Abstract
Background/purpose
As calcium silicate cements (CSCs) have been successfully used in various types of vital pulp therapy, many new CSC products have been developed. The aim of this study was to evaluate the biocompatibilities and mineralization potential of new CSC. The experimental materials were NeoMTA Plus and EndoSequence Root Repair Material-Fast Set Putty (ERRM-FS) which were compared to ProRoot MTA.
Materials and methods
In vitro, the effects of the new CSC on stem cells were evaluated. Each CSC was prepared for cell viability testing, alkaline phosphatase (ALP) assay, and calcium ion release assay. In vivo, the exposed pulp model was used for the partial pulpotomy procedure. Thirty-six teeth were treated with three materials: ProRoot MTA, NeoMTA Plus, or ERRM-FS. After four weeks, the teeth were extracted and processed for histologic analysis. Dentin bridge formation, pulp inflammation, and odontoblastic cell layer were evaluated and the area of newly formed calcific barrier of each group was measured.
Results
Three CSCs demonstrated similar cell viability on stem cells and the levels of ALP and calcium release were not significantly different between tested materials. ProRoot MTA and ERRM-FS showed better tissue healing process than NeoMTA Plus after partial pulpotomy, in terms of quality of calcific barrier and pulp inflammation. The outcomes from measuring newly formed calcific area demonstrated no significant differences between the materials.
Conclusion
NeoMTA Plus and ERRM-FS displayed similar biocompatibilities and mineralization potential compared to ProRoot MTA. Therefore, these new CSCs can be used as desirable alternatives to ProRoot MTA.
Keywords: Biocompatibility, Calcium silicate cement, Partial pulpotomy, Mineralization potential
Introduction
Calcium silicate cements (CSCs) mostly comprise dicalcium silicate (Ca2SiO4) and tricalcium silicate (Ca3SiO5). These particles primarily release calcium hydroxide and calcium silicate hydrate gel via a hydration reaction, eventually contributing to the healing process of the dentin-pulp complex.1 An ideal CSC has low toxicity, high biocompatibility, osteoinductive properties, high sealing ability with no discoloration of teeth. It should also be antibacterial, easy to manage, cost-effective, dimensionally stable and resistance to moisture.2,3
The most-well-known CSC is ProRoot MTA (Dentsply, Tulsa, OK, USA), which has been widely used in various types of vital pulp therapy since its introduction in the 1990s.4,5 Previous studies found that ProRoot MTA exhibited high levels of biocompatibility, sealing ability, and antibacterial activity, and the ability to form mineralized tissue.6,7 However, limitations of ProRoot MTA include tooth discoloration, prolonged setting time, and difficulty in manipulation.8 CSCs have therefore recently been developed with a focus on addressing the above limitations.
Among such current CSCs, NeoMTA Plus (NuSmile, Houston, TX, USA) is a powder-gel type CSC composed of an extremely fine powder of Ca3SiO5, Ca2SiO4, and tantalum oxide (Ta2O5), which was formulated to prevent tooth discoloration.9 According to the manufacturer, NeoMTA Plus can be used in vital pulp therapies such as pulp capping, and partial and complete pulpotomy. NeoMTA Plus can also be mixed into a putty form, which improves manageability relative to ProRoot MTA, which has a mud-like consistency. Previous studies found that NeoMTA Plus had stable discoloration and bioactivity characteristics, and it is therefore suggested as a suitable alternative to ProRoot MTA.10,11 However, the only previous in vivo study that we were aware of that has analyzed histologic pulpal reactions after a pulpotomy using NeoMTA Plus was that of Walsh et al.,12 who evaluated the healing potential of NeoMTA Plus compared with Quick-Set2 (Avalon Biomed, Bradenton, FL, USA) in a dog model. One of the limitations of that study was no attempt being made to quantify the differences in histologic features between the two materials. The findings would also have been more convincing if the authors had performed comparisons with ProRoot MTA.
EndoSequence Root Repair Material-Fast Set Putty (ERRM-FS; Brasseler USA, Savannah, GA, USA) is a premixed CSC comprising Ca3SiO5, Ca2SiO4, zirconium oxide (ZrO2), Ta2O5, calcium phosphate monobasic, and filler agents. As the name claims, ERRM-FS has a shorter setting time than the original EndoSequence BC Root Repair Material Putty (ERRM; Brasseler USA). ERRM-FS was primarily developed for root repairs but was also introduced as a pulp capping material. Most previous studies have focused on root repair rather than vital pulp therapy. ERRM-FS presented good marginal adaptation when it was used to seal immature root canals or for perforation in tooth models.13,14 ERRM-FS also exhibited good healing potential after root-end surgery in vivo.15 However, few studies have investigated the use of ERRM-FS in vital pulp therapy, and no animal studies have assessed the pulpal reaction after pulp capping using ERRM-FS. Further research on ERRM-FS is therefore needed to improve the understanding of the histologic response following pulp treatment.
The purpose of the present study was to determine the biocompatibilities of NeoMTA Plus and ERRM-FS compared with ProRoot MTA, and to evaluate the mineralization potential and histologic responses of dental pulp using in vitro and in vivo experiments.
Materials and methods
Isolation of cells and cell culture conditions
Considering the increasing use of CSC in deciduous teeth and differences in response to the materials of each stem cells,16 following two types of cells were used in all in vitro experiments: (1) stem cells from human exfoliated deciduous teeth (SHED) obtained from deciduous teeth, and (2) human dental pulp stem cells (DPSCs) obtained from permanent teeth. Both cell types were collected from extracted teeth under the guidelines approved by the institutional review board of the Yonsei dental hospital (#2-2019-0028). For primary cell cultures, pulp tissue was collected from the extracted teeth using a barbed broach (Mani, Utsunomiya, Japan) and added to 1.5-ml microtubes (Corning Life Science, Wujiang, China) mixed using 4 mg/ml dispase II (Sigma–Aldrich, St. Louis, MO, USA) and 3 mg/ml collagenase, Type I (Worthington Biochemical, Lakewood, NJ, USA). The specimen was reacted in a CO2 incubator at 37 °C for 30 min, and then filtered using a 70-μm cell strainer (BD Falcon, Lincoln Park, NJ, USA). Isolated SHED and DPSCs were cultured using alpha minimum essential medium (α-MEM; Thermo Fisher Scientific, Carlsbad, CA, USA) mixed with 10% fetal bovine serum, 1% penicillin streptomycin, 1% l-glutamine, and 0.2% amphotericin B (all from Invitrogen, Carlsbad, CA, USA) in a 5% CO2 incubator at 37 °C. Each type of stem cell was blended with cells from three donors before cell passage three and was used for the experiments before passage five.
Material extracts
Three CSCs were used in this study: ProRoot MTA, NeoMTA Plus, and ERRM-FS (Table 1). ProRoot MTA and NeoMTA Plus were mixed in accordance with the instructions of the manufacturer. ERRM-FS was provided as a premixed putty. Each mixture was shaped into 1.0-mm-thick and 8.0-mm-diameter discs using rubber molds, and then covered using an overhead projector (OHP) film (CG6000, 3 M Korea, Seoul, South Korea). Sample discs were placed in an incubator at 100% humidity and 37 °C for 24 h. After the OHP film was removed, the mixture was stored in the incubator for an additional day. The mixture discs were then removed from the rubber molds and sterilized using ethylene oxide. These discs were used to prepare the material extracts.
Table 1.
Chemical compositions of the three calcium silicate cements used in this study.
| Brand name | Manufacturer | Formulation | Composition | |
|---|---|---|---|---|
| ProRoot MTA | Dentsply Tulsa, OK, USA |
Powder liquid | Powder: | Tricalcium silicate |
| Dicalcium silicate | ||||
| Tricalcium aluminate | ||||
| Tetracalcium aluminoferrite | ||||
| Calcium sulfate dihydrate | ||||
| Bismuth oxide | ||||
| Liquid: | Distilled water | |||
| NeoMTA Plus | NuSmile Houston, TX, USA |
Powder gel | Powder: | Tricalcium silicate |
| Dicalcium silicate | ||||
| Tantalum pentoxide | ||||
| Gel: | Distilled water | |||
| Proprietary polymers | ||||
| ERRM-FS | Brasseler USA Savannah, GA, USA |
Premixed syringe | Tricalcium silicate | |
| Dicalcium silicate | ||||
| Tantalum pentoxide | ||||
| Zirconium oxide | ||||
| Proprietary fillers | ||||
| Thickening agents | ||||
ERRM-FS, EndoSequence Root Repair Material-Fast Set Putty.
Two discs were each placed in 50-ml conical tubes (SPL Life Sciences, Pocheon, South Korea) and the elute of each material was extracted using a cell culture medium (α-MEM) as the extraction vehicle. The tubes were stored in a 5% CO2 incubator at 37 °C for 3 days. The extracts were then collected using a 1-ml syringe (Koreavaccine, Ansan, South Korea), filtered through a 0.2-μm Minisart® syringe filter (Sartorius, Goettingen, Germany), and then stored at 4 °C.
Cell viability test
The Cell Counting Kit-8 (CCK8; Dojindo, Kumamoto, Japan) assay was performed to measure the cell viability according to the manual from the manufacturer. SHED and DPSCs were seeded in a 48-well plate at 2 × 108 cells/well, and when the cells reached a confluency of 70%, the cell culture medium was changed to various dilutions (1:1, 1:2, and 1:4) of the material extraction medium. After 3 days, CCK8 reagent was added dropwise to each well. After a further 1 h of incubation, cell viability was measured at 450 nm using a spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA).
Alkaline phosphatase assay
SHED and DPSCs were seeded at a density of 1 × 104 cells/well on a 12-well plate (BD Falcon). When the cell density reached about 50%, the existing culture medium was replaced with a material extracted medium (1:4 dilution), and the medium was then changed twice weekly. After 10 days, the cells were fixed in 10% neutral buffered formalin for 30 min at 4 °C and washed three times using phosphate buffered saline (Invitrogen). The ALP activity of the cells was then evaluated using the SensoLyte® pNPP Alkaline Phosphatase Assay Kit (AnaSpec, Fremont, CA, USA) at room temperature for 30 min, and quantified. A spectrophotometer was used to measure color changes at 405 nm. The standard value of ALP activity was also calculated by quantifying the total protein content at 562 nm using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). The negative control was the culture medium, and the positive control was odontogenic induction medium (α-MEM containing 10% FBS, 1% antibiotics, 0.1 μM dexamethasone, 2 mM β-glycerophosphate [Sigma–Aldrich], and 50 μM ascorbic acid 2-phosphate).
Calcium assay
The extraction medium of materials was placed in a 96-well plate (BD Falcon) and analyzed using the Calcium Assay Kit (Abcam, Cambridge, UK). The chromogenic solution and calcium assay buffer were treated using an extraction growth medium according to the instructions of the manufacturer and were reacted at room temperature for 10 min. Samples were then measured using a spectrophotometer at 575 nm, and the calcium concentration of each sample was subsequently normalized. The culture medium was used as a control.
Animals
Thirty-six teeth from two beagle dogs were selected for partial pulpotomy. Incisors and premolars in both the mandible and maxilla were used, with canines excluded due to the difficulty of their complete extraction. The beagles were 12 months old, and each animal had a healthy periodontium with normal dentition. Animal selection, care, and the surgical protocol were approved by the Institutional Animal Care and Use Committee of Yonsei University Health System, Seoul, South Korea (certification #2018–0212).
General anesthesia was induced by injecting tiletamine-zolazepam (10 mg/kg Zoletil 50, Virbac, Carros, France) and medetomidine hydrochloride (0.02 mg/kg, Provet Veterinary Products, Istanbul, Turkey). The animals then inhaled anesthetic isoflurane 2% (Hana Pharmaceutical, Hwaseong, South Korea). To prevent infection, cefazolin (30 mg/kg, Chong Kun Dang Pharmaceutical, Seoul, South Korea) was injected subcutaneously immediately before and after treatment, and cephalexin (30 mg/kg, Boryung Pharmaceutical, Seoul, South Korea) was administered intraorally for 7 days after the operation. At the same time, ketamine (0.5 mg/kg, Hana Pharmaceutical) and meloxicam (0.2 mg/kg, Boehringer Ingelheim Vetmedica, Ingelheim, Germany) were administered for postoperative pain control under the supervision of a trained veterinarian.
Partial pulpotomy procedure
An adequate lidocaine dose (2% lidocaine hydrochloride with epinephrine 1:100,000, Kwang Myung Pharmaceutical, Seoul, South Korea) was administered for local anesthesia. The 36 teeth were divided into ProRoot MTA, NeoMTA Plus, and ERRM-FS groups, with 6 incisors and 6 premolars per group. Coronal access was obtained using a sterile high speed carbide bur 330 (H7-314-008, Gebr. Brasseler GmbH, Lemgo, Germany) immediately before exposing the pulp tissue. The pulp tissue was then mechanically exposed to an approximate diameter of 1 mm using a sterile round carbide bur (Prima Classic RA4 no. 2, Prima Dental Group, Gloucester, UK) to standardize the exposure area in each tooth. The exposure site was rinsed using sterile saline, and bleeding was controlled by applying sterile wet cotton pellets with light pressure. ProRoot MTA and NeoMTA Plus were mixed in accordance with the instructions of the manufacturer. Since ERRM-FS was supplied packed in a premixed syringe, it did not need to be prepared. The material was placed at the exposed pulp site, and sterile wet cotton pellets were applied with light pressure. The access cavities were restored using reinforced glass ionomer cement (Ketac Molar, 3 M ESPE, St Paul, MN, USA). Standardized periapical radiographs were taken before and after treatment. The animals were sacrificed at 4 weeks after the operation.
Sample preparation and histologic analysis
The teeth were extracted using dental forceps and fixed in 10% buffered formalin for 48 h. Specimens were then decalcified in 10% EDTA (pH = 7.4; Thermo Fisher Scientific) for 6 weeks. The samples were embedded in paraffin, serially sectioned at a 3-μm thickness in the buccolingual plane of each tooth and stained with hematoxylin and eosin (HE). Photomicrographs for HE-stained slides were captured using a digital camera (Infinity 2-2, Lumenera, Ottawa, ON, Canada). The photographs to be analyzed were captured at fixed magnifications of×40 and × 100.
All slides were evaluated by three trained researchers. The procedure was conducted as a blind test with the photomicrographs randomly sorted using Microsoft Excel. The calcific bridge formation, pulpal inflammation, and odontoblast layer were graded using a previous reported scoring system (Table 2).17,18 The area of newly formed calcific barrier was measured using ImageJ software (version 1.8.0, National Institutes of Health, Bethesda, MD, USA). To guarantee the reproducibility of the results, each measurement was made three times, with the mean values used as the final data.
Table 2.
Scores in the histologic analysis of calcific barriers and dental pulp.
| Characterization | Score | |
|---|---|---|
| A. Calcific barrier continuity | ||
| Complete calcific barrier formation | 1 | |
| Partial/incomplete calcific barrier formation extending to more than half of the exposure site but not completely closing it | 2 | |
| Initial calcific barrier formation extending to no more than half of the exposure site | 3 | |
| No hard tissue deposition | 4 | |
| B. Calcific barrier morphology | ||
| Dentin or dentin-like irregular hard tissue | 1 | |
| Only irregular hard tissue deposition | 2 | |
| Only a thin layer of hard tissue deposition | 3 | |
| No hard tissue deposition | 4 | |
| C. Tubules in calcific barrier | ||
| No tubules present | 1 | |
| Mild (tubules present in <30% of the calcific barrier) | 2 | |
| Moderate to severe (tubules present in >30% of the calcific barrier) | 3 | |
| No hard tissue deposition | 4 | |
| D. Pulpal inflammation severity | ||
| Absent or very few inflammatory cells | 1 | |
| Mild (typically <10 inflammatory cells) | 2 | |
| Moderate (typically 10–25 inflammatory cells) | 3 | |
| Severe (typically >25 inflammatory cells) | 4 | |
| E. Pulpal inflammation extent | ||
| No inflammation | 1 | |
| Mild (inflammatory cells only next to the dentin bridge or the area of pulp exposure) | 2 | |
| Moderate (inflammatory cells observed in at least one-third of the coronal or mid pulp) | 3 | |
| Severe (all the coronal pulp is infiltrated or necrotic) | 4 | |
| F. Pulpal inflammation type | ||
| No inflammation | 1 | |
| Chronic inflammation | 2 | |
| Acute and chronic inflammation | 3 | |
| Acute inflammation | 4 | |
| G. Dental pulp congestion | ||
| No congestion | 1 | |
| Mild (enlarged blood vessels only next to the dentin bridge or exposed pulp area) | 2 | |
| Moderate (enlarged blood vessels observed in at least one-third of the coronal or mid pulp) | 3 | |
| Severe (all the coronal pulp is infiltrated with blood cells) | 4 | |
| H. Odontoblastic cell layer | ||
| Complete palisading cell pattern | 1 | |
| Partial/incomplete palisading cell pattern | 2 | |
| Presence of odontoblast-like cells only | 3 | |
| Absent | 4 | |
This scoring system is from Nowicka et al.17.
Statistical analysis
The statistical analyses of in vitro data were performed using IBM SPSS Statistics Software (SPSS version 26.0, Chicago, IL, USA). The Shapiro–Wilk test was used to assess normality, and the Mann–Whitney U test was employed to perform intergroup comparisons. All in vitro experiments were performed at least in triplicate.
The statistical analyses of the in vivo study were performed using R software (version 4.1.2). Normality of the data distribution was tested using the Shapiro–Wilk test. The nonparametric Kruskal–Wallis test followed by Dunn's post hoc test was used to compare the medians of the calcific barrier areas among the three groups. Significance was set at P < 0.05.
Results
Effect of the materials on cell viability
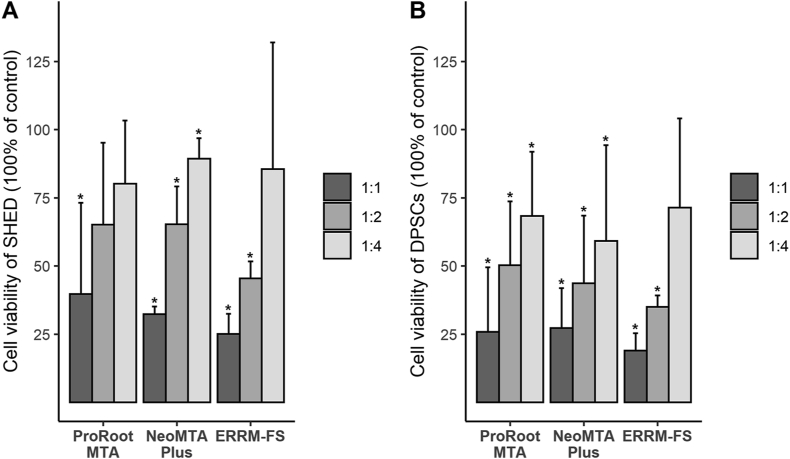
Fig. 1 shows the cell viability for different media concentrations in each group. With the undiluted extracts of ProRoot MTA, NeoMTA Plus, and ERRM-FS, both cell types presented low viability compared with the control group. In all three groups, cell viability steadily increased with dilution, although none reached 100%. ERRM-FS presented relatively low cell viability at 1:1 and 1:2 dilutions, but it increased sharply for the 1:4 dilution to reach a level that did not differ significantly from the control. The cell viability of SHED was higher than that of DPSCs at all concentrations of the three materials.
Figure 1.
Relative viability of cells exposed to the extracts of calcium silicate cements diluted to each concentration. The Y axis represents the relative percentage of cell count, compared to the control, which was set at 100%. The star symbol (∗) on the chart indicates the values that are significant differences from the control (P < 0.05) by Mann–Whitney analysis. Abbreviations: DPSCs, dental pulp stem cells; ERRM-FS, EndoSequence Root Repair Material-Fast Set Putty; SHED, stem cells from human exfoliated deciduous teeth.
Alkaline phosphatase and calcium assays
The levels of ALP activity and calcium concentration are presented in Fig. 2. SHED and DPSCs had significantly low ALP activity after exposure to each material compared with the control (cells cultured with osteogenic media). ProRoot MTA and ERRM-FS had mathematically higher ALP levels than NeoMTA Plus, but there were no significant differences (Fig. 2A).
Figure 2.
(A) ALP activities of stem cells in each calcium silicate cement extract. The Y axis represents the relative ALP activity, compared to the control (osteogenic media), which was set at 100% (B) Calcium concentration measured in material extracts. Data are presented as mean ± standard deviation. Abbreviations: ALP, alkaline phosphatase; DPSC, dental pulp stem cell; ERRM-FS, EndoSequence Root Repair Material-Fast Set Putty; SHED, stem cells from human exfoliated deciduous teeth.
An elevated level of calcium release was found in all three CSCs (Fig. 2B). ProRoot MTA and NeoMTA Plus differed significantly from the control (P > 0.05), but with no significant difference between them.
Histologic analysis
Histopathologic evaluations were performed on 12, 11, and 11 specimens with ProRoot MTA, NeoMTA Plus, and ERRM-FS, respectively. Two samples were excluded because of technical problems during extraction and histologic processing. The percentages of scores for calcific barriers, inflammation, and odontoblast of each group are listed in Table 3, Table 4, Table 5.
Table 3.
Scores for calcific barriers.
| Group | Score for calcific barrier continuity |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| ProRoot MTA | 66.67 (8/12) | 33.33 (4/12) | – | – |
| NeoMTA Plus | 27.27 (3/11) | 54.55 (6/11) | 18.18 (2/11) | – |
| ERRM-FS |
81.82 (9/11) |
18.18 (2/11) |
– |
– |
|
Score for calcific barrier morphology |
||||
| 1 |
2 |
3 |
4 |
|
| ProRoot MTA | 16.67 (2/12) | 75.00 (9/12) | 8.33 (1/12) | – |
| NeoMTA Plus | – | 54.55 (6/11) | 45.45 (5/11) | – |
| ERRM-FS |
18.18 (2/11) |
81.82 (9/11) |
– |
– |
|
Score for tubules in calcific barrier |
||||
| 1 |
2 |
3 |
4 |
|
| ProRoot MTA | 16.67 (2/12) | 50.00 (6/12) | 33.33 (4/12) | – |
| NeoMTA Plus | – | 36.36 (4/11) | 63.64 (7/11) | – |
| ERRM-FS | 9.09 (1/11) | 54.55 (6/11) | 36.36 (4/11) | – |
Data are % (number of teeth receiving the score)/(total number of teeth evaluated) values.
ERRM-FS, EndoSequence Root Repair Material-Fast Set Putty.
Table 4.
Scores for dental pulp inflammation.
| Group | Score for pulpal inflammation severity |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| ProRoot MTA | 41.67 (5/12) | 33.33 (4/12) | 16.67 (2/12) | 8.33 (1/12) |
| NeoMTA Plus | 27.27 (3/11) | 45.45 (5/11) | 27.27 (3/11) | – |
| ERRM-FS |
45.45 (5/11) |
45.45 (5/11) |
9.09 (1/11) |
– |
|
Score for pulpal inflammation extent |
||||
| 1 |
2 |
3 |
4 |
|
| ProRoot MTA | 41.67 (5/12) | 33.33 (4/12) | 16.67 (2/12) | 8.33 (1/12) |
| NeoMTA Plus | 27.27 (3/11) | 63.64 (7/11) | 9.09 (1/11) | – |
| ERRM-FS |
45.45 (5/11) |
54.55 (6/11) |
– |
– |
|
Score for pulpal inflammation type |
||||
| 1 |
2 |
3 |
4 |
|
| ProRoot MTA | 41.67 (5/12) | 33.33 (4/12) | 25.00 (3/12) | – |
| NeoMTA Plus | 27.27 (3/11) | 45.45 (5/11) | 27.27 (3/11) | – |
| ERRM-FS |
45.45 (5/11) |
45.45 (5/11) |
9.09 (1/11) |
– |
|
Score for dental pulp congestion |
||||
| 1 |
2 |
3 |
4 |
|
| ProRoot MTA | 8.33 (1/12) | 75.00 (9/12) | 16.67 (2/12) | – |
| NeoMTA Plus | 9.09 (1/11) | 72.73 (8/11) | 18.18 (2/11) | – |
| ERRM-FS | 18.18 (2/11) | 72.73 (8/11) | 9.09 (1/11) | – |
Data are % (number of teeth receiving the score)/(total number of teeth evaluated) values.
ERRM-FS, EndoSequence Root Repair Material-Fast Set Putty.
Table 5.
Scores for the odontoblastic cell layer.
| Group | Score for odontoblastic cell layer |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| ProRoot MTA | 8.33 (1/12) | 41.67 (5/12) | 50.00 (6/12) | – |
| NeoMTA Plus | 9.09 (1/11) | 18.18 (2/11) | 72.73 (8/11) | – |
| ERRM-FS | 18.18 (2/11) | 36.36 (4/11) | 45.45 (5/11) | – |
Data are % (number of teeth receiving the score)/(total number of teeth evaluated) values.
ERRM-FS, EndoSequence Root Repair Material-Fast Set Putty.
Calcific barrier formation
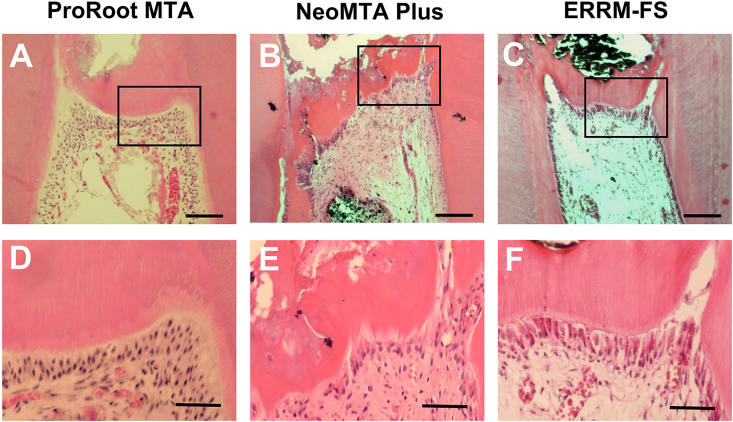
All ProRoot MTA and ERRM-FS samples showed complete or partial (more than one half of the exposure site) dentin bridge formation (Fig. 3, Table 3). Calcific barriers of ProRoot MTA and ERRM-FS exhibited relatively favorable morphology with lower incidences of tunnel defects (Fig. 3D, F) than NeoMTA Plus. In contrast, all of the specimens in the NeoMTA Plus group presented only irregular hard tissue formation (54.55%) or thin layers of hard tissue deposition (45.55%), among which mild (36.36%) to moderate (63.64%) tubules were present (Table 3).
Figure 3.
Hematoxylin-eosin staining for histologic evaluation of the newly formed calcific barriers after 4 weeks (A–C) shows the aspects of the calcific barriers for each test material (scale bars = 150 μm) (D–F) show magnified view of the boxed region of (A–C) respectively (scale bars = 50 μm). ERRM-FS, EndoSequence Root Repair Material-Fast Set Putty.
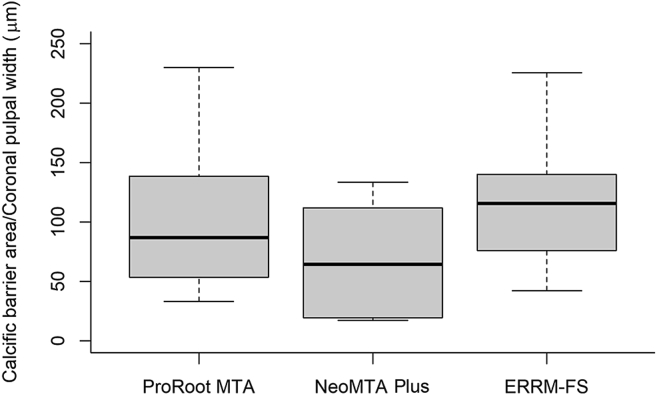
Fig. 4 presents quantitative comparisons of newly formed hard tissue among the groups. The measured area of newly formed calcific barrier was divided by the coronal pulpal width to exclude the effect of variations in tooth size and to provide objective comparisons.19 There were no significant differences between the three groups (P > 0.05).
Figure 4.
The comparison of areas of the newly formed calcific barrier for each material after 4 weeks. The y-axis is the value obtained by dividing the calcific barrier area by the width of coronal pulp of each tooth, for the objective comparisons (unit = μm). There were no statistically significant differences between groups. Statistical analysis with non-parametric Kruskal–Wallis test followed by Dunn's post hoc test was performed. ERRM-FS, EndoSequence Root Repair Material-Fast Set Putty.
Pulpal inflammation
The dental pulp in 41.67% of the ProRoot MTA specimens was found to be free of inflammation. The other specimens presented mild-to-moderate inflammation, with 1 of the 12 teeth exhibiting a severe inflammatory reaction. Most of the ERRM-FS specimens exhibited absent or mild inflammation (90.90%), whereas mild-to-moderate inflammation (72.72%) was found for NeoMTA Plus. Similar results were observed for dental pulp congestion, with no severe congestion in any of the three groups.
Odontoblastic cell layer
Evaluations of the shape of the formed odontoblast cell layer revealed that all specimens exhibited odontoblast or odontoblast-like cells. Palisade cell patterns (Table 5, Fig. 3D, F) were observed in 8.33% of ProRoot MTA, 9.09% of NeoMTA Plus, and 18.18% of ERRM-FS specimens.
Discussion
The properties and therapeutic effects of CSCs have been widely studied over the past 20 years. Favorable results were obtained using vital pulp therapy in human20 and animal18 studies, as well as in various in vitro studies.2,21 These positive findings have led to new CSC products being continuously developed with improved properties and reduced drawbacks compared with ProRoot MTA. Biodentine (Septodont, Saint-Maur-des-Fossés, France) and NeoMTA Plus exhibited reduced tooth staining,10 while RetroMTA (BioMTA, Seoul, South Korea) successfully shortened the setting time.16 NeoMTA Plus and ERRM-FS used in this study were putty-type CSCs that can improve their manageability in clinic applications.
ProRoot MTA, NeoMTA Plus, and ERRM-FS were tested to evaluate their cytocompatibility with SHED and DPSCs, and all three groups induced similar effects on both cell types. DPSCs isolated from permanent dentition have been widely used in various in vitro studies.17 In contrast, few studies have investigated SHED with calcium silicate materials. SHED have been characterized as multiple proliferative cells with the capacity to differentiate into several cell types such as osteoblasts, chondroblasts, and odontoblasts. SHED exhibit more extensive proliferation than do DPSCs,22 and they can be collected from a readily accessible source (exfoliate deciduous teeth). The functional differentiation and mineralization potentials of SHED exposed to Biodentine were investigated previously.23 However, we are not aware of SHED being investigated previously with newly developed CSCs such as NeoMTA Plus and ERRM-FS.
In addition to cell viability, osteogenic induction is considered an ideal characteristic of CSCs. ALP is a well-known marker of odontoblastic differentiation and plays an important role in mineral deposition.24 The results in the present study regarding ALP activities are consistent with that of a previous ALP analysis of ProRoot MTA and ERRM-FS in mouse DPSCs.25 Because high ALP levels alone are not directly correlated with the upregulation of odontogenic differentiation, other odontoblastic markers such as osteopontin, osteocalcin (OC), and dentin sialoprotein (DSP) should be evaluated.26 Nevertheless, the present study notably found that the calcific barrier areas in the three experimental groups in vivo were correlated with ALP levels in vitro. Although there were no significant differences in the areas of newly developed mineralization, and ProRoot MTA and ERRM-FS produced calcific areas of higher quality calcific than did NeoMTA Plus, which induced mild pulpal inflammation. Elevation of ALP induces the release of free phosphate ions, which may contribute to hydroxyapatite formation.27 Our study therefore suggests that the ALP level could be associated with the mineralization potential of CSCs.
The relatively low mineralization-inducing potential of NeoMTA Plus is supported by another study that demonstrated low osteogenic gene expression for NeoMTA Plus.28 NeoMTA Plus has larger contents of sulfur and aluminum than other CSCs,9 which could also explain the results of the present study. Another study found that human osteoblastic cells exposed to NeoMTA Plus had significantly lower cell viability and lower initial migration rates than for other CSCs.29 The authors of that study suggested that compounds released from the material into the environment could affect actin filament flow, and nonmuscle myosin II and adhesion molecule expression in osteoblastic cells. The most-feasible explanation is therefore that the specific component released from the proprietary gel of NeoMTA Plus affected its odontogenic potential; however, this explanation must be considered with some caution since there is insufficient evidence to explain the contradictory results of some other previous studies.12,30
CSCs mostly comprise calcium silicate derivatives, and calcium ions are released with hydration to form calcium hydroxide in a silicate matrix, which was also found in the present study. This reaction induces a highly alkaline environment for a long duration after setting.31 Furthermore, calcium ions play a vital role in the intracellular signaling cascade. According to a study of the signaling mediators related to CSC, extracellular calcium sensing receptors (CaSRs) are key mediators in CSC-induced regeneration of the dentin-pulp complex and alveolar bone.32 The authors of that study found that CSC activates diverse downstream CaSR pathways, and this cascade process requires the regulation of both calcium ion and pH.
No animal studies that we were aware of have investigated the effect of ERRM-FS as a pulp capping material, as was done in the present study. However, a previous study found more cementum-like tissue formation when the original ERRM was used in root-end microsurgery for beagle dogs.15 There have only been a few clinical case reports on ERRM-FS in vital pulp therapy. One case report found direct pulp capping on the mandibular molar, which was diagnosed as reversible pulpitis.33 At the follow-up visit 6 months later, the pulp remained vital without any negative symptoms. Another case report described the treatment of an immature mandibular molar using ERRM-FS.34 After regenerative endodontic procedures, all signs and symptoms were resolved completely at periodic follow-ups, and the affected tooth responded positively to vitality tests.
The fast setting time of ERRM-FS (approximately 20 min according to the manufacturer) not only shortens the chair time in dental treatment, but also reduces the risks of bacterial invasion and fluid inflow, which are related to its high sealing ability.14 The surface of ERRM-FS was also found to have a higher calcium/phosphate ratio than that of the EndoSequence BioCeramic Paste (Brasseler USA) or hydroxyapatite.35 Apatite precipitation is known to be important for sealing microscopic gaps between CSC and dentin walls, which leads to the tight adaptation of filling materials.36 Based on the combined results of this study and previous findings, it is reasonable to suggest that ERRM-FS is a promising alternative for other CSCs. However, since there have been few in vivo studies and no well-controlled clinical trials, further research is needed before the widespread use of ERRM-FS in vital pulp therapy can be promoted.
There were some limitations in this study. First, it is necessary to evaluate additional differentiation markers in order to further clarify the odontogenic differentiation potentials of different materials. For instance, immunohistochemical staining with OC and DSP can confirm odontogenic properties during the mineralization process.26 However, a study found that DSP alone cannot be regarded as a marker for verifying the presence of new odontoblasts.37 Various specific markers should therefore be applied together to more-accurately compare odontoblastic differentiation. Second, since pulp is an inflammation condition in most pulp treatment cases, an inflammation-related animal model is necessary to improve the understanding of this. Finally, the present data must be interpreted with caution because the sample may have been too small to allow significant differences to be detected; that is, the small sample reduced the statistical power of this study. Although nonparametric tests were used for comparisons between groups, different results might be obtained if the sample size is increased.
There are more CSCs now becoming available than before, but the clinical evidence supporting their use is not yet sufficient. This in vitro and in vivo study has revealed the biologic properties and mineralization potentials of NeoMTA Plus and ERRM-FS compared with ProRoot MTA, and there were no significant differences found between the materials. Thereby both new putty-type CSCs could be used as effective biomaterials in vital pulp therapy.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
The biospecimens and data used for this study were provided by the Biobank of Yonsei University Dental Hospital, a member of the Korea Biobank Network (KBN4_A04).
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1D1A1B07041657).
Contributor Information
Je Seon Song, Email: songjs@yuhs.ac.
Yooseok Shin, Email: densys@yuhs.ac.
References
- 1.Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007;40:462–470. doi: 10.1111/j.1365-2591.2007.01248.x. [DOI] [PubMed] [Google Scholar]
- 2.Parirokh M., Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review–part I: chemical, physical, and antibacterial properties. J Endod. 2010;36:16–27. doi: 10.1016/j.joen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Hasheminia M., Loriaei Nejad S., Asgary S. Sealing ability of MTA and CEM cement as root-end fillings of human teeth in dry, saliva or blood-contaminated conditions. Iran Endod J. 2010;5:151–156. [PMC free article] [PubMed] [Google Scholar]
- 4.Torabinejad M., Hong C.U., McDonald F., Pitt Ford T.R. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21:349–353. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- 5.Witherspoon D.E. Vital pulp therapy with new materials: new directions and treatment perspectives–permanent teeth. Pediatr Dent. 2008;30:220–224. [PubMed] [Google Scholar]
- 6.Torabinejad M., Parirokh M. Mineral trioxide aggregate: a comprehensive literature review–part II: leakage and biocompatibility investigations. J Endod. 2010;36:190–202. doi: 10.1016/j.joen.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Dawood A.E., Parashos P., Wong R.H.K., Reynolds E.C., Manton D.J. Calcium silicate-based cements: composition, properties, and clinical applications. J Investig Clin Dent. 2017;8 doi: 10.1111/jicd.12195. [DOI] [PubMed] [Google Scholar]
- 8.Parirokh M., Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review–part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400–413. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Tomás-Catalá C.J., Collado-González M., García-Bernal D., et al. Comparative analysis of the biological effects of the endodontic bioactive cements MTA-Angelus, MTA Repair HP and NeoMTA Plus on human dental pulp stem cells. Int Endod J. 2017;50(Suppl 2):e63–e72. doi: 10.1111/iej.12859. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri J. Staining potential of neo MTA plus, MTA plus, and biodentine used for pulpotomy procedures. J Endod. 2015;41:1139–1145. doi: 10.1016/j.joen.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 11.Quintana R.M., Jardine A.P., Grechi T.R., et al. Bone tissue reaction, setting time, solubility, and pH of root repair materials. Clin Oral Invest. 2019;23:1359–1366. doi: 10.1007/s00784-018-2564-1. [DOI] [PubMed] [Google Scholar]
- 12.Walsh R.M., Woodmansey K.F., He J., Svoboda K.K., Primus C.M., Opperman L.A. Histology of NeoMTA Plus and Quick-Set2 in contact with pulp and periradicular tissues in a canine model. J Endod. 2018;44:1389–1395. doi: 10.1016/j.joen.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran D., He J., Glickman G.N., Woodmansey K.F. Comparative analysis of calcium silicate-based root filling materials using an open apex model. J Endod. 2016;42:654–658. doi: 10.1016/j.joen.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Koç C., Aslan B., Ulusoy Z., Oruçoğlu H. Sealing ability of three different materials to repair furcation perforations using computerized fluid filtration method. J Dent Res Dent Clin Dent Prospects. 2021;15:183–187. doi: 10.34172/joddd.2021.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen I., Karabucak B., Wang C., et al. Healing after root-end microsurgery by using mineral trioxide aggregate and a new calcium silicate-based bioceramic material as root-end filling materials in dogs. J Endod. 2015;41:389–399. doi: 10.1016/j.joen.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang C.M., Kim S.H., Shin Y., et al. A randomized controlled trial of ProRoot MTA, OrthoMTA and RetroMTA for pulpotomy in primary molars. Oral Dis. 2015;21:785–791. doi: 10.1111/odi.12348. [DOI] [PubMed] [Google Scholar]
- 17.Nowicka A., Lipski M., Parafiniuk M., et al. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J Endod. 2013;39:743–747. doi: 10.1016/j.joen.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Lee H., Shin Y., Kim S.O., Lee H.S., Choi H.J., Song J.S. Comparative study of pulpal responses to pulpotomy with ProRoot MTA, RetroMTA, and TheraCal in dogs' teeth. J Endod. 2015;41:1317–1324. doi: 10.1016/j.joen.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Kwon W., Kim I.H., Kang C.M., Kim B., Shin Y., Song J.S. Comparative study of pulpal responses to ProRoot MTA, Vitapex, and Metapex in canine teeth. J Dent Sci. 2021;16:1274–1280. doi: 10.1016/j.jds.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aeinehchi M., Eslami B., Ghanbariha M., Saffar A.S. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-capping agents in human teeth: a preliminary report. Int Endod J. 2003;36:225–231. doi: 10.1046/j.1365-2591.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- 21.Chang S.W., Lee S.Y., Ann H.J., Kum K.Y., Kim E.C. Effects of calcium silicate endodontic cements on biocompatibility and mineralization-inducing potentials in human dental pulp cells. J Endod. 2014;40:1194–1200. doi: 10.1016/j.joen.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura S., Yamada Y., Katagiri W., Sugito T., Ito K., Ueda M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endod. 2009;35:1536–1542. doi: 10.1016/j.joen.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 23.Dahake P.T., Panpaliya N.P., Kale Y.J., Dadpe M.V., Kendre S.B., Bogar C. Response of stem cells from human exfoliated deciduous teeth (SHED) to three bioinductive materials - an in vitro experimental study. Saudi Dent J. 2020;32:43–51. doi: 10.1016/j.sdentj.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y.X., Ma Z.F., Huo N., et al. Porcine tooth germ cell conditioned medium can induce odontogenic differentiation of human dental pulp stem cells. J Tissue Eng Regen Med. 2011;5:354–362. doi: 10.1002/term.321. [DOI] [PubMed] [Google Scholar]
- 25.Machado J., Johnson J.D., Paranjpe A. The effects of Endosequence root repair material on differentiation of dental pulp cells. J Endod. 2016;42:101–105. doi: 10.1016/j.joen.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Papagerakis P., Berdal A., Mesbah M., et al. Investigation of osteocalcin, osteonectin, and dentin sialophosphoprotein in developing human teeth. Bone. 2002;30:377–385. doi: 10.1016/s8756-3282(01)00683-4. [DOI] [PubMed] [Google Scholar]
- 27.Kuru L., Griffiths G.S., Petrie A., Olsen I. Alkaline phosphatase activity is upregulated in regenerating human periodontal cells. J Periodontal Res. 1999;34:123–127. doi: 10.1111/j.1600-0765.1999.tb02231.x. [DOI] [PubMed] [Google Scholar]
- 28.Abu Zeid S.T., Alamoudi N.M., Atta H.M., Edrees H., Saleh A.M. Osteogenic differentiation of stem cells treated with fast set NeoMTA Plus. Int J Pharmaceut Res Allied Sci. 2018;7:77–85. [Google Scholar]
- 29.Santiago M.C., Gomes-Cornélio A.L., de Oliveira L.A., Tanomaru-Filho M., Salles L.P. Calcium silicate-based cements cause environmental stiffness and show diverse potential to induce osteogenesis in human osteoblastic cells. Sci Rep. 2021;11 doi: 10.1038/s41598-021-96353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanomaru-Filho M., Andrade A.S., Rodrigues E.M., et al. Biocompatibility and mineralized nodule formation of Neo MTA Plus and an experimental tricalcium silicate cement containing tantalum oxide. Int Endod J. 2017;50(Suppl 2):e31–e39. doi: 10.1111/iej.12780. [DOI] [PubMed] [Google Scholar]
- 31.Duarte M.A., Demarchi A.C., Yamashita J.C., Kuga M.C., Fraga Sde C. pH and calcium ion release of 2 root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:345–347. doi: 10.1067/moe.2003.12. [DOI] [PubMed] [Google Scholar]
- 32.Kim J.M., Choi S., Kwack K.H., Kim S.Y., Lee H.W., Park K. G protein-coupled calcium-sensing receptor is a crucial mediator of MTA-induced biological activities. Biomaterials. 2017;127:107–116. doi: 10.1016/j.biomaterials.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 33.Debelian G., Trope M. The use of premixed bioceramic materials in endodontics. G Ital Endod. 2016;30:70–80. [Google Scholar]
- 34.Lee S., Park Y.T., Setzer F.C. Combined regenerative and vital pulp therapies in an immature mandibular molar: a case report. J Endod. 2020;46:1085–1090. doi: 10.1016/j.joen.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Abu Zeid S.T., Alamoudi R.A., Abou Neel E.A., Mokeem Saleh A.A. Morphological and spectroscopic study of an apatite layer induced by fast-set versus regular-set EndoSequence root repair materials. Materials. 2019;12:3678. doi: 10.3390/ma12223678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han L., Okiji T., Okawa S. Morphological and chemical analysis of different precipitates on mineral trioxide aggregate immersed in different fluids. Dent Mater J. 2010;29:512–517. doi: 10.4012/dmj.2009-133. [DOI] [PubMed] [Google Scholar]
- 37.Zhu X., Wang Y., Liu Y., Huang G.T., Zhang C. Immunohistochemical and histochemical analysis of newly formed tissues in root canal space transplanted with dental pulp stem cells plus platelet-rich plasma. J Endod. 2014;40:1573–1578. doi: 10.1016/j.joen.2014.05.010. [DOI] [PubMed] [Google Scholar]