also a transport route for amphiphilic β-lactams with larger partition coefficient, such as nafcillin, cloxacillin, and benzyl-penicillin, of which the hydrophobic side chains are more likely to immerse in the outer leaflet of the inner membrane, while the β-lactam ring would still remain in the periplasm (63, 64). In general, it has been proposed that compounds with low molecular weight and low polar surface area are likely to enter the AcrB through CH1 (62). The CH2 pathway starting at the lateral cleft between the PC1 and PC2 interface is located about 15 Å above the putative membrane plane (36, 48, 49, 57). The location of CH2 seems to be suitable for capturing substrates in the periplasm. Although the substrate specificity of CH2 is still elusive, studies show that CH2 prefers high molecular weight drugs, such as erythromycin, rifampicin, minocycline, and doxorubin-dimer, in the classes of microlides, ansamycins, tetracyclines, and anthracyclines (36, 49, 57, 65). A site-directed mutagenesis revealed that planar aromatic cations with low molecular weight, such as ethidium bromide (EtBr), benzalkonium chloride (BZK), berberine (BER), and rhodamine 6G (R6G), are the preferred substrates for CH3 in the central cavity of TMD in the inner membrane (33). The fourth channel (CH4) comprised of the TM1/TM2 groove has been proposed based on several molecular modeling studies, as well as functional and structural analyses, and it has been shown that carboxylated molecules, such as fusidic acid, oxacillin, cloxacillin, dicloxacillin, and piperacillin, can be transported through CH4 (60, 62).
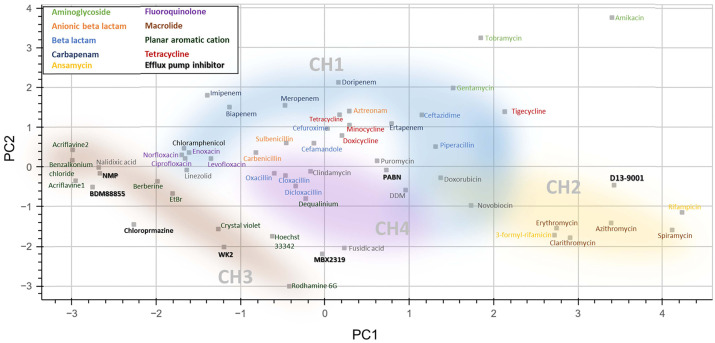
The substrate preference or specificity of each access channel appears to go toward certain physicochemical and structural properties of the substrates. For example, when some of the known substrates for AcrAB−TolC are projected in a principal component analysis (PCA) plot with respect to their molecular weight, hydrophobicity, and charge distribution, as well as their size and shape indicated by minimal and maximal projection areas, the drugs preferred by each channel are roughly clustered together, implying that the physicochemical and structural properties of the substrates play an important role in determining whether they can be recognized and transported through a certain channel in AcrB (Fig. 2) (11, 13). Hydrophilic aminoglycosides known to be unfavorable substrates for AcrAB−TolC are clustered away from the well-known AcrAB−TolC substrates in the plot (66). The PCA plot is made with 6 chemical features (molecular weight, hydrophilic-lipophilic Balance, logP, topological polar surface area, and minimum projection area and maximum projection area obtained from the UCSF ChimeraX); however, it is apparent that these 6 features are not sufficient to perfectly rationalize the substrate preference of each channel. For example, tetracycline, minocycline, and doxycycline, the drugs in the tetracycline class, have been reported as the substrates for CH2 (36). However, they are instead positioned close to the substrates that more likely use CH1 (Fig. 2). Piperacillin is also found to be apart from the other carboxylated β-lactams (oxacillin, cloxacillin, and dicloxacillin), although it has been shown that these carboxylated β-lactams are translocated through CH4 (60). Of note, piperacillin seems to indeed be somewhat different than other carboxylated β-lactams, behaving differently in the site-directed mutant (L300A) that is supposed to have altered CH4 function (60). To extend our comprehension of each channel’s substrate specificity, it would be necessary to integrate more chemical features of the substrates, including the presence of certain functional groups, such as carboxyl, amino, and hydroxyl groups that likely affect the substrate preferences between the access channels (67).
Fig. 2.
A principal component analysis (PCA) plot of AcrAB−TolC substrates and inhibitors. A PCA plot made with physicochemical properties (molecular weight, hydrophilic-lipophilic Balance, logP, topological polar surface area, minimum projection area, maximum projection area obtained from the UCSF ChimeraX, (93, 94) of 50 molecules that have been tested as substrates as well as 7 inhibitors (bold) for AcrAB−TolC. Potential access channels are roughly depicted based on the molecules with the reported dedicate access channels (57, 60, 62, 63, 66, 95-97).
Once the substrates enter the AcrAB−TolC complex through different access channels, they either move through PBP to DBP (CH1 and CH2), or directly end up at DBP (CH3 and CH4) (62). The hydrophobic environment in DBP, comprising mainly phenylalanine, but also isoleucine, valine, alanine, and asparagine side chains, seems to be opted for taking in all the known AcrAB−TolC substrates as an anteroom to enter the exit funnel connected to AcrA−TolC (11, 68). Overall, the substrate poly-specificity of AcrAB−TolC is the outcome of a complex interplay between the physicochemical and structural properties of the substrate molecules, and the molecular features of the AcrAB−TolC complex.
The substrate polyspecificity of AcrAB−TolC with different drug access channels would be a great benefit, granting them the capability to simultaneously cope with various structurally unrelated antibiotics in different classes. Of note, the strong cooperative efflux between different drugs, such as chloramphenicol and cefamandole, and the simultaneous extrusion of the CH3-preferred drugs and the CH1- or CH2-preferred drugs have been observed (62, 69). In the current clinical setting, combination therapies with more than one antibiotic have become a routine procedure, due to increased antibiotic resistance (70). In-depth understanding of the substrate polyspecificity mechanism for AcrAB−TolC, a major efflux pump in Gram-negative bacteria, might enable the development of a better strategy for a more effective combination therapy.
AcrAB−TolC INHIBITORS
AcrAB−TolC-associated multidrug resistance has been a significant clinical implication for the treatment failure of antibiotics against Gram-negative bacterial infections (71-74). In addition, the involvement of AcrAB−TolC in other cellular functions, such as biofilm formation and pathogenicity, in Gram-negative bacteria makes the pump an attractive target for the development of new strategies to combat antibiotic resistant infections (21-25). As such, inhibition of AcrAB−TolC can be an effective way to enhance the effectiveness of antibiotics and reduce the occurrence of antibiotic resistance. Various AcrAB−TolC inhibitors, including small molecules and peptides, have been identified (13, 26, 75).
Phenylalanine-Arginine-β-Naphtylamide (PAβN) is a broad-spectrum efflux pump inhibitor that has the inhibitory activity for various efflux pumps in Gram-negative bacteria, including MexAB−OprM, MexCD−OprJ, MexEF−OprN, AdeABC, and AcrAB−TolC. PAβN was initially identified through a 200,000 small molecule combination screening with levofloxacin against MexAB−OprM, MexCD−OprJ, or MexEF−OprN overexpressing Pseudomonas aeruginosa (76). Similar to PAβN, D13−9001, a 4-oxo-4H-pyrido[1,2-a]pyrimidine derivative, was initially identified through high-throughput screening against MexAB−OprM of Pseudomonas aeruginosa, and subsequently shown to have AcrAB−TolC inhibitory activity along with a co-crystallization with AcrB in DBP (77).
There are also inhibitors that specifically target AcrAB−TolC: 1-(1-naphtylmethyl)-piperazine (NMP), MBX2319, WK2, and BDM88855. An arylpiperazine, NMP, was identified in a high throughput screening against an AcrAB overexpressing E. coli strain, and has shown over 4-fold reduced MICs for levofloxacin, linezolid, clarithromycin, oxacillin, rifampin, chloramphenicol, and tetracycline (78). A 2H-benzo[h]chromene derivative, WK2, is identified as an AcrB targeting inhibitor, conferring increased susceptibility to chloramphenicol, erythromycin, tetraphenylphosphonium, and levofloxacin, but no effect for rifampicin (79). MBX2319 is a pyranopyridine molecule, and potentiates various antibiotics, including fluoroquinolones, β-lactams, and chloramphenicol against a wild-type E. coli strain, as well as ciprofloxacin resistant strains (80). An electron cryo-tomography study suggested that the mechanism of AcrAB− TolC inhibition by MBX2319 was likely due to MBX2319-mediated restriction of the conformational cycle in the pump (65). Similarly, the recently identified inhibitor, BDM88855 is also proposed to prevent the conformational cycle of the pump that is critical for the function of AcrAB−TolC. BDM88855 aids significant reduction of MIC values for oxacillin, linezolid, novobiocin, fusidic acid, pyridomycin, and chloramphenicol in a wild-type E. coli strain (81). The effect of BDM88855 seems to decrease for erythromycin, ciprofloxacin, piperacillin, tetracycline, triclosan, and ampicillin, and essentially have no effect for ceftazidime, streptomycin, aztreonam, and gentamicin (81). Interestingly, the position of BDM88855 is distant from most of these lesser (or no) effect compounds in the PCA plot (Fig. 2). Similarly, PAβN in particular showed better effects over NMP for clarithromycin and rifampin, which are positioned closer to PAβN than NMP in the PCA plot (Fig. 2). This observation implies that consideration of the physicochemical properties of the inhibitors, as well as antibiotics, potential combination partners, could provide a good strategy leading to the successful development of AcrAB−TolC inhibitors.
More recently, new strategies have been explored to identify AcrAB−TolC inhibitors, such as interfering with the pump assembly. Recent studies demonstrated that designed peptides that can mimic TMs of AcrB interrupt AcrB trimerization, leading to the inhibition of AcrAB−TolC mediated drug efflux (82). In another way to disrupt the efflux pump assembly, AcrA binding molecules, such as NSC series compounds, have been developed, and have shown potentiation activity for certain antibiotics, including novobiocin and erythromycin (83, 84). Recent articles have comprehensively reviewed some of the AcrAB−TolC inhibitors, describing their structure-activity relationships, chemical synthesis, mode of action, and in vitro and in vivo activity, as well as their pharmacological properties (26). Endeavors to develop effective AcrAB−TolC inhibitors are being carried on continuously identifying new molecules, and the development will be facilitated with new approaches such as molecular docking studies and virtual screenings (85-88). Further development of new molecules for clinical uses faces several challenges. Cytotoxicity has been a major reason for scarce in vivo data that demonstrate efficacy of efflux pump inhibitors (EPIs) (89, 90). The pharmacokinetics (PK) and pharmacodynamics (PD) of EPIs are other challenges. As in combination therapeutics of β-lactams and β-lactamase inhibitors, EPIs need to be able to reach the site of action, penetrate bacterial cells, and remain stable in the presence of efflux pumps along with companion antibiotics, necessitating careful evaluation of their PK and PD compatibilities (91). Systemic investigation of all identified AcrAB−TolC inhibitors regarding their cytotoxicity as well as PK and PD along with physicochemical properties will provide crucial information for successful development of effective AcrAB−TolC inhibitors, which will be of great assistance for antibiotics for the battle against antibiotic resistant bacterial infections.
CLOSING REMARKS
The AcrAB−TolC efflux pump is one of the major efflux pumps found in Gram-negative bacteria, and most likely is the main efflux pump contributing to multidrug resistance, especially in Enterobacteriae (11, 13). The pump is capable of extruding a wide range of structurally diverse compounds from the bacterial cell, including many antibiotics, thus reducing their intracellular concentrations, and rendering them ineffective. Studies over several decades have greatly extended our understanding of AcrAB−TolC in terms of its structure, substrates, and operational mechanism. However, there are many more gaps to fill to fully understand the nature of its sophisticated operation mechanism, as we glimpsed in this review, such as its substrate recognition process by the different access channels. The extrusion of molecules through AcrAB−TolC seems to be elaborately controlled by certain rules orchestrating the molecular features of the AcrAB−TolC protein complex and the diverse physicochemical properties of the substrates. In-depth comprehension of the mechanisms of AcrAB−TolC efflux pump-mediated antibiotic resistance will lead to the development of new strategies to combat antibiotic-resistant infections, such as the development of inhibitors that can effectively block the function of AcrAB−TolC, as well as new antibiotics that are less susceptible to AcrAB−TolC-mediated resistance.
Funding Statement
ACKNOWLEDGEMENTS This work was supported by the National Research foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020K1A4A7A02095129/2023M3A9G6057281 to S.J.).
Footnotes
CONFLICTS OF INTEREST
The author has no conflicting interests.
REFERENCES
- 1.Antimicrobial Resistance C. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 3.Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 4.Arzanlou M, Chai WC, Venter H. Intrinsic, adaptive and acquired antimicrobial resistance in Gram-negative bacteria. Essays Biochem. 2017;61:49–59. doi: 10.1042/EBC20160063. [DOI] [PubMed] [Google Scholar]
- 5.Langevin AM, El Meouche I, Dunlop MJ. Mapping the role of AcrAB−TolC efflux pumps in the evolution of antibiotic resistance reveals Near-MIC treatments facilitate resistance acquisition. mSphere. 2020;5:e01056–20. doi: 10.1128/mSphere.01056-20.e68fc3648e9644aab996e33f75d80c68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernando-Amado S, Blanco P, Alcalde-Rico M, et al. Multidrug efflux pumps as main players in intrinsic and acquired resistance to antimicrobials. Drug Resist Updat. 2016;28:13–27. doi: 10.1016/j.drup.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Li XZ, Plesiat P, Nikaido H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev. 2015;28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan KA, Liu Q, Henderson PJ, Paulsen IT. Homologs of the Acinetobacter baumannii AceI transporter represent a new family of bacterial multidrug efflux systems. mBio. 2015;6:e01982–14. doi: 10.1128/mBio.01982-14.3ea846d49c5e4c02aff7e7b3d6dc70bf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alnaseri H, Arsic B, Schneider JE, et al. Inducible expression of a resistance-nodulation-division-type efflux pump in Staphylococcus aureus provides resistance to linoleic and arachidonic acids. J Bacteriol. 2015;197:1893–1905. doi: 10.1128/JB.02607-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schindler BD, Frempong-Manso E, DeMarco CE, et al. Analyses of multidrug efflux pump-like proteins encoded on the Staphylococcus aureus chromosome. Antimicrob Agents Chemother. 2015;59:747–748. doi: 10.1128/AAC.04678-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du D, Wang-Kan X, Neuberger A, et al. Multidrug efflux pumps: structure, function and regulation. Nat Rev Microbiol. 2018;16:523–539. doi: 10.1038/s41579-018-0048-6. [DOI] [PubMed] [Google Scholar]
- 12.Nikaido H. RND transporters in the living world. Res Microbiol. 2018;169:363–371. doi: 10.1016/j.resmic.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobylka J, Kuth MS, Muller RT, Geertsma ER, Pos KM. AcrB: a mean, keen, drug efflux machine. Ann N Y Acad Sci. 2020;1459:38–68. doi: 10.1111/nyas.14239. [DOI] [PubMed] [Google Scholar]
- 14.Alav I, Kobylka J, Kuth MS, et al. Structure, assembly, and function of tripartite efflux and type 1 secretion systems in gram-negative bacteria. Chem Rev. 2021;121:5479–5596. doi: 10.1021/acs.chemrev.1c00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puzari M, Chetia P. RND efflux pump mediated antibiotic resistance in Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa: a major issue worldwide. World J Microbiol Biotechnol. 2017;33:24. doi: 10.1007/s11274-016-2190-5. [DOI] [PubMed] [Google Scholar]
- 16.Lorusso AB, Carrara JA, Barroso CDN, Tuon FF, Faoro H. Role of efflux pumps on antimicrobial resistance in Pseudomonas aeruginosa. Int J Mol Sci. 2022;23:15779. doi: 10.3390/ijms232415779.ad26716b1fa14047a802ce460de71f28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wieczorek P, Sacha P, Hauschild T, Zorawski M, Krawczyk M, Tryniszewska E. Multidrug resistant Acinetobacter baumannii--the role of AdeABC (RND family) efflux pump in resistance to antibiotics. Folia Histochem Cytobiol. 2008;46:257–267. doi: 10.2478/v10042-008-0056-x. [DOI] [PubMed] [Google Scholar]
- 18.Su CC, Yin L, Kumar N, et al. Structures and transport dynamics of a Campylobacter jejuni multidrug efflux pump. Nat Commun. 2017;8:171. doi: 10.1038/s41467-017-00217-z.51b9ec914f01445795f1807120926c42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolla JR, Su CC, Do SV, et al. Crystal structure of the Neisseria gonorrhoeae MtrD inner membrane multidrug efflux pump. PLoS One. 2014;9:e97903. doi: 10.1371/journal.pone.0097903.34a340589890428cbd269a72a7133032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikaido H, Zgurskaya HI. AcrAB and related multidrug efflux pumps of Escherichia coli. J Mol Microbiol Biotechnol. 2001;3:215–218. [PubMed] [Google Scholar]
- 21.Yamasaki S, Wang LY, Hirata T, Hayashi-Nishino M, Nishino K. Multidrug efflux pumps contribute to Escherichia coli biofilm maintenance. Int J Antimicrob Agents. 2015;45:439–441. doi: 10.1016/j.ijantimicag.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Bay DC, Stremick CA, Slipski CJ, Turner RJ. Secondary multidrug efflux pump mutants alter Escherichia coli biofilm growth in the presence of cationic antimicrobial compounds. Res Microbiol. 2017;168:208–221. doi: 10.1016/j.resmic.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Perez A, Poza M, Fernandez A, et al. Involvement of the AcrAB−TolC efflux pump in the resistance, fitness, and virulence of Enterobacter cloacae. Antimicrob Agents Chemother. 2012;56:2084–2090. doi: 10.1128/AAC.05509-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webber MA, Bailey AM, Blair JM, et al. The global consequence of disruption of the AcrAB−TolC efflux pump in Salmonella enterica includes reduced expression of SPI-1 and other attributes required to infect the host. J Bacteriol. 2009;191:4276–4285. doi: 10.1128/JB.00363-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langevin AM, Dunlop MJ. Stress introduction rate alters the benefit of AcrAB−TolC efflux pumps. J Bacteriol. 2018;200:e00525–17. doi: 10.1128/JB.00525-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Compagne N, Vieira Da Cruz A, Muller RT, Hartkoorn RC, Flipo M, Pos KM. Update on the discovery of efflux pump inhibitors against critical priority gram-negative bacteria. Antibiotics (Basel) 2023;12:180. doi: 10.3390/antibiotics12010180.c36548178e0f4e1bb0879082e17f1a6c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alenazy R. Drug efflux pump inhibitors: a promising approach to counter multidrug resistance in gram-negative pathogens by targeting AcrB protein from AcrAB-TolC multidrug efflux pump from Escherichia coli. Biology (Basel) 2022;11:1328. doi: 10.3390/biology11091328.52d794c104344d899b5ab555ea2cee3f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du D, Wang Z, James NR, et al. Structure of the AcrAB−TolC multidrug efflux pump. Nature. 2014;509:512–515. doi: 10.1038/nature13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daury L, Orange F, Taveau JC, et al. Tripartite assembly of RND multidrug efflux pumps. Nat Commun. 2016;7:10731. doi: 10.1038/ncomms10731.eba793de8123457e92c40e3142a2ad4f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen M, Shi X, Yu Z, et al. In situ structure of the AcrAB−TolC efflux pump at subnanometer resolution. Structure. 2022;30:107–113. doi: 10.1016/j.str.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Fan G, Hryc CF, et al. An allosteric transport mechanism for the AcrAB−TolC multidrug efflux pump. Elife. 2017;6:24905. doi: 10.7554/eLife.24905.714d43d2f1284de5b3691b0f864d7a20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su CC, Li M, Gu R, et al. Conformation of the AcrB multidrug efflux pump in mutants of the putative proton relay pathway. J Bacteriol. 2006;188:7290–7296. doi: 10.1128/JB.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zwama M, Yamasaki S, Nakashima R, Sakurai K, Nishino K, Yamaguchi A. Multiple entry pathways within the efflux transporter AcrB contribute to multidrug recognition. Nat Commun. 2018;9:124. doi: 10.1038/s41467-017-02493-1.cf87738eec424a5bb6b49a27e8fde79a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu W, Fu Z, Xu GG, et al. Structure and activity of lipid bilayer within a membrane-protein transporter. Proc Natl Acad Sci U S A. 2018;115:12985–12990. doi: 10.1073/pnas.1812526115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller RT, Travers T, Cha HJ, Phillips JL, Gnanakaran S, Pos KM. Switch loop flexibility affects substrate transport of the AcrB efflux pump. J Mol Biol. 2017;429:3863–3874. doi: 10.1016/j.jmb.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Eicher T, Cha HJ, Seeger MA, et al. Transport of drugs by the multidrug transporter AcrB involves an access and a deep binding pocket that are separated by a switch-loop. Proc Natl Acad Sci U S A. 2012;109:5687–5692. doi: 10.1073/pnas.1114944109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajapaksha P, Pandeya A, Wei Y. Probing the dynamics of AcrB through disulfide bond formation. ACS Omega. 2020;5:21844–21852. doi: 10.1021/acsomega.0c02921.dc16cea4299d43bfac7a59ce64d3638a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koronakis V, Sharff A, Koronakis E, Luisi B, Hughes C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature. 2000;405:914–919. doi: 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- 39.Hobbs EC, Yin X, Paul BJ, Astarita JL, Storz G. Conserved small protein associates with the multidrug efflux pump AcrB and differentially affects antibiotic resistance. Proc Natl Acad Sci U S A. 2012;109:16696–16701. doi: 10.1073/pnas.1210093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeong H, Kim JS, Song S, et al. Pseudoatomic Structure of the tripartite multidrug efflux pump AcrAB-TolC reveals the intermeshing cogwheel-like interaction between AcrA and TolC. Structure. 2016;24:272–276. doi: 10.1016/j.str.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Du D, Neuberger A, Orr MW, et al. Interactions of a bacterial RND transporter with a transmembrane small protein in a lipid environment. Structure. 2020;28:625–634. doi: 10.1016/j.str.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi X, Chen M, Yu Z, et al. In situ structure and assembly of the multidrug efflux pump AcrAB−TolC. Nat Commun. 2019;10:2635. doi: 10.1038/s41467-019-10512-6.28c29f6371ef4ce0931bd60ea29799b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koronakis V, Andersen C, Hughes C. Channel-tunnels. Curr Opin Struct Biol. 2001;11:403–407. doi: 10.1016/S0959-440X(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 44.Koronakis V. TolC--the bacterial exit duct for proteins and drugs. FEBS Lett. 2003;555:66–71. doi: 10.1016/S0014-5793(03)01125-6. [DOI] [PubMed] [Google Scholar]
- 45.Zgurskaya HI, Krishnamoorthy G, Ntreh A, Lu S. Mechanism and function of the outer membrane channel TolC in multidrug resistance and physiology of Enterobacteria. Front Microbiol. 2011;2:189. doi: 10.3389/fmicb.2011.00189.f2db5e4fa66f47bfaeba620f3e96d8e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikolosko J, Bobyk K, Zgurskaya HI, Ghosh P. Conformational flexibility in the multidrug efflux system protein AcrA. Structure. 2006;14:577–587. doi: 10.1016/j.str.2005.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neuberger A, Du D, Luisi BF. Structure and mechanism of bacterial tripartite efflux pumps. Res Microbiol. 2018;169:401–413. doi: 10.1016/j.resmic.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Seeger MA, Schiefner A, Eicher T, Verrey F, Diederichs K, Pos KM. Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism. Science. 2006;313:1295–1298. doi: 10.1126/science.1131542. [DOI] [PubMed] [Google Scholar]
- 49.Murakami S, Nakashima R, Yamashita E, Matsumoto T, Yamaguchi A. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature. 2006;443:173–179. doi: 10.1038/nature05076. [DOI] [PubMed] [Google Scholar]
- 50.Seeger MA, Diederichs K, Eicher T, et al. The AcrB efflux pump: conformational cycling and peristalsis lead to multidrug resistance. Curr Drug Targets. 2008;9:729–749. doi: 10.2174/138945008785747789. [DOI] [PubMed] [Google Scholar]
- 51.Murakami S, Nakashima R, Yamashita E, Yamaguchi A. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature. 2002;419:587–593. doi: 10.1038/nature01050. [DOI] [PubMed] [Google Scholar]
- 52.Fischer N, Kandt C. Porter domain opening and closing motions in the multi-drug efflux transporter AcrB. Biochim Biophys Acta. 2013;1828:632–641. doi: 10.1016/j.bbamem.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 53.Eicher T, Seeger MA, Anselmi C, et al. Coupling of remote alternating-access transport mechanisms for protons and substrates in the multidrug efflux pump AcrB. Elife. 2014;3:e03145. doi: 10.7554/eLife.03145.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yue Z, Chen W, Zgurskaya HI, Shen J. Constant pH molecular dynamics reveals how proton release drives the conformational transition of a transmembrane efflux pump. J Chem Theory Comput. 2017;13:6405–6414. doi: 10.1021/acs.jctc.7b00874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Webber A, Ratnaweera M, Harris A, Luisi BF, Ntsogo Enguene VY. A model for allosteric communication in drug transport by the AcrAB−TolC tripartite efflux pump. Antibiotics (Basel) 2022;11:52. doi: 10.3390/antibiotics11010052.d6bd12af81b64471bf2db86eb25f2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sennhauser G, Amstutz P, Briand C, Storchenegger O, Grutter MG. Drug export pathway of multidrug exporter AcrB revealed by DARPin inhibitors. PLoS Biol. 2007;5:e7. doi: 10.1371/journal.pbio.0050007.7cacaacc48224fc39899d8ee3e619613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakashima R, Sakurai K, Yamasaki S, Nishino K, Yamaguchi A. Structures of the multidrug exporter AcrB reveal a proximal multisite drug-binding pocket. Nature. 2011;480:565–569. doi: 10.1038/nature10641. [DOI] [PubMed] [Google Scholar]
- 58.Husain F, Nikaido H. Substrate path in the AcrB multidrug efflux pump of Escherichia coli. Mol Microbiol. 2010;78:320–330. doi: 10.1111/j.1365-2958.2010.07330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Husain F, Bikhchandani M, Nikaido H. Vestibules are part of the substrate path in the multidrug efflux transporter AcrB of Escherichia coli. J Bacteriol. 2011;193:5847–5849. doi: 10.1128/JB.05759-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tam HK, Malviya VN, Foong WE, et al. Binding and transport of carboxylated drugs by the multidrug transporter AcrB. J Mol Biol. 2020;432:861–877. doi: 10.1016/j.jmb.2019.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oswald C, Tam HK, Pos KM. Transport of lipophilic carboxylates is mediated by transmembrane helix 2 in multidrug transporter AcrB. Nat Commun. 2016;7:13819. doi: 10.1038/ncomms13819.fa3b0c6a09c14ed89a4c26f47061ebab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tam HK, Foong WE, Oswald C, Herrmann A, Zeng H, Pos KM. Allosteric drug transport mechanism of multidrug transporter AcrB. Nat Commun. 2021;12:3889. doi: 10.1038/s41467-021-24151-3.6482f47ba7254e868df9dbcc9afdc705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kobayashi N, Tamura N, van Veen HW, Yamaguchi A, Murakami S. Beta-Lactam selectivity of multidrug transporters AcrB and AcrD resides in the proximal binding pocket. J Biol Chem. 2014;289:10680–10690. doi: 10.1074/jbc.M114.547794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schuster S, Kohler S, Buck A, et al. Random mutagenesis of the multidrug transporter AcrB from Escherichia coli for identification of putative target residues of efflux pump inhibitors. Antimicrob Agents Chemother. 2014;58:6870–6878. doi: 10.1128/AAC.03775-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sjuts H, Vargiu AV, Kwasny SM, et al. Molecular basis for inhibition of AcrB multidrug efflux pump by novel and powerful pyranopyridine derivatives. Proc Natl Acad Sci U S A. 2016;113:3509–3514. doi: 10.1073/pnas.1602472113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nikaido H, Basina M, Nguyen V, Rosenberg EY. Multidrug efflux pump AcrAB of Salmonella typhimurium excretes only those beta-lactam antibiotics containing lipophilic side chains. J Bacteriol. 1998;180:4686–4692. doi: 10.1128/JB.180.17.4686-4692.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ornik-Cha A, Wilhelm J, Kobylka J, et al. Structural and functional analysis of the promiscuous AcrB and AdeB efflux pumps suggests different drug binding mechanisms. Nat Commun. 2021;12:6919. doi: 10.1038/s41467-021-27146-2.0fa39d64c8884cc2b68766a6f47a6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vargiu AV, Nikaido H. Multidrug binding properties of the AcrB efflux pump characterized by molecular dynamics simulations. Proc Natl Acad Sci U S A. 2012;109:20637–20642. doi: 10.1073/pnas.1218348109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kinana AD, Vargiu AV, Nikaido H. Some ligands enhance the efflux of other ligands by the Escherichia coli multidrug pump AcrB. Biochemistry. 2013;52:8342–8351. doi: 10.1021/bi401303v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davis K, Greenstein T, Viau Colindres R, Aldridge BB. Leveraging laboratory and clinical studies to design effective antibiotic combination therapy. Curr Opin Microbiol. 2021;64:68–75. doi: 10.1016/j.mib.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blair JM, Smith HE, Ricci V, Lawler AJ, Thompson LJ, Piddock LJ. Expression of homologous RND efflux pump genes is dependent upon AcrB expression: implications for efflux and virulence inhibitor design. J Antimicrob Chemother. 2015;70:424–431. doi: 10.1093/jac/dku380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piddock LJ. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev. 2006;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blair JM, Bavro VN, Ricci V, et al. AcrB drug-binding pocket substitution confers clinically relevant resistance and altered substrate specificity. Proc Natl Acad Sci U S A. 2015;112:3511–3516. doi: 10.1073/pnas.1419939112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang L, Shi H, Zhang L, et al. Emergence of two AcrB substitutions conferring multidrug resistance to Salmonella spp. Antimicrob Agents Chemother. 2023;65:e01589–20. doi: 10.1128/AAC.01589-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Opperman TJ, Nguyen ST. Recent advances toward a molecular mechanism of efflux pump inhibition. Front Microbiol. 2015;6:421. doi: 10.3389/fmicb.2015.00421.564b416639ef4639993c51c3dade2ee5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Renau TE, Leger R, Flamme EM, et al. Inhibitors of efflux pumps in Pseudomonas aeruginosa potentiate the activity of the fluoroquinolone antibacterial levofloxacin. J Med Chem. 1999;42:4928–4931. doi: 10.1021/jm9904598. [DOI] [PubMed] [Google Scholar]
- 77.Nakashima R, Sakurai K, Yamasaki S, et al. Structural basis for the inhibition of bacterial multidrug exporters. Nature. 2013;500:102–106. doi: 10.1038/nature12300. [DOI] [PubMed] [Google Scholar]
- 78.Bohnert JA, Kern WV. Selected arylpiperazines are capable of reversing multidrug resistance in Escherichia coli overexpressing RND efflux pumps. Antimicrob Agents Chemother. 2005;49:849–852. doi: 10.1128/AAC.49.2.849-852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y, Alenazy R, Gu X, et al. Design and structural optimization of novel 2H-benzo[h]chromene derivatives that target AcrB and reverse bacterial multidrug resistance. Eur J Med Chem. 2021;213:113049. doi: 10.1016/j.ejmech.2020.113049. [DOI] [PubMed] [Google Scholar]
- 80.Opperman TJ, Kwasny SM, Kim HS, et al. Characterization of a novel pyranopyridine inhibitor of the AcrAB efflux pump of Escherichia coli. Antimicrob Agents Chemother. 2014;58:722–733. doi: 10.1128/AAC.01866-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ple C, Tam HK, Vieira Da Cruz A, et al. Pyridylpiperazine-based allosteric inhibitors of RND-type multidrug efflux pumps. Nat Commun. 2022;13:115. doi: 10.1038/s41467-021-27726-2.2d5e83c99b5c4265bf969d47fed84ba5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jesin JA, Stone TA, Mitchell CJ, Reading E, Deber CM. Peptide-based approach to inhibition of the multidrug resistance efflux pump AcrB. Biochemistry. 2020;59:3973–3981. doi: 10.1021/acs.biochem.0c00417. [DOI] [PubMed] [Google Scholar]
- 83.Abdali N, Parks JM, Haynes KM, et al. Reviving antibiotics: efflux pump inhibitors that interact with AcrA, a membrane fusion protein of the AcrAB−TolC multidrug efflux pump. ACS Infect Dis. 2017;3:89–98. doi: 10.1021/acsinfecdis.6b00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Darzynkiewicz ZM, Green AT, Abdali N, et al. Identification of binding sites for efflux pump inhibitors of the AcrAB−TolC Component AcrA. Biophys J. 2019;116:648–658. doi: 10.1016/j.bpj.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grimsey EM, Fais C, Marshall RL, et al. Chlorpromazine and amitriptyline are substrates and inhibitors of the AcrB multidrug efflux pump. mBio. 2020;11:e00465–20. doi: 10.1128/mBio.00465-20.c0b86e8c528248f1b64fee868c1d93ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sobhy MK, Mowafy S, Lasheen DS, Farag NA, Abouzid KAM. 3D-QSAR pharmacophore modelling, virtual screening and docking studies for lead discovery of a novel scaffold for VEGFR 2 inhibitors: design, synthesis and biological evaluation. Bioorg Chem. 2019;89:102988. doi: 10.1016/j.bioorg.2019.102988. [DOI] [PubMed] [Google Scholar]
- 87.Phan TV, Nguyen VT, Nguyen CH, et al. Discovery of AcrAB−TolC pump inhibitors: virtual screening and molecular dynamics simulation approach. J Biomol Struct Dyn. 2023:1–18. doi: 10.1080/07391102.2023.2175381. [DOI] [PubMed] [Google Scholar]
- 88.Abdel-Halim H, Al Dajani A, Abdelhalim A, Abdelmalek S. The search of potential inhibitors of the AcrAB−TolC system of multidrug-resistant Escherichia coli: an in silico approach. Appl Microbiol Biotechnol. 2019;103:6309–6318. doi: 10.1007/s00253-019-09954-1. [DOI] [PubMed] [Google Scholar]
- 89.Lomovskaya O, Bostian KA. Practical applications and feasibility of efflux pump inhibitors in the clinic-a vision for applied use. Biochem Pharmacol. 2006;71:910–918. doi: 10.1016/j.bcp.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 90.Bolla JM, Alibert-Franco S, Handzlik J, et al. Strategies for bypassing the membrane barrier in multidrug resistant Gram-negative bacteria. FEBS Lett. 2011;585:1682–1690. doi: 10.1016/j.febslet.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 91.Crass RL, Pai MP. Pharmacokinetics and Pharmacodynamics of beta-Lactamase Inhibitors. Pharmacotherapy. 2019;39:182–195. doi: 10.1002/phar.2210. [DOI] [PubMed] [Google Scholar]
- 92.Reading E, Ahdash Z, Fais C, et al. Perturbed structural dynamics underlie inhibition and altered efflux of the multidrug resistance pump AcrB. Nat Commun. 2020;11:5565. doi: 10.1038/s41467-020-19397-2.f67d2dabeba6419d899fd8c49aa914be [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pettersen EF, Goddard TD, Huang CC, et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 2021;30:70–82. doi: 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goddard TD, Huang CC, Meng EC, et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 2018;27:14–25. doi: 10.1002/pro.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nikaido H, Pages JM. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol Rev. 2012;36:340–363. doi: 10.1111/j.1574-6976.2011.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang-Kan X, Blair JMA, Chirullo B, et al. Lack of AcrB efflux function confers loss of virulence on Salmonella enterica Serovar Typhimurium. mBio. 2017;8:e00968–17. doi: 10.1128/mBio.00968-17.91114a33f62a45c7933725d7402ec642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Atzori A, Malviya VN, Malloci G, et al. Identification and characterization of carbapenem binding sites within the RND-transporter AcrB. Biochim Biophys Acta Biomembr. 2019;1861:62–74. doi: 10.1016/j.bbamem.2018.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.