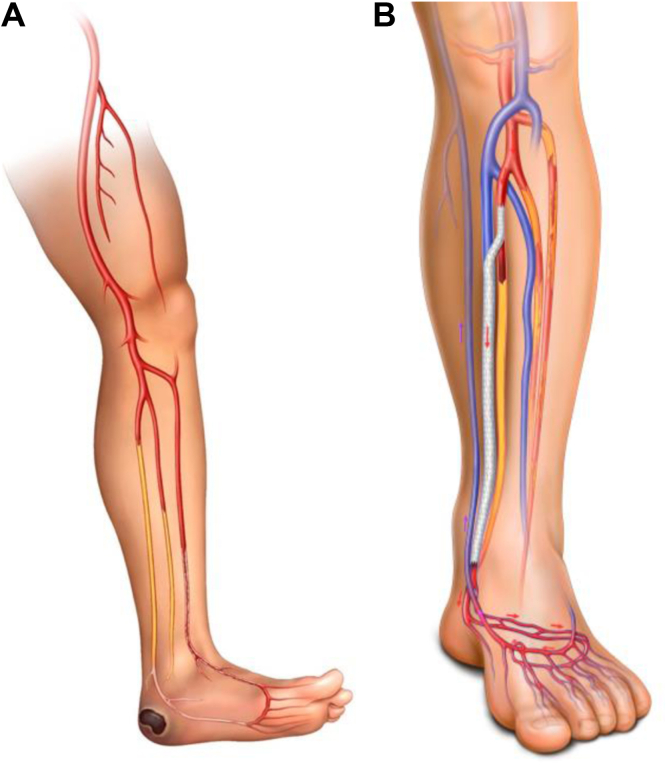
Abstract
We report on two venous arterialization (VA) techniques for treatment of CLTI in patients traditionally considered as having no treatment options for standard arterial endovascular or surgical bypass procedures. Screening and the preprocedural workup findings are outlined as deciding factors in determining a patient’s fitness for the two techniques, with a focus on careful preprocedure arterial duplex ultrasound and assessment for vein suitability. Cardiac and infection screening are also factors in determining patient suitability for VA. In addition, radiographic assessment for the presence of medial artery calcification, which is used as a marker of technical difficulty and is a predictor of poor outcomes, is required. Ultimately, anatomic factors are used to determine the decision between hybrid superficial VA and or endovascular deep VA. Those with an occluded anterior tibial artery and suitable great saphenous vein are prioritized to hybrid superficial VA, and those with an occluded posterior tibial artery to endovascular deep VA. Both procedures are described in detail in this report of vascular and surgical techniques.
Keywords: Chronic limb-threatening ischemia, CLTI, Endovascular deep vein arterialization, Hybrid superficial vein arterialization, Vascular surgery, Venous arterialization
Open or endovascular venous arterialization (VA) for the management of severe no-option CLTI (noCLTI) has been previously reported.1 Historically, VA has lacked rigorous scientific backing and technical standardization. Recently, retrospective studies have provided encouraging data results and suggested technical standardization.2,3 Moreover, the PROMISE II (percutaneous deep vein arterialization for the treatment of late-stage chronic limb-threatening ischemia) prospective study has completed enrollment with encouraging results recently presented.4
NoCLTI is a definition in progress. Generally, these are patients with extreme arterial disease who have no standardized arterioarterial reconstructions available. These patients present with extensive disease of the pedal anatomy, commonly have severe pedal calcification patterns with high medial artery calcification (MAC) scores, a poor autologous conduit for bypass, and a moderate-to-severe degree of frailty.5,6 Additionally, most of these patients have longstanding diabetes, and a good proportion have chronic kidney disease. This complex patient subgroup carries high amputation and mortality rates.7, 8, 9 The VA procedure appears to offer hope for appropriately selected patients with adequate anatomy for arterialization.3,10 In the present report, we describe our current preoperative algorithm, surgical techniques, and surveillance program.
Preoperative workup
The findings from the patient’s history and physical examination alone can result in a high suspicion of noCLTI status and should be promptly followed by a noninvasive workup for confirmation and to determine the type of arterialization of choice. All patients should undergo evaluation using the Society for Vascular Surgery WIfI (wound, ischemia, foot infection) staging system, cardiac screening, foot radiography, and comprehensive arterial and venous duplex ultrasound (DUS) before undergoing diagnostic angiography.11
Cardiac screening
Cardiac complications can have deleterious effects, especially among patients with vascular pathology.12 Patients with moderate-to-severe right heart failure and elevated pulmonary artery pressure or a low ejection fraction are judiciously reviewed before undergoing arterialization treatment. In general terms, the procedure will increase the preload and, thus, increase the risk of high-output heart failure.
Radiographic assessment
Foot radiographs in anteroposterior and lateral projections are used to evaluate (1) bone structure, (2) infection, and (3) MAC score. The MAC score, proposed by Ferraresi et al6 aims to evaluate the arterial distribution of calcification in the inframalleolar vessels. A high MAC score has been demonstrated to be a strong predictor of unplanned podiatric and vascular reinterventions and major amputations13; therefore, patients with high MAC scores are considered poor candidates for traditional revascularization methods and, instead, are considered for VA procedures.
DUS examination
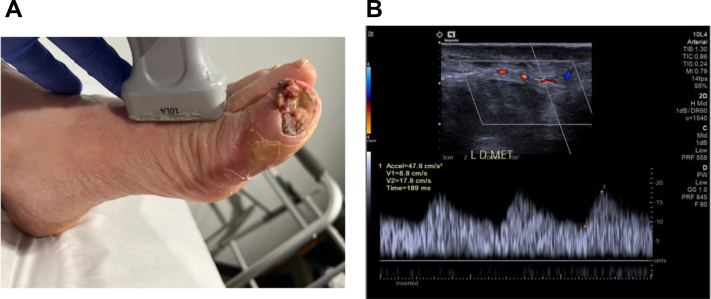
Preoperative DUS is an important part of our workup for arterialization. The baseline protocol and advanced disease of patients with complex CLTI can be challenging and could require additional training for the imaging studies to be standardized. A complete baseline arterial DUS examination is performed. Specific details are documented, including the proximal tibial artery patency length and location of the occlusions. A detailed evaluation of the pedal anatomy includes the pedal acceleration time (PAT),14 confirmation of pedal artery occlusions, and, most importantly, the PAT in the pedal artery closest to the wound bed (Fig 1).15
Fig 1.
Photograph (A) and scan (B) showing abnormal class 3 pedal acceleration time (PAT) in the calcified first dorsal metatarsal artery.
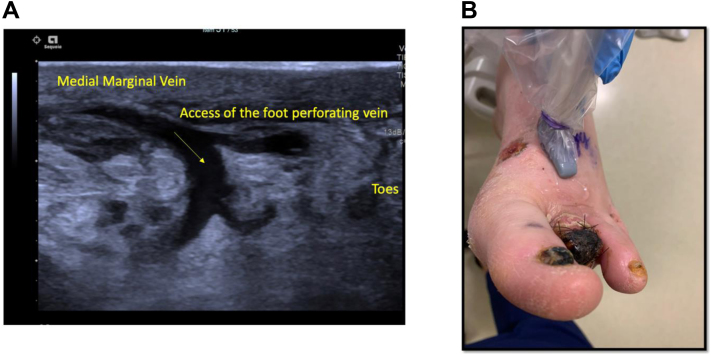
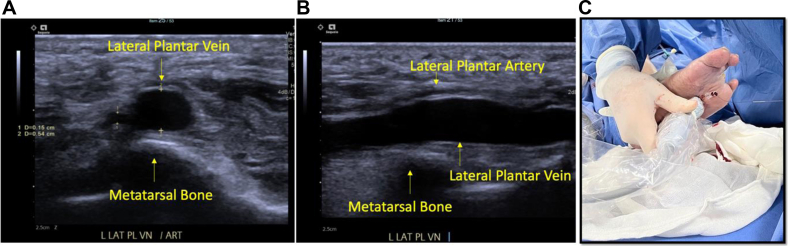
Comprehensive venous mapping is paramount for surgical decision-making. The superficial and deep venous systems are evaluated in detail. The great saphenous vein (GSV) and medial marginal vein (MMV) are evaluated for patency, quality, and size.16 Specifically, the MMV is studied in the transverse view to visualize the venous tributaries and pathway of the venous system. The foot perforating vein between the MMV and lateral plantar vein (LPV) is identified and marked with a square color box and low color scale. The foot perforating vein angles are evaluated to determine its usefulness as possible distal dorsal access (Fig 2). The LPV is also assessed for patency, quality, and size. The optimal view of the LPV is on the posterior, mid foot in a transverse view (Fig 3). Subclinical deep vein thrombosis or post-thrombotic syndrome are scenarios that can limit the possibility of successful VA.
Fig 2.
Long axis view (A) and photograph (B) of the foot perforating vein evaluated for possible venous access.
Fig 3.
A, Transverse view of the lateral plantar vein (LPV) with imaging landmarks. B, Long axis view of the LPV with imaging landmarks. C, LPV access with the probe held in long axis.
Diagnostic angiography
Diagnostic angiography should be used to confirm the preoperative workup findings. If the endovascular VA is selected for treatment, it should be pursued in the same setting to avoid delay.
Procedure
Selection of endovascular deep VA vs hybrid superficial VA
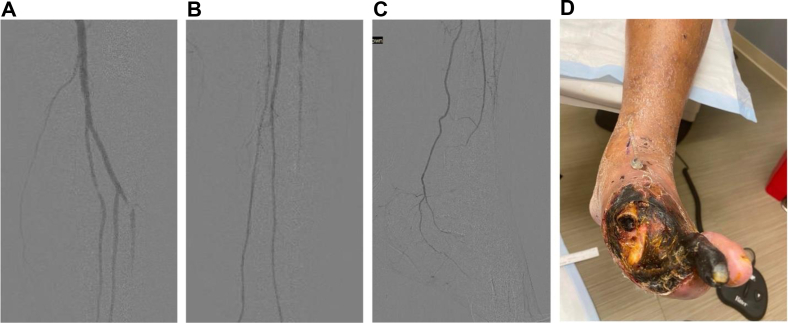
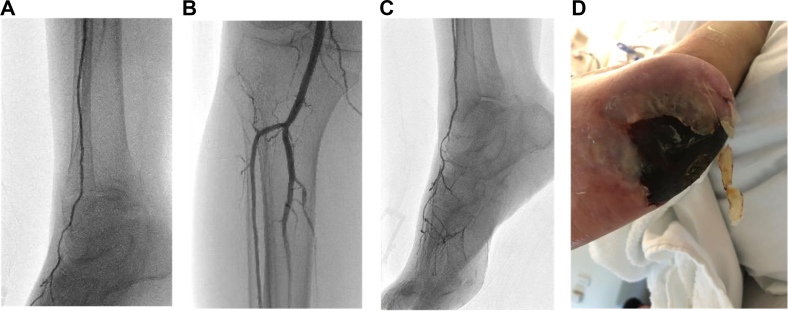
The decision to perform endovascular deep VA (eDVA) vs hybrid superficial VA (HySA) primarily depends on the anatomic distribution of the arterial and venous disease. In patients with an occluded anterior tibial artery and suitable GSV, HySA is preferred. However, for patients with an occluded posterior tibial artery, regardless of GSV status, eDVA is preferred (Figs 4 and 5).
Fig 4.
A-D, Scans and photograph showing occlusive pattern of the forefoot. This pattern is best suited to performing hybrid superficial venous arterialization (HySA). During an HySA procedure, there is no disruption of the posterior tibial artery (PTA) inflow because the anastomosis is performed end-to-side.
Fig 5.
A-D, Scans and photograph showing occlusive pattern of the hindfoot, also called orphan heel. This pattern is best suited to performing endovascular deep venous arterialization (eDVA). Using the posterior tibial artery (PTA) as inflow would not alter the baseline hemodynamics.
HySA procedure
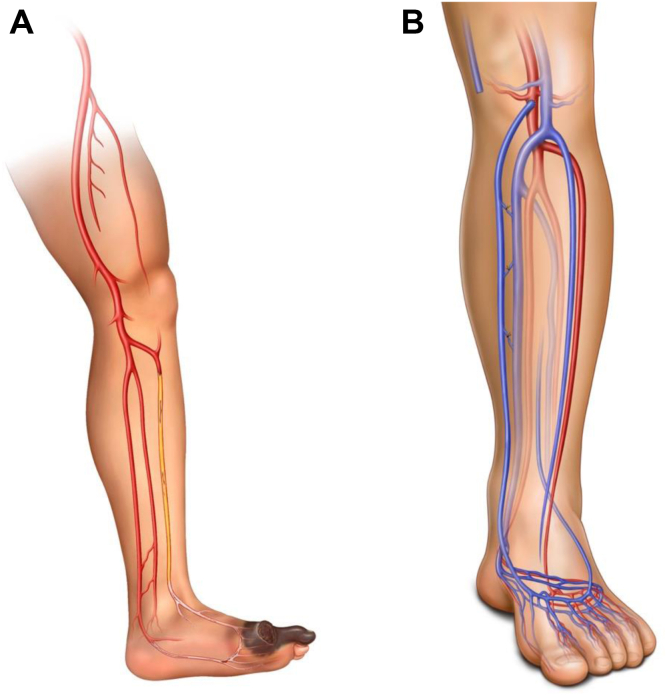
Candidates for HySA will have primary outflow via the posterior tibial artery (PTA). These patients are not ideal candidates for eDVA. In the process of creating the endovascular VA, all the flow to the PTA requires diversion to act as the donor artery for the DVA, eliminating all antegrade arterial flow and potentially creating profound distal ischemia. For HySA, inflow is obtained from the most distal and least diseased artery, usually the below-knee popliteal artery (Fig 6). The conduit of choice is the GSV. Once the decision has been made to proceed with HySA, patients continue receiving dual antiplatelet therapy (DAPT) or start taking DAPT, which is continued after surgery.
Fig 6.
Hybrid superficial venous arterialization (HySA). A, Diagram showing disease distribution. B, Diagram showing reconstruction after the procedure.
Step 1
The patient is brought to the operating room, and the leg is prepared and draped in the usual sterile fashion as for lower extremity bypass. Intraoperative ultrasound is used to mark the GSV course into the foot via the MMV and its tributaries.
Step 2
Exposure of the below-knee popliteal artery is begun by making a standard incision, with care not to injure the GSV. After the below-knee popliteal artery is controlled, attention is placed on the GSV. The GSV must be mobilized sufficiently to not be under any tension as it courses from the popliteal artery into the in situ space.
Step 3
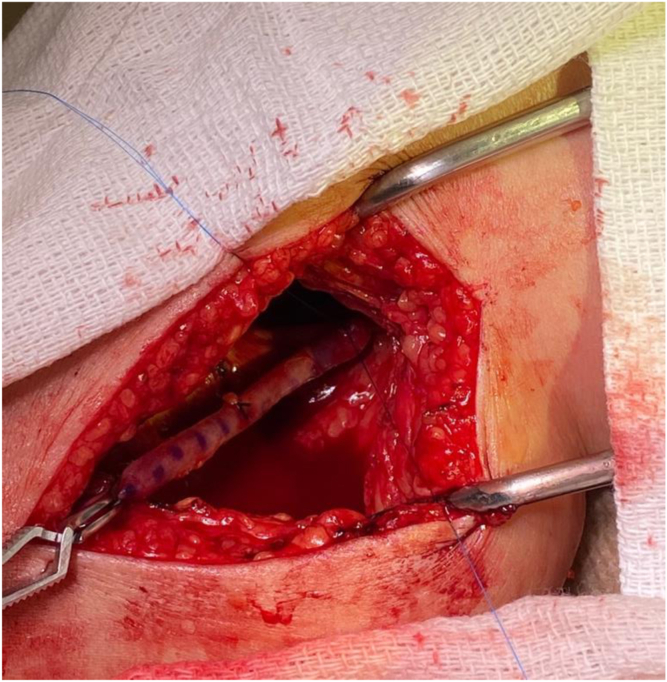
Therapeutic heparin is given to an activated clotting time of 250 to 300 seconds, and the below-knee popliteal flow is controlled with vessel loops or clamps. After the arteriotomy (usually done with a 4-mm punch), an end-to-side anastomosis is created (Fig 7).
Fig 7.
Intraoperative photograph showing end-to-side great saphenous vein (GSV) anastomosis to the below-knee popliteal artery.
Step 4
Using ultrasound, the medial marginal vein is found, and percutaneous access is obtained using a micropuncture kit at the most distal aspect of the vein with an adequate diameter before branching into the foot. After the access is selected, a 4F or 5F Terumo slender sheath (Terumo Interventional Systems) is inserted and flushed with heparin.
Step 5
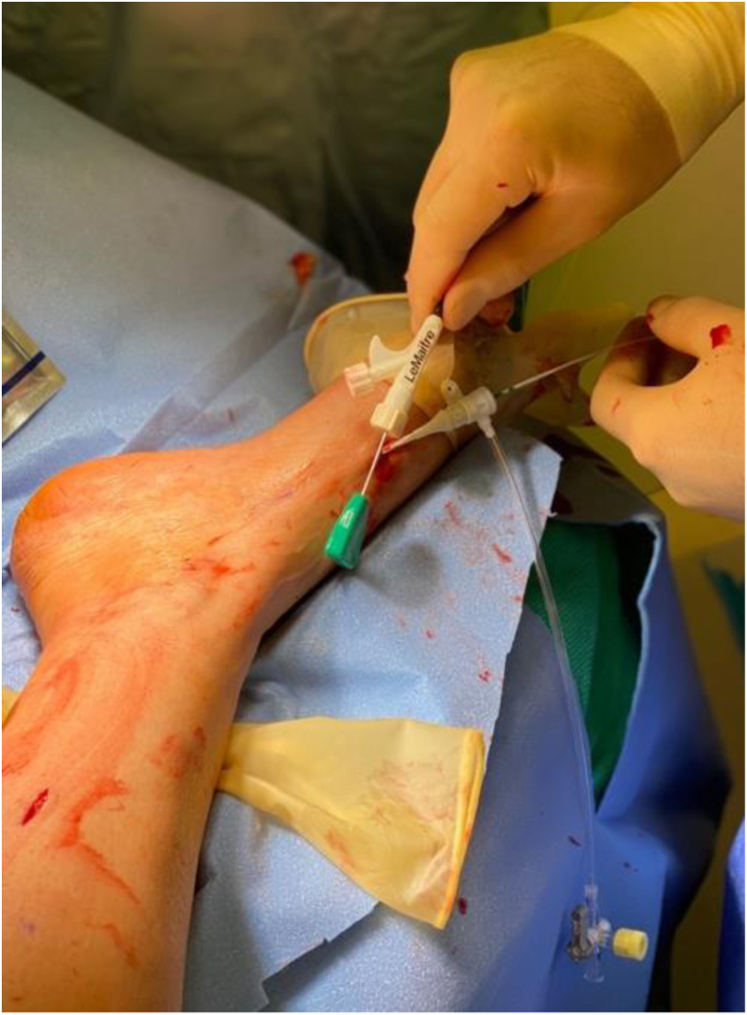
A LeMaitre flexible valvulotome (LeMaitre Vascular) is inserted via the sheath and advanced in retrograde fashion to a safe point distal to the anastomosis on the venous side (Fig 8). The valvulotome is the deployed and pulled back slowly to perform valve lysis.
Fig 8.
Intraoperative photograph showing LeMaitre flexible valvulotome (LeMaitre Vascular) inserted via a sheath in the medial marginal vein (MMV).
Step 6
Ultrasound is used to visualize the proximal anastomosis and evaluate for any retained valves at this level. Next, any large GSV tributaries or perforators are visualized under ultrasound guidance and ligated using 2-0 or 3-0 silk ties after small skip incisions.
Step 7
Angiography should be performed via an antegrade sheath of the ipsilateral common femoral artery if available to confirm forward and brisk flow through the GSV and MMV, with flow maintained through the distal arterial vessels of the PTA. Occasionally, retained pedal venous valves could require lysis using standard angioplasty tools (ie, balloons, cutting balloons, scoring balloons), specifically, to cross the tarsal vein valve and create forward flow into the digital veins. Depending on the intraoperative course and/or the operating room capacities (eg, C-arm, hybrid room), this step can be deferred as a planned reintervention.
Step 8
Flow is also confirmed in the arterialized GSV using Doppler ultrasound (volume flow goal, >50 mL/min or <250 mL/min). Heparin is reversed and the sheath removed. Hemostasis of this site is achieved with manual pressure. The below-knee popliteal artery exposure is closed in layers using interrupted Vicryl suture, with care not to injure the GSV.
eDVA procedure
As previously stated, eDVA is considered for patients with noCLTI who have occluded posterior tibial circulation in which the arterial stump can serve as the donor for creation of the arterialization (Fig 9). In this manner, any existing antegrade arterial flow will not be disrupted by eDVA. For all our cases, general anesthesia is preferred to avoid movement and both patient and operator comfort. Although this procedure can also be performed under a lower extremity block with sedation, we recommend against the use of sedation only, because it can be very painful for the patient when preparing the venous segment before stenting. In similar fashion to HySA, DAPT is started or continued before the procedure and continued postoperatively. If the patient has any prothrombotic syndrome (or reintervention is required secondary to occlusion), therapeutic anticoagulation and single antiplatelet therapy should be considered.
Fig 9.
Endovascular deep venous arterialization (eDVA). A, Diagram showing disease pattern. B, Diagram showing reconstruction after the procedure.
The following eDVA description refers to “off the shelf” items. The LimFlow system (LimFlow Inc) is an investigational device aiming to create eDVA. It involves using proprietary arterial and venous catheters that simplify and optimize the arterial–venous crossing, in addition to tapered covered stents and an antegrade over-the-wire valvulotome for ease of valve lysis. Current use of the device in the United States is limited to centers participating in the PROMISE III study.
Step 1
After the induction of general anesthesia, the bilateral groin and entire target extremity are prepared and draped in the usual sterile fashion. Antegrade ultrasound-guided arterial access is established in the index limb using a micropuncture kit, and a 5F sheath is inserted. Diagnostic angiography of the limb is performed, and 5F sheath is exchanged for a 6F × 45-cm sheath. At this point, heparin is given to achieve a therapeutic activated clotting time of 250 to 300 seconds.
Step 2
An elastic tourniquet is applied to the level of the ankle to allow for venous engorgement. Using a radial access kit, the LPV is accessed at the level of the fourth and fifth mid-metatarsal using longitudinal plantar ultrasound guidance and a V-18 wire (Boston Scientific). A 4F sheath is then inserted, and diagnostic venography is performed.
Step 3
Using the V-18 wire, the venous plexus is traversed to the level of the tibioperoneal trunk. The 4F sheath is exchanged for a long 4F × 55-cm sheath.
Step 4
Attention is then given to the below-knee area at the level of the tibioperoneal trunk. The image intensifier is brought to an oblique angle to separate the tibia and fibula. A single diagnostic angiogram is performed in sequence to determine an adequate area of crossing. In the same run, the arterial angiogram is performed first, quickly followed by venography from the 4F venous sheath.
Step 5
The adequate donor vessel (usually the PTA) is cannulated using a Command ES 0.014-in. wire (Abbot Vascular) and predilated with a 3- to 3.5-mm balloon to accommodate the reentry device that will be used for crossing.
Step 6
A reentry device of choice is then inserted; we elect for an Outback catheter (Cordis Medical) because it is a less bulky device and fits through a 6F sheath. Crossing from the arterial to the venous side is achieved, typically at 4 to 10 cm from the vessel ostium where the venous and arterial segments are closest to each other and relatively free of arterial atherosclerosis or significant calcification.
Step 7
From the venous side, a 9- to 15-mm Atrieve snare (Argon Medical Devices) is inserted to the desired area of crossing. Before crossing, vasodilators are given (200 mg of nitroglycerin, 2.5 mg of verapamil, and heparinized saline) to dilate the vein as much as possible and obtain a maximum snare diameter.
Step 8
The reentry system needle is deployed, and the Command ES 0.014-in. wire is advanced simultaneously as the snare is pulled back on the venous side. The area of crossing is marked with a hemostat outside the body to record its placement for stenting in the ensuing steps. After the wire is through-and-through, the 4- × 55-cm sheath is removed and replaced with the short 4F sheath from the initial access.
Step 9
A 3- to 3.5-mm cutting balloon is then used at the level of arterial-to-venous crossing to dilate this area. We prefer a cutting balloon because typically the arterial wall is calcified, and the balloon allows for controlled dilation and mitigation of additional vessel trauma. Venography is performed, and any segment with persisting venous valves is also treated with the cutting balloon.
Step 10
A 5- × 220-mm balloon is then advanced, and the venous segment is treated with care not to expand the balloon in the arterial side. It is not uncommon to have venous rupture with contrast extravasation during this step.
Step 11
A 5- × 250-mm Viabahn covered stent (W.L. Gore & Associates) is advanced to the venous segment. Distal deployment is crucial. The objective is to cover the largest branches to mitigate flow steal, with care not to cover any important venous branches that constitute the venous pedal loop.
Step 12
Often, a 250-mm covered stent will not be long enough, and an additional covered stent will be used to extend into the arterial side. (The arterial section of the covered stent should not exceed >3 cm in length.)
Step 13
The venous side of the crossing stent is postdilated using the prior 5- × 220-mm balloon. Because the crossing stent is self-expanding and sized for the larger diameter vein, it is constrained in the smaller diameter arterial segment at the point of crossover, with additional covered stent fabric running the risk of not being well opposed to the arterial wall. Therefore, the arterial side of the crossing stent (inflow segment) is reinforced with a balloon-expandable drug-eluting coronary stent extending from the arterial side into the venous point of crossover. The venous side of the coronary stent is postdilated to 4 to 5 mm.
Step 14
Completion angiography of the eDVA is performed. It is important to observe the late filling of the metatarsal venules, because these will provide perfusion information to the area of interest. If the distal venules are not visualized, we proceed to cannulate them with a 0.014-in. platform wire and perform angioplasty using a 2-mm balloon to allow for valve disruption and provide antegrade and superficial venous flow.
Step 15
In summary, the postoperative volume flows are measured; we aim for 50 to 250 mL/min.17 Simultaneously, we also measure the PAT to have a baseline measurement from the procedure. The venous sheath is removed, and manual pressure is applied. The arterial sheath is removed, and a closure device is routinely used. Heparin is reversed using protamine.
Postoperative surveillance and wound management
Clinically significant postoperative edema is not commonly seen with either technique if the final flow volumes remain low (80-250 mL/min). To control the flow volumes, two main elements are considered: the proximal donor site diameter and distal outflow resistance. Specifically, during the initial procedure, it is important to avoid too large of a proximal arteriotomy. Moreover, outflow through the various venous vessels of the foot can be modified using selective coil embolization to minimize large early outflow vessels that can lead to increased volume flow, ischemic steal, and preclusion of flow to the distal forefoot. Despite the work to control the volume flows, mild swelling and/or pulsatile postoperative pain can still be a concern and should be managed with compression wrapping and analgesics.
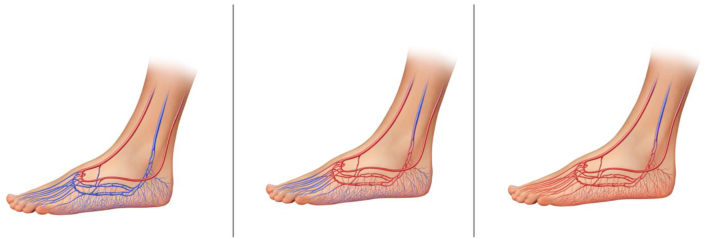
It appears that perfusion with arterialization increases over time secondary to venous dilation, which leads to valvular incompetence and further vascular recruitment.1 Prior studies have demonstrated that increments in transcutaneous oxygen tension and clinically evident wound healing will occur, on average, 6 to 8 weeks postoperatively.18 This “maturation time” is an important concept to fully put into practice, because operators must defer foot reconstruction (Fig 10).
Fig 10.
Diagrams showing perfusion changes over time with arterialization that lead to valvular incompetence and further vascular recruitment.
Specifically, unless intractable pain or infection are present, most podiatric-related procedures are deferred for a minimum of 6 weeks. If clear clinical signs are present that revascularization has occurred, the deferral time is flexible. We evaluate all patients on a weekly basis. The first postoperative DUS scan occurs 1 week after the procedure. We exclude the ankle brachial index and toe brachial index and tailor the DUS to which VA was performed. Documentation of the inflow, anastomosis, and outflow velocities and flow volumes with attention to the pedal venous flow volumes and PAT is performed. The use of bedside Doppler ultrasound evaluation is standard, with DUS performed only if significant changes have occurred in the clinical condition. In general, postoperative surveillance will be further dictated by the patient's course during this intensive follow-up period. Therefore, we believe these procedures should be reserved for high-volume CLTI centers with a multidisciplinary limb salvage team in place to support the challenging postoperative care.
Discussion
To date, management of noCLTI has been suboptimal with the current standard treatment strategies. The amputation rates for CLTI patients range from 22% to 34%,19,20 with amputation rates for noCLTI patients reported at 40% as early as 6 months.21 As VA increases in favor to treat noCLTI, adequate understanding of the technique and physiology becomes crucial to achieving standardization and improving outcomes.
Contemporary results for arterialization are encouraging and provide an avenue of treatment for this otherwise helpless population. Limb salvage rates at 12 months range from 70% to 81%, with complete wound healing rates of 46% to 68%.3,22,23 Furthermore, our group demonstrated that although eDVA had a higher reintervention rate, no difference was found in the outcomes for wound healing or limb salvage between HySA and eDVA.3 Although most of the literature are small retrospective cohort studies, the LimFlow system has been approved to standardize eDVA. The PROMISE I trial was an early feasibility study using the LimFlow system, which showed a 97% technical success rate and 70% amputation-free survival in their 32-patient cohort.23 The PROMISE II prospective study has now concluded, and the results shared are encouraging.4 Overall, larger studies with long-term results are still warranted to validate this procedure. However, adequate steps are underway to keep improving this technique.
Conclusions
Patients with noCLTI have a dismal prognosis with high amputation rates and associated high mortality. The feasibility of VA has improved owing to further understanding of the technique and improvements in endovascular technology. We have presented detailed steps in the creation of arterialization with the aim of standardizing this method to treat this population. Further research is necessary; however, the limb salvage results appear acceptable.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Clair D., Gibbons M. A review of percutaneous deep vein arterialization for the treatment of nonreconstructable chronic limb threatening ischemia. Semin Vasc Surg. 2021;34:188–194. doi: 10.1053/j.semvascsurg.2021.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Ho V.T., Gologorsky R., Kibrik P., et al. Open, percutaneous, and hybrid deep venous arterialization technique for no-option foot salvage. J Vasc Surg. 2020;71:2152–2160. doi: 10.1016/j.jvs.2019.10.085. [DOI] [PubMed] [Google Scholar]
- 3.Miranda J.A., Pallister Z., Sharath S., et al. Early experience with venous arterialization for limb salvage in no-option patients with chronic limb-threatening ischemia. J Vasc Surg. 2022;76:987–996.e3. doi: 10.1016/j.jvs.2022.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Shishehbor M.H., Powell R.J., Montero-Baker M.F., et al. Transcatheter arterialization of deep veins in chronic limb-threatening ischemia. N Engl J Med. 2023;388:1171–1180. doi: 10.1056/NEJMoa2212754. [DOI] [PubMed] [Google Scholar]
- 5.Kim T.I., Vartanian S.S., Schneider P.A. A review and proposed classification system for the No-option patient with chronic limb-threatening ischemia. J Endovasc Ther. 2021;28:183–193. doi: 10.1177/1526602820963911. [DOI] [PubMed] [Google Scholar]
- 6.Ferraresi R., Ucci A., Pizzuto A., et al. A novel scoring system for small artery disease and medial arterial calcification is strongly associated with major adverse limb events in patients with chronic limb-threatening ischemia. J Endovasc Ther. 2021;28:194–207. doi: 10.1177/1526602820966309. [DOI] [PubMed] [Google Scholar]
- 7.Bradbury A.W., Ruckley C.V., Fowkes F.G., Forbes J.F., Gillespie I., Adam D.J. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 8.Verwer M.C., Wijnand J.G., Teraa M., et al. External validation of the Vascular Quality Initiative prediction model for survival in no-option chronic limb-threatening ischemia patients. J Vasc Surg. 2020;72:1659–1666. doi: 10.1016/j.jvs.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Stoyioglou A., Jaff M.R. Medical treatment of peripheral arterial disease: a comprehensive review. J Vasc Interv Radiol. 2004;15:1197–1207. doi: 10.1097/01.RVI.0000137978.15352.C6. [DOI] [PubMed] [Google Scholar]
- 10.Ferraresi R., Casini A., Losurdo F., et al. Hybrid foot vein arterialization in No-option patients with critical limb ischemia: a preliminary report. J Endovasc Ther. 2019;26:7–17. doi: 10.1177/1526602818820792. [DOI] [PubMed] [Google Scholar]
- 11.Mills J.L., Sr., Conte M.S., Armstrong D.G., et al. Society for Vascular Surgery Lower Extremity Guidelines Committee The society for vascular surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI) J Vasc Surg. 2014;59:220–234.e1-2. doi: 10.1016/j.jvs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Flu W.J., Schouten O., van Kuijk J.P., Poldermans D. Perioperative cardiac damage in vascular surgery patients. Eur J Vasc Endovasc Surg. 2010;40:1–8. doi: 10.1016/j.ejvs.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Liu I.H., Wu B., Krepkiy V., et al. Pedal arterial calcification score is associated with the risk of major amputation in chronic limb-threatening ischemia. J Vasc Surg. 2022;75:270–278.e3. doi: 10.1016/j.jvs.2021.07.235. [DOI] [PubMed] [Google Scholar]
- 14.Sommerset J., Karmy-Jones R., Dally M., Feliciano B., Vea Y., Teso D. Plantar acceleration time: a novel technique to evaluate arterial flow to the foot. Ann Vasc Surg. 2019;60:308–314. doi: 10.1016/j.avsg.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Teso D., Sommerset J., Dally M., Feliciano B., Vea Y., Jones R.K. Pedal acceleration time (PAT): a novel predictor of limb salvage. Ann Vasc Surg. 2021;75:189–193. doi: 10.1016/j.avsg.2021.02.038. [DOI] [PubMed] [Google Scholar]
- 16.Darling R.C., 3rd, Kupinski A.M. In situ bypass: technical considerations. Preoperative evaluation of veins. Semin Vasc Surg. 1993;6:155–158. [PubMed] [Google Scholar]
- 17.Schreve M.A., Huizing E., Kum S., de Vries J.P.M., de Borst G.J., Ünlü Ç. Volume flow and peak systolic velocity of the arteriovenous circuit in patients after percutaneous deep venous arterialization. Diagnostics (Basel) 2020;10:760. doi: 10.3390/diagnostics10100760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt A., Schreve M.A., Huizing E., et al. Midterm outcomes of percutaneous deep venous arterialization with a dedicated system for patients with No-option chronic limb-threatening ischemia: the ALPS multicenter study. J Endovasc Ther. 2020;27:658–665. doi: 10.1177/1526602820922179. [DOI] [PubMed] [Google Scholar]
- 19.Marston W.A., Davies S.W., Armstrong B., et al. Natural history of limbs with arterial insufficiency and chronic ulceration treated without revascularization. J Vasc Surg. 2006;44:108–114. doi: 10.1016/j.jvs.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Steffen M.W., Undavalli C., Asi N., et al. The natural history of untreated severe or critical limb ischemia. J Vasc Surg. 2015;62:1642–1651. doi: 10.1016/j.jvs.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 21.Norgren L., Hiatt W.R., Dormandy J.A., Nehler M.R., Harris K.A., Fowkes F.G. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45:S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 22.Kum S., Tan Y.K., Schreve M.A., et al. Midterm outcomes from a pilot study of percutaneous deep vein arterialization for the treatment of No-option critical limb ischemia. J Endovasc Ther. 2017;24:619–626. doi: 10.1177/1526602817719283. [DOI] [PubMed] [Google Scholar]
- 23.Clair D.G., Mustapha J.A., Shishehbor M.H., et al. Promise I: early feasibility study of the LimFlow System for percutaneous deep vein arterialization in no-option chronic limb-threatening ischemia: 12-month results. J Vasc Surg. 2021;74:1626–1635. doi: 10.1016/j.jvs.2021.04.057. [DOI] [PubMed] [Google Scholar]