Abstract
Background/purpose
Recurrent aphthous stomatitis (RAS) is one of the most prevalent oral mucosa diseases with unknown etiology. Reduced glutathione (GSH) is a major intracellular non-protein physiological antioxidant, and it has been demonstrated that GSH deficiency may be related to cardiovascular, immune, and diabetes. The purpose of this investigation was to evaluate the potential roles of GSH, oxidized glutathione (GSSG), and glutathione reductase (GR) in the etiopathogenesis of minor recurrent aphthous stomatitis (MiRAS).
Materials and methods
The study comprised 87 patients with idiopathic MiRAS and 90 race-, age-, and gender-matched healthy individuals. The spectrophotometric method was used to determine serum GSH and GSSG concentrations as well as GR activity. The GSSG/GSH ratios were subsequently computed. For statistical evaluation, the independent sample t test, Pearson's chi-square test, Mann–Whitney U test, Kruskal–Wallis H test, and Binary logistic regression analysis were used.
Results
The serum GSSG level, GR activity and GSSG/GSH ratio were statistically higher in MiRAS patients, whereas the concentration of serum GSH was significantly decreased. With the exception of GR, serum GSSG, GSH, and GSSG/GSH were all significantly associated with MiRAS. Serum GSSG can be regarded as a risk factor, whereas serum GSH and GSSG/GSH maybe considered as protective factors against the occurrence of MiRAS.
Conclusion
GSSG may be a potential danger factor to MiRAS and GSH may be a protective factor, while GR may not play an important role in the aetiopathogenesis of MiRAS.
Keywords: Glutathione reductase, Oxidized glutathione, Oxidative stress, Recurrent aphthous stomatitis, Reduced glutathione
Introduction
Recurrent aphthous stomatitis (RAS) is the most common oral mucosal disease with uncertain etiology, characterized by recurrent painful ulcers on nonkeratinized or keratinized oral mucosa in humans of all genders, ages, races and geographical regions. It has been found that RAS lesion occurs mostly in children, teenagers, women, and individuals from better socioeconomic strata.1,2
Based on clinical symptoms, RAS has been categorized into three categories: minor RAS (MiRAS), major RAS (MaRAS), and herpetiform RAS (HU).3,4 MiRAS, which accounts for 80% of RAS cases, is characterised by small, shallow, round or ovoid ulcers with necrotic centers, yellowish-white fibrinopurulent pseudomembranes, circumscribed margins, and erythematous haloes. It typically affects nonkeratinized oral mucosae such as the labial mucosa, buccal mucosa, ventral surface of the tongue, and the floor of the mouth, and can heal spontaneously within 7–14 days without scarring.4
Trauma, nutritional deficiencies, psychological factors, genetic predisposition, poor eating habits, sleep deprivation, bacterial and viral antigens, anemia, allergies, hormone imbalance, and some immune diseases (Behcet's disease, Crohn's disease, pharyngitis and adenitis syndrome) have all been identified as predisposing factors for RAS.3,5 All of the aforementioned conditions are thought to have the direct or indirect capacity to disrupt the balance between oxidant and antioxidant systems in humans, which may eventually contribute to oxidative stress with the accumulation of excess reactive oxygen species (ROS), potentially accelerating the occurrence of toxic reactions and the damage of human cell tissue.6
Glutathione, a biologically active tripeptide including glutamate, cysteine, and glycine, exists in biological samples in the reduced form and oxidized form, which constitute the glutathione redox couple.7,8 Reduced glutathione (γ-L-glutamyl-L-cysteinyl-glycine, GSH) has the greatest abundance of sulfhydryl groups and is a major intracellular nonprotein physiological antioxidant for reactive oxygen species (ROS) detoxification in all living organisms.8,9 It has been proved that the deficiency of GSH may be connected with many human diseases including cardiovascular, immune, diseases of aging, arthritis and diabetes.10 Under oxidative conditions, two GSH molecules donate one electron each and convert into oxidized glutathione (glutathione disulfide, GSSG), which can be either reduced back to GSH by the action of glutathione reductase (GR) or excreted into the extracellular environment by an ATP-dependent transport system located in the plasma membrane to prevent the potentially toxic effects of intracellular excessive GSSG accumulation.11
The GSSG/GSH ratio is an important and stable biological indicator for indicating the status of the thiol redox homeostasis. A increased GSSG/GSH ratio has been suggested to be important contributing factors to many human diseases such as pneumonitis, diabetes, cataracts, chronic renal failure, malignancy, neurodegenerative diseases, Parkinson's disease, Alzheimer's disease, and so on.11 Glutathione reductase (GR), an important antioxidative enzyme in the cell with a redox active disulphide at its active site, is essential for the GSH redox cycle in order to maintain adequate intracellular levels of GSH by catalyzing the nicotinamide adenine dinucleotide phosphate (NADPH) - dependent reduction of GSSG to GSH.12,13
To the best of our knowledge, there are only a few published articles about the serum levels of GSH and GSSG in RAS patients with active lesions.6,14,15 However, there is no previous information on the potential contribution of GR activity to the pathogenesis of RAS in the literature. In order to determine whether these physiological biomarkers stated above contribute to the etiopathogenesis of RAS, this study compared the serum GSH and GSSG concentrations and GR activity in Chinese MiRAS patients with active lesions to those in healthy individuals and investigated the relevance between those biomarkers mentioned above and MiRAS.
Materials and methods
This cross-sectional study was designed and conducted in accordance with the World Medical Association Declaration of Helsinki and was approved by the Ethics Committee of Bethune International Peace Hospital (Project No. 2014-12-9). A power analysis was performed to determine the minimum number of participants for this study, with an alpha value (type 1 error) of 0.05 and power (type 2 error) of 0.9. To avoid missing visits and get a higher precision, we enlarged the theoretical sample size by 20% as the final sample size, which is 177. Each subject provided written informed consent after thoroughly understanding the research objectives and procedures. The recruitment period was 18 months from July 2020 to December 2021.
Patients with minor aphthous ulcers and a medical history of recurrence of similar idiopathic ulcers at least three times per year were recruited from the Department of Stomatology of the 980th Hospital of the PLA Joint Logistics Support Force (Bethune International Peace Hospital). Only those with aphthaes diameters less than 1 cm were included in the study. Clinical examination and evaluation of oral mucosa were performed by an experienced oral medicine specialist throughout the study. The final diagnosis was based on a detailed clinical history and typical clinical symptoms as described by Manfredini et al.2 and Bilodeau et al.3 The subjective complaints of MiRAS patients recruited were documented, including the number of ulcers per attack, the frequency of ulcerative episodes per year, and the duration of each ulcer attack.
Control subjects were chosen from those who had undergone routine medical examinations at the same hospital and had never experienced an idiopathic oral ulcer incident. On the basis of gender and age, control volunteers were matched to MiRAS patients. Age, gender, marital status, allergy history, and place of residence (rural or urban) were recorded for all participants.
All participants had no symptoms of active inflammation and were not under a therapeutic regimen of immunomodulatory agents, multivitamins, steroids, or other antioxidant supplements for the previous three months. We excluded subjects with a history of trauma, diabetes mellitus, respiratory disease, liver or kidney disease, cardiovascular disorders, rheumatoid arthritis, Behçet's disease, inflammatory bowel disease, celiac disease, chronic diarrhea, pemphigus vulgaris, cicatricial pemphigoid, viral enanthems, periodontitis, oral lichen planus, and other oral mucosal diseases. Furthermore, we also excluded those participants who were pregnant, nursing, or had a history of smoking, alcoholism, or intravenous drug abuse.
Following an overnight fast, 5 mL of venous blood was drawn from the antecubital vein with a sterile disposable syringe and transferred into a vacutainer without anticoagulant in the morning hours (8:00–10:00). In order to obtain serum, all blood samples were centrifuged at 3000g for 10 min at 4 °C and stored at −80 °C until analysis.
The GR activity and serum concentrations of GSH and GSSG were measured in this study using a spectrophotometer (Genesys10 UV Scanning UV/VIS Spectrophotometer, Shimadzu Co., Tokyo, Japan) according to the manufacturer's recommendations of commercial colorimetric assay kits (GSH-GSSG/GR Detection Kit, Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
The serum concentrations of GSH and GSSG were determined following GSH- 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB) recycling assay described by Giustarini et al.11 and Sairam et al.16 GSH reacts with DTNB to form 5-thio-2-nitrobenzoic acid (TNB) and GS-TNB, which can then be reduced back to GSH by GR and NADPH. The serum concentration of total GSH (T-GSH) was estimated according to the rate of TNB formation at 412 nm. After adding a –SH masking agent to the blood sample, the concentration of GSSG was measured, and the concentration of GSH was calculated by subtracting the serum level of GSSG from T-GSH.11 Both of the biomarkers were expressed in μmol/L. The GSH/GSSG ratio was subsequently calculated as a result.
GR activity was evaluated on the basis of NADPH consumption durning the reduction of GSSG at 340 nm, just as described by Villa-Correa et al.13 and Sairam et al..16 The molar ratio of GSSG reduced to NADPH oxidized during the process was 1:1, and the results indicating GR activity were represented as U/L.
All data were analyzed using the Statistical Package for the Social Sciences version 25.0 software for Windows (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as the mean ± standard deviation or median (interquartile range) according to the results of the Kolmogorov–Smirnov test, which was used to evaluate the level of data normality. Independent sample t-test was used for comparing those data with normal distribution. The Mann–Whitney U test and Kruskal–Wallis H test were performed for non-normally distributed variables. Categorical variables were shown as number (percentage) and were compared using Pearson's chi-square test. Binary logistic regression analysis was used to show the relevance between those investigated biomarkers and MiRAS. A two-sided P-value less than 0.05 was considered to be statistically significant.
Results
A total of 87 MiRAS patients (38 males, 49 females) and 90 race-, age- and sex-matched healthy controls (42 males, 48 females) were recruited in this study. MiRAS patients were 29.87 (5.02) years old on average, while healthy controls were 30.91 (6.47) years old. There was no significant difference between MiRAS patients and healthy volunteers with respect to age (P = 0.232) and gender (P = 0.690). Other possible confounders, such as marital status, allergy history, and place of residence, did not differ statistically between the groups (Table 1).
Table 1.
Demographics and potential confounders of MiRAS patients and healthy controls.
| Variable | Total | Healthy controls (n = 90) | MiRAS patients (n = 87) | P-value |
|---|---|---|---|---|
| Age(years)a | 29.87 ± 5.02 | 30.91 ± 6.47 | 0.232 | |
| Genderb | ||||
| Male | 80 | 42 | 38 | 0.690 |
| Female | 97 | 48 | 49 | |
| Marital statusb | ||||
| Married | 120 | 58 | 62 | 0.332 |
| Single | 57 | 32 | 25 | |
| Allergy history b | ||||
| Yes | 21 | 8 | 13 | 0.213 |
| No | 156 | 82 | 74 | |
| Place of residenceb | ||||
| Rural | 111 | 58 | 53 | 0.628 |
| Urban | 66 | 32 | 34 | |
MiRAS: minor recurrent aphthous stomatitis.
Data are expressed as mean ± standard deviation. Student's t test was used.
Data are expressed as number of patients. Pearson's chi-square test was used.
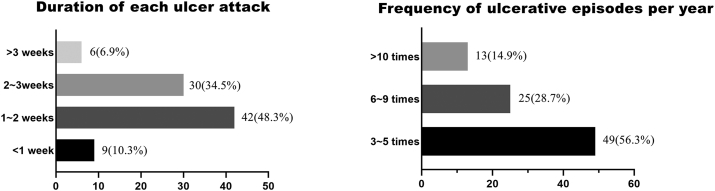
There were 73 MiRAS patients (83.9%) who had one ulcer per attack and 14 (16.1%) who had two or more ulcers per incident. The duration of each ulcer attack in most MiRAS patients included in this study was 1–3 weeks, and the frequency of recurrence was mostly 3–5 times per year (Fig. 1).
Fig. 1.
Subjective complaints of MiRAS patients about the recurring ulcer.
MiRAS: minor recurrent aphthous stomatitis.
The serum levels of GSSG (P < 0.001), GSSG/GSH (P < 0.001) and GR activity (P = 0.002) were statistically increased in MiRAS patients, whereas the serum GSH level (P < 0.001) was significantly decreased (Table 2).
Table 2.
Serum levels of GSSG, GSH, GSSG/GSH, and GR activity in MiRAS patients and healthy controls.
| Parameters | Healthy controls (n = 90) | MiRAS patients (n = 87) | P-value |
|---|---|---|---|
| GSSG (μmol/L)a | 29.94 (16.01) | 34.37 (9.07) | <0.001 |
| GSH (μmol/L)a | 44.32 (15.10) | 35.26 (8.95) | <0.001 |
| GR (U/L)a | 405.79 (150.69) | 430.87 (72.67) | 0.002 |
| GSSG/GSHa | 0.68 (0.51) | 0.97 (0.35) | <0.001 |
MiRAS: minor recurrent aphthous stomatitis; GSSG: oxidized glutathione; GSH: reduced glutathione; GR: glutathione reductase.
Data are expressed as median (interquartile range). Kruskal–Wallis H test and Mann–Whitney U tests were used.
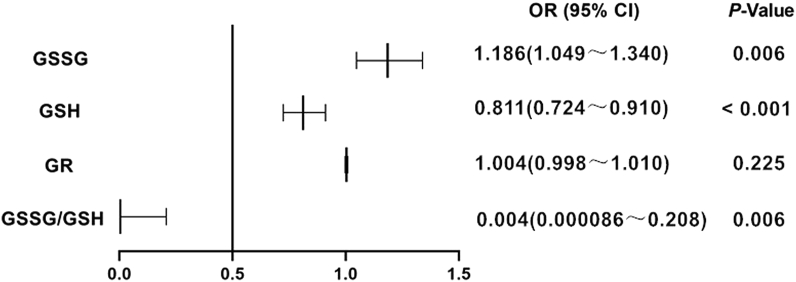
The results of binary logistic regression analysis has been presented in Table 3 and Fig. 2. According to the Hosmer–Lemeshow test, the logistic regression model had a better goodness of fit (P = 0.765). Serum GSSG, GSH, and GSSG/GSH were all significantly associated with MiRAS (P < 0.001), with the exception of GR (P = 0.225). MiRAS risk was statistically increased by serum GSSG (OR = 1.186), which can be regarded as a potential risk factor. Serum GSH and GSSG/GSH, as protective factors, reduced the incidence of MiRAS by 18.9% (OR = 0.811) and 99.6% (OR = 0.004), respectively.
Table 3.
Binary logistic regression analysis for glutathione-related biomarkers in MiRAS patients.
| 95% CI for OR |
|||||||
|---|---|---|---|---|---|---|---|
| Parameters | β | SE | Wald value | OR | Lower bound | Upper bound | P-value |
| GSSG (μmol/L) | 0.170 | 0.062 | 7.448 | 1.186 | 1.049 | 1.340 | 0.006 |
| GSH (μmol/L) | −0.209 | 0.058 | 12.783 | 0.811 | 0.724 | 0.910 | <0.001 |
| GR (U/L) | 0.004 | 0.003 | 1.475 | 1.004 | 0.998 | 1.010 | 0.225 |
| GSSG/GSH | −5.464 | 1.987 | 7.561 | 0.004 | 8.6 × 10−5 | 0.208 | 0.006 |
β: regression coefficient; SE: standard error; OR: odds ratio; CI: confidence interval; MiRAS: minor recurrent aphthous stomatitis; GSSG: oxidized glutathione; GSH: reduced glutathione; GR: glutathione reductase.
Fig. 2.
Odds ratio of serum GSSG, GSH, GR and GSSG/GSH for MiRAS patients.
OR: odds ratio; CI: confidence interval; MiRAS: minor recurrent aphthous stomatitis; GSSG: oxidized glutathione; GSH: reduced glutathione; GR: glutathione reductase.
Discussion
RAS is a prevalent, recurring, inflammatory, and ulcerative disease of the oral mucosa with a prevalence ranging from 5% to 66% in different countries.17 Even though the condition is not life-threatening, the presence of painful ulcers makes eating and speaking difficult, decreasing the quality of life for those who have active aphtha.5 It has been proved that the peak of RAS episodes is between 10 and 29 years of age and the recurrent frequency and severity decrease until the age of 40.3,5 The volunteers recruited in this study ranged in age from 43 to 19, with a mean of 30.4, which is consistent with the above epidemiological findings.
Under normal physiological circumstances, there is a favorable state of dynamic equilibrium between oxidants and antioxidant defences. When excessive reactive oxygen species (ROS) are mainly generated by the respiratory redox chain in mitochondria, and can't be eliminated by antioxidant defense mechanisms, the balance will be damaged and oxidative stress occurs, which may result in cell injury or even organ damage.18 It has been proved that oxidative stress may participate in a wide range of diseases including atherosclerosis, paraquat poisoning, chronic obstructive pulmonary disease (COPD), systemic inflammatory response syndrome (SIRS), idiopathic pulmonary fibrosis, type 2 diabetes mellitus, Alzheimer disease, hypertension and cancer.19
GSH is a low molecular-weight tripeptide providing antioxidant protection against oxidative damage and play a crucial role in cellular redox homeostasis.9,12 In the current investigation, there were significantly increased serum GSSG and decreased serum GSH in MiRAS patients compared with healthy subjects, which were consistent with the results of Bagan et al.14 As an important intracellular nonenzymatic antioxidant, GSH can be used to scavenge those excessive intracellular ROS and be converted into GSSG, resulting in higher concentration of intracellular GSSG and lower concentration of intracellular GSH. To keep the stable intracellular condition between GSSG and GSH, extracellular GSH may be transported into the intracellular environment to supplement consumed GSH, and increased intracellular GSSG also can be excreted into the extracellular environment except for being reduced back to GSH by GR, which may be used to explain the results of this study. However, Avci et al. demonstrated that the levels of GSH, determined by high-performance liquid chromatography, in patients with RAS were significantly higher than in healthy controls.15 The factors leading to the contradictory results may be different sample size, laboratory methods, subjects recruited with different racial background, dietary, and life habit. In the study, it also had been demonstrated by the binary logistic regression analysis that GSSG may be a risk factor with higher OR and GSH maybe a protective factor with lower OR in the etiopathogenesis of MiRAS.
GR is an essential enzyme for recycling oxidized glutathione to its reduced form.13,20 The potential role of GR in the pathogenesis of RAS was evaluated for the first time, which showed that GR activity was statistically increased in serum samples from MiRAS patients, but there was no significant correlation between MiRAS and GR, indicating that GR may not play an important role in the aetiopathogenesis of MiRAS. Increased GR activity can be viewed as a normal physiological response to supplement intracellular GR consumption in the GSH redox cycle.
Three limitations were identified in the current investigation. First, it had been demonstrated that those biomarkers were associated with MiRAS, but the cross-sectional design makes it difficult to evaluate whether there was a causative connection between them. Second, the majority of the participants in the study came from local area or surrounding cities, therefore the findings only represented the serum levels of GSSG, GSH, GSSG/GSH, and GR activity of this age group of the province's population. Third, the study did not take into account several other underlying factors, such as oral habits, sleeping duration, periodontal health conditions, and emotional stress status, which will be considered in our future study.
With those limitations, the findings of this study suggest that the primary thiol redox system of the cell, which is employed to defend the cell from oxidative stress, is disrupted with increased serum GSSG and decreased serum GSH. In addition, GSSG may be a potential danger factor to MiRAS and GSH may be a protective factor, while GR may not play an important role in the aetiopathogenesis of MiRAS.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
This study was supported by Open Project from State Key Laboratory of Military Stomatology (No. 2017KB02).
References
- 1.Halboub E., Al-Maweri S.A., Parveen S., et al. Zinc supplementation for prevention and management of recurrent aphthous stomatitis: a systematic review. J Trace Elem Med Biol. 2021;68 doi: 10.1016/j.jtemb.2021.126811. [DOI] [PubMed] [Google Scholar]
- 2.Manfredini M., Guida S., Giovani M., et al. Recurrent aphthous stomatitis: treatment and management. Dermatol Pract Concept. 2021;11 doi: 10.5826/dpc.1104a99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilodeau E.A., Lalla R.V. Recurrent oral ulceration: etiology, classification, management, and diagnostic algorithm. Periodontol 2000. 2019;80:49–60. doi: 10.1111/prd.12262. [DOI] [PubMed] [Google Scholar]
- 4.Chiang C.P., Yu-Fong Chang J., Wang Y.P., Wu Y.H., Wu Y.C., Sun A. Recurrent aphthous stomatitis-etiology, serum autoantibodies, anemia, hematinic deficiencies, and management. J Formos Med Assoc. 2019;118:1279–1289. doi: 10.1016/j.jfma.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z., Cao H., Xiong J., et al. Recent advances in the aetiology of recurrent aphthous stomatitis (RAS) Postgrad Med. 2022;98:57–66. doi: 10.1136/postgradmedj-2020-139421. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed S.A., Altaei T., Ahmed T. Comparative study of the antioxidant effects of lavender and flax oils in recurrent aphthous ulceration treatment. J Bagh Coll Dent. 2020;32:42–50. [Google Scholar]
- 7.Prasai P.K., Shrestha B., Orr A.W., Pattillo C.B. Decreases in GSH:GSSG activate vascular endothelial growth factor receptor 2 (VEGFR2) in human aortic endothelial cells. Redox Biol. 2018;19:22–27. doi: 10.1016/j.redox.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marushchak M., Maksiv K., Krynytska I., Stechyshyn I. Glutathione antioxidant system of lymphocytes in the blood of patients in a setting of concomitant chronic obstructive pulmonary disease and arterial hypertension. Pol Merkur Lek. 2019;47:177–182. [PubMed] [Google Scholar]
- 9.Öngöz Dede F., Bozkurt Doğan Ş., Balli U., Avci B., Durmuşlar M.C., Baratzade T. Glutathione levels in plasma, saliva and gingival crevicular fluid after periodontal therapy in obese and normal weight individuals. J Periodontal Res. 2016;51:726–734. doi: 10.1111/jre.12349. [DOI] [PubMed] [Google Scholar]
- 10.Jain S.K., Micinski D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem Biophys Res Commun. 2013;437:7–11. doi: 10.1016/j.bbrc.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giustarini D., Tsikas D., Colombo G., et al. Pitfalls in the analysis of the physiological antioxidant glutathione (GSH) and its disulfide (GSSG) in biological samples: an elephant in the room. J Chromatogr B: Anal Technol Biomed Life Sci. 2016;1019:21–28. doi: 10.1016/j.jchromb.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogłodek E.A. The role of PON-1, GR, IL-18, and OxLDL in depression with and without posttraumatic stress disorder. Pharmacol Rep. 2017;69:837–845. doi: 10.1016/j.pharep.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Villa-Correa Y.A., Isaza-Guzmán D.M., Tobón-Arroyave S.I. Influence of periodontal clinical status on salivary levels of glutathione reductase. J Periodontol. 2016;87:716–724. doi: 10.1902/jop.2016.150618. [DOI] [PubMed] [Google Scholar]
- 14.Bagan J., Saez G., Tormos C., et al. Oxidative stress and recurrent aphthous stomatitis. Clin Oral Invest. 2014;18:1919–1923. doi: 10.1007/s00784-013-1181-2. [DOI] [PubMed] [Google Scholar]
- 15.Avci E., Akarslan Z.Z., Erten H., Coskun-Cevher S. Oxidative stress and cellular immunity in patients with recurrent aphthous ulcers. Braz J Med Biol Res. 2014;47:355–360. doi: 10.1590/1414-431X20143714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sairam T., Patel A.N., Subrahmanian M., et al. Evidence for a hyper-reductive redoxin a sub-set of heart failure patients. J Transl Med. 2018;16:130. doi: 10.1186/s12967-018-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng B., Zeng X., Liu S., Zou J., Wang Y. The efficacy of probiotics in management of recurrent aphthous stomatitis: a systematic review and meta-analysis. Sci Rep. 2020;10 doi: 10.1038/s41598-020-78281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z., Li S., Fang H. Enzymatic antioxidants status in patients with recurrent aphthous stomatitis. J Oral Pathol Med. 2017;46:817–820. doi: 10.1111/jop.12547. [DOI] [PubMed] [Google Scholar]
- 19.Forman H.J., Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 2021;20:689–709. doi: 10.1038/s41573-021-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trivedi S., Lal N., Mahdi A.A., Singh B., Pandey S. Association of salivary lipid perxidation levels antioxidant enzymes and chronic periodontitis. Int J Periodontics Restor Dent. 2015;35:e14–e19. doi: 10.11607/prd.2079. [DOI] [PubMed] [Google Scholar]