Abstract

A recent suggestion that acetamide, CH3C(O)NH2, could be readily formed on water–ice grains by the acid induced addition of water across the C≡N bond has now been shown to be credible. Computational modeling of the reaction between R–CN (R = H, CH3) and a cluster of 32 molecules of water and one H3O+ proceeds catalytically to form first a hydroxy imine R–C(OH)=NH and second an amide R–C(O)NH2. Quantum mechanical tunneling, computed from small-curvature estimates, plays a key role in the rates of these reactions. This work represents the first reasonable effort to show, in general, how amides can be formed from nitriles and water, which are abundant substrates, reacting on a water–ice cluster containing catalytic amounts of hydrons in the interstellar medium with consequential implications toward the origins of life.
Introduction
The so-called peptide bond, −C(O)NH–, which is found in amides connects amino acids to peptides—of paramount importance to present day life on Earth. Unsurprisingly, how, when, and where peptide bond formation arose is of immediate interest in prebiotic astrochemistry, tackling that challenging question: the origins of life.1,2
The simplest amides, formamide, HC(O)NH2, and acetamide, CH3C(O)NH2, are common constituents of star-forming regions in our galaxy3,4 but apparently propionamide, CH3CH2C(O)NH2, is not.5 Adande et al.3 have shown, based on their observations of formamide toward star-forming regions of dense molecular clouds, that the compound could have been brought to Earth by exogenous delivery in substantial amounts of ∼0.18 mmol m–2 in a single impact.
The formation routes to formamide are still unclear; some have suggested gas-phase pathways via formaldehyde and amidogen:6,7
but disputed by Song and Kästner8 and convincingly refuted by Douglas et al.9 or surface reactions10 by the hydrogenation of isocyanic acid, HNCO, and on carbon monoxide–ammonia ices:11
| 1 |
| 2 |
| 3 |
and more recently by metal-ion mediated substitution reactions:12
where M = Na+,K+,Mg+,Mg2+, and Al+ and X = H, OH, and CH2OH but the evidence for all these is underwhelming. A comprehensive summary has been given recently by Chuang et al.13 during the course of their laboratory work on the formation of formamide in water- and carbon monoxide-rich water ices with ammonia as the substrate. Vacuum ultraviolet (VUV) irradiation of water-rich and CO-rich ammonia ices at 10 K with compositions H2O:CO:NH3 = 10:5:1, CO:NH3 = 4:1 and CO:NH3 = 0.6:1 have shown that formamide is preferentially formed, although mechanistically the situation is complicated with no clear indication as to actual formation routes.13 Indirect evidence for the formation routes of formamide based on the stratified distribution of the molecules HNCO and H2CO, putative parents of HCONH2, in the atmosphere of the HH 212 protostellar disk, appears to rule out HNCO as a parent.14
Most recently Marks et al. have carried out experiments involving acetaldehyde (ethanal) ammonia mixtures undergoing irradiation with simulated galactic cosmic rays.15 They report the formation of glycinal, HC(O)CH2NH2, and acetamide and their tautomers HC(OH)CHNH2 and H2C=C(OH)NH2. The compounds arise, they suggest, as the result of barrierless radical–radical recombination reactions:
This is somewhat surprising since tautomerization of acetamide would normally be expected to lead to the imidic acid CH3C(OH)=NH.
Studies of comets have indicated that the early Solar Nebula had nitriles (cyanides) such as HCN and CH3CN in abundance.16,17 It is currently assumed that reactions in the bulk or on the surface of water–ice grains are the most likely formation routes for complex organic molecules which are then liberated into the gas-phase by UV irradiation, electron and cosmic ray bombardment, via thermal shocks or grain–grain collisions.18−20
In a computational study of the direct reaction HC≡N + H2O → HC(OH)=NH neither the presence of a second H2O as a catalyst or as a spectator or as a reactant was sufficient to reduce the high barriers encountered, in excess of 220 kJ mol–1, which thereby rule out the possibility of it occurring in cold molecular clouds.21 The most pertinent previous work simulated a 33 H2O molecule cluster and showed that HC≡N cannot react with HC(O)NH2 under interstellar ice conditions because of large energy barriers. Any reactivity that was feasible proceeded through the CN• radical.22 These studies are in accord with the statement that “nitriles are curious functional groups because they are kinetically rather inert while thermodynamically quite unstable”.23
Ultraviolet (UV) photolysis of water methyl cyanide ices at 20 K (H2O:CH3CN = 20:1) does give rise to the formation of CH3C(O)NH2, and its isomer N-methyl formamide CH3NHCHO, but many other products as well.24
A consideration of the various suggested reactions in the literature led one of us to suggest that H3O+ induced water addition to nitriles on water–ice grains was likely to provide the most probable route.25 It is known that H3O+ exists in the ISM and even in other galaxies, that it reacts in water–ices, and that it has the potential to drive subsequent reaction.26−29 Note that although there is no direct experimental spectroscopic evidence for the presence of H+ in the ISM, our own Sun emits a “solar wind” of particles which are 95% H+ and 4% He2+. In addition “cosmic rays” mainly consist of high-energy protons. The capture of H+ by a water–ice grain in a one-time event would provide the necessary acid functionality.
Woon has very recently reviewed what is known about cation reactions
in ices from quantum chemical cluster studies, highlighting the novel
and more efficient pathways, vis-à-vis the gas-phase, that
HCO+, 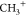 , and C+ (but not H3O+) undergo and appealed for experimental confirmation.30 Laboratory experiments have shown that bombardment
of water ice samples on a copper substrate at 10 K by 60 keV O6+ ions yields a number of secondary ions including (H2O)n·H+ with n = 1 → 8, although these ions are perhaps more accurately
denoted as (H2O)n−1·H3O+.31 The production
efficiency is much lower when crystalline ice is bombarded in comparison
with amorphous ice.
, and C+ (but not H3O+) undergo and appealed for experimental confirmation.30 Laboratory experiments have shown that bombardment
of water ice samples on a copper substrate at 10 K by 60 keV O6+ ions yields a number of secondary ions including (H2O)n·H+ with n = 1 → 8, although these ions are perhaps more accurately
denoted as (H2O)n−1·H3O+.31 The production
efficiency is much lower when crystalline ice is bombarded in comparison
with amorphous ice.
There is little direct evidence for the charge state of a typical water–ice grain; laboratory experiments of necessity greatly enhance molecular synthesis during electron bombardment of ices, thus perforce negatively charging the sample. For example, 1 keV electron irradiation of a 1:1 mixture of ammonia and methanol at 30 K led to the formation of formamide, formic acid, methyl formate, formaldehyde, and carbon monoxide and dioxide, as well as methane, but the electron flux is some 1010 times greater than the sample would experience in a cold dense molecular cloud.32 For the purposes of this work, we assume that the water–ice is neutral and acquires a positive charge on a one-time capture of H+ or indeed H3O+.
A primary consideration for suggesting acidified amorphous water–ice is the high mobility of the proton through the lattice; the transfer mechanism, via Grotthus hops, takes place on a subpicosecond time scale and with barriers of ≈1 kJ mol–1—these effectively increase the “collision rate” or encounter between reactant and H3O+.29,33 Since the proton is so free to move throughout the cluster, its actual location on the surface or in the interior, which is sometimes a consideration in discussions of reactions on water–ice grains, is much less relevant here. Note too that the diffusion of radicals on/in water–ice grains is likely to be very much slower.
The first step in the proposed addition of water is the protonation at nitrogen of the nitrile to form RC≡NH+, and in the case of HCN to form iminomethylium, HC≡NH+; this species has been widely detected in star-forming regions34−36 and in Titan’s atmosphere.37 Although it had been characterized as a precursor of HC≡N, in the most recent observations Fontani et al. show that it is formed from HC≡N or HC≡N+.38 Fundamental laboratory work to characterize the spectroscopic parameters for CH3C≡NH+ have been carried out but interstellar searches have not so far been successful.39
The hydronium-induced addition of water was seen as a two-step process with the first forming hydroxy imines or imidic acids RC(OH)=NH and the second converting these to amides RC(O)NH2; the latter reaction can occur either by intramolecular hydrogen-transfer via quantum-mechanical tunneling or by the further protonation of the nitrogen-atom followed by deprotonation from the O atom.
The actual formation of peptides as a byproduct was observed40 after the deposition of C atoms onto a CO + NH3 ice at 10 K and subsequent warming to 300 K. The authors argue that the initial reaction product is aminoketene, H2NCH=C=O, which polymerizes on warming yielding (−CH2–C(O)–NH−)n chains. While the experiments are compelling, the conditions are somewhat artificial since they consider pure CO + NH3 ices with substantial quantities of carbon atoms. The initial step H3N + C(3P0) → H3N---C is highly exothermic, ΔrH = −103 kJ mol–1, this is followed by a 1,2-H-transfer to H2N–C̈ H and finally reaction with CO to H2N–CH=C=O.
Interestingly Canepa41 in considering the survival rates of glycine, NH2CH2C(O)OH, embedded in micrometeorites undergoing atmospheric re-entry has shown that aminoketene, the product of dehydration, would also survive.
In this paper, we focus on a solid-state chemistry formation mechanism of R–C(OH)=NH and subsequently R–C(O)NH2. We perform electronic structure investigations of the energetics and also thermal rate constants calculations of this reaction mechanism. This information can then be used in astrochemical modeling and may prompt experimental laboratory confirmation.
Methods
Calculations were performed with the program Gaussian42 and used the long-range dispersion corrected density functional ωB97X-D together with the triple ζ basis set with added polarization and diffuse functions 6-311++G(d,p)43 with a factor of 0.96 applied to scale44 the zero-point energy. The functional is known as a range-separated hybrid generalized gradient approximation functional and is capable of modeling both short- and long-range interactions using a version of Grimme’s D2 dispersion treatment.45 The use of higher level theory for modeling a system with ≈35 “heavy” atoms would have exceeded our computational resources.
A system with thirty-two water molecules and one hydronium ion, H3O+, based on a cluster originally designed by Rimola et al.,46 with a total “volume” of ≈2,600 Å3, was chosen together with one reactant, either HCN or CH3CN. This choice represents a compromise between realistic ISM concentrations and computational effort, Figure 1.
Figure 1.

HCN embedded in acidified water.
All structures, the largest of which:
were fully optimized, and the harmonic frequencies were computed using DFT. Frequency calculations were performed in order to verify that all intermediates are true minima on the potential energy surface and that all transition states exhibit a single imaginary frequency. We studied all species in the reaction mechanism with the unrestricted ωB97XD/6-311++G(d,p) model chemistry. Gaussian 16 automatically includes an ultrafine integration grid in the DFT calculations in order to improve the accuracy of the results. The grid greatly enhances the accuracy at reasonable additional cost.
The reaction paths are computed using the intrinsic reaction coordinate (IRC) methodology47,48 to confirm the identities of the reactants and products for every transition state. IRC calculations require initial force constants of the transition state. Then, the first and second order energy derivatives are obtained to calculate the projected harmonic vibrational frequencies along each reaction path. The minimum energy paths (MEPs) were computed using the Page–McIver integrator with a gradient step size of 0.1 a0.49
Small curvature quantum mechanical and quantized-reactant-states tunneling calculations50,51 employed the PILGRIM software suite52 for the computation of rate constants via transition state theory53 (TST) and variational54 TST (VTST) necessitating calculations along the minimum energy path.
Results
Prior
to the first reaction steps we begin by considering a previously
published46 cluster of 33 water molecules,
[33(H2O)], to which a low energy Hydron H+ is
added in a highly exothermic process, ΔrH⊖(0 K) of −1, 065
kJ mol–1; this in turn can be dissipated throughout
the cluster and/or serves to drive subsequent reactions. Although
the whole process of energy dissipation is poorly understood Ferrero
et al.55 have shown in ab initio molecular dynamics simulations of the reactions 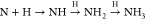 on water–ice surfaces that
60–90%
of the reaction energy may be absorbed by the ice cluster within 1
ps. If the energy is not dissipated quickly, then the newly formed
product will promptly dissociate; if the energy is dissipated too
quickly, then there is not enough to drive subsequent reactions.
on water–ice surfaces that
60–90%
of the reaction energy may be absorbed by the ice cluster within 1
ps. If the energy is not dissipated quickly, then the newly formed
product will promptly dissociate; if the energy is dissipated too
quickly, then there is not enough to drive subsequent reactions.
The only notable difference between the two clusters [33(H2O)] and [33(H2O)·(H+)], which is more realistically depicted as [32(H2O)·(H3O+)], are the three additional vibrational modes, two O–H asymmetric stretching vibrations near 2,400 cm–1 and a characteristic symmetric at 2,800 cm–1. It is to this cluster that the reactants HCN and CH3CN are then added.
First Step: Imidic Acid Formation
As originally envisaged the hydrolysis of HC≡N proceeded in three distinct phases:
| 4 |
| 5 |
| 6 |
the exothermic first step ΔrH⊖(0 K) = −20.5 kJ mol–1 simply a reflection of the larger proton affinity of HC≡N of 712.9 kJ mol–1 vis-à-vis H2O of 691.0 kJ mol–1.56 In a water cluster of an additional 32 H2O molecules, however, these later steps are elided since as H2O adds to the C atom it simultaneously transfers H to a neighboring O atom.
| 7 |
Overall reaction 7 is exothermic by −83 kJ mol–1, Table 2, which is considerably different from the gas-phase ΔrH⊖(0 K) = −21.9 kJ mol–1, reflecting the tighter binding of the hydrolyzed product in comparison to the reactant.
Table 2. Barrier Heights, E†, and Reaction Enthalpies, ΔrH(0 K)/kJ mol–1.
| reaction | E† | ΔrH(0 K) |
|---|---|---|
| initial protonation and hydroxy imine formation | ||
| HCN + 32H2O·H3O+ → HC(OH)NH + 31H2O·H3O+ | 78.5 | –82.8 |
| CH3CN + 32H2O·H3O+ → CH3C(OH)NH + 31H2O·H3O+ | 51.9 | –80.0 |
| intramolecular amide formation via 1,3[H]-transfer | ||
| HC(OH)NH(g) → HC(O)NH2(g) | 135.5 | –57.6 |
| CH3C(OH)NH(g) → CH3C(O)NH2(g) | 127.6 | –57.1 |
| HC(OH)NH + 31H2O·H3O+ → HC(O)NH2 + 31H2O·H3O+ | 168.8 | –81.5 |
| CH3C(OH)NH + 31H2O·H3O+ → CH3C(O)NH2 + 31H2O·H3O+ | 141.5 | –90.1 |
| intermolecular amide formation via protonation/deprotonation | ||
| HC(OH)NH + 31H2O·H3O+ → [HC(OH)NH2]+ + 32H2O | 1.6 | –75.5 |
| [HC(OH)NH2]+ + 32H2O → HC(O)NH2 + 31H2O·H3O+ | 14.6 | –4.83 |
The barrier to reaction 7, that is, protonation at the N atom, in the case of HCN is 78.5 kJ mol–1 and is even lower at 51.9 kJ mol–1 for acetonitrile.
Although Figure 1 shows the actual reaction structure, an oversimplified version, Figure 2, where the scaffold of water molecules has been deleted to highlight the important interactions, shows the parts played by four key water molecules. The first is the proton donor H3O+, the second is the “reactant” which will attack the C atom and also transfer a H+ to the “acceptor” water, while the “companion” H2O is less involved but nevertheless stabilizes the system through H···OH2 hydrogen-bonding; snapshots along the IRC path are shown in Figure 3 to the final product, methanimidic acid in its (E,Z) conformation with respect to the dihedrals ∠OCNH and ∠HOCN, respectively.
Figure 2.

Key elements of the reaction scheme for the first step.
Figure 3.
Structures along the IRC path for the first step.
Second Step: Amide Formation
We can distinguish between two different routes from the hydroxy imines to the corresponding amides which can proceed intramolecularly or intermolecularly.
Intramolecular Route
Once the α-hydroxy imines, R–C(OH)=NH, known as methanimidic and ethanimidic acids, are formed, then an intramolecular 1,3-H-transfer leads to formamide or acetamide, respectively; however, the barriers are considerable ranging from 136 or 128 kJ mol–1 in the gas-phase to 169 and 142 kJ mol–1 in this water-cluster. Clearly, surmounting such barriers is unfeasible at temperatures much less than 300 K except by tunneling.
Note that the presence of additional water molecules makes very little difference in the energetics of the process in comparison to the gas-phase. Exactly the same conclusion can be drawn from the gas-phase work of Darla et al. which showed in ωB97xD/aug–cc-pVTZ calculations that even in the presence of a “catalytic” water molecule the 1,3[H]-transfer faces a barrier of 131 kJ mol–1 or 132 kJ mol–1 with the additional water present as a “spectator”.21
The additional water molecules and H3O+ in our system of RC≡N + 32H2O + H3O+ only marginally affect the 1,3[H]-transfer reaction; this is seen in the energetics, as discussed above, and also from the values of the imaginary frequencies which are gas-phase, 20002 and 1,988 cm–1, and cluster, 1,953 and 1,916 cm–1, for HC(OH)NH ↔ HC(O)NH2 and CH3C(OH)NH ↔ CH3C(O)NH2 respectively.
In the case of the gas-phase RC(OH)NH ↔ RC(O)NH2 isomerization reaction, PILGRIM57 calculations at B3LYP/cc-pVTZ incorporating small-curvature tunneling yields rate constants, k, and half-lives, τ, as shown in Table 1; the ice-cluster values are probably not dissimilar. At the lowest temperatures, here ≤ 150 K, quantized reactant states tunneling is included. The barrier to reaction is higher at 131.5 kJ mol–1 for HC(OH)NH2 than the 123.9 kJ mol–1 for CH3C(OH)NH2 consequently the rate of isomerization to the appropriate amide is faster for ethanimidic acid. These are substantially faster rates of isomerization than our previous Multiwell values, Figure 4, which were based on Eckart tunneling;25 this makes the intramolecular route competitive with the intermolecular route, discussed below.
Table 1. Isomerization Rate Constants.
| HC(OH)NH → HC(O)NH2 |
CH3C(OH)NH → CH3C(O)NH2 |
|||
|---|---|---|---|---|
| T/K | k/s–1 | τ/days | k/s–1 | τ/days |
| 50 | 7.6 × 10–9 | 1,060 | 4.4 × 10–08 | 180 |
| 100 | 7.8 × 10–9 | 1,028 | 8.3 × 10–08 | 100 |
| 150 | 1.2 × 10–8 | 660 | 2.3 × 10–07 | 36 |
| 200 | 4.2 × 10–8 | 190 | 9.3 × 10–07 | 9 |
| 250 | 3.4 × 10–7 | 24 | 6.7 × 10–06 | 2 |
| 300 | 4.4 × 10–6 | 2 | 7.5 × 10–05 | 0.2 |
Figure 4.
Rate constants gas-phase tautomerization: Eckart (− – −) versus small-curvature tunneling (−). CH3C(OH)NH (red), HC(OH)NH (black).
A not dis-similar situation is considered by Concepción et al.58 in their work on the origin of the (E/Z) isomer ratio of imines in the ISM; thus they show that the less-stable (E) conformer of cyanomethanimine, RCH=NH where R is a C≡N group, rearranges to the (Z) conformer with dramatically increased rates over canonical transition-state theory values at temperatures of 250 K and below. The variational effect is small and the faster rates are ascribed to quantum tunneling; exactly the same as found here.
The detection of isotopically labeled compounds can be useful for tracing formation routes; specifically, deuteration has been used in this regard by Bianchi et al.59 in their study of CH3C≡N in the SVS13-A Class I hot corino. Unfortunately, such a tool is unavailable here since the hydrogen transferred has been sourced from the water–ice; however, if CH3C(OD)NH or CH3C(OH)ND were to be detected, then that might prompt other avenues of investigation.
Intermolecular Route
The same outcome, that is RC(OH)NH
→ RC(O)NH2, can come about by the attack of hydronium
at the N-atom leading similarly to R–C(OH)=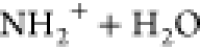 and attack by a further water molecule
at the O-atom leading to deprotonation, resulting in the final products
R–C(O)–NH2 + H3O+.
and attack by a further water molecule
at the O-atom leading to deprotonation, resulting in the final products
R–C(O)–NH2 + H3O+.
So this reaction follows the same course mechanistically as the first; first, protonation at the nitrogen atom, which has a tiny barrier of 1.6 kJ mol–1 (disappears when adding ZPE), is followed by H-abstraction from the OH group and transfer of H to a neighboring water and transfer of H to a second water, Figure 5, which just shows the active site. The reaction can be summarized as
and the barrier for this is low at 14.6 kJ mol–1. The results are summarized in Table 2 where E† is the zero-point corrected electronic energy and ΔrH is the reaction enthalpy, with both expressed in units of kJ mol–1.
Figure 5.

Intermolecular HC(OH)=NH → HC(O)NH2.
Implications
The original proposal,25 an earlier draft,60 and the work detailed in this paper have been deemed to be “highly original” which is demonstrably true since the very recent review of cation–ice astrochemical reactions does not include the hydronium ion among its reactants, although H3O+ does feature as a byproduct of said reactions.30
This originality is a mixed blessing since it means that one cannot appeal to previous precedent, and it can be argued that other processes may lead to the formation of amides and in particular that the barrier for the first step of 79 kJ mol–1 is too high. However, the direct reaction of the addition of water across the carbon–nitrogen triple bond has a much higher barrier of 220 kJ mol–1 so the catalytic reaction HCN + 32H2O ·H3O+ → HC(OH)NH + 31H2O ·H3O+ represents a considerable reduction in barrier height.
As noted in the
Introduction many schemes have been proposed for
the formation of amides in the ISM but none have been judged to be
convincing.22,66 The most often proposed are barrierless
radical–radical recombination reactions such as 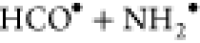 but this suffers from
a number of disadvantages
in comparison to the catalytic reaction. First, the two radicals must
diffuse toward each other in/on the water–ice and their rates
of diffusion are considerably slower than the “diffusion”
of H+ in the ice. Second, the absence of cross-products
such as HC(O)–HC(O) and N2H4, none of
which have been detected61 in the ISM,
presents a challenge to this viewpoint. Third, the rate of reaction,
but this suffers from
a number of disadvantages
in comparison to the catalytic reaction. First, the two radicals must
diffuse toward each other in/on the water–ice and their rates
of diffusion are considerably slower than the “diffusion”
of H+ in the ice. Second, the absence of cross-products
such as HC(O)–HC(O) and N2H4, none of
which have been detected61 in the ISM,
presents a challenge to this viewpoint. Third, the rate of reaction, 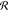 , is proportional
to the product of the
concentrations
, is proportional
to the product of the
concentrations  , both of which are very low, whereas for
the proposed catalytic reaction the concentration of water, which
is the actual reactant, in the ice is considerably greater.
, both of which are very low, whereas for
the proposed catalytic reaction the concentration of water, which
is the actual reactant, in the ice is considerably greater.
Since there has been very little work done previously on charged species reacting in/on water–ice grains, it might be argued that ion–molecule reactions known to occur barrierlessly in the gas-phase might be a guide to what happens in/on acidified water–ices. But this is not a reliable guide since protonation, attack by H2O and reformation of H3O+, is a concerted process according to our results; this is not the case in the gas-phase where these reactions occur in a stepwise manner. We note that KIDA, the Kinetic Database for Astrochemistry, lists many reactions leading to the formation of HCNH+; however, it does not include any reactions for HCNH+ + H2O other than the reverse reaction.63 For these reasons, we conclude that the reaction on water–ice is a considerably more efficient process and also a mechanism that is potentially applicable to all cyanides, vide infra, not just to hydrogen and methyl cyanides.
Since the hydronium ion is such a novel reactant, in the context of amide formation in interstellar space, can it be argued that H atoms are more likely to be involved? Although we have not attempted to model such a system, HCN + 33H2O·H•, it is known from theoretical work64 that HCN + H• → H2C=N• is preferred over HCN + H• → HCNH•. Further reactions with H• will lead, most probably, to amine formation, not amide.
The presence of other species
on the ice, such as, for example,
methanol, carbon dioxide, or ammonia, might outcompete and negate
the hydrolysis of RC≡N but this is a topic beyond the scope
of this article since modeling a system like [HCN·CH3OH·32H2O·H3O+]
would majorly exceed our resources. In very general terms, one can
surmise that the presence of ammonia on the water–ice would
be expected to mop up any H3O+ forming the very
stable ammonium ion, 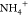 , leading to the cessation of any further
reaction—ammonia would act as a sink for H3O+. This argument is based on the very rough estimate that highly
exothermic reactions tend to have lower energy barriers than very
endothermic reactions, and, on the relative proton affinities62 of HCN and NH3 which are 713 and
854 kJ mol–1, respectively. Competition for H3O+ between substances with a lower proton affinity,
for example, CO2 at 594 kJ mol–1 on the
other hand would favor reaction with HCN.
, leading to the cessation of any further
reaction—ammonia would act as a sink for H3O+. This argument is based on the very rough estimate that highly
exothermic reactions tend to have lower energy barriers than very
endothermic reactions, and, on the relative proton affinities62 of HCN and NH3 which are 713 and
854 kJ mol–1, respectively. Competition for H3O+ between substances with a lower proton affinity,
for example, CO2 at 594 kJ mol–1 on the
other hand would favor reaction with HCN.
There are of course
other nitriles present in the ISM and one would
anticipate that a similar fate would befall them; Manna and Pal65 have detected the unfortunately named cyanamide,
or amino cyanide H2NC≡N, in the hot molecular core
G10.47 + 0.03 and outline three possible fates, degradation to H2N• + CN• by cosmic rays
and high-energy photons or by ion–neutral reactions: 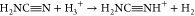 .
.
In an extensive computational study by Slate et al.66 into urea formation in the ISM, they concluded that closed shell reactions had prohibitive barriers but that a route involving charged species was feasible except that their starting point involved isocyanic acid HN=C=O with protonation at the O atom followed by addition of ammonia and subsequent deprotonation; a somewhat more complex sequence than that envisaged here but nevertheless comparable. Previously Brigiano et al. had considered ion–molecule, neutral–neutral, and radical reactions leading to the formation of urea but only in the gas-phase.67
Catalytic addition of water on ice-grains to cyanamide H2N–C≡N, which is known to exist in the ISM,68,69 would lead to the formation of carbonyl diamide, OC(NH2)2, better known as urea, which has been detected previously.70,71 In other work we have shown that the tautomerization reaction R–C(OH)NH → R–C(O)NH2 proceeds at a much faster rate in the gas-phase for R = H2N than for any of those imidic acids with R = H,HO,NC,HC(O),H3C,HOCH2,H2CCH, H3C(O),H2NCH2,C2H5 and CN.72 Urea is a key intermediate in biosynthetic pathways. and recent work outlines ways in which urea is of fundamental importance to the prebiotic chemistry that gave rise to life on our planet73—Darwin’s “warm little pond” scenario.74
Conclusion
The catalytic addition of acidified water to RC≡N triple bonds is shown to be a credible process on water clusters, which are impacted by hydrons H+. We refer in the paper to the very high barrier for the direct reaction HCN + H2O in excess of 220 kJ mol–1 and demonstrate that the presence of catalytic amounts of H3O+ dramatically reduces the barrier to reaction of 79 kJ mol–1 for HCN and 52 kJ mol–1 for CH3CN. The high mobility of the hydron through the cluster leads to the initial reactive step, protonation at the N atom in a facile manner. This effectively transforms a bimolecular reaction into a unimolecular process—thereby removing the “collisional handicap” which hampers all gas-phase reactions in the interstellar medium.
Concerted attack by water at carbon and abstraction of H+ yields the hydroxy imine RC(OH)NH which can then undergo a 1,3[H]-transfer reaction either intramolecularly or intermolecularly to the amide RC(O)NH2. Quantum-mechanical tunneling plays a key role in these processes.
The current paradigm which emphasizes radical–radical combination on grains, R1 + R2 → R1R2, as possible formation routes for complex organic molecules because such reactions are barrierless but the absence of the cross-products R1R1 and R2R2 and the low probabilities of the two radicals separately arriving on said grain may need to be reconsidered in the case of the formation of amides.
The recent review by Woon with its stress on the need to pay more attention to cation reactions in ices is shown to be prescient and his call for experimental confirmation timely.30
Acknowledgments
J.M.S. and B.K. thank the Irish Centre for High-End Computing, ICHEC, for the provision of computational resources (Projects nuig02, ngche102c, ngche115c, and ngast001b). The assistance by David Ferro-Costas (Univ. Santiago de Compostela), author of Pilgrim, is gratefully acknowledged.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Author Contributions
B.K. and J.M.S. contributed equally to this work.
The authors declare no competing financial interest.
References
- Kolesniková L.; Belloche A.; Koucký J.; Alonso E. R.; Garrod R. T.; Luková K.; Menten K. M.; Müller H. S. P.; Kania P.; Urban Š. Laboratory rotational spectroscopy of acrylamide and search for acrylamide and propionamide towards Sgr B2(N) with ALMA. A & A 2022, 659, A111. 10.1051/0004-6361/202142448. [DOI] [Google Scholar]
- Ligterink N. F. W.; Ahmadi A.; Luitel B.; Coutens A.; Calcutt H.; Tychoniec L.; Linnartz H.; Jørgensen J. K.; Garrod R. T.; Bouwman J. The prebiotic molecular inventory of Serpens SMM1: II. The building blocks of peptide chains. ACS Earth Space Chem. 2022, 6, 455–467. 10.1021/acsearthspacechem.1c00330. [DOI] [Google Scholar]
- Adande G. R.; Woolf N. J.; Ziurys L. M. Observations of Interstellar Formamide: Availability of a Prebiotic Precursor in the Galactic Habitable Zone. Astrobiol 2013, 13, 439–453. 10.1089/ast.2012.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B. A. 2021 Census of Interstellar, Circumstellar, Extragalactic, Protoplanetary Disk, and Exoplanetary Molecules. Astro J. 2022, 259, 30. 10.3847/1538-4365/ac2a48. [DOI] [Google Scholar]
- Schuessler C.; Remijan A.; Xue C.; Carder J.; Scolati H.; McGuire B. Searching for Propionamide (C2H5CONH2) Toward Sagittarius B2 at Centimeter Wavelengths. ApJ 2022, 941, 102. 10.3847/1538-4357/ac8668. [DOI] [Google Scholar]
- Barone V.; Latouche C.; Skouteris D.; Vazart F.; Balucani N.; Ceccarelli C.; Lefloch B. Gas-phase formation of the prebiotic molecule formamide: insights from new quantum computations. MNRAS 2015, 453, L31–L35. 10.1093/mnrasl/slv094. [DOI] [Google Scholar]
- Skouteris D.; Vazart F.; Ceccarelli C.; Balucani N.; Puzzarini C.; Barone V. New quantum chemical computations of formamide deuteration support gas-phase formation of this prebiotic molecule. MNRAS 2017, 468, slx012. 10.1093/mnrasl/slx012. [DOI] [Google Scholar]
- Song L.; Kästner J. Formation of the prebiotic molecule NH2CHO on astronomical amorphous solid water surfaces: accurate tunneling rate calculations. Phys. Chem. Chem. Phys. 2016, 18, 29278. 10.1039/C6CP05727F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas K. M.; Lucas D. I.; Walsh C.; West N. A.; Blitz M. A.; Heard D. E. The gas-phase reaction of NH2 with formaldehyde (CH2O) is not a source of formamide (NH2CHO) in interstellar environments. ApJl 2022, 937, L16. 10.3847/2041-8213/ac8cef. [DOI] [Google Scholar]
- Fedoseev G.; Chuang K.-J.; van Dishoeck E. F.; Ioppolo S.; Linnartz H. Simultaneous hydrogenation and UV-photolysis experiments of NO in CO-rich interstellar ice analogues; linking HNCO, OCN–, NH2CHO, and NH2OH. MNRAS 2016, 460, 4297–4309. 10.1093/mnras/stw1028. [DOI] [Google Scholar]
- Jones B. M.; Bennett C. J.; Kaiser R. I. Mechanistical studies on the production of formamide (H2NCHO) within interstellar ice analogs. Astro J. 2011, 734, 78. 10.1088/0004-637X/734/2/78. [DOI] [Google Scholar]
- Thripati S.; Ramabhadran R. O. Pathways for the Formation of Formamide, a Prebiotic Biomonomer: Metal-Ions in Interstellar Gas-Phase Chemistry. J. Phys. Chem. A 2021, 125, 3457–3472. 10.1021/acs.jpca.1c02132. [DOI] [PubMed] [Google Scholar]
- Chuang K.-J.; Jäger C.; Krasnokutski S. A.; Fulvio D.; Henning T. Formation of the simplest amide in molecular clouds: formamide (NH2CHO) and its derivatives in H2O-rich and CO-rich interstellar ice analogs upon VUV irradiation. Astro J. 2022, 933, 107. 10.3847/1538-4357/ac7320. [DOI] [Google Scholar]
- Lee C.-F.; Codella C.; Ceccarelli C.; López-Sepulcre A. Stratified Distribution of Organic Molecules at the Planet-Formation Scale in the HH 212 Disk Atmosphere. ApJ 2022, 937, 10. 10.3847/1538-4357/ac8c28. [DOI] [Google Scholar]
- Marks J. H.; Wang J.; Kleimeier N. F.; Turner A. M.; Eckhardt A. K.; Kaiser R. I. Prebiotic Synthesis and Isomerization in Interstellar Analog Ice: Glycinal, Acetamide, and Their Enol Tautomers. Angew. Chem. 2023, 135, e202218645 10.1002/ange.202218645. [DOI] [PubMed] [Google Scholar]
- Cordiner M. A.; Remijan A. J.; Boissier J.; Milam S. N.; Mumma M. J.; Charnley S. B.; Paganini L.; Villanueva G.; Bockelée-Morvan D.; Kuan Y.-J.; et al. Mapping the release of volatiles in the inner comae of comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON) using the Atacama large millimeter/submillimeter array. Astro J. 2014, 792, L2. 10.1088/2041-8205/792/1/L2. [DOI] [Google Scholar]
- Loomis R. A.; Cleeves L. I.; Oberg K. I.; Aikawa Y.; Bergner J.; Furuya K.; Guzman V. V.; Walsh C. The Distribution and Excitation of CH3CN in a Solar Nebula Analog. Astro J. 2018, 859, 131. 10.3847/1538-4357/aac169. [DOI] [Google Scholar]
- Colzi L.; Rivilla V. M.; Beltrán M. T.; Jiménez-Serra I.; Mininni C.; Melosso M.; Cesaroni R.; Fontani F.; Lorenzani A.; Sanchez-Monge A.; et al. The GUAPOS project ii. A comprehensive study of peptide-like bond molecules. A & A 2021, 653, A129. 10.1051/0004-6361/202141573. [DOI] [Google Scholar]
- Kalva̅ns K.; Silsbee K. Icy molecule desorption in interstellar grain collisions. MNRAS 2022, 515, 785–794. 10.1093/mnras/stac1792. [DOI] [Google Scholar]
- Minissale M.; Aikawa Y.; Bergin E.; Bertin M.; Brown W. A.; Cazaux S.; Charnley S. B.; Coutens A.; Cuppen H. M.; Guzman V.; Linnartz H.; et al. Thermal desorption of interstellar ices: A review on the controlling parameters and their implications from snowlines to chemical complexity. ACS Earth Space Chem. 2022, 6, 597–630. 10.1021/acsearthspacechem.1c00357. [DOI] [Google Scholar]
- Darla N.; Sharma D.; Sitha S. Formation of Formamide from HCN + H2O: A Computational Study on the Roles of a Second H2O as a Catalyst, as a Spectator, and as a Reactant. J. Phys. Chem. A 2020, 124, 165–175. 10.1021/acs.jpca.9b09924. [DOI] [PubMed] [Google Scholar]
- Rimola A.; Skouteris D.; Balucani N.; Ceccarelli C.; Enrique-Romero J.; Taquet V.; Ugliengo P. Can Formamide Be Formed on Interstellar Ice? An Atomistic Perspective. ACS Earth Space Chem. 2018, 2, 720–734. 10.1021/acsearthspacechem.7b00156. [DOI] [Google Scholar]
- Guthrie J. P.; Yim J. C.-H.; Wang Q. Hydration of nitriles: an examination in terms of No Barrier Theory. J. Phys. Org. Chem. 2014, 27, 27–37. 10.1002/poc.3226. [DOI] [Google Scholar]
- Bulak M.; Paardekooper D. M.; Fedoseev G.; Linnartz H. Photolysis of acetonitrile in a water-rich ice as a source of complex organic molecules: CH3CN and H2O: CH3CN ices. A & A 2021, 647, A82. 10.1051/0004-6361/202039695. [DOI] [Google Scholar]
- Simmie J. M. C2H5NO Isomers: From Acetamide to 1,2-Oxazetidine and Beyond. J. Phys. Chem. A 2022, 126, 924–936. 10.1021/acs.jpca.1c09984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootten A.; Mangum J. G.; Turner B. E.; Bogey M.; Boulanger F.; Combes F.; Encrenaz P. J.; Gerin M. Detection of Interstellar H3O+: A Confirming Line. Astrophys. J. 1991, 380, L79. 10.1086/186178. [DOI] [Google Scholar]
- Van der Tak F. F. S.; Aalto S.; Meijerink R. Detection of extragalactic H3O+. A & A 2008, 477, L5–L8. 10.1051/0004-6361:20078824. [DOI] [Google Scholar]
- Moon E. S.; Kang H.; Oba Y.; Watanabe N.; Kouchi A. Direct Evidence for Ammonium Ion Formation in Ice Through Ultraviolet-Induced Acid Base Reaction of NH3 with H3O+. Astrophys. J. 2010, 713, 906. 10.1088/0004-637X/713/2/906. [DOI] [Google Scholar]
- Lee D. H.; Kang H. Proton Transport and Related Chemical Processes of Ice. J. Chem. Phys. B 2021, 125, 8270–8281. 10.1021/acs.jpcb.1c04414. [DOI] [PubMed] [Google Scholar]
- Woon D. E. Quantum chemical cluster studies of cation-ice reactions for astrochemical applications: Seeking experimental confirmation. Acc. Chem. Res. 2021, 54, 490–497. 10.1021/acs.accounts.0c00717. [DOI] [PubMed] [Google Scholar]
- Martinez R.; Agnihotri A. N.; Boduch P.; Domaracka A.; Fulvio D.; Muniz G.; Palumbo M. E.; Rothard H.; Strazzulla G. Production of Hydronium Ion (H3O+) and Protonated Water Clusters (H2On ·H+) after Energetic Bombardment of Water Ice in Astrophysical Environments. J. Phys. Chem. A 2019, 123, 8001–8008. 10.1021/acs.jpca.9b05029. [DOI] [PubMed] [Google Scholar]
- Mason N. J.; Nair B.; Jheeta S.; Szymańska E. Electron induced chemistry: a new frontier in astrochemistry. Faraday Discuss. 2014, 168, 235–247. 10.1039/C4FD00004H. [DOI] [PubMed] [Google Scholar]
- Lee D. H.; Choi C. H.; Choi T. H.; Sung B. J.; Kang H. Asymmetric transport mechanisms of hydronium and hydroxide ions in amorphous solid water: Hydroxide goes Brownian while hydronium hops. J. Phys. Chem. Letts 2014, 5, 2568–2572. 10.1021/jz501235y. [DOI] [PubMed] [Google Scholar]
- Ziurys L. M.; Turner B. E. Detection of interstellar vibrationally excited HCN. Ap J. 1986, 302, L31. 10.1086/184631. [DOI] [PubMed] [Google Scholar]
- Schilke P.; Walmsley C. M.; Henkel C.; Millar T. J. Protonated HCN in molecular clouds. A & A 1991, 247, 487. [Google Scholar]
- Quénard D.; Vastel C.; Ceccarelli C.; Hily-Blant P.; Lefloch B.; Bachiller R. Detection of the HC3NH+ and HCNH+ ions in the L1544 pre-stellar core. MNRAS 2017, 470, 3194. 10.1093/mnras/stx1373. [DOI] [Google Scholar]
- Cravens T. E.; Robertson I. P.; Waite J. H.; Yelle R. V.; Kasprzak W. T.; Keller C. N.; Ledvina S. A.; Niemann H. B.; Luhmann J. G.; McNutt R. L.; et al. Composition of Titan’s ionosphere. Geophys. Res. Lett. 2006, 33, L07105. 10.1029/2005GL025575. [DOI] [Google Scholar]
- Fontani F.; Colzi L.; Redaelli E.; Sipilä O.; Caselli P. First survey of HCNH+ in high-mass star-forming cloud cores. A & A 2021, 651, A94. 10.1051/0004-6361/202140655. [DOI] [Google Scholar]
- Marimuthu A. N.; Huis in’t Veld F.; Thorwirth S.; Redlich B.; Brünken S. Infrared predissociation spectroscopy of protonated methyl cyanide, CH3CNH+. J. Mol. Spectrosc. 2021, 379, 111477. 10.1016/j.jms.2021.111477. [DOI] [Google Scholar]
- Krasnokutski S. A.; Chuang K. J.; Jager C.; Ueberschaar N.; Henning T. A pathway to peptides in space through the condensation of atomic carbon. Nature Astro 2022, 6, 381–386. 10.1038/s41550-021-01577-9. [DOI] [Google Scholar]
- Canepa C. A Model Study on the Dynamics of the Amino Acid Content in Micrometeoroids during Atmospheric Entry. Chemistry 2020, 2, 918–936. 10.3390/chemistry2040058. [DOI] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford CT, 2016. [Google Scholar]
- Chai J.-D.; Head-Gordon M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. 10.1039/b810189b. [DOI] [PubMed] [Google Scholar]
- NIST Computational Chemistry Comparison and Benchmark Database, NIST Standard Reference Database Number 101. Release 22;Johnson R. D., III, Ed.; 2022; http://cccbdb.nist.gov/.
- Grimme S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. 10.1002/jcc.20495. [DOI] [PubMed] [Google Scholar]
- Enrique-Romero J.; Rimola A.; Ceccarelli C.; Ugliengo P.; Balucani N.; Skouteris D. Reactivity of HCO with CH3 and NH2 on Water Ice Surfaces. A Comprehensive Accurate Quantum Chemistry Study. ACS Earth Space Chem. 2019, 3, 2158–2170. 10.1021/acsearthspacechem.9b00156. [DOI] [Google Scholar]
- Hratchian H. P.; Schlegel H. B. Accurate reaction paths using a Hessian based predictor–corrector integrator. J. Chem. Phys. 2004, 120, 9918. 10.1063/1.1724823. [DOI] [PubMed] [Google Scholar]
- Hratchian H. P.; Schlegel H. B. Using Hessian Updating To Increase the Efficiency of a Hessian Based Predictor-Corrector Reaction Path Following Method. J. Chem. Theo Comp 2005, 1, 61–69. 10.1021/ct0499783. [DOI] [PubMed] [Google Scholar]
- Page M.; McIver J. W. Jr On evaluating the reaction path Hamiltonian. J. Chem. Phys. 1988, 88, 922–935. 10.1063/1.454172. [DOI] [Google Scholar]
- Liu Y.-P.; Lynch G. C.; Truong T. N.; Lu D.-H.; Truhlar D. G.; et al. Molecular Modelling of the Kinetic Isotope Effect for the [1,5]-Sigmatropic Rearrangement of cis-1,3-Pentadiene. J. Am. Chem. Soc. 1993, 115, 2408. 10.1021/ja00059a041. [DOI] [Google Scholar]
- Wonchoba S. E.; Hu W.-P.; Truhlar D. G.. Reaction Path Approach to Dynamics at a Gas-Solid Interface: Quantum Tunnelling Effects for an Adatom on a Non-Rigid Metallic Surface. In Theoretical and Computational Approaches to Interface Phenomena Sellers H. L., Golab J. T., Eds.; Plenum, New York, 1994; p 1 . [Google Scholar]
- PILGRIM, v2021.5; https://github.com/cathedralpkg/pilgrim.
- Eyring H. The Activated Complex in Chemical Reactions. J. Chem. Phys. 1935, 3, 107–115. 10.1063/1.1749604. [DOI] [Google Scholar]
- Garrett B. C.; Truhlar D. G. Criterion of Minimum State Density in the Transition State Theory of Bimolecular Reactions. J. Chem. Phys. 1979, 70, 1593–1598. 10.1063/1.437698. [DOI] [Google Scholar]
- Ferrero S.; Pantaleone S.; Ceccarelli C.; Ugliengo P.; Sodupe M.; Rimola A. Where does the energy go during the interstellar NH3 formation on water ice? A computational study. ApJ 2023, 944, 142. 10.3847/1538-4357/acae8e. [DOI] [Google Scholar]
- Hunter E. P.; Lias S. G. Evaluated Gas Phase Basicities and Proton Affinities of Molecules: An Update. J. Phys. Chem. Ref. Data 1998, 27, 413–656. 10.1063/1.556018. [DOI] [Google Scholar]
- Ferro-Costas D.; Truhlar D. G.; Fernández-Ramos A. Pilgrim: A thermal rate constant calculator and a chemical kinetics simulator. Comp Phys. Comms 2020, 256, 107457. 10.1016/j.cpc.2020.107457. [DOI] [Google Scholar]
- Garcia de la Concepción J.; Jiménez-Serra I.; Carlos Corchado J.; Rivilla V. M.; Martín-Pintado J. The Origin of the (E/Z) Isomer Ratio of Imines in the Interstellar Medium. ApJL 2021, 912, L6. 10.3847/2041-8213/abf650. [DOI] [Google Scholar]
- Bianchi E.; Ceccarelli C.; Codella C.; et al. SOLIS XV. CH3CN deuteration in the Class I hot corino. A & A 2022, 662, A103. 10.1051/0004-6361/202141893. [DOI] [Google Scholar]
- Kerkeni B.; Simmie J. M.. Peptide bonds in the interstellar medium: facile auto-catalytic formation from nitriles on water–ice grains. arXiv; 2209.10929. [DOI] [PMC free article] [PubMed]
- Müller H. S. P.; Schlöder F.; Stutzki J.; Winnewisser G. The Cologne database for molecular spectroscopy, CDMS: a useful tool for astronomers and spectroscopists. J. Mol. Struct. 2005, 742, 215–227. 10.1016/j.molstruc.2005.01.027. [DOI] [Google Scholar]
- Hunter E. P. L.; Lias S. G. Evaluated Gas Phase Basicities and Proton Affinities of Molecules: An Update. J. Phys. Chem. Ref. Data 1998, 27, 413–656. 10.1063/1.556018. [DOI] [Google Scholar]
- Wakelam V.; Herbst E.; Loison J.-C.; Smith I. W. M.; et al. A Kinetic Database for Astrochemistry (KIDA). Ap J. S 2012, 199, 21. 10.1088/0067-0049/199/1/21. [DOI] [Google Scholar]
- Basiuk V. A.; Kobayashi K. DFT Study of HCN and NCCN Reactions With Hydrogen Species. Int. J. Quantum Chem. 2004, 99, 91. 10.1002/qua.20133. [DOI] [Google Scholar]
- Manna A.; Pal S. Detection of interstellar cyanamide (NH2CN) towards the hot molecular core G10.47 + 0.03. J. Astrophys. Astron. 2022, 43, 83. 10.1007/s12036-022-09868-x. [DOI] [Google Scholar]
- Slate E. C. S.; Barker R.; Euesden R. T.; Revels M. R.; Meijer A. J. H. M. Computational studies into urea formation in the interstellar medium. MNRAS 2020, 497, 5413–5420. 10.1093/mnras/staa2436. [DOI] [Google Scholar]
- Brigiano F. S.; Jeanvoine Y.; Largo A.; Spezia R. The formation of urea in space I. Ion–molecule, neutral-neutral, and radical gas-phase reactions. A & A 2018, 610, A26. 10.1051/0004-6361/201731610. [DOI] [Google Scholar]
- Turner B. E.; Liszt H. S.; Kaifu N.; Kisliakov A. G. Microwave Detection of Interstellar Cyanamide. Astro J. 1975, 201, L149–L152. 10.1086/181963. [DOI] [Google Scholar]
- Coutens A.; Willis E. R.; Garrod R. T.; Müller H. S. P.; Bourke T. L.; Calcutt H.; Drozdovskaya M. N.; Jørgensen J. K.; Ligterink N. F. W.; Persson M. V.; et al. First Detection of Cyanamide (H2NCN) towards Solar-type Protostars. A & A 2018, 612, A107. 10.1051/0004-6361/201732346. [DOI] [Google Scholar]
- Belloche A.; Garrod R. T.; Müller H. S. P.; Menten K. M.; Medvedev I.; Thomas J.; Kisiel Z. Re-exploring Molecular Complexity with ALMA (ReMoCA): interstellar detection of urea. A & A2019, 628, A10. See also A & A 2020, 637, C4. 10.1051/0004-6361/201935428e. [DOI] [Google Scholar]
- Jiménez-Serra I.; Martín-Pintado J.; Rivilla V. M.; Rodríguez-Almeida L.; Alonso Alonso E. R.; Zeng S.; Cocinero E. J.; Martín S.; Requena-Torres M.; Martín-Domenech R.; Testi L. Toward the RNA-World in the Interstellar Medium — Detection of Urea and Search of 2-Aminooxazole and Simple Sugars. Astrobiol. 2020, 20, 1048–1066. 10.1089/ast.2019.2125. [DOI] [PubMed] [Google Scholar]
- Würmel J.; Simmie J. M. Substituent effects in the tautomerisation of imidic acids R–C(OH)NH → R–C(O)NH2: kinetic implications for the formation of peptide bonds in the interstellar medium. Int. J. Chem. Kinet. 2023, 55, 381. 10.1002/kin.21642. [DOI] [Google Scholar]
- Menor Salván C.; Bouza M.; Fialho D. M.; Burcar B. T.; Fernández F. M.; Hud N. V. Prebiotic Origin of Pre-RNA Building Blocks in a Urea ”Warm Little Pond” Scenario. Chembiochem 2020, 21, 3504–3510. 10.1002/cbic.202000510. [DOI] [PubMed] [Google Scholar]
- Follmann H.; Brownson C. Darwin’s warm little pond revisited: from molecules to the origin of life. Naturwissenschaften 2009, 96, 1265–1292. 10.1007/s00114-009-0602-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.




