Abstract
Background and Aims
The evolving mutants of SARS‐CoV‐2 have made the COVID‐19 pandemic sustained for over 3 years. In 2022, BA.4 and BA.5 were the Omicron variants dominating the spread globally. Although COVID‐19 was no longer a Public Health Emergency of International Concern (PHEIC) as announced by WHO, the SARS‐CoV‐2 variants remain a challenge to global healthcare under the circumstances of withdrawal and loosening of personal protective behavior in the post‐quarantine era. This study aims to acknowledge the clinical characteristics caused by Omicron BA.4/BA.5 in COVID‐19 naive people and analyze possible factors affecting disease severities.
Methods
In this retrospective study, we report and analyze the clinical features of 1820 COVID‐19 patients infected with the BA.4/BA.5 Omicron variants of SARS‐CoV‐2 during a local outbreak that occurred in Macao SAR, China, from June to July 2022.
Results
A total of 83.5% of patients were symptomatic eventually. The most common symptoms were fever, cough, and sore throat. Hypertension, dyslipidemia, and diabetes mellitus were the leading comorbidities. There were significantly more elderly patients (p < 0.001), more patients with comorbidity (p < 0.001) and more patients without vaccination or not completing the series (p < 0.001) in the “Severe to Critical” group. All deceased patients were elderly with at least three comorbidities and were partial to totally dependent in their daily lives.
Conclusion
Our data are consistent with a milder disease caused by BA.4/5 Omicron variants in the general population, while patients with old age and comorbidities have developed severe to critical diseases. Complete vaccination series and booster doses are effective strategies to reinforce protection against severe diseases and avoid mortality.
Keywords: infectious diseases, public health, respiratory medicine, vaccines
1. BACKGROUND AND AIMS
The pandemic of coronavirus disease 2019 (COVID‐19), caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) infection, has remained a global health issue ever since World Health Organization defined it in early 2020. As of May 15, 2023, there were over 766 million confirmed cases of COVID‐19, with an estimated accumulated death of more than 6.9 million worldwide. 1 In 2022, Omicron variants (B.1.1.529) have become the dominant circulating Variants of Concern (VOC) after alpha, beta, gamma, and delta variants of SARS‐CoV‐2, following the report of its first sublineage BA.1 in November 2021 in South Africa. 2
In the first half of 2022, the two new lineages of Omicron (BA.4 and BA.5) have rapidly emerged and spread globally through their high transmissibility and immune escape capabilities. 3 , 4 , 5 , 6 Although less severe diseases and mortality of Omicron variants were reported, the increased infectivity can still lead to a relative proportion of mortality based on a more significant number of cases, which can add to healthcare and socioeconomic burdens. 6 , 7
Macao is one of China's Special Administrative Regions (SAR) located on the southeast coast of China. It is one of the world's densest regions (about 20,620 people per square kilometers) and is famous for tourism and resort. 8 Based on the close adherence to the strategic principles of “Preventing imported cases and rebound of the epidemic” and the “Dynamic Zero” policy by the government, almost all COVID‐19 confirmed cases were imported and import‐related cases since the first confirmed case diagnosed in January 2020. 9 , 10 Most city residents acquired immunities against the coronavirus through a vaccination program promoted by Health Bureau. It was until the first community outbreak in Macao, from June to July 2022 that the SARS‐CoV‐2 Omicron variants (BA.4/5) were the culprits, resulting in six deaths among 1820 cases. Therefore, we retrospectively report the demographic and clinical characteristics of COVID‐19 patients infected by Omicron BA.4/5 and analyze the possible predisposing factors affecting disease severity and mortality.
2. METHODS
2.1. Enrollment and data collection
We retrospectively collected the clinical data of 1821 patients diagnosed and managed by Centro Hospitalar Conde de São Januário (CHCSJ), which is the only public hospital in Macao that is responsible for handling patients with SARS‐CoV‐2 infections during a COVID‐19 outbreak in Macao between 18 June and July 31, 2022. The study was approved by the Hospital Medical Ethical Committee of CHCSJ, Health Bureau of Macao SAR, China. Written informed consent was waived due to the retrospective nature and use of anonymous data. We collected data, including medical records describing the patients' clinical symptoms, vaccination status, comorbidity, mortality, laboratory, radiological, and microbiological results, and so forth, from our electronic medical systems. All patients were diagnosed based on the positive results of qualitative real‐time reverse transcriptase‐polymerase chain reaction assay (qRT‐PCR) in respiratory specimens according to WHO technical guidance. SARS‐CoV‐2 sublineage classifications were performed among the patients in a random‐sampling aspect, and the results of all tested samples yielded either Omicron BA.4/5 or Omicron BA.5. Retrospective analysis was performed to define the clinical characteristics of patients infected by the Omicron subvariants, as well as the possible causes and risk factors of the deceased cases.
2.2. Severity classifications
Patients' severities were classified based on the “Guideline on the management of COVID‐19, trial version 9” published by the National Health Commission of the People's Republic of China. 11 “Mild” was defined as mild clinical symptoms with no signs of pneumonia in imaging; “Moderate” was defined as having clinical symptoms and signs of pneumonia in imaging; “Severe” was classified if one of the following was present: (a) dyspnea with a respiratory rate of ≥30 per minute, (b) O2 saturation ≤93%, and (c) PaO2/FiO2 ≤ 300 mmHg, (d) progressive exaggeration of clinical symptoms and lesions progressed >50% in lung imaging studies within 24–48 h; “Critical” was classified if one of the following was present: (a) respiratory failure requiring mechanical ventilation, (b) shock, and (c) coexisting multiple organ failure requiring close monitoring in intensive care unit (ICU).
2.3. Real‐time reverse transcriptase‐polymerase chain reaction assay (RT‐PCR) for SARS‐CoV‐2 and sublineage classification
Samples were taken from respiratory specimens, nasopharyngeal swabs (NPS) or oropharyngeal swabs (OPS) in all patients where available. Samples were tested for SARS‐CoV‐2 ORF1a/b gene, E gene and N2 gene with qRT‐PCR using the reagent kits of cobas®SARS‐CoV‐2 and Xpert®Xpert SARS‐CoV‐2, and the Cobas® 6800 System (Roche) and the GeneXpert®Infinity System (Cepheid) respectively following manufacturer's instructions. The results were interpreted according to the kit manual. Extraction of nucleic acid from the respiratory samples was performed using reagent kits NUCLISENS®easyMag Accessory product and the NUCLISENS®EasyMag system (BioMerieux) following the manufacturer's instructions when samples tested positive for SARS‐CoV‐2 nucleic acids. Amplifications of nucleic acids and SARS‐CoV‐2 sublineage classifications were performed for gene E484A, F486V, D3N, del H69/V70, A67V, P681H/R, K417N, L452R, N501Y, Y505H, D614G using reagent kits of LightCycler®Multiplex RNA Virus Master and VirSNiP SARS‐CoV‐2 and the LightCycler®480 real‐time PCR system (Roche) following manufacturer's instructions. The results were interpreted according to the kit manual.
2.4. Statistical analysis
Statistical analyses were conducted using SPSS version 25.0 (SPSS Inc). Continuous data were presented as either the mean ± standard deviation (SD) or the median and quartiles as appropriate, and dichotomous variables were presented as percentages. Unpaired Student's t tests, χ 2 tests and Fisher's Exact tests were used to compare the clinical characteristics (age, male, comorbidity, and COVID‐19 vaccination status) of the “Asymptomatic‐Mild‐Moderate” and “Severe‐Critical” groups, a p < 0.05 was considered statistically significant. Differences in percentages between groups of different disease severities and age, comorbidity, and doses of COVID‐19 vaccination were examined respectively using Spearman‐Rank correlation analysis (two‐sided) and a p < 0.01 was considered statistically significant.
3. RESULTS
3.1. Demographic characteristics
This report included 1820 hospitalized patients with positive COVID‐19 nucleic acid tests described as follows. After a review of clinical data, two patients were excluded from this study: one was due to persisting negative results of serial nucleic acid tests (NAT); the other one with history of positive NAT during entry into Macao from abroad in June and sublineage classification of BA.2 was classified as an imported case retested positive. One patient was included due to positive results in serial NATs during the inclusion period notified afterwards, resulting in 1820 patients classified in this outbreak for analysis. All patients (100%) were admitted either to the Public Health Clinical Center or to the isolation hotels after triage at the Special Emergency Room (SER) of CHCSJ or the Community Treatment Center (CTC) in the Macao East Asian Games Dome.
3.2. Clinical characteristics
The demographic and clinical information of these 1820 patients is summarized (Table 1). The median age of the patients was 40.1 years old (IQR: 30.9–54.0). Patients aged 60‐year‐old or above constitute about 17.1%. Eight hundred eighty‐two patients were male (48.5%). About one‐third of patients presented with symptoms initially at admission while over 80% of patients became symptomatic during the hospitalization course eventually. The most common symptoms were fever (66.6%), cough (35.3%), sore throat (26.9%), followed by headache (14.6%), rhinorrhea (8.9%), and myalgia (5.2%). Of these 1820 patients, more than one‐fourth (27.1%) of them had one or more coexisting internal medical conditions, such as hypertension, dyslipidemia and diabetes mellitus, and so forth. Only two patients had past SARS‐CoV‐2 infections.
Table 1.
Summary of demographic data of 1820 patients with SARS‐CoV‐2 infections in Macao from June 18 to July 31, 2022.
| Number of patients | 1820 |
|---|---|
| Age (median, IQR) | 40.1 (30.9–54.0) |
| <18‐year‐old (N, %) | 188 (10.3) |
| 18 to 59‐year‐old (N, %) | 1320 (72.5) |
| ≥60‐year‐old (N, %) | 312 (17.1) |
| Gender (male, N, %) | 882 (48.5) |
| Symptomatic at admission (N, %) | 706 (38.8) |
| Symptomatic during hospitalization (N, %) | 1520 (83.5) |
| Symptom and sign (N, %)a | |
| Body temperature ≥37.5°C | 1212 (66.6) |
| Cough | 642 (35.3) |
| Sore throat | 490 (26.9) |
| Rhinorrhea | 162 (8.9) |
| Nasal congestion | 31 (1.7) |
| Dyspnea | 26 (1.4) |
| Headache | 266 (14.6) |
| Dizziness | 71 (3.9) |
| Fatigue | 45 (2.5) |
| Myalgia | 94 (5.2) |
| Arthralgia | 8 (0.4) |
| Abdominal pain | 23 (1.3) |
| Nausea/vomit | 40 (2.1) |
| Diarrhea (≥3 times/day) | 28 (1.5) |
| Dysgeusia | 25 (1.4) |
| Past illness and comorbidity (N, %) | 494 (27.1) |
| Hypertension | 247 (13.6) |
| Dyslipidemia | 158 (8.7) |
| Diabetes mellitus | 108 (5.9) |
| Hyperuricemia/gout | 54 (3.0) |
| Coronary artery disease | 14 (0.8) |
| Heart failure | 2 (0.1) |
| Arrhythmia | 19 (1.0) |
| Valvular heart disease | 9 (0.5) |
| Cerebrovascular disease | 18 (1.0) |
| Asthma | 10 (0.5) |
| Chronic obstructive pulmonary disease (COPD) | 6 (0.3) |
| Bronchiectasis | 5 (0.3) |
| Pulmonary tuberculosis/sequela | 38 (2.1) |
| Pneumothorax | 3 (0.2) |
| Hepatitis B/Hepatitis C | 59 (3.2) |
| Fatty liver | 31 (1.7) |
| Cirrhosis | 2 (0.1) |
| Chronic kidney disease | 20 (1.1) |
| Thyroid disease | 40 (2.2) |
| Anemia | 30 (1.6) |
| Malignancy | 31 (1.7) |
| SARS‐CoV‐2 infection | 2 (0.1) |
| Others | 39 (2.1) |
| Smoker/ex‐smoker (N, %) | 37 (2.0) |
| Pregnancy (N/number of females, %) | 9/946 (1.0) |
| Number of doses of vaccination for SARS‐CoV‐2b (N, %) | |
| 0 dose | 337 (18.5) |
| 1 dose | 54 (3.0) |
| 2 doses | 688 (37.8) |
| 3 doses (booster) | 741 (40.7) |
| Severity classificationc (N, %) | |
| Asymptomatic | 280 (15.4) |
| Mild | 1338 (73.5) |
| Moderate | 184 (10.1) |
| Severe | 11 (0.6) |
| Critical | 7 (0.4) |
| Oxygen therapy (N, %) | 19 (1.0) |
| Mechanical ventilation support (N, %) | 1 (0.1) |
| Death (N, %) | 6 (0.3) |
| Antiviral therapy (N, %) | 215 (11.8) |
| Nirmatrelvir‐ritonavir | 125 (6.9) |
| Remdesivir | 2 (0.1) |
| Molnupiravir | 88 (4.8) |
The proportion is classified according to “Symptomatic during hospitalization.”
Types of vaccine: Inactivated vaccine: Sinopharm/BIBP COVILO, SINOVAC Coronavac; mRNA vaccine: BioNTech COMIRNATY/BNT162b2; protein subunit vaccine: Zhifei Longcom ZIFIVAX. Number of doses regarded as primary series: Two doses for inactivated and mRNA vaccine; three doses for protein subunit vaccine. Different types of vaccine may be selected as booster dose based on vaccination strategies in different districts.
“Guideline on the management of COVID‐19 (trial version 9)” published by the National Health Commission of the People's Republic of China.
In our cohort, about one‐fifth of the patients received none or incomplete primary series of vaccination for SARS‐CoV‐2. In contrast, more than 78% of patients have at least completed the primary series of COVID‐19 vaccination. Based on the severity classification criteria, there were 73.5% mild, 10.1% moderate, 0.6% severe, and 0.4% critical cases.
3.3. Disease severities
In this outbreak, patients with severe and critical disease severities were aged populations (Severe: ≥50‐year‐old; Critical: ≥70‐year‐old). Patients categorized as “Severe to Critical” had different clinical features compared to those who were “Asymptomatic to Mild to Moderate” (Table 2). In the “Severe to Critical” group, there were significantly more elderly patients (p < 0.001), more co‐existing comorbidities (p < 0.001) and less completion of the primary series of COVID‐19 vaccination compared to the “Asymptomatic to Mild to Moderate” group (p < 0.001). Further correlation analysis between disease severity and age, comorbidities, and vaccination status showed a weak positive trend between disease severity and age (Spearman's rho: 0.346) and that of comorbidity (Spearman's rho: 0.264) (Table 3).
Table 2.
Comparison of clinical data between COVID‐19 patients with asymptomatic‐mild‐moderate and severe‐critical disease severity.
| Total N = 1820 | Asymptomatic to mild to moderate N = 1802 | Severe to critical N = 18 | p Value* | |
|---|---|---|---|---|
| Proportion (%) | Proportion (%) | Proportion (%) | ||
| Age (mean ±SD, years) | 41.9 ± 18.3 | 41.5 ± 17.9 | 81.4 ± 14.0 | <0.001 |
| Male | 882/1820 (48.5) | 875/1802 (48.6) | 7/18 (38.9) | 0.414 |
| Comorbidity | 494/1820 (27.1) | 478/1802 (26.5) | 16/18 (88.9) | <0.001 |
| COVID‐19 vaccination status (none or not complete of primary series)a | 392/1820 (21.5) | 381/1802 (21.1) | 11/18 (61.1) | <0.001 |
| COVID‐19 vaccination status (complete of primary series)a | 1428/1820 (78.5) | 1421/1802 (78.9) | 7/18 (38.9) | <0.001 |
Number of doses regarded as completion of primary series: Inactivated vaccine: Two doses; mRNA vaccine: Two doses; protein subunit vaccine: Three doses.
p < 0.05: Significant.
Table 3.
Relationship between disease severity and age, comorbidities, and number of doses of vaccination of 1820 patients with SARS‐CoV‐2 infections in Macao from June 18 to July 31, 2022.
| Groups | Number of cases | Asymptomatic | Mild | Moderate | Severe | Critical | ρ | p Value* |
|---|---|---|---|---|---|---|---|---|
| Age (N, %) | 0.346 | <0.001 | ||||||
| <18 | 188 | 40 (21.3) | 144 (76.6) | 4 (2.1) | 0 (0.0) | 0 (0.0) | ||
| 18–59 | 1320 | 217 (16.4) | 1054 (79.8) | 47 (3.6) | 2 (0.2) | 0 (0.0) | ||
| ≥60 | 312 | 23 (7.4) | 140 (44.9) | 133 (42.6) | 9 (2.9) | 7 (2.2) | ||
| Comorbidity (N, %) | 0.264 | <0.001 | ||||||
| No | 1326 | 229 (17.3) | 1036 (78.1) | 59 (4.4) | 2 (0.2) | 0 (0.0) | ||
| Yes | 494 | 51 (10.3) | 302 (61.1) | 125 (25.3) | 9 (1.8) | 7 (1.4) | ||
| Doses of vaccination (N, %) | –0.078 | 0.001 | ||||||
| 0 | 337 | 60 (17.8) | 214 (63.5) | 54 (16.0) | 7 (2.1) | 3 (0.9) | ||
| 1 | 54 | 12 (22.2) | 37 (68.5) | 4 (7.4) | 1 (1.9) | 0 (0.0) | ||
| 2 | 688 | 88 (12.8) | 517 (75.1) | 77 (11.2) | 2 (0.3) | 4 (0.6) | ||
| 3 (booster)a | 741 | 120 (16.2) | 571 (77.1) | 49 (6.6) | 1 (0.1) | 0 (0.0) |
Number of doses regarded as completion of primary series: Inactivated vaccine: Two doses; mRNA vaccine: Two doses; protein subunit vaccine: Three doses.
p < 0.01: Significant.
3.4. Laboratory parameters
Initial laboratory studies, including hemogram, biochemical, and inflammatory markers after admission of cases categorized as “Severe to Critical” are summarized in Table 4. A substantial portion (72.2%) of the patients demonstrated abnormal levels of lymphocytes with signs of lymphocytopenia (mean ± SD: 1.0 ± 0.5). Elevation of lactate dehydrogenase (mean ± SD: 246.1 ± 71.5) and C‐reactive protein levels (mean ± SD: 2.9 ± 3.2) were also noted in a considerable proportion of this subgroup. Less than half of the patients in this subgroup presented with elevated aminotransaminase and creatine kinase levels. Early presentation of the above laboratory parameters may indicate possible severe disease progression.
Table 4.
Initial laboratory data of COVID‐19 patients with “Severe‐Critical” disease severity and “Mortal cases” in Macao from June 18 to July 31, 2022.
| Severe‐critical (N = 18) | Mortal cases (N = 6) | |||||
|---|---|---|---|---|---|---|
| Normal range | Abnormal values | Mean ± SD (minimum–maximum) | Patients with abnormal values (N, %) | Mean ± SD (minimum–maximum) | Patients with abnormal values (N, %) | |
| White blood cells, ×109/L | 4.3–10.0 | >10.0 | 6.1 ± 2.3 (3.0–12.0) | 1/18 (5.6) | 5.4 ± 2.2 (3.7~8.3) | 0 (0) |
| Neutrophils, ×109/L | 1.9–7.3 | >7.3 | 4.3 ± 2.2 (1.6–9.8) | 2/18 (11.1) | 3.9 ± 2.2 (2.0–6.8) | 0 (0) |
| Lymphocytes, ×109/L | 1.5–4.0 | <1.5 | 1.0 ± 0.5 (0.3–1.8) | 13/18 (72.2) | 0.7 ± 0.3 (0.3–1.1) | 6/6 (100.0) |
| Hemoglobin, g/L | 12.0–17.0 | <12.0 | 12.6 ± 1.7 (8.4–14.8) | 5/18 (27.8) | 11.3 ± 2.1 (8.4–13.7) | 3/6 (50.0) |
| Platelets, ×109/L | 100.0–400.0 | <100.0 | 174.1 ± 56.7 (124.0–374.0) | 0 (0) | 162 ± 38 (124.0–227.0) | 0 (0) |
| Prothrombin time, sec | 11.7 | >11.7 | 11.9 ± 1.3 (9.9–15.0) | 8/17 (47.1)a | 11.0 ± 0.7 (9.9–11.8) | 1/6 (16.7) |
| Activated partial thromboplastin time, sec | 29.8 | >29.8 | 34.6 ± 6.8 (26.6–57.6) | 15/17 (88.2)a | 32.4 ± 3.5 (28.4–38.7) | 5/6 (83.3) |
| Alanine aminotransferase (ALT), U/L | ≤33.0 | >33.0 | 22.2 ± 14.6 (7.0–52.0) | 3/18 (16.7) | 12.7 ± 6.1 (7.0–24.0) | 0 (0) |
| Aspartate aminotransaminase (AST), U/L | ≤32.0 | >32.0 | 42.2 ± 41.6 (13.0–180.0) | 8/18 (44.4) | 23.5 ± 9.8 (13.0–39.0) | 1/6 (16.7) |
| Urea, mmol/L | 2.5–7.7 | >7.7 | 7.0 ± 6.0 (3.0–29.4) | 2/18 (11.1) | 9.5 ± 9.8 (4.4–29.4) | 1/6 (16.7) |
| Creatinine, mmol/L | 52.0–94.0 | >94.0 | 95.6 ± 54.9 (42.0–276.0) | 6/18 (33.3) | 114.8 ± 83.9 (42.0–276.0) | 2/6 (33.3) |
| Lactate dehydrogenase (LDH), U/L | 135.0–220.0 | >220.0 | 246.1 ± 71.5 (151.0–424.0) | 12/17 (70.6)a | 218.0 ± 71.2 (151.0–346.0) | 3/6 (50.0) |
| Creatine kinase (CK), U/L | <180.0 | ≥180.0 | 488.1 ± 1241.1 (30.0–5221.0) | 4/18 (22.2) | 86.0 ± 48.8 (30.0–167.0) | 0 (0) |
| C‐reactive protein (CRP), mg/dL | <0.5 | ≥0.5 | 2.9 ± 3.2 (0.1–11.4) | 13/18 (72.2) | 2.2 ± 4.5 (0.1–11.4) | 2/6 (33.3) |
| Procalcitonin (PCT), ng/mL | <0.06 | ≥0.06 | 0.27 ± 0.63 (0.03–2.69) | 14/17 (82.4)a | 0.12 ± 0.09 (0.03–0.26) | 5/6 (83.3) |
N = 17 due to data unavailable.
3.5. Management
All patients were administered standardized medications for symptomatic treatment after triage at the SER of CHCSJ or the CTC, including antipyretics, analgesics, antihistamines, mucolytics, and antiemetic agents as needed base. Patients who were old age (≥60‐year‐old), had comorbidities (especially poor‐controlled), unvaccinated, pregnant, infants/children, had possible risk of progression to severe disease, and so forth, would be admitted to the Public Health Clinical Center for further observation and management. Oral antiviral treatments were prescribed to individuals with risk factors for progression to severe disease according to Pneumologists' evaluation regarding the Guideline on the management of COVID‐19 (trial version 9) published by the National Health Commission of the People's Republic of China. 11 Three types of antiviral regimens were available: (1) Nirmatrelvir‐ritonavir; (2) Remdesivir; (3) Molnupiravir. A total of 215 patients (11.8%) were prescribed antiviral regimens as follows: Nirmatrelvir‐ritonavir (6.9%), Molnupiravir (4.8%), and Remdesivir (0.1%) (Table 1). Nineteen (1.0%) patients required oxygen therapy, including both at baseline and this admission. One patient was intubated with mechanical ventilation support due to respiratory failure. Out of the seven critical cases, six patients were deceased.
3.6. Features of critical and mortal cases
There were six deceased patients in this outbreak (Supporting Information: 1). All six patients were older than 85, with a mean age of 92.5 years old. Five of them were female. The duration of hospitalization ranged from 3 to 13 days, for which 50% were dead in the first week of admission and the other half in the second week. All six patients had multiple underlying comorbidities, including hypertension, dyslipidemia, diabetes mellitus, and so forth. Over 80% of the patients were totally dependent (Barthel Index for Activities of Daily Living score <20). 12 Half of the patients were unvaccinated for COVID‐19. A substantial portion of patients suffered from aggravation of underlying comorbidities, related complications and secondary infections. All of them were prescribed antiviral regimens and empirical antibiotics. Molnupiravir was selected for patients with existing or impending renal impairment. Invasive resuscitations were not performed under the consensus of the patients' families' Do‐Not‐Resuscitate (DNR) preferences.
Chest computed tomography (CT) scans were performed in patients admitted to the Public Health Clinical Center. Selected CT images of some critical cases were demonstrated in Figure 1. Small ground glass opacities (GGO) were noted on Day 3 and Day 8 of two death cases, as illustrated (Figure 1A,B). More prominent peripheral GGO with superimposed intralobular reticulations could be observed on Day 12 of another death case (Figure 1C). The lung involvement of the mortal cases was less severe than expected, and sequential changes of chest imaging were not available due to cases expiring within the first 2 weeks. Multiple infiltrates and GGO in both lungs were demonstrated in the critical case who received intubation with mechanical ventilation support for respiratory failure (Figure 1D).
Figure 1.
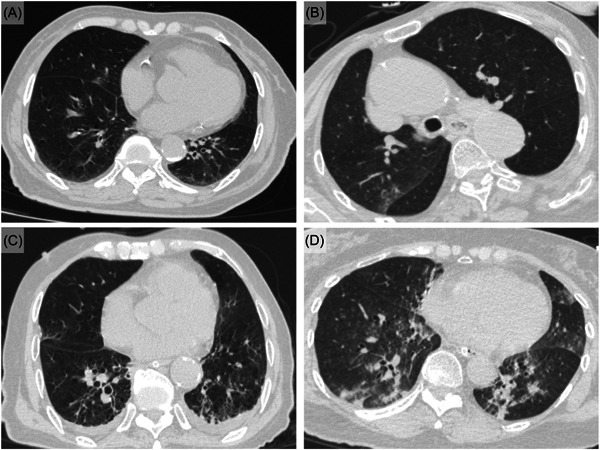
Chest computed tomographic (CT) images of critical cases of COVID‐19 patients in Macao from June 18 to July 31, 2022. Day 3 of hospitalization (88‐year‐old female, third death case): Small ground glass opacities (GGO) in right middle lobe. (B) Day 8 of hospitalization (86‐year‐old female, fifth death case): Small patch of GGO in right lung. (C) Day 12 of hospitalization (93‐year‐old male, sixth death case): Small peripheral GGO with intralobular reticulations and minimal pleural effusions in bilateral basal lungs. (D) Day 2 of hospitalization (71‐year‐old female on mechanical ventilation): multiple infiltrates and ground glass opacities in both lungs.
4. DISCUSSION
COVID‐19 Omicron variant infections became the dominant virus in the COVID‐19 pandemic worldwide since its first emergence in South Africa in early 2022. 13 BA.4 and BA.5 subvariants have rapidly replaced the previous Omicron variant as the dominating lineages globally in 2022. 3 Differing from other countries or regions, Macao had a low prevalence of local COVID‐19 infections and no local outbreak since the beginning of the pandemic due to the efficacy of government strategies. 14 It was regarded as the first COVID‐19 local outbreak in this special administrative region (SAR) of China with Omicron variants as the opponents. Therefore, most patients' immunities against COVID‐19 were acquired from effective vaccination only instead of previous natural infections. These novel variants were believed to be causing less severe diseases but more contagious, leading to rapid spread with a large number of infections which still caused a burden to the healthcare systems and the city. 3
Although COVID‐19 was no longer a Public Health Emergency of International Concern (PHEIC) announced on May 5, 2023 by WHO, the SARS‐CoV‐2 variants remain a challenge to global healthcare under the circumstances of withdrawal and loosening of personal protective behavior in the post‐quarantine era. 15 This study aims to acknowledge the clinical characteristics caused by Omicron BA.4/BA.5 in COVID‐19 naive people and analyze possible factors affecting disease severities, which is crucial for identifying severe and critical cases for medical resources allocation and decreasing mortality in healthcare frontier practice. The impact of age, comorbidities, and vaccination status towards disease severities was demonstrated in this cohort.
Early data reported that runny nose, headache, and fatigue were the most common symptoms of SARS‐CoV‐2 Omicron infection. 16 Our data was contrary to these reports that fever (66.6%), cough (35.3%), and sore throat (26.9%) were more common, which is more compatible with more involvement of the upper respiratory tract noted in another observational study of the Delta and Omicron variants. Change of cellular tropism with higher viral loads in the upper respiratory tract may provide some explanations. 17 , 18 Smell and taste abnormalities and gastrointestinal symptoms were less common in this study of Omicron variants. 18 , 19 Besides, nearly one‐third of SARS‐CoV‐2 Omicron infections were reported asymptomatic. 20 However, it was demonstrated from our cohort that over 80% of patients presented with symptoms during the hospitalization course, eventually indicating a presymptomatic status. The difference can be affected by the timing of data acquisition, for which our data included symptoms at both initial and during the course. Also, the impact of clinical differences between ethnicities, different sublineages (BA.4/BA.5), vaccination rates and types must be considered.
At the beginning of Omicron arising in South Africa, studies revealed that Omicron infection was associated with significantly shorter hospitalization and reduced severity and mortality, especially compared to the delta variants. 7 , 21 , 22 Over 80% of the Omicron infected cases in our cohort were asymptomatic or with mild severity, which echoed the findings from the early Omicron variants. About one per cent of patients in our cohort developed severe and critical disease severities, with six finally deceased. Further exploration of these two subgroups of patients in this outbreak was performed.
Older age is associated with increased mortality, and the elderly are more susceptible to increased disease severity of COVID‐19, correspondingly to the population prevalence of comorbid conditions in the prevaccine era. 23 , 24 , 25 Omicron BA.4 and BA.5 demonstrate the same trend in our study (Tables 2 and 3). The proportion of older people (≥60‐year‐old) constituted up to 17% in this outbreak, with three‐quarters of these patients having underlying comorbidities. Over 90% of patients younger than 60‐year‐old were asymptomatic or developed mild disease only. There was no critical case with age less than 60‐year‐old. All patients in the “Severe‐to‐Critical” group were aged 50‐year‐old or older, with a significantly older mean age (81.3‐year‐old) than that of the “Asymptomatic‐Mild‐Moderate” group (41.5‐year‐old) (p < 0.001). Weak immunity, other organ dysfunction, and increased frequency of comorbidities contribute to higher susceptibility in ageing populations.
Multiple comorbidities and underlying conditions have been associated with severe illness in COVID‐19 patients. 24 , 25 Our study echoed the findings in Omicron variants. About one‐fourth of patients had at least one comorbidity. Among all patients, hypertension, dyslipidemia, and diabetes mellitus were the first three leading comorbidities, aligning with results from a meta‐analysis. There were significantly more patients with comorbidities in the “Severe‐to‐Critical” group (p < 0.001). Decreased innate immunity response and lymphocyte functions were present in patients with comorbidities, and they are more susceptible to inflammation and infection due to underlying metabolic conditions such as diabetes mellitus and chronic liver disorder, and so forth. 24 , 26 , 27 , 28 It warrants the awareness of possible disease progression in those with old age and multiple comorbidities, even with the prevalence of mild severities in the general populations of Omicron infections.
Some laboratory abnormalities have been associated with worse outcomes in COVID‐19 infections. 29 Lymphopenia and neutrophilia were reported as features of severe COVID‐19 illness. 30 A substantial portion (about 70%) of our Omicron COVID‐19 patients with “Severe‐to‐Critical” severity and all mortal cases had decreased lymphocytes while neutrophilia was not observed in the initial hemogram. Besides, elevated C‐reactive protein (CRP), lactate dehydrogenase (LDH), D‐dimer, and prolonged prothrombin time (PT) are predictors of disease progression in critically ill patients. 31 , 32 In our study, a relatively high proportion of patients with “Severe‐to‐Critical” severity presented with prolonged activated partial thromboplastin time (APTT) far more than that of PT. Elevated LDH, CRP and procalcitonin levels were reflected in the same group upon admission, compatible with severity prediction. 33 Over 80% of mortal cases had elevated PCT levels upon admission. Despite being a predictor of severe course, PCT was applicable for detecting secondary bacterial infections and guiding the use of antibiotics. 34 , 35 The interpretation and serial measurements of the above laboratory parameters could have a crucial role on early identifying severe or critical cases.
Common chest CT abnormalities in COVID‐19 include ground glass opacities (GGO) with or without consolidations involving bilateral lower lobes and peripheral distribution. 36 Omicron variants seem to yield less typical pneumonia with a lower consolidation rate and less peripheral but more random distribution than the ancestors. 37 , 38 These features were mainly in line with our cases. Usually, abnormalities develop throughout the illness after symptoms onset and disease progression, while the only intubated case in this outbreak presented multiple GGO in bilateral lungs on Day 2 of hospitalization (Figure 1). Notably, the lung involvement of the mortal cases was less severe than expected in the initial chest CT imaging, for which most of them suffered from secondary infections due to underlying old age and multiple comorbidities (Supporting Information: 1).
The treatment approach towards COVID‐19 evolves rapidly as clinical data emerge, and the mainstays are to target the virus and to modulate the immune response. A number of drugs used for other conditions have been repurposed to deal with COVID‐19. 39 Antiviral therapies (Nirmatrelvir‐ritonavir, Remdesivir, Molnupiravir) were administered in most severe cases and all of the critical cases according to Pneumologists' evaluation and guidelines at that time. Dexamethasone and other glucocorticoids were used on some of the moderate‐severe‐critical patients based on clinical assessment and the living WHO guidelines. 40 The role and indications of the above regimens and various adjunctive immunomodulators were already well‐established.
There were six mortal cases (0.3%) out of these 1820 COVID‐19 cases. All cases were older than 85‐year‐old, which complies with the highest age‐specific mortality in the age group ≥80‐year‐old in Hong Kong after introducing the Omicron variant in early 2022. 41 They were almost totally ADL dependent (except one was partially dependent) with at least three comorbid factors. Ageing, accompanied by the coexistence of a variety of chronic diseases and the decline and dysregulation of immune systems, can result in different complications after COVID‐19 infections. 42 Risks of COVID‐19 deaths were suggested to be associated with pre‐existing comorbidity, dementia, and impairment in ADL. 33 , 43 Upon admission, most mortal patients presented with mild symptoms and minimal or no pulmonary involvement in chest imaging. Poor intake with easy choking and aspiration occurred before respiratory failure were noted in two totally dependent and one partially dependent elderlies. Follow‐up chest CT of these patients showed severe pneumonia, parallel with clinical deterioration after the episodes. COVID‐19 pneumonia usually becomes progressive and peaks in imaging from Day 5 to 13 after disease onset, 44 while the rapid change of imaging in the elderly was within the first few days of disease onset. Although bilateral subpleural patches of ground‐glass opacity (GGO) can be presented by both COVID‐19 pneumonia and aspiration pneumonia, 45 the rapid deterioration within the first week of disease onset highlighted choking and aspiration as possible causes of mortality. This experience raised the early alert of patients' intake status and early prevention of choking, for which enteral feeding or decompression utilizing a nasogastric tube in optimal timing is worth further review. Dehydration was noted in our patients with poor appetite due to sore throat. It may further aggravate the underlying comorbidities of diabetes mellitus and result in complications such as diabetic ketoacidosis. Intensive blood glucose control is encouraged in COVID‐19 patients. Some other serious complications, such as acute kidney injury, gastrointestinal bleeding, and delirium, were also observed, especially in the cases with relevant history. A systematic inflammatory response caused by SARS‐CoV‐2 virus infection has been identified nowadays. 46 The broad involvement of additional organ damage may be related to the abundant expression of the Angiotensin‐converting enzyme 2 (ACE2) receptor. 47 BA.4 and BA.5 were known to have distinguishing mutations in the spike(S) protein, leading to enhanced viral fitness and ACE2 receptor binding despite immunity evasion. 3 Although available evidence suggests milder disease severity in Omicron BA.4/5, the systematic inflammatory response in vulnerable patients with predisposing factors to severe diseases infected by Omicron variants, may still result in mortality and warrant early attention.
The COVID‐19 vaccination program was started in early 2021 in Macao, and two types of vaccines (Inactivated vaccine: Sinopharm and mRNA vaccine: BNT162b2) were available. 48 Completing two doses was considered a complete primary series for the general population. By the end of June 21, 2022, a rough estimate of over 86% of the population finished the primary series. 48 , 49 About three‐quarters of the patients in our cohort finished the primary series. Provided with a higher probability of immunity escape of the highly transmissible Omicron variants, durability and waning of immunity by vaccination were questioned, as well as the efficacy of vaccinations toward Omicron variants. 3 , 4 Although neutralizing resistance and waning neutralizing‐antibody titers of vaccination reduced vaccine‐induced immune protection, cellular responses induced by SARS‐CoV‐2 vaccines still appear to protect against severe disease. 4 Most of the patients' immunity against COVID‐19 in this study was acquired from effective vaccination instead of previous natural infection owing to the practical measures and policies adopted by the Macao government since the beginning of the pandemic. 14 There were significantly more patients who received none or incomplete primary series (61.1%) to present with severe to critical diseases than those who have completed primary series (38.9%) from our data (p < 0.001). Half of the deceased cases were unvaccinated. Vaccination is essential in decreasing mortality in the post‐Omicron era, especially with booster doses.
This study has several limitations. First, the number of patients in this study was not large enough that patient numbers in some subgroups were even smaller under the premise of lessening disease severity caused by Omicron. Second, sublineage classifications, evaluation of serum antibodies, laboratory parameters, and imaging studies were only available for some patients in this study due to the limited time and medical resources allocations on outbreak control. Third, the length of hospitalization was lacking because of the difficulty of estimation since patients were not discharged to the community according to clinical improvement but also under public health policies. Finally, post‐discharge follow‐up and long‐term outcomes of the Omicron BA.4/5 infections in this cohort are unavailable.
5. CONCLUSION
Most of the patients infected by the Omicron variants of SARS‐CoV‐2 presented with mild disease severity under the circumstances of people with acquired immunity against COVID‐19 from effective vaccination only. Patients with old age and comorbidities are still vulnerable to developing severe to critical diseases with complications of predisposing medical illnesses that result in mortality. Vaccination and booster doses effectively reinforce protection, prevent severe diseases, and decrease mortality to counteract the rapidly evolving mutants of SARS‐CoV‐2.
AUTHOR CONTRIBUTIONS
Hou Hon Cheong: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; validation; writing—original draft; writing—review & editing. Fong I Sio: Data curation; investigation; project administration; validation; writing—original draft. Chi Chung Chan: Data curation; investigation; project administration; validation; writing—original draft; writing—review & editing. Seong In Neng: Data curation; investigation; visualization; writing—original draft. Ip Pio Sam: Data curation; investigation; validation; writing—original draft. Teng Cheang: Conceptualization; project administration; writing—original draft. Weng Ieong Tou: Formal analysis; methodology; validation. Hong San Lei: Investigation; project administration. Tan Fong Cheong: Project administration. Edmundo Patricio Lopes Lao: Conceptualization; project administration; supervision. Tak Hong Cheong: Conceptualization; project administration; supervision; writing—review & editing. Cheong U Kuok: Conceptualization; supervision. Iek Long Lo: Conceptualization; supervision.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Hospital Medical Ethical Committee of Centro Hospitalar Conde de São Januário, Health Bureau of Macao SAR, China.
TRANSPARENCY STATEMENT
The lead author Iek Long Lo affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
Cheong HH, Sio FI, Chan CC, et al. Clinical characteristics of COVID‐19 patients infected by the Omicron variants in Macao, China: a cross‐sectional study. Health Sci Rep. 2023;6:e1361. 10.1002/hsr2.1361
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. WHO . WHO Coronavirus (COVID‐19) Dashboard. World Health Organization. https://covid19.who.int/ [Google Scholar]
- 2. WHO . Tracking SARS‐CoV‐2 Variants. World Health Organization. https://www.who.int/activities/tracking-SARS-CoV-2-variants [Google Scholar]
- 3. Tallei TE, Alhumaid S, AlMusa Z, et al. Update on the omicron sub‐variants BA. 4 and BA. 5. Rev Med Virol. 2022;33:e2391. 10.1002/rmv.2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeng QL, Lv YJ, Liu XJ, et al. Clinical characteristics of omicron SARS‐CoV‐2 variant infection after non‐mRNA‐based vaccination in China. Front Microbiol. 2022;13:901826. 10.3389/fmicb.2022.901826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Callaway E. What Omicron's BA. 4 and BA. 5 variants mean for the pandemic. Nature. 2022;606(7916):848‐849. 10.1038/d41586-022-01730-y [DOI] [PubMed] [Google Scholar]
- 6. Mohapatra RK, Kandi V, Sarangi AK, et al. The recently emerged BA. 4 and BA. 5 lineages of Omicron and their global health concerns amid the ongoing wave of COVID‐19 pandemic–correspondence. Int J Surg. 2022;103:106698. 10.1016/j.ijsu.2022.106698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and outcomes of hospitalized patients in South Africa during the COVID‐19 Omicron wave compared with previous waves. JAMA. 2022;327(6):583‐584. 10.1001/jama.2021.24868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Macao SAR. Detailed Results of the 2021 Population Census. Government of Macao Special Administrative Region‐Statistics and Census Service. http://www.dsec.gov.mo/Statistic/Demographic/PopulationCensus/2021%E4%BA%BA%E5%8F%A3%E6%99%AE%E6%9F%A5%E5%88%9D%E6%AD%A5%E7%B5%90%E6%9E%9C.aspx?lang=en-US [Google Scholar]
- 9. Macao Health Bureau . Special Webpage Against Epidemics. Health Bureau, Macao SAR Government. https://www.ssm.gov.mo/apps1/PreventCOVID-19/en.aspx#clg22916 [Google Scholar]
- 10. Lo IL, Lio CF, Cheong HH, et al. Evaluation of SARS‐CoV‐2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID‐19 in Macau. Int J Biol Sci. 2020;16(10):1698‐1707. 10.7150/ijbs.45357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Health Commission of the People's Republic of China . Guideline on the Management of COVID‐19, Trial Version 9. National Health Commission of the People's Republic of China. http://www.nhc.gov.cn/yzygj/s7653p/202203/b74ade1ba4494583805a3d2e40093d88/files/ef09aa4070244620b010951b088b8a27.pdf [Google Scholar]
- 12. Mahoney FI, BARTHEL DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14(2):61‐65. [PubMed] [Google Scholar]
- 13. Tegally H, Moir M, Everatt J, et al. Emergence of SARS‐CoV‐2 Omicron lineages BA. 4 and BA. 5 in South Africa. Nature Med. 2022;28(9):1785‐1790. 10.1038/s41591-022-01911-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lio CF, Cheong HH, Lei CI, Lo IL, Lam C, Leong IH. Minimizing the risk of community spread of COVID‐19 via institutional quarantine of high‐risk travelers with serial viral RNA testing: a successful experience from Macao SAR, China. World J Clin Cases. 2020;8(13):2674‐2678. 10.12998/wjcc.v8.i13.2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. WHO . Coronavirus Disease (COVID‐19) Pandemic. World Health Organization. https://www.who.int/europe/emergencies/situations/covid-19 [Google Scholar]
- 16. Iacobucci G. Covid‐19: runny nose, headache, and fatigue are commonest symptoms of omicron, early data show. BMJ. 2021;375:n3103. 10.1136/bmj.n3103 [DOI] [PubMed] [Google Scholar]
- 17. Meng B, Abdullahi A, Ferreira IATM, et al. Altered TMPRSS2 usage by SARS‐CoV‐2 Omicron impacts infectivity and fusogenicity. Nature. 2022;603(7902):706‐714. 10.1038/s41586-022-04474-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Menni C, Valdes AM, Polidori L, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS‐CoV‐2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID study. Lancet. 2022;399(10335):1618‐1624. 10.1016/S0140-6736(22)00327-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coelho DH, Reiter ER, French E, Costanzo RM. Decreasing incidence of chemosensory changes by COVID‐19 variant. Otolaryngol Head Neck Surg. 2023;168(4):704‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shang W, Kang L, Cao G, et al. Percentage of asymptomatic infections among SARS‐CoV‐2 omicron variant‐positive individuals: a systematic review and meta‐analysis. Vaccines. 2022;10(7):1049. 10.3390/vaccines10071049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davies M‐A, Morden E, Rousseau P, et al. Outcomes of laboratory‐confirmed SARS‐CoV‐2 infection during resurgence driven by Omicron lineages BA. 4 and BA. 5 compared with previous waves in the Western Cape Province, South Africa. Int J Infect Dis. 2023;127(22):63‐68. 10.1016/j.ijid.2022.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS‐CoV‐2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399(10323):437‐446. 10.1016/S0140-6736(22)00017-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sorensen RJ, Barber RM, Pigott DM, et al. Variation in the COVID‐19 infection‐fatality ratio by age, time, and geography during the pre‐vaccine era: A systematic analysis. Lancet. 2022;399(10334):1469‐1488. 10.1016/S0140-6736(21)02867-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barek MA, Aziz MA, Islam MS. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID‐19 cases: a meta‐analysis with 55 studies and 10014 cases. Heliyon. 2020;6(12):e05684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584(7821):430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Odegaard JI, Chawla A. Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harbor Perspect Med. 2012;2(3):a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lim S, Bae JH, Kwon H‐S, Nauck MA. COVID‐19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17(1):11‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feng G, Zheng KI, Yan Q‐Q, et al. COVID‐19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol. 2020;8(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liao D, Zhou F, Luo L, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID‐19: a retrospective cohort study. Lancet Haematol. 2020;7(9):e671‐e678. 10.1016/S2352-3026(20)30217-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al‐Saadi EAKD, Abdulnabi MA. Hematological changes associated with COVID‐19 infection. J Clin Lab Anal. 2022;36(1):e24064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen R, Sang L, Jiang M, et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID‐19 patients in China. J Allergy Clin Immunol. 2020;146(1):89‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Henry BM, Aggarwal G, Wong J, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID‐19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020;38(9):1722‐1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dadras O, SeyedAlinaghi S, Karimi A, et al. COVID‐19 mortality and its predictors in the elderly: a systematic review. Health Sci Rep. 2022;5(3):e657. 10.1002/hsr2.657 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Vazzana N, Dipaola F, Ognibene S. Procalcitonin and secondary bacterial infections in COVID‐19: association with disease severity and outcomes. Acta Clin Belg. 2022;77(2):268‐272. [DOI] [PubMed] [Google Scholar]
- 35. Pink I, Raupach D, Fuge J, et al. C‐reactive protein and procalcitonin for antimicrobial stewardship in COVID‐19. Infection. 2021;49(5):935‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus disease 2019 (COVID‐19) CT findings: a systematic review and meta‐analysis. J Am Coll Radiol. 2020;17(6):701‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ito N, Kitahara Y, Miwata K, Okimoto M, Takafuta T. Comparison of COVID‐19 pneumonia during the SARS‐CoV‐2 Omicron wave and the previous non‐Omicron wave in a single facility. Respir Investig. 2022;60(6):772‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoon SH, Lee JH, Kim B‐N. Chest CT findings in hospitalized patients with SARS‐CoV‐2: delta versus Omicron variants. Radiology. 2023;306(1):252‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hossen MS, Barek MA, Jahan N, Safiqul Islam M. A review on current repurposing drugs for the treatment of COVID‐19: reality and challenges. SN Compr Clin Med. 2020;2:1777‐1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for covid‐19. BMJ. 370. 10; 2020:m3379. 10.1136/bmj.m3379 [DOI] [PubMed] [Google Scholar]
- 41. Smith DJ, Hakim AJ, Leung GM, et al. COVID‐19 mortality and vaccine coverage—Hong Kong special administrative region, China, January 6, 2022–March 21, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(15):545‐548. 10.15585/mmwr.mm7115e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li Q, Zhao C. A review of the current status of clinical management of COVID‐19 in the elderly. Med Sci Monit. 2021;27:e930278. 10.12659/MSM.930278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Antos A, Kwong ML, Balmorez T, Villanueva A, Murakami S. Unusually high risks of COVID‐19 mortality with age‐related comorbidities: an adjusted meta‐analysis method to improve the risk assessment of mortality using the comorbid mortality data. Infect Dis Rep. 2021;13(3):700‐711. 10.3390/idr13030065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hani C, Trieu NH, Saab I, et al. COVID‐19 pneumonia: a review of typical CT findings and differential diagnosis. Diagn Interv Imaging. 2020;101(5):263‐268. 10.1016/j.diii.2020.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zarei F, Reza J, Sefidbakht S, Iranpour P, Haghighi RR. Aspiration pneumonia or COVID‐19 infection: a diagnostic challenge. Academic Radiol. 2020;27(7):1046. 10.1016/j.acra.2020.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bourgonje AR, Abdulle AE, Timens W, et al. Angiotensin‐converting enzyme 2 (ACE2), SARS‐CoV‐2 and the pathophysiology of coronavirus disease 2019 (COVID‐19). J Pathol. 2020;251(3):228‐248. 10.1002/path.5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Macao Health Bureau . COVID‐19 Vaccine Information Page. Health Bureau of Macao SAR Government. https://www.ssm.gov.mo/apps1/covid19vaccine/en.aspx#clg18772 [Google Scholar]
- 49. Novel Coronavirus Response and Coordination Centre . Closure of Macao SAR Public Services From 22nd to 24th of June; Antigen Testing for all Macao Residents and Nucleic Acid Testing for Key Resident Groups. Novel Coronavirus Response and Coordination Centre. https://www.gov.mo/zh-hant/news/902088/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.


