Abstract
Leg ulcers are a major complication of sickle cell disease (SCD). They are particularly challenging to treat and innovative therapies are needed. We previously showed that the healing of SCD ulcers is delayed because of decreased angiogenesis. During pregnancy, fetal microchimeric cells (FMC) transferred to the mother are recruited to maternal wounds and improve angiogenesis. After delivery, FMC persist in maternal bone marrow for decades. Here, we investigated whether fetal cells could also improve SCD ulcers in the post-partum setting. We found that skin healing was similarly improved in post-partum mice and in pregnant mice, through increased proliferation and angiogenesis. In a SCD mouse model that recapitulates refractory SCD ulcers, we showed that the ulcers of post-partum SCD mice healed more quickly than those of virgin mice. This was associated with the recruitment of fetal cells in maternal wounds where they harbored markers of leukocytes and endothelial cells. In a retrospective cohort of SCD patients, using several parameters we found that SCD women who had ever had a baby had less of a burden related to leg ulcers compared to nulliparous women. Taken together, these results indicate that healing capacities of FMC are maintained long after delivery and may be exploited to promote wound healing in post-partum SCD patients.
Introduction
Sickle cell disease (SCD), one of the most common genetic diseases around the world,1 leads to leg ulcers in 2% to up to 40% of affected patients.2,3 These ulcers are usually long-lasting, recurrent, and difficult to treat, causing major disabilities and impairing quality of life.4 Treatment of SCD leg ulcers remains a challenge and innovative treatments are urgently needed.4 The prevalence of leg ulcers in SCD patients increases with age, and these ulcers have been associated with the level of anemia.5 In addition, the risk of developing leg ulcers is higher in patients with hyperhemolysis and is associated with pulmonary hypertension, priapism and stroke.6,7 Accordingly, most authors consider that the release of free hemoglobin by hemolysis is responsible for skin ulcers through a reduction of endothelial nitric oxide bioavailability leading to the development of vasoconstriction and endothelial activation.1,8 However, the association of leg ulcers with hyperhemolytic phenotypes has been challenged in sub-
Saharan African SCD patients.9 In addition, pure hemolytic disorders, such as paroxysmal nocturnal hemoglobinuria, are usually not associated with leg ulcers.10 Besides, several cutaneous disorders displaying vasoconstriction and vasculopathy with endothelial activation, such as cryoglobulinemia and vasculitis, may lead to leg ulcers, although these usually resolve in weeks or months upon specific treatment of the causative disease.11 Thus, the exact etiopathogenic mechanisms leading to SCD ulcers remain incompletely understood. Our group previously hypothesized that skin wound healing could be specifically delayed in SCD, leading to ulcers after an initial trauma. We found that wound healing is impaired in a transgenic mouse model of SCD harboring a mutated form of human β-globin (SAD mice).12 Wound healing is a complex process that involves several cell types, both resident in the skin and also circulating cells recruited to the wound.13,14 Using SAD mice, we showed a decreased recruitment of bone marrow-derived endothelial progenitor cells to cutaneous wounds, leading to impaired angiogenesis within the wound bed, which could be partially rescued by local injections of SDF-1α/CXCL12.12
During pregnancy, fetal cells are transferred to maternal blood and enter the maternal bone marrow niche where they persist for decades.15 We previously showed that fetal microchimeric cells (FMC) can be recruited to maternal wounds during pregnancy, through the CCR2/CCL2 pathway, and play a crucial role in maternal skin repair.16,17 They can differentiate into endothelial cells able to form blood vessels and secrete pro-angiogenic factors, including CXCL1, which stimulate maternal angiogenesis.16,17 Triggering the recruitment of FMC to participate in maternal wound healing is a promising strategy as compared with conventional cell therapies. However, this would require the FMC to have a sustained capacity to promote maternal skin repair over time and after delivery. In this work, we explored whether healing was improved in post-partum mice as is the case during pregnancy, and characterized the properties of circulating FMC after delivery. We also assessed the repair capacities of FMC in post-partum SAD mice. Finally, we investigated the course of leg ulcers and their complications in parous women with SCD.
Methods
Mice
Male transgenic mice expressing enhanced green fluorescence protein (eGFP) were obtained from Riken Laboratories (C57BL/6-Tg(CAG-EGFP)C14-Y01-FM131Osb) and mated to 6- to 8-week-old wildtype C57BL/6 females (Janvier Labs). SAD-1 (SAD) transgenic mice are hemizygous knock-in mice with a mutated form of human β-globin.18 This work was performed in accordance with European Community guidelines and approved by an Institutional Animal Care and Use Committee under the license APA-FIS#32354-202102040946663. Surgical wounds and measurements of wound surface were performed as previously described.17
Antibodies
The primary antibodies used were as follows: rabbit anti-K14 (1:1000; Biolegend), rabbit anti-Ki67 (1:200; Abcam), rat anti-CD31 (1:40; BD Biosciences), rat anti-F4/80 (1:250; Abcam), rat anti-GR-1 (1:250; eBiosciences), rat anti-CD45 (1:200; BD Biosciences) and rabbit anti-GFP (1:200; ABclonal). Alexa Fluor-conjugated antibodies (ThermoFisher Scientific) were used at 1:1000 as secondary antibodies. Nuclei were counterstained with 0.3 µg/mL DAPI (SigmaAldrich).
Clinical study
We conducted a retrospective, single-center, cohort study using routinely collected data in compliance with good clinical practice and the Declaration of Helsinki. According to French law, formal ethics committee approval was not required for this study. Female SCD patients aged ≥18 years with at least one current or previous leg ulcer were recruited from the Red Blood Cell Genetic Diseases Unit (Hôpital Mondor, Créteil, France) between January 2020 and September 2021. Patients’ medical histories, treatments, laboratory data and information related to leg ulcers were extracted from medical files. Patients did not undergo a specific medical examination for this study.
Statistical analysis and reproducibility
Statistical analyses were performed with the statistical software Prism 8 (GraphPad). When required, normality of the data was tested with the Shapiro-Wilk test and a statistical method to correct for multiple comparisons was used.
Data availability
The RNA-sequencing datasets produced in this study are available in Online Supplementary Tables S1-S4.
Supplementary methods
Further details of the study methods are available in the Online Supplementary File.
Results
Improvement of wound healing is sustained in C57BL/6 post-partum mice
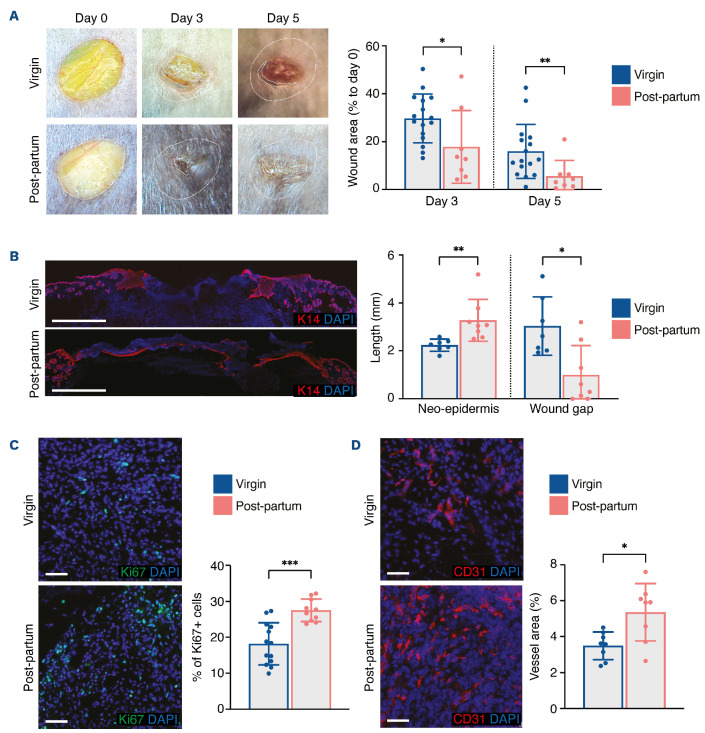
To evaluate how pregnancy affects skin wound healing, we mated virgin C57BL/6 females with homozygous males expressing eGFP. We then performed back skin excisional wounds on pregnant mice at gestational day E15.5 and on age-matched virgin littermates. Wound closure was significantly accelerated in pregnant mice (Online Supplementary Figure S1A). Re-epithelialization, as measured by the length of the K14+ neo-epidermis, was improved in pregnant mice (Online Supplementary Figure S1B). Ki67+ proliferating cells and CD31+ blood vessels were significantly increased in the wound bed of pregnant mice (Online Supplementary Figure S1C, D). As expected, expression of Vegfa, Vegfr1 and Vegfr2 was significantly elevated in wounds of pregnant mice, whereas levels of expression of Vegfc and Vegfr3, implicated in lymphatic angiogenesis, were not (Online Supplementary Figure S2A). Lastly, we found that infiltration by F4/80+ macrophages was not different between virgin and pregnant mice, while GR1+ neutrophils were slightly increased in pregnant mice (Online Supplementary Figure S1B, C). These results confirm that pregnancy promotes wound healing, as we had previously observed.17 We then performed back skin excisional wounds on postpartum mice 12 weeks after their last gestation and on age-matched virgin littermates. We observed a significant improvement of wound closure kinetics in post-partum mice (Figure 1A). The epidermal gap was wider, while the K14+ tongue was smaller in virgin mice than in post-partum mice (Figure 1B). The proliferation index was significantly increased (Figure 1C), and CD31+ blood vessels were more abundant in the wound beds of post-partum mice (Figure 1D). We also observed the presence of eGFP+ FMC in postpartum wounded skin, confirming the survival of these cells in the post-partum condition (Online Supplementary Figure S3A). Together, these results indicate that the improvement of wound healing observed in pregnant mice is sustained in post-partum mice months after the last pregnancy and is associated with efficient recruitment of FMC to the wound.
Figure 1.
Improvement of skin wound healing is sustained in post-partum mice. (A) Representative images of wounds at days 0, 3, and 5, and planimetry of wound area at each time point relative to the original wound area. (B) Representative images and measurement of anti-K14 labeling of neo-epidermal tongues and wound gap at day 5. (C) Representative images and quantification of Ki67+ cells in the wound bed at day 5. (D) Representative images of CD31+ cells and quantification of vessel area in the wound bed at day 5. Scale bars represent 1000 mm (B) or 50 µm (C, D). In (B-D), nuclei were counterstained with DAPI. In (A-D), four 6-mm excisional wounds were performed in virgin mice (n=4) or post-partum mice (n=2). Data are presented as means with standard deviations and individual values. Statistical analyses were performed with two-tailed t tests with the Welch correction whenever required (A [day 3], C-D) or Mann-Whitney test (A [day 5], B). *P<0.05; **P<0.005; ***P<0.0005.
Fetal microchimeric cells display features of hematopoietic progenitor cells
To explore the properties of long-term engrafted FMC, we mated C57BL/6 females with males expressing the eGFP transgene to induce the transfer of eGFP+ FMC to the mothers. Eight weeks after delivery, we harvested bone marrow cells from post-partum females, seeded them at low density in EGM-2 medium, and measured colony size after 9 days. We showed that eGFP+ hematopoietic cells grew in colonies that proliferate significantly more than their adult eGFP– counterparts (Online Supplementary Figure S4A). eGFP+ cells expressed CD45, CD11b and CD31, while a minority expressed CD34 similarly to the adult eGFP– hematopoietic cells (Online Supplementary Figure S4B).
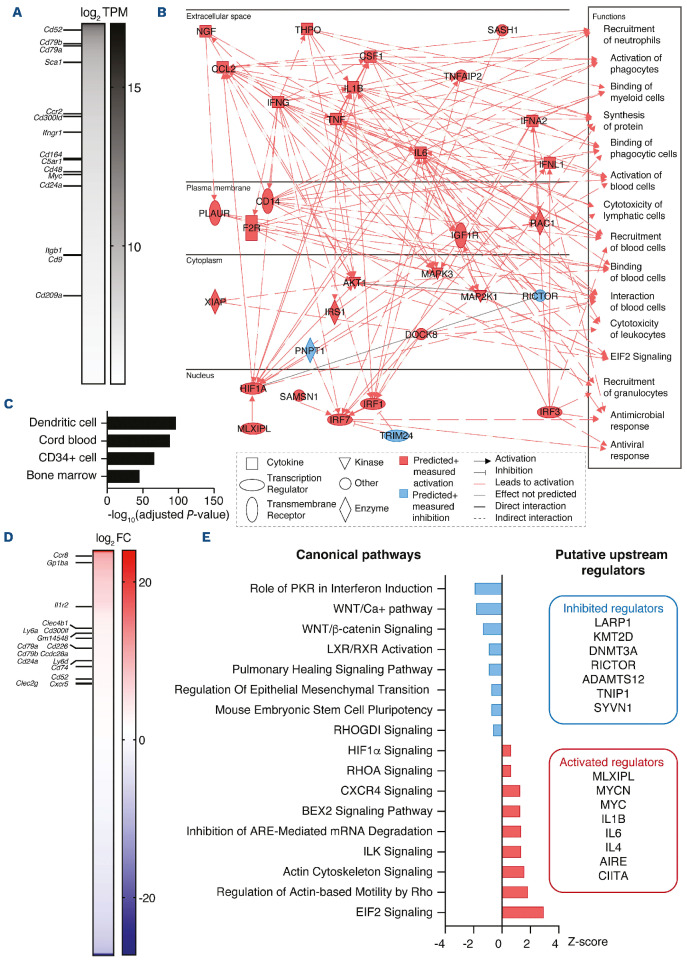
We then sorted eGFP+ FMC circulating in wounded and unwounded post-partum mice and analyzed them by RNA sequencing (Figure 2). We first analyzed the transcriptome of circulating FMC from unwounded mice to characterize the main genes expressed at steady state (Figure 2A). This revealed a high level of expression of membrane receptors previously reported to be enriched in circulating FMC of wounded pregnant mice, such as Cd52, Cd79b, Ccr2, Cd300ld, and Ifngr1.17 Genes associated with hematopoietic stem cells, such as Sca1 and Myc, were also highly expressed in post-partum FMC (Figure 2A). Transcriptional analysis of the post-partum FMC transcriptome using ingenuity pathway analysis showed an enrichment for functions related to immune response, such as “recruitment of neutrophils”, “activation of phagocytes” or “binding of myeloid cells” in parallel with a high expression of cytokine and chemokine genes such as Ccl2, Ifng, Il1b, Il6, and Tnf (Figure 2B). Besides, the ARCHS4 database identified these cells as “dendritic cells”, “cord blood”, “CD34+ cells” or “bone marrow” (Figure 2C). We also analyzed eGFP+ FMC from peripheral blood of post-partum mice after creating a cutaneous wound. This enabled us to describe the fetal cells specifically responding to a maternal wound. When compared to circulating fetal cells at steady state, there were 487 differentially expressed genes (Figure 2D). Several plasma membrane receptor genes were enriched in FMC from wounded post-partum mice, including Ccr8, Il1r2 and Cxcr5, suggesting that several pathways amplify the recruitment of these cells to damaged skin (Figure 2D). Canonical pathway analysis showed a significant enrichment of pathways related to CXCR4 signaling and to integrin signaling and cell motility, through ILK, RHOA and the actin cytoskeleton, in post-partum FMC upon wounding (Figure 2E). In parallel, we observed an underrepresentation of WNT/β-catenin pathways and the stem cell pluripotency program suggesting that wound-mobilized FMC start to acquire a differentiated fate during their journey to the damaged skin. Activated upstream regulators were predicted to be MYCN, MYC and several cytokines (IL1B, IL6, IL4), while inhibited regulators included mTOR-related regulators (LARP1, RICTOR) and methyltransferases (KMT2D, DNMT3A) (Figure 2E). These results indicate a greater clonogenic capacity of FMC as compared to their adult counterparts and reveal that circulating FMC are transcriptionally modified upon wounding to favor cell motility and response to immune attractants.
Wound healing is improved in post-partum SAD mice displaying altered skin healing
We next explored the properties of FMC in post-partum females in the context of delayed wound healing as observed in SAD mice.12
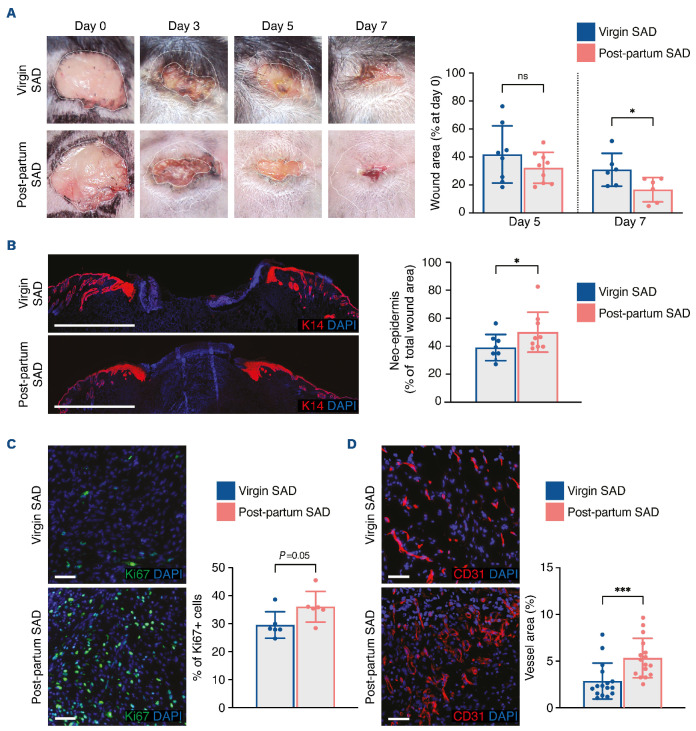
We performed back skin excisional wounds on post-partum SAD mice 12 weeks after their last gestation and on agematched virgin SAD mice. We noted a reduction of the wound area at day 7 after wounding (Figure 3A). Of note, the wound size was also reduced at day 5 after the wound was created, but the difference was not statistically significant (Figure 3A). In agreement, the neo-epidermal tongue was longer (Figure 3B) and Ki67+ cells were increased in the granulation tissue (Figure 3C) of post-partum SAD mice as compared with those in virgin littermates. Interestingly, we observed a significant increase in vessel density in post-partum SAD mice suggesting improved recruitment of endothelial progenitors in these mice (Figure 3D). We also found efficient recruitment of eGFP+ FMC in the granulation tissue of post-partum mice (Online Supplementary Figure S3B).
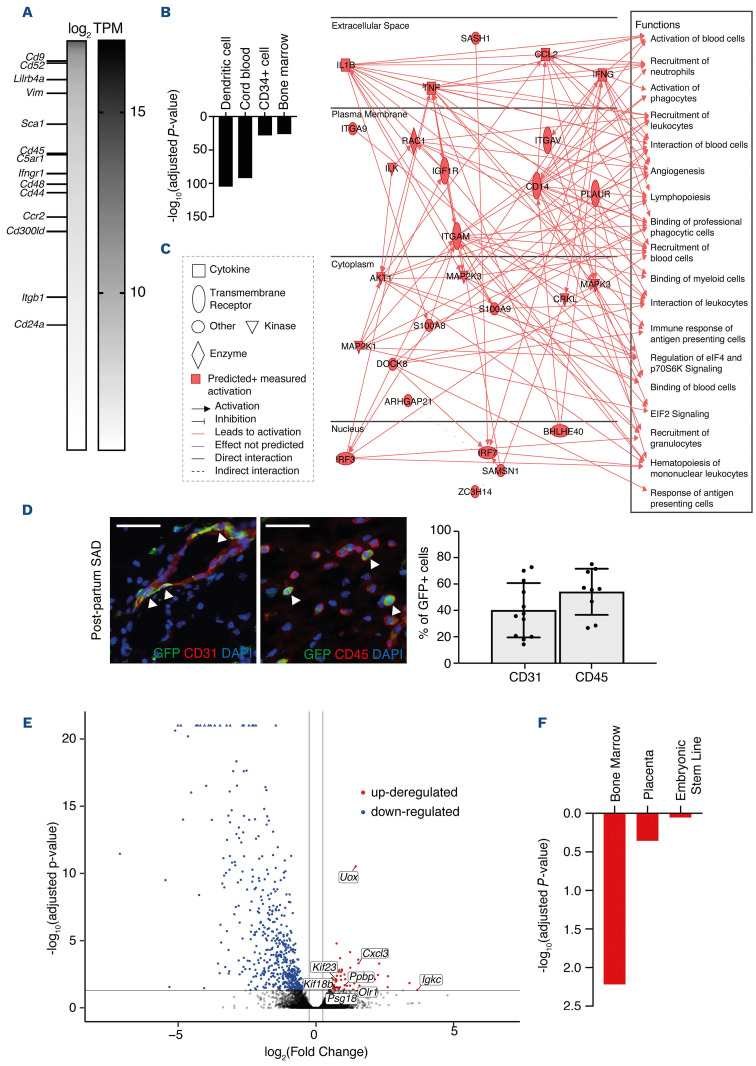
We then assessed the gene expression profile of cutaneous FMC by performing RNA sequencing analysis of sorted eGFP+ cells from day 3 wounds of post-partum SAD mice (Figure 4A-C). We observed high levels of FMC-enriched membrane receptors and genes associated with hematopoietic stem cells (Figure 4A), as previously found in steady-state circulating FMC (Figure 2A), suggesting their common origin. Besides, “dendritic cell”, “cord blood”, “CD34+ cells” and “bone marrow” signatures were also identified by ARSCHS4 in wound-associated FMC (Figure 4B). Ingenuity pathway analysis showed enrichment for functions related to immune response and angiogenesis (Figure 4C). In order to better identify the characteristics of fetal cells in SAD wounds, we performed immunostaining on wound sections of SAD mice at day 3 after wounding. We observed that eGFP+ cells displayed a phenotype of CD31+ endothelial cells and CD45+ leukocytes (Figure 4D). To analyze the wound changes in relation to fetal cell trafficking, we performed RNA sequencing analysis comparing skin wounds from virgin and post-partum SAD mice at day 3 after wounding (Figure 4E, F). We found 72 significantly upregulated genes and 540 downregulated genes (Figure 4E). Using the Mouse Gene Atlas we identified several factors associated with bone marrow and placenta signatures in upregulated genes (Figure 4F), likely to reflect the contribution of skin-recruited FMC upon wounding. Collectively, these results demonstrate that FMC are able to improve wound healing several months after gestation in a SCD mouse model displaying severely altered healing.
Figure 2.
Fetal microchimeric cells are related to hematopoietic progenitors and can be mobilized upon injury. (A) Heatmap showing genes, ordered by transcripts per million (TPM) and with TPM >50 expressed in eGFP+ fetal microchimeric cells (FMC) harvested from peripheral blood of unwounded post-partum mice. No comparison was performed in order to display the main genes expressed by circulating FMC at steady state. Membrane receptors previously reported to be enriched in blood FMC of wounded pregnant mice17 are shown. Data are presented as log2 TPM. (B) Graphical summary obtained upon transcriptional analysis, using ingenuity pathway analysis (IPA) software, of blood FMC harvested from unwounded post-partum mice. Only transcripts with at least 50 TPM were kept for the analysis. (C) ARCHS4 enrichment analysis of the 500 most expressed genes in FMC in unwounded post-partum mice. (D) Heatmap showing the significantly (adjusted P<0.05) differentially expressed genes in FMC in post-partum mice with or without wounds. Upregulated membrane receptors are indicated. Data are presented as log2 fold change. (E) Statistically significant (adjusted P<0.05) upregulated and downregulated canonical pathways and upstream regulators were determined using IPA software from differentially expressed genes in blood FMC from wounded post-partum mice as compared with unwounded counterparts. Canonical pathways are expressed as a Z score. In (A-E), RNA sequencing was performed in post-partum mice left unwounded (n=3) or 1 day after performing one 6-mm cutaneous wound (n=3 mice).
Leg ulcer burden in sickle cell disease patients is decreased during the post-partum period
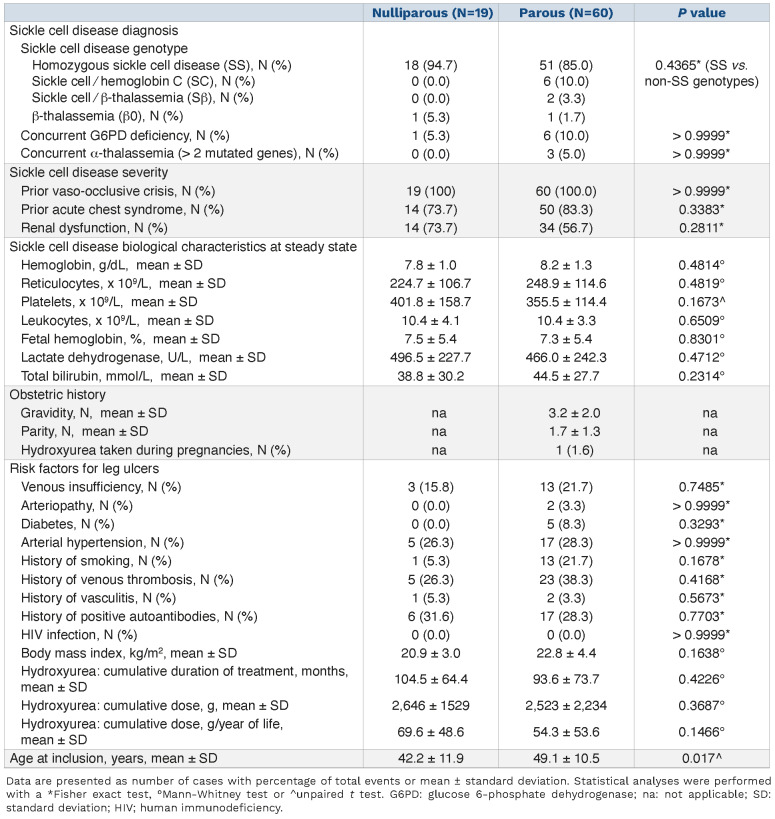
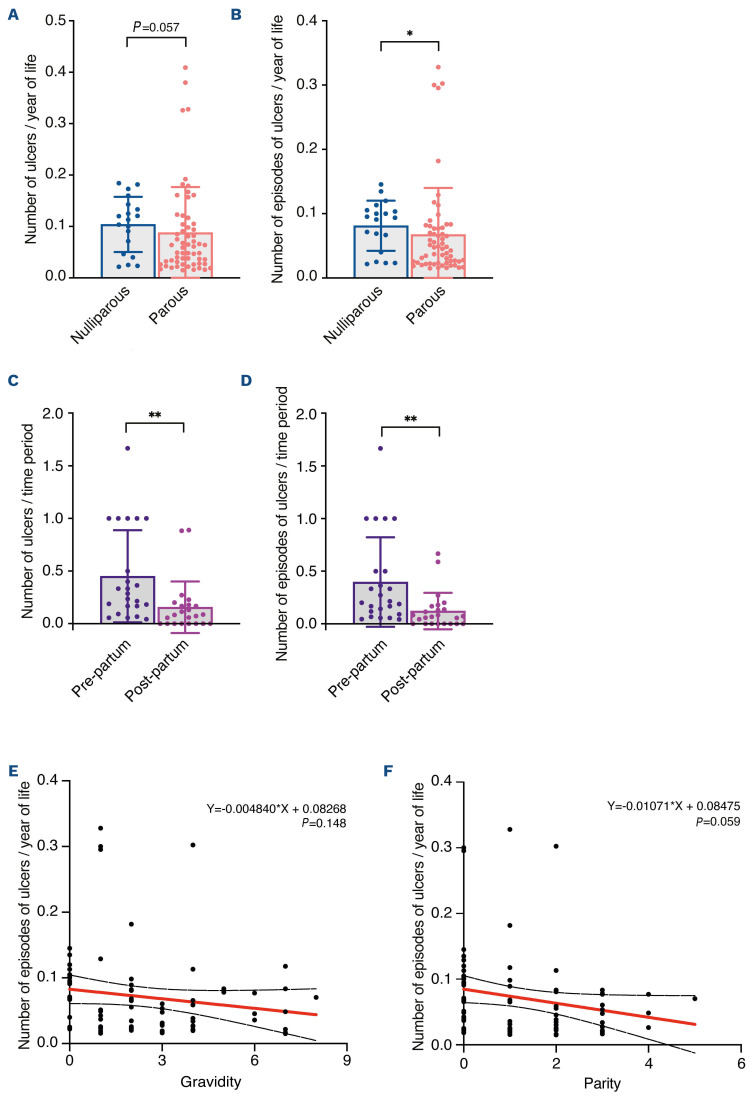
Complete healing of leg ulcers of SCD patients has been reported during pregnancy,19,20 leading to the hypothesis that this could represent a favorable effect of fetal cell transfer to the mother. As FMC have been described to persist at least several decades after pregnancy in women,15 we investigated the prevalence and severity of leg ulcers in a cohort of female patients followed for a genetic red blood cell disease in Mondor SCD referral center (Paris, France). We identified 79 women who presented with leg ulcers. Nineteen patients were nulliparous, while 60 had had at least one pregnancy. Most of these patients had SCD with a homozygous SS genotype (Table 1). In the nulliparous and parous groups we investigated different clinical and biological parameters related to disease severity such as history of vaso-occlusive crises, acute chest syndrome or renal dysfunction, and levels of hemoglobin, reticulocytes, leukocytes, lactate dehydrogenase, total bilirubin and fetal hemoglobin. We previously showed that SCD patients with leg ulcers have SCD hemolytic complications more frequently than do patients without ulcers.2,7 Accordingly, patients in this cohort had clinical and biological features of severe disease. However, none of these parameters was statistically different between nulliparous and parous women with ulcers (Table 1). We then assessed the prevalence of other risk factors for leg ulcers including venous insufficiency, arteriopathy, diabetes, arterial hypertension, vein thrombosis, vasculitis, human immunodeficiency virus infection, autoimmunity, history of smoking, and body mass index (Table 1). Again, there were no differences between the two groups. In addition, exposure to hydroxyurea, as measured by the cumulative duration of treatment and dose, was not different between nulliparous and parous SCD patients ruling out an effect of this parameter on leg ulcers (Table 1). Of note, hydroxyurea was suspended during pregnancies in all women but one. However, parous SCD patients were significantly older (by 6.9 years) than nulliparous women (Table 1). We therefore measured the prevalence of leg ulcers after adjustment for age and found that parous SCD patients had a decreased total number of ulcers (P=0.057) and fewer episodes of ulcers (P=0.012) (Figure 5A, B). We also compared the adjusted rate of ulcers between the pre-partum and postpartum periods in parous SCD patients who had had leg ulcers before their first pregnancy. This analysis revealed a significantly reduced total number of leg ulcers (P=0.003) and episodes of leg ulcers (P=0.004) in the post-partum period as compared with the pre-partum period (Figure 5C, D). We also observed a tendency to fewer episodes of ulcers with increased parity, but not gravidity (Figure 5E, F). We then explored eight items reflecting the severity of leg ulcers: need for hospitalization or sick leave, rate of leg ulcer-associated infection and depression, and the following therapeutic options required to treat leg ulcers: morphine, skin graft, bosentan/ilomedin and red blood cell exchange transfusion. Apart from the last, all these items were less frequent in parous SCD patients than in nulliparous ones. However, differences were only statistically significant for morphine use and bosentan or ilomedin treatment (Online Supplementary Figure S1A). In conclusion, these results show a better overall outcome of leg ulcers in parous SCD patients than in nulliparous ones, which could result from FMC mobilization and participation in skin tissue repair.
Discussion
Our work provides evidence that skin wound healing is improved in female mice during and after pregnancy through enhanced vascular angiogenesis and cell proliferation. This was also observed in a mouse model of SCD in which wound healing is severely delayed. We demonstrated that cutaneous wounds healed faster in post-partum SAD mice than in virgin littermates. Finally, in SCD female patients with leg ulcers, we found that the course of the ulcers was improved, as assessed through several parameters. We found that parous women had significantly fewer episodes of leg ulcers throughout their lifetime, particularly during the post-partum period as compared with the pre-partum period, and that their ulcers were less severe. Our data therefore indicate that post-partum females, including those suffering from SCD, heal better than nulliparous ones. While the transfer of FMC to mothers increases steadily during pregnancy, it decreases swiftly after delivery.21,22 In women, around 40-470 fetal cells per 106 maternal cells were found in maternal organs during or shortly after pregnancy,23 whereas this rate drops to 2-10 fetal cells per 106 maternal bone marrow cells 30 to 50 years after delivery.15 Despite this reduction, FMC seem able to exit their niche in the marrow and reach an injured maternal tissue.24 In this study, we observed an efficient recruitment of FMC to the wound bed of post-partum mice both during normal and altered healing and demonstrated that these cells, despite their low number, may have a beneficial role during skin wound healing long after pregnancy.
Figure 3.
Skin wound healing is improved in post-partum SAD mice with delayed cutaneous healing. (A) Representative images of wounds at days 0, 3, 5 and 7, and planimetry of wound area at days 5 and 7 relative to the original wound area. (B) Representative images of wounds labeled with anti-K14 antibody at day 7. The size of the neo-epidermis relative to the total wound area is provided. (C) Representative images and quantification of Ki67+ cells in the wound bed at day 7. (D) Representative images of CD31+ cells and quantification of vessel area in the wound bed at day 7. Scale bars represent 1000 mm (B) or 50 mm (C, D). In (B-D), nuclei were counterstained with DAPI. In (A-D), one 8-mm excisional wound was performed in virgin mice (n=4) or post-partum SAD mice (n=5). Data are presented as means with standard deviations and individual values. Statistical analyses were performed with two-tailed t tests with the Welch correction whenever required (A, C) or Mann-Whitney test (B, D). ns: not statistically significant; *P<0.05; ***P<0.0005.
Figure 4.
Characterization of fetal microchimeric cells in wounds in post-partum SAD mice. (A) Heatmap showing genes, ordered by transcripts per million (TPM) and with TPM >50, expressed in eGFP+ fetal microchimeric cells (FMC) sorted from digested wounds of post-partum SAD mice at day 3. No comparison was performed in order to display the main genes expressed by FMC recruited in wounds. Membrane receptors previously reported to be enriched in blood FMC of wounded pregnant mice17 are shown. Data are presented as log2 TPM. (B) ARCHS4 enrichment analysis of the 500 most expressed genes in sorted eGFP+ FMC of post-partum SAD wounds. (C) Graphical summary obtained upon transcriptional analysis, using ingenuity pathway analysis (IPA) software, of sorted eGFP+ FMC from harvested wounds of post-partum SAD mice at day 3 after the injury. (D) Representative images of CD31+ and CD45+ cells co-stained with GFP in wound beds of post-partum SAD mice. White arrowheads show double-stained cells. The quantification of double-stained cells at day 5 is shown. Nuclei were counterstained with DAPI. Data are presented as means ± standard deviations and individual values. Scale bars represent 50 mm. (E) Volcano plot showing differentially expressed genes between wounds of post-partum and virgin SAD mice. Blue dots and red dots represent, respectively, downregulated and upregulated genes in wounds in post-partum mice compared with those in virgin mice. The labeled genes correspond to selected bone marrow and placental genes. (F) Mouse Gene Atlas enrichment analysis of upregulated genes in wounds in post-partum SAD mice as compared with wounds in virgin SAD mice. In (A-C, E and F), one 6-mm excisional wound was performed in post-partum SAD mice (n=2 mice for A-C, and n=3 mice for E and F) or virgin SAD mice (n=2 mice). In (D), one 8-mm excisional wound was performed in virgin mice (n=4) or post-partum SAD mice (n=5).
Leg ulcers represent a frequent and severe complication of SCD associated with an overall decreased survival.25 Life quality is impaired in SCD patients with leg ulcers, and can be evaluated through the substantial rates of depression, severe pain requiring class III opioid treatment, as well as the prolonged evolution and frequent relapses of the skin ulcers.3,4 Despite extensive efforts, treating leg ulcers remains challenging with high rates of failure or relapse regardless of the cause.26 Stem cell therapy has gained a lot of attention in recent years.27 Two pilot studies evaluating therapies using autologous adipose-derived stem cells28 or bone marrow mononuclear cells29 to treat SCD ulcers showed favorable outcomes. Besides, in another genetic disease leading to severe skin ulcers, namely recessive dystrophic epidermolysis bullosa, recent phase I/II clinical trials found partial efficacy of intravenous infusions of allogeneic ABCB5+ dermal mesenchymal stem cells (MSC),30 human umbilical cord blood-derived MSC,31 or bone marrow-derived MSC.32 While these studies acknowledged the current good tolerance of these donor-derived cell preparations, beneficial effects were only transient despite high concentrations of infused MSC (1x106-4x106 cells per kg of body weight).30–32 This is in agreement with these cells’ primary function as microenvironment modulators, mainly through paracrine factors and extracellular vesicle release, but highlights their limited long-term engraftment.33
Considering the multiple and complex steps needed for stem cell-based therapy as well as its transient effect, an alternative strategy for regenerative medicine consists in triggering FMC recruitment. Fetal cell microchimerism occurs very early during pregnancy and can be detected in the mothers as soon as 6 weeks of gestation.21 Several groups have demonstrated that FMC include CD34+CD38–and CD34+CD38+ hematopoietic progenitors,35,36 CD34+CD31+ endothelial progenitors,37 and CD45–CD14–CD68–CD34–SH2+Vimentin+Collagen type I– MSC.38 A few studies also documented the expression of pluripotent stem cell markers such as Oct-4, Nanog, Rex1 and Sox2 in FMC.39,40 While these cells enter the maternal bone marrow niche at steady state, they are able to respond to a maternal injury, proliferate and migrate to the damaged organ where they have a multilineage potential,41 as demonstrated in the brain, thyroid, lungs, heart, liver, gut, kidney, bone and skin in mice as in humans.42-44 We previously showed that CD34+CD11b+CD31+ FMC are recruited to the granulation tissue of maternal skin wounds in pregnant mice where they contribute to maternal repair similarly to adult marrow cells. During the early stages of wound healing, FMC mainly differentiate into CD45+ leukocytes, while at later stages, they mostly differentiate into αSMA+ mural cells and CXCL1-secreting VWF+ endothelial cells that are able to form fetal-derived vessels connected to the maternal circulation.16,17 We were able to show here that circulating FMC contained progenitors capable of forming colonies with a higher potential than their adult counterparts. In addition, fetal cells recruited to cutaneous wounds differentiate into leukocytes and endothelial cells in post-partum SAD mice. These results indicate that FMC are still potent contributors to maternal skin repair after parturition, opening the way for a new therapeutic option to treat delayed wound healing in post-partum women.
The relevance of these murine results needs to be confirmed in SCD patients. However, the course and severity of leg ulcers in SCD remain difficult to assess, as retrospective studies have specific biases that may confuse data analysis. We therefore chose to study multiple outcome measurements collected from a single center to reduce variability. As a consequence, we cannot be certain that our cohort is fully representative of the general population of female SCD patients, but it is rather representative of SCD patients with ulcers. Indeed, we confirmed a higher rate of SCD hemolytic complications in our patients as previously published in SCD patients with leg ulcers.2,7 Our data indicate that the burden related to leg ulcers is reduced in parous SCD female patients, as measured by the age-adjusted total number of ulcers and ulcer episodes. We found no significant difference between nulliparous and parous SCD women in other risk factors for leg ulcers, including venous insufficiency, arteriopathy, diabetes, obesity, smoking, vasculitis, human immunodeficiency virus infection, or autoimmunity. Besides, there was no difference in the exposure to hydroxyurea. This treatment has been associated with anti-angiogenic effects,45 and with an increased risk for leg ulcers.46 Importantly, in parous SCD patients, there was a significant reduction in total number of leg ulcers and in episodes of leg ulcers during the post-partum period as compared with the pre-partum period. Taken together, these findings support a beneficial effect of pregnancy on the course of SCD leg ulcers, at least partly through feto-maternal microchimerism. Concordantly, male SCD patients are more likely to have leg ulcers than women.5,7 A demonstration of the presence of FMC in post-partum SCD ulcers would have been important; however, it was not possible to obtain this information for ethical reasons, since biopsies worsen leg ulcers, especially in SCD patients. We observed that circulating and wound-recruited FMC in post-partum mice have a transcriptional profile close to that of cord blood cells and CD34+ progenitors. Whether these fetal cells consist of a homogeneous population of multipotent progenitors or a mixture of different progenitors with different potentialities has yet to be investigated. Post-partum FMC express high levels of several cytokine and transmembrane receptors, including Ccr2 mRNA, whose expression we previously found to be enhanced in circulating FMC in pregnant females upon injury.17 This suggests that long-term engrafted fetal progenitors may be poised to respond to a maternal injury. Since SCD ulcers are very severe and frequently resistant to usual therapies the improvement we observed in parous women appears a major result. We previously showed the possibility of amplifying FMC recruitment using low doses of chemokines that selectively recruit fetal cells;17 this could be an interesting therapeutic strategy in SCD to expand the healing effect of FMC. Self-regenerating properties are likely to explain why a very low number of fetal stem cells is able to rescue healing. While triggering the recruitment and amplification of FMC in situ appears a valid strategy in postpartum women, one would need to identify which FMC types are better at supporting wound repair in order to target them specifically. However, this strategy is not possible in nulliparous women and men, but using other fetal-derived products could be an option in these populations. We could explore pro-healing molecules secreted by FMC and present in the fetal secretome as new treatments for skin wounds. Another interesting option could be to use fetal stem cells from other sources, as demonstrated with human umbilical cord blood-derived MSC.31 Lastly, human amniotic fluid stem cells have been shown to accelerate wound healing by enhancing re-epithelialization and reducing fibrotic scarring.48,49
Table 1.
Characteristics of the patients included in the study.
Figure 5.
The frequency of leg ulcers was lower in parous women with sickle cell disease than in nulliparous women with sickle cell disease. (A, B) Total number of ulcers (A) and total number of episodes of ulcers (B) expressed as a normalized ratio over the age of each patient in the groups of nulliparous and parous patients with sickle cell disease (SCD). (C, D) Total number of ulcers (C) and total number of episodes of ulcers (D) expressed as a normalized ratio over the duration of pre-partum (from the first leg ulcer to the first pregnancy) and post-partum (from the first pregnancy to the last follow-up date) periods for each parous SCD patient who developed leg ulcers before the first pregnancy. (E, F) Correlation of normalized number of episodes of ulcers/year of life with gravidity (E) and parity (F). The linear regression curve is in red and its 95% confidence interval is in dashed black. Nineteen SCD patients with leg ulcers were included in the nulliparous group and 60 in the parous group. For the analysis of pre-partum and post-partum periods, 24 SCD patients were included. Data are presented as means ± standard deviations and individual values (A-D) or individual values (E, F). Statistical analyses were performed with a Mann-Whitney test (A, B), Wilcoxon matched-pairs signed rank test (C, D) or simple linear regression (E, F). ns: not statistically significant; *P<0.05; **P<0.005.
In conclusion, our work indicates that pregnancy leads to improved skin repair in mice, as well as in women suffering from SCD, likely through the recruitment of FMC. As FMC are detectable in about 63% of all women in western countries,50 FMC-based therapy could represent a new and original advantage for cutaneous, and presumably extra-cutaneous repair, in women who have previously been pregnant, even in the case of a miscarriage. As the beneficial effects of parity observed in mice studies or in human epidemiological studies remain limited, such a strategy would need a good way to stimulate FMC mobilization to the injured tissue.17 This requires future studies to characterize the FMC repertoire precisely in order to offer safe and effective options to amplify their recruitment and harness their full therapeutic potential in SCD patients.
Supplementary Material
Acknowledgments
We are grateful to all members of the Cutaneous Biology Laboratory for helpful discussions. We are grateful to the Genom’IC platform and animal facility of Institut Cochin, the flow cytometry facilities of Institut Curie and the imaging centers of Institut Cochin and Centre de Recherche St Antoine for technical support. We particularly thank Benjamin Saintpierre and Lucie Adoux for their help with RNA-sequencing experiments and analyses.
Funding Statement
Funding: This work was funded by grants to SA from ANR (19-CE17-0025-04-NatStem) and to BO from INSERM-Fondation Bettencourt Schueller (R20011KS).
References
- 1.Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Prim. 2018;4(1):18010. [DOI] [PubMed] [Google Scholar]
- 2.Halabi-Tawil M, Lionnet F, Girot R, Bachmeyer C, Lévy PP, Aractingi S. Sickle cell leg ulcers: a frequently disabling complication and a marker of severity. Br J Dermatol. 2007;158(2):339-344. [DOI] [PubMed] [Google Scholar]
- 3.Senet P, Blas-Chatelain C, Levy P, et al. Factors predictive of leg-ulcer healing in sickle cell disease: a multicentre, prospective cohort study. Br J Dermatol. 2017;177(1):206-211. [DOI] [PubMed] [Google Scholar]
- 4.Halabi-Tawil M, Lionnet F, Girot R, Bachmeyer C, Lévy PP, Aractingi S. Sickle cell leg ulcers: a frequently disabling complication and a marker of severity. Br J Dermatol. 2008;158(2):339-344. [DOI] [PubMed] [Google Scholar]
- 5.Koshy M, Entsuah R, Koranda A, et al. Leg ulcers in patients with sickle cell disease. Blood. 1989;74(4):1403-1408. [PubMed] [Google Scholar]
- 6.Kato GJ, McGowan V, Machado RF, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107(6):2279-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nolan VG, Adewoye A, Baldwin C, et al. Sickle cell leg ulcers: associations with haemolysis and SNPs in Klotho, TEK and genes of the TGF-beta/BMP pathway. Br J Haematol. 2006;133(5):570-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris CR. Mechanisms of vasculopathy in sickle cell disease and thalassemia. Hematology. 2008;2008(1):177-185. [DOI] [PubMed] [Google Scholar]
- 9.Dubert M, Elion J, Tolo A, et al. Degree of anemia, indirect markers of hemolysis, and vascular complications of sickle cell disease in Africa. Blood. 2017;130(20):2215-2223. [DOI] [PubMed] [Google Scholar]
- 10.Rietschel RL. Skin lesions in paroxysmal nocturnal hemoglobinuria. Arch Dermatol. 1978;114(4):560. [PubMed] [Google Scholar]
- 11.Shanmugam VK, Angra D, Rahimi H, McNish S. Vasculitic and autoimmune wounds. J Vasc Surg Venous Lymphat Disord. 2017;5(2):280-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen VT, Nassar D, Batteux F, Raymond K, Tharaux P-L, Aractingi S. Delayed healing of sickle cell ulcers is due to impaired angiogenesis and CXCL12 secretion in skin wounds. J Invest Dermatol. 2016;136(2):497-506. [DOI] [PubMed] [Google Scholar]
- 13.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99(1):665-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Donoghue K, Chan J, De La Fuente J, et al. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364(9429):179-182. [DOI] [PubMed] [Google Scholar]
- 16.Nassar D, Droitcourt C, Mathieu-d’Argent E, Kim MJ, Khosrotehrani K, Aractingi S. Fetal progenitor cells naturally transferred through pregnancy participate in inflammation and angiogenesis during wound healing. FASEB J. 2012;26(1):149-157. [DOI] [PubMed] [Google Scholar]
- 17.Castela M, Nassar D, Sbeih M, Jachiet M, Wang Z, Aractingi S. Ccl2/Ccr2 signalling recruits a distinct fetal microchimeric population that rescues delayed maternal wound healing. Nat Commun. 2017;8:15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trudel M, Saadane N, Garel MC, et al. Towards a transgenic mouse model of sickle cell disease: hemoglobin SAD. EMBO J. 1991;10(11):3157-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Droitcourt C, Khosrotehrani K, Girot R, Aractingi S. Healing of sickle cell ulcers during pregnancy: a favourable effect of foetal cell transfer? J Eur Acad Dermatology Venereol. 2008;22(10):1256-1257. [DOI] [PubMed] [Google Scholar]
- 20.Cornbleet T. Spontaneous healing of sickle-cell anemia ulcer in pregnancy. J Am Med Assoc. 1952;148(12):1025-1026. [DOI] [PubMed] [Google Scholar]
- 21.Ariga H, Ohto H, Busch MP, et al. Kinetics of fetal cellular and cell-free DNA in the maternal circulation during and after pregnancy: implications for noninvasive prenatal diagnosis. Transfusion. 2001;41(12):1524-1530. [DOI] [PubMed] [Google Scholar]
- 22.Fujiki Y, Johnson KL, Tighiouart H, Peter I, Bianchi DW. Fetomaternal trafficking in the mouse increases as delivery approaches and is highest in the maternal lung. Biol Reprod. 2008;79(5):841-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rijnink EC, Penning ME, Wolterbeek R, et al. Tissue microchimerism is increased during pregnancy: a human autopsy study. Mol Hum Reprod. 2015;21(11):857-864. [DOI] [PubMed] [Google Scholar]
- 24.Cómitre-Mariano B, Martínez-García M, García-Gálvez B, et al. Feto-maternal microchimerism: memories from pregnancy. iScience. 2022;25(1):103664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minniti CP, Eckman J, Sebastiani P, Steinberg MH, Ballas SK. Leg ulcers in sickle cell disease. Am J Hematol. 2010;85(10):831-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aleksandrowicz H, Owczarczyk-Saczonek A, Placek W. Venous leg ulcers: advanced therapies and new technologies. Biomedicines. 2021;9(11):1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raghuram AC, Yu RP, Lo AY, et al. Role of stem cell therapies in treating chronic wounds: a systematic review. World J Stem Cells. 2020;12(7):659-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farina Junior JA, De Santis GC, Orellana MD, et al. Autologous adipose-derived stem cell for painful leg ulcers in patients with sickle cell disease. A preliminary study. Br J Haematol. 2019;186(3):e47-e50. [DOI] [PubMed] [Google Scholar]
- 29.Meneses JVL, Fortuna V, de Souza ES, et al. Autologous stem cell-based therapy for sickle cell leg ulcer: a pilot study. Br J Haematol. 2016;175(5):949-955. [DOI] [PubMed] [Google Scholar]
- 30.Kiritsi D, Dieter K, Niebergall-Roth E, et al. Clinical trial of ABCB5+ mesenchymal stem cells for recessive dystrophic epidermolysis bullosa. JCI Insight. 2021;6(22):e151922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SE, Lee S-J, Kim S-E, et al. Intravenous allogeneic umbilical cord blood–derived mesenchymal stem cell therapy in recessive dystrophic epidermolysis bullosa patients. JCI Insight. 2021;6(2):e143606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rashidghamat E, Kadiyirire T, Ayis S, et al. Phase I/II open-label trial of intravenous allogeneic mesenchymal stromal cell therapy in adults with recessive dystrophic epidermolysis bullosa. J Am Acad Dermatol. 2020;83(2):447-454. [DOI] [PubMed] [Google Scholar]
- 33.Krampera M, Le Blanc K. Mesenchymal stromal cells: putative microenvironmental modulators become cell therapy. Cell Stem Cell. 2021;28(10):1708-1725. [DOI] [PubMed] [Google Scholar]
- 34.Kinder JM, Stelzer IA, Arck PC, Way SS. Immunological implications of pregnancy-induced microchimerism. Nat Rev Immunol. 2017;17(8):483-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996;93(2):705-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guetta E, Gordon D, Simchen MJ, Goldman B, Barkai G. Hematopoietic progenitor cells as targets for non-invasive prenatal diagnosis: detection of fetal CD34+ cells and assessment of post-delivery persistence in the maternal circulation. Blood Cells Mol Dis. 2003;30(1):13-21. [DOI] [PubMed] [Google Scholar]
- 37.Parant O, Dubernard G, Challier JC, et al. CD34+ cells in maternal placental blood are mainly fetal in origin and express endothelial markers. Lab Investig. 2009;89(8):915-923. [DOI] [PubMed] [Google Scholar]
- 38.O’Donoghue K. Identification of fetal mesenchymal stem cells in maternal blood: implications for non-invasive prenatal diagnosis. Mol Hum Reprod. 2003;9(8):497-502. [DOI] [PubMed] [Google Scholar]
- 39.Mikhail MA, M’Hamdi H, Welsh J, et al. High frequency of fetal cells within a primitive stem cell population in maternal blood. Hum Reprod. 2008;23(4):928-933. [DOI] [PubMed] [Google Scholar]
- 40.Cismaru CA, Soritau O, Jurj A-M, et al. Isolation and characterization of a fetal-maternal microchimeric stem cell population in maternal hair follicles long after parturition. Stem Cell Rev Rep. 2019;15(4):519-529. [DOI] [PubMed] [Google Scholar]
- 41.Khosrotehrani K. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA. 2004;292(1):75. [DOI] [PubMed] [Google Scholar]
- 42.Bianchi DW, Khosrotehrani K, Way SS, MacKenzie TC, Bajema I, O’Donoghue K. Forever connected: the lifelong biological consequences of fetomaternal and maternofetal microchimerism. Clin Chem. 2021;67(2):351-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boddy AM, Fortunato A, Wilson Sayres M, Aktipis A. Fetal microchimerism and maternal health: a review and evolutionary analysis of cooperation and conflict beyond the womb. Bioessays. 2015;37(10):1106-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cismaru CA, Pop L, Berindan-Neagoe I. Incognito: are microchimeric fetal stem cells that cross placental barrier real emissaries of peace? Stem Cell Rev Rep. 2018;14(5):632-641. [DOI] [PubMed] [Google Scholar]
- 45.Lopes FCM, Ferreira R, Albuquerque DM, et al. In vitro and in vivo anti-angiogenic effects of hydroxyurea. Microvasc Res. 2014;94:106-113. [DOI] [PubMed] [Google Scholar]
- 46.Sirieix M-E, Debure C, Baudot N, et al. Leg ulcers and hydroxyurea. Arch Dermatol. 1999;135(7):818-820. [DOI] [PubMed] [Google Scholar]
- 47.Ritzel RM, Patel AR, Spychala M, et al. Multiparity improves outcomes after cerebral ischemia in female mice despite features of increased metabovascular risk. Proc Natl Acad Sci U S A. 2017;114(28):E5673-E5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fukutake M, Ochiai D, Masuda H, et al. Human amniotic fluid stem cells have a unique potential to accelerate cutaneous wound healing with reduced fibrotic scarring like a fetus. Hum Cell. 2019;32(1):51-63. [DOI] [PubMed] [Google Scholar]
- 49.Sun Q, Li F, Li H, et al. Amniotic fluid stem cells provide considerable advantages in epidermal regeneration: B7H4 creates a moderate inflammation microenvironment to promote wound repair. Sci Rep. 2015;5(1):11560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gilmore GL, Haq B, Shadduck RK, Jasthy SL, Lister J. Fetal-maternal microchimerism in normal parous females and parous female cancer patients. Exp Hematol. 2008;36(9):1073-1077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-sequencing datasets produced in this study are available in Online Supplementary Tables S1-S4.