Abstract
Objective:
Methotrexate (MTX) remains the first-choice disease-modifying therapy in rheumatoid arthritis (RA). However, clinical response is variable, and no reliable predictive biomarkers of efficacy currently exist. In this study, plasma metabolomic profiling is evaluated as a tool to identify pretreatment biomarkers of MTX response in RA.
Methods:
Plasma collected from RA patients initiating MTX therapy (n=20) were analyzed by metabolomic profiling totaling 648 identified metabolites. Pretreatment metabolomic profiles were compared based on clinical response after 16-weeks of MTX therapy. Clinical response to MTX was defined by a clinically meaningful reduction in disease activity score in 28 joints (DAS28-ESR) of greater than 1.2.
Results:
Pretreatment plasma levels of 19 metabolites were found to differ (p<0.05) between RA patients based on response to MTX at 16-weeks. Spearman’s correlation of pretreatment plasma metabolite levels with change in DAS28-ESR over the treatment period further supported three of the identified metabolites as associated with MTX response (p<0.05). The identified metabolite levels were all found to be lower in RA patients responsive to MTX but were not found to be intercorrelated. Receiver operating characteristic analysis of each of the identified metabolites, alone or in combination, demonstrated an excellent discrimination between responders and non-responders based on pretreatment plasma levels of nornicotine (AUC=0.84), N-methylisoleucine (AUC=0.82), 2,3-dihydroxybutanoic acid (AUC=0.82), and a combination biomarker panel score (AUC=0.98).
Conclusion:
Pretreatment plasma metabolomic profiling identified multiple metabolites associated with early response to MTX therapy in RA and represents a promising approach for the identification of clinical biomarkers of MTX response in RA.
Keywords: metabolomics, rheumatoid arthritis, methotrexate, biomarker, plasma, metabolome
Graphical Abstract

Introduction
Despite the expansion of disease modifying therapies available for the treatment of rheumatoid arthritis (RA), methotrexate (MTX) remains the first-choice agent for the treatment of RA based on its safety, efficacy, and cost (1). However, up to two-thirds of RA patients fail to achieve an adequate response in the first 6 months following initiation of MTX resulting in the need for treatment escalation (2). Recognizing that early disease control through initiation of effective disease modifying therapy is an important predictor of clinical outcomes in RA, a need exists for the identification of biomarkers that are predictive of response to therapy that will allow for early stratification of patients and individualization of drug therapy in RA (1).
Advances in high-resolution mass spectrometry and small-molecule metabolite identification has allowed for the rapid screening and characterization of the metabolome in diverse biological samples. Metabolomics allows for the measurement of hundreds to thousands of exogenous and endogenous low molecular weight compounds and provides insight into the biochemical status of a biological system in relation to a given disease state or in association with a therapeutic intervention (3). Previous work in our group has employed metabolomics to elucidate disease-related biochemical changes in RA, providing a better understanding of the metabolic signature of RA, and of the biochemical response to MTX (4). In this work, analysis of the pretreatment plasma metabolome in RA patients is used to identify potential clinical biomarkers associated with early treatment response to MTX.
Patients and Methods
Patients
Plasma samples were obtained from a cohort of RA patients initiating MTX (n=20) that participated in a 16-week open-label study involving sites within the Rheumatology Arthritis Investigational Network (NCT03414502). Plasma collected prior to the initiation of MTX was analyzed in this work. Patients received 15 mg/week of MTX orally or subcutaneously and 1 mg/day of folic acid orally daily upon enrollment. MTX was escalated to 20 mg/week in patients not reaching clinical remission by 8 weeks, as tolerated. Eligibility for participation in this study included: ≥19 years of age, fulfillment of the 1987 American College of Rheumatology criteria for RA, no prior exposure to MTX within the last 12 months, no or stable doses of prednisone for the last 2 weeks (not to exceed 10 mg/day of prednisone) and receiving no or stable doses of non-steroidal anti-inflammatory drugs for the last week (5). Patients excluded from the study included patients with other rheumatic diseases, pregnant or lactating women, women of childbearing age not practicing an effective method of contraception, patients with a history of alcohol abuse who are unwilling to abstain or limit alcohol consumption, and patients with cytopenias or abnormal hepatic or renal function at the time of screening. All patients provided informed consent and all studies were conducted under the University of Nebraska Medical Center Institutional Review Board approved protocols.
Clinical Data
Clinical data were collected on all study participants prior to initiation of MTX and at their 16-week follow up visit. Data included the patient’s global assessment (0–100 mm visual analog scale), 28-joint swollen and tender joint counts (SJC; TJC), and erythrocyte sedimentation rate (ESR, mm/hr). From these components, the DAS28-ESR was calculated (6). The primary response outcome in this study was defined as a clinically significant improvement in disease activity based on reduction in the DAS28-ESR of greater than 1.2 (1).
Metabolomics Analysis
Plasma samples were analyzed by the National Institutes of Health (NIH) West Coast Metabolomics Center at the University of California, Davis (Davis, CA, USA). Plasma was analyzed using three independent analytical methods for the relative quantification of intermediates of primary metabolism, biogenic amines, and lipids (7). Briefly, samples were prepared using a biphasic liquid-liquid extraction protocol standardized for metabolomic profiling in plasma and serum using the three analytical platforms in this study. Peaks were identified by retention time and mass spectral data from MassBank of North America. Peak height intensity tables were curated by the NIH West Coast Metabolomics Center and submitted to Metabolomics Workbench (https://www.metabolomicsworkbench.org/) under Project ID 2895. The raw peak intensity data were normalized using a standard normalization procedure (7). Metabolites observed in more than one analytical platform were combined by mean normalization to ensure equal weighting in a given platform. Normalized peak height intensities were uploaded into MetaboAnalyst (Version 5.0, McGill University; Montreal, Quebec, Canada), processed by logarithmic transformation and auto-scaled according to unit variance. Metabolomic data were evaluated by univariate analysis to identify metabolites differentiating responders and non-responders based on a p-value threshold of 0.05.
Enrichment Analysis
The fold-change and p-values for metabolites associated with response to MTX were processed using MetaMapp 2020 and visualized in Cytoscape (Version 3.7.2, Institute for Systems Biology, Seattle, Washington, DC, USA). The metabolic network map was generated based upon chemical similarity utilizing the Kyoto Encyclopedia of Genes and Genomes metabolic network database and Tanimoto substructure similarity coefficients (8).
Statistical Analysis
Spearman’s rank correlation analysis was used to determine correlation coefficients (ρ) and evaluate associations between continuous variables. Unpaired group comparisons were conducted by Wilcoxon rank-sum testing. Data analysis and statistical testing was performed using JMP Pro 15 (SAS Institute, Cary, NC). Performance of each individual marker was assessed by receiver operating characteristic (ROC) analysis (9) by calculation of empirical area under the ROC analyses curve (AUC). Markers were combined using the linear term of a logistic regression model with a logit link function. AUCs are reported as 95% bootstrap-based confidence intervals with two-sided p-values and the optimal operating point was determined based on the maximization of the Youden index (10).
Results
Patient Demographic and Clinical Data
Study participants included patients with RA initiating MTX (n=20). The median [IQR] age was 53 [38,66] years and was 70% female (n=14). The median [IQR] time from symptom onset to diagnosis was 9 [4,14] months, and the time from diagnosis to initiation of MTX was 0 [0,3] months. The median [IQR] DAS28-ESR was 4.9 [4.1,6.0] prior to initiating MTX and 3.0 [1.7,4.7] at 16-weeks following the initiation of MTX (p=0.0007). Eleven patients (55%) had a significant response to MTX at 16-weeks. The pretreatment demographic and clinical data in patients responsive or non-responsive to MTX at 16-weeks are provided for comparison (Table 1). There were no statistically significant differences in the pretreatment demographic or clinical measures of disease between RA patients based on response at 16-weeks, including SJC, TJC, patient global assessment, and DAS28-ESR. As expected, the change in DAS28-ESR over the 16-week treatment period (ΔDAS28-ESR) was significantly greater for those responsive to MTX at 16-weeks.
Table 1.
Pretreatment demographic and clinical data for the study population.
| Pretreatment | Non-Responders | Responders | p-value |
|---|---|---|---|
| Subjects, n | 9 | 11 | — |
| Female sex, n (%) | 6 (67%) | 8 (73%) | 1.00 |
| Current Smoker, n (%) | 3 (33%) | 1 (9%) | 0.28 |
| Age (years) | 59 [43,75] | 49 [24,64] | 0.30 |
| Time from Symptoms to Diagnosis (mo) | 7.5 [3.5,16] | 9 [3.5,22.5] | 1.00 |
| Time from Diagnosis to MTX Initiation (mo) | 0 [0,4.5] | 0 [0,6] | 1.00 |
| SJC (0–28) | 4 [3,9] | 7 [6,15] | 0.32 |
| TJC (0–28) | 4 [2,9] | 7 [5,18] | 0.09 |
| ESR (mm/hr) | 19 [14,34] | 25 [16,33] | 0.42 |
| Patient Global Assessment (0–100mm) | 49 [37,61] | 44 [34,65] | 0.70 |
| DAS28-ESR | 4.6 [3.9,5.5] | 5.7 [4.7,6.2] | 0.11 |
| ΔDAS28-ESR | −0.50 [−0.75,0.35] | −3.6 [−4.6,−2.7] | 0.002 |
RA patients were characterized as “Responders” and “Non-Responders” based on a reduction in DAS28-ESR of greater than or less than 1.2. respectively. Data is presented as median [IQR] unless otherwise noted. Swollen Joint Count (SJC); Tender Joint Count (TJC); Erythrocyte Sedimentation Rate (ESR); Disease Activity Score (DAS28-ESR); Change in DAS28-ESR (ΔDAS28-ESR).
Pretreatment plasma metabolic markers associated with response to MTX
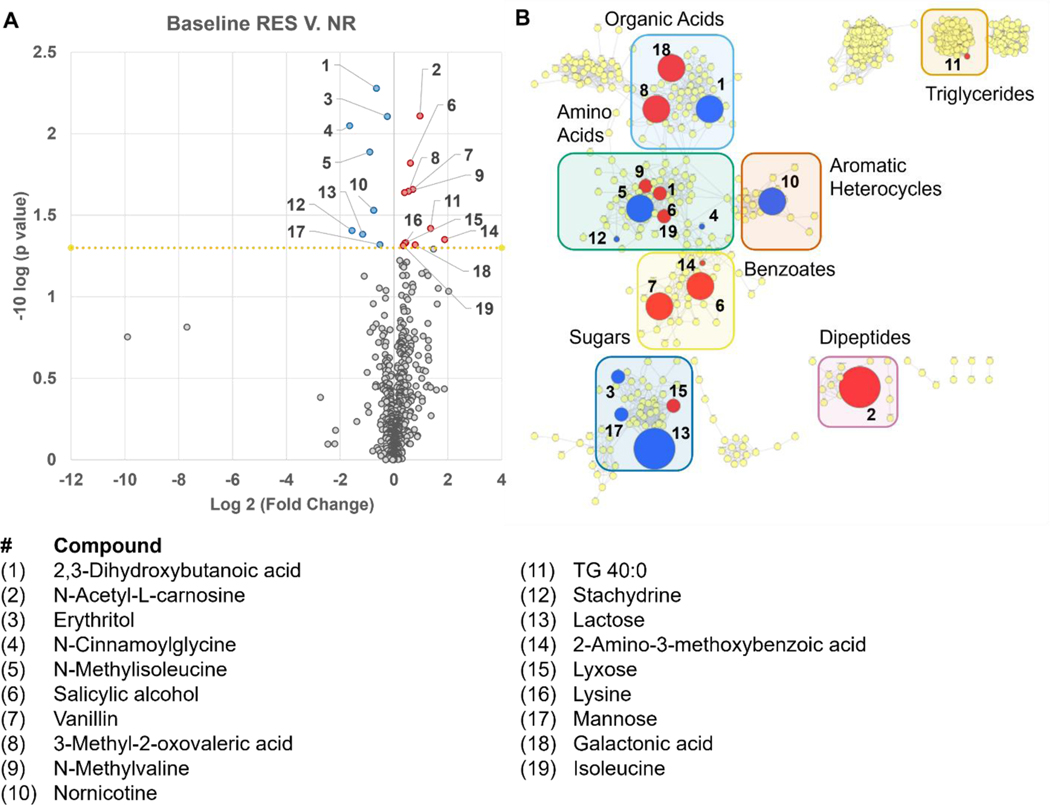
Pretreatment plasma metabolomic profiles were measured and compared based on MTX response at 16-weeks (Figure 1A). Due to the small sample size employed, the large number of metabolites analyzed, and the discovery-focused approach in this proof-of-concept analysis, no filtering of metabolites based on covariance or corrections for multiple testing were applied. Of the 648 plasma metabolites measured, 19 significantly differed based on patient response to MTX (p<0.05). Identified metabolites were mapped based on chemical structural similarity and biochemical pathways (Figure 1B). The resulting network map was divided into clusters and labeled by metabolite class. Several classes of metabolites were identified, including triglycerides (n=1), benzoates (n=3), dipeptides (n=1), aromatic heterocycles (n=1), organic acids (n=3), amino acids (n=4), and sugars (n=4). One substrate-product pair was identified and represented the methylation of isoleucine to form N-methylisoleucine. Response was associated with increased plasma levels of isoleucine and reduced levels of N-methylisoleucine.
Figure 1.
Pretreatment plasma metabolomic differences associated with MTX response at 16-weeks. Pretreatment plasma levels of 648 identified metabolites were compared between patients based on their response to MTX therapy at 16-weeks. Response to MTX was defined as a reduction in DAS28-ESR of greater than 1.2. Differences in the pretreatment plasma metabolome are presented as a (A) volcano plot and a (B) metabolic network map identifying metabolites found to be significantly (p<0.05) increased (red) or decreased (blue) in the pretreatment plasma of patients responsive to MTX. The metabolic network map is divided into clusters using the community cluster tool and labeled by class of metabolite. The node size on the metabolic network map is directly proportional to the measured fold-change.
Pretreatment plasma metabolites as biomarkers of MTX efficacy
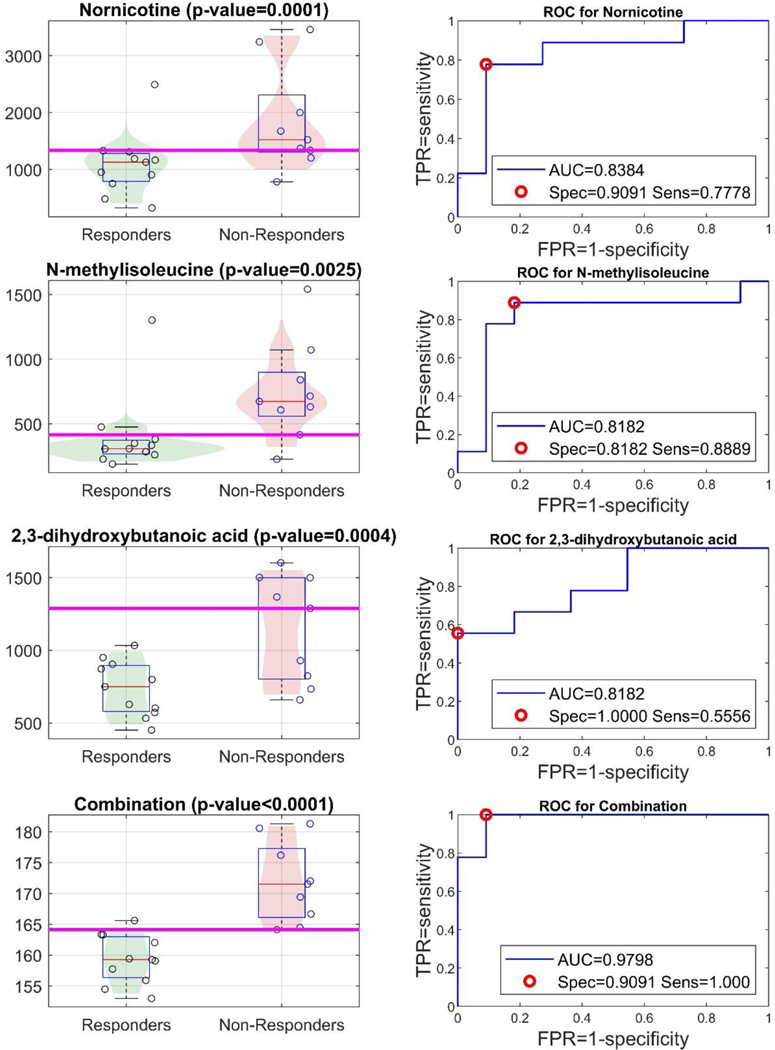
Recognizing that the small sample size limited the application of corrections for multiple testing and increased the risk of false positives, a separate regression analysis approach was applied to filter the identified metabolites and increase the confidence in their relationship with disease response to MTX. The relationship between pretreatment plasma levels of the 19 identified metabolites and ΔDAS28-ESR over the 16-week treatment period for the study cohort was evaluated by Spearman’s rank correlation analysis. Pretreatment levels of three of the 19 metabolites identified significantly correlated with ΔDAS28-ESR over the 16-week treatment period and included: N-methylisoleucine (ρ=0.54, p=0.02), 2,3-dihydroxybutanoic acid (ρ=0.51, p=0.02), and nornicotine (ρ=0.50, p=0.02). Lower pretreatment plasma levels of each of these metabolites were associated with a greater reduction in DAS28-ESR over the 16-week treatment period, and no significant correlation was observed between pretreatment plasma levels of the metabolites.
ROC analyses were conducted to evaluate the ability of the proposed pretreatment plasma biomarkers to discriminate between responders and non-responders at 16-weeks (Figure 2). The Youden optimal point for nomicotine and 2,3-dihydroxybutanoic acid were found to provide high specificities and relatively lower sensitivities, and N-methylisoleucine provided a high sensitivity with a relatively lower specificity. Recognizing the lack of intercorrelation of the biomarkers and the potential complementary tradeoff in sensitivity and specificity, the three biomarkers were combined to create a combination score to evaluate their combined performance in differentiating responders and non-responders.
Figure 2.
Pretreatment plasma metabolites as potential discriminatory biomarkers of MTX response at 16 weeks. Performance of pretreatment plasma metabolite levels based on MTX response at 16 weeks by univariate analysis for nornicotine, N-methylisoleucine, 2,3-dihydroxybutanoic acid, and by multivariate analysis using a combination pseudo-score (marker-panel). Receiver operating characteristic (ROC) curves and the Youden-optimal operating points are illustrated (right panel). For each biomarker, or combination, the distribution (box-plots and actual scores) along with the Youden-based optimal cutoff is illustrated on the left panel.
Plasma nornicotine levels, smoking status, and MTX response
Nornicotine plasma levels were assessed based on smoking status, as nornicotine is a metabolite of nicotine, and levels were found to be 1.5-fold higher in smokers (p=0.10). Patients were stratified based on smoking status, and nornicotine plasma levels were compared between responders and non-responders. In non-smokers, median plasma nornicotine levels were 1.3-fold higher in non-responders (p=0.07), and in smokers, median plasma nornicotine levels were 1.7-fold higher in non-responders (p=0.37).
Discussion
In this study, plasma metabolomic profiling was applied towards the identification of pretreatment metabolites associated with MTX efficacy in RA to demonstrate its potential as a biomarker discovery tool. Differences in the plasma metabolome between responders and non-responders to MTX were identified using a metabolomic analysis that included 648 metabolites representing a diversity of metabolic classes. With a sample size of 20 RA patients initiating MTX, we identified 19 metabolites in pretreatment plasma that significantly differed between patients based on response to MTX after 16-weeks of therapy. Evaluation of the relationship between pretreatment plasma metabolite levels and change in DAS28-ESR over the treatment period resulted in a reduction in the number of metabolites of interest to three: nornicotine, N-methylisoleucine, and 2,3-dihydroxybutanoic acid. ROC analysis was used to evaluate the ability of the identified pretreatment plasma biomarkers to discriminate between MTX responders and non-responders resulting in individual AUCs of greater than 0.80 and a multi-biomarker ROC with an AUC of 0.98. The excellent discrimination between MTX responders and non-responders based on pretreatment plasma metabolomic markers in this work supports the application of metabolomics as a biomarker discovery tool for individualized therapy in the treatment of RA. Although these results show great promise, we acknowledge the sample size is small and thus these findings need to be subjected to external validation.
Metabolomic profiling has been used to evaluate metabolic changes associated with disease activity in RA and a limited number of studies have applied this technique to identify predictive biomarkers of MTX response in RA (11,12,13). While this study is limited by the number of patients compared to previous studies, it represents the broadest coverage of the plasma metabolome and includes a diversity of metabolites not included in prior studies. Similar to the previous studies this work did not attempt to correct for multiple testing in the identification of significant metabolites but will need to be a consideration as this approach is applied to larger discovery and validation cohorts. Recognizing the risk of false positives, a complementary regression analysis between baseline metabolite levels and absolute change in DAS28-ESR over the 16-week treatment period was used here. We recognize this is an imperfect approach as ΔDAS28-ESR and clinical response based on a reduction in the DAS28-ESR of greater than 1.2 are highly related. However, the consistency in the relationship of nornicotine, N-methylisoleucine, and 2,3-dihydroxybutanoic acid with response to MTX across the two analyses increases the confidence in the relationship of baseline levels of these metabolites with early response to MTX. Previous works have identified intermediates of energy metabolism, amino acids, nucleotides, and products of gut microbial metabolism as potential predictors of MTX response (11,12,13). Although the 19 metabolites identified in this study can be broadly categorized within these metabolic classes, there was limited overlap in individual metabolites identified, with the exception of the association of increased levels of lysine with response to MTX (13). Previous targeted analysis of erythrocyte folate concentrations has identified low pretreatment folate concentrations as predictive of MTX non-response (14). Unfortunately, folate levels were not measured in these patients as part of their clinical care and none of the circulating forms of folate were measured in the metabolomics platform employed in this study.
Mapping of the 19 identified metabolites demonstrated differences in various biochemical classes, including: triglycerides, benzoates, dipeptides, aromatic heterocycles, organic acids, amino acids, and sugars. Among the amino acids identified were the substrate-product pair, isoleucine and N-methylisoleucine. Although decreased levels of isoleucine have been previously observed in active RA (15), we are the first to find higher pretreatment plasma levels associated with MTX response. Further, we found that decreased levels of the product of isoleucine methylation (i.e., N-methylisoleucine) is associated with MTX response, suggesting that reduced N-methyltransferase mediated metabolism of isoleucine may be associated with MTX responsiveness in RA. N-methylation of isoleucine is not known to occur through endogenous N-methyltransferase activity, but likely represents enzymatic biotransformation of isoleucine by the gut microbiota (16). These findings are supported by previous work in the collagen-induced arthritis mouse model that found decreases in N-methylisoleucine associated with therapeutic response to MTX (17). Despite the efficiency of protein metabolism and amino acid absorption in the small intestine a substantial amount of protein/peptide enter the large intestine and are metabolized to free amino acids with free isoleucine concentrations ranging from 1.0 to 15.0 mmol/g of feces (18). Within the large intestine amino acids are subject to metabolism by the microbiota resulting in the formation of numerous bacteria-derived metabolites. Although the mass of N-methylisoleucine normally formed by the gut microbiota hasn’t been quantified, it has been found to be sensitive to diet and is increase in individuals following transition to a Mediterranean diet (19). Methylation of amino acids represents an approach to increase the bioavailability of peptides through enhanced passive diffusion and increased metabolic stability (20), however the relative bioavailability of N-methylisoleucine relative to isoleucine is not known. Together, these findings support evidence that the gut microbiome may play an important role in the pharmacological activity of MTX in the treatment of RA (21).
In addition to N-methylisoleucine, lower pretreatment plasma concentrations of 2,3-dihydroxybutanoic acid and nornicotine were also found to be associated with MTX response at 16 weeks. As a human metabolite, 2,3-dihydroxybutanoic acid was first identified in the plasma of patients with juvenile-onset Type 1 diabetes as an organic acid metabolite of threonine metabolism (22). Subsequent studies in acute myelogenous leukemia patients identified 2,3-dihydroxybutanoic acid as an endogenous plasma biomarker of isocitrate dehydrogenase activity (23). Although isocitrate dehydrogenase hasn’t been implicated in RA, isocitrate dehydrogenase activity is important in the activation and proliferation of mitogen stimulated lymphocytes in vitro and in vivo (24). As a result, reduced plasma 2,3-dihydroxybutanoic acid levels may represent reduced isocitrate dehydrogenase activity associated with reduced activation and proliferation of lymphocytes in RA patients responsive to MTX.
Nornicotine is a circulating metabolite found as a minor alkaloid in tobacco that can also be formed through the endogenous biotransformation of nicotine and represents a biomarker of smoking (25). Smoking has been associated with reduced response to MTX (26) and is the most well-established environmental risk factor for RA (27). Therefore, it was not surprising that lower plasma nornicotine levels were associated with improved response to MTX. Plasma nornicotine levels were elevated in patients identifying as current smokers but failed to reach statistical significance. Stratification of patients based on smoking status demonstrated a similar relationship between pretreatment plasma nornicotine levels and response to MTX in smokers and non-smokers, with higher levels of nornicotine associated with non-response to MTX, although statistical significance was not reached in these subgroups. Elevated levels of nornicotine in those found non-responsive to MTX may indicate patients have not been honest regarding smoking status or the potential influence of environmental exposure, such as secondhand smoke or alternative forms of nicotine intake/exposure. As a biomarker of smoking or smoke exposure, elevated nornicotine levels may be indicative of a persistent proinflammatory state that is associated with smoking, increasing susceptibility to RA, and reducing the pharmacological response to MTX (26). Nornicotine levels may also represent a biomarker of altered MTX pharmacokinetics, as smoking has been previously found to be associated with reduced tissue accumulation of MTX and its bioactive polyglutamate metabolites (28).
The data presented here is intended to be hypothesis generating. Metabolomic analyses have been shown to be sensitive to diet and levels of physical activity which were not able to be controlled for in this study (4). The power of this study is limited due to its small sample size and large number of variables tested for. In order to draw further conclusions these analyses must be applied to larger and more diverse cohorts that will allow for application of false discovery rate corrections and validation in independent cohorts. Nevertheless, the data presented in this study serves as preliminary evidence of the ability to use metabolomic analysis as a discovery tool in the identification of predictive biomarkers of therapeutic success of MTX in RA.
Acknowledgements
The authors would like to acknowledge Kelly Paglia and the Fiehn Laboratory at the NIH West Coast Metabolomics Center for help in the design and conduct of the metabolomics analysis. The authors would also like to acknowledge Bartlett Hamilton for assistance in coordination of sample and clinical data collection and curation.
Funding:
This research was supported by the University of Kansas and a CTSA grant from NCATS awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute, KL2TR002367 (R.F.), and the Kansas Institute of Precision Medicine Centers of Biomedical Research Excellence grant from NIGMS awarded to the University of Kansas Medical Center, P20GM130423 (R.F.). Further support includes the VA Merit Award, I01 BX0046000 (T.M.), the U.S Department of Defense, PR200793 (T.M), and grants from the NIAAA, R25AA020818 (T.M), NIGMS, U54GM115458 (T.M), NIAMS, P50AR60772 (T.M), the National Institutes of Health, R35HL155460, R33HL154123 (R.G), VA CSR&D IK2 CX002203 (BRE).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no financial or personal conflicts of interested related to the contents of this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.England BR, Tiong BK, Bergman MJ, Curtis JR, Kazi S, Mikuls TR, O’Dell JR, Ranganath VK, Limanni A, Suter LG, Michaud K. 2019 Update of the American College of Rheumatology Recommended Rheumatoid Arthritis Disease Activity Measures. Arthritis Care Res (Hoboken). 2019. Dec; 71(12):1540–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreland LW, O’Dell JR, Paulus HE, Curtis JR, Bathon JM, St Clair EW, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheum. 2012. Sep; 64(9): 2824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guma M, Tiziani S, Firestein GS. Metabolomics in rheumatic diseases: desperately seeking biomarkers. Nat Rev Rheumatol. 2016. May;12(5):269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medcalf MR, Bhadbhade P, Mikuls TR, O’Dell JR, Gundry RL, Funk RS. Plasma Metabolome Normalization in Rheumatoid Arthritis Following Initiation of Methotrexate and the Identification of Metabolic Biomarkers of Efficacy. Metabolites. 2021; 11(12):824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 Revised Criteria for the Classification of Rheumatoid Arthritis. Arthritis Rheum. 1988. Mar; 31(3):315–24. [DOI] [PubMed] [Google Scholar]
- 6.van Riel PL. The development of the disease activity score (DAS) and the disease activity score using 28 joint counts (DAS28). Clin Exp Rheumatol. 2014. Sep-Oct; 32(5 Suppl 85) :S-65–74. [PubMed]
- 7.Fiehn O. Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr Protoc Mol Biol. 2016. Apr; 114:30.4.1–30.4.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaženović I, Kind T, Ji J, Fiehn O. Software Tools and Approaches for Compound Identification of LC-MS/MS Data in Metabolomics. Metabolites. 2018. May 10; 8(2):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pepe MS. (2003) The Statistical Evaluation of Medical Tests for Classification and Prediction. Oxford University Press, New York.
- 10.Youden WJ. Index for rating diagnostic tests. Cancer. 1950. Jan; 3(1):32–5. [DOI] [PubMed] [Google Scholar]
- 11.Gosselt HR, Muller IB, Jansen G, van Weeghel M, Vaz FM, Hazes JMW, Heil SG, de Jonge R. Identification of Metabolic Biomarkers in Relation to Methotrexate Response in Early Rheumatoid Arthritis. J Pers Med. 2020. Dec 10; 10(4):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Chen Z, Yang S, Wang Y, Yu L, Zhang B, Rao Z, Gao J, Tu S. (1)H NMR-based metabolomic analysis for identifying serum biomarkers to evaluate methotrexate treatment in patients with early rheumatoid arthritis. Exp Ther Med. 2012. Jul; 4(1):165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teitsma XM, Yang W, Jacobs JWG, Pethö-Schramm A, Borm MEA, Harms AC, Hankemeier T, van Laar JM, Bijlsma JWJ, Lafeber FPJG. Baseline metabolic profiles of early rheumatoid arthritis patients achieving sustained drug-free remission after initiating treat-to-target tocilizumab, methotrexate, or the combination: insights from systems biology. Arthritis Res Ther. 2018. Oct 15; 20(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Rotte MC, de Jong PH, Pluijm SM, Calasan MB, Barendregt PJ, van Zeben D, van der Lubbe PA, de Sonnaville PB, Lindemans J, Hazes JM, de Jonge R. Association of low baseline levels of erythrocyte folate with treatment nonresponse at three months in rheumatoid arthritis patients receiving methotrexate. Arthritis Rheum. 2013. Nov; 65(11):2803–13. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Chen J, Hu C, Xie Z, Li H, Wei S, Wang D, Wen C, Xu G. Exploration of the serum metabolite signature in patients with rheumatoid arthritis using gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2016. Aug 5; 127:60–7. [DOI] [PubMed] [Google Scholar]
- 16.Yajima T, Mason K, Katz E. Biogenetic origin of the D-isoleucine and N-methyl-L-alloisoleucine residues in the actinomycins. Antimicrob Agents Chemother. 1976. Feb; 9(2):224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salamoun YM, Polireddy K, Cho YK, Medcalf MR, Funk RS. Methotrexate Disposition, Anti-Folate Activity, and Metabolomic Profiling to Identify Molecular Markers of Disease Activity and Drug Response in the Collagen-Induced Arthritis Mouse Model. Metabolites. 2022; 12(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zawadzki A, Thiele M, Suvitaival T, Wretlind A, Kim M, Ali M, Bjerre AF, Stahr K, Mattila I, Hansen T, Krag A, Legido-Quigley C. High-Throughput UHPLC-MS to Screen Metabolites in Feces for Gut Metabolic Health. Metabolites. 2022. Feb 25; 12(3):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu C, Sawrey-Kubicek L, Beals E, Rhodes CH, Houts HE, Sacchi R, Zivkovic AM. Human gut microbiome composition and tryptophan metabolites were changed differently by fast food and Mediterranean diet in 4 days: a pilot study. Nutr Res. 2020 May;77:62–72. doi: 10.1016/j.nutres.2020.03.005. Epub 2020. Mar 26. PMID: . [DOI] [PubMed] [Google Scholar]
- 20.Räder AFB, Reichart F, Weinmüller M, Kessler H. Improving oral bioavailability of cyclic peptides by N-methylation. Bioorg Med Chem. 2018. Jun 1;26(10):2766–2773. [DOI] [PubMed] [Google Scholar]
- 21.Artacho A, Isaac S, Nayak R, Flor-Duro A, Alexander M, Koo I, Manasson J, Smith PB, Rosenthal P, Homsi Y, Gulko P, Pons J, Puchades-Carrasco L, Izmirly P, Patterson A, Abramson SB, Pineda-Lucena A, Turnbaugh PJ, Ubeda C, Scher JU. The Pretreatment Gut Microbiome Is Associated With Lack of Response to Methotrexate in New-Onset Rheumatoid Arthritis. Arthritis Rheumatol. 2021. Jun; 73(6):931–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kassel DB, Martin M, Schall W, Sweeley CC. Urinary metabolites of L-threonine in type 1 diabetes determined by combined gas chromatography/chemical ionization mass spectrometry. Biomed Environ Mass Spectrom. 1986. Oct; 13(10):535–40. [DOI] [PubMed] [Google Scholar]
- 23.Idle JR, Seipel K, Bacher U, Pabst T, Beyoğlu D. (2R,3S)-Dihydroxybutanoic Acid Synthesis as a Novel Metabolic Function of Mutant Isocitrate Dehydrogenase 1 and 2 in Acute Myeloid Leukemia. Cancers (Basel). 2020. Oct 1; 12(10):2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, Luo H, Vinay P, Wu J. Molecular cloning of the cDNA of mouse mitochondrial NADP-dependent isocitrate dehydrogenase and the expression of the gene during lymphocyte activation. J Cell Biochem. 1996. Mar 1;60(3):400–10. [DOI] [PubMed] [Google Scholar]
- 25.Jacob P 3rd, Yu L, Shulgin AT, Benowitz NL. Minor tobacco alkaloids as biomarkers for tobacco use: comparison of users of cigarettes, smokeless tobacco, cigars, and pipes. Am J Public Health. 1999. May; 89(5):731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Floris A, Perra D, Cangemi I, Congia M, Chessa E, Angioni MM, Mangoni AA, Erre GL, Mathieu A, Piga M, Cauli A. Current smoking predicts inadequate response to methotrexate monotherapy in rheumatoid arthritis patients naïve to DMARDs: Results from a retrospective cohort study. Medicine (Baltimore). 2021. Apr 30; 100(17):e25481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, Kumagai S. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2010. Jan;69(1):70–81. [DOI] [PubMed] [Google Scholar]
- 28.Stamp LK, O’Donnell JL, Chapman PT, Zhang M, Frampton C, James J, Barclay ML. Determinants of red blood cell methotrexate polyglutamate concentrations in rheumatoid arthritis patients receiving long-term methotrexate treatment. Arthritis Rheum. 2009. Aug;60(8):2248–56. [DOI] [PubMed] [Google Scholar]