Abstract
Medical internal radiation dosimetry constitutes a fundamental aspect of diagnosis, treatment, optimization, and safety in nuclear medicine. The MIRD committee of the Society of Nuclear Medicine and Medical Imaging developed a new computational tool to support organ-level and suborgan tissue dosimetry (MIRDcalc, version 1). Based on a standard Excel spreadsheet platform, MIRDcalc provides enhanced capabilities to facilitate radiopharmaceutical internal dosimetry. This new computational tool implements the well-established MIRD schema for internal dosimetry. The spreadsheet incorporates a significantly enhanced database comprising details for 333 radionuclides, 12 phantom reference models (International Commission on Radiological Protection), 81 source regions, and 48 target regions, along with the ability to interpolate between models for patient-specific dosimetry. The software also includes sphere models of various composition for tumor dosimetry. MIRDcalc offers several noteworthy features for organ-level dosimetry, including modeling of blood source regions and dynamic source regions defined by user input, integration of tumor tissues, error propagation, quality control checks, batch processing, and report-preparation capabilities. MIRDcalc implements an immediate, easy-to-use single-screen interface. The MIRDcalc software is available for free download (www.mirdsoft.org) and has been approved by the Society of Nuclear Medicine and Molecular Imaging.
Keywords: dosimetry, radiobiology, radionuclide therapy, research methods, dosimetry, MIRD, software
Methods for radiopharmaceutical dosimetry, that is, estimation of absorbed radiation dose, have evolved gradually since the 1950s. Accurate dose assessment requires an accounting of both the spatial and temporal pharmacokinetics of an administered radiopharmaceutical. This characterization defines the biodistribution of a radiopharmaceutical which is used to estimate absorbed dose to the patient. Measurement-based data may be obtained from clinical quantitative imaging (1). The MIRD schema and supporting software represent the standard method for calculating absorbed radiation doses resulting from given biodistributions of administered radiopharmaceuticals (2).
Medical internal radionuclide dosimetry comes with unique challenges; clinicians rely on supporting software and user expertise to make dose calculations timely and practical. Existing software tools vary significantly in design and function and are typically based on different models and assumptions. These tools vary in complexity, ranging from simple lookup tables (3) to computationally intensive Monte Carlo–based radiation transport models (4).
Dosimetry can be performed at different scales, including at the whole-body, organ, suborgan, voxel, and cellular levels. Organ-level dosimetry may stand out in one respect because it provides a balance of relatively personalized dosimetry that can be derived with practical methods based on quantitative PET and SPECT imaging. Calculating absorbed dose to specific organs and tissues allows one to contextualize the dosimetry against known, commonly accepted organ-specific dosimetric thresholds for tissue reactions (5).
Organ-level dosimetry software has been used in the field for many years. For example, the OLINDA/EXM software (6) based on MIRDOSE (developed in the mid-1980s), demonstrated to the nuclear medicine community the utility of software tools for implementing a standardized dosimetry schema. Other organ-level dosimetry software tools include IDAC-Dose 2.1, which is freely available and is used internally within International Commission on Radiological Protection (ICRP) task group 36 on radiopharmaceuticals (7). A companion article is provided which compares dose calculations using the different available software (8). Other software tools extend dosimetric analysis beyond the organ level, including voxel Monte Carlo, kernel convolution, cellular-level, and microdosimetric (9–12) applications. Across the variety of tools, no solution is demonstrably superior across all use cases (13). A contemporary summary of dosimetry software options can be found in chapter 6 of MIRD primer 2022 (14).
The dose calculations provided in MIRDcalc software are based on the well-established MIRD schema and other methods needed for calculating absorbed radiation doses (2). Thus, MIRDcalc was inspired by MIRDOSE and OLINDA/EXM, with attention to their generalizable suitability and practical application. MIRDcalc uses the organ-level dosimetry paradigm as the starting point from which to innovate, exemplified with new features that include error propagation and dynamic source regions. MIRDcalc itself is not a complete package for absorbed dose calculation workflows. It is a robust tool to support the computational aspect of a dosimetry protocol, given an input of time-integrated activity coefficients (TIACs) of the radiopharmaceutical in organs and tissues and specification of a pertinent anatomic model.
The MIRDcalc software project was undertaken to meet the needs of the community for validated, open-source, flexible, and freely accessible dosimetry tools. MIRDcalc is immediately available to physicists, biomedical researchers, and health-care colleagues worldwide, reducing the time required for, and variability of, dosimetry-related computations. The MIRDcalc project also provides a framework for further development and community cooperation and collaboration.
MIRDCALC SOFTWARE
Organ-Level Dosimetry
The MIRD schema for absorbed dose calculation was originally formulated in the 1960s (2,15) as the computational basis for performing dosimetry with models of representative organ geometry, presented in the form of stylized anthropomorphic digital phantoms. The main equations and standardized nomenclature are described in MIRD pamphlet 21 (16) and MIRD primer 2022 (11).
The MIRD framework allows for the logical separation of tasks in the process of calculating absorbed dose. Computationally intensive processing can be performed a priori to establish reference individual- and radiopharmaceutical-specific dose calculation models, which may later be used with subject-specific biodistributions to provide individualized absorbed dose estimates.
The parameters that characterize the dosimetry model are called S values, which quantitatively relate the mean absorbed dose rate to a target organ (or region) per unit of activity in a given source organ (or region). When a user inputs a time-integrated activity distribution, S values stored within the software, based on an anthropomorphic model, are used to quickly estimate radiation doses using the standard MIRD equations (given in the companion article (8)). MIRDcalc provides the models, S values, and interface for performing the absorbed dose calculations.
Absorbed dose estimates calculated in this manner have a limitation because they are usually based on anatomic models of reference individuals (phantoms) of specified sex and age. Models may be extrapolated to represent specific patients. Reference S values account for all dosimetrically relevant anatomy, including regions with complex microstructure (such as bone marrow). MIRDcalc uses a well-established methodology for scaling reference S values for patient-mass–specific absorbed dose calculations (17). MIRDcalc also offers a unique feature to support global scaling of all regions between reference phantoms, on the basis of total-body mass.
Development of Radionuclide S Values
The radionuclide S values within the MIRDcalc program were developed according the MIRD schema. The S-value components for the photon, electron/positron, α-particle, and α-recoil components of the radionuclide decay scheme were computed as…
| Eq. 1 |
where is the specific absorbed fraction (SAF) for radiation particle i emitted in source region and irradiating target region , whereas and are, respectively, the energies and yields of radiation emitted during radionuclide decay as taken from the MIRD monograph on radionuclide data and decay schemes (18).
The full energy spectrum for both β-particles and positrons was used in lieu of considering only their mean energies. The β-particle component of the S value was computed as
| Eq. 2 |
where E is the energy of emission, ranging from zero to endpoint energy , and and are, respectively, the energy-dependent yield and SAF.
All S values were computed using a Python script with SAF interpolation through particle energies using piecewise cubic Hermite interpolation polynomials. For α-recoils, the SAF values were interpolated at a 2-MeV α-particle, an approach previously adopted by the ICRP (19). S values for members of α-emitter decay chains were computed and reported independently for the parent radionuclide and all individual progeny.
For the ICRP reference adult phantoms within MIRDcalc, ICRP publication 133 (19) was used as the source of all SAF values in the computation of S values. These 2 phantoms—reference adult male and reference adult female—are fully described in ICRP publication 110 (20). For the pediatric reference phantoms, as described in ICRP publication 143 (21), SAF values for photons and electrons were taken primarily from the work presented by Schwarz et al. (22,23) with subsequent adjustments for source region blood content when . SAF values that were not fully covered in the 2 articles by Schwarz et al., including SAFs for α-particles, SAFs for localized regions in the respiratory and alimentary tracts, and SAFs for intraskeletal sources and targets, were taken from transport studies conducted by ICRP task group 96, which are fully described in a forthcoming ICRP publication (24).
Platform
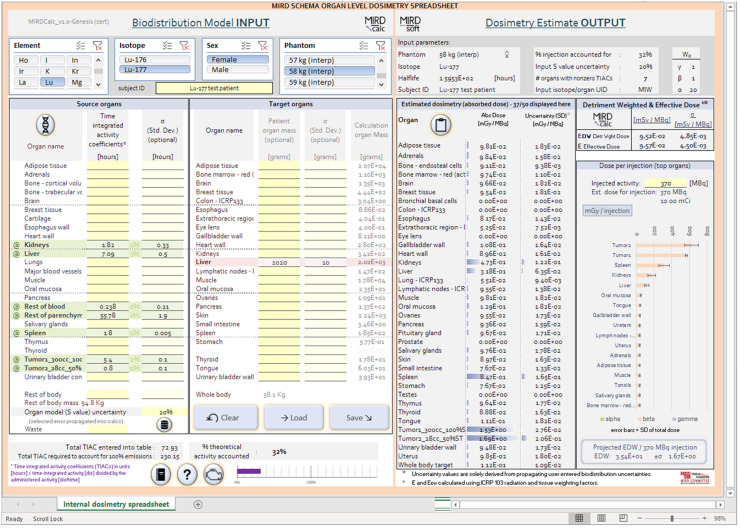
The platform of our software is Microsoft Excel, with additional interface features supported with Visual Basic coding, and is compiled as an executable application for Microsoft Windows operating systems. Advances in Excel over the last decade, including the PowerPivot tools, have made this project possible. The PowerPivot tools allow seamless integration of large reference datasets, such as the reference individual-specific S values, into MIRDcalc. The Excel platform has several advantages over traditional compiled code: the software is familiar, accessible, and easy to install; the platform is highly developed for broad use across industries and supports easy integration of complex computational and visual functions; the tools can be built to fit on an intuitive, user-friendly single-screen interface (Fig. 1); the interface responds instantly to user interaction; the software includes visual graphics that check data integrity, conditional formatting, and selective cell protection; inputs and outputs are inherently formatted for easy access and integration; and the calculations are easy to access for educational purposes and for quality control with transparency. The only part of the code that will not be available to users is the Visual Basic patches, but this portion of the code provides support only for executable protection and input and output functionality.
FIGURE 1.
Screenshot of MIRDcalc software.
User Manual
MIRDcalc comes with a comprehensive user manual in searchable portable document file format, available within the software and online (https://mirdsoft.org/mirdcalc). The manual covers all relevant elements of software use, including the topics presented in this article. The manual also provides background theory to help users understand basic concepts of radiopharmaceutical internal dosimetry.
Classic Use
Analogous to other software, MIRDcalc allows users to select a radionuclide and reference patient model and enter the TIACs that characterize the radiopharmaceutical biodistribution in a subject. Given this input information, an organ-level dosimetry report is generated on-screen and may be copied to the clipboard or exported. An example classic absorbed dose calculation is provided in Supplement A (supplemental materials are available at http://jnm.snmjournals.org) (25–30).
Software Validation
MIRDcalc version 1.0 was beta tested and validated by benchmarking against published references (8). The results of MIRDcalc were compared with reference values in ICRP publication 128 (3) using biokinetic data for 71 radiopharmaceuticals. Absorbed dose coefficients estimated with MIRDcalc were systematically compared against dose coefficients derived using other software; the absorbed dose coefficients are the dose quantity per organ or tissue that, when multiplied by administered activity, provide the organ or tissue dose estimates. The dosimetry computed with MIRDcalc agreed closely with results from another dosimetry software implementing the ICRP publication 133 reference adult SAFs, IDAC-Dose 2 (19), and showed partial agreement with dose coefficients derived using stylized or hybrid phantoms with reference masses derived from ICRP publication 89 (31) in OLINDA/EXM version 2.0 (32).
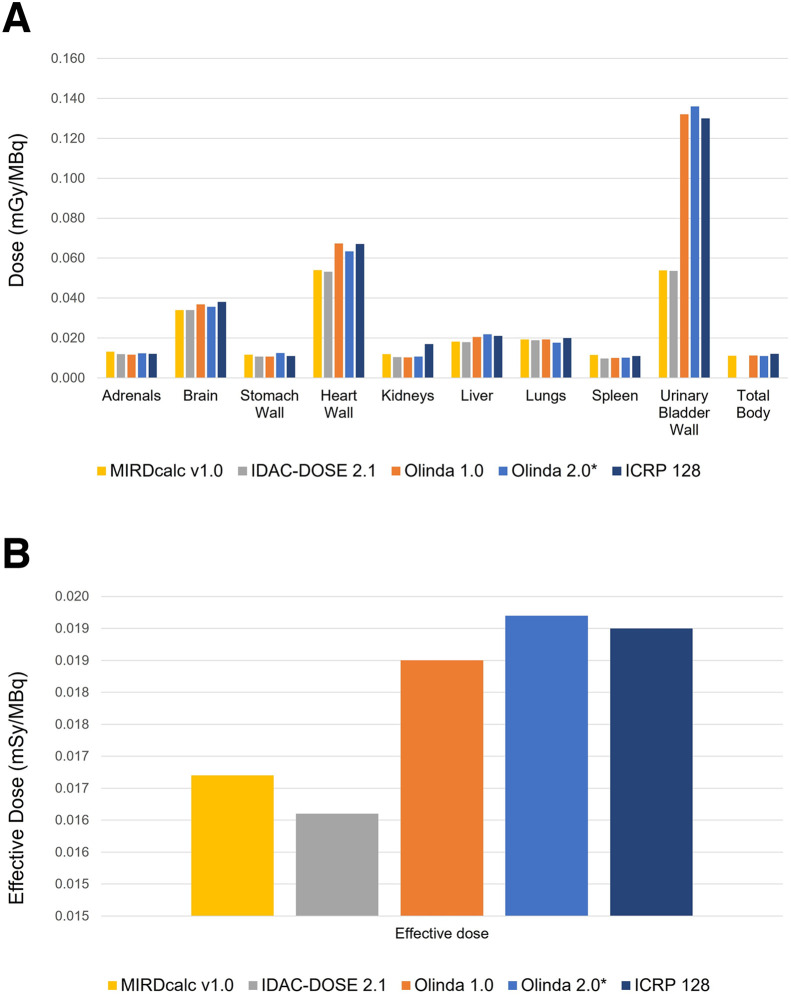
A comparison of different organ-level dosimetry software calculations, derived using a standard 18F-FDG biodistribution published in ICRP publication 128, calculated for an adult male anthropomorphic model, is presented in Fig 2. The figure illustrates general concordance between organ-absorbed doses and the effective dose calculations across different software platforms. It also demonstrates a certain degree of variability in dose calculations in reported relevant organs, largely within 20%. Systematic differences are also seen in the bladder wall dose, and an analysis of this issue is presented in the Design Considerations section below.
FIGURE 2.
Comparison of different organ-level dosimetry software calculations for typical 18F-FDG case, calculated for adult male anthropomorphic model. (A) Absorbed dose calculations for various organs. (B) Effective dose presented by different software.
Innovative Features
Single-Screen Interface
MIRDcalc provides full absorbed dose calculation tools on a single-screen interface, facilitating usability, inspection, and interpretation of results (Fig. 1).
Free Distribution
MIRDcalc is freely downloadable from the MIRDsoft.org website, together with other dosimetry-related tools developed within the MIRDsoft initiative.
Quality Control Graphics: Real-Time Dose Calculations
MIRDcalc provides useful quality control and safety check metrics and graphics to protect against user error. These include checks on activity accounted for and ability to account for activity excreted and eliminated. MIRDcalc also displays a unique isotope/input organ identification code.
Fully Accessible Calculations (Open Source)
Dose calculations are performed within an accessible spreadsheet that permits a user to review all math operations. Users may edit and change values in designated input cells, whereas the other portions of the interface are locked to prevent code corruption.
Comprehensive Case Documentation
Subject dose calculations may be saved in a comma-separated-value file format to document output results, as well as input parameters and phantom S values.
Uncertainty Propagation
MIRDcalc propagates estimated uncertainty values of radiopharmaceutical biodistribution parameters and organ masses to calculate an associated uncertainty for calculated absorbed doses. Users may optionally enter custom uncertainties for TIACs or organ masses. Users may also select a global S-value uncertainty (coefficient of variation), which is propagated to all calculations that are derived from the S values; this feature may ideally be used to insert a global uncertainty that provides a general accounting of the expected mismatch between anthropomorphic reference models and any given patient geometry. The uncertainties entered are propagated through all TIAC-to-dose calculations using the Joint Committee for Guides in Metrology Guide to the Expression of Uncertainty in Measurement (33) generalized schema for propagating uncertainties. To propagate the error of parameters when these are added or subtracted () or multiplied or divided ( in the dosimetry calculations, Equations 3 and 4 are used (assuming only 2 variables are combined in the operation), respectively.
| Eq. 3 |
| Eq. 4 |
where A and B are real variables, with SDs and , respectively; is covariance. Both Equations 3 and 4 include a covariance term at the end. In the context of dosimetry, this term is complex and derived from many physical and biologic variables. To provide a generally applicable and implementable tool, MIRDcalc assumes that the covariance of all variables in the dosimetry calculations is zero, with justification from Guide to the Expression of Uncertainty in Measurement clause F.1.2.1.c (33). Thus, the propagation of error equations implemented in all but one situation in MIRDcalc are shown in Equations 5 and 6.
| Eq. 5 |
| Eq. 6 |
Zero covariance, however, is not assumed in uncertainty calculation for effective dose, which includes male and female dose averaging. In this instance, the covariance between male and female doses is set to be 1, as the two are highly correlated. For users interested in performing more complex error propagation calculations, MIRDcalc outputs all data required for in-house calculations in the standard MIRDcalc output files.
Incorporating uncertainty propagation into dosimetry calculations remains a relatively new concept for the field of internal dosimetry (34). The community has yet to reach consensus and develop standards for how to properly use this information. MIRDcalc includes this feature to promote the development of a standard for error integration in the field. However, all uncertainty estimates presented by MIRDcalc are based on the above-mentioned covariance assumptions and are entirely dependent on uncertainty values provided by the user. Reported uncertainty values of the outputs are therefore only as accurate as the user’s data and assumptions.
Mass Accounting with New Source and Target Regions
MIRDcalc accounts for the complete subject using published values for reference man and woman (19,20) and pediatric phantoms (21). Two additional source regions were added: heart contents and major blood vessels. A detailed accounting of tissue masses can be found in Supplement B to this article.
Users are also given access to new suborgan combination target regions, which aggregate dose to subregions defined in ICRP publication 133: colon, extrathoracic region, lung, lymphatic nodes, and whole-body target. A description of these regions can be found in the original reference from the ICRP (19) and the MIRDcalc user manual.
Dynamic Source Regions
MIRDcalc introduces 3 dynamic source regions: rest of body, rest of blood, and rest of parenchyma. This option allows users to assign TIAC values across entire body tissues.
TIACs entered into the “rest-of” regions are distributed among unaccounted-for organs and tissues, weighted by mass. Unaccounted-for source regions are defined as the nonoverlapping source regions for which the user has not explicitly assigned a coefficient. The rest-of regions are dynamic; as the user assigns TIACs to static source organs, the total mass of the rest-of regions adjusts accordingly. This strategy is modeled after the additive approach described by the ICRP for handling other tissues as presented in ICRP publication 133, Equation 2.10 (19). MIRDcalc distributes the dynamic TIAC into nonassigned organs rather than updating SAFs.
The explicit makeup of the subregions that comprise the MIRDcalc-defined rest-of regions are presented in the MIRDcalc user manual.
Blood Source Model
Modeling blood separate from soft tissues and bone can be difficult but important since the biokinetics of blood are different from the biokinetics of tissue-bound activity. Although other dosimetry approaches assume parenchyma and blood within each organ as a single region, MIRDcalc offers the option to treat the different biokinetics separately. Coefficients entered for the source regions may be associated with either the combined blood and parenchyma in each source region (accommodating imaging-derived input) or parenchyma only to accommodate pharmacokinetic models that address tissue parenchyma and total-body blood separately. MIRDcalc keeps track of the separate blood and parenchyma masses accounted for in the regions of user-entered TIACs and removes them from the rest-of-body, rest-of-blood, and rest-of-parenchyma input terms as appropriate. These rest-of terms can then be used to distribute radionuclides into unaccounted-for tissues.
Coefficients associated with the blood may be assigned to organ regions (defining blood and parenchyma), blood regions (heart content and major blood vessels), rest-of-body source region (distributes to all unaccounted-for blood and soft and hard tissues), and separate rest-of-blood and rest-of-parenchyma source regions (which separately distribute TIACs to the blood and parenchyma, respectively).
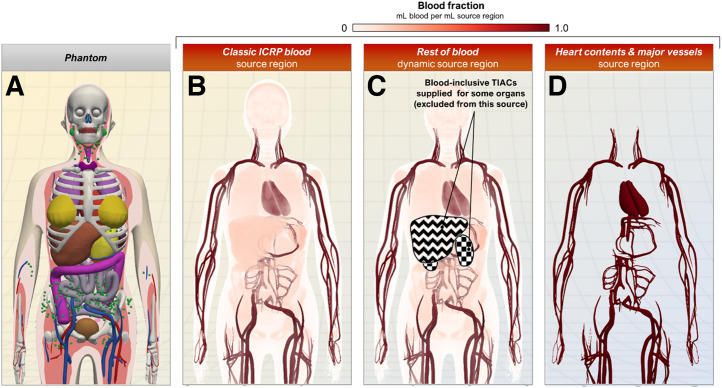
The blood source region models provided in MIRDcalc are depicted in Figure 3. The choice of regions to use depends on the user’s activity measurement methods and assumptions. For example, if a user defines the remainder (or unaccounted-for activity) from imaging, a background measurement derived from a background region or volume of interest measured from images may be used for the rest of body TIAC, accounting for both remainder blood and remainder parenchyma. Alternatively, if blood activity measurements are available from specimens or blood pool imaging, the input values may be entered for separate blood and parenchyma inputs.
FIGURE 3.
MIRDcalc blood source options support different strategies for defining source region TIACs. (A) Example phantom (ICRP 15-y-old female) shown for anatomic reference. (B) Classic ICRP blood source region accounts for entire volume of blood in phantom and spatially overlaps with volumes of blood-perfused source regions. (C) Rest-of-blood source region accounts for total remaining blood after input of blood-inclusive TIACs into various organs. (D) Heart contents and major vessel source regions do not overlap with parenchyma of any source regions.
Integrated Spheric Tumor Dosimetry
MIRDcalc supports simplistic tumor dosimetry by calculating the self-dose that a sphere receives from uniformly incorporated activity. MIRDcalc allows selection of sphere volume, volume uncertainty, tissue composition (bone/soft-tissue mixture), radionuclide, TIAC, and TIAC uncertainty. These dose calculations are based on S values provided by Olguin et al. (35). MIRDcalc implementation utilizes S-value interpolation or extrapolation of published data points for user-specified volumes. Interpolation and extrapolation are accomplished via log–log interpolation between the 2 nearest sampling points. Propagation of uncertainty in tumor dosimetry is estimated from the inherent uncertainty of the tissue volume and coefficient values, as described in Equation 67 of Gear et al. (34).
MIRDcalc tumor dosimetry is performed independently from organ dosimetry. Only tumor self-dose is provided; cross-organ contributions from all other source regions are not included in the tumor dose calculation.
Command Line and Batch Processing
MIRDcalc supports 2 methods of running absorbed dose calculations: via the user interface or via spool processing. The batch-processing feature allows MIRDcalc dose calculations to run without an interface or user interaction and supports batch processing of population data.
Organ Mass Interpolation
It is recognized that organ masses from specific subjects may not match those from the standard phantoms, causing an error in organ dose estimation. A first-order approximation for correcting these differences was presented in MIRD pamphlet 11 and is implemented in MIRDcalc (36). Specifically, it was recommended that the impact of differences in nonreference organ sizes can be accounted for through scaling the self-dose by the ratio of the organ masses to a constant power. The value of the power was set to −2/3 for photon self-dose scaling and −1 for electron and α-self-dose scaling. Estimations of cross-dose contributions are unchanged when using user-modified organ masses.
The option to add associated uncertainty to the user-modified mass is also available. This will impact only mass scaling calculations and therefore are relevant only for organs that have had their mass changed.
Whole-Phantom Mass Interpolation
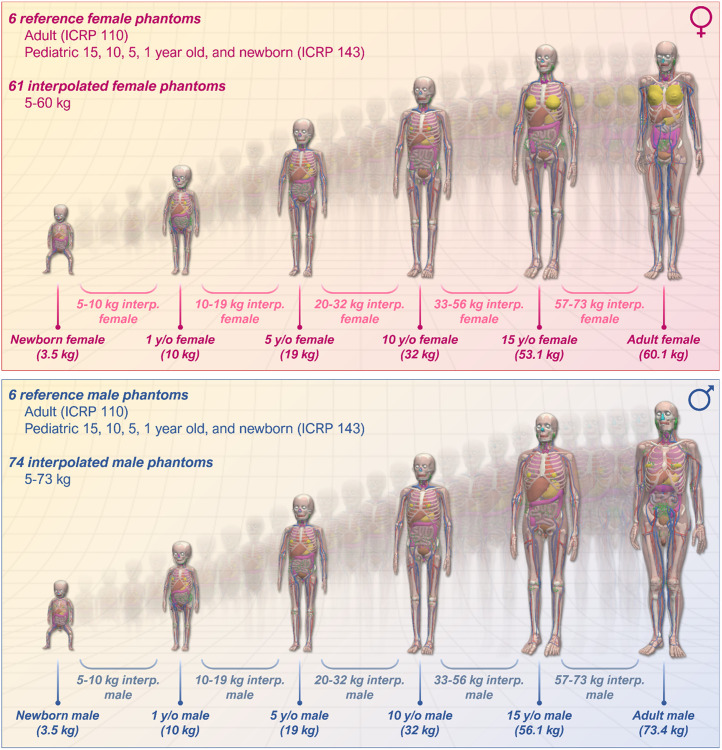
The user may select among multiple phantom models for dose calculations; these include the ICRP phantom series (newborn, 1-y-old, 5-y-old, 10-y-old, 15-y-old, and adult male and female) (20,21). In addition, the user may select a phantom model based on weight. A representation of the MIRDcalc phantom library is shown in Figure 4. Selection of a weight-based phantom will load a linearly interpolated mass phantom model from the 2 closest-by-mass phantoms in the ICRP reference series. Reference region masses are scaled linearly, and S values are interpolated with log–log scaling, relative to the user-selected phantom mass (identified by the phantom name) and the 2 closest ICRP reference individuals.
FIGURE 4.
Visualization of MIRDcalc phantom library, including 12 ICRP reference phantoms (publications 110 and 143), and graphical representation of MIRDcalc interpolation feature.
ICRP Publication 128 Case Library
The ICRP published reference biokinetic data in the form of TIACs for radiopharmaceuticals (ICRP publication 128) (3). These reference coefficients were incorporated into MIRDcalc case library, formatted for direct import. Processing of these files is performed using the batch-processing capability and is the basis of the comparisons reported in the companion validation article (8).
Effective Dose, Detriment-Weighted Dose, and Risk Index
In addition to absorbed doses, MIRDcalc also calculates the detriment-weighted dose and effective dose (19,37). Effective dose is a risk-related weighted construct based on organ doses. It is calculated using radiation-weighting factors and tissue-weighting factors described in ICRP publication 103 (38). Effective dose is specific to the ICRP reference individuals and is averaged over both male and female phantoms.
The detriment-weighted dose is also a risk-related weighted sum of organ-absorbed doses calculated using radiation-weighting factors and tissue-weighting factors described in ICRP publication 103 (38). Detriment-weighted dose is calculated similarly to effective dose but is specific to the phantom model used for dose calculations (single sex, potentially modified organ masses) (37).
MIRDcalc calculates a risk index, a ratio of the estimated added risk of cancer from specific radiation exposure relative to the estimated natural risk of cancer according to a concept known as lifetime attributable risk of cancer (National Cancer Institute’s Radiation Risk Assessment Tool (39)). The baseline natural incidence of cancer in a population with an absence of radiation exposure was derived from the Surveillance, Epidemiology, and End Results database (40) as defined in the Radiation Risk Assessment Tool. The risk index was recently introduced in the literature (41,42). The intent behind the concept of risk index was to provide an alternative to effective dose for risk assessment. However, the validity and applicability of this quantity is limited by its dependence on the linear no-threshold model, a radiation dose risk model whose applicability at low radiation doses continues to be questioned (43,44). It also ignores the potential benefit that a diagnostic or therapeutic radiopharmaceutical may have.
DESIGN CONSIDERATIONS
The specialty of internal dosimetry is evolving, and the number of software tools has increased. Software packages provide different results and should therefore be compared and scrutinized. For the organ-level dosimetry tools, most discrepancies come from the differences in phantom models; MIRDcalc uses the ICRP publication 133 reference adult series and SAFs based on the ICRP publication 143 pediatric phantoms (Fig. 3) (21) and MIRD decay schemes (18), whereas OLINDA/EXM version 2.0 uses RADAR phantoms (45,46).
In comparing the similar dosimetry software packages, we can take note some differences. First, calculated absorbed doses to the urinary bladder were factors of 2–3 times higher in ICRP publication 128 than in MIRDcalc because of advances in the stylized dosimetry model used to compute absorbed fractions of energy deposited in the bladder wall from emissions in the urinary bladder contents (47,48). Next, MIRDcalc spheric tumor β-dosimetry was modeled using the entire β-energy spectrum, rather than the mean β-emission energy. Finally, the skeletal target (endosteum) was redefined in ICRP publication 110, enlarged to a thickness of 50 μm from 10 μm (49). This change produced significant differences, relative to other organs, for calculated SAFs and S values for charged particles emitted within skeletal tissues (19,50).
The MIRDcalc software and the MIRDsoft.org webspace provide platforms for continued expansion and evolution of community dosimetry software tools. Future improvements will include new utilities for curve fitting, pregnant phantom/fetus dosimetry, and suborgan dosimetry. We can also expect to see an analogous CT dosimetry software, MIRDct, to be released shortly. These additions will complement the already-released MIRDcell software, which performs cellular and multicellular dosimetry and bioeffect modeling (11)
SUMMARY AND CONCLUSIONS
MIRDcalc represents a new software solution for medical internal radiation dosimetry. The software was developed by the MIRD committee of the Society of Nuclear Medicine and Molecular Imaging to benefit an international user community. MIRDcalc implements the basic MIRD schema, equations, and nuclear databases with molecular imaging data for applications to patient-specific dosimetry and radionuclide therapy planning.
As clinical nuclear medicine evolves with the ability to acquire more detailed quantitative molecular imaging data on radiopharmaceutical uptake, distribution, and biokinetics, the need has intensified for more powerful and more accurate computational tools for calculating radiation dose to organs, suborgan regions, tissues, and tumors. Integration of advanced quantitative imaging data with more flexible and detailed anatomic models, such as those recently developed by the ICRP, necessitated development of software that could make best use of these models. New radionuclide applications also suggested the need for an expanded and updated nuclear emissions database. Although greater complexity may provide additional key features and capabilities, the need remained to provide the user community with a software platform that was readily available, simple to execute, and affordable.
The design of a next-generation computing package necessarily focused first on addressing the technical gaps, weaknesses in, deficiencies with, and elements missing from all other available software platforms. In response to calls for improvements in personalization, standardization, and contextualization of dosimetry calculations, MIRDcalc increases user ability to incorporate custom organ sizes obtained from medical imaging, to interpolate between standard models, and to customize biokinetic modeling to more accurately calculate a full suite of organ and tissue doses. Accordingly, the MIRDcalc developers collaborated closely with the leadership of ICRP Task Group 96, a group which has been responsible for producing anatomically realistic reference voxel phantoms, dosimetric models, and specific absorbed fractions for use in internal dosimetry. Going forward, MIRDcalc will be revised and updated as scientific progress in nuclear medicine physics dose modeling continues.
DISCLOSURE
The MIRDcalc software aids a user in performing dose calculations for a variety of diagnostic and therapeutic isotopes. MIRDcalc is intended for educational and research use only. MIRDcalc has not been approved by the U.S. Food and Drug Administration and is not intended for clinical use or use as a medical device. MIRDcalc and any results generated from its use are not substitutes for medical diagnosis, advice, or treatment of specific medical conditions. A physician should always be consulted for any health problem or medical condition. This research was funded in part through the NIH/NCI Cancer Center support grant P30 CA008748 and NIH U01 EB028234. Lukas Carter acknowledges support from the Ruth L. Kirschstein NRSA postdoctoral fellowship (NIH F32 EB025050). No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
This work was done in collaboration with the Society of Nuclear Medicine and Molecular Imaging MIRD Committee: Vikram Adhikarla, Rachael M. Barbee, Wesley E. Bolch, Yuni K. Dewaraja, William D. Erwin, Darrell R. Fisher, Roger W. Howell, Adam L. Kesner, Richard Laforest, Joseph G. Rajendran, George Sgouros, and Pat B. Zanzonico (chair). We thank Dr. Pat Zanzonico from Memorial Sloan Kettering Cancer Center for providing guidance and support to the MIRDcalc development effort. We thank Dr. Keith Eckerman from Oak Ridge National Laboratory for help in validating the MIRDcalc S values. We thank Dr. Jazmin Schwartz from Memorial Sloan Kettering Cancer Center for providing statistics support.
Contributor Information
SNMMI MIRD Committee:
Adhikarla Vikram, Vikram Adhikarla, Rachael M. Barbee, Wesley E. Bolch, Yuni K. Dewaraja, William D. Erwin, Darrell R. Fisher, Robert F. Hobbs, Roger W. Howell, Adam L. Kesner, Richard Laforest, Joseph G. Rajendran, George Sgouros, and Pat B. Zanzonico
REFERENCES
- 1. Siegel JA, Thomas SR, Stubbs JB, et al. MIRD pamphlet no. 16: techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med. 1999;40(suppl):37S–61S. [PubMed] [Google Scholar]
- 2. Loevinger R, Budinger TF, Watson EE. MIRD Primer for Absorbed Dose Calculations. Society of Nuclear Medicine and Molecular Imaging; 1991. [Google Scholar]
- 3. Mattsson S, Johansson L, Svegborn LS, et al. ICRP publication 128: radiation dose to patients from radiopharmaceuticals: a compendium of current information related to frequently used substances. Ann ICRP. 2015;44:1–321. [DOI] [PubMed] [Google Scholar]
- 4. Villoing D, Marcatili S, Garcia MP, Bardiès M. Internal dosimetry with the Monte Carlo code GATE: validation using the ICRP/ICRU female reference computational model. Phys Med Biol. 2017;62:1885–1904. [DOI] [PubMed] [Google Scholar]
- 5. ICRP publication 41: nonstochastic effects of ionizing radiation. Ann ICRP. 1984;14:1–33. [PubMed] [Google Scholar]
- 6. Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–1027. [PubMed] [Google Scholar]
- 7. Andersson M, Johansson L, Eckerman K, Mattsson S. IDAC-Dose 2.1, an internal dosimetry program for diagnostic nuclear medicine based on the ICRP adult reference voxel phantoms. EJNMMI Res. 2017;7:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carter LM, Ramos JCO, Olguin EA, et al. MIRD pamphlet no. 28, part 2: comparative evaluation of MIRDcalc dosimetry software across a compendium of diagnostic radiopharmaceuticals. J Nucl Med. In press. [DOI] [PMC free article] [PubMed]
- 9. Vergara Gil A, Amato E, Auditore L, et al. OpenDose3D: a free, collaborative 3D Slicer module for patient-specific dosimetry. Eur J Nucl Med Mol Imaging. 2020;47(suppl 1):S314–S315. [Google Scholar]
- 10. Capala J, Graves SA, Scott A, et al. Dosimetry for radiopharmaceutical therapy: current practices and commercial resources. J Nucl Med. 2021;62(suppl 3):3S–11S. [DOI] [PubMed] [Google Scholar]
- 11. Katugampola S, Wang J, Rosen A, Howell RW. MIRD pamphlet no. 27: MIRDcell V3, a revised software tool for multicellular dosimetry and bioeffect modeling. J Nucl Med. 2022;63:1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaziri B, Wu H, Dhawan AP, Du P, Howell RW. MIRD pamphlet no. 25: MIRDcell V2.0 software tool for dosimetric analysis of biologic response of multicellular populations. J Nucl Med. 2014;55:1557–1564. [DOI] [PubMed] [Google Scholar]
- 13. Chiesa C, Bardiès M, Zaidi H. Voxel-based dosimetry is superior to mean absorbed dose approach for establishing dose-effect relationship in targeted radionuclide therapy. Med Phys. 2019;46:5403–5406. [DOI] [PubMed] [Google Scholar]
- 14. Bartlett RM, Bolch WE, Brill AB, et al. MIRD Primer 2022: A Complete Guide to Radiopharmaceutical Dosimetry. Society of Nuclear Medicine and Molecular Imaging; 2022. [Google Scholar]
- 15. Loevinger R. A schema for absorbed-dose calculations for biologically-distributed radionuclides. J Nucl Med. 1968;9(suppl 1):9–14. [PubMed] [Google Scholar]
- 16. Bolch WE, Eckerman KF, Sgouros G, Thomas SR. MIRD pamphlet no. 21: a generalized schema for radiopharmaceutical dosimetry—standardization of nomenclature. J Nucl Med. 2009;50:477–484. [DOI] [PubMed] [Google Scholar]
- 17. Petoussi-Henss N, Bolch WE, Zankl M, Sgouros G, Wessels B. Patient-specific scaling of reference S-values for cross-organ radionuclide S-values: what is appropriate? Radiat Prot Dosimetry. 2007;127:192–196. [DOI] [PubMed] [Google Scholar]
- 18. Eckerman KF, Endo A. MIRD: Radionuclide Data and Decay Schemes. 2nd ed. Society of Nuclear Medicine and Molecular Imaging; 2008. [Google Scholar]
- 19. Bolch WE, Jokisch D, Zankl M, et al. ICRP publication 133: the ICRP computational framework for internal dose assessment for reference adults: specific absorbed fractions. Ann ICRP. 2016;45:5–73. [DOI] [PubMed] [Google Scholar]
- 20. Menzel HG, Clement C, DeLuca P. ICRP Publication 110: adult reference computational phantoms. Ann ICRP. 2009;39:1–164. [DOI] [PubMed] [Google Scholar]
- 21. Bolch WE, Eckerman K, Endo A, et al. ICRP publication 143: paediatric reference computational phantoms. Ann ICRP. 2020;49:5–297. [DOI] [PubMed] [Google Scholar]
- 22. Schwarz BC, Godwin WJ, Wayson MB, et al. Specific absorbed fractions for a revised series of the UF/NCI pediatric reference phantoms: internal photon sources. Phys Med Biol. 2021;66:035006. [DOI] [PubMed] [Google Scholar]
- 23. Schwarz BC, Godwin WJ, Wayson MB, et al. Specific absorbed fractions for a revised series of the UF/NCI pediatric reference phantoms: internal electron sources. Phys Med Biol. 2021;66:035005. [DOI] [PubMed] [Google Scholar]
- 24. Jokisch DW, Bolch WE, Schwarz BC, et al. Specific absorbed fractions for reference paediatric individuals. Ann ICRP. In press. [Google Scholar]
- 25. Silberstein EB, Alavi A, Balon HR, et al. The SNMMI practice guideline for therapy of thyroid disease with 131I 3.0. J Nucl Med. 2012;53:1633–1651. [DOI] [PubMed] [Google Scholar]
- 26. Hobbs RF, Wahl RL, Lodge MA, et al. 124I PET-based 3D-RD dosimetry for a pediatric thyroid cancer patient: real-time treatment planning and methodologic comparison. J Nucl Med. 2009;50:1844–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thierens HM, Monsieurs MA, Bacher K. Patient dosimetry in radionuclide therapy: the whys and the wherefores. Nucl Med Commun. 2005;26:593–599. [DOI] [PubMed] [Google Scholar]
- 28. Benua R, Leeper R. A method and rationale for treating metastatic thyroid carcinoma with the largest safe dose of 131I. Front Thyroidol. 1986;2:1317–1321. [Google Scholar]
- 29. Hänscheid H, Lassmann M, Luster M, et al. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J Nucl Med. 2006;47:648–654. [PubMed] [Google Scholar]
- 30. Sgouros G, Song H, Ladenson PW, Wahl RL. Lung toxicity in radioiodine therapy of thyroid carcinoma: development of a dose-rate method and dosimetric implications of the 80-mCi rule. J Nucl Med. 2006;47:1977–1984. [PMC free article] [PubMed] [Google Scholar]
- 31. ICRP publication 89: basic anatomical and physiological data for use in radiological protection: reference values. Ann ICRP. 2002;32:5–265. [PubMed] [Google Scholar]
- 32. Stabin M, Farmer A. OLINDA/EXM 2.0: the new generation dosimetry modeling code [abstract]. J Nucl Med. 2012;53(suppl 1):585. [Google Scholar]
- 33. Working Group 1 of the Joint Committee for Guides in Metrology. Evaluation of Measurement Data: Supplement 2 to the “Guide to the Expression of Uncertainty in Measurement”—Extension to Any Number of Output Quantities. Bureau International des Poids et Mesures; 2011. [Google Scholar]
- 34. Gear JI, Cox MG, Gustafsson J, et al. EANM practical guidance on uncertainty analysis for molecular radiotherapy absorbed dose calculations. Eur J Nucl Med Mol Imaging. 2018;45:2456–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olguin E, President B, Ghaly M, Frey E, Sgouros G, Bolch WE. Specific absorbed fractions and radionuclide S-values for tumors of varying size and composition. Phys Med Biol. 2020;65:235015. [DOI] [PubMed] [Google Scholar]
- 36. Snyder WS, Ford MR, Warner GG, Watson SB. MIRD Pamphlet No. 11: “S,” Absorbed Dose per Unit Cumulated Activity for Selected Radionuclides and Organs. Society of Nuclear Medicine and Molecular Imaging; 1975. [Google Scholar]
- 37. Kofler C, Domal S, Satoh D, Dewji S, Eckerman K, Bolch WE. Organ and detriment-weighted dose rate coefficients for exposure to radionuclide-contaminated soil considering body morphometries that differ from reference conditions: adults and children. Radiat Environ Biophys. 2019;58:477–492. [DOI] [PubMed] [Google Scholar]
- 38. ICRP publication 103: the 2007 recommendations of the International Commission on Radiological Protection. Ann ICRP. 2007;37:1–332. [DOI] [PubMed] [Google Scholar]
- 39. Berrington de Gonzalez A, Iulian Apostoaei A, Veiga LH, et al. RadRAT: a radiation risk assessment tool for lifetime cancer risk projection. J Radiol Prot. 2012;32:205–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. SEER Cancer Statistics Review (CSR), 1975–2018. National Cancer Institute website. https://seer.cancer.gov/csr/1975_2018/. Published April 15, 2021. Accessed April 25, 2023.
- 41. O’Reilly SE, Plyku D, Sgouros G, et al. A risk index for pediatric patients undergoing diagnostic imaging with 99mTc-dimercaptosuccinic acid that accounts for body habitus. Phys Med Biol. 2016;61:2319–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brown JL, Sexton-Stallone B, Li Y, et al. Body morphometry appropriate computational phantoms for dose and risk optimization in pediatric renal imaging with Tc-99m DMSA and Tc-99m MAG3. Phys Med Biol. 2020;65:235026. [DOI] [PubMed] [Google Scholar]
- 43. Siegel JA, Brooks AL, Fisher DR, et al. A critical assessment of the linear no-threshold hypothesis: its validity and applicability for use in risk assessment and radiation protection. Clin Nucl Med. 2019;44:521–525. [DOI] [PubMed] [Google Scholar]
- 44. Shore RE, Dauer LT, Beck HL, et al. NCRP Commentary No. 27: Implications of Epidemiologic Studies for the Linear-Nonthreshold Model and Radiation Protection. National Council on Radiation Protection and Measurement; 2018. [Google Scholar]
- 45. Segars WP, Lalush DS, Frey EC, Manocha D, King MA, Tsui BM. Improved dynamic cardiac phantom based on 4D NURBS and tagged MRI. IEEE Trans Nucl Sci. 2009;56:2728–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Segars PW, Tsui BM. MCAT to XCAT: the evolution of 4-D computerized phantoms for imaging research—computer models that take account of body movements promise to provide evaluation and improvement of medical imaging devices and technology. Proc IEEE Inst Electr Electron Eng. 2009;97:1954–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carter LM, Choi C, Krebs S, et al. Patient size-dependent dosimetry methodology applied to 18F-FDG using new ICRP mesh phantoms. J Nucl Med. 2021;62:1805–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stabin MG, Siegel JA. Physical models and dose factors for use in internal dose assessment. Health Phys. 2003;85:294–310. [DOI] [PubMed] [Google Scholar]
- 49. ICRP publication 110: adult reference computational phantoms. Ann ICRP. 2010;39:1–164. [DOI] [PubMed]
- 50. Bolch WE, Shah AP, Watchman CJ, et al. Skeletal absorbed fractions for electrons in the adult male: considerations of a revised 50-μm definition of the bone endosteum. Radiat Prot Dosimetry. 2007;127:169–173. [DOI] [PubMed] [Google Scholar]