Visual Abstract
Keywords: 161Tb, specifications, DOTATOC, GMP compliant, automated
Abstract
161Tb is an interesting radionuclide for application in the treatment of neuroendocrine neoplasms’ small metastases and single cancer cells because of its conversion and Auger-electron emission. Tb has coordination chemistry similar to that of Lu; therefore, like 177Lu, it can stably radiolabel DOTATOC, one of the leading peptides used for the treatment of neuroendocrine neoplasms. However, 161Tb is a recently developed radionuclide that has not yet been specified for clinical use. Therefore, the aim of the current work was to characterize and specify 161Tb and to develop a protocol for the synthesis and quality control of 161Tb-DOTATOC with a fully automated process conforming to good-manufacturing-practice guidelines, in view of its clinical use. Methods: 161Tb, produced by neutron irradiation of 160Gd in high-flux reactors followed by radiochemical separation from its target material, was characterized regarding its radionuclidic purity, chemical purity, endotoxin level, and radiochemical purity (RCP) in analogy to what is described in the European Pharmacopoeia for no-carrier-added 177Lu. In addition, 161Tb was introduced into a fully automated cassette-module synthesis to produce 161Tb-DOTATOC, as used for 177Lu-DOTATOC. The quality and stability of the produced radiopharmaceutical in terms of identity, RCP, and ethanol and endotoxin content were assessed by means of high-performance liquid chromatography, gas chromatography, and an endotoxin test, respectively. Results: 161Tb produced under the described conditions showed, as the no-carrier-added 177Lu, a pH of 1–2, radionuclidic purity and RCP of more than 99.9%, and an endotoxin level below the permitted range (175 IU/mL), indicating its appropriate quality for clinical use. In addition, an efficient and robust procedure for the automated production and quality control of 161Tb-DOTATOC with clinically applicable specifications and activity levels, that is, 1.0–7.4 GBq in 20 mL, was developed. The radiopharmaceutical’s quality control was also developed using chromatographic methods, which confirmed the product’s stability (RCP ≥ 95%) over 24 h. Conclusion: The current study demonstrated that 161Tb has appropriate features for clinical use. The developed synthesis protocol guarantees high yields and safe preparation of injectable 161Tb-DOTATOC. The investigated approach could be translated to other DOTA-derivatized peptides; thus, 161Tb could be successfully applied in clinical practice for radionuclide therapy.
In recent years, peptide receptor radionuclide therapy (PRRT) has emerged as an option in metastatic or nonresectable neuroendocrine neoplasms (NENs) expressing high levels of somatostatin receptor (1,2). DOTATOC and DOTATATE are most commonly used for PRRT in NENs (3). In particular, in the neuroendocrine tumors therapy phase 3 randomized trial (4), 177Lu-DOTATATE treatment was confirmed as effective in tumor control with only minor side effects. It was approved by the European Medicines Agency in 2017 and the U.S. Food and Drug Administration in 2018 (Lutathera; Advanced Accelerator Applications) for the treatment of well-differentiated gastroenteropancreatic NENs (5,6). Despite its efficacy, studies have since shown partial remission of no more than 50%, with complete response of no more than 18% (4,7,8). The radionuclide 161Tb (Mean β− energy, 154 keV [100%]; half-life, 6.96 d) (9,10) is proposed as a potential alternative to 177Lu (Mean β− energy, 134 keV [100%]; half-life, 6.65 d) (11) because of their similar physical decay characteristics with regard to β−-particle emission, suitability for PRRT, and concomitant emission of photons, which can be used for SPECT imaging purposes (Table 1) (12,13). In addition, Tb has similar coordination chemistry to Lu (14); therefore, it can be stably coordinated with a DOTA chelator and respective tumor-targeting peptides, for example, DOTATOC or DOTATATE. 161Tb is regarded as superior to 177Lu because it coemits a substantial number of conversion and Auger electrons (∼12 e−, ∼37 keV per decay for 161Tb, ∼1 e− and ∼1.0 keV per decay for 177Lu, respectively) (9,11,13), which would be more effective in the treatment of the smallest metastases, as well as single cancer cells (13,15–19). 161Tb production for radiopharmaceutical application was developed and is regularly performed at the Paul Scherrer Institute. It is produced by neutron irradiation of enriched 160Gd targets. The separated 161Tb product yields 161TbCl3 in a quality suitable for highly specific radiolabeling, which is useful for preclinical applications (13,18–20). At a clinical level, phantom studies were performed that demonstrated the feasibility of imaging 161Tb in high resolution when using low-energy, high-resolution collimators (21); a first-in-humans study was also conducted (22). The next step in the development of this radionuclide toward medical application is its introduction into drug manufacture under good manufacturing practice (GMP) to be able to demonstrate the higher efficacy of 161Tb-based radiopharmaceuticals than of 177Lu-labeled counterparts in clinical trials. An approach to demonstrate the sufficient quality of the 161TbCl3 solution for clinical purposes was to compare it with the specifications of commercially available no-carrier-added 177LuCl3, because the latter is approved for the preparation of several radiopharmaceuticals for clinical studies (4,23). One purpose of this study was to characterize 161Tb for clinical use. After this assessment, a protocol for the production of 161Tb-DOTATOC was developed, conforming to GMP principles, on an automated synthesis module.
TABLE 1.
161Tb Specifications Until Shelf-Life Expiration Date (9 Days After End of Separation)
| Characteristic | Test | 161TbCl3 specification |
|---|---|---|
| Appearance | Visual inspection | Clear and colorless solution |
| Identity | γ-spectrometry (keV) | 74.6 ± 1, 87.9 ± 1, 103.1 ± 1, 106.1 ± 1, 292.4 ± 1, and 550.3 ± 1 |
| pH | pH paper | 1–2 |
| Chemical purity | ICP-MS (μg/GBq) | Cu: <1.0 |
| Fe: <0.5 | ||
| Pb: <0.5 | ||
| Zn: <1.0 | ||
| Sterility | Not required | |
| Bacterial endotoxins | LAL test | <175 IU/mL (injectable dose) |
| Radionuclidic purity | γ-spectrometry | 160Tb ≤ 0.1% |
| RCP | TLC | ≥99.0% as 161TbCl3 |
ICP-MS = inductively coupled plasma–mass spectrometry; LAL = Limulus amebocyte lysate; TLC = thin-layer chromatography.
MATERIALS AND METHODS
161Tb Production and Quality Control (QC)
161Tb was produced by neutron irradiation of enriched 160Gd2O3 targets (98.2% enrichment; Isoflex; Supplemental Table 1 [supplemental materials are available at http://jnm.snmjournals.org]), via the 160Gd(n,γ)161Gd→161Tb nuclear reaction at the South African Fundamental Atomic Research Installation (South African Nuclear Energy Corp.; ∼2·1014 n·cm−1·s−1 neutron flux) or the Institut Laue-Langevin’s High Flux Reactor (∼1·1015 n·cm−1·s−1 neutron flux). The quartz ampoule, containing the irradiated target material, was processed at the Paul Scherrer Institute for radiochemical separation of the produced 161Tb from the target material, as described previously (20). An approach to demonstrate the high quality of the 161TbCl3 product was to compare it with the specifications of commercially available no-carrier-added 177Lu, approved for the preparation of radiopharmaceuticals for clinical studies, described in the European Pharmacopoeia (Ph. Eur.) monograph (24).
Identity, pH, and Radionuclidic Purity
The identification and radionuclidic purity of 161Tb were examined by γ-spectrometry using a high-purity germanium detector (Canberra), in combination with the InterWinner software package (version 7.1; Itech Instruments; supplemental materials and Supplemental Fig. 1). The choice of γ-rays used for a reliable radionuclide identification accounted for possible interferences from other radionuclides that could create artifacts (supplemental materials). The pH of the 161TbCl3 solution was assessed by means of pH paper.
Chemical Purity
The chemical purity of the 161TbCl3 solution was assessed via inductively coupled plasma–mass spectrometry (Element HR; Thermo Fisher Scientific; supplemental materials). Four batches of 161Tb were analyzed after the radiochemical purification for contents of Cu, Fe, Pb, Zn, Gd, and Tb, as described in the Ph. Eur. monograph for 177Lu (24). The purity was also investigated in all 7 161Tb productions performed during this study by means of apparent molar activity (AMA) determination through hand-operated labeling of the product to DOTATOC (supplemental materials), as described elsewhere (13,20).
Endotoxin Level
Because 161Tb is considered a radiopharmaceutical precursor, the bacterial endotoxin level needs to be tested. Pyrogens were tested according to Ph. Eur. standards using a chromogenic Limulus amebocyte lysate test (supplemental materials) (25). In the evaluation of the endotoxin level, international units per milliliter were used, considering that the unit used in the Ph. Eur. monograph is international units per volume, indicating the maximum volume to be used for the preparation of a single patient dose, which corresponds to 1 mL of 161Tb (maximum volume at the end of separation).
Radiochemical Purity (RCP)
The RCP of 3 161Tb batches was assessed by thin-layer chromatography, as described in the Ph. Eur. monograph for 177Lu (supplemental materials) (24). Quantification of the thin-layer chromatography signals with the indicated software enabled the determination of the area of the peak of 161Tb in ionic form (Rf = 0.4–0.7), over the total activity.
Automated Synthesis of 161Tb-DOTATOC
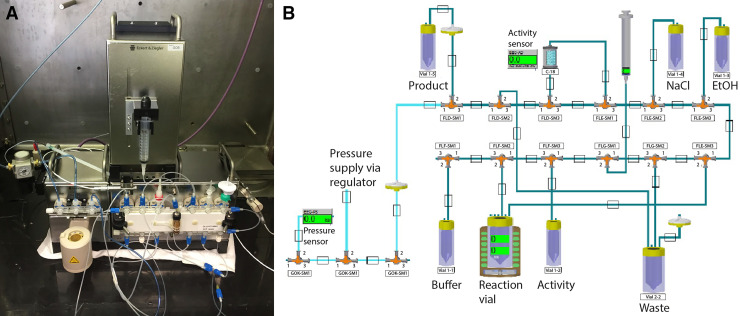
Ten syntheses of 161Tb-DOTATOC were performed using a Modular-Lab PharmTracer fully automated cassette synthesis system (Eckert & Ziegler; Fig. 1A), installed in a hot cell so that high activities of the radionuclide could be manipulated with no radiation dose exposure to the operator. For each synthesis, the sterile synthesis cassettes were prepared with the necessary reagents and attached to the Modular-Lab’s synthesis module (supplemental materials). A synthesis protocol was adequately adapted and optimized, and the production of 161Tb-DOTATOC was run by the Modular-Lab PharmTracer software (Modular-Lab SoftPLC; Eckert & Ziegler), which provided a graphical display of each step and the progress of the synthesis together with audit trail as required by GMP (Fig. 1B) (26,27). To ensure sterility, the final product was finally passed through a 0.22-μm filter (Medical Millex-GV Syringe Filter, Hydrophilic Polyvinylidene Fluoride, γ-sterilized; Merck Millipore). At the end of the synthesis, the final product activity and the residual radioactivity remaining in the principal components (activity vial, reaction vial, C18 cartridge, and waste) were measured (Isomed Dose Calibrator 2010) (28). Aliquots were withdrawn from the total volume for QC purposes.
FIGURE 1.
(A) Eckert & Ziegler Modular-Lab PharmTracer fully automated cassette synthesis system. (B) Graphical display of cassette components and synthesis process.
161Tb-DOTATOC QC
High-Performance Liquid Chromatography (HPLC)
QC was performed to determine the RCP of 161Tb-DOTATOC, as well as the identification of the compound and possible impurities, by means of HPLC (Dionex P680 LGP Pump). First, the retention times of the product peaks in the radiochromatogram and UV chromatogram were assessed (Supplemental Figs. 2 and 3). Eventually, during the QC of 161Tb-DOTATOC, 20 μL of the synthesis product were analyzed by means of HPLC to estimate the RCP of 161Tb-DOTATOC from the HPLC radiodetector chromatogram and to identify the compound and possible impurities in the UV chromatogram.
Further evaluations of the method were performed to investigate the suitability and robustness of the QC. In particular, the linearity, limit of detection, and limit of quantification for both the radiodetector and the UV detector were assessed (Supplemental Fig. 4), together with the setup of a system suitability test (Supplemental Fig. 5).
Gas Chromatography
The ethanol content in the synthesis product was assessed by means of gas chromatography. The quantification of ethanol was based on the acetonitrile internal standard (supplemental materials). The linearity of the gas chromatography method was assessed by linear regression analysis for 6 ethanol concentrations between 1% and 15% (Supplemental Fig. 6).
Sterility and Endotoxin Level
Although the synthesis was not performed under GMP-compliant clean conditions, the pyrogenicity of the product and the integrity of the sterilization filter were evaluated to assess the suitability of the methods and to prove the acceptable quality of 161Tb-DOTATOC. Pyrogens were tested according to Ph. Eur. standards with a chromogenic Limulus amebocyte lysate test, as described earlier for the endotoxin test on the 161TbCl3 solution (25).
To ensure the integrity of the sterilization filter used at the end of the synthesis, a bubble point test was developed. The threshold value of the pressure depends on the matrix of the sterilized solution; therefore, it was assessed for the product in question (supplemental materials) (29). The bubble point test was then performed on the filter used during the synthesis of the batch tested for bacterial endotoxins.
Stability
The stability of the synthesis product, 161Tb-DOTATOC, was tested in both normal and stress conditions. To establish the stability of the radiolabeled peptide, all synthesized batches of 161Tb-DOTATOC were stored at room temperature and the RCP was assessed 3 and 24 h after synthesis. In addition, 1 batch of DOTATOC radiolabeled with high activity (8.77 GBq) was stored at room temperature for 96 h and the RCP was assessed by means of HPLC every 24 h with the method described earlier. After 96 h at room temperature, the product was incubated at 40 °C for an additional 72 h and HPLC analyses were conducted every 24 h.
RESULTS
161Tb Production and QC
Multiple productions of 161Tb were analyzed, compared with the Ph. Eur. monograph for 177Lu, and found to comply with all requirements (Table 1).
Radionuclidic Purity
The content of the long-lived impurity 160Tb (half-life, 72.3 d) (30) was less than 0.005% of the total 161Tb activity at end of bombardment (EOB), as reported previously by our group (20). Three weeks after EOB, the level of 160Tb was still no more than 0.03% (Supplemental Fig. 1; Supplemental Table 2). Therefore, the specification for radionuclidic purity, which must apply until the end of shelf life, was prudently proposed for 160Tb at a level of 0.1%. With this level of impurity, the additional radiation dose to the patient was estimated to be about 0.4% (supplemental materials) (31).
Chemical Purity
The content of Cu, Fe, Pb, and Zn measured in the 161Tb samples was far below the limit for metal impurities reported for 177Lu (Supplemental Table 3). The Gd content was less than 0.4 ppm. Moreover, 6 of the 7 161Tb productions were tested for AMA and successfully labeled DOTATOC at 100 MBq/nmol. Even so, the 161Tb production that allowed labeling of only the lower AMA was sufficiently pure to be used for 161Tb-DOTATOC synthesis (Table 2). Thanks to Tb quantification, the specific activity was also calculated and resulted in values greater than 3.5 GBq/μg.
TABLE 2.
161Tb Activity at EOS 161Tb Activity and Measured Before and at End of Synthesis for Each 161Tb-DOTATOC Production
| Activity EOS (GBq) | Labeling yield at 100 MBq/nmol AMA (%) | Synthesis | Initial activity (GBq) | Bulk product activity (GBq) | Production yield (%) |
|---|---|---|---|---|---|
| 13.4 | 99.9 | 1 | 1.29 | 1.12 | 86.8 |
| 2 | 1.16 | 1.08 | 93.1 | ||
| 15.3 | 99.9 | 3 | 1.19 | 1.13 | 95.0 |
| 15.2 | 100 | 4 | 8.80 | 8.77 | 99.7 |
| 5.78 | 68.0 | 5 | 1.33 | 1.25 | 94.0 |
| 6 | 2.87 | 2.79 | 97.2 | ||
| 11.7 | 99.9 | 7 | 9.26 | 8.92 | 96.3 |
| 11.8 | 99.7 | 8 | 9.17 | 9.08 | 99.0 |
| 14.3 | 100 | 9 | 9.39 | 9.26 | 98.6 |
| 10 | 1.56 | 1.50 | 96.2 |
EOS = end of separation.
Endotoxin Level
The endotoxin content of the 3 batches tested was less than 1.20, 1.00, and 1.65 IU/mL, respectively, considerably lower than the requirement of less than 175 IU/mL.
RCP
In all productions tested, the peak of 161Tb in ionic form (Rf = 0.4–0.7) over the total activity (161Tb-diethylenetriaminepentaacetic acid, Rf = 0.9) was above 99.0%, thereby allowing the RCP of 161Tb to be established as greater than 99.0%.
Automated Synthesis of 161Tb-DOTATOC
Ten preparations of 161Tb-DOTATOC were performed and form part of this work. For all syntheses, the 161Tb-labeled radioactivity at the shelf-life expiration date was in the range from 1 to 7.4 GBq ± 10%, which was the target to obtain a product comparable to a patient dose for 177Lu-DOTATOC (32). In particular, 5 syntheses produced 161Tb-DOTATOC with the minimum activity, 4 syntheses produced 161Tb-DOTATOC with the maximum activity, and 1 synthesis produced 161Tb-DOTATOC with intermediate activity (Table 2). The AMA was maintained at a constant of approximately 50 MBq/nmol, varying the peptide amount from 27 μg to a maximum of 200 μg (32). The duration of the process was approximately 45 min, and the total volume of the bulk product at the end of the synthesis was 24 mL, of which 4 mL were intended for QC and 20 mL were intended as final product. The final formulation was established in analogy to that of 177Lu-DOTATOC (Table 3).
TABLE 3.
Composition of 161Tb-DOTATOC (bulk product)
| Characteristic | Data |
|---|---|
| Range of 161Tb activity (shelf-life expiration date) | 1–7.4 GBq |
| Volume | 24 mL |
| DOTATOC and metal complexes | 1 μg/37 MBq (maximum, 200 μg) |
| Calcium-DTPA | 19.5 mg (0.1 mL, 195 mg/mL) |
| Ascorbic acid | 250 mg (2.5 mL, 100 mg/mL) |
| Ethanol | 1.25 mL (2.5 mL, ethanol 50%) |
| Saline | 19 mL |
DTPA = diethylenetriaminepentaacetic acid.
All syntheses produced 161Tb-DOTATOC within ±10% of stated parameters.
Residual radioactivity was detected in the activity vial, the reaction vial, the C18 cartridge, and the waste (Supplemental Table 4). Unlike the bulk product and initial activity measurements, which were performed with appropriate calibration for each container (28), the residual activity measurements were not comparable to one another, or to the initial activity, because of the distinct geometries of the samples. Nevertheless, the measurements indicated losses mainly in the waste or because of bound activity on the C18 cartridge. However, a total yield of more than 85% of 161Tb-DOTATOC, in reference to the initial 161Tb activity, was determined (Table 2).
161Tb-DOTATOC QC
The synthesis product complied with the established specifications (Table 4).
TABLE 4.
161Tb-DOTATOC Specifications Until Shelf-Life Expiration Date
| Characteristic | Test | Specification |
|---|---|---|
| Appearance | Visual inspection | Clear, colorless solution, without visible particles |
| pH | pH paper | 4–8 |
| Activity concentration | Dose calibrator | 50–370 MBq/mL |
| RCP | HPLC | ≥95% |
| 161Tb-DOTATOC identity | HPLC | Peaks at defined RT in radio- and UV chromatograms |
| Impurities and degradation products | HPLC | Each impurity ≤ 100 μg/patient dose |
| Ethanol content | GC | <10% |
| Bacterial endotoxin level | LAL test | <175 IU/20 mL |
| Filter integrity (matrix-based) | bubble point test | Bubble point > 2.75 bar |
| Shelf life | 24 h |
RT = retention time; GC = gas chromatography.
HPLC
The results of the HPLC analysis of the synthesis products demonstrated that the RCP of 161Tb-DOTATOC was, on average, approximately 98% at the end of synthesis (n = 10; 98.2% ± 0.82; Supplemental Table 5), which meets the 95% specification level normally applied to these types of products (Fig. 2) (33,34).
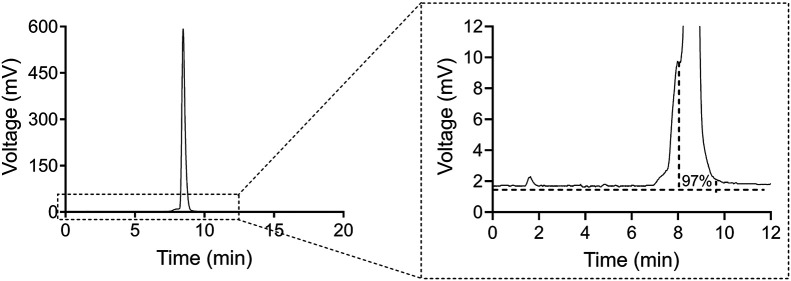
FIGURE 2.
Representative HPLC radiochromatogram of high-activity batch of 161Tb-DOTATOC with RCP of 97.0% (1.6-min retention time would indicate 161Tb-diethylenetriaminepentaacetic acid, whereas 8.5 min indicates 161Tb-DOTATOC).
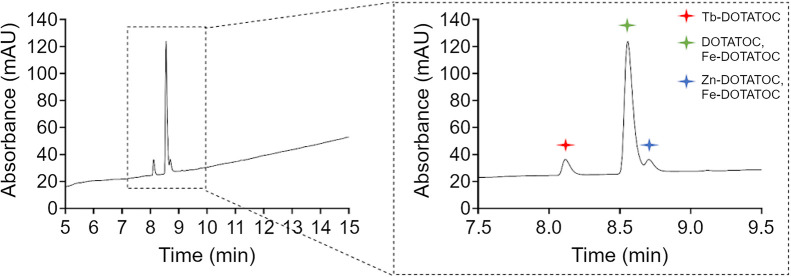
The UV chromatograms showed low levels or complete nonappearance of impurities. The peaks of the unlabeled peptide (DOTATOC), together with those of Tb-DOTATOC, Fe-DOTATOC, and Zn-DOTATOC, established during the development of the HPLC method, were identified during the QC of the synthesis product and considered product peaks (Fig. 3).
FIGURE 3.
Representative HPLC UV chromatogram obtained during QC of synthesis product. Product peaks (DOTATOC, Tb-DOTATOC, Fe-DOTATOC, and Zn-DOTATOC) were identified, and no other peaks (impurities) were observed. milli Absorbance Unit.
Gas Chromatography
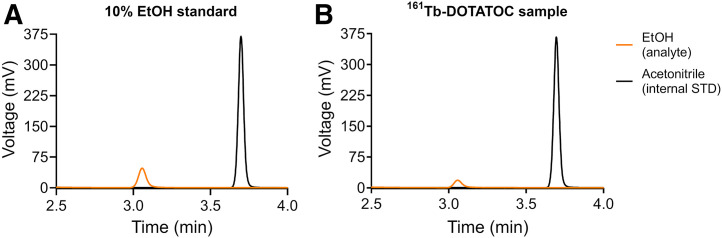
The ethanol content in the synthesis product was demonstrated to be less than 10%, which is the acceptable limit for radiopharmaceutical preparation for intravenous injection (35). In particular, the area of the ethanol peak in the analyzed sample of the synthesis product was normalized to the internal standard peak area and then compared with the normalized area of the ethanol peak in the 10% ethanol standard solution. In Figure 4, the ethanol peaks of the standard solution and the synthesis product are visually compared, with the lower ethanol content in the synthesis product displayed.
FIGURE 4.
Representative gas chromatograms of 10% ethanol standard solution (A) and of sample of synthesis product (B). In both solutions, acetonitrile was added as internal standard (STD).
Sterility and Endotoxin Level
The synthesis resulted in a clear, colorless, particle-free product with a low level of bacterial endotoxins in the batch tested (<6.15 international units/mL). This endotoxin content was compliant with the specification for radiopharmaceutical products defined by the Ph. Eur. monograph (≤175 international units/20 mL) (25,33).
Although the sterility of the final product requires further validation, the integrity of the sterilization filter used at the end of the synthesis was successfully proven with a bubble point test. In particular, the bubble point was less than 2.75 bar, as specified for the product matrix (Supplemental Table 6).
Stability
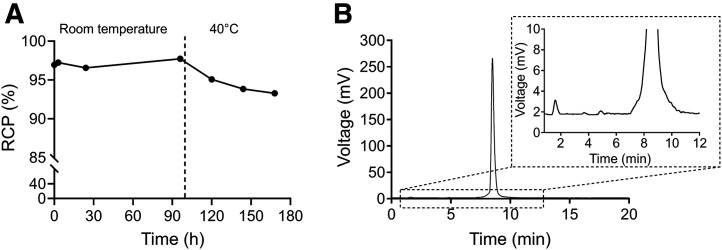
161Tb-DOTATOC presented RCP of more than 95% up to 24 h for all batches tested (n = 9; 97.6% ± 1.0 at 24 h; Supplemental Table 5). In addition, the RCP results of the batch selected for the stability test under stress conditions indicated that 161Tb-DOTATOC was highly stable at room temperature for up to 4 d (Fig. 5A). When the product was incubated at 40 °C, it demonstrated RCP of less than 95% over a period of 48 h, with increased released 161Tb and radiolysis (Fig. 5B).
FIGURE 5.
(A) 161Tb-DOTATOC (8.77-GBq batch) stability for 4 d at room temperature and additional 3 d at 40 °C. (B) HPLC radiochromatogram of 161Tb-DOTATOC after 48 h at 40 °C (RCP of 93.8%).
DISCUSSION
The aim of this work was to characterize 161Tb for clinical use, creating a list of parameters that can be the basis for future official specifications and monographic standards. The study compared 177Lu specifications with 161Tb features, showing that the latter comply with all requirements reported in the Ph. Eur. monograph for 177Lu (24). With regard to radionuclidic purity, long-lived 160Tb (half-life, 72.3 d) (30) was found as an impurity; it is coproduced by the 159Tb(n,γ)160Tb nuclear reaction, and the multistep reactions on the residual 159Tb, 158Gd, and 157Gd present as impurities in the target material. However, the content did not exceed 0.005% of the total 161Tb activity at EOB. Three weeks after EOB, the ingrowth of 160Tb was still no more than 0.03%. These results were observed in several productions after the irradiation of various amounts of target material with different neutron fluxes. According to previous studies by our group (20), the quality of 161Tb in terms of radiolabeling yield and AMA was comparable to that of commercially available 177Lu for 9 d after end of separation. This would prudently set the specifications to a shelf life of 9 d after end of separation and a radionuclidic purity of at least 99.9%. Because the latter would be true until 21 d after EOB, to comply with these specifications, the separation cannot be performed later than 12 d after EOB. However, these assumptions may no longer appear to be true if the target material has a different enrichment level from that used in this study; hence, more research is foreseen to examine this subject in greater detail. In addition, our earlier reports on the radiolabeling yield were supported by the results from this study with regard to 161Tb chemical purity and RCP. RCP tests showed that more than 99% of 161Tb was present in the chloride form, which is the expected chemical form after radiochemical separation, ensuring its direct use for radiolabeling. In terms of chemical impurities, the tested metals complied with the same requirement as stated for 177Lu. Moreover, only trace amounts of Gd were measured, implying the success of the radiochemical separation from the target material to provide 161Tb with a quality suitable for clinics. The specific activity resulted in more than 3.5 GBq/μg, which is comparable to what was achieved for commercially available no-carrier-added 177Lu (36). Because 161TbCl3 solution is not intended for direct administration to humans or for use in kit-type products at this stage of development, sterility does not need to be guaranteed (24). However, the product needs to be nonpyrogenic; therefore, pyrogens were tested according to Ph. Eur. standards with a chromogenic Limulus amebocyte lysate test (25), resulting in values below the recommended level.
Taking the preceding observations into consideration, one can see that 161Tb-labeled radiopharmaceuticals can easily be produced and made GMP-compliant for routine clinical use. The production, including synthesis and QC, of 161Tb-DOTATOC, the 161Tb equivalent of the somatostatin analogs currently used with 177Lu in clinical practice (37,38), was demonstrated. An Eckert & Ziegler cassette synthesis module, with a modified sequence, was applied to radiolabel DOTATOC with levels of 161Tb radioactivity suitable for a patient dose comparable to that used for 177Lu-DOTATOC, that is, 7.4 GBq in 20 mL (32). Ten productions were performed, yielding approximately 50 MBq/nmol AMA with up to 9.3 GBq of 161Tb-DOTATOC produced, which was enough to guarantee the required activity dose for a shelf life of 24 h. Precise activity measurement of 161Tb, which emits γ-radiation mainly with energies below 100 keV, becomes inaccurate with radionuclide dose calibrators used without appropriate calibration factors. An intensive investigation for the calibration of the dose calibrators with the geometries used in the manufacturing process was conducted in collaboration with the certified Institut de Radiophysique and is reported elsewhere (28). The 24 h-shelf life was established to reduce the activity use during the development work, but according to the stability studies, it could easily be extended for up to 4 d. The design of the synthesis system was based on disposable and sterilized flow paths that, together with the final filtration through a 0.22-mm filter, are expected to deliver a pharmaceutically adequate product (33,34). In addition, during the synthesis, 161Tb-DOTATOC was passed through a C18 cartridge to guarantee the removal of unreacted 161Tb. However, the final formulation contained diethylenetriaminepentaacetic acid to ensure the coordination of possible traces of released 161Tb, which is then quickly eliminated via renal excretion, preventing bone uptake of activity after the administration (39). The final product was then tested for RCP and ethanol and endotoxin content, which were determined to be in the acceptable ranges for pharmaceutical preparations. A tendency toward a lower RCP was observed in batches with high activity levels, which may be attributed to the greater activity concentration that caused the peptide to undergo more radiolysis. However, the RCP values for all products were far higher than the criterion for RCP of more than 95%. As a result of these assessments, further purification of the peptide was not required.
CONCLUSION
In the present work, it was demonstrated that 161Tb shows quality standards comparable to those of 177Lu, implying its suitability for potential clinical use. In addition, an efficient and robust procedure for the automated production and QC of 161Tb-DOTATOC was developed using an automated cassette synthesis module and appropriate analytic techniques. The process described was evaluated in accordance with existing guidelines and quality standards. This will form the foundation of monographic standards for this nuclide and further attempts to introduce the use of Tb radioisotopes in clinical applications.
DISCLOSURE
This work was financially supported by the Swiss National Science Foundation (200021_188495) and the NET Research Foundation Petersen Award. No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
We acknowledge Prof. Dr. Jan Rijn Zeevaart for the supervision and arrangement of the neutron irradiations at the South Africa Fundamental Atomic Research Installation facility, as well as Colin C. Hillhouse for technical support in the radiochemical separations. We thank David E. Schmid and Valeria Farsaci for the quality assurance/QC support and Rolf Hesselmann for regulatory counsel. We also thank Matthias Martin, Dr. Peter Sprung, and Adelheid Fankhauser for the inductively coupled plasma–mass spectrometry analysis and evaluations, as well as Dr. Francesca Borgna for her contribution in the previous work that led to this project. We are grateful to Prof. Dr. Peter Bernhard for his support in the dosimetry calculations.
KEY POINTS
QUESTION: Why is it important to develop a robust production of 161Tb and derived radiopharmaceuticals for the treatment of NENs?
PERTINENT FINDINGS: The newly developed radionuclide 161Tb was characterized for clinical use, and fully automated synthesis of 161Tb-DOTATOC was achieved, showing positive results regarding the safety and quality of the product.
IMPLICATIONS FOR PATIENT CARE: These findings would form the foundation of the introduction of 161Tb for clinical applications in the treatment of NENs, which could improve the outcome of the therapy because of its conversion and Auger electron emission targeting small metastases and single cancer cells.
REFERENCES
- 1. Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. [DOI] [PubMed] [Google Scholar]
- 2. Camus B, Cottereau AS, Palmieri LJ, et al. Indications of peptide receptor radionuclide therapy (PRRT) in gastroenteropancreatic and pulmonary neuroendocrine tumors: an updated review. J Clin Med. 2021;10:1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kam BLR, Teunissen JJM, Krenning EP, et al. Lutetium-labelled peptides for therapy of neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2012;39(suppl 1):S103–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177Lu-DOTATATE for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Committee for Medicinal Products for Human Use. Assessment Report: Lutathera. European Medicines Agency; 2017:1–132. EMA/506460/2017. [Google Scholar]
- 6. Food and Drug Administration (FDA), Department of Health and Human Services. NDA Approval 208700 (reference ID 4212675). FDA website. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/208700Orig1s000Approv.pdf. Published January 26, 2018. Accessed May 3, 2023.
- 7. van Essen M, Krenning EP, Bakker WH, De Herder WW, Van Aken MO, Kwekkeboom DJ. Peptide receptor radionuclide therapy with 177Lu-octreotate in patients with foregut carcinoid tumours of bronchial, gastric and thymic origin. Eur J Nucl Med Mol Imaging. 2007;34:1219–1227. [DOI] [PubMed] [Google Scholar]
- 8. Sansovini M, Severi S, Ambrosetti A, et al. Treatment with the radiolabelled somatostatin analog 177Lu-DOTATATE for advanced pancreatic neuroendocrine tumors. Neuroendocrinology. 2013;97:347–354. [DOI] [PubMed] [Google Scholar]
- 9. Reich CW. Nuclear data sheets for A=161. Nucl Data Sheets (NY NY). 2011;112:2497–2713. [Google Scholar]
- 10. Durán MT, Juget F, Nedjadi Y, et al. Determination of 161Tb half-life by three measurement methods. Appl Radiat Isot. 2020;159:109085. [DOI] [PubMed] [Google Scholar]
- 11. Kondev FG. Nuclear data sheets for A=177. Nucl Data Sheets (NY NY). 2019;159:1–412. [Google Scholar]
- 12. Lehenberger S, Barkhausen C, Cohrs S, et al. The low-energy β− and electron emitter 161Tb as an alternative to 177Lu for targeted radionuclide therapy. Nucl Med Biol. 2011;38:917–924. [DOI] [PubMed] [Google Scholar]
- 13. Müller C, Reber J, Haller S, et al. Direct in vitro and in vivo comparison of 161Tb and 177Lu using a tumour-targeting folate conjugate. Eur J Nucl Med Mol Imaging. 2014;41:476–485. [DOI] [PubMed] [Google Scholar]
- 14. Amoroso AJ, Fallis IA, Pope SJA. Chelating agents for radiolanthanides: applications to imaging and therapy. Coord Chem Rev. 2017;340:198–219. [Google Scholar]
- 15. Hindié E, Zanotti-Fregonara P, Quinto MA, Morgat C, Champion C. Dose deposits from 90Y, 177Lu, 111In, and 161Tb in micrometastases of various sizes: implications for radiopharmaceutical therapy. J Nucl Med. 2016;57:759–764. [DOI] [PubMed] [Google Scholar]
- 16. Bernhardt P, Benjegård SA, Kölby L, et al. Dosimetric comparison of radionuclides for therapy of somatostatin receptor-expressing tumors. Int J Radiat Oncol Biol Phys. 2001;51:514–524. [DOI] [PubMed] [Google Scholar]
- 17. Bernhardt P, Svensson J, Hemmingsson J, et al. Dosimetric analysis of the short-ranged particle emitter 161Tb for radionuclide therapy of metastatic prostate cancer. Cancers (Basel). 2021;13:2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Müller C, Umbricht CA, Gracheva N, et al. Terbium-161 for PSMA-targeted radionuclide therapy of prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46:1919–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borgna F, Haller S, Monné Rodriguez JM, et al. Combination of terbium-161 with somatostatin receptor antagonists: a potential paradigm shift for the treatment of neuroendocrine neoplasms. Eur J Nucl Med Mol Imaging. 2022;49:1113–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gracheva N, Müller C, Talip Z, et al. Production and characterization of no-carrier-added 161Tb as an alternative to the clinically-applied 177Lu for radionuclide therapy. EJNMMI Radiopharm Chem. 2019;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marin I, Rydèn T, Van Essen M, et al. Establishment of a clinical SPECT/CT protocol for imaging of 161Tb. EJNMMI Phys. 2020;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baum RP, Singh A, Kulkarni HR, et al. First-in-human application of terbium-161: a feasibility study using 161Tb-DOTATOC. J Nucl Med. 2021;62:1391–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. European Directorate for the Quality of Medicines and Health Care. Lutetium (177Lu) solution for radiolabelling. In: European Pharmacopoeia. EDQM; 2020:1218–1219. [Google Scholar]
- 25. European Directorate for the Quality of Medicines and Health Care. Bacterial endotoxins. In: European Pharmacopoeia. EDQM; 2020:209–213. [Google Scholar]
- 26. European Commission. Good manufacturing practice medicinal products for human and veterinary use. Annex 11: computerised systems. EudraLex. 2011;4:2005–2007. [Google Scholar]
- 27. Food and Drug Administration (FDA), Department of Health and Human Services. Guidance for Industry: Part 11, Electronic Records; Electronic Signatures. FDA; 2003:1–9. [Google Scholar]
- 28. Juget F, Talip Z, Nedjadi Y, et al. Precise activity measurements of medical radionuclides using an ionization chamber: a case study with terbium-161. EJNMMI Phys. 2022;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hayashi K, Douhara K, Kashino G. Evaluation of the bubble point test of a 0.22-μm membrane filter used for the sterilizing filtration of PET radiopharmaceuticals. Ann Nucl Med. 2014;28:586–592. [DOI] [PubMed] [Google Scholar]
- 30. Nica N. Nuclear data sheets for A=160. Nucl Data Sheets (NY NY). 2021;176:1–428. [Google Scholar]
- 31. Chauvin M, Borys D, Botta F, et al. OpenDose: open-access resource for nuclear medicine dosimetry. J Nucl Med. 2020;61:1514–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bodei L, Mueller-Brand J, Baum RP, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. European Directorate for the Quality of Medicines and Health Care. General monograph: radiopharmaceutical preparations. In: European Pharmacopoeia. EDQM; 2020:884–887. [Google Scholar]
- 34. European Commission. Good Manufacturing Practice Medicinal Products for Human and Veterinary Use Annex 3 Manufacture of Radiopharmaceuticals. EudraLex; 2008:1–8. [Google Scholar]
- 35. Serdons K, Verbuggen A, Bormans G. The presence of ethanol in radiopharmaceutical injections. J Nucl Med. 2008;49:2071. [DOI] [PubMed] [Google Scholar]
- 36. Committee for Medicinal Products for Human Use. Assessment Report: EndolucinBeta. European Medicines Agency. 2016:1–58. EMA/404078/2016.
- 37. Baum RP, Kluge AW, Kulkarni HR, et al. [177Lu-DOTA]0-D-Phe1-Tyr3-octreotide (177Lu-DOTATOC) for peptide receptor radiotherapy in patients with advanced neuroendocrine tumours: a phase-II study. Theranostics. 2016;6:501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uccelli L, Boschi A, Cittanti C, et al. 90Y/177Lu‐DOTATOC: from preclinical studies to application in humans. Pharmaceutics. 2021;13:1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Breeman WAP, van der Wansem K, Bernard BF, et al. The addition of DTPA to [177Lu-DOTA0, Tyr3]octreotate prior to administration reduces rat skeleton uptake of radioactivity. Eur J Nucl Med Mol Imaging. 2003;30:312–315. [DOI] [PubMed] [Google Scholar]