Abstract
Background
Focal segmental glomerulosclerosis (FSGS) is frequently associated with heavy proteinuria and progressive renal failure requiring dialysis or kidney transplantation. However, primary FSGS also has a ~40% risk of recurrence of disease in the transplanted kidney (rFSGS). Multiple circulating factors have been identified to contribute to the pathogenesis of primary and rFSGS including soluble urokinase-type plasminogen activator receptor (suPAR) and patient-derived CD40 autoantibody (CD40autoAb). However, the downstream effector pathways specific to individual factors require further study. The tumor necrosis factor, TNF pathway activation by one or more circulating factors present in the sera of patients with FSGS has been supported by multiple studies.
Methods
A human in vitro model was used to study podocyte injury measured as the loss of actin stress fibers. Anti-CD40 autoantibody was isolated from FSGS patients (recurrent and non-recurrent) and control patients with ESRD due to non-FSGS related causes. Two novel human antibodies—anti-uPAR (2G10) and anti-CD40 antibody (Bristol Meyer Squibb, 986090) were tested for their ability to rescue podocyte injury. Podocytes treated with patient derived antibody were transcriptionally profiled using whole human genome microarray.
Results
Here we show that podocyte injury caused by sera from FSGS patients is mediated by CD40 and suPAR and can be blocked by human anti-uPAR and anti-CD40 antibodies. Transcriptomic studies to compare the molecules and pathways activated in response to CD40 autoantibody from rFSGS patients (rFSGS/CD40autoAb) and suPAR, identified unique inflammatory pathways associated with FSGS injury.
Conclusions
We identified several novel and previously described genes associated with FSGS progression. Targeted blockade of suPAR and CD40 pathways with novel human antibodies showed inhibition of podocyte injury in FSGS.
Keywords: CD40, soluble urokinase-type plasminogen activator receptor (suPAR), focal segmental glomerulosclerosis (FSGS), autoantibody, kidney transplantation
Highlight box.
Key findings
• We have identified two potential therapeutic molecules for rFSGS treatment—a human anti-uPAR antibody (2G10) and a humanized anti-CD40 blocking antibody (Bristol Meyer Squibb, 986090) that reverse podocyte injury associated with FSGS in cultured podocytes.
• We identified genes and transcriptional pathways specific to podocyte injury from patient-derived CD40 autoantibodies (rFSGS/CD40autoAb) and suPAR.
What is known and what is new?
• Several studies have suggested that multiple circulating factors may contribute to the progression and recurrence of FSGS and our study supports this view.
• We have identified novel molecules uniquely associated with two proposed circulating factors, rFSGS/CD40autoAb and suPAR.
What is the implication, and what should change now?
• The study suggests that targeting unique pathways for FSGS in a patient specific manner might be beneficial. We also identify two new antibodies that can be further tested in pre-clinical and clinical models for treatment of FSGS.
Introduction
Focal segmental glomerulosclerosis (FSGS) is the most common primary glomerular disorder causing end-stage renal disease in the United States (1). It is frequently associated with severe proteinuria (>3.5 g/day in adults and >1 g/day in children), scarring of the glomerulus and loss of terminally differentiated podocytes. The etiology of FSGS is diverse including genetic mutations in podocyte genes, adverse drug interactions, toxic insults and viral infections. However, in the majority of FSGS cases, the etiology remains unknown (idiopathic). Whatever the cause, the injury is primarily directed at the podocytes (2). Podocytes are highly differentiated cells of the renal glomerulus consisting of a cell body, major processes and foot processes (FPs). FPs form a characteristic interdigitating pattern with FPs of neighboring podocytes, leaving in between the filtration slits that are bridged by the glomerular slit diaphragm (3). The slit diaphragm and the apical and basal membrane domains of podocytes are connected to each other by a dynamically regulated actin-based cytoskeleton, which is critical for the maintenance of the glomerular filtration barrier. It is reported that idiopathic FSGS results from disorganization of the podocyte cytoskeleton and slit diaphragm with consequent foot process effacement, podocyte hypertrophy, detachment from the glomerular basement membrane (GBM) and loss with migration into the Bowman space (4). Idiopathic FSGS recurs after transplant in about 40% of adult and pediatric patients, in some cases within a few hours or days after kidney transplantation (5). These clinical observations have given rise to the idea that FSGS is associated with circulating factors generated after cellular or humoral immune reactions. The first experimental evidence of a circulating factor was provided in a seminal study demonstrating that sera from patients with FSGS increased glomerular permeability to albumin in an in vitro model of glomerular permeability using isolated rat glomeruli (6). Since then, further studies from several groups have shown that sera from FSGS patients causes loss of stress fibers and activation of β3 integrin and sites of focal adhesion (7,8). Microarray studies have also demonstrated that genes associated with cytokine signaling and apoptosis are activated in podocytes exposed to sera from FSGS patients (9,10).
Some of the proposed circulating factors that may increase glomerular permeability are soluble urokinase-like plasminogen activator receptor (suPAR), cardiotrophin-like-cytokine-1 (CLC-1), vascular permeability factor (VPF) and hemopexin (11). We recently described a panel of autoantibodies in sera from FSGS patients to predict the risk of recurrence after transplant (7). Furthermore, we showed interaction between two proposed circulating factors in augmenting podocyte injury in vitro and in vivo. Administration of suPAR and anti-CD40 antibodies isolated from rFSGS patients (rFSGS/CD40autoAb) caused an increase in proteinuria in mice and enhanced podocyte injury in an in vitro cell culture model of immortalized human podocytes (7,12). Our study suggested that anti-uPAR and anti-CD40 therapies may be effective in slowing down or reversing the renal injury leading to recurrence after transplant.
CD40 is a costimulatory molecule of the tumor necrosis factor (TNF) receptor (TNFR) superfamily. CD40 and its ligand (CD40L/CD154) play a fundamental role in both humoral and cell-mediated immunity (13). CD40 is constitutively expressed on professional antigen-presenting cells (B cells, dendritic cells, and macrophages) and can be induced on several non-hematopoietic cells including the parenchymal cells of the kidney (14). CD40L is expressed mainly by activated T cells and platelets but can also be secreted into the blood by platelets as a soluble protein (sCD40L) (15). A role for CD40/CD40L signaling in the development of proteinuria in various disease settings has been demonstrated. sCD40L has been shown to directly act on podocytes and cause loss of nephrin in culture. Additionally, sCD40L increases albumin permeability in isolated rat glomeruli that can be inhibited by pre-treatment with inhibitors of CD40/CD40L interaction (16). Recently, the efficacy of an anti-mouse CD40 antagonist antibody in reversal of proteinuria in two Systemic Lupus Erythematosus (SLE)-prone mouse strains was demonstrated (14).
The urokinase plasminogen activator receptor (uPAR) is a membrane-bound receptor for urokinase plasminogen activator (uPA) which regulates plasminogen-mediated extracellular proteolysis. uPAR has three domains and suPAR represents a soluble form of uPAR (17). uPAR is expressed on several cell types such as active leukocytes, endothelial cells and podocytes. The role of suPAR in the development of FSGS has been extensively studied. In cultured podocytes, membrane-bound uPAR binds and activates αvβ3 integrin and small GTPases Rac1 and Cdc42 promoting motility (18). Similarly, suPAR binds and activates β3 integrin in podocytes and high doses of suPAR in mice cause proteinuria (8). This podocytopathic effect of suPAR is dependent upon its ability to bind and activate β3 integrin as no proteinuria and foot process effacement was observed in mice expressing suPAR that is incapable of β3 integrin binding (sPlaurE134A) (8). A recent Mendelian randomization links suPAR as a causal mediator of kidney and heart disease (19).
In this study we have tested and confirmed the ability of two novel humanized antibodies—anti-uPAR (2G10) and anti-CD40 (kindly provided by Bristol Meyer Squibb, 986090), to reverse podocyte injury in an in vitro model of cultured human podocytes. 2G10, was isolated from a fragment-antigen binding (Fab) phage display library using soluble, recombinant human uPAR as the antigen to biopan the library (20). This antibody competes with uPA for uPAR binding to inhibit cell invasion in vitro and in a preclinical model of aggressive breast cancer (21,22). BMS-986090 is a dimeric anti-human CD40 VH antagonist domain antibody formatted with a human IgG4 Fc tail (dAb-huIgG4), and an antagonist for CD40-CD40L mediated signaling. We showed that anti-uPAR and anti-CD40 antibodies are both efficacious in inhibiting podocyte injury caused by sera from different rFSGS patients, highlighting their role in FSGS pathogenesis. In addition, these results also suggest that multiple factors might contribute to and/or synergize in the development and/or maintenance of podocyte injury in primary FSGS. A combination of one or more circulating factors in FSGS has been proposed as triggers for the early activation of the TNF pathway resulting in podocyte injury (9). Elevated serum TNF levels have been described in patients with nephrotic syndrome (23) and a beneficial role for TNF suppression has been observed in a subset of pediatric FSGS patients (24). While glomerular TNF but not serum TNF is associated with loss of eGFR, activation of the TNF pathway in podocytes treated with sera from FSGS patients has been demonstrated repeatedly (25,26). We have performed global transcriptomic profiling of podocytes treated with two proposed circulating factors in FSGS- rFSGS/CD40autoAb, suPAR and combination of rFSGS/CD40autoAb with suPAR and compared them to TNF treated podocytes to identify common and unique pathways for these different podocytopathic factors, and their potential contribution to FSGS progression and recurrence. We present this article in accordance with the MDAR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3670/rc).
Methods
Collection of patient sera
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of University of California San Francisco (No. 14-13573) and informed consent was taken from all the patients. Sera from FSGS patients, healthy individuals and patients with end-stage renal disease due to non-FSGS related causes (non-FSGS) were collected. FSGS patients were further characterized as primary FSGS, based on the exclusion of causes of secondary FSGS, and biopsy confirmed diagnosis of FSGS recurrence after transplantation, together with clinical FSGS recurrence with proteinuria, edema and hypoalbuminemia (1). The demographic information for all the patients is provided in Table S1.
Measurement of suPAR, anti-CD40 antibody and total IgG content in serum
Anti-CD40 antibody in sera was measured using a previously optimized Meso Scale Discovery platform (7). Briefly, patient serum was added to a 96-well MULTI-ARRAY plate (Meso Scale Discovery) coated with CD40 protein (Abcam) and allowed to bind at room temp for 2hr. After washing, SULFO-tag anti-human secondary antibody (MSD) was added, and the plate was read on a MESO Quickplex imager (Meso Scale Discovery, Rockville, MD, USA). suPAR levels were measured using a commercial quantikine ELISA kit (R&D Systems). Total IgG in human sera was measured using a commercial human IgG ELISA kit (Fisher Scientific) according to manufacturer’s protocol.
Cell culture and treatment with anti-uPAR or anti-CD40 antibodies
Immortalized human podocytes have been previously described (27). Briefly, primary human podocytes were immortalized by transfection with the temperature sensitive SV40 T gene. These cells proliferate at the “permissive” temperature (33 ℃) and are considered undifferentiated. After transferring to the “nonpermissive” temperature (37 ℃), they enter growth arrest and by day 10-14 express markers of differentiated podocytes in vivo, such as nephrin, podocin, CD2 associated protein (CD2AP), synaptopodin, and known molecules of the slit diaphragm ZO-1, α, β, and γ-catenin, and P-cadherin. The podocytes were cultured in RPMI medium supplemented with insulin, transferrin, selenium, sodium pyruvate (ITS-A, Gibco #513000), 10% FBS and penicillin/streptomycin. After differentiation, cells were either left untreated or treated with 4% sera from patients for 24 hours. For rescue of stress fibers, podocytes were treated with patient sera in the presence of a control antibody (human IgG, Invitrogen #02-7102) or anti-uPAR antibody (2G10) or anti-CD40 antibody (BMS, 986090) at 1 µg/mL for 24 h. The 2G10 antibody was prepared as previously described (20). Following treatment, podocytes were fixed in 4% paraformaldehyde (PFA)/Sucrose, permeabilized with 0.3% Triton X-100 and stained with rhodamine-conjugated phalloidin to visualize stress fibers. Cells were imaged by Lecia SP5 confocal microscopy and number of cells with intact stress fibers was counted.
Podocyte treatment with proposed circulating factors
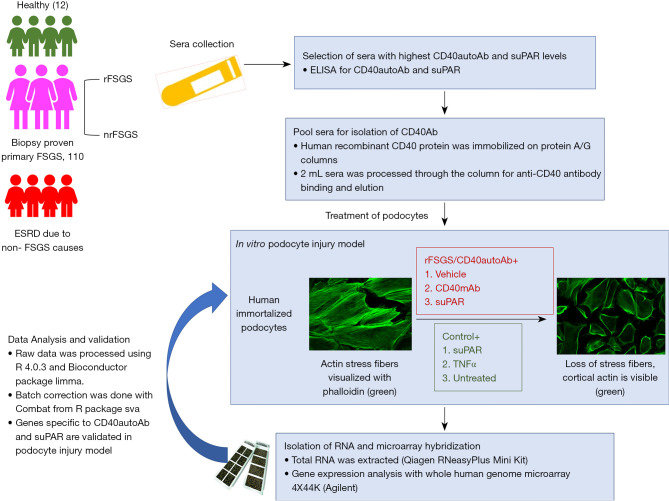
Immortalized podocytes were cultured and differentiated as described above. Primary podocytes were isolated from healthy renal nephrectomy tissue as described previously (27). CD40Ab was purified from pooled plasma of rFSGS patients (rFSGS/CD40autoAb). Differentiated podocytes were treated with patient purified CD40Ab alone (10 µg/mL) or together with suPAR (1 µg/mL) or a commercially available mouse monoclonal CD40Ab (R&D mAb, 5 µg/mL) for 24 h. Podocytes were also treated with suPAR alone (1µg/mL), TNFα (0.1 µg/mL) for 24 h. Figure 1 outlines the study design.
Figure 1.
Study design for the transcriptomic profiling of podocytes. Serum/plasma was collected from ESRD patients with biopsy proven primary FSGS or ESRD due to non-FSGS causes. Primary FSGS group included patients that underwent biopsy proven rFSGS and patients that did not recur after renal transplantation (nrFSGS). Sera from healthy individuals was collected as control. Levels of CD40autoAb and suPAR were measured with ELISA and rFSGS patients with highest CD40autoAb levels were selected for further experiments. For the transcriptomic profiling, CD40autoAb was purified from pooled sera of rFSGS patients with highest autoantibody levels (rFSGS/CD40autoAb). Differentiated podocytes in culture were treated with rFSGS/CD40autoAb (10 µg/mL) in the presence of suPAR (1 µg/mL), or commercial mouse monoclonal anti CD40 antibody (CD40mAb, R&D Tech, 5 µg/mL) or suPAR alone, TNF alone or left untreated. RNA was extracted according to the manufacturer’s protocol and subjected to Agilent 44k microarray. For validation, differentiated human podocytes in culture were treated with sera from patients or healthy control and loss of stress fibers was rescued with novel blocking antibodies against uPAR and CD40. ESRD, end-stage renal disease; FSGS, focal segmental glomerulosclerosis; rFSGS, recurrence of FSGS; nrFSGS, nonrecurrent FSGS; CD40autoAb, anti-CD40 autoantibody; suPAR, soluble urokinase-type plasminogen activator receptor; ELISA, enzyme-linked immunosorbent assay; TNF, tumor necrosis factor.
RNA isolation and microarray hybridization
RNA was extracted from podocytes using the RNeasyPlus Mini Kit (Qiagen) following manufacturer’s instructions. RNA integrity was assessed spectrophotometrically using NanoDrop (Thermo Scientific) and ensuring that the 260/280 ratio was approximately 2.0. A whole Human Genome Microarray 4X44K (Agilent Technologies) was used for gene expression analysis. Images were scanned with a dual-laser microarray scanner (Agilent C, Agilent Technologies) and data was extracted using Feature Extraction (FE) software (Agilent Technologies).
Data processing
The raw data were processed using R language version 4.0.3 (28) and the Bioconductor (29) package limma (30). Processing steps included background correction, log2-transformation, and Loess normalization. The cell culture source and day of microarray hybridization were recognized as a source of non-biological data variability using principal component analysis (PCA). The combat approach from the R package sva (31) was used to eliminate the technical factors.
Differential gene expression analysis
The differential gene expression analysis was performed using the R package limma (30). The cell culture source and microarray hybridization date were used as covariates in the linear model.
Pathway analysis
The gene sets were analyzed for enrichment in Gene Ontology biological pathways using the R package clusterProfiler (32). Genes with more than 1.2 log fold change were included in the analysis and enriched pathways with q-value <0.05 were accepted as significant.
Statistical analysis
Statistical analyses for all studies except microarray were conducted using GraphPad Prism version 9.0.2 for Windows, GraphPad Software, San Diego, CA, USA. Number of stress fiber positive podocytes were compared between groups by one-way ANOVA followed by Dunnett’s multiple comparisons test. For microarray studies, differentially expressed genes were identified using a P value less than 0.05 adjusted with the Benjamini-Hochberg multiple testing correction approach and >1.2-fold change.
Results
suPAR and anti-CD40 antibody levels are higher in pre transplant sera from rFSGS patients
suPAR, anti-CD40 antibody and total human IgG concentration in sera of each patient were measured as described and the values are provided in Table 1. Both suPAR and anti-CD40Ab levels were at least two-fold higher in each patient as compared to that in healthy individuals as reported in a published study (33), and data from our group (Rashmi et al., unpublished results).
Table 1. Soluble urokinase-type plasminogen activator receptor (suPAR) and CD40 auto antibody (CD40autoAb) levels in sera from focal segmental glomerulosclerosis (FSGS) patients with biopsy proven recurrence after kidney transplant.
| Circulating factor | Patient 1 | Patient 2 | Patient 3 | Mean level reported in healthy individuals |
|---|---|---|---|---|
| suPAR (ng/mL) | 5.17 | 8.934 | 6.806 | 2.1 |
| CD40 (ng/μL) | 563.03 | 1,041.89 | 980.55 | 214.1 |
| Total IgG (ng/μL) | 5,253.69 | 6,208.20 | 11,626.74 | 8,324.01 |
| CD40 /IgG | 0.11 | 0.17 | 0.08 | 0.02 |
Co-treatment with anti-uPAR antibody or anti-CD40 antibody inhibits podocyte injury caused by sera from rFSGS patients
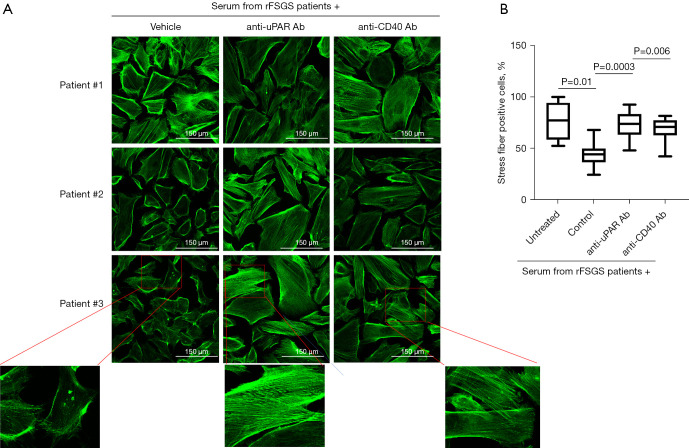
Differentiated podocytes were treated with sera (4% final concentration in media) from patients with rFSGS after kidney transplantation (n=3) and controls with non-recurrent FSGS (nrFSGS, n=1) and end stage renal disease due causes other than FSGS (non-FSGS, n=1) for 24 hours. The podocyte actin cytoskeleton was visualized by labeling with rhodamine-conjugated phalloidin as described (methods). Cells were imaged by confocal microscopy and the number of cells with intact stress fibers were counted. As shown in Figure S1, sera from rFSGS but not controls (nrFSGS and non-FSGS patients), caused a 50% reduction in the percentage of cells positive for stress fiber. Next, differentiated podocytes were treated as above with sera from rFSGS patients along with a human IgG for control or anti-CD40 antibody (Bristol Meyer Squibb, 986090) or anti-uPAR antibody (2G10) and stress fiber positive cells were counted as before. As shown in Figure 2, 2G10 was able to rescue the loss of stress fibers in all rFSGS sera treated podocytes, restoring podocyte morphology to that seen in untreated/control sera treated cells. Similarly, the number of stress fiber positive cells upon co-treatment with anti-CD40 antibody and sera from rFSGS patients, was higher than podocyte treatment with patient serum alone (81.1±10.9 vs. 53.4±12.2). Control human IgG had no effect on the number of stress fiber positive cells (Figure S1). These results support a role for CD40 and uPAR signaling in the podocytopathy of rFSGS.
Figure 2.
Blocking anti CD40 and anti uPAR antibodies rescue the actin depolarization in podocytes induced by sera from FSGS patients with recurrence of FSGS after renal transplant (rFSGS). (A) Representative images displaying stress fibers in cultured human podocytes stained with rhodamine conjugated phalloidin (green) after treatment with pre-transplant sera from three rFSGS patients. Podocyte were cultured in the presence of sera (4% final concentration) with or without pre-treatment with anti uPAR or anti CD40 blocking antibody. (B) Quantification of stress fiber positive podocytes (minimum 100 cells per condition were counted). Treatment with sera from rFSGS patients caused significant depolarization of stress fibers as determined by number of stress fiber positive cells (30%, 59% and 49% reduction with respected to untreated podocytes respectively). Pre-treatment of podocytes with novel blocking anti CD40 antibody or anti uPAR antibody (1 µg/mL) before addition of patient sera rescued stress fibers. FSGS, focal segmental glomerulosclerosis; rFSGS, recurrence of FSGS.
Identification of genes and pathways dysregulated in response to specific circulating factors
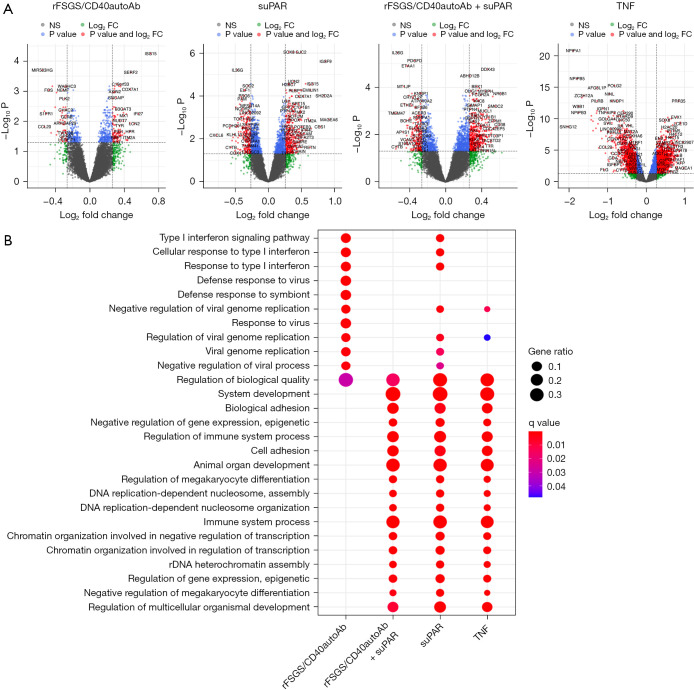
Recently it has been proposed that regardless of the nature of one or more circulating factors present in the serum of patients with FSGS, the renal intrinsic activation of the TNF pathway contributes to disease pathogenesis and/or progression of FSGS (25). We have previously shown that suPAR and anti-CD40 autoantibody isolated from FSGS patients (rFSGS/CD40autoAb) alone or in combination can cause podocyte injury in vitro as well as in mice. However, the injury was more pronounced in mice when suPAR and rFSGS/CD40autoAb were used in combination (7). Therefore, we sought to deconvolute the pathways and markers associated with each of the proposed circulating factors by microarray analysis. Figure S2 shows that the podocyte injury response to rFSGS/CD40autoAb is CD40 signaling specific as it can be reversed by a commercial monoclonal anti-CD40 antibody (CD40mAb) that blocks CD40 signaling. We found differential expression of 645 genes in response to treatment with patient derived rFSGS/CD40autoAb. Upregulated genes such as ISG15, IFI27 and LCN2 (Figure 3A) are involved in the regulation several pathways including type I interferon signaling pathway, cytokine-mediated signaling pathway as well as endothelial and epithelial cell migration (Figure 3B). suPAR treatment alone resulted in the differential expression of 1,737 genes primarily involved in the negative regulation of megakaryocyte development, cell surface receptor signaling and DNA replication-dependent nucleosome assembly among others (Figure 3A,3B). This is consistent with a major role for suPAR in the platelet biology. Combined administration of patient-derived rFSGS/CD40autoAb and suPAR resulted in 1,783 gene expression changes. The gene enrichment showed regulation of megakaryocyte differentiation consistent with suPAR treatment. However, the addition of rFSGS/CD40autoAb did result in additional transcriptional changes (FAP, VIT, ITGA7 among others) leading to pathways associated with cell adhesion (Figure 3). The largest set of transcriptionally altered genes was observed in this TNF-treated group (5,094 genes) presumably due a more widespread effect of TNF treatment, but many of these pathways were also observed with rFSGS/CD40autoAb and suPAR treatment. The significant pathways associated with TNF treatment were broadly associated with the regulation of the immune system. In addition, TNF also induced the expression of APOC1, APOE, PLA2G2A and APOA1 associated with protein-lipid complex remodeling (Figure 3A,3B). A role for renal TNF in lipid dependent podocyte injury has been previously described (25). Website: https://cdn.amegroups.cn/static/public/atm-22-3670-1.xlsx provides a comprehensive list of all differentially expressed genes in response to each stimulus.
Figure 3.
Identification of molecular pathways activated in podocytes exposed to various stimuli. Microarray was performed on RNA isolated from cultured human podocytes exposed to anti-CD40 autoantibody derived from FSGS patients with recurrence of FSGS after kidney transplant (rFSGS/CD40autoAb), suPAR, a combination of suPAR and rFSGS/CD40autoAb or TNF, as described in materials and methods, was conducted. Raw data was processed using R language version 4.0.3 to obtain gene expression profiles after each treatment followed by differential gene expression and gene expression KEGG pathway analysis. (A) For each treatment volcano plot of significantly altered genes with greater than 1.2-fold change compared to untreated are depicted. (B) Results of Gene set enrichment in Gene Ontology Pathways. Differential gene analysis was performed using R package limma (2) with a P value 0.05 adjusted with Benjamini-Hochberg multiple testing correction approach. NS, genes with P value not significant. suPAR, soluble urokinase-type plasminogen activator receptor; TNF, tumor necrosis factor.
Identification of genes and pathways uniquely activated by CD40Ab and suPAR
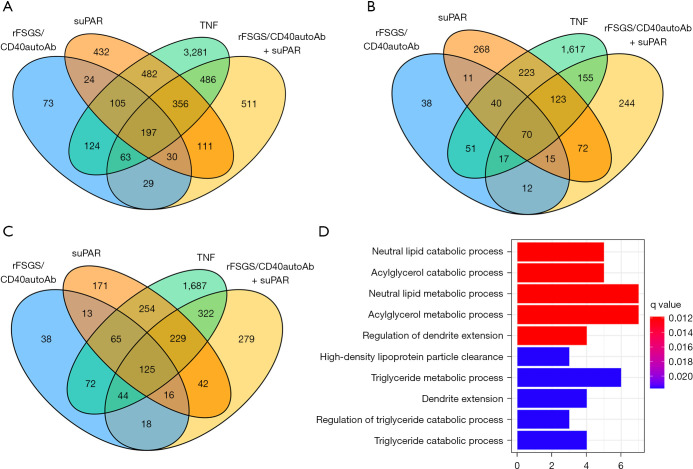
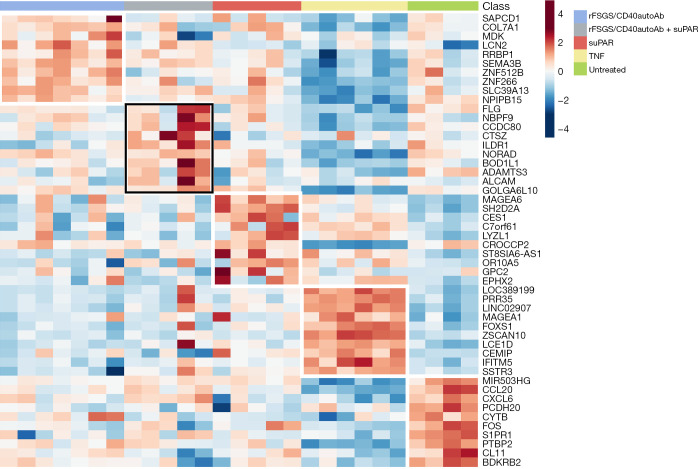
As stated above, many of the genes that are transcriptionally altered in response to various stimuli are non-specific to FSGS. After conducting the differential gene expression, unique genes were selected using the Gene list Venn diagram software. There are 73 and 432 genes uniquely altered in response to rFSGS/CD40autoAb or suPAR alone respectively. Simultaneous treatment of podocytes with suPAR and rFSGS/CD40autoAb caused alterations in 511 unique genes. The largest subset of 3,281 uniquely altered genes was associated with TNF stimulation (Figure 4A). Figure 4B displays the significantly downregulated genes across various treatments. Interestingly, the 70 genes that are downregulated across all treatments did not lead to any known pathway enrichment. 125 upregulated genes were common to all stimulations and associated with neutral lipid catabolic process, regulation of dendrite extension among others (Figure 4C,4D). The top ten unique genes activated in response to each factor has been shown in the heatmap in Figure 5. Uniquely activated genes in response to combined treatment with suPAR and rFSGS/CD40autoAb included the TNF receptor superfamily member 25 (TNFRSF25) which is a well-known mediator of NFκB to regulate apoptosis (34).
Figure 4.
Characterization of genes and pathways uniquely activated by anti-CD40 autoantibody (CD40autoAb) and suPAR. (A) Venn diagram showing all, common and unique genes transcriptionally altered in podocytes in response to treatment with CD40autoAb purified from sera of FSGS patients with recurrence of FSGS after kidney transplant (rFSGS/CD40autoAb), suPAR, combination of rFSGS/CD40autoAb with suPAR or TNF alone as described in materials and methods. (B) Profile of downregulated genes in podocytes after each treatment shows that 70 genes were downregulated across all treatments. However, gene enrichment analysis did not reveal any significant pathway enrichment. (C) Upregulated genes in podocytes after aforementioned stimulations shows 125 common upregulated genes stimulated with rFSGS/CD40autoAb, suPAR or rFSGS/CD40autoAb and suPAR together. (D) Pathways associated with genes upregulated in C. Differentially expressed gene sets were analyzed for enrichment in Gene Ontology biological pathways. Genes with more than 1.2 log fold change were included in the analysis and enriched pathways with q-value <0.05 were accepted as significant. X-axis represents the number of differentially expressed genes corresponding to the pathway indicated on Y-axis. FSGS, focal segmental glomerulosclerosis; suPAR, soluble urokinase-type plasminogen activator receptor; TNF, tumor necrosis factor.
Figure 5.
Identification of unique genes activated in response to various stimuli. To generate the stimulus-specific gene list, differential gene expression was conducted as described previously and unique genes were selected using the Gene list Venn diagram software. rFSGS, recurrence of focal segmental glomerulosclerosis; suPAR, soluble urokinase-type plasminogen activator receptor; TNF, tumor necrosis factor.
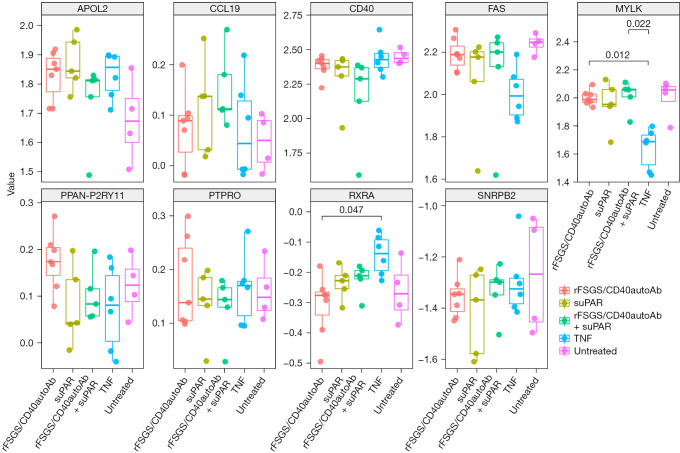
CD40 expression is downregulated in podocytes after treatment with suPAR and rFSGS-CD40Ab
Delville et al. proposed a panel of seven antibodies (CD40, PTPRO, CGB5, FAS, P2RY11, SNRPB2 and APOL2) in the pre-transplant sera of FSGS patients that could predict posttransplant FSGS recurrence with 92% accuracy (7). We tested if any of these genes were transcriptionally altered after podocyte injury in our study. Interestingly, CD40 levels were downregulated after treatment with TNF, rFSGS/CD40autoAb or suPAR and could be rescued after treatment with commercial CD40 monoclonal antibody (Figure 6).
Figure 6.
Transcriptomic regulation of antigens previously described to be associated with recurrence of FSGS after kidney transplant (Delville et al.) (7). The expression level of indicated genes was compared in podocytes treated with anti-CD40 autoantibody purified from sera of FSGS patients that recur after kidney transplant (rFSGS/CD40autoAb), suPAR, the combination of rFSGS/CD40autoAb and suPAR and TNF alone. FSGS, focal segmental glomerulosclerosis; suPAR, soluble urokinase-type plasminogen activator receptor; TNF, tumor necrosis factor.
Discussion
In this study, we aimed to further elucidate the role of CD40 and suPAR in FSGS and its recurrence through podocyte injury mechanisms. Here, we validate two novel antibodies against CD40 and suPAR as potential therapeutics for reversing podocyte injury associated with rFSGS. Furthermore, we identify molecules and pathways uniquely associated with rFSGS/CD40autoAb, or suPAR or their combination. The specific blockade of each of these pathways can be further evaluated for abrogating podocyte injury. Despite the morbidity of rFSGS, there are as yet, no specific therapies for the prevention or rapid reversal of podocyte injury after recurrence of FSGS after kidney transplantation. Therefore, an unmet need exists to develop and understand the mechanisms of novel therapeutic approaches to prevent or slow FSGS disease progression. suPAR and autoantibodies to several antigens have been proposed as biomarkers and possible therapeutic targets, but the heterogeneity of FSGS and the variability of patient specific responses to therapies have made substantive advances in therapeutic management extremely challenging.
There are several established in vitro and in vivo models to study podocyte injury and rescue after treatment (35). Other in vivo models include rodents such as transgenic and heterologous expression mouse and rat models. Pathogenesis is studied in terms of levels of proteinuria, renal histology including number of podocytes and foot process effacement. However, these studies are costly and time-consuming. Zebrafish has emerged as another attractive in vivo model due to the presence of mesonephros and 70% gene conservation with humans (36). Other model organisms to study podocyte biology are Caenorhabditis elegans and Drosophila melanogaster which do not have a corresponding glomerular ortholog structure but contain orthologs of podocyte integral protein and orthologs of slit diaphragm proteins. These models have been successfully used to study signaling pathways in renal function (37,38). For example, a recent study showed the beneficial effect of suPAR blockade (39). In vitro models include increasing use of organoids but reproducibility remains an issue (40). Here, we attempt to understand podocyte injury in FSGS by the use of an established human podocyte in vitro model (7,12), treated with human autoantibodies, suPAR and blocking target-specific antibodies. This model is relevant as the regulation of the podocyte actin cytoskeleton is crucial for the maintenance of glomerular filtration function and injury results in a shift towards increased motility of in vitro podocytes, reflected by foot process effacement in vivo (18). Podocyte injury and the effect of therapeutics on the injury can be easily studied in this model by imaging the actin cytoskeleton for the presence of stress fibers, migration assays and mechanistic studies (41). This study supports that there is a pathogenic role for several circulating factors in rFSGS sera as contractile stress fibers in cultured podocytes are lost after treatment with whole sera and CD40autoAb isolated from rFSGS patients, but not with sera from FSGS patients that do not recur after kidney transplant. We assessed two human blocking antibodies—anti-uPAR antibody (2G10) and anti-CD40 antibody (BMS-986090), and both were found to prevent podocyte injury as mediated by rFSGS patient sera. Earlier studies have proposed that a full complex of uPA-uPAR binds to and activates β3-integrin on podocyte foot processes (7,8,18,42). This results in the activation of small GTPase Rac, induction of podocyte migratory phenotype and FSGS like phenotype. The anti-uPAR antibody, 2G10 blocks uPA-uPAR interaction suggesting that 2G10 mediated inhibition of podocytopathy progression in our study involves an altered conformation of uPAR that cannot bind to and activate β3-integrin. At this time, the mechanism of rFSGS/CD40autoAb mediated podocyte injury as well as that of the antagonistic anti-CD40 antibody-mediated rescue of podocyte stress fiber loss induced by patient sera is not understood. The therapeutic effect of commercial monoclonal CD40 blocking antibody as opposed to the pathogenic effect of activating rFSGS/CD40autoAb isolated from patients has suggested that the two antibodies activate different signaling pathways and likely compete with CD40L for CD40 binding (7,12). In this study, different transcriptional changes in human podocytes to these CD40 antibodies can be observed. We have previously shown that higher concentrations of pre-transplant suPAR and rFSGS/CD40autoAb confer an increased risk of recurrence of FSGS after transplantation (7,43). We hypothesize that CD40autoAb synergizes with suPAR in causing podocyte injury by directly facilitating its binding with β3-integrin, the antagonistic anti-CD40 antibody might cause a conformational change in CD40 thus interfering with the synergy.
To further understand the molecular mechanism of podocyte injury induced by each of the aforementioned pathogenic factors and the pathways targeted by uPAR or CD40 blockade, we profiled the podocyte transcriptome after different stimulatory conditions. TNFα-treated podocytes served as a pre-established model of inflammatory injury and demonstrated dominant expression of multiple Apolipoprotein family members (APOC1, APOE and APOA1) (25). This may be a non-specific final common pathway for podocyte injury from different triggers. Apolipoproteins have previously been reported in the context of FSGS as a modified form of apolipoprotein A1 (ApoA-Ib) was detected in the urine of relapsing FSGS patients and later validated in an independent cohort as a potential biomarker to predict FSGS recurrence regardless of proteinuria status (44). Individuals with genetic variants in the apolipoprotein L1 gene (APOL1) have a greatly increased risk of end-stage renal disease, focal segmental glomerulosclerosis (FSGS), and human immunodeficiency virus (HIV)-associated nephropathy (45-47). suPAR plasma levels have been shown to independently modify APOL1 risk with lower suPAR levels being nephroprotective even in the presence of two APOL1 risk alleles (48). In addition, the involvement of ApoL1 as a non-specific final common pathway for podocytopathy is supported by studies showing that dysregulation in ApoL1 levels can lead to autophagic cell death (49-51). While the role of autophagic cell death in the development of FSGS or other podocytopathies are speculative at this time, there are studies suggesting a connection (52-54). Deletion of the autophagy associated E1-like activating enzyme, autophagy related 5 (ATG5) in podocytes and tubular epithelial cells results in FSGS like phenotype in mice (53). Our earlier studies show that podocyte injury in response to combined suPAR and rFSGS/CD40autoAb is greater than the individual administration of each factor (7,12). Individual stimulation with rFSGS/CD40autoAb causes predominant activation of type I interferon signaling and genes involved in podocyte foot process effacement such as Nck2 (55). However, suPAR alone primarily results in upregulation of genes involved in megakaryocyte differentiation. Common pathways perturbed across both suPAR and rFSGS/CD40Ab individual stimulations were associated with neutral lipid catabolic processes, cell adhesion mediated by integrins and included genes such as lipocalin 2 (LCN2). LCN2 has been proposed as a blood and urine biomarker for acute kidney injury (AKI) (56). However, our data suggests that when combined with suPAR, rFSGS/CD40Ab mediates the induction of TNF receptor superfamily member 25 (TNFRSF25) and several apolipoproteins, especially APOL2. APOL2 forms a tripartite complex with αvβ3 complex in the presence of suPAR causing podocyte injury (48). Therefore, it is likely that the formation of CD40autoAb and increased suPAR may be components of the two different hits which may be needed for the development or augmentation of renal injury mechanisms leading to FSGS.
As stated earlier, multiple pathogenic factors have been proposed for the recurrence of FSGS including suPAR, CLCF-1, CD40 axis and apolipoprotein A-Ib (57). However, further validation work is needed to assess the accuracy and predictability of these biomarkers for the recurrence of FSGS. Delville et al. (7) proposed a panel of seven antibodies (CD40, PTPRO, CGB5, FAS, P2RY11, SNRPB2 and APOL2) in the pre-transplant sera of FSGS patients that could predict posttransplant FSGS recurrence with 92% accuracy. While there can be many reasons for the formation of autoantibodies, we tested if any of these genes were transcriptionally altered after podocyte injury in our study. We found that many of the apolipoproteins were dysregulated as described above but none of the other markers were transcriptionally altered apart from CD40. Interestingly, CD40 levels were downregulated after treatment with TNF, rFSGS/CD40autoAb or suPAR and could be rescued after treatment with commercial CD40 monoclonal antibody. This suggests that CD40-CD40L signaling is required for the maintenance of podocyte structure function and pathogenic factors act by abrogating this signaling albeit through different mechanisms.
In summary, through this study, we have been able to make several interesting observations related to the role of suPAR and CD40 and their antibodies in the recurrence of FSGS. By utilizing a human in vitro podocyte injury model, we examined the impact of two pathogenic factors (CD40 autoantibodies and suPAR) and their respective blockade antibodies on podocyte injury and alterations in podocyte mRNA levels. We can observe the synergy between rFSGS/CD40autoAb and suPAR in the development of podocyte injury and pathways that maybe of relevance to that injury in rFSGS. Additional in vivo studies focusing on suPAR, CD40autoAb and other pathogenic factors as well as new target-specific blocking antibodies for therapeutics will help further elucidate and unravel the different and myriad mechanisms of pathogenicity of rFSGS after kidney transplantation. Several methods for in vivo translational analysis to identify biomarkers and therapeutic targets in glomerular diseases are continuously being developed including isolation of glomeruli, laser capture microdissection, ribosomal purification followed by transcriptional or proteomic characterization (58). Recently we and others have described single cell transcriptomic as well as near single cell proteomic characterization of native and disease kidney (59-61). We and several other groups are using several independent approaches to understand the onset and progression of FSGS. One approach is to understand the recurrence of FSGS after transplant which is the focus of our previous publications identifying the upregulation of antibodies against autoantigens as well as the current study (7,12). Additionally, we have recently completed another study that characterizes the single-cell transcriptome of bone marrow cells from FSGS patients to identify the identity and source of pathogenic circulatory factors leading to renal injury in FSGS (Rashmi et al.). In closing, it has become evident through published studies that recurrent FSGS is a disease of a dysregulated immune response. Targeted intervention to disrupt this response harbors potential for more effective treatment options.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors acknowledge help from Mr. Dane Munar and Jim Cimino for manuscript submission.
Funding: The work presented in this manuscript was supported by funds from the NIDDK/NIH DK DK109720 to MS. AAS was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brazil (CAPES-Finance Code 001).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of University of California San Francisco (No. 14-13573) and informed consent was taken from all the patients.
Footnotes
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3670/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3670/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3670/coif). MMS is a founder of technology for the assessment of kidney injury, owned by the regents, University of California. As the founder she has financial or non-financial interests in Nephrosant. JR reports that he receives consulting fees from Reata, Novateur Ventures, Walden Biosciences, Biomarin, Astellas, Massachusetts General Hospital, Genentech, Up to Date, Merck, Insceptionsci, GL, Visterra, Aclipse and MantraBio. JR also has several patents: US20110212083-Role of soluble uPAR in the Pathogenesis of Proteinuric Kidney Disease, S9867923-Reducing Solucble Rokinase Receptor in the Circulation, JP2016530510-Non-Glycoslyated suPAR Biomarkers and Uses thereof, US20160296592-Methods/Compositions for the Treatment of Proteinuric Diseases, US9144594-Dynamin Mediated Diseases and US8809386-Dynamin Ring Stabilizers. JR is on Scientific advisory board of Walden Biosciences and has stocks/stock options at Walden Biosciences and Aclipse. The other authors have no conflicts of interest to declare.
References
- 1.D'Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med 2011;365:2398-411. 10.1056/NEJMra1106556 [DOI] [PubMed] [Google Scholar]
- 2.Mundel P. Podocytes and the quest for precision medicines for kidney diseases. Pflugers Arch 2017;469:1029-37. 10.1007/s00424-017-2015-x [DOI] [PubMed] [Google Scholar]
- 3.Faul C, Asanuma K, Yanagida-Asanuma E, et al. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 2007;17:428-37. 10.1016/j.tcb.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 4.Reggiani F, Ponticelli C. Focal segmental glomerular sclerosis: do not overlook the role of immune response. J Nephrol 2016;29:525-34. 10.1007/s40620-016-0272-y [DOI] [PubMed] [Google Scholar]
- 5.Vinai M, Waber P, Seikaly MG. Recurrence of focal segmental glomerulosclerosis in renal allograft: an in-depth review. Pediatr Transplant 2010;14:314-25. 10.1111/j.1399-3046.2009.01261.x [DOI] [PubMed] [Google Scholar]
- 6.Savin VJ, Sharma R, Sharma M, et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med 1996;334:878-83. 10.1056/NEJM199604043341402 [DOI] [PubMed] [Google Scholar]
- 7.Delville M, Sigdel TK, Wei C, et al. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med 2014;6:256ra136. 10.1126/scitranslmed.3008538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei C, El Hindi S, Li J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 2011;17:952-60. 10.1038/nm.2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otalora L, Chavez E, Watford D, et al. Identification of glomerular and podocyte-specific genes and pathways activated by sera of patients with focal segmental glomerulosclerosis. PLoS One 2019;14:e0222948. 10.1371/journal.pone.0222948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panigrahi S, Pardeshi VC, Chandrasekaran K, et al. Expression profiling of cultured podocytes exposed to nephrotic plasma reveals intrinsic molecular signatures of nephrotic syndrome. Clin Exp Pediatr 2021;64:355-63. 10.3345/cep.2020.00619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savin VJ, Sharma M, Zhou J, et al. Multiple Targets for Novel Therapy of FSGS Associated with Circulating Permeability Factor. Biomed Res Int 2017;2017:6232616. 10.1155/2017/6232616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei C, Sigdel TK, Sarwal MM, et al. Circulating CD40 autoantibody and suPAR synergy drives glomerular injury. Ann Transl Med 2015;3:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elgueta R, Benson MJ, de Vries VC, et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 2009;229:152-72. 10.1111/j.1600-065X.2009.00782.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perper SJ, Westmoreland SV, Karman J, et al. Treatment with a CD40 Antagonist Antibody Reverses Severe Proteinuria and Loss of Saliva Production and Restores Glomerular Morphology in Murine Systemic Lupus Erythematosus. J Immunol 2019;203:58-75. 10.4049/jimmunol.1900043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henn V, Steinbach S, Büchner K, et al. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood 2001;98:1047-54. 10.1182/blood.V98.4.1047 [DOI] [PubMed] [Google Scholar]
- 16.Doublier S, Zennaro C, Musante L, et al. Soluble CD40 ligand directly alters glomerular permeability and may act as a circulating permeability factor in FSGS. PLoS One 2017;12:e0188045. 10.1371/journal.pone.0188045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roldan AL, Cubellis MV, Masucci MT, et al. Cloning and expression of the receptor for human urokinase plasminogen activator, a central molecule in cell surface, plasmin dependent proteolysis. EMBO J 1990;9:467-74. 10.1002/j.1460-2075.1990.tb08132.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei C, Möller CC, Altintas MM, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med 2008;14:55-63. 10.1038/nm1696 [DOI] [PubMed] [Google Scholar]
- 19.Hindy G, Tyrrell DJ, Vasbinder A, et al. Increased soluble urokinase plasminogen activator levels modulate monocyte function to promote atherosclerosis. J Clin Invest 2022;132:e158788. 10.1172/JCI158788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duriseti S, Goetz DH, Hostetter DR, et al. Antagonistic anti-urokinase plasminogen activator receptor (uPAR) antibodies significantly inhibit uPAR-mediated cellular signaling and migration. J Biol Chem 2010;285:26878-88. 10.1074/jbc.M109.077677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeBeau AM, Duriseti S, Murphy ST, et al. Targeting uPAR with antagonistic recombinant human antibodies in aggressive breast cancer. Cancer Res 2013;73:2070-81. 10.1158/0008-5472.CAN-12-3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harel ET, Drake PM, Barfield RM, et al. Antibody-Drug Conjugates Targeting the Urokinase Receptor (uPAR) as a Possible Treatment of Aggressive Breast Cancer. Antibodies (Basel) 2019;8:54. 10.3390/antib8040054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joy MS, Gipson DS, Powell L, et al. Phase 1 trial of adalimumab in Focal Segmental Glomerulosclerosis (FSGS): II. Report of the FONT (Novel Therapies for Resistant FSGS) study group. Am J Kidney Dis 2010;55:50-60. 10.1053/j.ajkd.2009.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peyser A, Machardy N, Tarapore F, et al. Follow-up of phase I trial of adalimumab and rosiglitazone in FSGS: III. Report of the FONT study group. BMC Nephrol 2010;11:2. 10.1186/1471-2369-11-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedigo CE, Ducasa GM, Leclercq F, et al. Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury. J Clin Invest 2016;126:3336-50. 10.1172/JCI85939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung CF, Kitzler T, Kachurina N, et al. Intrinsic tumor necrosis factor-α pathway is activated in a subset of patients with focal segmental glomerulosclerosis. PLoS One 2019;14:e0216426. 10.1371/journal.pone.0216426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saleem MA, O'Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 2002;13:630-8. 10.1681/ASN.V133630 [DOI] [PubMed] [Google Scholar]
- 28.R Core Team. R: A language and environment for statistical computing. 2018. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- 29.Huber W, Carey VJ, Gentleman R, et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods 2015;12:115-21. 10.1038/nmeth.3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015;43:e47. 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leek JT, Johnson WE, Parker HS, et al. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012;28:882-3. 10.1093/bioinformatics/bts034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu G, Wang LG, Han Y, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 2012;16:284-7. 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chew-Harris J, Appleby S, Richards AM, et al. Analytical, biochemical and clearance considerations of soluble urokinase plasminogen activator receptor (suPAR) in healthy individuals. Clin Biochem 2019;69:36-44. 10.1016/j.clinbiochem.2019.05.010 [DOI] [PubMed] [Google Scholar]
- 34.Jiang Y, Woronicz JD, Liu W, et al. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science 1999;283:543-6. 10.1126/science.283.5401.543 [DOI] [PubMed] [Google Scholar]
- 35.Hagmann H, Brinkkoetter PT. Experimental Models to Study Podocyte Biology: Stock-Taking the Toolbox of Glomerular Research. Front Pediatr 2018;6:193. 10.3389/fped.2018.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroeger PT, Jr, Wingert RA. Using zebrafish to study podocyte genesis during kidney development and regeneration. Genesis 2014;52:771-92. 10.1002/dvg.22798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganner A, Neumann-Haefelin E. Genetic kidney diseases: Caenorhabditis elegans as model system. Cell Tissue Res 2017;369:105-18. 10.1007/s00441-017-2622-z [DOI] [PubMed] [Google Scholar]
- 38.Helmstädter M, Huber TB, Hermle T. Using the Drosophila Nephrocyte to Model Podocyte Function and Disease. Front Pediatr 2017;5:262. 10.3389/fped.2017.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Method of the Year 2020: spatially resolved transcriptomics. Nat Methods 2021;18:1. 10.1038/s41592-020-01042-x [DOI] [PubMed] [Google Scholar]
- 40.Hale LJ, Howden SE, Phipson B, et al. 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat Commun 2018;9:5167. 10.1038/s41467-018-07594-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol 2002;13:3005-15. 10.1097/01.ASN.0000039661.06947.FD [DOI] [PubMed] [Google Scholar]
- 42.Zhang B, Shi W, Ma J, et al. The calcineurin-NFAT pathway allows for urokinase receptor-mediated beta3 integrin signaling to cause podocyte injury. J Mol Med (Berl) 2012;90:1407-20. 10.1007/s00109-012-0960-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei C, Trachtman H, Li J, et al. Circulating suPAR in two cohorts of primary FSGS. J Am Soc Nephrol 2012;23:2051-9. 10.1681/ASN.2012030302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puig-Gay N, Jacobs-Cacha C, Sellarès J, et al. Apolipoprotein A-Ib as a biomarker of focal segmental glomerulosclerosis recurrence after kidney transplantation: diagnostic performance and assessment of its prognostic value - a multi-centre cohort study. Transpl Int 2019;32:313-22. 10.1111/tri.13372 [DOI] [PubMed] [Google Scholar]
- 45.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010;329:841-5. 10.1126/science.1193032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedman DJ, Kozlitina J, Genovese G, et al. Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol 2011;22:2098-105. 10.1681/ASN.2011050519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 2011;22:2129-37. 10.1681/ASN.2011040388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayek SS, Koh KH, Grams ME, et al. A tripartite complex of suPAR, APOL1 risk variants and αvβ3 integrin on podocytes mediates chronic kidney disease. Nat Med 2017;23:945-53. 10.1038/nm.4362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wan G, Zhaorigetu S, Liu Z, et al. Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. J Biol Chem 2008;283:21540-9. 10.1074/jbc.M800214200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar V, Ayasolla K, Jha A, et al. Disrupted apolipoprotein L1-miR193a axis dedifferentiates podocytes through autophagy blockade in an APOL1 risk milieu. Am J Physiol Cell Physiol 2019;317:C209-25. 10.1152/ajpcell.00538.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhaorigetu S, Wan G, Kaini R, et al. ApoL1, a BH3-only lipid-binding protein, induces autophagic cell death. Autophagy 2008;4:1079-82. 10.4161/auto.7066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Venkatachalam MA. Could Autophagic Exhaustion Be a Final Common Pathway for Podocytopathy in FSGS? J Am Soc Nephrol 2015;26:999-1001. 10.1681/ASN.2014090919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawakami T, Gomez IG, Ren S, et al. Deficient Autophagy Results in Mitochondrial Dysfunction and FSGS. J Am Soc Nephrol 2015;26:1040-52. 10.1681/ASN.2013111202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yildirim D, Bender O, Karagoz ZF, et al. Role of autophagy and evaluation the effects of microRNAs 214, 132, 34c and prorenin receptor in a rat model of focal segmental glomerulosclerosis. Life Sci 2021;280:119671. 10.1016/j.lfs.2021.119671 [DOI] [PubMed] [Google Scholar]
- 55.Buvall L, Rashmi P, Lopez-Rivera E, et al. Proteasomal degradation of Nck1 but not Nck2 regulates RhoA activation and actin dynamics. Nat Commun 2013;4:2863. 10.1038/ncomms3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanda J, Mori K, Kawabata H, et al. An AKI biomarker lipocalin 2 in the blood derives from the kidney in renal injury but from neutrophils in normal and infected conditions. Clin Exp Nephrol 2015;19:99-106. 10.1007/s10157-014-0952-7 [DOI] [PubMed] [Google Scholar]
- 57.Shoji J, Mii A, Terasaki M, et al. Update on Recurrent Focal Segmental Glomerulosclerosis in Kidney Transplantation. Nephron 2020;144 Suppl 1:65-70. 10.1159/000510748 [DOI] [PubMed] [Google Scholar]
- 58.Grgic I, Hofmeister AF, Genovese G, et al. Discovery of new glomerular disease-relevant genes by translational profiling of podocytes in vivo. Kidney Int 2014;86:1116-29. 10.1038/ki.2014.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Menon R, Otto EA, Hoover P, et al. Single cell transcriptomics identifies focal segmental glomerulosclerosis remission endothelial biomarker. JCI Insight 2020;5:e133267. 10.1172/jci.insight.133267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rashmi P, Sur S, Sigdel TK, et al. Multiplexed droplet single-cell sequencing (Mux-Seq) of normal and transplant kidney. Am J Transplant 2022;22:876-85. 10.1111/ajt.16871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sigdel TK, Piehowski PD, Roy S, et al. Corrigendum: Near-Single-Cell Proteomics Profiling of the Proximal Tubular and Glomerulus of the Normal Human Kidney. Front Med (Lausanne) 2020;7:625788. 10.3389/fmed.2020.625788 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as