ABSTRACT
BACKGROUND:
Severe traumatic injuries not only constitute an important population of pediatric intensive care unit (PICU) but they also play a major role in mortality and morbidity. Mortality risk assessment of traumatic injuries in the PICU is a delicate issue as it influences the treatment decisions. BIG score (Base Deficit +[2.5 × INR] + [15-GCS]) and the Pediatric Trauma Score (PTS) are utilized in pediatric trauma centers for the assessment of trauma severity. In this research, we aimed to elucidate the predictivity of trauma severity scores, the PRISM-3 (pediatric risk of mortality), and admission laboratory parameters in pediatric patients with high-energy traumas.
METHODS:
Children who had been exposed to high-energy polytraumas between 2018 and 2020 and treated in a tertiary care PICU were included in this retrospective analysis. Newly developed mental or motor disabilities, post-traumatic acquired epilepsy, requirement for tracheostomy, and/or extremity loss at PICU discharge were defined as morbidity. The PTS, the BIG score, PRISM-3 score, and admission laboratory parameters were utilized for mortality and morbidity prediction.
RESULTS:
A total of 155 patients were included in the study. The median age of the participants were 66 months (25–134). The origin of trauma was fall from height in 45.2% (n=70) of the subjects and traffic accident 54.8% (n=85) of the cases. New morbidities had occurred in 8.7% (n=13) and 3.2% (n=5) of the patients deceased in the ICU. The results of logistic regression analysis indicated that BIG score (p=0.01), PTS (p=0.003), PRISM-3 (p=0.02), admission D-dimer (p=0.01), and albumin levels (p=0.001) were significantly associated with mortality. The receiver operating characteristics curve analysis denoted that BIG score (cutoff >21.5, area under the curve [AUC]: 0.984 95% CI: 0.943–0.988), PRISM-3 score (cutoff >18, AUC: 0.997 95% CI: 0.970–1), the PTS (cutoff ≤3, AUC: 0.969 95% CI: 0.928–0.990), admission albumin level (cutoff ≤3 g/dL, AUC: 0.987 95% CI: 0.953–0.998), and D-dimer level (cutoff >13,100 mcg/L, AUC: 0.776 95% CI: 0.689–0.849) all had high predictive values for mortality.
CONCLUSION:
Regarding the results of this research, one can conclude that BIG score is a strong predictor of mortality and morbidity in high-energy pediatric traumas. Although PRISM-3 score has a similar predictive capability, the earlier and easier calculation assets of BIG score positions itself as a more useful and powerful predictor for mortality and morbidity in pediatric high-energy traumas.
Keywords: BIG score, high-energy trauma, mortality, pediatric intensive care unit, pediatric trauma
INTRODUCTION
Traumatic injuries are still one of the leading causes of death in children.[1] Preventive measures and education of families can reduce traumatic accidents. High-energy traumas can be observed in falls from height, traffic accidents, or during war by high amount of kinetic energy transfer to the body that cause serious damage to the tissues.[2] Children are more likely to be exposed to high-energy trauma than adults, due to the low body mass index and high body surface area/weight ratio. High-energy polytraumas have the potential to develop serious complications during the acute and subsequent phases such as acute respiratory distress syndrome and brain edema in children even if no serious findings are observed in the initial evaluation.[3,4] Therefore, follow-up of the patients may be necessary in the pediatric intensive care unit (PICU). Prediction of outcomes such as mortality or morbidity is another important task for ICUs, because it requires patient-based decisions, affects intensive care quality assessment, and provides the classification of cases according to mortality or morbidity risks in academic research. In this study, we aimed to elucidate the predictivity of trauma severity scores, the PRISM-3 score (pediatric risk of mortality), and admission laboratory parameters in PICU patients with high-energy traumas.
MATERIALS AND METHODS
Children who had been exposed to high-energy polytraumas between 2018 and 2020 and treated in a tertiary care PICU were included in this retrospective cross-sectional study. The research was conducted in a 12-bed PICU of a training and research hospital, which admits approximately 500 surgical and medical patients per year. Children younger than 18 years of age and hospitalized after acute-onset traumatic injuries in the PICU had been enrolled in the study. Fall from height (>3 m) and high-risk traffic accidents were considered as high-energy polytraumas.[5] Individuals older than 18 years of age and with other traumas have not been included. In addition, if the interval between traumatic event and the hospital admission was more than 24 h, the patient was excluded from the study.
Patient demographics, PRISM-3 score, PTS, BIG score, trauma type, mechanical ventilation requirement, admission procalcitonin level, C-reactive protein, lactate dehydrogenase (LDH), creatinine kinase, white blood cells, platelets, D-dimer, INR, base deficit, length of PICU stay, morbidities, and survival at PICU discharge were retrospectively collected from the hospital database.
The pediatric trauma score (PTS), the BIG score (Base Deficit +[2.5 × INR] + [15–GCS]), and the pediatric risk of mortality III-24 score (PRISM-3) were utilized for determining the severity of disease and outcome prediction.[6,7] Glasgow Coma Scale (GCS) was used to assess consciousness quantitatively and pediatric GCS was preferred for subjects under 2 years of age. Newly developed mental or motor disabilities (paresis or plegia), post-traumatic acquired epilepsy, requirement for tracheostomy, or extremity loss at PICU discharge were defined as morbidity.
The ethics committee approval has been granted on 2019 and protocol number: 2019–2020/17.
Statistical Analysis
Continuous variables were expressed as the median and interquartile range (25–75 percentiles). Categorical variables were expressed as n (%). Statistical comparison of continuous variables between two independent groups was performed using the Mann–Whitney U-test. Statistical comparisons of categorical variables were performed using the Chi-square test or Fisher’s exact test. Single logistic regression analysis was used for the determination of significant parameters for mortality and morbidity. Statistically significant logistic regression analysis results were presented as odds ratio (OR), 95% confidence interval, and P-value. Receiver operating characteristics (ROC) curve analysis was performed to analyze the predictive value of variables. The Youden index was used to identify cutoff values. Results were expressed as a cutoff value, area under the ROC curve area under the curve (AUC), sensitivity, specificity, and p-value. P<0.05 was considered statistically significant. Statistical analysis was performed through Statistical Package for the Social Sciences (SPSS) for Windows version 23 and MedCalc for Windows version 14.8.1.
RESULTS
There were 1290 PICU admissions during the study period and 167 (12.9%) of them were due to traumatic injuries and 155 of 167 patients were classified as acute high-energy traumas. The median age was 66 months (25–134) and the female-to-male ratio was 50/105. The origin of trauma was fall from height in 45.2% (n=70) of the subjects and traffic accident 54.8% (n=85) of the cases (21 in a vehicle and 64 out of a vehicle). The median PRISM-3 score was 6 (5–12), PTS was 8 (5–10), GCS was 12 (9–14), and the BIG score was 9.4 (6.8–5.1).
Forty-four (28.4%) patients had intrathoracic or abdominal hemorrhages and 39 (25.2%) patients had intracranial hemorrhages. The most common intracranial hemorrhage type was subdural in 13 (33.3%) of the cases followed by epidural in 9 (23.1%), subarachnoid in 9 (23.1%), and parenchymal in 8 (20.5%) patients.
Laboratory values at admission were as follows; C-reactive protein 2.65 mg/L (1–11), procalcitonin 0.44 ng/mL (0.12–1.97), albumin 4.2 g/dL (3.7–4.5), LDH 481 U/L (344–758), creatine kinase 405 U/L (192–880), D-dimer 3692 mcg/L (1213–9657), white blood cells 15.2×103/mm3 (12.1–19.4), platelets 282×103/mm3 (220–340), INR 1.15 (1–1.5), and base deficit was 3.1 (2.1–5.1).
D-dimer was significantly higher (9657 ng/mL [4601–2062]) in the patients with intracranial hemorrhage compared to others (2813 ng/mL [986–6363]) (p<0.001). However, no significance has been achieved between patients with and without abdominal or thoracic hemorrhage (p=0.96).
Forty-three (27.7%) patients required invasive mechanical ventilation during their PICU stay. Thirteen (8.7%) patients had morbidities at PICU discharge and neurologic deficits were the most common (n=12, 8%) one. Other morbidities were elaborated as post-traumatic epilepsy in 5 (3.3%) patients, tracheostomy need in 3 (2%) patients, and extremity loss in 1 (0.6%) patient.
The median length of PICU stay was 3 days (2–7). Five (3.3%) of the patients were deceased in the ICU (Table 1). Logistic regression analysis results for the dependent variables mortality and morbidity are presented in Table 2. Receiver operating curve analysis results are presented in Table 3.
Table 1.
Features of survived and non-survived patients
| Survived n=150 | Non-Survived n=5 | p | |
|---|---|---|---|
|
|
|
||
| Median (25–75p) | Median (25–75p) | ||
| Age (months) | 65 (25–132) | 125 (54–136) | 0.49 |
| BIG score | 9.2 (6.7–15.8) | 30.4 (25.9–32.05) | <0.001 |
| Pediatric Trauma Score | 8 (6-10) | -1 (-1–2) | <0.001 |
| Glasgow Coma Scale | 12.5 (10–14) | 3 (3–3) | 0.002 |
| Pediatric Risk of Mortality Score | 6 (5–9) | 34 (26–45) | <0.001 |
| Intraabdominal or intratorasic hemorrhage, n (%) | 43 (28.7) | 1 (20) | 0.6 |
| Intracranial hemorrhage, n (%) | 37 (24.7) | 2 (40) | 0.6 |
| C-reactive protein (mg/dL) | 2.8 (1–11.4) | 1.3 (1.2–1.7) | 0.54 |
| D-Dimer (mcg/L) | 3646 (1213–8780) | 18451 (13686–36850) | 0.03 |
| Albumin (g/dL) | 4.25 (3.8–4.5) | 2.4 (1.7–2.7) | <0.001 |
| Base deficit (meq/L) | 3.1 (2.1–5) | 12.3 (9.2–13.9) | <0.001 |
| International normalized ratio | 1.1 (1–1.4) | 1.9 (1.8–2.2) | 0.002 |
| Lactate dehydrogenase (U/L) | 481 (343–756) | 478 (390–813) | 0.8 |
| Creatinin kinase (U/L) | 388 (191–876) | 855 (542–1853) | 0.13 |
| Procalcitonin (ng/ml) | 0.45 (0.12–1.89) | 0.12 (0.03–12.06) | 0.671 |
| White blood cells (x103/mm3) | 15.2 (12.1–19.4) | 11.2 (9.6–15.3) | 0.26 |
| Platelets (x103/mm3) | 283 (223–340) | 133 (64–211) | 0.015 |
Table 2.
Single variable logistic regression analysis results for the dependent variables morbidity and mortality
| Morbidity | Mortality | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| p | OR | %95 CI | p | OR | %95 CI | |||
| Intracranial hemorrhage (yes/no) | <0.001 | 13.5 | 3.49 | 52.63 | 0.446 | |||
| GCS | 0.994 | 0.995 | ||||||
| BIG score | 0.001 | 1.720 | 1.261 | 2.346 | 0.01 | 1.768 | 1.138 | 2.746 |
| PTS | 0.000 | 0.547 | 0.422 | 0.710 | 0.003 | 0.468 | 0.285 | 0.768 |
| PRISM-3 score | 0.023 | 1.451 | 1.222 | 1.724 | 0.024 | 1.647 | 1.067 | 2.543 |
| D-Dimer 500* (mcg/L) | 0.019 | 1.026 | 1.004 | 1.049 | 0.011 | 1.032 | 1.007 | 1.058 |
| Albumin 0.1** (g/dL) | <0.001 | 0.827 | 0.746 | 0.918 | 0.001 | 0.747 | 0.634 | 0.881 |
| PCT (ng/mL) | 0.792 | 0.027 | 1.094 | 1.010 | 1.184 | |||
| PLT50*** (x103/mm3) | 0.865 | 0.005 | 0.422 | 0.232 | 0.767 | |||
Age, intracranial hemorrhages, intrathoracic or abdominal hemorrhages, Glasgow coma score (GCS), Pediatric trauma score (PTS), Pediatric risk of mortality (PRISM-3), C-reactive protein, D-Dimer, Albumin, Lactate dehydrogenase, Creatinin kinase, Procalcitonin (PCT), White blood cells and Platelets were included to the single variable logistic regression analysis. Only statistically significant variables were presented.
Calculated for each 500 mcg/L D-dimer change.
Calculated for each 0.1 g/dL Albumin change.
Calculated for each 50x103/mm3 Platelet change.
Table 3.
Receiver operating curve characteristics analysis results for the mortality and morbidity
| Cut-Off Value | AUC | %95 CI for AUC | Sensitivity | Specificity | p | ||
|---|---|---|---|---|---|---|---|
| Mortality | PTS | ≤3 | 0.969 | 0.928-0.990 | 100 | 90 | <0.001 |
| BIG score | >21.5 | 0.984 | 0.943-0.998 | 100 | 94.2 | <0.001 | |
| PRISM score | >18 | 0.997 | 0.970-1 | 100 | 98.6 | <0.001 | |
| D-Dimer (mcg/L) | >13100 | 0.776 | 0.689-0.849 | 80 | 84.5 | 0.03 | |
| Albumin (g/dL) | ≤3 | 0.987 | 0.953-0.998 | 100 | 95.8 | <0.001 | |
| Morbidity | PTS | ≤5 | 0.940 | 0.889-0.972 | 100 | 83.9 | <0.001 |
| BIG score | >17.8 | 0.970 | 0.921-0.993 | 100 | 91.6 | <0.001 | |
| PRISM score | >9 | 0.917 | 0.861-0.956 | 92.3 | 82.4 | <0.001 | |
| D-Dimer (mcg/L) | >2150 | 0.755 | 0.664-0.832 | 100 | 39 | 0.001 | |
| Albumin (g/dL) | ≤3.8 | 0.774 | 0.697-0.839 | 69.2 | 76.6 | <0.001 |
CI: Confidence Interval; AUC: Area under the curve; PTS: Pediatric Trauma Score; PRISM: Pediatric risk of mortality.
DISCUSSION
Traumatic injuries are still one of the most important causes of mortality in childhood age. Most of the fatal traumas in children are due to high-energy traumas. This study investigated the general characteristics of pediatric high-energy traumas and factors associated with mortality and morbidity in PICU. The key findings of this study were relevant to outcome-associated factors. The BIG score appeared to be one of the most sensitive predictors of mortality and the most sensitive predictor of morbidity. PTS was also considered as a sensitive marker for morbidity outcomes of pediatric high-energy traumas. Not surprisingly, PRISM-3 score was the most sensitive predictor of mortality in our study. Admission albumin levels indicated high predictive performance on mortality (AUC: 0.987) and morbidity (AUC: 0.774). Despite the significant difference of GCS between survived and deceased patients, there was no significant relationship in logistic regression analysis between GCS and intensive care mortality or morbidity.
The BIG score was designed to assess disease severity and to predict trauma mortality in the military setting.[8] The performance of the BIG score in ROC curve analysis for mortality prediction has been assessed in different studies. In the original study, the AUC of the ROC curve for mortality was calculated as 0.890 and it has been reported that it was better than all other trauma severity scoring systems assessed in the study.[8] In recent years, evidence about the mortality prediction performance of the BIG score has been growing for pediatric trauma victims. Davis et al.[9] have reported that the BIG score can accurately predict mortality (AUC: 0.950) in pediatric blunt trauma patients in the emergency setting. They have also stated that the BIG score had an excellent predictive performance (AUC: 0.920) in patients followed in the ICU. In another study, Muisyo et al.[10] have emphasized that BIG score had an excellent (AUC: 0.940) and comparable performance to PRISM-3 (0.960), pediatric index of mortality-2 (PIM-2) (AUC: 0.970), and pediatric logistic organ dysfunction scores (AUC: 0.930). In our study, BIG score presented very good predictivity (AUC: 0.984) for mortality like PRISM-3 and PTS. However, the disadvantage of PRISM-3 score could be elaborated as it should be calculated at the end of the first 24th h. Therefore, BIG score seems to be a useful mortality predictor and disease severity score for pediatric trauma victims because it can be calculated easily on admission with basic laboratory and physical examination findings.
Prediction of morbidity in pediatric trauma victims as an outcome has not been evaluated comprehensively in the previous literature. It is because morbidity does not describe clearly like mortality. This study interpreted that morbidity prediction can be possible with BIG and PTS trauma scoring systems. However, the increased use of quantitative morbidity scoring systems like the Functional Status Score will be able to identify morbidity predictors more accurately in the future.[11]
The PTS was developed by Tepas et al. in 1987.[12] The total PTS ranges between 6 and 12 and the PTS <=8 is considered as severe trauma.[6] In a study by Chabok et al.,[13,14] it has been reported that PTS is not a significant predictor for the mortality of pediatric trauma in the emergency setting. However, ROC analysis of the same study showed that PTS had good performance (AUC: 0.949, 95% CI: 0.908–0.991) for predicting mortality as indicated in our study (AUC: 0.969). GCS has also been shown as a significant mortality predictor in the same study, unlike our results. In the emergency setting, admission GCS is a more raw variable than PICU admission, because, during the emergency follow-up, patients who have low or deteriorating GCS are almost always sedated and intubated before ICU even if emergency admission GCS was not low. Therefore, GCS of intensive care admission concludes a bimodal distribution (Fig. 1), unlike the emergency admission, and statistical significance was not detected even if all mortalities and morbidities were observed in the low GCS patients.
Figure 1.
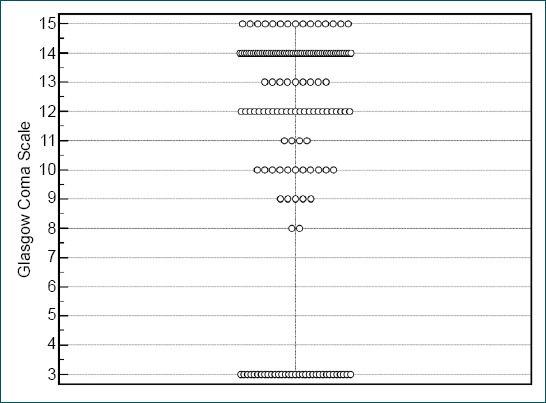
Bimodal distribution of Glasgow Coma Scales of the patients. Each circle represents a patient in the study.
D-dimer assay measures cross-linked fibrin degradation products and it is one of the most sensitive biomarkers of fibrinolysis.[10] It is known that traumatic disorders are associated with hypercoagulability and high D-dimer values.[15] Investigators interested in pediatric traumatic brain injury processed D-dimer to predict intracranial hemorrhages. Similar D-dimer cutoff values were published in different studies and normal D-dimer levels (<500 ng/mL) can exclude intracranial hemorrhages in almost all pediatric patients with isolated head trauma and prevent unnecessary head CT imaging.[16–18] In our study, patients had severe high-energy polytrauma and D-dimer levels were higher than previously mentioned studies. Therefore, we think that D-dimer cannot be a reliable marker to separate patients with intracranial hemorrhage from the others in high-energy polytrauma patients. It has been reported that high D-dimer and low albumin levels on emergency admission can predict poor outcomes in severe trauma patients.[19–21] This study elaborated that high D-dimer and low albumin levels were important mortality and morbidity predictors in high-energy pediatric polytraumas.
The most important limitation of this study could be attributed to its retrospective nature. Trauma-related morbidities can usually resolve completely over weeks or months, so the morbidities on intensive care discharge do not reflect permanent disabilities in this population. The strength of this study lies beneath the fact that it is one of the few articles of evaluating the relationship between trauma scores and mortality in pediatric high-energy traumas in PICU.
Conclusion
Regarding the results of this research, one can conclude that BIG score is a strong predictor of mortality and morbidity in high-energy pediatric traumas. Although PRISM-3 score has a similar predictive capability, the earlier and easier calculation assets of BIG score positions itself as a more useful and powerful predictor for mortality and morbidity in pediatric high-energy traumas.
Footnotes
Ethics Committee Approval: This study was approved by the Antalya Training and Research Hospital Clinical Research Ethics Committee (Date: 12.09.2019, Decision No: 20/17).
Peer-review: Internally peer-reviewed.
Authorship Contributions: Concept: H.S.K., E.A.O.; Design: H.S.K., E.A.O.; Supervision: H.S.K., E.A.O.; Resource: H.S.K., E.A.O.; Materials: H.S.K., E.A.O.; Data: H.S.K., E.A.O.; Analysis: H.S.K., E.A.O.; Literature search: H.S.K., E.A.O.; Writing: H.S.K.; Critical revision: H.S.K., E.A.O.
Conflict of Interest: None declared.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019:A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–22. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abelsson A, Rystedt I, Suserud BO, Lindwall L. Learning high-energy trauma care through simulation. Clin Simul Nurs. 2018;17:1–6. [Google Scholar]
- 3.De Roulet A, Burke RV, Lim J, Papillon S, Bliss DW, Ford HR, et al. Pediatric trauma-associated acute respiratory distress syndrome:Incidence, risk factors, and outcomes. J Pediatr Surg. 2019;54:1405–10. doi: 10.1016/j.jpedsurg.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Beer-Furlan A, Soares M, Teixeira M, Paiva WS. Delayed unilateral traumatic brain swelling in a child. J Pediatr Neurosci. 2014;9:169–71. doi: 10.4103/1817-1745.139345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasser SM, Hunt RC, Faul M, Sugerman D, Pearson WS, Dulski T, et al. Guidelines for field triage of injured patients:Recommendations of the national expert panel on field triage, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:1–20. [PubMed] [Google Scholar]
- 6.Kaufmann CR, Maier RV, Rivara FP, Carrico CJ. Evaluation of the pediatric trauma score. JAMA. 1990;263:69–72. [PubMed] [Google Scholar]
- 7.Pollack MM, Patel KM, Ruttimann UE. PRISM III:An updated pediatric risk of mortality score. Crit Care Med. 1996;24:743–52. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Borgman MA, Maegele M, Wade CE, Blackbourne LH, Spinella PC. Pediatric trauma bıg score:Predicting mortality in children after military and civilian trauma. Pediatrices. 2011;127:e892–7. doi: 10.1542/peds.2010-2439. [DOI] [PubMed] [Google Scholar]
- 9.Davis AL, Wales PW, Malik T, Stephens D, Razik F, Schuh S. The big score and prediction of mortality in pediatric blunt trauma. J Pediatr. 2015;167:593–8. doi: 10.1016/j.jpeds.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 10.Muisyo T, Bernardo EO, Camazine M, Colvin R, Thomas KA, Borgman MA, et al. Mortality prediction in pediatric trauma. J Pediatr Surg. 2019;54:1613–6. doi: 10.1016/j.jpedsurg.2018.08.045. [DOI] [PubMed] [Google Scholar]
- 11.Pollack MM, Holubkov R, Glass P, Dean JM, Meert KL, Zimmerman J, et al. Functional status scale:New pediatric outcome measure. Pediatrices. 2009;124:e18–28. doi: 10.1542/peds.2008-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tepas JJ, Mollitt DL, Talbert JL, Bryant M. The pediatric trauma score as a predictor of injury severity in the injured child. J Pediatr Surg. 1987;22:14–8. doi: 10.1016/s0022-3468(87)80006-4. [DOI] [PubMed] [Google Scholar]
- 13.Anıl M, Sarıtaş S, Bıcılıoğlu Y, Gökalp G, Can FK, Anıl AB. The performance of the pediatric trauma score in a pediatric emergency department:A prospective study. J Pediatr Emerg Intensive Care Med. 2017;4:1–7. [Google Scholar]
- 14.Yousefzadeh-Chabok S, Kazemnejad-Leili E, Kouchakinejad-Eramsadati L, Hosseinpour M, Ranjbar F, Malekpouri R, et al. Comparing pediatric trauma score, Glasgow coma scale, and ınjury severity score for mortality prediction in traumatic children. Turk J Trauma Emerg Surg. 2016;22:328–32. doi: 10.5505/tjtes.2015.83930. [DOI] [PubMed] [Google Scholar]
- 15.Adam SS, Key NS, Greenberg CS. D-dimer antigen:Current concepts and future prospects. Blood. 2009;113:2878–87. doi: 10.1182/blood-2008-06-165845. [DOI] [PubMed] [Google Scholar]
- 16.Ryan ML, Van Haren RM, Thorson CM, Andrews DM, Perez EA, Neville HL, et al. Trauma induced hypercoagulablity in pediatric patients. J Pediatr Surg. 2014;49:1295–9. doi: 10.1016/j.jpedsurg.2013.11.050. [DOI] [PubMed] [Google Scholar]
- 17.Nozawa M, Mishina H, Tsuji S, Takayama JI. Low plasma D-dimer predicts absence of intracranial injury and skull fracture. Pediatr Int. 2020;62:22–8. doi: 10.1111/ped.14063. [DOI] [PubMed] [Google Scholar]
- 18.Langness S, Ward E, Halbach J, Lizardo R, Davenport K, Bickler S, et al. Plasma D-dimer safely reduces unnecessary CT scans obtained in the evaluation of pediatric head trauma. J Pediatr Surg. 2018;53:752–7. doi: 10.1016/j.jpedsurg.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Berger RP, Fromkin J, Rubin P, Snyder J, Richichi R, Kochanek P. Serum D-Dimer Concentrations are ıncreased after pediatric traumatic brain ınjury. J Pediatr. 2015;166:383–8. doi: 10.1016/j.jpeds.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sung J, Bochicchio GV, Joshi M, Bochicchio K, Costas A, Tracy K, et al. Admission serum albumin is predictive of outcome in critically ill trauma patients. Am Surg. 2004;70:1099–102. [PubMed] [Google Scholar]
- 21.Hayakawa M, Maekawa K, Kushimoto S, Kato H, Sasaki J, Ogura H, et al. High D-Dimer levels predict a poor outcome in patients with severe trauma, even with high fibrinogen levels on arrival. Shock. 2016;45:308–14. doi: 10.1097/SHK.0000000000000542. [DOI] [PubMed] [Google Scholar]


