Chimeric antigen receptor (CAR) therapy is a novel immunotherapy that is based on the genetic targeting and reprogramming of immune cells to rapidly provide effective immunity. CAR are synthetic receptors for antigen that typically comprise an extracellular antigen-recognition domain (most often consisting of an scFv derived from an antibody specific for the targeted cell-surface molecule) and a dual-signaling intracellular domain that initiates T-cell activation and augments T-cell functions through costimulatory signals provided by CD28 or 4-1BB cytoplasmic domains.1 Various extracellular scaffold and transmembrane elements may be interposed between the antigen-binding and signaling moieties. The targeting of CD19, a cell surface molecule found in most leukemias and non-Hodgkin lymphomas, has established the formidable potency of CAR T-cell therapy in the clinic2 and paved the way for a vast spectrum of potential CAR therapies for other hematologic malignancies, solid tumors, and several pathologies beyond cancer.3 Six CAR therapies have so far been approved in the US, four of which target CD19 and two B-cell maturation antigen (BCMA), an antigen commonly found in multiple myeloma. BCMA binds to its ligands, BAFF (B-cell activating factor) and APRIL (a proliferation-inducing ligand), and promotes survival in plasma cells. BCMA is a favorable CAR target owing to its restricted expression in B cells and plasma cells, including malignant plasma cells. The two CAR therapies that are approved for the treatment of refractory/relapsed multiple myeloma, idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel), consist of auto-logous T cells that are lentivirally transduced to express a CAR binding to BCMA through an scFv or two llama VHH elements and signaling through 4-1BB and CD3ζ cytoplasmic domains (Figure 1A). The remarkable response rates following BCMA CAR treatment led to US Food and Drug Administration (FDA) approval of ide-cel in March 2021 and cilta-cel in February 2022.
Challenges remain, in particular in terms of efficacy and access to therapy. Although initial response rates are excellent, many patients will eventually relapse. Furthermore, since most CAR products require autologous manufacturing and are thus personalized for each patient, commercial production in centralized facilities is both expensive, and possible delays impact CAR T-cell availability. In this issue of Haematologica, Asherie and co-workers describe their findings in a phase I dose escalation clinical trial with a BCMA CAR T-cell product, HBI0101, developed in-house and locally manufactured for the first time in Israel.4 The CAR molecule itself comprises an scFv derived from the C11D5.3 anti-BCMA monoclonal antibody, the hinge and transmembrane domains of CD8α and arrayed 4-1BB and CD3ξ cytoplasmic domains. The CAR design is similar in concept to idecabtagene vicleucel (ide-cel) but differs in using a γ-retroviral vector for its transduction to patient T cells. The clinical team enrolled 20 patients with relapsed and/or refractory multiple myeloma. The study shows a good safety profile (on the whole similar to other BCAM CAR T-cell phase I-II studies) and similar efficacy (albeit with shorter follow-up) to that initially reported with the later FDA-approved BCMA CAR T cells (75% overall response rate [ORR]; 85% ORR in the group given the highest CAR T-cell dose,4 compared with 85% in the phase I trial evaluating ide-cel5 and 97% in the phase 1b/II trial for cilta-cel6). The Jerusalem trial included nine patients who had relapsed after treatment with an anti-BCMA antibody, belantamab mafodotin, prior to receiving HBI0101, which the authors suggested may be associated with a less favorable response to CAR therapy, although there were no differences in BCMA levels or in the frequency of positive plasma cells compared to patients who had not been treated with belantamab mafodotin. The authors further suggest that CD56 expression in plasma cells may be a favorable prognostic biomarker as 70% of responders were positive for CD56 while non-responders were all CD56-negative. The Jerusalem trial is a small study with a median follow-up of 136 days, and findings need to be substantiated in a larger cohort and with longer follow-up.
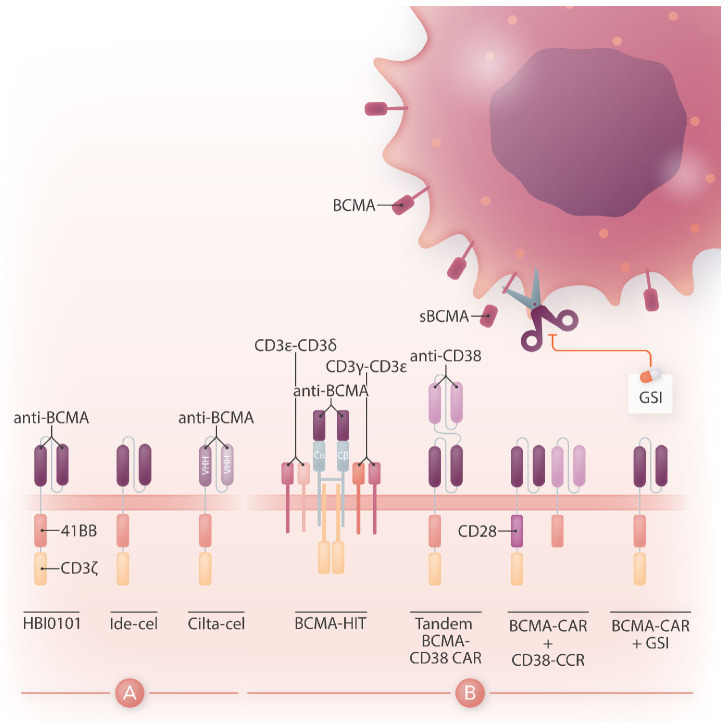
Figure 1.
Design of HBI0101 and new CAR strategies to target B-cell maturation antigen-low multiple myeloma. (A). HBI0101 is a novel chimeric antigen receptor (CAR) that incorporates 4-1BB and CD3ξ signaling moieties, similar to both US Food and Drug Administration-approved B-cell maturation antigen (BCMA) CAR. Cilta-cel comprises two llama-based VHH regions that bind to two different epitopes of BCMA and thereby increase overall binding affinity. (B) To minimize BCMA-low escape, several new CAR designs have been proposed. These include, from left to right, the use of a BCMA HIT receptor, a tandem CAR engaging CD38 and BCMA, a BCMA CAR co-expressed with a CD38 CCR and the use of y-secretase inhibitors to block the shedding of soluble BCMA. Ide-cel: idecabtagene vicleucel; cilta-cel: ciltacabtagene autoleucel; CCR: chimeric co-stimulatory receptor; VHH: variable domain on a heavy-chain; GSI: y-secretase inhibitor; sBCMA: soluble BCMA; HIT: HLA (human leukocyte antigen)-independent T-cell receptor (TCR); Cα/β: constant regions of αβ-TCR.
What makes this study so remarkable is how two academic groups came together to construct a CAR, set up a Current Good Manufacturing Practice production facility, and completed a clinical trial in record time. The laboratory of Cyrille Cohen at Bar-Ilan University built a BCMA CAR vector while Polina Stepensky and her team established the facilities and procedures for local CAR T-cell manufacturing at the Hadassah Medical Center. Having hatched the project in 2018, they opened the trial in February 2021, and had infused 20 subjects by December 2021. This exploit will likely inspire others in the academic world who are tempted to part take in projects to advance CAR therapy and may not have thought it possible.
While there is a need to meet the increasing demand for CAR T-cell therapy and shorten the vein-to-vein delivery time, it is also essential to improve CAR T-cell designs for multiple myeloma since most of the patients treated with BCMA CAR will eventually relapse. One mechanism behind treatment failure is antigen-low relapse.7 It is noteworthy that all current BCMA CAR in the clinic are of the 4-1BB type, which several studies have shown to be less sensitive to low antigen density than CD28-based CAR.8 Other strategies are under evaluation to more effectively target BCMA (Figure 1B). One is to stabilize BCMA on the cell surface by blocking its cleavage using a γ-secretase inhibitor.9 Another is to increase the avidity of CAR T cells for BCMA-positive cells by co-expressing along with the BCMA CAR a second scFv or chimeric co-stimulatory receptor binding to CD38.10 A bi-specific or tandem CAR engaging both BCMA and CD38 has also shown increased cytotoxicity against multiple myeloma cell lines.11 Finally, novel CAR designs co-opting the CD3 complex, such as the HIT receptor, also increase sensitivity to BCMA.12 All in all, BCMA targeted CAR T-cell therapy offers the prospect for improved outcome in heavily pre-treated patients with multiple myeloma. Increased accessibility to this therapy will benefit patients worldwide. Novel CAR designs introduced into T cells and other immune cell types that avert late relapse have the potential to further improve the efficacy of this therapy and will shape the future of CAR therapy for multiple myeloma.
References
- 1.Sadelain M, Rivière I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545(7655):423-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379(1):64-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin AG, Riviere I, Eshhar Z, Sadelain M. CAR T cells: building on the CD19 paradigm. Eur J Immunol. 2021;51(9):2151-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asherie N, Kfir-Erendeld S, Avni B, et al. Development and manufacture of novel locally produced anti-BCMA CAR T cells for the treatment of relapsed/refractory multiple myeloma: results from a phase I clinical trial l. Haematologica. 2023:108(7):1827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314-324. [DOI] [PubMed] [Google Scholar]
- 7.Roex G, Timmers M, Wouters K, et al. Safety and clinical efficacy of BCMA CAR-T-cell therapy in multiple myeloma. J Hematol Oncol. 2020;13(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamieh M, Dobrin A, Cabriolu A, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568(7750):112-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pont MJ, Hill T, Cole GO, et al. γ-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood. 2019;134(19):1585-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katsarou A, Sjostrand M, Naik J, et al. Combining a CAR and a chimeric costimulatory receptor enhances T cell sensitivity to low antigen density and promotes persistence. Sci Transl Med. 2021;13(623):eabh1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei H, Li C, Jiang H, et al. A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma. J Hematol Oncol. 2021;14(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansilla-Soto J, Eyquem J, Haubner S, et al. HLA-independent T cell receptors for targeting tumors with low antigen density. Nat Med. 2022;28(2):345-352. [DOI] [PMC free article] [PubMed] [Google Scholar]