Abstract
Background/purpose
The incidence and patient population of medication-related osteonecrosis of the jaw (MRONJ) has dramatically increased due to the use of drugs suppressing bone metastasis. However, its clinical treatment is still very difficult. The aim of this study was to evaluate the effectiveness and outcome of immediate fibular flap reconstruction for MRONJ in the mandible.
Materials and methods
Patients who underwent immediate fibular flap reconstruction for MRONJ in the mandible in our institution from 1990 to 2022 were screened and identified. Their demographics, drug history, symptoms, surgical parameters and follow-up data were collected and analyzed.
Results
In total, 25 patients with MRONJ stage 3 were included. The main cause of drug administration was osseous metastasis (88%), and zoledronate was the main drug. Pain, swelling (44%), pyorrhea (28%), extraoral fistulas (16%) and necrotic bone exposure (12%) were the main chief complaints. After segmental mandibulectomy, the fibular flap harvest was 9.73 ± 3.37 cm, and 18/25 (72%) were cut into two segments to reconstruct the mandible. Sixty-eight percent had an intraoral skin paddle placed. All of the flaps survived, and 21/25 (84%) of the soft tissue underwent primary healing. During follow-up, the alleviation of symptoms was effective, and there was no primary disease progression or death.
Conclusion
This is the largest comprehensive investigation of fibular flap reconstruction for MRONJ in the mandible, which is proved to be an alternative and effective treatment for managing advanced patients with MRONJ.
Keywords: Medication-related osteonecrosis of the jaw, Segmental mandibulectomy, Fibular flap, Microvascular reconstruction
Introduction
With the emergence of an increasing number of targeted drugs, the survival rate of many malignancies, such as lung cancer and breast cancer, has significantly improved. During treatment, prevention and control of bone metastasis are crucial for the long-term survival of patients. Bisphosphonates and anti-receptor activator of nuclear factor kappa-B ligand (RANKL) antibodies (denosumab) are drugs that widely inhibit bone resorption by suppressing osteoclasts, and they are administered to many patients with osteoporosis, bone metastases of cancer, and multiple myeloma.1 Currently, the pathophysiological mechanisms of medication-related osteonecrosis of the jaw (MRONJ) have not been fully elucidated. Inhibition of osteoclastic activity in bone resorption and remodeling and/or inhibition of angiogenesis plays a significant role in the occurrence of MRONJ.2 In addition to the number of patients at risk for bone metastasis, the population of elderly osteoporosis patients is rapidly growing with global aging.3 Active intervention in osteoporosis is of great significance for improving the quality of life.
Bisphosphonate (BP), as the core drug used to treat osteoporosis and bone metastasis, has been widely used in the clinic. Bisphosphonate-related jaw necrosis is one of the serious adverse complications of long-term oral or intravenous BPs and was first reported in 2003.4 The incidence of bisphosphonate-related jaw necrosis in osteoporosis patients was reported to be 0.01%∼0.04%, and the incidence of bisphosphonate-related jaw necrosis caused by intravenous injection of BPs in bone tumor patients was reported to be 0.88%∼1.15%.5 The occurrence of MRONJ will lead to treatment interruption of the primary disease and thus threaten the lives of patients. On the other hand, it will also seriously impair the basic physiological functions of patients, such as chewing, swallowing, breathing, etc. How to effectively handle MRONJ is an urgent clinical problem to be solved.
Currently, MRONJ is divided into three stages according to the American Association of Oral and Maxillofacial Surgeons (AAOMS).6 There are many methods for treating stages 1 and 2, such as debridement, antibiotics,7 buccal fat flap and submental island flap,8 L-platelet-rich fibrin (L-PRF), etc.9 However, the cure rate is unsatisfactory, only 33% for stage 1 and 24% for stage 2.10 The treatment of stage 3 MRONJ is difficult to handle with traditional methods, and an insufficient surgical margin will easily lead to relapse. Maxillofacial surgeons are often with advanced stages of MRONJ that require radical surgery, leading to compromised function and aesthetics and deterioration of the patient's quality of life.11 Segmental mandibulectomy will bring new dilemmas. MRONJ patients are often older and have poor general condition. Whether or how can a functional mandible be reconstructed after resection? How can the risks due to the patient's general condition and the need for a long, general anesthesia operation be balanced? Both are difficult in clinical decision-making.
To explore a safe and effective surgical strategy for stage 3 MRONJ, we conducted a clinical study on mandible reconstruction with a fibula flap in MRONJ patients. Through continuous follow-up, we found that this operation scheme was successful in all 25 patients, which significantly improved their quality of life and did not trigger the progression of the primary diseases. This study provides strong evidence for surgical intervention of stage 3 MRONJ in the mandible.
Materials and methods
Patients included and excluded
We retrospectively reviewed the medical records of patients with mandible MRONJ that underwent reconstruction with a fibular flap between 1990 and 2022 in our hospital. The patients with a diagnosis of osteoradionecrosis or chronic osteomyelitis were excluded. Patients who underwent reconstruction with a soft tissue flap were also excluded. All enrolled patients were followed up with panoramic radiography and/or computed tomography (CT). Long-term follow-up was regularly performed. This study was approved by the Ethics Committee of the Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine (SH9H-2021-TK472-1).
Data collection and analysis
All of the patient's clinical characteristics were reviewed, including age, sex, cause of drug administration, primary malignancy, type of drug, route of drug therapy, drug duration and holidays. Mandible lesion information included main symptoms, trigger factors and past treatment history. The surgery records were also reviewed, especially for the length of the fibular graft, the segment numbers, the location of the skin paddle, and the flap survival status. During the follow-up, the status of soft tissue healing and symptom alleviation, the condition of the primary disease and the survival status were carefully recorded.
Results
Indications for MRONJ reconstruction with a fibular flap
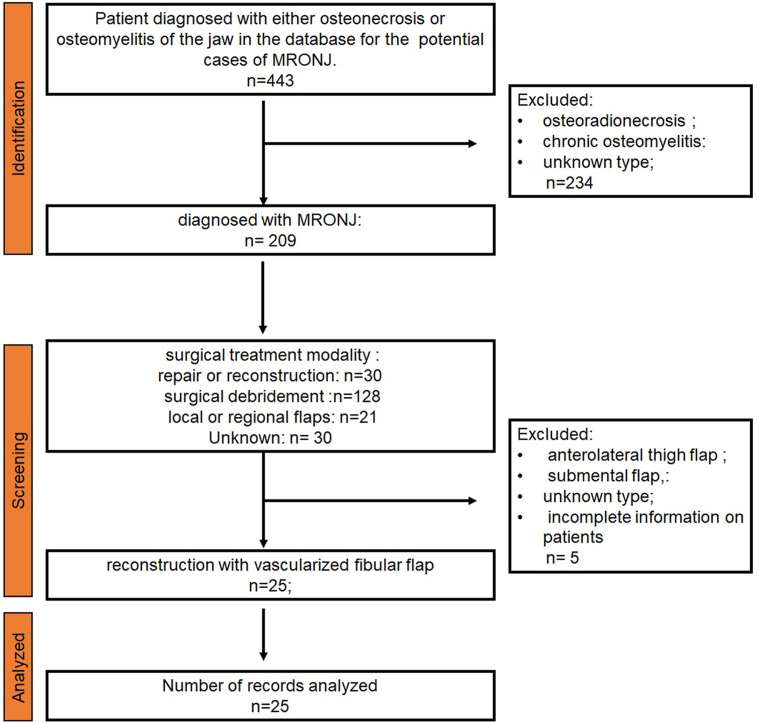
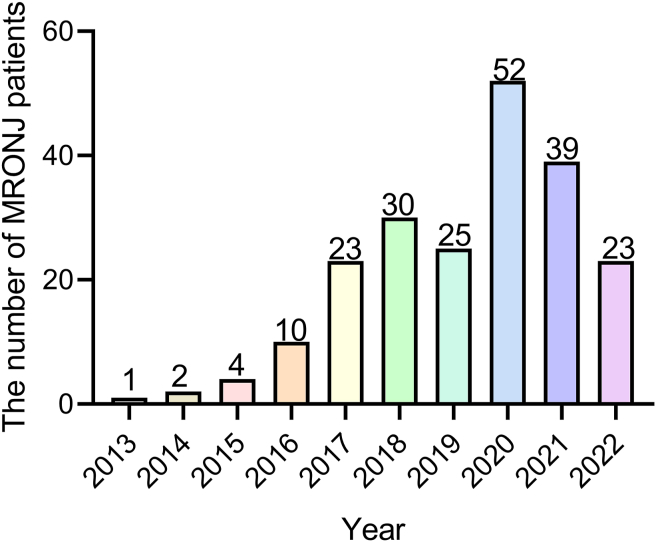
A total of 209 patients were diagnosed with MRONJ in our hospital, among which osteoradionecrosis and chronic osteomyelitis accounted for 234 cases. Almost half of them (128/209, 61.2%) underwent surgical debridement. Thirty patients (14.4%) were reconstructed with a microvascular flap. A total of 25 patients were reconstructed with vascularized fibular flaps and enrolled in this study. The identification and screening process is shown in the diagram (Fig. 1). Very interestingly, we found that the cases of MRONJ was increasing year by year. As for recent two years, the diagnosed cases slightly decreased since the impact of the COVID-19 pandemic (Fig. 2).
Fig. 1.
Flow diagram of the identification and screening of patients.
Fig. 2.
Cases of diagnosed MRONJ patients per year.
The basic clinical characteristics of the MRONJ patients
The 25 patients had a mean age of 63 ± 8.7 years and comprised 9 men and 16 women (Table 1). Osseous metastasis accounted for the majority (88%) of drug administration. Breast cancer (41%), lung cancer (23%) and prostate cancer (14%) were the main malignancies causing bone metastasis. Zoledronate (88%) was the most frequent drug inhibiting bone absorption, and intravenous injection (76%) was the most common route. The drug administration duration was 2.79 ± 2.16 years, and 88% had a drug holiday of less than 90 days. For the mandible lesion, pain and swelling (44%) were the most common chief complaints, followed by pyorrhea 28%, extraoral fistulas 16%, and necrotic bone exposure 12%. Only 14% of patients were triggered by tooth extraction, while 88% had no precipitating factor. Antibiotic therapy, curettage and no treatment had similar proportions in their past treatment history.
Table 1.
Clinical characteristics of MRONJ patients (n = 25).
| Clinical characteristics | No of patients (%) or mean ± SD |
|---|---|
| Age (years) | 63 ± 8.7 |
| Sex | |
| male | 9 (36%) |
| female | 16 (64%) |
| The cause of drug administration | |
| Osteoporosis | 2 (8%) |
| Osseous metastasis | 22 (88%) |
| Others | 1 (4%) |
| The location of primary malignancy | |
| Breast | 9 (41%) |
| Lung | 5 (23%) |
| Prostate | 3 (14%) |
| Kidney | 2 (9%) |
| Melanoma | 1 (4.3%) |
| Thyroid | 1 (4.3%) |
| Esophagus | 1 (4.3%) |
| Type of drugs | |
| Zoledronate | 22 (88%) |
| Alendronate | 1 (4%) |
| Denosumab | 1 (4%) |
| Ibandronate | 1 (4%) |
| Route of drug therapy | |
| Orally | 5 (20%) |
| intravenous | 19 (76%) |
| Subcutaneous injection | 1 (4%) |
| Drug administration duration(year) | 2.79 ± 2.16 |
| Drug holiday | |
| No. <90 days | 22 (88%) |
| Yes. ≥90 days | 3 (12%) |
| The mandible lesion characteristics | |
| Main symptoms | |
| Pain, swelling | 11 (44%) |
| Necrotic bone exposure | 3 (12%) |
| Extraoral Fistulas | 4 (16%) |
| pyorrhea | 7 (28%) |
| Trigger factors | |
| Spontaneous | 21 (84%) |
| Extraction | 4 (16%) |
| Past treatment history | |
| Antibiotic therapy | 8 (32%) |
| Curettage | 8 (32%) |
| None | 9 (36%) |
The clinical characteristics of the mandibular reconstruction and postoperative follow-up
A fibular flap is an ideal approach to reconstruct mandible defects.12 The length of the fibula graft harvested during the operation was 9.71 ± 3.37 cm (range: 4.6–18.6 cm) (Table 2). A total of 18/25 (72%) fibular grafts were cut into two segments to restore the mandibular radian, while 24% were cut into three segments. The skin paddle of the fibular flap was sutured into the intraoral mucosa (68%), and the others were extraoral. All free fibular flaps survived, and most of them (84%) showed primary healing. The secondary healing patients (4/25) had undergone approximately two weeks of dressing changes. All patients were strictly followed for 11.4 ± 9.0 months (range: 0.5–30 months). All of the patients had significant symptom alleviation and life quality improvement. No cases of new malignancy or worsening of preexisting malignancy were observed among the participants. To date, all patients are alive. Next, we will introduce two representative cases of fibular reconstruction for stage 3 MRONJ.
Table 2.
Clinical characteristics of mandibular reconstruction and postoperative follow-up.
| Clinical characteristics | No of patients (%) or mean ± SD |
|---|---|
| The length of fibula graft (cm) | 9.71 ± 3.37 |
| The fibular segment | |
| One | 1 (4%) |
| Two | 18 (72%) |
| Three | 6 (24%) |
| The location of skin paddle | |
| Intraoral | 17 (68%) |
| Extraoral | 8 (32%) |
| Flap survival | |
| Yes | 25 (100%) |
| No | 0 (0%) |
| The status of soft tissue healing | |
| Primary healing | 21 (84%) |
| Secondary healing | 4 (16%) |
| Follow-up time (months) | 11.4 ± 9.0 |
| The alleviation of symptoms | |
| Yes | 25 (100%) |
| No | 0 (0%) |
| The status of primary disease | |
| Stable | 25 (100%) |
| Progressive | 0 (0%) |
| Survival status | |
| Live | 25 (100%) |
| Dead | 0 (0%) |
Case 1
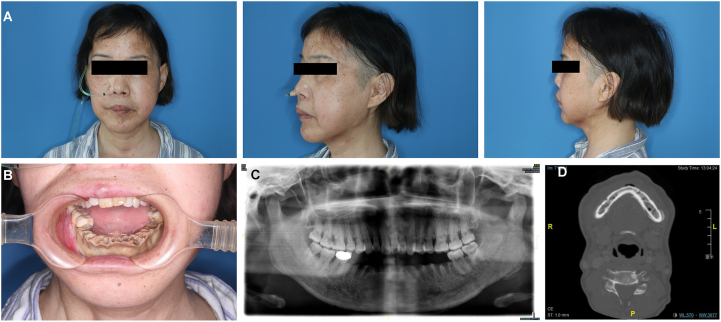
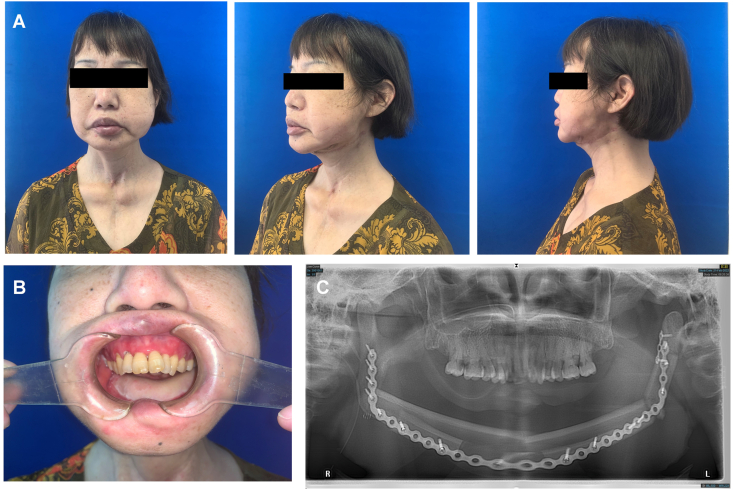
The first patient, a 53-year-old woman, suffered from follicular thyroid cancer with systemic metastasis. Zoledronic acid was orally administered to inhibit bone metastasis. One year ago, dead bone exposure in the mandible and tooth loosening began to appear, which made it unable for her to eat and a gastric tube was placed (Fig. 3A). Preoperative photos show extensive yellow bone exposure of the mandible, and all teeth involved in the exposed bone area fell out (Fig. 3B). Panoramic pantomogram and spiral CT showed extensive bone destruction of the mandible, sequestration and obvious buccal periosteal reaction (Fig. 3C and D).
Fig. 3.
Preoperative examination of a 53-year-old woman diagnosed with MRONJ in the mandible. (A) Anteroposterior and lateral photos before the operation. (B) The intro-oral photos show dead bone exposure. (C, D) Panoramic and CT images showing bone sequestrum and the periosteal reaction at the mandible.
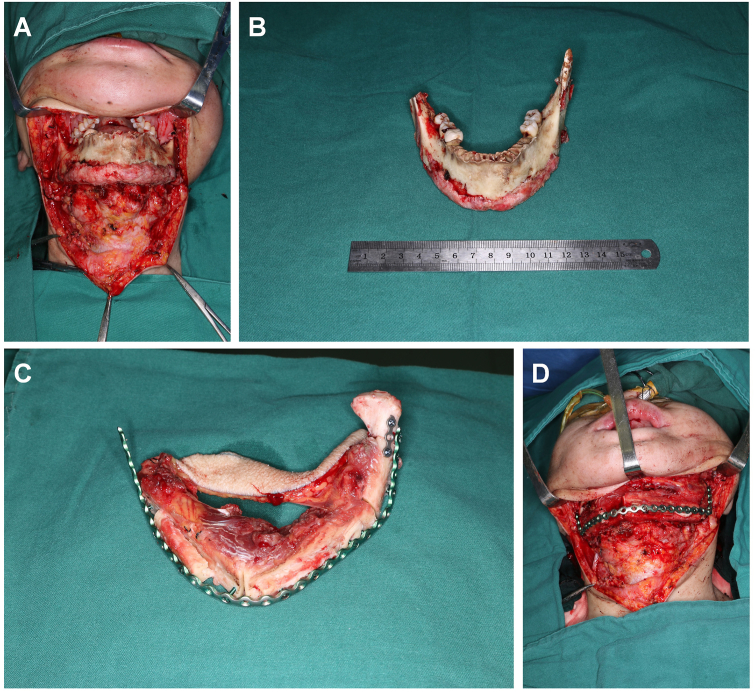
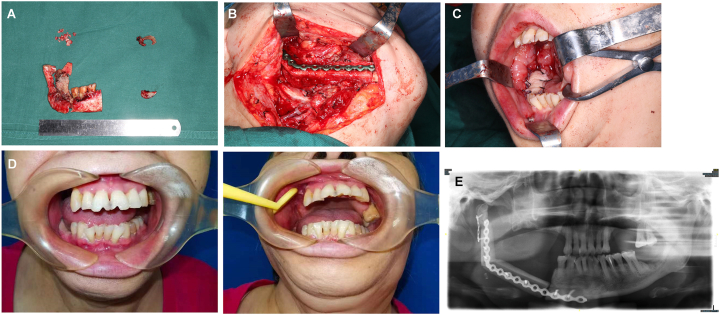
During the operation, a submandibular incision from the right to the left angle of the mandible was designed to expose the diseased bone fully (Fig. 4A). Only the anterior mandibular ramus and condyle were preserved on both sides, and the others were removed (Fig. 4B). Meanwhile, the fibular myocutaneous flap was cut into 3 segments, the reconstruction titanium plate was shaped and fixed, and the skin paddle was sutured in the mouth (Fig. 4C and D). One month after the operation, the patient resumed normal mouth food intake, and the skin paddle survived well (Fig. 5A and B). From the panoramic pantomogram, we found that the transplanted fibula was fixed and had healed well (Fig. 5C). This patient suffered from bilateral mandible destruction, which underwent an almost total mandibular reconstruction with a longer fibular flap. This represented a very difficult surgery, while the operation was successful and the patient begin to seek dental implant to restore occlusal function.
Fig. 4.
Intraoperative photograph of patient 1. (A, B) The pathological mandible was exposed and resected. (C, D) The fibular flap was shaped and fixed with a mandibular titanium plate.
Fig. 5.
The postoperative follow-up of patient 1. (A) Anteroposterior and lateral photos after the operation. (B) The intro-oral photos show healing oral mucosa. (C) Panoramic image showing the mandible reconstruction.
Case 2
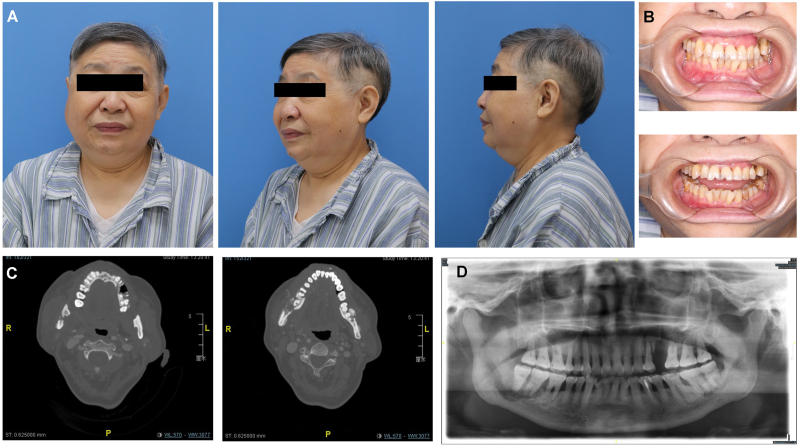
Another patient, a 64-year-old woman, was administered bisphosphonate via intravenous injection since bone metastasis of breast cancer. After one year of medication, swelling and pain of the right mandible with limited mouth opening began to appear, which did not improve after anti-infection treatment (Fig. 6A). Preoperative examination showed that the degree of mouth opening was less than one transverse finger (Fig. 6B). Spiral CT showed bone destruction and periosteal reaction in the bilateral mandibular angle area and maxilla (Fig. 6C). The panoramic scan showed dead bone formation in the right mandibular angle and ramus, spreading to the inferior alveolar nerve canal (Fig. 6D). During the operation, the bone in the right mandible was found to be severely damaged with pathological fracture. Meanwhile, dead bone was also removed from the bilateral maxilla and the opposite mandible (Fig. 7A). A fibular flap was harvested, shaped and reconstructed to the right mandibular body and ramus, and the skin island was placed in the mouth (Fig. 7B and C). The postoperative follow-up showed that the patient's mouth opening was significantly improved, and the soft tissue of the flap healed well (Fig. 7D). Postoperative panoramic film showed that the condyle was in the glenoid fossa and that the fibula was well fixed and healed (Fig. 7E). This case was a representative one that the lesion simultaneously affected four quadrants of maxilla and mandible. While the fibular flap was used to reconstruct the worst side.
Fig. 6.
The preoperative details of a 64-year-old MRONJ patient. (A) Anteroposterior and lateral photos before the operation. (B) The intraoral images show the limitation of mouth opening. (C, D) CT and panoramic images showing lesions in both the mandible and maxilla.
Fig. 7.
Intraoperative and postoperative photographs of patient 2. (A) All of the pathological bone was removed and is shown. (B) A fibular flap was shaped to reconstruct the right mandible defect. (C) The skin island of the flap was sutured to seal the oral cavity. (D) The intraoral photos after the operation show healing and mouth opening improvement. (E) Panoramic image showing the right mandible reconstruction.
Discussion
Advanced-stage MRONJ is a difficult issue to be handled clinically. How to balance a long, challenging surgery with a patient's generally poor overall condition makes it difficult to devise a suitable surgical strategy. Here, our study demonstrated that fibular flap reconstruction after segmental mandibulectomy in MRONJ patients is safe and effective. Vascularized fibular flap reconstruction provides a margin guarantee for complete resection of the pathological bone to reduce the risk of relapse. This strategy can also restore the facial profile and enhance the patient's quality of life. This study provides new evidence for surgical intervention and strengthens our confidence in the treatment of MRONJ.
For segmental mandibulectomy of MRONJ, anterolateral thigh flap (ALTF) is the main reconstruction strategy. However, a truncated mandible without effective support will inevitably lead to facial deviation and occlusion disorder. Bowe et al. reported 30 consecutive patients who underwent reconstruction of a segmental mandibulectomy defect using a bridging mandibular reconstruction plate and ALTF.13 In the follow-up in our department, there were also 4 cases of ALTF reconstruction. Although this flap had advantages for soft tissue reconstruction, it could not restore the patient's facial shape and occlusal relationship.
It has also been reported that a reconstruction plate can be applied to fix and support the free bone ends.14 It can restore the facial profile to a certain extent, but there is long-term stress fatigue and fracture risk of the titanium plate. Once the titanium plate breaks, it inevitably leads to plate exposure and reoperation. Moreover, Mitsunobu et al. reported that 13 patients underwent segmental mandibulectomy using free fibula flap transplantation or just with a metal plate or no reconstruction.,15 just focal debridement.
Individual cases reconstructed with fibular flaps for MRONJ patients have been previously reported.16,17 The previous case reports of fibular flap reconstruction required larger samples for validation and investigation. The vascularized fibula used in our study can effectively support the facial appearance and restore normal oral function. The skin paddle of the fibular flap can effectively seal the mouth and provide a closed physical environment for bone reconstruction. The first patient achieved satisfactory primary healing after surgery. After follow-up, the patient raised the demand for dental implantation, suggesting that the initial goal of fibular reconstruction is to restore the facial appearance, and the ultimate goal is occlusal reconstruction.
According to our results, the length of the mandible defect in MRONJ patients is approximately 9 cm, and the two-segment fibula can meet most of the intraoperative requirements. In terms of surgical techniques alone, the operation is not very difficult. However, accurate evaluation of patients' general conditions, especially preoperative vascular evaluation, is crucial to the success of the operation. In addition to the effects of the bisphosphate, chemotherapy using antiangiogenic drugs can easily lead to vascular endothelial damage.18
Currently, we routinely use preoperative ultrasound to analyze the hemodynamics of the recipient vessels, such as the external maxillary artery and the superior thyroid artery. For the donor, the peroneal artery and posterior tibial artery should be assessed to determine whether they share a common trunk, as well as to determine the perforator location of the skin paddle. High-frequency color Doppler ultrasound can quantitatively evaluate and accurately mark the peroneal artery and vein and perforators before fibular flap transplantation.19
Moreover, identifying the extent of necrotic bone is a critical step to avoid recurrence. Generally, bone bleeding and color can distinguish healthy and pathological bone. Of course, the VELscope system inducing doxycycline bone fluorescence by emitting blue light (400–460 nm) is applied to assist with the mandibulectomy.20,21 Moreover, 18F-fluoride positron emission tomography-computed tomography (PET-CT) imaging was conducted in all patients suffering from malignancies to exclude fibula metastasis and evaluate their general condition, which was emphasized in a similar clinical trial.22 Adequate preoperative preparation and stable microsurgery techniques are key points for the success of the operation. However, the active treatment of patients' primary diseases and other chronic diseases after surgery plays a decisive role in whether the patients can achieve primary healing. In our study, there were also 4/25 (84%) patients with secondary healing due to diabetes and advanced age.
Certainly, there still exist some limitations of this study. First, the treatment experience originated from a single center. This retrospective analysis is vulnerable to self-selection bias and recall bias inherent to this study design. Second, the sample size and follow-up time were still not sufficient. Long-term follow-up can improve the surgeon's confidence in performing such surgery.
In summary, this is the largest comprehensive investigation of fibular flap reconstruction for MRONJ in the mandible, which has proven to be an effective treatment strategy. This study will change our concept of the treatment of MRONJ from simple debridement to functional reconstruction, which will greatly improve the quality of life and long-term survival.
Declaration of competing interest
The authors declare no conflicts of interest with respective to the research and publication of this article.
Acknowledgments
This study was supported by grants from the Project of National Natural Science Foundation of China (grant No. 82272983, 82173451, 82172897), The Innovative Research Team of High-level Local Universities in Shanghai (SHSMU-ZLCX20212300, SHSMU-ZDCX20212500), Shanghai “Rising stars of medical talents”-specialist program.
References
- 1.Satcher R.L., Zhang X.H. Evolving cancer-niche interactions and therapeutic targets during bone metastasis. Nat Rev Cancer. 2022;22:85–101. doi: 10.1038/s41568-021-00406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.On S.W., Cho S.W., Byun S.H., Yang B.E. Various therapeutic methods for the treatment of medication-related osteonecrosis of the jaw (MRONJ) and their limitations: A narrative review on new molecular and cellular therapeutic approaches. Antioxidants. 2021;10:680. doi: 10.3390/antiox10050680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rachner T.D., Khosla S., Hofbauer L.C. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marx R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 5.Mavrokokki T., Cheng A., Stein B., Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg. 2007;65:415–423. doi: 10.1016/j.joms.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 6.Ruggiero S.L., Dodson T.B., Fantasia J., et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 7.Montebugnoli L., Felicetti L., Gissi D.B., Pizzigallo A., Pelliccioni G.A., Marchetti C. Biphosphonate-associated osteonecrosis can be controlled by nonsurgical management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:473–477. doi: 10.1016/j.tripleo.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Myoken Y., Kawamoto T., Fujita Y., Toratani S. Simultaneous defect reconstruction in stage 3 medication-related osteonecrosis of the maxilla and mandible using the buccal fat flap and submental island flap: Case report. J Dent Sci. 2022;17:1066–1068. doi: 10.1016/j.jds.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouland C., Meuleman N., Widelec J., et al. Case reports of medication-related osteonecrosis of the jaw (MRONJ) treated with uncultured stromal vascular fraction and L-PRF. J Stomatol Oral Maxillofac Surg. 2021;122:212–218. doi: 10.1016/j.jormas.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Rupel K., Ottaviani G., Gobbo M., et al. A systematic review of therapeutical approaches in bisphosphonates-related osteonecrosis of the jaw (BRONJ) Oral Oncol. 2014;50:1049–1057. doi: 10.1016/j.oraloncology.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Sarmiento L.A. Resolution without surgery of an advanced stage of medication-related osteonecrosis of the jaw (MRONJ) in a patient who could not suspend her treatment for osteoporosis. Oral Oncol. 2019;99 doi: 10.1016/j.oraloncology.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Dowgierd K., Pokrowiecki R., Wolanski W., et al. Analysis of the effects of mandibular reconstruction based on microvascular free flaps after oncological resections in 21 patients, using 3D planning, surgical templates and individual implants. Oral Oncol. 2022;127 doi: 10.1016/j.oraloncology.2022.105800. [DOI] [PubMed] [Google Scholar]
- 13.Bowe C., Butler D., Dhanda J., Gulati A., Norris P., Bisase B. Lateral segmental mandibulectomy reconstruction with bridging reconstruction plate and anterolateral thigh free flap: a case series of 30 consecutive patients. Br J Oral Maxillofac Surg. 2021;59:91–96. doi: 10.1016/j.bjoms.2020.08.054. [DOI] [PubMed] [Google Scholar]
- 14.Wilde F., Hendricks J., Riese C., Pausch N.C., Schramm A., Heufelder M. Bone regeneration without bone grafting after resection of a segment of the mandible to treat bisphosphonate-related osteonecrosis of the jaw. J Oral Maxillofac Surg. 2011;69:2657–2662. doi: 10.1016/j.joms.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 15.Otsuru M., Soutome S., Hayashida S., Rokutanda S., Yanamoto S., Umeda M. A preliminary clinical study of segmental mandibulectomy on medication-related osteonecrosis of the jaw. J Dent Sci. 2022;17:444–450. doi: 10.1016/j.jds.2021.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mücke T., Haarmann S., Wolff K.D., Hölzle F. Bisphosphonate related osteonecrosis of the jaws treated by surgical resection and immediate osseous microvascular reconstruction. J Cranio-Maxillo-Fac Surg. 2009;37:291–297. doi: 10.1016/j.jcms.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Nocini P.F., Saia G., Bettini G., et al. Vascularized fibula flap reconstruction of the mandible in bisphosphonate-related osteonecrosis. Eur J Surg Oncol. 2009;35:373–379. doi: 10.1016/j.ejso.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Beth-Tasdogan N.H., Mayer B., Hussein H., Zolk O., Peter J.U. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst Rev. 2022;7:Cd012432. doi: 10.1002/14651858.CD012432.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong J., Jia Y.P., Luo W.D., Li C.J. Preoperative high-frequency color Doppler ultrasound assessment of the blood vessels of the fibular myocutaneous flap. J Plast Reconstr Aesthetic Surg. 2022;75:3964–3969. doi: 10.1016/j.bjps.2022.08.045. [DOI] [PubMed] [Google Scholar]
- 20.Wehrhan F., Weber M., Neukam F.W., Geppert C.I., Kesting M., Preidl R.H.M. Fluorescence-guided bone resection: A histological analysis in medication-related osteonecrosis of the jaw. J Cranio-Maxillo-Fac Surg. 2019;47:1600–1607. doi: 10.1016/j.jcms.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Otto S., Schnödt E.M., Haidari S., et al. Autofluorescence-guided surgery for the treatment of medication-related osteonecrosis of the jaw (MRONJ): a retrospective single-center study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131:519–526. doi: 10.1016/j.oooo.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Sim I.W., Borromeo G.L., Tsao C., et al. Teriparatide promotes bone healing in medication-related osteonecrosis of the jaw: A placebo-controlled, randomized trial. J Clin Oncol. 2020;38:2971–2980. doi: 10.1200/JCO.19.02192. [DOI] [PubMed] [Google Scholar]