ABSTRACT
BACKGROUND:
Delayed autologous nerve graft reconstruction is inevitable in devastating injuries. Delayed or prolonged repair time has deleterious effects on nerve grafts. We aimed improving and accelerating nerve graft reconstruction process in a rat long nerve defect model with loop nerve graft prefabrication particularly to utilize for injuries with tissue loss.
METHODS:
Twenty-four Sprague-Dawley rats were allocated into three groups. 1.5 cm long peroneal nerve segment was excised, reversed in orientation, and used as autologous nerve graft. In conventional interpositional nerve graft group (Group 1), nerve defects were repaired in single-stage. In loop nerve graft prefabrication group (Group 2), grafts were sutured end-to-end (ETE) to the proximal peroneal nerve stumps. Distal ends of the grafts were sutured end-to-side to the peroneal nerve stumps 5 mm proximal to the ETE repair sites in first stage. In second stage, distal ends of the prefabricated grafts were transposed and sutured to distal nerve stumps. In staged conventional interpositional nerve graft group (Group 3), grafts were sutured ETE to proximal peroneal nerve stumps in first stage. Distal ends of the grafts and nerve stumps were tacked to the surrounding muscles until the final repair in second stage. Follow-up period was 4 weeks for each stage in Groups 2 and 3, and 8 weeks for Group 1. Peroneal function index (PFI), electrophysiology, and histological assessments were conducted after 8 weeks. P<0.05 was considered significant for statistical analysis.
RESULTS:
PFI results of Group 1 (−22.75±5.76) and 2 (−22.08±6) did not show statistical difference (p>0.05). Group 3 (−33.64±6.4) had a statistical difference compared to other groups (p<0.05). Electrophysiology results of Group 1 (16.19±2.15 mV/1.16±0.21 ms) and 2 (15.95±2.82 mV/1.17±0.16 ms) did not present statistical difference (p>0.05), whereas both groups had a statistical difference compared to Group 3 (10.44±1.96 mV/1.51±0.15 ms) (p<0.05). Axon counts of Group 1 (2227±260.4) and 3 (2194±201.1) did not have statistical difference (p>0.05), whereas both groups had significantly poor axon counts compared to Group 2 (2531±91.18) (p<0.05).
CONCLUSION:
Loop nerve graft prefabrication improved axonal regeneration without delay. Loop prefabrication can accelerate prolonged regeneration time for the injuries indicating a delayed nerve reconstruction. Higher axon counts derived with loop nerve prefabrication may even foster its investigation in immediate long nerve defect reconstructions in further studies.
Keywords: Loop nerve prefabrication, nerve graft, nerve injury, nerve repair
INTRODUCTION
In a peripheral nerve injury, outcomes of proper primary end-to-end (ETE) nerve repair are better than a nerve graft reconstruction.[1] However, tension has a deleterious effect on recovery, and bridging strategies may be inevitable to reconstruct a defect. Numerous conduits are investigated to repair the nerve defects.[2,3] Still autologous nerve grafts remain the treatment of choice.[4,5] Endoneural structures of nerve grafts serve as a scaffold with appropriate milieu to guide the ingrowing axons.[4,6,7]
Regeneration potential of a nerve graft is critically associated with its diameter, length, and vascular supply.[8] Intraneural fibrosis and axonal growth retardation are inevitable for a long and thick nerve graft. Although nerve grafts with large diameter face difficulties in revascularization and end-up with fibrosis,[8] axon fibers originating from proximal nerve stump require a large conduit; because, the number of regenerating axons determine the functional outcome.[4] Nerve grafts placed without paying attention to the inadequate blood supply of recipient body sites or with lack of soft-tissue support are prone to fail.[8]
In devastating soft-tissue injuries, such as battlefield wounds, delayed nerve repair after sufficient soft-tissue healing is necessary for a successful conventional interpositional nerve grafting.[4,5,8] However, prolonged or delayed repair time has a deleterious effect on nerve regeneration and end organ re-innervation.[7–9] Prolonged denervation diminishes the Schwann cell ability to support axonal regeneration. In chronically denervated Schwann cells neurotrophic factor expression progressively and irreversibly decrease.[10,11] Thus, the methods promoting the regenerative capacity of the delayed nerve grafting merit further investigation.
End-to-side (ETS) neurorrhaphy, as a proven nerve reconstruction alternative, induces collateral sprouting from axons of donor nerve to distal nerve stump.[12,13]
In this study, we investigated the feasibility of loop nerve graft prefabrication through the combination of ETE and ETS coaptation concepts in an experimental two-stage peripheral long nerve defect repair model to enable immediate reconstruction in devastating injuries.
We aimed to overcome the delay in nerve repair process and prevent the retardation in nerve regeneration particularly for injuries with soft-tissue loss indicating a delayed nerve grafting.
MATERIALS AND METHODS
Eight weeks old 24 Sprague-Dawley rats (250–300 g) were allocated to three experimental groups (n=8). Institutional animal care and utilization committee approved the Study design.
Surgical Design
Same surgeons performed the surgical procedures under a Zeiss OPMI 7 (Jena, Germany) operating microscope using sterile techniques. Intraperitoneal ketamine (100 mg/kg) and chlorpromazine (5 mg/kg) were administered for anesthesia. Thiopental sodium (100 mg/kg) was applied for euthanasia. In all groups, right peroneal nerves were exposed and dissected free of neighboring connective tissue and separated by internal neurolysis from the sciatic nerve. A 1.5 cm long segment of the peroneal nerve was excised to create a nerve defect. Harvested nerve segments were reversed in direction and used as interpositional autologous nerve graft in all defect reconstructions.
In Group 1, the nerve grafts were sutured ETE to the proximal and distal peroneal nerve stumps as a conventional interpositional nerve graft in a single stage (Fig. 1). In Group 2, loop nerve graft prefabrication repair was performed in two stages. In first stage, the nerve grafts were reversed in direction and proximal end of the nerve graft was sutured ETE to the proximal peroneal nerve stump. To build up the nerve loop, the distal end of the nerve graft was sutured ETS to the proximal peroneal nerve stump 5 mm proximal to the ETE repair site, after removal of a 1 mm diameter size window on the epineurium and perineurium (Fig. 2). <10% of the total axons of the nerve were transected during the ETS repair. In second stage, 4 weeks after the nerve loop prefabrication, the grafts were exposed surgically and ETS neurorrhaphies were transected. The distal end of the prefabricated loop nerve grafts were transposed and coapted to the distal nerve stump (Fig. 3). In Group 3, staged conventional interpositional nerve graft repair was performed. In first stage, the nerve grafts were sutured ETE to the proximal peroneal nerve stumps. However, the distal end of the nerve grafts and the distal nerve stumps was capped with epineural cuff to prevent unintended communications. The capped nerve stumps were tacked to the surrounding muscles without a nerve repair to prevent retraction. In second stage, 4 weeks after the initial proximal ETE repair, the nerve grafts were exposed surgically and the distal ends of the nerve grafts were sutured to the distal nerve stump (Fig. 4).
Figure 1.

Peroneal nerve segment excised and reversed as a conventional interpositional autologous nerve graft (a), nerve defect repaired with conventional interpositional autologous nerve graft (b), and schematic illustration for the single stage conventional interpositional nerve graft repair (c).
Figure 2.

Epineurotomy with delicate axotomy for end-to-side coaptation.
Figure 3.

First stage of loop peroneal nerve graft prefabrication with end-to-end and end-to-side coaptation (a). Schematic illustration for first stage of loop peroneal nerve graft prefabrication. Blue arrows indicate the end-to-side axonal sprouting to the distal end of the nerve graft. Red arrows represent the end-to-end axonal sprouting to the proximal end of the nerve graft (b). End-to-side coaptation is transected and the distal end of the nerve graft is transposed to the distal nerve stump in second stage (c). Schematic illustration for second stage of the loop nerve graft prefabrication. Red arrow indicates the regular pathway for the axonal regeneration. Blue arrow indicates the axonal sprouting from the end-to-side coaptation site that regenerated and refreshed the distal end of the nerve graft. The reverse axonal sprouting direction observed at the distal end of the nerve graft is consistent with reversing the nerve graft orientation after the harvest (d).
Figure 4.

Staged conventional interpositional nerve graft repair. In first stage, proximal end of the nerve graft is coaptated to the proximal nerve stump. Distal end of the graft and distal nerve stump is not coaptated (a). Schematic illustration of the staged interpositional nerve grafting. The red arrow shows the axonal sprouting direction (b). In second stage, distal end of the nerve graft and distal nerve stump are coaptated (c). The red arrow in the illustration shows the constant regeneration pathway in conventional nerve graft repair (d).
Total follow-up period was 8 weeks; however, the period was split into half for each stage in Groups 2 and 3. Walking track analysis and electrophysiology studies were conducted for functional assessment before euthanasia. Histological assessment was performed for nerve samples.
Walking-track Analysis
At the 8 week, animals were given conditioning trials in an 8.2×42 cm walking-track covered with a paper to capture footprints of hind limbs dipped in ink. The operated right footprints were compared with the non-operated left footprints. Footprints were assessed according to the Bain-Mackinnon-Hunter (BMH) peroneal function index formula (PFI). The PFI is a quantitative functional assessment measure for rat peroneal nerve injury. After the peroneal nerve injury, print length gets shorter compared to control side. According to BMH formula, lower PFI value represents poor functional recovery.
Print length and toe spread were measured for operated experimental and healthy control feet. Experimental toe spread (ETS), normal toe spread (NTS), experimental print length (EPL), and normal print length (NPL) values were used in BMH-PFI formula (BMH-PFI = 174.9 ((EPL-NPL)/NPL) + 80.3 ((ETS-NTS)/NTS).[14]
Electrophysiological Analysis
After the walking-track trial, reconstructed peroneal nerves were surgically exposed and electrophysiological analysis was performed to evaluate the nerve conduction responses. Platinum electrode was placed 5 mm proximal to the proximal coaptation site on the right peroneal nerve. Evoked compound motor response was recorded from anterior tibial muscle with a needle electrode. Peroneal nerve was stimulated with a constant current pulse of 0.1 milliseconds. Recorded latency (velocity) and peak action potential (amplitude) values were compared.
Histomorphometric Assessment
After the euthanasia 0.5 cm long reconstructed peroneal nerve segment, specimens were taken from 1 cm distal to the proximal coaptation line for histomorphometric evaluation. The samples were first fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer, and 1% osmium tetroxide was used for post fixation. The samples were, then, embedded in Epon812 at 60°C. Subsequently, the tissue blocks were cut on a Leica Ultracut-R ultramicrotome. Semi-thin sections (1 μm) from the proximal ends of the biopsy specimens were stained with toluidin blue, examined, and photographed under an Olympus B × 51 light microscope. Total myelinated axons in the entire cross-section of the samples were counted from the micrographs with a magnification of ×100. Electron microscopy samples were prepared from the same specimens.
Statistical Analysis
A computer-based statistical program was used to analyze the results. Descriptive tests, Kruskal–Wallis and Mann–Whitney U tests, were used to assess the walking-track analysis, histomorphometry, and electrophysiology results. P<0.05 was considered as significant.
RESULTS
Animals survived without any sign of infection, foot ulceration or automutilation. Nerve stumps and nerve coaptation sites did not show any unintended communication or neuroma formation. The coaptation sites were free of scar tissue and integrated to each other.
Walking-track Analysis
There was no statistical difference between PFI results of conventional interpositional nerve graft group (Group 1) (−22.75±5.76) and loop nerve graft prefabrication group (Group 2) (−22.08±6) (p>0.05), whereas Group 2 presented a higher PFI value. The poorest mean PFI value was recorded in staged interpositional nerve graft repair group (Group 3) (−33.64±6.4) with a statistically significant difference compared to other groups (p<0.05) (Fig. 5 and Table 1).
Figure 5.
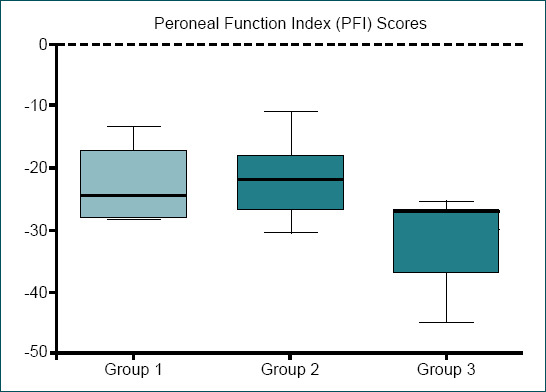
Peroneal function index (PFI) scores.
Table 1.
Peroneal Function Index (PFI) scores
| Group | |||
|---|---|---|---|
|
| |||
| 1 | 2 | 3 | |
| PFI scores - Mean (SD) | -22.75 (5.76) | -22.08 (6) | -33.64 (6.4) |
P>0.05 betwen groups 1 and 2, p<0.05 between groups 1 and 3, p<0.05 between groups 2 and 3. Kruskal-Wallis test and Mann-Whitney U test. SD: Standard deviation.
Electrophysiological Analysis
Comparison of nerve conduction study results between conventional interpositional nerve graft group (Group 1) and loop nerve graft prefabrication group (Group 2) did not present any statistical difference (p>0.05), whereas both groups had a statistical difference compared to staged interpositional nerve graft repair group (Group 3) (p<0.05). The nerve conduction amplitudes for Groups 1, 2, and 3 were 16.19±2.15 mV, 15.95±2.82 mV, and 10.44±1.96 mV, whereas the velocity values were 1.16±0.21 ms, 1.17±0.16 ms, and 1.51±0.15 ms, respectively (Figs. 6 and 7, Table 2).
Figure 6.

Nerve conduction study amplitude results.
Figure 7.

Nerve conduction study velocity results.
Table 2.
Nerve conduction study results
| Group | |||
|---|---|---|---|
|
| |||
| 1 | 2 | 3 | |
| Amplitude (mV) - Mean (SD) | 16.19 (2.15) | 15.95 (2.82) | 10.44 (1.96) |
| Velocity (ms) - Mean (SD) | 1.16 (0.21) | 1.17 (0.16) | 1.51 (0.15) |
P>0.05 betwen groups 1 and 2, p<0.05 between groups 1 and 3, p<0.05 betwen groups 2 and 3. Kruskal-Wallis test and Mann-Whitney U test. SD: Standard deviation.
Histomorphometric Assessment
Myelinated axons, Schwann cells, and fibroblasts in connective tissue were assessed. The highest axon count was determined in loop nerve graft prefabrication group (Group 2) (2531±91.18), and morphologically myelinated fibers were well organized with large diameters in Group 2. The lowest axon count was seen in staged interpositional nerve graft repair group (Group 3) (2194±201.1). There was no statistical difference between conventional interpositional nerve graft group (Group 1) (2227±260.4) and Group 3 (p>0.05), whereas statistically both groups had significantly poor axon counts compared to loop nerve graft prefabrication group (Group 2) (p<0.05) (Fig. 8 and 9, Table 3).
Figure 8.

Light (a-c) and electron (d-f) micrographs. (a) Conventional single stage interpositional nerve graft repair group. Black arrow: Myelinated nerve fiber, arrowhead: Schwann cell, asterisk: connective tissue, white arrow: Fibroblast. (b) Loop peroneal nerve graft prefabrication group. Black arrow: Myelinated nerve fiber, arrowhead: Schwann cell, asterisk: connective tissue. (c) Staged interpositional nerve graft repair group. Black arrow: Myelinated nerve fiber, arrowhead: Schwann cell, asterisk: connective tissue, white arrow: fibroblast. (d) Conventional single stage interpositional nerve graft repair group; a: axoplasm, m: myelin sheath, n: nucleus of Schwann cell, Sc: cytoplasm of Schwann cell. (e) Loop peroneal nerve graft prefabrication group; a: axoplasm, m: myelin sheath, Sc: cytoplasm of Schwann cell, c: collagen fibers, asterisk: vacuole in the axoplasm. (f) Staged interpositional nerve graft repair group; a: axoplasm, m: myelin sheath, Sc: cytoplasm of Schwann cell, n: nucleus of Schwann cell, asterisk: vacuole in the axoplasm.
Figure 9.

Myelinated axon count results.
Table 3.
Number of myelinated axons
| Group | |||
|---|---|---|---|
|
| |||
| 1 | 2 | 3 | |
| Number of axons - Mean (SD) | 2227 (260.4) | 2531 (91.18) | 2194 (201.1) |
P<0.05 betwen groups 1 and 2, p>0.05 between groups 1 and 3, p<0.05 betwen groups 2 and 3. Kruskal-Wallis test and Mann-Whitney U test. SD: Standard deviation.
DISCUSSION
Following a peripheral nerve injury, proximal axons start to elongate after a brief time of dormancy. Single proximal regenerating axon sprouts multiple offspring fibers. Axon fibers degenerate except for the ones establishing a functional synapse. Neural elements of distal nerve segment or graft proceed to Wallerian degeneration after injury. In degeneration process, Schwann cells form the bands of Büngner and guide the regenerating axons along residual endoneural tubes and basement membranes. Axonal regeneration is dependent on an ongoing anterograde and retrograde exchange of growth and neurotrophic factors between the axon and target tissue. After a nerve transection, neurotrophic factors are produced at the site of nerve injury by the Schwann cells, and axonal growth cones sprout distally toward the neurotrophic factor source.[4,7,15]
Regenerating axon sprouts demonstrate both tissue and end organ specificity through neurotropism process.[16] Sprouts of proximal nerve fibers respond to the interference of degenerating myelin and Schwann cell derived neurotrophic factors across a limited gap to grow toward the distal nerve segment.[4,15] To enable the neurotropism and neurotrophic effect of distal nerve segment, nerve injury requires immediate primary repair or interpositional nerve graft reconstruction unless essential to provide a healthy wound bed with sufficient blood supply.[4]
An avulsion, crush, or blast injury can hinder revascularization of a nerve. The nerve damage can also spread proximal and distal to the injury site in time. Thus, in a soft-tissue defect, the primary nerve repair or bridging a nerve gap with an immediate ETE interpositional nerve graft are doomed to fail. For open wounds due to soft-tissue avulsion, infected wounds, crush, or blast injuries, secondary intention or late reconstruction may be inevitable. In such devastating injuries, proximal and distal ends of the nerve are temporarily tacked together without repair. Nerve repair is postponed until providing a well-vascularized recipient bed. In delayed re-exploration, the ultimate extent of nerve injury usually necessitates nerve grafting.[4,5,8] However, overall success in nerve repair deteriorates with an increase in graft length and prolonged regeneration time.[10,17,18] Shorter nerve grafts yield faster and better recovery. Re-innervation specificity decreases in correlation with prolonged recovery time in longer nerve grafts.[18,19] Delay in axonal regeneration or limited intraneural blood supply result in a scarred endoneural tube and a fibrotic distal nerve segment, either in the graft or distal stump of the nerve. Regenerating axonal units cannot penetrate to the fibrotic bands of Büngner. Eventually, the nerve regeneration is arrested.[7–9,20]
Unfavorable functional recovery in longer nerve grafts is also related with axonal fiber misdirection and diminished pruning effectiveness. Disorganized regenerating axonal fibers become aberrant throughout a long nerve graft.[18,19] Nerve reconstruction strategies need to provide guidance cues to improve pruning effectiveness and organization capacity of axonal fiber regeneration.
The outcomes of nerve graft reconstruction are directly associated with the number of axons traversing the graft.[21] Target tissue alterations diminished neurotropism with less neurotrophin production, multiple nerve coaptation sites, vascular impairment, Schwann cell senescence, endoneural collagenization, and scarring prevent guidance properties of nerve grafts. Axonal fiber misdirection rate increases.[11,21,22] Accordingly, the growing proximal axon cone cannot follow the bands of Büngner and match with distal nerve stump.[23–25] Eventually, the axon count repopulating the nerve graft decreases.
Conventional interpositional autologous nerve grafts possess aforementioned technical and physiological restrictions and are compelled to regenerate from only proximal ETE coaptation site. Thus, the distal nerve stump or distal end of the graft faces the endoneual collagenization even before the regenerating axons reach to distal segment particularly in prolonged regeneration time or in delayed nerve repair.
Viterbo popularized ETS neurorrhaphy concept in research.[12,13] Yüksel[26] successfully regenerated a sectioned peripheral nerve with ETS repair through a healthy nerve trunk. Ulkur[27] prefabricated a nerve graft between two healthy nerve trunks using ETS repair technique. However, prefabricating a nerve graft before the onset of an injury is not feasible. Besides transferring the prefabricated nerve graft into the defect site as a free avascular graft may not be a convenient option.
Still favorable outcomes in ETS neurorrhaphy studies encouraged us to adapt its features into loop nerve graft prefabrication. Neurotrophic factors released from a nerve graft can facilitate collateral axonal sprouting from a nerve trunk after an ETS coaptation.[28,29] In nerve loop, both ends of the graft provide chemotactic guidance through neurotropism. The axons sprouting from ETS and ETE coaptation sites regenerate mainly the proximal and in less-rate the distal ends of the loop nerve graft simultaneously.
Revascularization and axonal ingrowth from both ends of the loop graft starts at the initial temporary recipient site without delay and subsequently, if necessary after repairing the soft-tissue defect, prefabricated distal end of the loop graft is transferred to the distal nerve stump in second stage without compromising the established re-vascularization and nerve regeneration at the ETE coaptation site. Exploiting the waiting time of late nerve repair with the nerve loop prefabrication can be beneficial for the injuries with significant soft-tissue loss.
Loop nerve prefabrication in a well-vascularized milieu with ETS repair enables collateral sprouting, supplies trophic factors, prevents Schwann cell senescence and further fibrosis of the endoneural tubes, and eliminates axonal regeneration arrest at the distal end of the graft before the onset of final reconstruction in second stage. In contrast to conventional nerve graft, the nerve loop prefabrication preserves the graft viability, enables organized axonal fiber regeneration, and increases regenerating axon count in a nerve graft without delaying the nerve reconstruction.
Nerve defects are not left unattended provided that an early nerve graft repair is feasible. However, in nerve injuries with soft-tissue loss immediate conventional nerve graft repair may not be possible. Staged nerve graft repair with initial proximal ETE coaptation and delayed distal ETE coaptation may be an alternative for reconstruction. According to electrophysiology, PFI and axon count results of this study, staged repair did not warrant the best healing outcome. Thus, delayed conventional nerve graft repair may be mandatory only after achieving a complete soft-tissue reconstruction. Immediately after the nerve injury, loop nerve graft prefabrication can be performed at a healthy, well-vascularized soft-tissue area to sustain the nerve healing instead of waiting for a delayed conventional nerve graft repair after fulfilling the soft-tissue reconstruction. In loop nerve graft repair increased number of axon fibers penetrating to the graft simultaneously from both proximal and distal ends, concomitantly promote regeneration by exploiting the waiting time until a complete soft-tissue repair is achieved.
In ETS nerve repair, injurious donor coaptation is necessary for improved recipient nerve reinnervation.[30] We performed epineurotomy and perineurotomy with delicate axotomy to promote collateral axonal sprouting into the nerve loop. However, less than 10% of the axons were affected during the ETS repair. Thus, retrograde regenerating axons through the ETS repair were refreshing the distal end of the nerve loop while stealing less than 10% of the anterograde regenerating axons of the loop.
The axon count and functional assessment results of the study demonstrated that the regenerating axon loss after the detachment of the ETS repair in the loop group did not deteriorate the healing compared to other groups. Regeneration capacity of the loop nerve prefabrication far more exceeded the regeneration capacity of the ETE repair alone, through promoting the physiologic environment of the graft.
Single stage repair in conventional autologous nerve graft group and two-stage repair in the loop nerve graft prefabrication group constitutes a bias against the loop group. However, axon count results of the loop nerve prefabrication group were better, although the repair was two-staged. The difference in axon count can be attributed to both the fibrosis of the Büngner bands in conventional nerve graft group, and the improved regeneration capacity in the loop group.
Crush, blast, or avulsion injuries may inevitably necessitate multi-stage procedures for reconstruction; thus, two-stage loop nerve graft prefabrication repair can easily be adapted to multi-stage reconstructions. A given extend of nerve defect in combination with a tissue loss particularly indicating a late nerve reconstruction can benefit from the loop nerve prefabrication. Functional motor nerve recovery of vascularized composite tissue allotransplantation cases, such as forehand and face transplantations, are predominantly retarded due to the delayed nerve healing. Nerve regeneration processes of hand and face allotransplantations may also benefit from loop nerve graft prefabrication method.
The ETS repair is considered to replenish the neurotrophic factors at the distal end of the loop nerve graft and halt the subtle progress of intraneural collagenization and Schwann cell senescence and support axonal regeneration.
Conclusion
Loop nerve graft prefabrication improved axonal regeneration in autogenous nerve grafts through ETS collateral sprouting without delay. Prolonged regeneration time or delayed nerve repair process for the injuries indicating a late nerve reconstruction can benefit from the loop nerve graft prefabrication. Higher axon counts acquired in loop nerve graft without delay may foster its evaluation in immediate long nerve defect reconstructions, particularly for the defects in close proximity to the end organ.
Murine nerve defect is not an optimal translational model. Based on the findings of this study, further experimental studies focusing on more fundamental molecular markers of nerve regeneration can be designed with larger animals.
Acknowledgment
The authors would like to thank Dr. M. Tansel Kendirli for his technical support about Electrophysiological Analysis.
Footnotes
Ethics Committee Approval: This study was approved by the Marmara University Animal Experiments Local Ethics Committee (Date: 15.02.2013, Decision No: 07.2013.mar).
Peer-review: Internally peer-reviewed.
Authorship Contributions: Concept: S.Ö., F.E.; Design: S.Ö., F.E.; Supervision: S.Ö., F.E., C.C.; Resource: S.Ö., F.E.; Materials: S.Ö., C.C., M.A.E.; Data: S.Ö., F.E., M.A.E., S.Ş.; Analysis: .Ö., F.E., M.A.E.; Literature search: S.Ö., C.C.; Writing: S.Ö., F.E., S.Ş.; Critical revision: S.Ö., F.E., S.Ş.
Conflict of Interest: None declared.
Financial Disclosure: This study received Gulhane institutional scientific research project support.
REFERENCES
- 1.Valero-Cabré A, Tsironis K, Skouras E, Navarro X, Neiss WF. Peripheral and spinal motor reorganization after nerve injury and repair. J Neurotrauma. 2004;21:95–108. doi: 10.1089/089771504772695986. [DOI] [PubMed] [Google Scholar]
- 2.Eren F, Öksüz S, Küçükodaci Z, Kendirli MT, Cesur C, Alarçin E, et al. Targeted mesenchymal stem cell and vascular endothelial growth factor strategies for repair of nerve defects with nerve tissue implanted autogenous vein graft conduits. Microsurgery. 2016;36:578–85. doi: 10.1002/micr.22401. [DOI] [PubMed] [Google Scholar]
- 3.Siemionow M, Bozkurt M, Zor F. Regeneration and repair of peripheral nerves with different biomaterials:Review. Microsurgery. 2010;30:574–88. doi: 10.1002/micr.20799. [DOI] [PubMed] [Google Scholar]
- 4.Mackinnon SE, Colbert SH. Principles and techniques of peripheral nerve repair, grafts, and transfers. In: Thorne CH, editor. Grabb and Smith's Plastic Surgery. 7th ed. Philadelphia: Lippincott Williams &Wilkins; 2014. pp. 77–86. [Google Scholar]
- 5.Birch R. Nerve repair. In: Wolfe SW, editor. Green's operative hand surgery. 6th ed. Philadelphia: Churchill Livingstone; 2011. pp. 1035–74. [Google Scholar]
- 6.Mirsky R, Jessen KR, Brennan A, Parkinson D, Dong Z, Meier C, et al. Schwann cells as regulators of nerve development. J Physiol Paris. 2002;96:17–24. doi: 10.1016/s0928-4257(01)00076-6. [DOI] [PubMed] [Google Scholar]
- 7.Sunderland S. Nerves and Nerve Injuries. 2nd ed. Edinburgh: Churchill Livingstone; 1978. pp. 31–80. [Google Scholar]
- 8.Breidenbach WC, Terzis JK. Vascularized nerve grafts:An experimental and clinical review. Ann Plast Surg. 1987;18:137–46. doi: 10.1097/00000637-198702000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Remensnyder J. Physiology of nerve healing and nerve grafts. In: Krizek TJ, editor. Symposium on basic science of plastic surgery. St. Louis: CV Mosby; 1976. pp. 214–41. [Google Scholar]
- 10.Sulaiman OA, Gordon T. Role of chronic Schwann cell denervation in poor functional recovery after nerve injuries and experimental strategies to combat it. Neurosurgery. 2009;65:A105–14. doi: 10.1227/01.NEU.0000358537.30354.63. [DOI] [PubMed] [Google Scholar]
- 11.Saheb-Al-Zamani M, Yan Y, Farber SJ, Hunter DA, Newton P, Wood MD, et al. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp Neurol. 2013;247:165–77. doi: 10.1016/j.expneurol.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viterbo F, Trindade JC, Hoshino K, Mazzoni Neto A. Latero-terminal neurorrhaphy without removal of the epineural sheath. Experimental study in rats. Rev Paul Med. 1992;110:267–75. [PubMed] [Google Scholar]
- 13.Jaberi FM, Abbas BP, Nezhad ST, Tanideh N. End-to-side neurorrhaphy:An experimental study in rabbits. Microsurgery. 2003;23:359–62. doi: 10.1002/micr.10142. [DOI] [PubMed] [Google Scholar]
- 14.Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–38. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Lundborg G, Dahlin L, Danielsen N, Zhao Q. Trophism, tropism, and specificity in nerve regeneration. J Reconstr Microsurg. 1994;10:345–54. doi: 10.1055/s-2007-1006604. [DOI] [PubMed] [Google Scholar]
- 16.Brushart TM, Seiler WA., 4th Selective reinnervation of distal motor stumps by peripheral motor axons. Exp Neurol. 1987;97:289–300. doi: 10.1016/0014-4886(87)90090-2. [DOI] [PubMed] [Google Scholar]
- 17.Weber RV, Mackinnon SE. Bridging the neural gap. Clin Plast Surg. 2005;32:605–16. doi: 10.1016/j.cps.2005.05.003. viii. [DOI] [PubMed] [Google Scholar]
- 18.Bertelli JA, Taleb M, Mira JC, Ghizoni MF. Variation in nerve autograft length increases fibre misdirection and decreases pruning effectiveness:An experimental study in the rat median nerve. Neurol Res. 2005;27:657–65. doi: 10.1179/016164105X18494. [DOI] [PubMed] [Google Scholar]
- 19.Choi D, Raisman G. After facial nerve damage, regenerating axons become aberrant throughout the length of the nerve and not only at the site of the lesion:An experimental study. Br J Neurosurg. 2004;18:45–8. doi: 10.1080/02688690410001660454. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Terenghi G, Hall SM. Effects of delayed re-innervation on the expression of c-erbB receptors by chronically denervated rat Schwann cells in vivo. Glia. 1997;20:333–47. doi: 10.1002/(sici)1098-1136(199708)20:4<333::aid-glia6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Beer GM, Burg D, Zehnder A, Seifert B, Steurer M, Grimaldi H, et al. Functional, electrophysiologic, and morphometric evaluation of nerve regeneration from coaptation on regenerated nerve fibers:Experimental study in rabbits. J Reconstr Microsurg. 2004;20:159–66. doi: 10.1055/s-2004-820773. [DOI] [PubMed] [Google Scholar]
- 22.Maragh H, Meyer BS, Davenport D, Gould JD, Terzis JK. Morphofunctional evaluation of fibrin glue versus microsuture nerve repairs. J Reconstr Microsurg. 1990;6:331–7. doi: 10.1055/s-2007-1006838. [DOI] [PubMed] [Google Scholar]
- 23.Hall S. Nerve repair:A neurobiologist's view. J Hand Surg Br. 2001;26:129–36. doi: 10.1054/jhsb.2000.0497. [DOI] [PubMed] [Google Scholar]
- 24.Sunderland S, Bradley KC. Endoneurial tube shrinkage in the distal segment of a severed nerve. J Comp Neurol. 1950;93:411–20. doi: 10.1002/cne.900930305. [DOI] [PubMed] [Google Scholar]
- 25.Sunderland S, Bradley KC. Denervation atrophy of the distal stump of a severed nerve. J Comp Neurol. 1950;93:401–9. doi: 10.1002/cne.900930304. [DOI] [PubMed] [Google Scholar]
- 26.Yüksel F, Ulkür E, Baloğlu H, Celíköz B. Nerve regeneration through a healthy peripheral nerve trunk as a nerve conduit:A preliminary study of a new concept in peripheral nerve surgery. Microsurgery. 2002;22:138–43. doi: 10.1002/micr.21741. [DOI] [PubMed] [Google Scholar]
- 27.Ulkur E, Karagoz H, Celikoz B, Turan P, Arbak S, Yapar M. Nerve graft prefabrication:Preliminary study. J Reconstr Microsurg. 2008;24:137–45. doi: 10.1055/s-2008-1076087. [DOI] [PubMed] [Google Scholar]
- 28.Okajima S, Terzis JK. Ultrastructure of early axonal regeneration in an end-to-side neurorrhaphy model. J Reconstr Microsurg. 2000;16:313–23. doi: 10.1055/s-2000-7339. [DOI] [PubMed] [Google Scholar]
- 29.Lundborg G, Zhao Q, Kanje M, Danielsen N, Kerns JM. Can sensory and motor collateral sprouting be induced from intact peripheral nerve by end-to-side anastomosis?J Hand Surg Br. 1994;19:277–82. doi: 10.1016/0266-7681(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 30.Brenner MJ, Dvali L, Hunter DA, Myckatyn TM, Mackinnon SE. Motor neuron regeneration through end-to-side repairs is a function of donor nerve axotomy. Plast Reconstr Surg. 2007;120:215–23. doi: 10.1097/01.prs.0000264094.06272.67. [DOI] [PubMed] [Google Scholar]


