Abstract
The ubiquitin E3 ligase substrate adapter cereblon (CRBN) is a target of thalidomide and lenalidomide1, therapeutic agents used in the treatment of haematopoietic malignancies2-4 and as ligands for targeted protein degradation5-7. These agents are proposed to mimic a naturally occurring degron; however, the structural motif recognized by the thalidomide-binding domain of CRBN remains unknown. Here we report that C-terminal cyclic imides, post-translational modifications that arise from intramolecular cyclization of glutamine or asparagine residues, are physiological degrons on substrates for CRBN. Dipeptides bearing the C-terminal cyclic imide degron substitute for thalidomide when embedded within bifunctional chemical degraders. Addition of the degron to the C terminus of proteins induces CRBN-dependent ubiquitination and degradation in vitro and in cells. C-terminal cyclic imides form adventitiously on physiologically relevant timescales throughout the human proteome to afford a degron that is endogenously recognized and removed by CRBN. The discovery of the C-terminal cyclic imide degron defines a regulatory process that may affect the physiological function and therapeutic engagement of CRBN.
Cereblon (CRBN) is a conserved protein that functions as a substrate recognition adapter in the CRL4CRBN E3 ubiquitin ligase complex. The immunomodulatory drugs (IMIDs) thalidomide, lenalidomide and pomalidomide bind CRBN and modulate the selection of protein substrates for ubiquitination and degradation1, which partially underlies the pleiotropic effects of the IMIDs, including their therapeutic efficacy in multiple myeloma2,3 and del(5q) myelodysplastic syndrome4, and their teratogenic effects observed during development8-10. However, the endogenous substrate selection mechanisms of CRBN have remained unknown. The conservation of CRBN across species11 and its association with neurological development12 implies its participation in a vital biological process that may be affected during therapy.
E3 ubiquitin ligase complexes select proteins for degradation through the recognition of degrons, specific amino acid sequences embedded in a substrate that promote ubiquitination and degradation. Short sequences at the N terminus of the substrate were the first degrons to be discovered13, and E3 ligases that recognize C-terminal degrons have been reported recently14. Degrons may also be generated by post-translational modifications (PTMs), such as the recognition of proline oxidation by the E3 ligase VHL15. Additionally, small molecules that chemically induce degrons exist in nature (for example, the plant hormone auxin), which are reminiscent of the activity of the IMIDs16. Thus, IMID-based degraders may chemically mimic the endogenous recognition element of the thalidomide-binding domain of CRBN by one of these mechanisms (Fig. 1a).
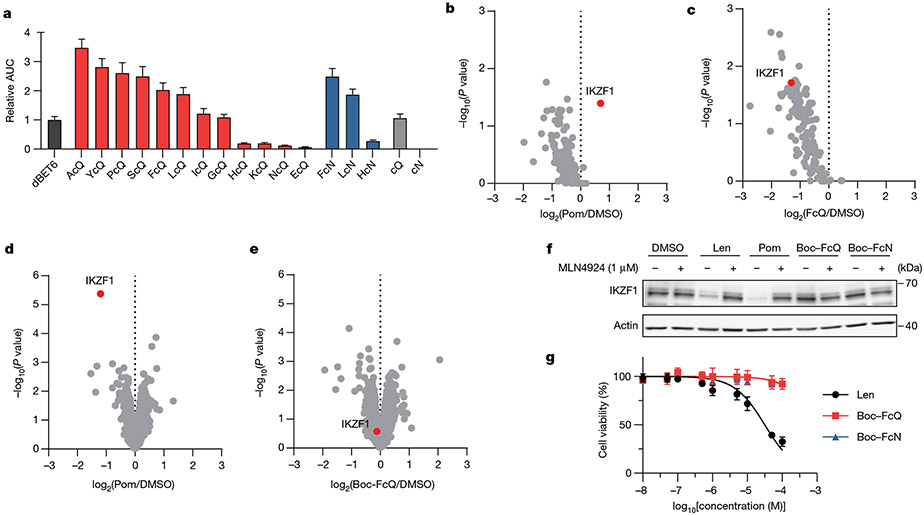
Fig. 1 ∣. Cyclic imide dipeptides functionally engage cereblon in targeted protein degradation.
a, CRBN is engaged by thalidomide and lenalidomide in a manner that may mimic the biological ligand. Models of CRBN engagement by either a small molecule or PTM-based degron. Examples of neosubstrate and known targets used here are shown in parentheses. Ub, ubiquitin. b, The structure of thalidomide and candidate structures for the CRBN degron. c, The structure of dBET6 and candidate dipeptide degraders JQ1–XcN and JQ1–XcQ for functional engagement of CRBN and target protein degradation in cells. d, Western blot analysis of BRD4 after treatment of HEK293T cells with the indicated glutarimide degrader over 1–100 nM. The normalized intensity of the BRD4 band is shown below the blot. e, Western blot of BRD4 after treatment of wild-type (WT) or CRBN-knockdown (CRBN-KD; using short hairpin RNA) HEK293T cells with the indicated degrader. f, Western blot of BRD4 after treatment of HEK293T cells with one of the three epimers of JQ1–FcQ at 100 nM. g, Western blot of BRD4 after co-treatment of HEK293T cells with JQ1–FcQ and lenalidomide or Boc–FcQ. h, Western blot analysis of BRD4 levels after treatment of HEK293T cells with different concentrations of the indicated aspartimide degrader. All degradation assays were performed with 4 h incubation. All western blot data are representative of at least two independent replicates. For uncropped western blot images, see Supplementary Fig. 6.
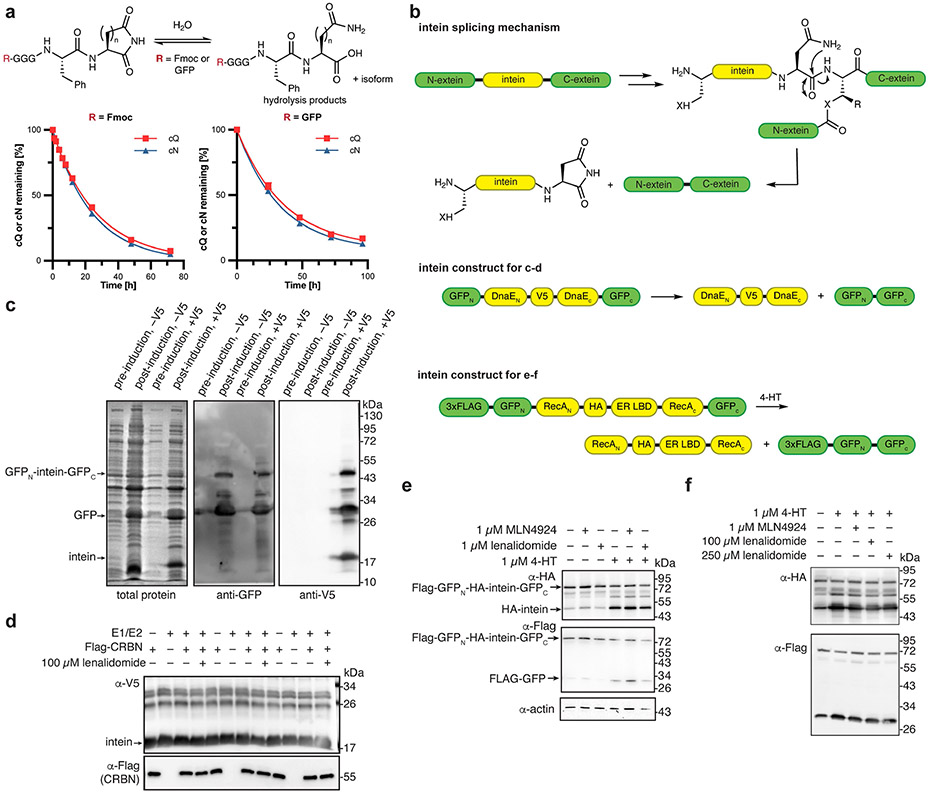
Efforts to identify a degron for the thalidomide-binding domain of CRBN have sought either to discover substrates that compete for IMID binding or to reveal ligands using an in vitro structure-focused approach. Substrates for the thalidomide-binding domain of CRBN that compete for binding with thalidomide include MEIS2 (ref.17) and amyloid precursor protein18, but a degron has not been identified within these substrates. Structure-focused approaches capitalize on the finding that thalidomide binds to CRBN by creating contacts through the glutarimide ring17,19, which suggests that the degron recognized by CRBN could be derived from nucleotide or amino acid sources with the potential for a similar binding pattern (Fig. 1b). Although several uridine derivatives can bind to the thalidomide-binding domain of CRBN in vitro20,21, limited connection of these ligands to cellular activity has been reported. We hypothesized that thalidomide may mimic PTMs such as pyroglutamate or C-terminal cyclic imides that arise from cyclized glutamine (cQ) or cyclized asparagine (cN) (Fig. 1b and Extended Data Fig. 1a). These PTMs are generated by enzymes22, spontaneous cyclization during protein ageing23-26, or during specific protein splicing events27-29 (for example, intein excision). C-terminal cyclic imides may therefore be overlooked PTMs if proteins bearing these modifications are indeed recognized and degraded by CRBN.
Substitution of IMIDs in degraders
A major challenge for the discovery of a physiologically relevant degron for the thalidomide-binding domain of CRBN is the identification of a minimal functional chemical motif from a vast array of potential cellular substrates. To circumvent this challenge, we first set out to identify biomimetic ligands that functionally engage CRBN using a targeted protein degradation strategy30 to report on engagement of CRBN in the CRL4CRBN complex in cells. Inspired by the flexible conversion of the bromodomain BRD4 inhibitor JQ1 to a degrader by functionalization with a thalidomide analogue5-7 (for example, dBET6), we designed analogous degraders that replaced thalidomide with potential biomimetic ligands for CRBN (Fig. 1c). We expected that successful substitution of thalidomide with a biologically relevant structure would promote degradation of BRD4 and thus give a functional readout of CRBN engagement in cells.
Our initial evaluation of uridine-based or pyroglutamate probes revealed no functional engagement of CRBN leading to degradation of BRD4 (Extended Data Fig. 1b-e). Ensuing evaluation of 15 candidates eventually revealed a set of functional degraders consisting of JQ1 linked to a dipeptide with a C-terminal glutarimide (JQ1–XcQ; Fig. 1c). Degradation of BRD4 with JQ1–XcQ minimally requires the dipeptide motif for functional engagement of CRBN (Extended Data Fig. 1d,e). Substitution of the dipeptide degrader at the variable N − 1 position (X) with each of the twenty amino acids showed that all of the dipeptide degraders promoted degradation of BRD4 in a dose-dependent manner (1 nM–10 μM), except for JQ1–DcQ and JQ1–EcQ, and that non-polar and aromatic amino acid side chains promoted the most efficient degradation (Fig. 1d and Extended Data Fig. 2a).
On the basis of these data and the molecular similarity to thalidomide, the dipeptide FcQ was selected for further evaluation. Treatment of HEK293T wild-type or CRBN-knockdown cells31 with dBET6 or JQ1–FcQ showed that degradation of BRD4 was CRBN-dependent (Fig. 1e). Epimerization of JQ1–FcQ at each of the three stereocentres inactivated the degrader, indicating that the natural stereochemistry of the amino acids promotes optimal recognition by CRBN and degradation of BRD4 (Fig. 1f). The addition of lenalidomide or Boc-protected FcQ (Boc–FcQ) competitively inhibited BRD4 degradation by JQ1–FcQ or dBET6 (Fig. 1g and Extended Data Fig. 2b). Both degraders similarly depleted BRD4 within 90 min in a cullin-dependent manner (Extended Data Fig. 2c). Collectively, these data establish glutarimide-based dipeptides are successful substitutes for thalidomide and functionally engage CRBN in cells.
We also evaluated whether the C-terminal aspartimide is a functional substitute of thalidomide, given their chemical similarity. We constructed three aspartimide-based dipeptide degraders for evaluation and observed that degradation of BRD4 in cells was similar to its degradation by glutarimide-based dipeptide degraders (Fig. 1c,h and Extended Data Fig. 2d). Closer investigation of JQ1–FcN showed that degradation of BRD4 was dependent on CRBN, completed within 2 h in HEK293T cells, and competitively inhibited by lenalidomide and the dipeptide Boc–FcN (Fig. 1h and Extended Data Fig. 2e-g). These data indicate that aspartimide-based dipeptides are analogous to glutarimide-based dipeptides in terms of their cellular engagement of CRBN. We thus focused our efforts on further characterization of both five- and six-membered C-terminal cyclic imides as degrons recognized by CRBN.
Dipeptide degraders are ligands for CRBN
We next investigated the propensity of the different degraders to directly engage CRBN and mediate ternary complex formation with BRD4, as cellular degradation efficiency by chemical degraders is a composite of cellular accessibility, ternary complex engagement and orientation of the complex for ubiquitination7,32. We co-immunoprecipitated the target protein BRD4 from a stable HEK293T cell line31 (HEK-CRBN) with Flag–CRBN upon treatment with dipeptide degraders with a range of activities (for example, JQ1–FcQ, JQ1–FcN, JQ1–DcQ and JQ1–EcQ), indicative of successful engagement of CRBN in cells (Extended Data Fig. 3a). These data imply that CRBN has a broad and flexible ligand scope for C-terminal cyclic imide ligands.
To directly evaluate the engagement of CRBN, we used photolenalidomide33 to perform a photo-affinity labelling displacement assay with CRBN–DDB1 (Extended Data Fig. 3b-d). Significant displacement was measured with lenalidomide, the dipeptides (FcQ, FcN and DcQ), and the pentapeptide GGGFcQ, but not with a dipeptide bearing pyroglutamate (FpE). By contrast, displacement by uridine metabolites was not generalizable. Subsequently, we evaluated the ternary complex mediated by the dipeptide degraders with recombinant GST–BRD4 and His6–CRBN–DDB1 and found that all dipeptide degraders induced ternary complex formation (Fig. 2a and Extended Data Fig. 4a,b). Measurement of ternary complex formation in cells using a bioluminescence resonance energy transfer assay (NanoBRET) revealed similar trends (Extended Data Fig. 4d,e). Thus, all of the dipeptide degraders directly engage CRBN in vitro and in cells and the efficiency of ternary complex formation broadly corresponds with the observed cellular activity.
Fig. 2 ∣. Engagement of CRBN and ternary complex formation by dipeptide degraders, but not the dipeptides alone.
a, The relative area under the curve (AUC) from AlphaScreen for ternary complex formation between GST–BRD4(BD2) and His6–CRBN–DDB1 in the presence of the indicated degrader normalized to dBET6. AlphaScreen data are representative of three replicates. Data are mean ± s.e.m. b,c, Quantitative proteomics of co-immunoprecipitated proteins from lysates of HEK-CRBN cells overexpressing IKZF1 after 2 h incubation with 1 μM pomalidomide (Pom) (b) or FcQ (c). Protein concentrations were normalized to the amount of CRBN in each channel. One-way ANOVA with Tukey’s honest significant difference (HSD) post hoc test. d,e, Quantitative proteomics of MM.1S cells after 10 h treatment with 10 μM pomalidomide (d) or Boc–FcQ (e). P-values by t-test (background) method. f, Western blot of IKZF1 after treatment with 10 μM of the indicated compound in MM.1S cells. Western blot data are representative of three independent replicates. Len, lenalidomide. g, Cell viability (MTT) assay of the indicated compounds after treatment of MM.1S cells for five days. Data are mean ± s.d. (n = 4 biologically independent samples). All proteomics experiments were performed in biological triplicates. For uncropped western blot images, see Supplementary Fig. 7.
Substrate recruitment by CRBN ligands
We next investigated whether the cyclic imide-containing dipeptides can chemically induce substrate recruitment for ubiquitination by the CRL4CRBN E3 ligase complex, which would be analogous to the mechanism of thalidomide and its derivatives (Fig. 1a). To predict whether the dipeptides would recruit substrates such as thalidomide34, we performed molecular modelling with the dipeptide degraders in the ternary complex with CRBN and BRD4 (Extended Data Fig. 5a). These models indicated that dipeptides are unlikely to stabilize the same β-hairpin motifs as the IMIDs, but may mediate interactions with distinct substrates. We thus co-immunoprecipitated CRBN from HEK-CRBN cells overexpressing IKZF1 in the presence of pomalidomide, FcQ, FcN or a longer peptide, GGGFcQ, but found that no additional substrates significantly co-immunoprecipitated with any of the evaluated peptide ligands (Fig. 2b,c, Extended Data Fig. 5b-d and Supplementary Table 1).
To evaluate whether the peptides independently promote substrate degradation in cells, we examined the minimal motif necessary for competitive inhibition of BRD4 degradation by JQ1–FcQ in cells and found that the Boc-protected dipeptide (Boc–FcQ) was the most effective competitor (Extended Data Fig. 5e). Although the Boc-protecting group is bulky, we evaluated potential substrates in the multiple myeloma cell line MM.1S by global proteomics. Treatment with pomalidomide showed selective degradation of IKZF1, but no examined substrates consistently showed a change in level upon treatment with either Boc–FcQ or Boc–FcN2,3 (Fig. 2d-f, Extended Data Fig. 5f and Supplementary Table 2). IKZF1 expression levels were similar across treatments (Extended Data Fig. 5g). The differences in substrate degradation extended to differences in anti-proliferative effects, as MM.1S cells were sensitive to lenalidomide treatment (half-maximal inhibitory concentration (IC50) = 31.6 μM), but not to Boc–FcQ or Boc–FcN (IC50 > 100 μM) (Fig. 2g). The absence of independent substrate degradation further translated to high target protein selectivity in depleting BRD2 and BRD4 from the degrader JQ1–FcQ (Extended Data Fig. 5h and Supplementary Table 2). Protein expression levels of BRD2 and BRD4 were similar across treatments (Extended Data Fig. 5i). Together, these data indicate that the peptide ligands examined here do not engage or degrade chemically induced substrates in vitro or in MM.1S or HEK293T cells.
Cyclic imides are substrates for CRBN
To characterize whether the dipeptides FcQ and FcN behave as degrons following incorporation into a protein substrate, we used the sortase system35 to install a C-terminal cyclic imide on GFP–LPETG–His6 and afford semi-synthetic GFP bearing C-terminal cyclic imide motifs (for example, GFP–FcQ or GFP–FcN) (Fig. 3a). We also engineered controls, including GFP–GGG, GFP–Me with an inactive methylated glutarimide, and GFP–FQ and GFP–FN bearing the parent uncyclized amino acid.
Fig. 3 ∣. C-terminal cyclic imides are degrons that promote CRBN-dependent ubiquitination and degradation.
a, The sortase system used to generate degron-tagged GFP from GFP–LPETG–His6. b, In vitro ubiquitination of GFP tagged with C-terminal cyclic imide with K0 ubiquitin. c, Quantification of ubiquitinated protein band intensity in the experiment shown in b across three replicates. d, In vitro ubiquitination of GFP tagged with uncyclized C-terminal glutamine or asparagine with K0 ubiquitin. e, Quantification of ubiquitinated protein band intensity in experiment shown in d across three replicates. f, Flow cytometry analysis of GFP in wild-type or CRBN-knockout (KO) HEK293T cells 6 h after electroporation with GFP tagged with the indicated peptide. g, Flow cytometry analysis of GFP in HEK293T cells 6 h after electroporation with GFP tagged with the indicated peptide. GFP–His6, GFP–His6 (no sortase treatment). h, Flow cytometry analysis of the GFP in HEK293T cells 6 h after electroporation with GFP tagged with the indicated peptide, with or without lenalidomide competition (100 μM). Data are mean ± s.d. One-way ANOVA with Šídák’s multiple comparisons test. P-values are shown. Western blot and flow cytometry data are representative of three independent replicates. For uncropped western blot images, see Supplementary Fig. 7.
The engineered GFPs were evaluated as substrates for the CRL4CRBN complex by examining their susceptibility to the priming step of ubiquitination in vitro. The CRL4CRBN complex isolated from HEK-CRBN cells selectively transferred lysine-free (K0) ubiquitin to GFP–FcQ and GFP–FcN, and was competitively inhibited by co-treatment with lenalidomide (Fig. 3b,c). By contrast, GFP–FQ and GFP–FN were not modified by the CRL4CRBN complex, indicating that the cyclic imide is required (Fig. 3d,e).
We further assessed whether the cyclic imide is a degron for CRBN in cells by introducing the modified GFPs into HEK293T cells by electroporation. Analysis of cellular GFP levels after 6 h revealed significantly lower GFP levels when GFP–FcQ or GFP–FcN was delivered to cells relative to GFP–Me (Fig. 3f). The rapid degradation of GFP–FcQ and GFP–FcN was commuted on genetic knockout of CRBN, co-treatment with lenalidomide, or replacement of cQ or cN with the acyclic Q or N, respectively (Fig. 3f-h). Furthermore, tailoring the C terminus of GFP with PcQ, LcQ or LcN accelerated depletion of GFP relative to GFP–His6 in HEK293T cells and was rescued by lenalidomide competition (Extended Data Fig. 6a). Depletion of GFP bearing a C-terminal cyclic imide was additionally observed across cell lines and species (Extended Data Fig. 6b,c). These data indicate that recognition of the C-terminal cyclic imide degron by CRBN is conserved across cell types and species and that CRBN flexibly accepts various amino acid residues adjacent to the cyclic imide.
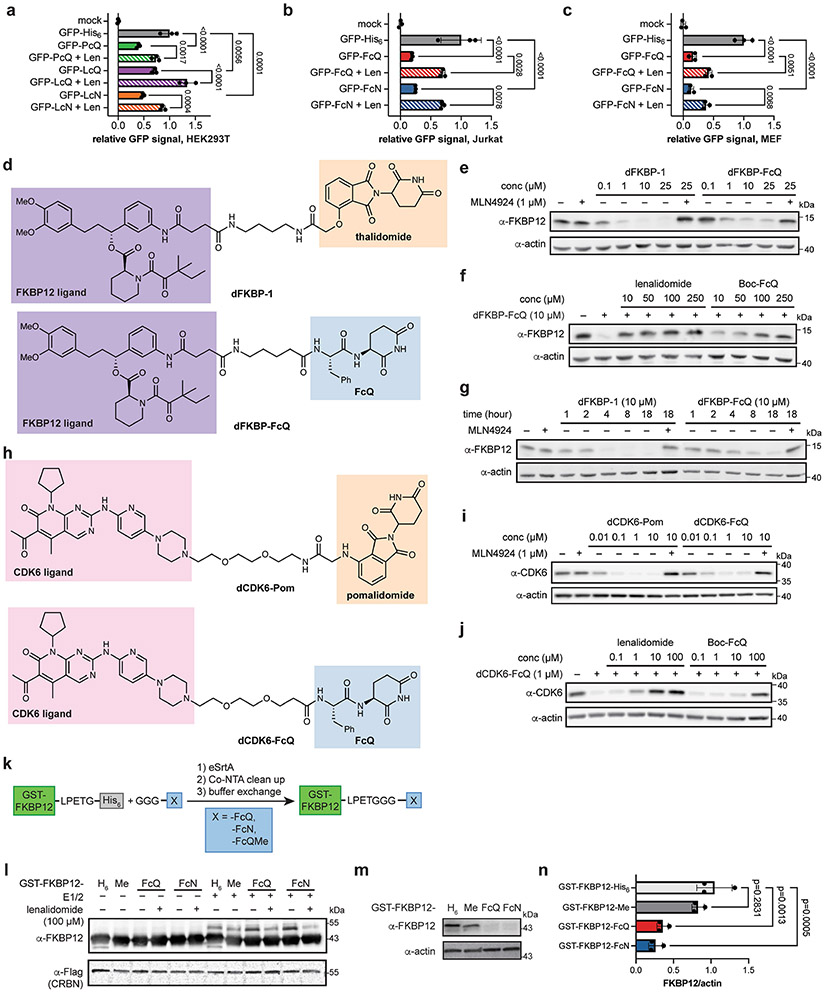
If thalidomide is mimicking the C-terminal cyclic imide degron, we would expect the degron to readily substitute for thalidomide in separate small molecule degraders and proteins. The thalidomide moiety of dFKBP-15 and the pomalidomide moiety of dCDK6–Pom36 were readily substituted with FcQ to afford active CRBN-dependent dipeptide degraders for the respective protein target (Extended Data Fig. 6d-j). The cyclic imides were additionally transferrable to the C terminus of GST–FKBP12 as a separate engineered substrate that promoted CRBN-dependent ubiquitination and degradation in vitro and in cells (Extended Data Fig. 6k-n). These data demonstrate that the C-terminal cyclic imides are transferrable chemical motifs that act as degrons recognized by CRBN to promote degradation of modified proteins in cells.
Prevalence of C-terminal cyclic imides
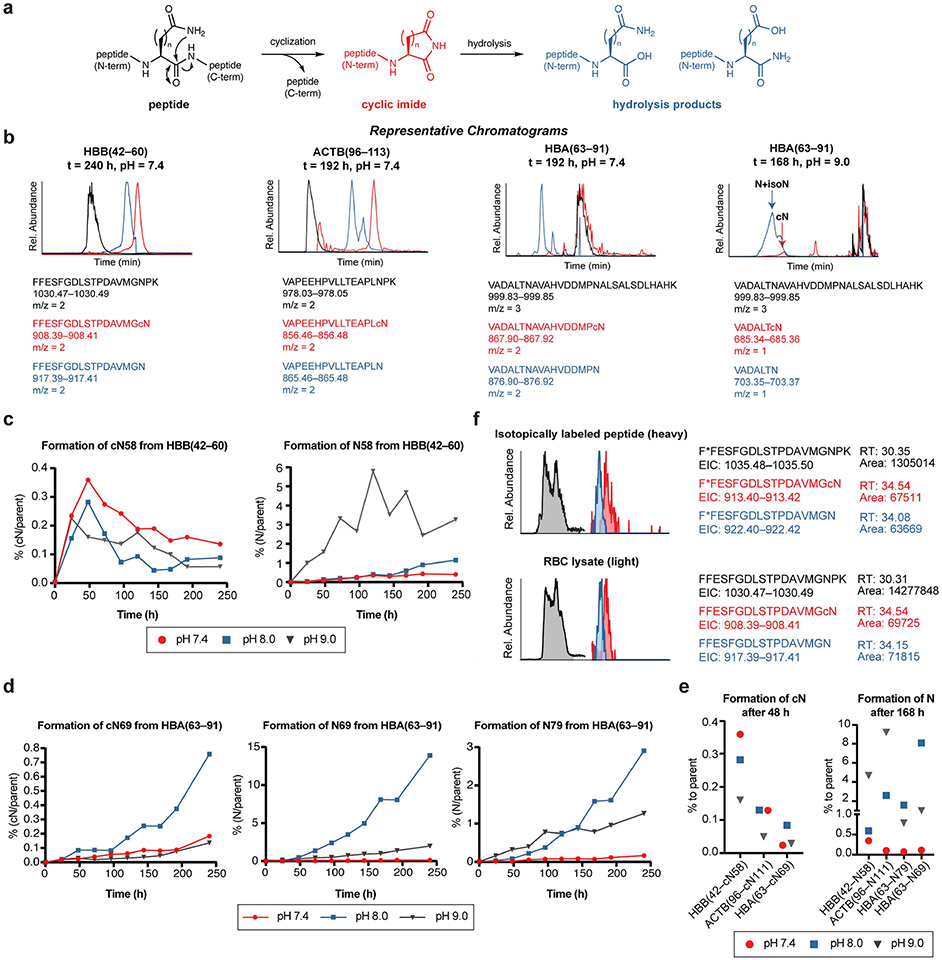
We next sought evidence for the natural occurrence of C-terminal cyclic imide PTMs in the proteome. Cyclic imides are formed spontaneously during asparagine or glutamine deamidation and an analogous process wherein nucleophilic attack from the amide side chain results in protein cleavage and reveals the degron for CRBN (Fig. 4a). Although cyclic imides undergo subsequent hydrolysis, we found that C-terminal cyclic imides have half-lives (t1/2) at 37 °C in PBS as follows: t1/2 for cQ, 18.4 h; t1/2 for cN, 16.7 h; t1/2 for GFP–FcQ, 24.9 h; and t1/2 for GFP–FcN, 23.0 h–well within the degradation timeframe of most known substrates of CRBN induced by the IMIDs (2–8 h in cells) (Extended Data Fig. 7a).
Fig. 4 ∣. C-terminal cyclic imides are PTMs that form readily in vitro.
a, Schematic of cyclic imide formation, which reveals a degron for CRBN, and the subsequent hydrolysis product. b, Unique proteins and peptides with at least one cN or cQ modification in CPTAC global proteomics datasets. c, Frequency chart of the sequence alignment flanking the amino acids in poitions −5 to +5 relative to cN and cQ sites observed in CPTAC datasets. Alignment generated by weblogo.berkeley.edu. d, Schematic of HBA and HBB sequences. Peptides with cQ or cN modifications observed with trypsin or chymotrypsin digestion and mass spectrometry analysis are underlined: global datasets, blue; RBC tryptic peptides, pink; RBC chymotryptic peptides, orange; recombinant HBB tryptic peptides, brown. Modification sites are shown in red. e, In vitro time course of the formation of ACTB(96–cN111) and ACTB(96–N111) from the synthetic peptide representing ACTB(96–113). f, In vitro time course of the formation of HBB(42–cN58) and HBB(42–N58) from full-length recombinant HBB protein. Quantification was performed by selected ion monitoring. Data are mean ± s.d. (n = 3 biologically independent samples). Unpaired two-tailed t-test; P-values are shown. g, Quantification of HBB(42–cN58) and HBB(42–N58) from HBB(42–60) peptide and recombinant HBB protein after 144 h of incubation. h, Quantification of HBB(42–cN58) and HBB(42–N58) in two RBC donors by selected ion monitoring.
The relatively long half-lives of C-terminal cyclic imide PTMs indicate their availability for recognition by CRBN and that the actual prevalence of these modifications may be higher than previously recognized. Spontaneous formation of C-terminal cyclic imides has been most prevalently observed with inteins, which promote excision through a specific protein fold27. However, examination of two inteins in vitro and in cells indicated that these proteins are not generally a source of CRBN substrates, which may be owing to the hindered C terminus resulting from intein excision (Extended Data Fig. 7b-f).
To detect where these modifications may be occurring in human proteomes, we analysed global proteomics datasets from the NCI7 cell line panel and six primary human tissues collected by the Clinical Proteomic Tumor Analysis Consortium (CPTAC) for C-terminal cyclic imides37-43. We identified several hundred proteins with more than 1,0 unique cN or cQ modification sites across these datasets (Fig. 4b and Supplementary Table 3). These modification sites occurred at a rate of 0.8% relative to fully tryptic peptides by spectral counting in the NCI7 dataset. Sequence alignment of these modification sites revealed an apparent consensus sequence (Fig. 4c). We additionally searched for semi-tryptic peptides with C-terminal N or Q that may represent hydrolysed cyclic imide sites and found more than 20,000 putative cleavage sites derived from 6,800 proteins (Extended Data Fig. 8a and Supplementary Table 3). Mapping the most frequently observed C-terminal cyclic imide modification sites to protein structure indicates that these sites are largely solvent-exposed.
C-terminal cyclic imides derived from haemoglobin subunits α (HBA) and β (HBB) were two of the most frequently observed proteins in these analyses. Haemoglobin is expressed globally across tissue types, but is particularly abundant in red blood cells (RBCs), which have a lifespan of approximately 120 days, but do not express CRBN44. Therefore, cyclic imides formed on haemoglobin may accumulate and not be degraded in vivo. Indeed, CRBN was not observed in RBCs (Extended Data Fig. 8b). To confirm the existence of cN and cQ modifications, we digested recombinant HBB with trypsin at 47 °C for 1 h and mapped 6 cN or cQ sites across the protein (Fig. 4d). A similar evaluation of RBC lysates from two healthy donors revealed three unique peptides bearing a C-terminal cN modification that appeared at retention times independent of the parent tryptic peptide and were fully responsive to base treatment performed prior to mass spectrometry analysis, which induces hydrolysis of the C-terminal cyclic imide (Fig. 4d, Extended Data Fig. 8c-e and Supplementary Table 4). Digestion of RBC lysates with the orthogonal protease chymotrypsin revealed two modification sites on HBA that were also observed in the CPTAC global proteomics datasets (Fig. 4d, Extended Data Fig. 8d). These data indicate that C-terminal cyclic imides are present in authentic human samples and were not formed during sample preparation or analysis.
To further extend the observation of C-terminal cyclic imides in aged proteins, we used bovine eye lenses, as crystallins undergo deamidation to afford cyclic imide modifications during ageing45,46. We mapped 22 modification sites that were responsive to base treatment and differentiable from the corresponding tryptic peptides by their retention times (Extended Data Fig. 8h,i and Supplementary Table 4). The identification of more than 1,000 cN and cQ modification sites across proteins in human tissues, RBCs and bovine eye lenses substantially expands the map of these modifications in the human proteome and implies that they are more prevalent than previously understood.
Formation of the cyclic imide degron
To verify the ready formation of C-terminal cyclic imide modifications, we evaluated three synthetic peptides from proteins bearing four of the most frequently observed modification sites: HBB(42–60), HBA(63–91), and ACTB(96–113) (highlighted in red and blue, Fig. 4d). These peptides were monitored for internal cleavage and formation of the C-terminal cyclic imide, followed by downstream hydrolysis at 37 °C, over a pH range of 7.4–9.0 (Extended Data Fig. 9a). The fragmentation spectra from the synthetic peptides and their cleavage products were identical to those assigned by global proteomics. Furthermore, the extracted ion chromatograms for the parent peptide, the cyclic imide-bearing fragment, and its hydrolysed products all showed distinct retention times, apart from the cyclic imide fragment at HBA cN79, where the hydrolysis product HBA N79 was readily measured (Extended Data Fig. 9b). The spontaneous formation of the cyclic imide fragment was observed from each peptide after 24 h and largely plateaued after 48 h. By contrast, the abundance of the hydrolysed forms of the cyclic imide fragment for all four sites increased continuously over ten days in a pH-dependent manner (Fig. 4e, Extended Data Fig. 9c-e and Supplementary Table 5).
We next investigated the rate of formation of HBB cN58 on recombinant HBB using selected ion monitoring (SIM). To generate the internal standard, the synthetic heavy HBB(42*–60) peptide (* indicates residue with heavy isotope) was incubated at 37 °C for 48 h to form HBB(42*–cN58) and HBB(42*–N58). We found that recombinant HBB possessed 0.10% of HBB cN58 and 0.15% of HBB N58, which increased on incubation to amounts analogous to those observed with the HBB(42–60) peptide (Fig. 4f,g). The levels of the modification observed in vitro are approximately tenfold greater than that observed from RBC digests, where the full haemoglobin complex is presumably intact (Fig. 4h and Extended Data Figs. 8g and 9f). Collectively, these data indicate that whereas the C-terminal cyclic imide concentration may plateau at a low level for any individual peptide or protein sequence, the corresponding hydrolysed fragments accumulate over time if there is no mechanism for removal of protein fragments bearing the C-terminal cyclic imide.
Regulation of cyclic imides by CRBN
To demonstrate that a protein bearing an adventitiously formed cN or cQ modification affords a degron recognized by the thalidomide-binding domain of CRBN, we examined frequently measured modification sites for compatibility with expressed protein ligation and selected recombinant HBB(1–cQ132) for closer analysis. The degron-modified HBB(1–cQ132) and the methylated control HBB(1–Me132) were prepared via a ligation and deselenization sequence (Fig. 5a and Extended Data Fig. 10a). In vitro ubiquitination of HBB(1–cQ132) with lysine-free (K0) ubiquitin in the presence of the CRL4CRBN complex showed enhancement of di- and tri- mono-ubiquitination events in a manner that is inhibited by lenalidomide or methylation of the degron (Fig. 5b and Extended Data Fig. 10b). These ubiquitination events mapped to three K-ε-GG peptides by ubiquitin site analysis using mass spectrometry, and a significantly higher level of K(66, 67)-ε-GG peptide (modified at both sites) was detected in HBB(1–cQ132) without lenalidomide treatment (Extended Data Fig. 10c and Supplementary Table 6). These data demonstrate that when the C-terminal cyclic imide modification is formed adventitiously on a protein such as HBB(1–cQ132), the modification promotes CRBN-dependent ubiquitination of the protein.
Fig. 5 ∣. CRBN regulates recombinant and endogenous substrates bearing C-terminal cyclic imides.
a, Schematic of HBB(1–cQ132) (HBB native sequence with C-terminal glutarimide at residue 132 instead ofglutamine) and its methylated derivative HBB(1–Me132). b, Quantification of mono-, di- and tri-mono-ubiquitinated HBB band intensity from in vitro ubiquitination of HBB(1–cQ132) or HBB(1–Me132) with K0 ubiquitin. Ordinary one-way ANOVA with Šídák’s multiple comparisons test. Data are mean ± s.d. (n = 3 biologically independent samples). c, Volcano plot of peptide groups bearing C-terminal cyclic imides in HEK293T cells treated without or with 200 μM lenalidomide over 48 h. Upregulated proteins at 1% FDR (red) or 5% FDR (pink); ACTB(96–cN111) peptide (blue). One-way ANOVA with Tukey’s HSD post hoc test. d, Volcano plot of peptide groups bearing C-terminal cyclic imides in MM.1S cells treated with DMSO or 200 μM lenalidomide over 48 h. Upregulated proteins at 1% FDR (red) or 5% FDR (pink); ACTB(96–cN111) peptide (blue). One-way ANOVA with Tukey’s HSD post hoc test. e,f, Quantification of ACTB(96–cN111) and ACTB(96–N111) in HEK293T (e) or MM.1S (f) cells treated with DMSO or 200 μM lenalidomide over 48 h, using SIM. Data are mean ± s.d. (n = 4 biologically independent samples). Unpaired two-tailed t-test; P-values are shown.
To investigate whether the level of C-terminal cyclic imides across proteins is dependent on the availability of the thalidomide-binding domain of CRBN in cells, we performed a quantitative proteomics experiment to compare the global proteome obtained from HEK293T cells before and after CRBN knockout or treatment with lenalidomide using exogenous GFP as an internal control. We found 39 unique peptides bearing C-terminal cyclic imides and observed most of these modified peptides increase when CRBN is knocked out or the thalidomide-binding domain of CRBN is inhibited by lenalidomide (Fig. 5c, Extended Data Fig. 10d and Supplementary Table 7). A similar increase in 34 out of 36 peptides bearing a C-terminal cyclic imide was identified in MM.1S cells after treatment with lenalidomide (Fig. 5d and Supplementary Table 7). Additionally, peptides bearing C-terminal asparagine and glutamine increase in both cell lines upon CRBN knockout or inhibition (Extended Data Fig. 10e,f and Supplementary Table 7).
We selected ACTB cN111 for closer evaluation to more sensitively detect and quantify these changes (Fig. 4c, highlighted in blue). SIM quantification of ACTB cN111 and N111 from HEK293T or MM.1S cell lysates spiked with heavy ACTB peptides (ACTB(96*–113), ACTB (96*–cN111) and ACTB(96*–N111)) as an internal standard showed a 25–36% and 76–77% increase in the modifications relative to the parent peptide after 48 h treatment with lenalidomide (Fig. 5e,f and Extended Data Fig. 10g). Thus, these data align with a model where the thalidomide-binding domain of CRBN promotes the ubiquitination and eventual degradation of endogenous substrates bearing the C-terminal cyclic imide degron to prevent the unwanted accumulation of these fragments and their hydrolysis products over time.
Discussion
We used a targeted protein degradation strategy to discover that previously underappreciated C-terminal cyclic imide modifications possess properties that are prototypical for a degron and are physiologically recognized on endogenous substrates by the thalidomide-binding domain of CRBN. Observation of the C-terminal cyclic imide modification on thousands of sites across the proteome indicates that these modifications may represent an overlooked form of protein damage that is generated adventitiously at susceptible asparagine and glutamine residues. A model for the C-terminal cyclic imide modification as a form of protein damage is reminiscent of another form of spontaneous protein damage, the isoaspartate PTM, which arises from spontaneous deamidation at asparagine, and conservation of the cellular machinery (for example, PIMT) to protect organisms from this form of protein damage is particularly important in the brain47. Similarly, loss of the thalidomide-binding domain of CRBN is associated with intellectual disability12. The deleterious effects of this form of protein damage may therefore be most pronounced in vivo, where C-terminal cyclic imide-bearing proteins, and particularly their downstream hydrolysed products, may accumulate to a greater degree. Whether the hydrolysed products are removed separately if they are not intercepted by CRBN is an area for future investigation. Although CRBN probably has additional endogenous substrates and functions, these observations are congruent with a model in which CRBN is conserved to regulate the removal of the C-terminal cyclic imide, thus preventing the deleterious accumulation of protein fragments.
Future studies on the genesis of C-terminal cyclic imides and comparison to the IMIDs will enable a more robust definition of the physiological function of CRBN, and may lead to the identification of biomarkers, rationalization of clinical effects or the discovery of induced substrates that are affected by ligand engagement of CRBN. For example, the contribution of the epimers of the C-terminal cyclic imide to the biological function of the degron and the physiological role of CRBN, in comparison to the separate enantiomers of the IMIDs, is an area of future evaluation. In addition to a spontaneous internal cyclization mechanism, C-terminal cyclic imide modifications may form by other means, as several reported substrates of CRBN have protein termini that end in N or Q residues and our analysis of large proteomics datasets shows many instances of C-terminal cyclic imides found at the naturally occurring C termini of proteins. Evaluation of the role of C-terminal cyclic imides will be accelerated by the development of specific methods to visualize and map these PTMs. As C-terminal cyclic imide sites and substrates become more clearly defined, further studies will elucidate the implications of these PTMs and their roles in protein regulation and cellular signalling with respect to mechanisms regulated by CRBN.
Methods
Cell culture
Human-derived cell lines were cultured in DMEM or RPMl 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1× penicillin-streptomycin. Mouse embryonic fibroblast (MEF) cells were cultured in DMEM supplemented with 15% heat-inactivated FBS. Cells were grown at 37 °C in a humidified atmosphere with 5% CO2. For the collection of cell pellets, cells were dissociated and collected by two PBS washes and centrifugation at 500g, 24 °C, 3 min, followed by a final PBS wash of the pellets. The pellets were flash frozen with liquid nitrogen and stored at −80 °C until use.
Generation of CRBN-knockout HEK293T cells by CRISPR–Cas9
CRBN CRISPR–Cas9 knockout plasmids mix, consisting of three CRBN-specific guide RNAs (gRNAs) and HDR plasmids, were purchased from Santa-Cruz Biotechnology. The HDR plasmid contains puromycin resistance and RFP genes to be inserted into the DSB site. Transfection was performed using similar procedure as described by the manufacturer. Wild-type HEK293T cells were seeded in 6-well plates with DMEM + 10% FBS without antibiotics to reach 90% confluency at the time of transfection. For each well, 0.5 μg CRBN CRISPRαCas9 knockout plasmid and 0.5 μg HDR plasmid were diluted in 150 μl Opti-MEM I and 4 μl Lipofectamine 3000 was then added. This mix was added into another 150 μl Opti-MEM I containing 4 μl Lipofectamine 3000 and incubated for 20 min. The mixture was added dropwise into each well and incubated for 48 h. Selection was performed with 4 μg ml−1 puromycin for 7 days. The top 1% RFP-expressing population was the selected by sorting and the sorted cells were expanded and validated for CRBN knockout by western blotting.
In vivo degradation assay of substrates
HEK293T or Jurkat cells (1.5 × 106) were seeded in DMEM or RPMI supplemented with 10% FBS and 1× penicillin-streptomycin and incubated at 37 °C, 5% CO2 for 30 min, then treated with compounds of interest and incubated at 37 °C, 5% CO2 for the indicated time prior to collection and lysis. If noted, cells were treated with 1 μM MLN4924 for 1 h before treatment with compounds following the initial 30 min incubation. All compounds were dissolved in DMSO, and the final DMSO concentration after addition of the compound to the cells did not exceed 0.2% (v/v).
Immunoprecipitation with bivalent compounds
For in vivo Flag-tag co-immunoprecipitation, 5.0 × 106 HEK-CRBN cells were seeded and incubated at 37 °C, 5% CO2 for 30 min, and then were treated with 1 μM MLN4924 for 1 h. Compounds of interest were added to a final concentration of 25 μM and the cells were incubated at 37 °C, 5% CO2 for 2 h. The cells were collected and lysed in 1× protease/phosphatase inhibitor/1× non-denaturing lysis buffer (500 μl) and clarified by centrifugation (21,000 g, 4 °C, 10 min). The soluble portion of the lysate was collected and 200 μl of lysate was incubated with of protein G magnetic beads for 20 min (20 μl) to minimize the non-specific binding. The solution was collected and was then incubated with anti-Flag M2 magnetic beads (40 μl) on a tube rotator at 4 °C for 1.5 h. The magnetic beads were washed with 1μ non-denaturing lysis buffer (5× 500 μl). Then, the enriched proteins were eluted by addition of 40 μl of 2× SDS–PAGE loading buffer and heated at 95 °C for 5 min prior to western blot analysis.
For in vitro Flag-tag co-immunoprecipitation, 5.0 × 106 HEK-CRBN cells were collected by centrifugation, lysed in the same lysis buffer (400 μl), and cleared by centrifugation. The soluble portion of the lysate (200 μl) was incubated with MLN4294 and compound of interest (final concentration: 1 μM) at 4 °C for 2 h. Then, the mixture was added to pre-washed anti-Flag M2 magnetic beads (40 μl) and incubated on a tube rotator at 4 °C for 1.5 h. The wash and elution were performed similarly to in vivo Flag-tag co-immunoprecipitation.
Photo-affinity labelling displacement assay
Reactions were prepared by combining 19.5 μl of CRBN-DDB1 (Creative Biolabs) in 0.1% Triton-X 100/PBS and 0.5 μl of DMSO, photo-lenalidomide, or the pre-mixed solution of a competitor with photo-lenalidomide (final concentrations: 1.41 μM CRBN–DDB1, 1 μM photo-lenalidomide, and 100 μM competitor). Reactions were incubated in dark for 30 min at 24 °C, followed by photoirradiation for 30 s at 4 °C via Dymax. The eluents were then tagged with azide-fluor 488 via the CuAAC reaction by adding a pre-mixed cocktail (final concentrations: 25 μM azide-fluor 488, 100 μM THPTA, 1 mM freshly prepared CuSO4, 2 mM freshly prepared sodium ascorbate), followed by incubation in dark for 1 h at 24 °C. The reactions were quenched by the addition of pre-cooled acetone (100 μl) and incubated for 2 h at −80 °C. The protein precipitates were isolated by centrifugation (21,000 g, 4 °C, 15 min) and the supernatant was discarded. Each protein pellet was air-dried for 10 min at 24 °C with the lid open, and then resuspended in 2% SDS in 1× PBS (20 μl). 5× SDS–PAGE loading buffer (5 μl) was added, and the samples heated at 95 °C for 5 min prior to western blot analysis and in-gel fluorescence imaging.
AlphaScreen
AlphaScreen buffer (3× stock solution: 150 mM HEPES pH 7.4, 600 mM NaCl, 0.3% w/v BSA, 3 mM TCEP) was prepared fresh. A 3× stock solution of each compound (60 μM, 3% DMSO final/1× AlphaScreen buffer) and a series of twofold serial dilutions, in 3% DMSO/1× AlphaScreen buffer, were prepared fresh for each experiment. A solution of 750 nM His6–CRBN–DDB1, 375 nM GST–BRD4(BD2)/1×AlphaScreen buffer (5 –l) was added to each well of a 384-well Optiplate. Then, compound (5 –l) was added, with each concentration assayed in triplicate. As a positive control, 10 μl 100 nM GST-His6 was added to three wells. The plate was sealed with TopSeal-A PLUS, centrifuged (200g, 25 °C, 1 min), and incubated at 25 °C for 1 h. Under low light, the seal was removed and 5 μl of 60 –g ml−1 AlphaScreen Glutathione Donor beads, 60 –g ml−1 AlphaLISA Nickel Chelate Donor beads/1× AlphaScreen buffer was added to each well. The plate was sealed with TopSeal-A PLUS, centrifuged (200g, 25 °C, 1 min), and incubated at 25 °C for 1 h. The seal was removed and the plate was analysed. Prior to analysis, the plate reader was calibrated with a plate containing 15 μl of 20 μg ml−1 Omnibeads/1× AlphaScreen buffer in the corner wells. Analysis was performed in Graphpad Prism, using the vehicle-treated wells as the baseline, fitting the signal from each compound to a Gaussian curve, and calculating the AUC. AUC results from each plate were normalized to dBET6.
NanoBRET assay
Four million HEK293T cells were seeded in a 100 mm TC-treated dish in 10 ml DMEM supplemented with 10% FBS. Cells were incubated 18–24 h (37 °C, 5% CO2). The transfection mix, containing 12 μg HaloTag-CRBN fusion vector, 0.12 μg NanoLuc-BRD4 FL fusion vector, 1.2 ml Opti-MEM I reduced serum media without phenol red, and 12 μl TransIT-Pro, was incubated at 25 °C for 15 min prior to dropwise addition to the culture. Cells were incubated 20 h (37 °C, 5% CO2). Cells were trypsinized and washed, then resuspended at 2× 105 cells ml−1 in Opti-MEM I reduced serum media without phenol red. For no-ligand samples, 5.5 μl DMSO was added per 5.5 ml cell suspension. For +ligand samples, 5.5 μl of 0.1 mM HaloTag NanoBRET 618 Ligand was added per 5.5 ml cell suspension. In an opaque white TC-treated 96-well plate, cells were reseeded, with 100 μl cell suspension per well. Cells were incubated 18–24 h (37 °C, 5% CO2). From a 50 mM stock in DMSO, MG132 was diluted to 50 μM in Opti-MEM I reduced serum media without phenol red and 25 μl was added to each well. Cells were incubated 30 min (37 °C, 5% CO2). From 1 mM stocks in DMSO, compounds being analysed were diluted to 6 μM in Opti-MEM I reduced serum media without phenol red and 25 μl was added to each well, with each compound dosed in triplicate in cells with or without ligand. Cells were incubated 2 h (37 °C, 5% CO2). Nanoglo substrate was diluted to 4×, from a 500× stock, in Opti-MEM I reduced serum media without phenol red, and 50 μ! was added to each well. Within 10 min, luminescence at 450 nm and 618 nm (15 nm bandpass filters, 1 s integration time) was read on an SpectraMax i3x plate reader. BRET ratios were calculated as the luminescence at 618 nm divided by the luminescence at 450 nm, and corrected BRET ratios were calculated by subtracting the BRET ratio −ligand from the BRET ratio +ligand for each compound.
Computational modelling
Computational models were generated in MOE version 2020.09. The IMID ligand from the indicated crystal structure was adapted to the indicated dipeptide and the adapted complex was subjected to energy minimization with Amber10:EHT force field followed by preparation with Protonate 3D.
Immunoprecipitation with monovalent compounds
Samples were prepared in technical triplicate for each condition. HEK-CRBN cells (80% confluence, 10 cm dishes) were transiently transfected with pcDNA3.1-IKZF1-EPEA. The transfection mix, containing 10 μg plasmid, 1 ml Opti-MEM I reduced serum media, and 10 μl TransIT-Pro, was incubated at 25 °C for 15 min prior to dropwise addition to the culture. Cells were harvested 48 h after transfection and lysed in 1× protease/phosphatase inhibitor/1× non-denaturing lysis buffer (500 μl) and clarified by centrifugation (21,000 g, 4 °C, 10 min). Clarified lysate (250 μl) was incubated with 100× compound stock (2.5 μl, final concentration: 1 μM) or DMSO on a tube rotator at 4 °C for 2 h. The solution was collected and then incubated with anti-Flag M2 magnetic beads on a tube rotator at 4 °C for 1 h. The magnetic beads were washed with 1% Triton-100 in TBS (6× 1 ml). The enriched proteins were eluted by the addition of 125 μl 5% SDS/50 mM triethylammonium bicarbonate (TEAB) and heated at 95 °C for 10 min, and the supernatant was kept for proteomics sample preparation.
Preparation of immunoprecipitated samples for quantitative proteomics
Immunoprecipitated samples were reduced by addition of dithiothreitol (20 mM) at 24 °C for 30 min then alkylated by addition of iodoacetamide (40 mM) and incubation in the dark at 24 °C for 30 min. The samples were desalted and digested using a S-Trap micro48,49. Samples were acidified by the addition of phosphoric acid to a final concentration of 1.2%. S-Trap buffer (90% methanol, 0.1 M TEAB, pH 7.1, 165 μl) was then added. Each sample was transferred to a S-Trap micro column. Using a vacuum manifold, the columns were washed with S-Trap buffer (3 × 150 μl). To digest the S-trap-bound proteins, 1 μg of trypsin resuspended in 25 μl 50 mM TEAB, pH 8.0 was added to each column and incubated at 47 °C for 2 h without rotation. The digested peptides were eluted by sequential addition of 50 mM TEAB, pH 8.0 (40 μl), 0.2% formic acid (40 μl), and 0.2% formic acid, 50% acetonitrile/water (40 μl), with each elution collected by centrifugation (4,000 g, 24 °C, 1 min) in a clean Eppendorf tube. The eluted samples were concentrated to dryness in a vacufuge and resuspended in 25 μl ddH2O. For each resuspended sample, 10 μl was taken for labelling with TMTpro 16-plex reagent (10 μl, 3 replicates × 5 conditions = 15 channels used) at 24 °C for 1 h. Hydroxylamine (5%, 5 μl) was added to each sample to quench the TMT reagent, and the samples were incubated at 24 °C for 15 min. The TMT-labelled samples were combined and dried in a vacufuge. The dried sample was resuspended in 300 μl 0.1% trifluoroacetic acid (TFA) and fractionated to 5 fractions using a Pierce high pH reversed-phase peptide fractionation kit. The peptides were eluted sequentially by 5% acetonitrile/0.1% triethylamine (TEA), followed by 10%, 20%, 35% and 50% acetonitrile/0.1% TEA. The first fraction (5% acetonitrile/0.1% TEA) was excluded from LC–MS/MS analysis. The other 4 fractions were concentrated to dryness and each sample was resuspended in 20 μl of 0.1% formic acid prior to LC–MS/MS analysis.
Global quantitative proteomics sample preparation
Global proteomics samples were prepared in biological triplicate for each condition. 1.5 × 106 HEK293T or MM.1S cells were seeded in 6-well plates and incubated at 37 °C, 5% CO2 for 30 min. Small molecules of interest were then added, and cells were incubated at 37 °C, 5% CO2 for the indicated time. Cells were collected according to the described general procedure, lysed by probe sonication (5 s on, 3 s off, 15 s in total, 11% amplitude) in lysis buffer (5% SDS in 50 mM TEAB, pH 7.55), and cleared by centrifugation (21,000 g, 4 °C, 10 min). After protein quantification by BCA protein assay, the lysates were diluted to 1 mg ml−1 with the lysis buffer. The diluted lysates (100 μl) were reduced by addition of dithiothreitol (20 mM) at 24 °C for 30 min then alkylated by addition of iodoacetamide (40 mM) and incubation in the dark at 24 °C for 30 min. The samples were desalted and digested using a S-Trap micro48,49. Samples were acidified by the addition of phosphoric acid to a final concentration of 1.2%. S-Trap buffer (90% methanol, 0.1 M TEAB, pH 7.1, 900 μl) was then added. Each sample was transferred to a S-Trap micro column. Using a vacuum manifold, the columns were washed with S-Trap buffer (3 × 150 μl). To digest the S-trap-bound proteins, 4 μg of trypsin/Lys-C mix resuspended in 40 μl 50 mM TEAB pH 8.0 was added to each column and incubated at 37 °C for 16 h without rotation. The digested peptides were eluted by sequential addition of 50 mM TEAB pH 8.0 (40 μl), ddH2O (40 μl) and 0.2% formic acid, 50% acetonitrile/water (35 μl), with each elution collected by centrifugation (4,000 g, 24 °C, 1 min) in a clean Eppendorf tube. The eluted samples were concentrated to dryness in a vacufuge and resuspended in ddH2O. For each resuspended sample, 5 μl was taken for labelling with TMT reagent (2 μl) at 24 °C for 1 h such that the combined total protein was 100 μg. Hydroxylamine (50%, 1.2 μl) was added to each sample to quench the TMT reagent, and the samples were incubated at 24 °C for 15 min. The TMT-labelled samples were combined and dried in a vacufuge. The dried sample was resuspended in 300 μl 0.1% TFA and fractionated to 20 fractions using a Pierce high pH reversed-phase peptide fractionation kit. The peptides were eluted sequentially by 4% acetonitrile/0.1% TEA through 20% acetonitrile/0.1% TEA in 1% acetonitrile increments (17 fractions), followed by 25%, 30% and 50% acetonitrile/0.1% TEA (all 300 μl). The first fraction (4% acetonitrile/0.1% TEA) was excluded from LC–MS/MS analysis. The other fractions were concentrated to dryness and each sample was resuspended in 20 μl of 0.1% formic acid prior to LC–MS/MS analysis.
Proteomics mass spectrometry acquisition procedures for protein level quantification
Desalted and fractionated samples were resuspended in 0.1% formic acid/water (20 μl per sample). The samples (10 μl) were loaded onto a C18 trap column (3 cm, 3 μm particle size C18 Dr. Maisch 150 μm internal diameter) and then separated on an analytical column (50 cm PharmaFluidics, Belgum) at 0.2 μl min−1 with a Thermo Scientific Ultimate 3000 system connected in line to a Thermo Scientific Orbitrap Fusion Lumos Tribrid. The column oven temperature was maintained at 35 °C. Peptides were eluted using a multi-step gradient at a flow rate of 0.2 μl min−1 over 180 min (0–15 min, 2–10% acetonitrile in 0.1% formic acid/water; 15–150 min, 10–40%; 150–170 min, 40–95%; 170–180 min, 95%) for immunoprecipitation proteomics and 90 min (0–15 min, 7% acetonitrile in 0.1% formic acid/water; 15–65 min, 7–37%; 65–75 min, 37–95%; 75–85 min, 95%; 85–90 min, 95–2%) for global proteomics. The electrospray ionization voltage was set to 2.2 kV and the capillary temperature was set to 275 °C. Dynamic exclusion was enabled with a mass tolerance of 10 ppm and exclusion duration of 90 or 150 s. MS1 scans were performed over 410–1,800 m/z at resolution 120,000. HCD fragmentation was performed on the top ten most abundant precursors exhibiting charge states from two to five at a resolving power setting of 60,000 and fragmentation energy of 37 or 38% in the Orbitrap. CID fragmentation was applied with 35% collision energy, and resulting fragments were detected using the normal scan rate in the ion trap.
Mass spectrometry data analysis for protein level quantification
Analysis was performed in Thermo Scientific Proteome Discoverer version 2.4.1.15. The raw data were searched against SwissProt human (Homo sapiens) protein database (19 August 2016; 20,156 total entries) and contaminant proteins using the Sequest HT algorithm. Searches were performed with the following guidelines: spectra with a signal-to-noise ratio greater than 1.5; mass tolerance of 10–20 ppm for the precursor ions and 0.02 Da (HCD) and 0.6 Da (CID) for fragment ions; full trypsin digestion; 2 missed cleavages; variable oxidation on methionine residues (+15.995 Da); static carboxyamidomethylation of cysteine residues (+57.021 Da); static TMT labelling (+226.163 Da for TMT 10-plex or +304.207 Da for TMTpro 16-plex) at lysine residues and N termini. The TMT reporter ions were quantified using the reporter ions quantifier node and normalized to the amount of CRBN for immunoprecipitation proteomics and total peptide amount for global proteomics. Peptide spectral matches (PSMs) were filtered using a 1% false discovery rate (FDR) using Percolator. PSMs were filtered to PSMs in only one protein group with an isolation interference under 70%. For the obtained proteome, the data were further filtered to include only master proteins with high protein FDR confidence and exclude all contaminant proteins. For immunoprecipitation proteomics, the ratios and P-values were obtained from Proteome Discoverer (P-values were calculated by one-way ANOVA with Tukey’s HSD post hoc test). For global proteomics, the data were further processed according to the methods of Huber and co-workers50. The model incorporates dependence of the variance on the mean intensity and a variance-stabilizing data transformation. In brief, missing abundances were filled in with minimum noise level computed by taking the minimum for each channel in control and minimum for each channel in treatment. A set of 2,000 centroids were generated at random from the absolute maximum in the control and treatment and the absolute minimum in control and treatment, and a minimum noise level was generated using a K-means clustering method. If one abundance was missing, then the instance was filled with the geometric mean of the PSM for control or treatment. If all abundances were missing for control and treatment or the variance between existing abundances was above 30%, the PSM was removed. P-values for the abundance ratios were calculated using the t-test (background) method.
Cell viability assay
MM.1S cells (2.0 × 104) were seeded in each well of a 96-well plate containing 100 μl RPMI supplemented with 10% FBS and 1× penicillin-streptomycin. The compound of interest was added to each well to a final concentration of 10 nM–100 μM from 100× stock solutions in 4% DMSO/PBS (1 μl). Samples were incubated at 37 °C, 5% CO2 for 5 days. Each well was treated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT, 4 mg ml−1, 10 μl), and the treated plate was incubated at 37 °C, 5% CO2 for 3 h. The formazan crystals were solubilized by the addition of 100 μl of 10% SDS, 0.01 M HCl per well, and the plate was allowed to incubate at 37 °C overnight. The absorbance at 570 nm was measured to quantify the formazan generated in each well. The blank was defined by wells containing media and MTT reagent without any cells. For each treatment well, the cell viability was calculated by subtracting the blank value and normalizing to the average absorbance of the vehicle control wells (cells treated with 4% DMSO/PBS only).
RT-qPCR
Cells were treated with the indicated drugs in biological triplicate and collected as described in the general procedures. Total RNA was extracted using the Monarch total RNA miniprep kit with on-column DNase digestion. Quantitative PCR with reverse transcription (RT–qPCR) were performed using the Luna universal one-step RT–qPCR kit using the supplied protocol. The relative mRNA level was calculated using the 2(−ΔΔCt) method with ACTB as the reference gene. Primers 7–14 were used.
Intact protein mass spectrometry
An Agilent PLRP-S column (50 mm length, 5 μm particle size, 4.6 mm ID, 1,000 Å pore size) was used. The column was maintained at 70 °C during the run. For each sample, 10 μl protein solution was either injected directly through a union and eluted with 0.1% formic acid/60% acetonitrile/water or injected onto the column and eluted using the following method with mobile phases A (0.1% formic acid/water) and B (0.1% formic acid/acetonitrile). Prior to the gradient, the column was maintained at 0% B for 2 min to wash salts. Then, a linear gradient was applied over 10 min to a final concentration of 100% B. The column was maintained at 100% B for 1 min to wash before changing to 0% B over 0.1 min. Then, the column was re-equilibrated at 0% B for 5.9 min prior to the next run. The data was internally calibrated using sodium formate clusters injected at the end of each run. The data was analysed using Bruker Compass DataAnalysis (v. 4.3), and deconvoluted using the maximum entropy algorithm between selected mass ranges (27–29 kDa for GFP or 25–40kDa for GST–FKBP12). Modification masses used included: GFP fluorophore formation = −20.0256 Da, dehydration = −18.0153 Da, methylation = +14.0266 Da.
Protein overexpression and purification of SrtA, GFP–LPETG and GST–FKBP12–LPETG
The procedure for overexpression and purification of SrtA was adapted from refs. 51,52. BL21 (DE3) cells transformed with pET29-eSrtA were used to inoculate overnight cultures of LB + 50 μg ml−1 kanamycin, which were then incubated at 37 °C with shaking at 200 rpm for approximately 16 h. For each large-scale overexpression, kanamycin was added to 750 ml autoclaved LB to a final concentration of 50 μg ml−1 and the culture was inoculated with overnight culture diluted 1:100. The overexpression cultures were incubated at 37 °C with shaking at 200 rpm until the A600 was approximately 0.5–0.8, at which point IPTG was added to a final concentration of 0.1 mM and the temperature was reduced to 30 °C. The cultures were incubated for 3 h prior to collecting the cells by centrifugation, flash freezing with liquid nitrogen, and storing at −80 °C. Up to 2 pellets, each from a 750 ml overexpression, were purified simultaneously. To purify protein, cell pellets were thawed on ice or in cool water, then resuspended in 10 ml of 0.1 mg ml−1 lysozyme, 1:1,000 benzonase, 6 mM MgCl2/B-PER per pellet. Lysates were incubated at 25 °C with shaking for 15 min, then were clarified by centrifugation (15,000 g, 4 °C, 10 min), and syringe filtration (0.45 μm). The His-tagged protein was crudely purified on a Ni-bound 1 ml HiTrap Chelating HP column using standard methods, equilibrating and washing with 25 mM imidazole/PBS and eluting with a gradient to 500 mM imidazole/PBS. Protein-containing fractions, as determined by A280, were concentrated to approximately 1 ml and further purified on a S75 10/300 GL column, pre-equilibrated and run with TBS. Protein-containing fractions not eluting with the dead volume were collected and concentrated to approximately 100 μM with a 10 kDa MWCO spin concentrator, with protein concentration determined by measuring the A280 (ε = 14,440 M−1 cm−1). The protein was aliquoted, flash frozen with liquid nitrogen, and stored at −80 °C.
The protocol for overexpression and purification of GFP–LPETG was adapted from ref. 35. pET28a-GFP-LPETG was constructed by adding the sequence encoding TGGSLPETG–His6 to the C terminus of GFP in pET28a:GFP. BL21 (DE3) cells transformed with pET28a-GFP-LPETG were used to inoculate overnight cultures of LB + 50 μg ml−1 kanamycin, which were then incubated at 37 °C with shaking at 200 rpm for approximately 16 h. For each large-scale overexpression, kanamycin was added to 750 ml autoclaved LB to a final concentration of 50 μg ml−1 and the large-scale overexpression cultures were inoculated with overnight culture diluted 1:100. The overexpression cultures were incubated at 37 °C with shaking at 200 rpm until the A600 was approximately 1, at which point IPTG was added to a final concentration of 0.45 mM and the temperature was reduced to 20 °C. The cultures were incubated for approximately 16 h prior to collecting the cells by centrifugation, flash freezing with liquid nitrogen, and storing at −80 °C. Up to 3 pellets, each from a 750 ml overexpression, were purified simultaneously. To purify protein, cell pellets were thawed on ice or in cool water, then resuspended in 8.3 ml of 25 mM imidazole, 1× protease inhibitor, 1% Triton-X 100/PBS per pellet. Lysates were combined and sonicated (30 s on, 10 s off, 5 min total, 25% amplitude) on ice. Lysates were clarified by centrifugation (20,000 g, 4 °C, 10 min) and syringe filtration (0.45 μm). The His-tagged protein was crudely purified on a Ni-bound 1 ml HiTrap Chelating HP column using standard methods, equilibrating and washing with 25 mM imidazole/PBS and eluting with a gradient to 500 mM imidazole/PBS. Protein-containing fractions, as determined by A280, were concentrated to approximately 1 ml and further purified on a S75 10/300 GL column, pre-equilibrated and run with TBS. Protein-containing fractions not eluting with the dead volume were collected and concentrated to approximately 200 μM with a 10 kDa MWCO spin filter, then diluted to 50 μM with TBS, with protein concentration determined by measuring the A488 (ε = 55,000 M−1 cm−1). The protein was aliquoted, flash frozen with liquid nitrogen, and stored at −80 °C.
pGEX2T-FKBP12-LPETG (Plasmid 5) was constructed from pGEX2T-FKBP12 (Plasmid 4) using primers 3 and 4 to remove the internal His6 tag and primers 5 and 6 to add the C-terminal LPETG–His6 tag. Overexpression in Rossetta 2 (DE3) cells was performed as previously described for pGEX2T-FKBP1253. Up to 3 pellets, each from a 750 ml overexpression, were purified simultaneously. To purify protein, cell pellets were thawed on ice or in cool water, then resuspended in 8.3 ml of 25 mM imidazole, 1 mM PMSF, 1% Triton-X 100/PBS per pellet. Lysates were combined and sonicated (30 s on, 10 s off, 5 min total, 25% amplitude) on ice together. Lysates were clarified by centrifugation (20,000 g, 4 °C, 10 min) and syringe filtration (0.45 μm). The His-tagged protein was crudely purified on a Ni-bound 1 ml HiTrap Chelating HP column using standard methods, equilibrating and washing with 25 mM imidazole/PBS and eluting with a gradiant to 500 mM imidazole/PBS. Protein-containing fractions, as determined by A280, were concentrated to approximately 1 ml and further purified on a S75 10/300 GL column, pre-equilibrated and run with TBS. Protein-containing fractions not eluting with the dead volume were collected and concentrated to approximately 500 μM with a 10 kDa MWCO spin filter, with protein concentration determined by measuring the A280 (ε = 53,080 M−1 cm−1). The protein was aliquoted, flash frozen with liquid nitrogen, and stored at −80 °C.
Sortase reaction
Conditions for the sortase reaction were adapted from refs. 35,54. For each reaction, 10 μl of 50 μM GFP–LPETG or 100 μM GST–FKBP12–LPETG was combined with 2 μl of 10 mM peptide substrate in 37 μM of 100 mM Tris pH 7.5, 150 mM NaCl, 5 mM CaCl2. Then, 1 μl of 97 μM eSrtA was added and the reaction was mixed by flicking. The reaction was allowed to incubate at 25 °C for 1 h. Non-reacted proteins and eSrtA was removed by adding 25 μl of washed His-purification Dynabeads and incubating with inversion for 15 min. The supernatant was collected using a magnetic tube rack. To remove excess peptide substrate, a 0.5 ml Zeba 7 kDa desalting column was equilibrated three times with 300 μl of experiment buffer (centrifuging 1,500 g, 25 °C, 1 min for each equilibration). The sample was added to the equilibrated column, which was centrifuged (1,500 g, 25 °C, 2 min). The flowthrough was collected. The final concentration of GFP was determined by measuring the A488 (ε = 55,000 M−1 cm−1). For in cellulo experiments, the reaction was performed on a larger scale, with the volume of Dynabeads reduced to be equal to the volume of 50 μM GFP–LPETG or 100 μM GST–FKBP12–LPETG used. The larger-scale reactions were desalted using a 5 ml Zeba 7 kDa spin desalting column then concentrated using a 3 or 10 kDa MWCO spin concentrator. If not used immediately, proteins were flash frozen with liquid nitrogen and stored at −80 °C until use.
In vitro ubiquitination of C-terminally tagged proteins or HBB(1–cQ132) and HBB(1–Me132)
Five million HEK-CRBN cells were collected per pellet, flash frozen with liquid nitrogen, and stored at −80 °C until use. Approximately 1 pellet per 1.5 samples was thawed on ice, and each pellet was resuspended in 1× protease inhibitor cocktail/Pierce IP lysis buffer (250 μl). Lysates were incubated on ice for 10 min, then were clarified by centrifugation (21,000 g, 4 °C, 10 min). The soluble portions of the lysates were collected, with 1 ml lysate per tube, and 150 μl of TBS-washed anti-Flag M2 beads were added. Samples were incubated at 4 °C on a roller for 1 h. Using a magnetic tube rack, the beads were collected and washed 3× with 1 ml TBS. Each sample was then eluted by adding 100 μl of 100 ng μl−1 3× Flag peptide/TBS and incubating at 4 °C on a roller for 1 h. The eluent was collected. Substrate proteins were diluted to 5–7.5 μM in PBS, using the same protein concentration for all samples in each experiment, and 25× stocks of small molecules were prepared in 2.5% DMSO/PBS. Ubiquitination master mixes were prepared at 2× with and without E1 and E2 enzymes. Final concentrations (1×) of the master mix components were 0.2 μM UBE1, 2 μM UbcH5a, 1 mM UbcH5c, 400 μg ml−1 K0 ubiquitin, 1 μM ubiquitin aldehyde, 1× Mg-ATP, 1× E3 ligase buffer, 10 μM MG132, 100 nM MG101. Reactions were prepared by combining 6.25 μl Flag eluent, 5.25 μl of target protein (6.25 μl if no small molecule competition was performed in the experiment), 1 μl of 25× small molecule stock in 2.5% DMSO/PBS (final concentration: 100 μM), and 12.5 μl of ubiquitination master mix in PCR tubes. Reactions were incubated at 30 °C for 90 min then were stopped by the addition of 6.25 μl of 5× SDS–PAGE loading buffer. Samples were heated at 95 °C for 5 min prior to analysis by SDS–PAGE and western blotting.
Cellular degradation of C-terminally tagged proteins
Cells were grown to 80–90% confluency in FBS-supplemented media without antibiotics prior to electroporation. Cells were detached by trypsinization as necessary and washed with PBS, then resuspended in PBS and counted. For each sample, an equal number of cells (7 × 105–2.5 × 106) were aliquoted into a 1.7 ml tube and pelleted. Electroporation mixes were prepared for each sample type. Electroporation mixes for 100 μl tips were prepared by combining 311 μl Neon buffer R with 3.6 μl DMSO or 100× compound or, for experiments without small molecule competition, 315 μl Neon buffer R and 45 μl of 50–60 μM protein in PBS or TBS, using the same protein concentration for all samples in each experiment, or buffer alone for control samples. All protein concentrations were determined by nanodrop, using the same extinction coefficient for all proteins of the same type. Electroporation mixes for 10 μl tips were prepared for each sample type by combining43.25 μl Neon buffer R with 0.5 μl DMSO or 100× compound and 6.25 μl of 50 μM protein in PBS, or TBS for control samples. Immediately prior to each electroporation, the PBS was removed from the pelleted cells and the pellet was resuspended in 110 μl or 12 μl of electroporation mix (for 100 and 10 μl tips, respectively). The sample was taken up into a tip attached to a Neon pipette, and the pipette tip was submerged in a Neon cuvette containing 3 ml Neon buffer E2. The sample was then electroporated (HEK293T cells 800 V, 25 ms, 2 pulses;Jurkat cells 1,325 V, 10 ms, 3 pulses; MEF cells 1350 V, 30 ms, 1 pulse). The cells were then dispensed into 10 volumes of warmed PBS. This process was repeated for each sample, with each tip used for 3 electroporation cycles. Cells were then pelleted by centrifugation and the supernatant was removed. Cells were resuspended in 0.5 ml or 50 μl trypsin-EDTA solution (for 100 and 10 μl tips, respectively) and incubated at 37 °C for 5 min. Trypsinization was quenched by the addition of 0.5 ml DMEM with DMSO or 1,000× compound stock added to the media and cells electroporated with 100 μl tips were again pelleted. The supernatant was removed. Each sample was resuspended in 1 ml DMEM and transferred to a well of a 12-well plate, with DMSO or 1,000× compound stock added to the media. Cells electroporated with 10 μl tips were transferred directly to a 24-well plate. The samples were then incubated at 37 °C, 5% CO2 until 6 h after electroporation. Cells were detached by trypsinization, agitation, and/or scraping, collected by centrifugation, and washed with PBS. For analysis of GFP levels, each sample was resuspended in 500 μl PBS with 50 nM SYTOX Blue or 10 μl 0.5 mg ml−1 propidium iodide added to allow for exclusion of dead cells. Cells were analysed by flow cytometry (mCherry, Pacific Blue, and FITC on LSRII or dsRed and FITC on Fortessa). At least 9,600 events were analysed for each sample. Relative GFP level was determined by subtracting the arithmetic mean GFP signal among live cells in the control sample from the arithmetic mean GFP signal among live cells for each sample, then normalizing the resulting values to the arithmetic mean for GFP–His6 or GFP–Me. For FKBP12 samples, cells were collected by centrifugation 6 h after electroporation and were lysed in 1% SDS, 1× protease inhibitor/PBS by brief electroporation (5 s, 10% amplitude). Protein concentration was normalized by BCA assay, and samples were analysed by western blotting.
Mutagenesis, overexpression and in vitro ubiquitination of Npu DnaE
A V5 tag was added to pSARSF505 (Plasmid 6) using primers 15 and 16 to obtain pSARSF505-V5 (Plasmid 7). BL21 (DE3) cells transformed with pSARSF505 and pSARSF505-V5 were used to inoculate overnight cultures of LB media + 50 μg ml−1 kanamycin, which were then incubated at 37 °C with shaking at 200 rpm for approximately 16 h. For each small-scale overexpression, kanamycin was added to 50 ml LB media to a final concentration of 50 μg ml−1. The cultures were inoculated with overnight culture diluted 1:100 and incubated at 37 °C with shaking at 200 rpm until the A600 was approximately 0.5, at which point IPTG was added to a final concentration of 1 mM. The cultures were incubated for approximately 3 h prior to collecting the cells by centrifugation, flash freezing with liquid nitrogen, and storing at −80 °C. For in vitro ubiquitination, each pellet from a small-scale overexpression was lysed in 1% NP-40, 1× protease inhibitor cocktail/PBS (1 ml). Samples were sonicated (10 s on, 5 s off, 1 min total, 25% amplitude) and clarified by centrifugation (10 min, 21,000 g, 4 °C). The soluble portion of the lysate was used for in vitro ubiquitination without purification.
4-Hydroxytamoxifen-induced intein splicing
A haemagglutinin (HA) tag was added to 37-2 (Plasmid 8) using primers 16 and 18 to obtain 37-2–HA (Plasmid 9). The N − 1 residue was mutated from H to F using primers 19 and 20 to obtain 37-2–HA(HtoF) (Plasmid 10). HEK293T (0.5 × 106) cells were seeded in 1 ml antibiotic-free DMEM supplemented with 10% FBS per well in a 12-well tissue culture-treated plate and allowed to adhere overnight at 37 °C, 5% CO2. Cells were transfected with 1 μg DNA per well using TransIT-Pro transfection reagent (1 μl per 1 μg DNA) and incubated at 37 °C, 5% CO2 for 24 h. Cells were pre-treated with DMSO, MLN4924, or lenalidomide from 400× stocks and incubated at 37 °C, 5% CO2 for 1 h prior to addition of DMSO or 4-hydroxytamoxifen (4-HT) from a 400× stock. Cells were incubated 24 h, then lysed in the plate with 150 μl of 1× protease inhibitor/M-PER per well. Samples were clarified by centrifugation. Loading buffer was added to 1× and samples were heated 5 min at 95 °C prior to analysis by western blotting.
Time-course study of hydrolysis of GFP–FcQ and GFP–FcN
The indicated proteins were diluted to 1 μM in PBS (120 μl), with each condition prepared in triplicate. Samples were incubated in a sand bath pre-heated at 37 °C, and 20 μl was taken from each sample at t = 0 h (immediately after sample preparation), 24 h, 48 h, 72 h, and 96 h. Samples were flash frozen in liquid nitrogen and stored at −80 °C until analysis by intact protein mass spectrometry. The maximum peak intensity within 2 Da of the expected masses of the cyclizedand hydrolysed forms of each protein was used to determine the per cent cQ or cN remaining relative to the starting proportion.
Preparation of RBC and bovine lens lysates
Whole blood samples were obtained from Stanford Blood Center, and the IRB-approved human subjects’ involvement in our research project. Whole blood (−10 ml) was pelleted by centrifugation (500g) at 24 °C for 10 min. The plasma supernatant was aspirated and the cell pellet was washed with 150 mM NaCl in PBS, pH 7.4 (1 × 5 ml) and PBS (2 × 5 ml). The washed RBCs were then resuspended in PBS (5 ml), aliquoted, flash frozen with liquid nitrogen, and stored at −80 °C until use. RBCs (50 μl) were resuspended in 5% SDS in 50 mM TEAB, pH 7.55 (400 μl) and clarified by centrifugation (21,000 g, 4 °C, 10 min). An equivalent suspension of RBCs (50 μl) was prepared in 400 μl non-SDS lysis buffer (25 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40 and 5% glycerol) for protein concentration measurement. The concentration of RBC lysate was measured by NanoDrop using the oxy-haemoglobin custom method (https://assets.thermofisher.com/TFS-Assets/MSD/Application-Notes/nanodrop-one-onec-custom-method-hemoglobin-measurements-T144.pdf). As both conditions gave homogenous lysates and SDS led to the degradation of haem55, the protein concentration of RBCs in 5% SDS, 50 mM TEAB was determined by that of the equivalent suspension.
Fresh bovine eyes were obtained from Nebraska Scientific (PZ7K486F). Lenses were extracted from fresh whole bovine eyes and rinsed with PBS prior to flash freezing with liquid nitrogen. Each frozen lens was then ground to a powder using a mortar and pestle cooled with liquid nitrogen. The frozen powder was stored at −80 °C. To prepare lysate, 100 mg crushed lens powder was suspended in 5% SDS/50 mM TEAB pH 7.4. The sample was lysed by sonication (5 s on, 2 s off, 10 s total, 10% amplitude) and clarified by centrifugation (21,000 g, 4 °C, 10 min). Protein concentration was measured by BCA assay.
Proteomics for cyclic imide detection in RBCs and bovine lens
RBC samples were prepared in biological triplicate or quintuplicate, and bovine lens samples were prepared in quadruplicate for each condition. For trypsin digestion, lysates (100 μg per sample) were loaded on an S-trap micro column similarly to in ‘Global quantitative proteomics sample preparation’. To digest the S-trap-bound proteins, 2 μg of trypsin in 40 μl 50 mM TEAB, pH 7.4 was added to each column and incubated at 47 °C for 1 h without rotation. For chymotrypsin digestion, lysates were diluted to 1 mg ml−1 by 5% SDS, 50 mM TEAB, pH 7.55 and 100 μl of the lysates were taken to reduction and alkylation. The proteins were precipitated using methanol-chloroform precipitation. In brief, four volumes of chilled methanol, one volume of chloroform and three volumes of water were added sequentially to the lysates. The mixture was vortexed and centrifugated at 14,000 g, 5 min, 4 °C, and the supernatant aspirated. The protein pellet was washed with three volumes of methanol and centrifugated at 14,000 g, 5 min, 4 °C, and the resulting precipitated protein was air-dried. The protein was dissolved in 200 μl 50 mM HEPES, 10 mM CaCl2, pH 7.5, followed by the addition of 2.5 μg of chymotrypsin in 5 μl of 1 mM HCl. The digestion was allowed to proceed at 25 °C for 18 h without rotation and quenched by the addition of 10 μl of 10% formic acid. For both tryptic and chymotryptic peptides, approximately 40 μg of peptides in 10 μl ddH2O per condition was taken for labelling with TMT reagent (10 μl) at 24 °C for 1 h. TMT labelling was quenched by 1M Tris-Cl (5 μl, pH 7.6) instead of hydroxylamine to minimize the hydrolysis of cyclic imides. For base ablation of the cyclic imides, the dried, digested samples were incubated with 1% TEA/H2O (100 μl, pH 12.0) at 65 °C for 30 min. The base-treated samples were concentrated to dryness before TMT labelling. The combined sample after TMT labelling was resuspended in 0.1% TFA and 300 μl of solution containing approximately 100 μg peptides was taken to fractionation into 6 fractions (all 300 μl) at 5%, 10%, 15%, 20%, 25%, 35% and 50% acetonitrile/0.1% TEA using the Pierce high pH reversed-phase peptide fractionation kit. The first fraction (5% acetonitrile/0.1% TEA) was excluded from LC–MS/MS analysis. Immediately after the elution, each fraction was acidified by the addition of 5 μl of 10% formic acid. The fractions were concentrated to dryness and each sample was resuspended in 20 μl of 0.1% formic acid prior to LC–MS/MS analysis. Samples for cyclic imide detection were dried in a vacufuge set at 24 °C and the dried samples were stored at −20 °C or −80 °C to avoid long exposure to higher temperature.
Time-course study of formation of cyclic imide and its hydrolysis products in synthetic peptides
The synthetic peptides (20 μl, 5 mM stock in ddH2O) were incubated in 20 mM ammonium acetate buffer (380 μl) at pH 7.4, 8.0, or 9.0 (final concentration: 250 μM). Samples were incubated in a sand bath pre-heated to 37 °C, and 30 μl was taken from each sample at t = 0 h (collected immediately after resuspension in pH 7.4 buffer), 24 h, 48 h, 72 h, 96 h, 120 h, 144 h, 168 h, 192 h and 240 h. Immediately after collection, the samples were quenched by the addition of 0.75 μl of 10% formic acid and stored at −80 °C until analysis. The MS samples were prepared by mixing 2 μl of the peptides, 18 μl 0.1% formic acid, and 20 μl acetonitrile. Peptides were injected on a Thermo Orbitrap Fusion Lumos Tribrid or LTQ Orbitrap Velos and eluted using a multi-step gradient at a flow rate of 0.2 μl min−1 over 60 min (0–5 min, 5% acetonitrile in 0.1% formic acid/water; 5–52 min, 5–80%; 52–55 min, 80–98%; 55–60 min, 98%). The electrospray ionization voltage was set to 2 kV and the capillary temperature was set to 275 °C. MS1 scans were performed over 400–2,000 m/z at resolution of 120,000. The raw chromatograms were extracted with m/z of label-free parent peptide (1,030.47–1,030.49), cyclic imide (908.39–908.41), and hydrolysis products (917.39–917.41) for HBB(42–60); parent peptide (978.03–978.05), cyclic imide (856.46–856.48), and hydrolysis products (865.46–865.48) for ACTB(96–113); parent peptide (999.83–999.85), cyclic imide (867.90–867.92 or 685.34–685.36) and hydrolysis products (876.90–876.92 or 703.35–703.37) for HBA(63–91). For each species, only the peak area at the expected retention time was integrated and quantified using the Genesis peak integration algorithm on Xcalibur Qual Browser version 3.0.63.
Selected ion monitoring quantification of HBB parent and modified peptides in RBCs
RBC lysates (100 μg) were taken for reduction, alkylation and trypsin digestion as described in ‘Proteomics for cyclic imide identification in RBCs and bovine lens’. The peptides were desalted by Pierce peptide desalting spin columns following the manufacturer protocol. The dried samples were resuspended in 30 μl 0.1% formic acid. The isotopically labelled HBB(42*–60) (HBB*) was incubated in ammonium acetate buffer at pH 7.4, 37 °C for 48 h to generate the cyclic imide and hydrolysis products (final concentration: 250 μM). The MS samples were prepared by mixing 1 μl of the HBB* mixture, 10 μl of RBC digests, and 9 μl 0.1% formic acid. The sample (8 μl) was injected on a Thermo Orbitrap Fusion Lumos Tribrid and eluted using a multi-step gradient at a flow rate of 0.2 μl min−1 over 60 min (0–5 min, 5% acetonitrile in 0.1% formic acid/water; 5–52 min, 5–80%; 52–55 min, 80–98%; 55–60 min, 98%). The electrospray ionization voltage was set to 2 kV and the capillary temperature was set to 275 °C. MS1 scans were performed on the SIM mode to only look for m/z of 1,029.88–1,037.08, 908.30–915.50 and 917.31–924.51, and retention time window of 25–33 min, 33.5–50 min, and 33.5–50 min for the parent peptide, cyclic imide and hydrolysed products, respectively. Orbitrap resolution was set at 120,000, RF lens at 60%, maximum injection time at 300 ms and normalized AGC target at 2,000%. The raw chromatograms were extracted with m/z of light parent peptide (1,030.47–1,030.49), cyclic imide (908.39–908.41), and hydrolysis products (917.39–917.41) for RBC lysates and heavy parent peptide (1,035.48–1,035.50), cyclic imide (913.40–913.42), and hydrolysis products (922.40–922.42) for peptide standard. For each species, only the peak area with the expected retention time and isotope patterns was integrated and quantified using the ICIS peak integration algorithm on Xcalibur Qual Browser version 3.0.63. The quantification was performed as following: the mixture of HBB* (206.29 ng) was run on the full scan mode first (1) to obtain the absolute mass of unmodified parent peptide in the mixture. Then, HBB* was run alone on SIM mode to quantify the modified species (2) and lastly, the RBC digests spiked with HBB* mixture were run on the same SIM method to quantify the modified species in the RBC (3). The mass of HBB* injected was the same across all samples.
Where Area refers to the integrated peak area of extracted ion chromatograms; light mod refers to the light peptide bearing the cN or N modification, which originates from the sample; and heavy mod refers to the heavy peptide bearing the cN or N modification, which originates from the isotopically labelled standard.
Time-course study of formation of cyclic imide and its hydrolysis products in recombinant HBB
Recombinant human HBB protein (200 μg) was precipitated using methanol-chloroform precipitation to remove the storage buffer and allowed to air-dry. The dried pellet was resuspended in ammonium acetate buffer (200 μl, 20 mM) at pH 7.4 and aliquoted into 3 tubes (66 μl each) for biological triplicate. Samples were incubated in a sand bath pre-heated to 37 °C, and 15 μl was taken from each sample at t = 0 h (collected immediately after resuspension), 48 h, 96 h and 144 h. Immediately after collection, the samples were stored at −80 °C until analysis without acid quench. All the samples were dried on a vacufuge at once and resuspended in 23 μl of 5% SDS, 50 mM TEAB, pH 7.55. The samples were reduced by addition of dithiothreitol (1 μl of 240 mM stock) at 24 °C for 30 min then alkylated by addition of iodoacetamide (1 μl of 500 mM stock) and incubation in the dark at 24 °C for 30 min. The samples were acidified by the addition of phosphoric acid (2.5 μl of 27.5% stock) to a final concentration of 2.5%. S-Trap buffer (90% methanol, 0.1 M TEAB, pH 7.1, 165 μl) was then added. Each sample was transferred to a S-Trap micro column. Using a vacuum manifold, the columns were washed with S-Trap buffer (3 × 150 μl). To digest the S-trap-bound proteins, 2 μg of trypsin resuspended in 20 μl 50 mM TEAB pH 7.4 was added to each column and incubated at 47 °C for 1 h without rotation. The digested peptides were eluted by sequential addition of 50 mM TEAB pH 7.4 (40 μl), ddH2O (40 μl) and 0.2% formic acid, 50% acetonitrile/water (40 μl), with each elution collected by centrifugation (4,000 g, 24 °C, 1 min). The eluted samples were concentrated to dryness in a vacufuge, resuspended in 0.1% TFA (300 μl) and desalted by Pierce peptide desalting spin column and exactly following the manufacturer protocol. The desalted samples were dried by vacufuge and resuspended in 20 μl 0.1% formic acid. The HBB* mixture (1 μl) was spiked into the samples as the internal standard before injection. The samples were run using the same SIM method (except for minor adjustment of the RT window) and quantified similarly to in ‘Selected ion monitoring quantification of HBB parent and modified peptides in RBCs’. The P-values for the ratios were calculated by unpaired two-tailed t-tests.
The samples were also run with full scan mode, which were taken for analysis using Proteome Discoverer version 2.4.1.15. The raw data were searched against HBB (UniProt: P68871) using the Sequest HT algorithm. Searches were performed with the following guidelines: spectra with a signal-to-noise ratio greater than 1.5; mass tolerance of 20 ppm for the precursor ions and 0.02 Da (HCD) and 0.6 Da (CID) for fragment ions; semi-tryptic digestion with specificity at the peptide N terminus only; 2 missed cleavages; variable oxidation on methionine residues (+15.995 Da); static carboxyamidomethylation of cysteine residues (+57.021 Da); variable 13C915N labelling (+10.027 Da) on phenylalanine residues; variable dehydration on asparagine and glutamine residues at C terminus only (−18.015 Da). PSMs were filtered using a 1% or 5% FDR using Target Decoy PSM validator.
Generation of recombinant HBB(1–129) thioester
Constructs for Escherichia coli expression were derived from the plasmid pcDNA3.1-HBB-Flag and pTXB1-HSP27-AvaE-His6. Inserts corresponding to HBB(1–129) and backbone excluding HSP27 were amplified using overhang PCR with primers designed for Gibson Assembly using NEBuilder online tools. The inserts were then ligated with pTXB1-AvaE-His6 linear vector to generate recombinant plasmids using HiFi DNA Assembly Kit (NEB). Ligated DNAs were transformed into high efficiency DH5α competent cells (NEB) and the sequences validated by Sanger sequencing.
BL21(DE3) cells were transformed with the HBB(1–129)-AvaE-His6 plasmid. A starter culture was grown overnight at 37 °C for 16 h from a single colony. Terrific Broth (3 l, IBI Scientific) was then inoculated with the starter culture and grown to an A600 of 0.6–0.8 at 37 °C while being shaken at 225 rpm. Expression was induced with IPTG at a final concentration of 1 mM followed by incubation at room temperature for 16 h with shaking at 225 rpm. Cells were then collected at 6,000g, and the resulting pellets were resuspended in lysis buffer (20 mM NaH2PO4, 500 mM NaCl, 1 mM TCEP, 5 mM imidazole, and 2 mM phenylmethylsulfonyl fluoride, pH 7.0). Solid guanidine HCl was added to the slurry and the resulting solution was subjected to tip sonication on ice (30 s on, 30 s off, 5 min total, 70% amplitude) to break up the protein inclusion bodies. After clarification by centrifugation (20,000g, 4 °C, 30 min), the pH of the lysate was adjusted to 6.8 with 3 M NaOH. The resulting supernatants were then loaded onto Cobalt-NTA agarose beads (Genessee Scientific), incubated at 4 °C for 1 h with slight agitation, and then extensively washed (4 M urea, 20 mM NaH2PO4, 600 mM NaCl, 20 mM imidazole, pH 6.8) for 3 column volumes. The intein fusion protein was then eluted (4 M urea, 20 mM NaH2PO4, 600 mM NaCl, 1 mM TCEP, 250 mM imidazole, pH 6.8) and buffer exchanged 500-fold into DPBS buffer with 4 M urea by centrifugal filtering with 10K spin filters (Amicon Ultra, EMD Millipore) to remove excess imidazole. The intein fusion was then cleaved through transthioesterification by the addition of sodium mercaptoethanesulfonate (MESNa) to a final concentration of 250 mM at pH 6.8 prior to incubation at room temperature for 16 h. The cleaved AvaE–His6 intein and unreacted fusion protein were removed by loading the mixture onto Co-NTA agarose beads, incubating at 4 °C for 3 h. The final protein thioester was obtained by elution with washing buffer (20 mM NaH2PO4, 600 mM NaCl, 20 mM imidazole, pH 6.8), followed by further purification by reversed-phase liquid chromatography. Pure protein was characterized by analytical C4 RP-HPLC and ESI-MS. Purified proteins were lyophilized prior to storage.
Ligation and deselenization for generation of HBB(1–cQ132) and HBB(1–Me132)
HBB(1–129) thioester (1.5 mg, 0.11 μmol, 1 mM final concentration, 1 equivalent) and Sec–YcQ or Sec–YcQMe (0.2 mg, 0.36 μmol, 3 mM final concentration, 3 equivalents) were dissolved in 100 μl of degassed ligation buffer (6 M guanidine HCl, 100 mM phosphate, 100 mM l-ascorbic acid, 100 mM TCEP, 250 mM mercaptophenylacetic acid (MPAA), pH 7.0). The reaction was monitored by analytical C4 RP-HPLC and ligation was largely complete after 4 h at 25 °C with gentle agitation. The mixture was buffer exchanged 1,000-fold into 6 M guanidine HCl, 100 mM phosphate pH 6.0 to remove MPAA using 3K centrifugal filters (Amicon Ultra, Millipore). Dithiothreitol (DTT) was added to a final concentration of 25 mM and the mixture was incubated for 10 min to fully reduce the selenocysteine residues. Solid TCEP was then added to a final concentration of 100 mM and the pH was adjusted to 5.0. Analytical RP-HPLC and ESI-MS were used to monitor the conversion of selenocysteine to alanine. After 2 h, deselenization was deemed complete, and the final product was purified by semipreparative RP-HPLC on a C4 column. The purity and identity of the product were confirmed by analytical RP-HPLC and qTOF-ESI-MS, respectively. HBB(1–cQ132) was found as an inseparable mixture consistent with the expected protein product and another product bearing a RtoG mutation due to misreading of the codon in E. coli, as has been observed previously56. Similarly, HBB(1–Me132) was found to have the same mixture, as well as the oxidation of two methionine residues.
Ubiquitination site quantification of HBB(1–cQ132) and HBB (1–Me132) by mass spectrometry
In vitro ubiquitination was performed as described above with 30.0 μl Flag eluent, 25.2 μl of 33.5 μM HBB(1–cQ132) or HBB(1–Me132), 4.8 μl of 2.5% DMSO/PBS or 2.5 mM lenalidomide stock in 2.5% DMSO/PBS, and 60.0 μl of ubiquitination master mix in Eppendorf tubes. Samples for mass spectrometry analysis were prepared in technical quadruplicate for each condition. Reduction, alkylation and trypsin digestion were performed similarly to in ‘Preparation of immunoprecipitated samples for quantitative proteomics’. The eluted samples were concentrated to dryness in a vacufuge and resuspended in 60 μl ddH2O. For each resuspended sample, 4 aliquots of 10 μl were taken for labelling with 4 channels of TMTpro 16-plex reagent (10 μl, 4 replicates × 4 conditions = 16 channels used) at 24 °C for 1 h. Tris-Cl (1 M, 5 μl, pH 7.6) was added to each sample to quench the TMT reagent, and the samples were incubated at 24 °C for 15 min. The TMT-labelled samples were combined and dried in a vacufuge. The dried sample was resuspended in 600 μl 0.1% TFA and fractionated to 5 fractions using a Pierce high pH reversed-phase peptide fractionation kit (300 μl per column, 2 columns in total). The peptides were eluted sequentially by 5% acetonitrile/0.1% TEA, followed by 10%, 15%, 25% and 50% acetonitrile/0.1% TEA. The first fraction (5% acetonitrile/0.1% TEA) was excluded from LC–MS/MS analysis. The other four fractions were concentrated to dryness. The fractions with the same acetonitrile percentage were combined and resuspended in 30 μl of 0.1% formic acid prior to LC–MS/MS analysis. The LC–MS/MS method was same as that used for immunoprecipitation proteomics described in ‘Proteomics mass spectrometry acquisition procedures for protein level quantification’. Analysis was performed in Thermo Scientific Proteome Discoverer version 2.4.1.15. The raw data were searched against HBB (UniProt: P68871) using the Sequest HT algorithm. Searches were performed with the following guidelines: spectra with a signal-to-noise ratio greater than 1.5; mass tolerance of 20 ppm for the precursor ions and 0.02 Da (HCD) and 0.6 Da (CID) for fragment ions; semi-tryptic digestion with specificity at the peptide N terminus only; 2 missed cleavages; variable oxidation on methionine residues (+15.995 Da); static carboxyamidomethylation of cysteine residues (+57.021 Da); static TMTpro labelling (+304.207 Da) at N termini; static diglycine adduct (+114.043 Da) on lysine residues; variable dehydration on asparagine and glutamine residues at C terminus only (−18.015 Da). The TMT reporter ions were quantified using the reporter ions quantifier node and normalized to the amount of HBB. PSMs were filtered using a 1% or 5% FDR using the Target Decoy PSM validator. The abundances were normalized to the largest mean and the P-values were obtained by unpaired two-tailed t-tests.
Proteomics for cyclic imide detection in HEK293T and MM.1S cells
Samples were prepared in biological triplicate for each condition. 3 × 106 wild-type HEK293T cells or CRBN-knockout HEK293T cells with the same passage number (p19) were collected in separate tubes. For lenalidomide treatment, 1.5 × 106 wild-type HEK293T cells (p19) were seeded in 6-well plates and incubated at 37 °C for 1 h. The cells were treated with 200 μM lenalidomide for 24 h first. Then, the media was aspirated and fresh media containing 200 μM lenalidomide was added into each well and incubated for another 24 h (48 h treatment in total). The procedure was the same for MM.1S cells, except that 2 × 106 cells were initially seeded and cells were spun down at 300g, 24 °C, 4 min and resuspended in fresh media containing lenalidomide after 24 h. After protein quantification by BCA assay, the lysates were diluted to 1 mg ml−1 with the lysis buffer. To the diluted lysate (100 μl), 0.5 μg of GFP–LPETG was added as an internal standard (0.42 μl of 43 μM TBS stock), and the mixtures were taken for reduction and alkylation. The samples were loaded on S-trap micro columns similarly to in ‘Global quantitative proteomics sample preparation’. To digest the S-trap–bound proteins, 2.5 μg of trypsin in 40 μl 50 mM TEAB pH 7.4 was added to each column and incubated at 47 °C for 1 h without rotation. TMT labelling was performed as described in ‘Proteomics for cyclic imide detection in RBCs and bovine lens’. The combined sample after TMT labelling was resuspended in 900 μl 0.1% TFA and 300 μl was taken to fractionation into 18 fractions (all 300 μl) using the Pierce high pH reversed-phase peptide fractionation kit. The peptides were eluted sequentially by 5%, 6%, 8%, 10%, 11–20% (with 1% increments), 25%, 30%, 35% and 50% acetonitrile/0.1% TEA. Immediately after the elution, each fraction was acidified by the addition of 5 μl of 10% formic acid. The fractions were concentrated to dryness and each sample was resuspended in 20 μl of 0.1% formic acid prior to LC–MS/MS analysis.
Proteomics mass spectrometry acquisition procedures for cyclic imide detection
Desalted and fractionated samples were resuspended in 0.1% formic acid/water (20 μl per sample). The sample (2.0 μl) was loaded onto a C18 trap column (3 cm, 3 μm particle size C10 Dr. Maisch 150 μm internal diameter) and then separated on an analytical column (Thermo Scientific Acclaim PepMap 100, 2 μm particle size, 250 mm length, 75 μm internal diameter) at 0.2 μl min−1 with a Thermo Scientific Ultimate 3000 system connected in line to a Thermo Scientific Orbitrap Fusion Lumos Tribrid. The column temperature was maintained at 50 °C. Peptides were eluted using a multi-step gradient at a flow rate of 0.2 μl min−1 over 90 min (0–5 min, 2% acetonitrile in 0.1% formic acid/water; 5–7 min, 2–5%; 7–75 min, 5–40%; 75–85 min, 40–95%; 85–90 min, 95%) for HEK293T and MM.1S and 180 min (0–5 min, 2% acetonitrile in 0.1% formic acid/water; 5–15 min, 2–10%; 15–160 min, 10–40%; 160–170 min, 40–95%; 170–180 min, 95%) for RBC and bovine lens. The electrospray ionization voltage was set to 2 kV and the capillary temperature was set to 275 °C. Dynamic exclusion was enabled with a mass tolerance of 10 ppm and exclusion duration of 90 s. MS1 scans were performed over 410–2,000 m/z at resolution 120,000. HCD fragmentation was performed on the top ten most abundant precursors exhibiting a charge state from two to five at a resolving power setting of 60,000 and fragmentation energy of 37% in the Orbitrap. CID fragmentation was applied with 35% collision energy, and resulting fragments were detected using the normal scan rate in the ion trap.
Selected ion monitoring quantification of ACTB parent and modified peptides in HEK293T and MM.1S cells
Samples were prepared in biological quadruplicate for each condition. Wild-type HEK293T cells (1.5 × 106) (p20) or 2 × 106 MM.1S cells (p14) were seeded in 6-well plates and incubated at 37 °C for 1 h. The cells were treated with DMSO or 200μM lenalidomide for 24 h first. For HEK293T, the media was aspirated and fresh media containing 200 μM lenalidomide was added into each well and incubated for another 24 h. For MM.1S, the cells were centrifugated at 300 g, 24 °C, 4 min and the media aspirated. Cells were reseeded in 6-well plates with fresh media containing DMSO or 200 μM lenalidomide and incubated for another 24 h (48 h treatment in total). Cells were reseeded in 6-well plates with fresh media containing DMSO or 200 μM lenalidomide and incubated for another 24 h (48 h treatment in total). Lysate preparation, reduction/alkylation, and trypsin digestion were performed as described in ‘Proteomics for cyclic imide identification in HEK293T and MM.1S cells’. The dried peptide digests (100 μg) were fractionated into 8 fractions using the Pierce high pH reversed-phase peptide fractionation kit. The peptides were eluted sequentially by 10%, 12%, 14%, 16%, 18%, 20%, 25% and 50% acetonitrile/0.1% TEA. Immediately after the elution, each fraction was acidified by the addition of 5 μl of 10% formic acid. The fractions were concentrated to dryness and each sample was resuspended in 20 μl of 0.1% formic acid. The isotopically labelled ACTB(96*–113) (ACTB*) was incubated in ammonium acetate buffer at pH 7.4, 37 °C for 48 h to generate the cyclic imide and hydrolysis products (final concentration: 250 μM). Each fraction was spiked with 0.75 μl of the ACTB(96*–113) incubation mixture. Samples were first run on the SIM mode on LTQ Orbitrap Velos in search of light ACTB peptides and modified species, which were found in fraction 12% for HEK293T and fractions 12% and 14% for MM.1S. The same fractionation procedure was performed on all the samples. For HEK293T, fraction 12% was resuspended in 20 μl 0.1% formic acid + 0.75 μl ACTB* standard. For MM.1S, fractions 12% and 14% were dried and resuspended in 40 μl 0.1% formic acid + 1.5 μl ACTB* mixture in total. The sample (6 or 8 μl) was injected on a Thermo Orbitrap Fusion Lumos Tribrid and eluted using a multi-step gradient at a flow rate of 0.2 μl min−1 over 90 min (0–5 min, 5% acetonitrile in 0.1% formic acid/water; 5–65 min, 5–45%; 65–80 min, 45–98%; 80–90 min, 98%). The electrospray ionization voltage was set to 2 kV and the capillary temperature was set to 275 °C. MS1 scans were performed on SIM mode to only look for m/z of 977.44–982.64, 855.76–861.16 and 864.76–870.16, and retention time window of 25–40 min, 35–48 min, and 32–45 min for the parent peptide, cyclic imide and hydrolysed products, respectively. Orbitrap resolution was set at 120,000, RF lens at 60%, maximum injection time at 300 ms and normalized AGC target at 2,000%. The raw chromatograms were extracted with m/z of light parent peptide (978.03–978.05), cyclic imide (856.45–856.47), and hydrolysis products (865.45–865.47) for cell lysates and heavy parent peptide (981.03–981.05 for MM.1S and 981.04–981.06 for HEK293T), cyclic imide (859.45–859.47), and hydrolysis products (868.45–868.47 for MM.1S and 868.46–868.48 for HEK293T) for peptide standard. For each species, only the peak area with the expected retention time and isotope patterns was integrated and quantified using the ICIS peak integration algorithm on Xcalibur Qual Browser version 3.0.63. The quantification was performed as following: the mixture of ACTB* (91.89 ng) was run on the full scan mode first (1) to obtain the absolute mass of unmodified parent peptide in the mixture. Then, ACTB* was run alone on SIM mode to quantify the modified species (2) and lastly, the cell lysates spiked with ACTB* mixture were run on the same SIM method to quantify the modified species in the ACTB (3). For some samples of HEK293T, the mixture of ACTB* injected was 68.92 ng and the calculations of absolute masses were scaled accordingly (the ratios of modified species to parent peptide are unaffected). The P-values for the ratios were calculated by unpaired two-tailed t-tests.
Mass spectrometry data analysis for cyclic imide detection
Analysis was performed in Thermo Scientific Proteome Discoverer version 2.4.1.15. The raw data were searched against SwissProt human (H. sapiens) protein database (21 February 2019; 20,355 total entries) or SwissProt bovine (Bos taurus) protein database (19 August 2016; 5,997 entries) and contaminant proteins using the Sequest HT algorithm. Searches were performed with the following guidelines: spectra with a signal-to-noise ratio greater than 1.5; mass tolerance of 20 ppm for the precursor ions and 0.02 Da (HCD) and 0.6 Da (CID) for fragment ions; semi-tryptic or semi-chymotryptic (FLWY) digestion with specificity at the peptide N terminus only; 2 missed cleavages; variable oxidation on methionine residues (+15.995 Da); static carboxyamidomethylation of cysteine residues (+57.021 Da); static TMT labelling (+226.163 Da) at lysine residues and N termini; variable dehydration on asparagine and glutamine residues at C terminus only (−18.015 Da). The TMT reporter ions were quantified using the Reporter Ions Quantifier node and normalized to the intensity of GFP–LPETG peptides for HEK293T and MM.1S and the summed peptide intensity for RBC and bovine lens. PSMs were filtered using a 1% or 5% FDR using Target Decoy PSM validator. For the obtained peptide groups, the data were further filtered to include only peptides with 1% or 5% FDR, bearing the modification of dehydration on asparagine or glutamine residues, and derived from non-contaminant proteins to generate the list of peptides bearing C-terminal cyclic imide modifications. To generate the list of peptides bearing C-terminal glutamine or asparagine, the data were filtered to include only peptides with 1% or 5% FDR, bearing asparagine or glutamine residues at the peptide C terminus, and derived from non-contaminant proteins. The peptides that were mapped back to the protein C terminus were excluded. The P-values for the ratios were obtained by one-way ANOVA with Tukey’s HSD post hoc test from Proteome Discoverer.
Identification of C-terminal cyclic imide modification sites from public datasets
Data were analysed as described under ‘Mass spectrometry data analysis for cyclic imide detection’ with the following modifications. For the analysis of global proteomics datasets obtained from CPTAC, the RAW datasets were processed according to the methods in the respective publication for fully tryptic peptides and spectra that did not receive a confident peptide spectral match were searched for N-terminal semi-tryptic sequences and an additional dynamic modification of dehydration on asparagine or glutamine residues (−18.015 Da) at the C terminus of the peptide. The data were filtered with a 1% FDR using Percolator.
Extended Data
Extended Data Fig. 1 ∣. Examination of bifunctional degraders of BRD4 with candidate degrons for CRBN.
(a) Representative mechanisms of the formation of C-terminal cyclic aspartimide (cN) or glutarimide (cQ) and N-terminal pyroglutamate (pE) in vivo. (b) Structures of JQ1-uracil, JQ1-PEG-uracil, and JQ1-uridine. (c) Western blot of BRD4 after treatment with JQ1-uracil, JQ1-PEG-uracil, JQ1-uridine in HEK293T cells for 24 h. (d) Structures of JQ1-cQ, JQ1-cN, and JQ1-FpE. (e) Western blot of BRD4 after treatment with JQ1-cQ, JQ1-cN, JQ1-FpE in HEK293T cells for 4 h. All western blot data are representative of at least 2 independent replicates. For uncropped western blot images, see Supplementary Fig. 8.
Extended Data Fig. 2 ∣. Evaluation of JQ1-XcQ and JQ1-XcN degraders in targeted protein degradation of BRD4.
(a) Western blots of BRD4 levels after treatment of HEK293T cells with 100 nM, 1 μM, or 10 μM dBET6 for the 20 dipeptide degraders. Interestingly, the hook effect resulting in reduced degradation of BRD4 can be observed from dBET6 at 10 μM, but not from the dipeptide degraders. (b) BRD4 degradation with dBET6 over 4 h was competitively inhibited by lenalidomide or Boc-FcQ over a dose-dependent concentration (0.1–100 μM), indicating that the thalidomide-binding domain of CRBN is engaged by glutarimide ligands. (c) Levels of BRD4 over time in HEK293T cells treated with dBET6 or JQ1-FcQ. (d) Evaluation of JQ1-HcN in targeted protein degradation of BRD4 at various concentrations in HEK293T cells over 4 h. This probe was constructed given that protein splicing products (e.g., inteins) are typically promoted by a penultimate histidine. (e) Western blot of BRD4 after treatment of wild type (WT) or shRNA knockdown (CRBN KD) HEK293T cells with the indicated degrader. (f) Western blot of BRD4 after co-treatment of HEK293T cells with JQ1-FcN and lenalidomide or Boc-FcN. (g) Levels of BRD4 over time in HEK293T cells treated with JQ1-FcN. All western blot data are representative of 2 independent replicates. For uncropped western blot images, see Supplementary Fig. 8.
Extended Data Fig. 3 ∣. Co-immunoprecipitation and photo-affinity displacement assay with dipeptide degraders and ligands.
(a) Co-immunoprecipitation of endogenous BRD4 from HEK-CRBN in cells (left) or in lysates (right) after 2 h treatment with 25 μM or 1 μM of the indicated degrader. Western blot data are representative of 2 independent replicates. (b) Photo-affinity labeling displacement assay using photo-lenalidomide (pLEN) to visualize in-gel fluorescence imaging of CRBN/DDB1. Comparisons were performed using unpaired two-tailed t-tests. ns = not significant, * = p < 0.05, ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001. Data are presented as mean ± SD (n = 3 biologically independent samples). (c) Structure of photo-lenalidomide (pLEN) and schematic of photo-affinity labeling displacement assay using photo-lenalidomide (pLEN) to visualize in-gel fluorescence imaging of CRBN/DDB1. (d) Corresponding gel images after treatment of CRBN/DDB1 with 1 μM pLEN and 100 μM of the indicated competitor for 30 min prior to photo-affinity labeling and in-gel fluorescence imaging. Data shown include 3 biologically independent replicates. For uncropped western blot and gel images, see Supplementary Fig. 9.
Extended Data Fig. 4 ∣. Ternary complex formation of dipeptide ligands with CRBN and BRD4.
(a) Schematic showing the domains of CRBN, including the CULT domain with key residues for immunomodulatory drug binding highlighted, and AlphaScreen design. (b–c) AlphaScreen experiments performed with the indicated degrader compounds. The relative area under the curve reflected the cellular degradation trends, with several of the dipeptide degraders resulting in a signal greater than or comparable to dBET6. Interestingly, the inactive degraderJQ1-cQ promotes ternary complex formation equivalent to the active JQ1-GcQ, indicating that the N–1 amino acid residue positions the ternary complex in a more productive conformation. Data shown in (b) and (c) are each performed on a single 384-well plate; dBET6 was assayed on each plate as an internal control. Each condition was measured in triplicate. (d) Schematic of NanoBRET assay. (e) NanoBRET measurement of ternary complex formation induced by the indicated members of the degrader library as determined by acceptor:donor signal ratio, with background signal from no-ligand control for each degrader subtracted. Each condition was assayed in triplicate. Error bars represent mean ± SEM.
Extended Data Fig. 5 ∣. Characterization of the IMiDs and the indicated peptides in HEK293T cells and multiple myeloma MM.1S cells.
(a) Model of dipeptide degraders with CRBN built from 6BOY and 6H0F. Left: Model of JQ1-FcQ (yellow) by molecular modeling of dBET6 (orange) in the ternary complex with CRBN (grey) and BRD4 (blue). Middle: zoom-in of the CRBN binding pocket with thalidomide analog (orange), Ac-FcQ (yellow), or Ac-FcN (green). Right: Model of pomalidomide (orange), FcQ (yellow), and FcN (green) in the ternary complex with CRBN (grey) and IKZF1 (blue, space-filling mode). (b) Structures of Boc-cQ, Me-FcQ, Ac-FcQ, H2N-FcN or H2N-FcQ, Boc-FcN or Boc-FcQ, and GGGFcQ. (c–d) Quantitative proteomics of co-immunoprecipitated proteins from lysates of HEK-CRBN cells overexpressing IKZF1 after 2 h incubation with 1 μM (c) FcN or (d) GGGFcQ. Protein levels were normalized to the amount of CRBN in each channel. P-values for the abundance ratios were calculated by one-way ANOVA with TukeyHSD post-hoc test. (e) Competitive inhibition of BRD4 degradation by the indicated dipeptide degrader (JQ1-FcN, JQ1-FcQ) with the indicated compounds in HEK293T cells for 4 h. All western blot data are representative of 2 independent replicates. (f) Quantitative proteomics of MM.1S cells after treatment with 10 μM of Boc-FcN for 10 h. P-values for the abundance ratios were calculated using the t-test (background) method. (g) Protein expression levels of IKZF1 after treatment with the indicated compounds in MM.1S cells for 10 h. Data are presented as mean ± SD (n = 3 biologically independent samples). (h) Quantitative proteomics of HEK293T cells after treatment with 0.1 μM of JQ1-FcQ or dBET6 for 2 h. P-values for the abundance ratios were calculated using the t-test (background) method. (i) Protein expression levels of BRD2 and BRD4 after treatment with the indicated compounds in HEK293T cells for 2 h. Data are presented as mean ± SD (n = 3 biologically independent samples). All proteomics experiments were performed in biological triplicates. For uncropped western blot images, see Supplementary Fig. 10.
Extended Data Fig. 6 ∣. The C-terminal cyclic imide degron is transferrable.
(a-c) Flow cytometry analysis of the GFP levels in (a) HEK293T cells, (b) Jurkat or (c) MEF cells 6 h after electroporation with GFP tagged with the indicated peptide, with or without lenalidomide competition (100 μM). Comparisons were performed using an ordinary one-way ANOVA with Šídák’s multiple comparisons test, and p values are shown above comparison bars. (d) Structure of FKBP12 degraders dFKBP-1 and dFKBP-FcQ. (e) Western blot of FKBP12 levels after treatment of HEK293T cells with dFKBP-1 or dFKBP-FcQ over a 0.1–25 μM dose-response range. (f) Western blot of FKBP12 levels after co-treatment of HEK293T cells with dFKBP-FcQ and lenalidomide or Boc-FcQ. (g) Levels of FKBP12 over time in HEK293T cells treated with dFKBP-1 or dFKBP-FcQ. (h) Structure of CDK6 degraders dCDK6-Pom and dCDK6-FcQ. (i) Western blot of CDK4/6 levels after treatment of Jurkat cells with dCDK6-Pom or dCDK6-FcQ over a 0.01–10 μM dose-response range. (j) Western blot of CDK6 levels after co-treatment of Jurkat cells with dCDK6-FcQ and lenalidomide or Boc-FcQ. (k) Sortase system used to generate degron-tagged GST-FKBP12 from GST-FKBP12-LPETG-His6. (l) In vitro ubiquitination of FKBP12 tagged with C-terminal cyclic imide. FKBP12-H6 = FKBP12 with C-terminal His6 tag (no sortase treatment); FKBP12-Me = FKBP12 with C-terminal FcQMe; FKBP12-FcQ = FKBP12 with C-terminal FcQ; FKBP12-FcN = FKBP12 with C-terminal FcN. (m) Western blot of FKBP12 tagged with the indicated peptides 6 h after electroporation into HEK293T cells. (n) Quantification of Western blot in (m). Error bars represent mean ± SD. Comparisons were performed using an ordinary one-way ANOVA with Šídák’s multiple comparisons test. All western blot data are representative of at least 2 independent replicates. Flow cytometry data is representative of 3 independent replicates. For uncropped western blot images, see Supplementary Fig. 10.
Extended Data Fig. 7 ∣. The inteins Npu DnaE and Mtu RecA are not CRBN substrates.
(a) Left: hydrolysis of cyclic imides Fmoc-GGGFcQ or Fmoc-GGGFcN in PBS at 37 °C. (n = 3 biologically independent samples, error bars, which fall within symbols, represent mean ± SD). Right: hydrolysis of C-terminal cyclic imides on GFP-FcQ or GFP-FcN in PBS at 37 °C (n = 3 biologically independent samples, error bars, which fall within symbols, represent mean ± SD). (b) Schematic of the intein splicing mechanism with the penultimate step in intein excision that generates a C-terminal aspartimide highlighted, and the two intein constructs before and after splicing. X = O or S, R = H or CH3. (c) Analysis of expression and splicing of Npu DnaE in E. coli with and without a V5 tag inserted in the intein. (n = 1 biologically independent replicate) (d) In vitro ubiquitination of V5-tagged cell lysate generated in c (n = 3 biologically independent replicates, all shown). (e, f) Effect of MLN4924 or lenalidomide pretreatment on 4-hydroxytamoxifen (4-HT)-induced splicing of GFP with HA-tagged Mtu RecA intein in HEK293T cells. For both e and f, data shown are representative of 2 biologically independent replicates. For uncropped western blot images, see Supplementary Fig. 11.
Extended Data Fig. 8 ∣. Analysis of C-terminal cQ/cN modifications on hemoglobin derived from red blood cells and beta-crystallin derived from bovine lens.
(a) Unique proteins and peptides that carry a semi-tryptic terminal N or Q from global proteomics datasets. (b) Western blot of red blood cell (RBC) lysates from two donors in comparison to HEK293T lysate. RBCs do not express CRBN. (c) Representative MS2 spectra of HBB(42–cN58) and HBA(63–cN79) detected in RBC lysates. Western blot is representative of 2 independent replicates. (d) Comparison of peptide spectral matches (PSMs) for selected hemoglobin subunits and actin observed in global proteomics datasets, recombinant hemoglobin beta (HBB) protein, and red blood cell (RBC) lysates. HBA(63–cN69) was observed by extracted ion chromatogram in the MS1. Sites selected for further analysis are highlighted in red or blue. (e) Quantification of the three major peptide groups bearing a C-terminal cyclic imide from RBC samples with or without base treatment. Data are presented as mean ± SD (n = 3 biologically independent samples). The noted p-values were obtained by one-way ANOVA with TukeyHSD post-hoc test from Proteome Discoverer. (f) Quantification of the two major chymotryptic peptide groups bearing C-terminal cyclic imides in RBC samples with or without base treatment. Data are presented as mean ± SD (n = 5 biologically independent samples). The noted p-values were obtained by one-way ANOVA with TukeyHSD post-hoc test from Proteome Discoverer. (g) Quantification of the absolute masses and percentages of HBB(42–60), HBB(42–cN58), and HBB(42–N58) in RBC samples using selected ion monitoring. (h) Ion intensity chromatograms extracted for the masses of the cyclic imide fragment and the corresponding tryptic peptide for three cyclic imide-bearing peptide groups identified in bovine lens. (i) Quantification of these peptide groups validates the sensitivity of the cyclic imide modifications to base treatment. Data are presented as mean ± SD (n = 4 biologically independent samples). The noted p-values were obtained by one-way ANOVA with TukeyHSD post-hoc test from Proteome Discoverer. For uncropped western blot images, see Supplementary Fig. 11.
Extended Data Fig. 9 ∣. Analysis of C-terminal cQ/cN and Q/N modifications on synthetic peptides.
(a) Scheme of cyclic imide formation in a peptide and subsequent hydrolysis to afford the truncated C-terminal glutamine or asparagine fragments. (b) Overlay of representative extracted ion chromatograms of each peptide at the masses corresponding to parent peptide (black), cyclic imide fragment (red), and its hydrolyzed products (blue). The two constitutional isomers formed via hydrolysis of the cyclic imide fragment were not distinguished in our study. (c–d) In vitro time course for formation of the cyclic imide fragment and the hydrolysis products at the indicated position on the synthetic peptide after incubation. (e) Percentage of the formed cyclic imide fragment and the hydrolysis products at the indicated residue relative to the parent synthetic peptide at different pH. (f) Ion intensity chromatogram extracted for the masses of the corresponding species for RBC lysates and the mixture of isotopically labeled HBB(42*–60) containing the modifications. The RBC samples were spiked with the peptide mixture and ran on the selected ion monitoring mode to validate the overlap of retention times and amplify the signal of selected ions.
Extended Data Fig. 10 ∣. Analysis of substrates bearing C-terminal cQ/cN and Q/N modifications in vitro and in cells.
(a) Schematic of expressed protein ligation using HBB(1–129) thioester and Sec-YcQ or Sec-YcQMe to generate HBB(1–cQ132) or HBB(1–Me132). (b) Western blot of in vitro ubiquitination of HBB proteins in triplicate. Unfortunately neither the HBB[1–cQ132] nor recombinant HBB was amenable to electroporation, which precluded our ability to evaluate CRBN-dependent degradation in cells. (c) TMT-based quantification of K-ε-GG sites identified from the in vitro ubiquitination samples using mass spectrometry. Data are presented as mean ± SD (n = 4 technical replicates). Comparisons were performed using unpaired two-tailed t-tests and p-values are noted. (d) Volcano plots of peptide groups bearing C-terminal cyclic imides in WT HEK293T or CRBN CRISPR/Cas9 knockout HEK293T over 48 h. Upregulated proteins at 1% FDR = red, 5% FDR = pink. ACTB(96–cN111) peptide = blue. P-values for the abundance ratios were calculated by one-way ANOVA with TukeyHSD post-hoc test. (e) Volcano plots of peptide groups bearing C-terminal glutamine or asparagine (protein terminus excluded) in WT HEK293T, CRBN KO HEK293T, or HEK293T treated with 200 μM lenalidomide over 48 h. Upregulated peptide groups at 1% FDR = red, 5% FDR = pink, consistent with a model where blockade of degradation of substrates bearing the C-terminal imide allows the modifications to be hydrolyzed. P-values for the abundance ratios were calculated by one-way ANOVA with TukeyHSD post-hoc test. (f) Volcano plots of peptide groups bearing C-terminal glutamine or asparagine (protein terminus excluded) in MM.1S treated with DMSO or 200 μM lenalidomide over 48 h. Upregulated peptide groups at 1% FDR = red, 5% FDR = pink. P-values for the abundance ratios were calculated by one-way ANOVA with TukeyHSD post-hoc test. (g) Ion intensity chromatogram extracted for the masses of the corresponding species for HEK293T lysates and the mixture of isotopically labeled ACTB(96*–113) containing the modifications. The cell samples were spiked with the peptide mixture and ran on the selected ion monitoring mode to validate the overlap of retention times and amplify the signal of selected ions. For uncropped western blot images, see Supplementary Fig. 11.
Supplementary Material
Acknowledgements
We thank Y. Amako, D. Miyamoto and A. D’Souza for helpful discussions; S. Trager, B. Budnik, R. Robinson and Z. Niziolek for technical support; and the Agilent Center of Excellence in Biomolecular Characterization for instrumentation. His6–CRBN–DDB1 was a gift from Bristol Myers Squibb. Some data used in this publication were generated by the Clinical Proteomic Tumor Analysis Consortium (NCI/NIH). Support from the Ono Pharma Foundation (C.M.W.), Sloan Research Foundation (C.M.W.), Camille–Dreyfus Foundation (C.M.W.), the Blavatnik Biomedical Accelerator at Harvard University (C.M.W.), the National Institutes of Health (R01GM114537, M.R.P.), Japan Society for the Promotion of Science (S.I.) and National Science Foundation (H.A.F.) is gratefully acknowledged.
Footnotes
Competing interests Harvard University filed a PCT patent application on 13 April 2022 covering the chemical structures described in this Article and their use. C.M.W., S.I., H.A.F. and W.X. are listed as inventors on this patent. The Woo laboratory receives support from Merck and Ono Pharmaceuticals. All other authors declare no competing interests.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41586-022-05333-5.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41586-022-05333-5.
Data availability
All data generated or analysed during this study are available in the main text or the Supplementary Information. Uncropped, full western blot images and gels are provided in Supplementary Figs. 6-12. Proteomics data have been deposited to the PRIDE repository with the dataset identifiers PXD025413, PXD030091 and PXD034248. Source data are provided with this paper.
References
- 1.Ito T et al. Identification of a primary target of thalidomide teratogenicity. Science 327, 1345–1350 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Krönke J et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343, 301–305 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu G et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of ikaros proteins. Science 343, 305–309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krönke J et al. Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS. Nature 523, 183–188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winter GE et al. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 348, 1376–1381 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu J et al. Hijacking the E3 ubiquitin ligase cereblon to efficiently target BRD4. Chem. Biol 22, 755–763 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowak RP et al. Plasticity in binding confers selectivity in ligand-induced protein degradation. Nat. Chem. Biol 14, 706–714 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donovan KA et al. Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane radial ray syndrome. eLife 7, e38430 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matyskiela ME et al. SALL4 mediates teratogenicity as a thalidomide-dependent cereblon substrate. Nat. Chem. Biol 14, 981–987 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Asatsuma-Okumura T et al. p63 is a cereblon substrate involved in thalidomide teratogenicity. Nat. Chem. Biol 15, 1077–1084 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Lupas AN, Zhu H & Korycinski M The thalidomide-binding domain of cereblon defines the CULT domain family and is a new member of the β-tent fold. PLoS Comput. Biol 11, e1004023 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JJ, Pucilowska J, Lombardi RQ & Rooney JP A mutation in a novel ATP-dependent Lon protease gene in a kindred with mild mental retardation. Neurology 63, 1927–1931 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachmair A, Finley D & Varshavsky A In vivo half-life of a protein is a function of its amino-terminal residue. Science 234, 179–186 (1986). [DOI] [PubMed] [Google Scholar]
- 14.Koren I et al. The eukaryotic proteome is shaped by E3 ubiquitin ligases targeting C-terminal degrons. Cell 173, 1622–1635.e1614 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maxwell PH et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Gray WM, Kepinski S, Rouse D, Leyser O & Estelle M Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 414, 271–276 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Fischer ES et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 512, 49–53 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Prete D, Rice RC, Rajadhyaksha AM & D’Adamio L Amyloid precursor protein (APP) may act as a substrate and a recognition unit for CRL4CRBN and Stub1 E3 ligases facilitating ubiquitination of proteins involved in presynaptic functions and neurodegeneration. J. Biol. Chem 291, 17209–17227 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamberlain PP et al. Structure of the human Cereblon–DDB1–lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat. Struct. Mol. Biol 21, 803–809 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Hartmann MD et al. Thalidomide mimics uridine binding to an aromatic cage in cereblon. J. Struct. Biol 188, 225–232 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Boichenko I, Deiss S, Bar K, Hartmann MD & Hernandez Alvarez B A FRET-based assay for the identification and characterization of cereblon ligands. J. Med. Chem 59, 770–774 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Schilling S et al. Glutaminyl cyclase inhibition attenuates pyroglutamate Aβ and Alzheimer’s disease-like pathology. Nat. Med 14, 1106–1111 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Geiger T & Clarke S Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J. Biol. Chem 262, 785–794 (1987). [PubMed] [Google Scholar]
- 24.Voorter CE, de Haard-Hoekman WA, van den Oetelaar PJ, Bloemendal H & de Jong WW Spontaneous peptide bond cleavage in aging alpha-crystallin through a succinimide intermediate. J. Biol. Chem 263, 19020–19023 (1988). [PubMed] [Google Scholar]
- 25.Tyler-Cross R & Schirch V Effects of amino acid sequence, buffers, and ionic strength on the rate and mechanism of deamidation of asparagine residues in small peptides. J. Biol. Chem 266, 22549–22556 (1991). [PubMed] [Google Scholar]
- 26.Brennan TV & Clarke S Effect of adjacent histidine and cysteine residues on the spontaneous degradation of asparaginyl- and aspartyl-containing peptides. Int. J. Pept. Protein Res 45, 547–553 (1995). [DOI] [PubMed] [Google Scholar]
- 27.Paulus H Protein splicing and related forms of protein autoprocessing. Annu. Rev. Biochem 69, 447–496 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Mills KV, Manning JS, Garcia AM & Wuerdeman LA Protein splicing of a Pyrococcus abyssi intein with a C-terminal glutamine. J. Biol. Chem 279, 20685–20691 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Shah NH & Muir TW Inteins: natur’s gift to protein chemists. Chem. Sci 5, 446–461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakamoto KM et al. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl Acad. Sci. USA 98, 8554–8559 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Nguyen T et al. Glutamine triggers acetylation-dependent degradation of glutamine synthetase via the thalidomide receptor cereblon. Mol. Cell 61, 809–820 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith BE et al. Differential PROTAC substrate specificity dictated by orientation of recruited E3 ligase. Nat. Commun 10, 131 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin Z et al. Development of photolenalidomide for cellular target identification. J. Am. Chem. Soc 144, 606–614 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Sievers QL et al. Defining the human C2H2 zinc finger degrome targeted by thalidomide analogs through CRBN. Science 362, eaat0572 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li M et al. Discovery and characterization of a peptide that enhances endosomal escape of delivered proteins in vitro and in vivo. J. Am. Chem. Soc 137, 14084–14093 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Jiang B et al. Development of dual and selective degraders of cyclin-dependent kinases 4 and 6. Angew. Chem. Int. Ed 58, 6321–6326 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang B et al. Proteogenomic characterization of human colon and rectal cancer. Nature 513, 382–387 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mertins P et al. Proteogenomics connects somatic mutations to signalling in breast cancer. Nature 534, 55–62 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen TW et al. APOBEC3A is an oral cancer prognostic biomarker in Taiwanese carriers of an APOBEC deletion polymorphism. Nat. Commun 8, 465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark DJ et al. Evaluation of NCI-7 cell line panel as a reference material for clinical proteomics. J. Proteome Res 17, 2205–2215 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao Q et al. Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma. Cell 179, 561–577.e522 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Clark DJ et al. Integrated proteogenomic characterization of clear cell renal cell carcinoma. Cell 179, 964–983.e931 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang LB et al. Proteogenomic and metabolomic characterization of human glioblastoma. Cancer Cell 39, 509–528 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bryk AH & Wisniewski JR Quantitative analysis of human red blood cell proteome. J. Proteome Res 16, 2752–2761 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Takata T, Oxford JT, Demeler B & Lampi KJ Deamidation destabilizes and triggers aggregation of a lens protein, βA3-crystallin. Protein Sci. 17, 1565–1575 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilmarth PA et al. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J. Proteome Res 5, 2554–2566 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim E, Lowenson JD, MacLaren DC, Clarke S & Young SG Deficiency of a protein-repair enzyme results in the accumulation of altered proteins, retardation of growth, and fatal seizures in mice. Proc. Natl Acad. Sci. USA 94, 6132–6137 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ludwig KR, Schroll MM & Hummon AB Comparison of in-solution, FASP, and S-Trap based digestion methods for bottom-up proteomic studies. J. Proteome Res 17, 2480–2490 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.HaileMariam M et al. S-Trap, an ultrafast sample-preparation approach for shotgun proteomics. J. Proteome Res 17, 2917–2924 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Huber W, von Heydebreck A, Sultmann H, Poustka A & Vingron M Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics 18, S96–104 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Chen I, Dorr BM & Liu DR A general strategy for the evolution of bond-forming enzymes using yeast display. Proc. Natl Acad. Sci. USA 108, 11399–11404 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Broguiere N, Formica FA, Barreto G & Zenobi-Wong M Sortase A as a cross-linking enzyme in tissue engineering. Acta Biomater. 77, 182–190 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Flaxman HA, Chang CF, Wu HY, Nakamoto CH & Woo CM A binding site hotspot map of the FKBP12–rapamycin–FRB ternary complex by photoaffinity labeling and mass spectrometry-based proteomics. J. Am. Chem. Soc 141, 11759–11764 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Popp MW, Antos JM & Ploegh HL Site-specific protein labeling via sortase-mediated transpeptidation. Curr. Protoc. Protein Sci 10.1002/0471140864.ps1503s56 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salehi N et al. Heme degradation upon production of endogenous hydrogen peroxide via interaction of hemoglobin with sodium dodecyl sulfate. J. Photochem. Photobiol. B 133, 11–17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carbon J & Curry JB Genetically and chemically derived missense suppressor transfer RNA’s with altered enzymic aminoacylation rates. J. Mol. Biol 38, 201–216 (1968). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are available in the main text or the Supplementary Information. Uncropped, full western blot images and gels are provided in Supplementary Figs. 6-12. Proteomics data have been deposited to the PRIDE repository with the dataset identifiers PXD025413, PXD030091 and PXD034248. Source data are provided with this paper.