Abstract
Background/purpose
Fibrosis is present in various physiologic and pathologic conditions of salivary glands (SGs). This study aimed to identify novel biomarkers of SG fibrosis by next-generation sequencing.
Materials and methods
We established the SG fibrosis mouse model by excretory main duct ligation. Next-generation sequencing, differentially expressed gene (DEG) analysis, and gene set enrichment analysis was performed by comparing ligated and control SGs. We used algorithms of Cytohubba, molecular complex detection, Lasso logistic regression, and support vector machine to identify the key biomarkers. Selected key biomarkers were verified by the polymerase chain reaction and immunohistochemistry. We also retrieved and analyzed the key gene expression in the fibrosis of the heart, liver, lung, and kidney to ensure the generalization of key biomarkers in SG fibrosis.
Results
Both interlobular and intralobular fibrosis was confirmed in the ligated SGs, with improved expressions of collagen I and transforming growth factor β. Next-generation sequencing identified 2666 upregulated DEGs and 336 downregulated DEGs, which were highly enriched in the extracellular matrix-related pathways. Multiple algorithms identified 15 key biomarkers in SG fibrosis, including Thrombospondin-1 (THBS1) and Prolyl 4-Hydroxylase Subunit Alpha 3 (P4HA3). The mRNA and protein expression of THBS1 and P4HA3 was verified in mice. THBS1 was also highly expressed in lung and kidney fibrosis, whereas P4HA3 was upregulated in liver fibrosis.
Conclusion
THBS1 and P4HA3 may be potential biomarkers for SG fibrosis. They may be also applicable in the diagnosis of multi-organ fibrosis.
Keywords: Sialadenitis, Salivary gland diseases, Fibrosis, Transcriptome, Gene expression profiling, ADAMTS proteins
Introduction
Fibrous tissue repair is a complex physiological process after external stimuli and injuries. Under normal conditions, fibrosis ceases after the inflammation subsides and the injury is repaired. However, continuous fibrosis results in abnormal accumulation of fibrous connective tissue in the extracellular matrix, which leads to structural damage and dysfunction of tissues and organs.1,2 Fibrotic injuries are common in various organs, such as pulmonary, renal, and hepatic fibrosis, etc.3, 4, 5
In the salivary glands (SGs), fibrosis often occurs in a variety of pathological conditions, such as chronic inflammation, aging, duct obstruction, irradiation, and autoimmune diseases including immunoglobulin G4–related sialadenitis, and Sjögren's syndrome.6, 7, 8 The abnormal accumulation of fibrous connective tissue in the extracellular matrix leads to the shrinking of the glandular vesicles, stiffening of the gland, impaired salivary gland function, and reduced salivary flow rate.9 However, Current treatment strategies for SG fibrosis remain limited and symptomatic, and promising treatment targets may be needed for biological treatment in the future.
Therefore, in this study, we constructed a mouse model of SG fibrosis by duct ligation and performed next-generation sequencing to identify novel biomarkers of SG fibrosis.
Methods and materials
Animal model
This study was approved by the institutional board of Sichuan University West China Hospital of Stomatology (WCHSIRB-CT-2021-048). Twenty 8-week-old male C57BL/6J mice, weighing approximately 20 g, were purchased from Chengdu Dossy Experimental Animals Co. Ltd (Chengdu, China). The mice were weighed and subsequently anesthetized with Avertin (T48402, Sigma-Aldrich, St. Louis, MO, USA; 0.75 mg/g mouse weight). The SG fibrosis mouse model was established by creating a submandibular incision, dissecting the dominant duct, and ligating the ducts on day 0.11 In the control group, the ducts were exposed but not ligated.
Saliva flow rate (SFR) measurement
After anesthesia, saliva collection was performed following subcutaneous injection of pilocarpine (P6503, Sigma-Aldrich) at 0.25 mg/kg body weight to stimulate saliva secretion. Saliva was collected with a dry cotton roll for 30 min and transferred into 1.5 mL pre-weighed Eppendorf tubes. The saliva volume was estimated on the basis of the difference in cotton weight before and after collection. SFR was calculated by dividing the estimated saliva volume by the collection time. SFR was measured before surgery and before sacrifice on day 7. When measuring SFR before sacrifice, the duct was deligated to ensure that the saliva on the experimental side could be measured.
Histopathology and immunohistochemistry (IHC)
Mouse SG samples were fixed in formalin, paraffin-embedded, and stained with Masson's trichrome, as previously described.10 The percentage of positive staining in Masson was measured using ImageJ software (https://cnij.imjoy.io/). Immunohistochemistry was performed on the formalin-fixed tissue sections using a microwave-based antigen retrieval method. The primary antibodies used in this immunohistochemistry included TGF-β (3711S; Cell Signaling Technology, Boston, MA, USA), COL 1 (1310-01; SouthernBiotech, Birmingham, AL, USA), THBS1 (ab267388; Abcam, Cambridge, UK), and P4HA3 (23185-1-AP; Proteintech, Chicago, IL, USA).
Quantitative real-time PCR (qRT-PCR)
Total RNA from mouse SG samples was isolated using TRIzol reagent (15596026; Thermo Fisher Scientific, Waltham, MA, USA). Total RNA (1 μg) was used for synthesizing the first strand of cDNA using a TaqMan MicroRNA Reverse Transcription kit (RR047A, Takara, Shiga, Japan). Real-time PCR was performed using SYBR Green (RR820A, Takara) on a 7900HT Fast Real-Time PCR system (4329001, Applied Biosystems, Waltham, MA, USA). The detailed methods and primers of GAPDH were previously described.10 Other primers included:
| COL-1 | Forward | 5′-GGGGAGATAACGGTGTGTTTG-3′ |
| Reverse | 5′-CGGGGATCAGGTTGGCATT-3′ | |
| P4HA3 | Forward | 5′-GAGTACCGCATCAGCAAA AG-3′ |
| Reverse | 5′-CCCTCCGATTCCATAGTTCAC-3′ |
The relative expression levels of target genes were normalized with GAPDH and calculated using the 2−ΔΔCt method.
Transcriptome sequencing and analysis
Total RNA was isolated using TRIzol reagent (15596026; Thermo Fisher Scientific, Waltham, MA, USA) and genomic DNA was removed with the TURBO DNase I kit (AM2238; Thermo Fisher Scientific, Waltham, MA, USA). RNA concentration and quality were assessed with the Agilent Bioanalzyer (Agilent, Santa Clara, CA). Library construction was prepared using the TruSeq RNA sample Preparation Kit (Illumina), following the manufacturer's protocol for RNA input quantity relative to RNA quality. Sequencing was performed on Illumina HiSeq2500 (Illumina, Santa Clara, CA) to generate 100-bp paired-end reads. DESeq2 R package was used to normalize the original read count matrix for a unified expression level and define the differential expression of genes (DEGs). If the criterion of |log2 (fold change)| > 1 and P < 0.05, we could consider it statistically significant. Principal Component Analysis (PCA) was then performed. We converted Ensembl ID (downloaded from http://asia.ensembl.org) into gene names with Perl language. Heatmaps were created by the pheatmap R package. R software (version 4.0.5) and the limma R package were employed to deal with data. Images were generated by the ggplot2 R package. GO and KEGG enrichment was implemented on these upregulated DEGs with clusterProfiler R package.
Identification of candidate key genes
The STRING database (http://string-db.org) was used to perform protein-protein interaction (PPI) analysis. Interactions among DEGs were shown with the assistance of Cytoscape (version 3.7.1). CytoHubba plugin was utilized to identify hub genes. Molecular complex detection (MCODE) was used to find the part with a highly connected area with a degree cut-off of 10, a node score cut-off of 0.2, a k-core of 2, and a maximum depth of 100. We applied two machine learning approaches, including Support Vector Machine (SVM) and LASSO logistic regression, to identify candidate genes. For the SVM, we employed the multiclass version of SVM recursive feature elimination (SVM-RFE) as the feature selection method, which was implemented using the e1071 R package. LASSO was implemented using the glmnet R package, with an alpha value equal to 1. We assessed the performance based on tenfold cross-validation.
Validation of biomarkers’ function related to fibrosis
Transcriptome data for fibrosis of cardiac muscle, liver, kidney, and lung or relevant conditions in various models were obtained from the NCBI GEO database (http://www.ncbi.nlm.nih.gov/geo). Transcriptomes of GSE196656, GSE197112, GSE66494, GSE180415, GSE98892, GSE152329, GSE221598, and GSE97546 were retrieved and analyzed. The receiver operating characteristic (ROC) curve was used for testing the diagnostic power of biomarkers. The area under the curve (AUC) and 95% confidence intervals (CIs) were calculated and visualized by heatmap.
Results
Ligation of the main duct induced SG hyposalivation and fibrosis
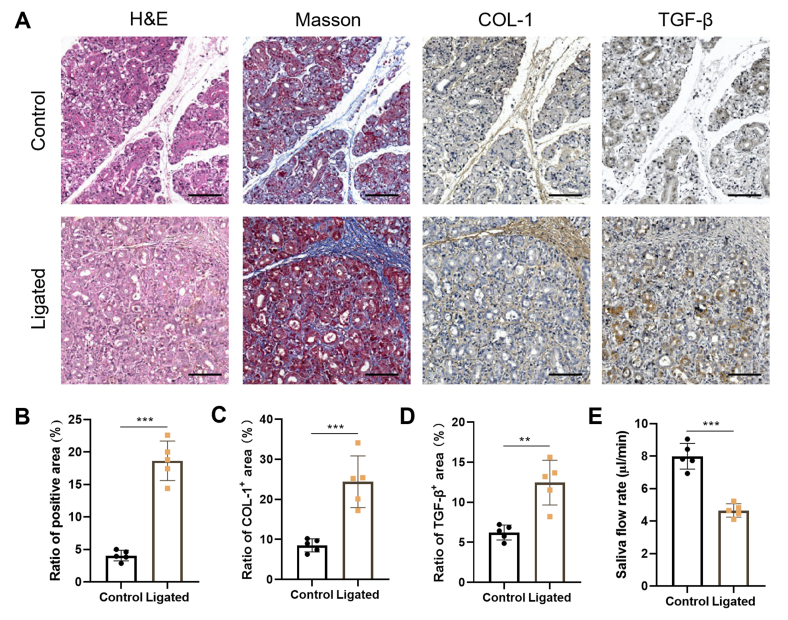
Before the SGs were prepared for transcriptional sequencing, we would like to first confirm that duct ligation successfully induced SG fibrosis. To verify whether the ligation-induced saliva hyposalivation was attributable to the SG fibrosis, we observed the SG sections by H&E and Masson staining, which suggested that collagen deposited both in the interlobular and intralobular matrix (Fig. 1A and B). As COL-1 and TGF-β were well-recognized biomarkers of fibrosis, we further checked the expression of these two proteins, which showed a significant upregulation in the ligated SG (Fig. 1C and D). Unilateral ligation reduced the saliva flow (P < 0.05) (Fig. 1E).
Fig. 1.
Duct ligation in mice leads to SG fibrosis. (A) The representative H&E staining, Masson trichrome staining, and immunohistochemistry staining of COL-1 and TGF-β on SG sections on day 7. (bar = 100 μm) (B) The ratio of the positive area on Masson trichrome staining. (C) The ratio of the positive area on immunohistochemistry staining of COL-1. (D) The ratio of the positive area on immunohistochemistry staining of TGF-β. (E) The saliva flow rate (SFR) on day 7. ∗∗, P < 0.01, ∗∗∗, P < 0.001).
Next-generation sequencing revealed differentially expressed genes in ligated SGs
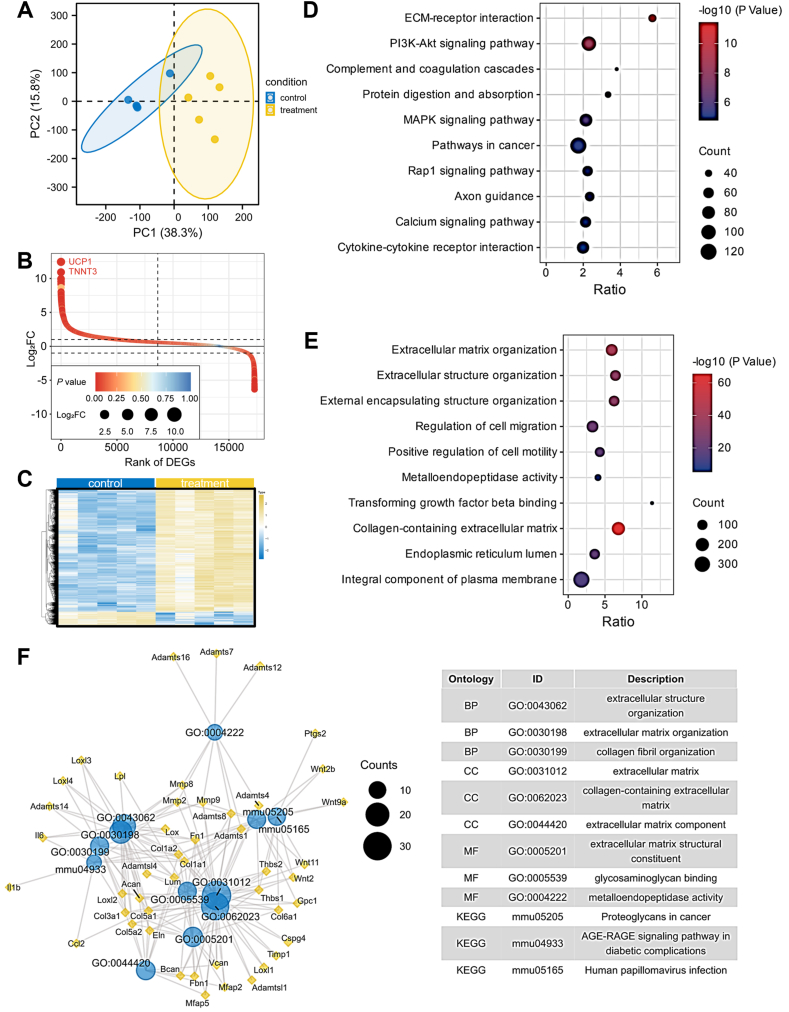
We performed the next-generation sequencing in healthy and ligated mouse SGs. Principal component analysis showed a clear separation between the healthy and ligated mouse SGs (Fig. 2A), suggesting the effectiveness of ligation. Under the criterion of |log2 (fold change)| > 1 and P < 0.05, 3002 differentially expressed genes (DEGs) were filtered, including 2666 upregulated and 336 downregulated genes (Fig. 2B and C). To explore the potential biological functions of these DEGs, we performed GO and KEGG enrichment analyses. The KEGG analysis identified 59 enriched pathways, including ECM-receptor interaction, PI3K-Akt signaling pathway, complement and coagulation cascades, protein digestion and absorption, and MAPK signaling pathway (Fig. 2D). The GO analysis identified a total of 333 biological processes, 53 cellular components, and 72 molecular functions were enriched. The GO term results exhibited that DEGs were mainly involved in the extracellular matrix organization, regulation of cell migration, metalloendopeptidase activity, transforming growth factor beta (TGF-β) binding, and collagen-containing extracellular matrix (Fig. 2E).
Fig. 2.
Characteristics of differentially expressed genes and enriched pathways in SG fibrosis. (A) Principal component analysis for next-generation sequencing. (B) DEGs ranked according to their Log2FC. The sizes of the dots represent the number of Log2FC, and the color means the P value. (C) A heatmap of the DEGs between SG fibrosis and control. (D) KEGG enrichment of upregulated DEGs. (E) GO enrichment of upregulated DEGs. (F) Interaction network of DEGs and enriched pathways. Yellow color indicates proteins. Blue color indicates enriched pathways. DEGs, differentially expressed genes; FC, fold change.
Machine learning algorithms facilitate the selection of key genes of SG fibrosis
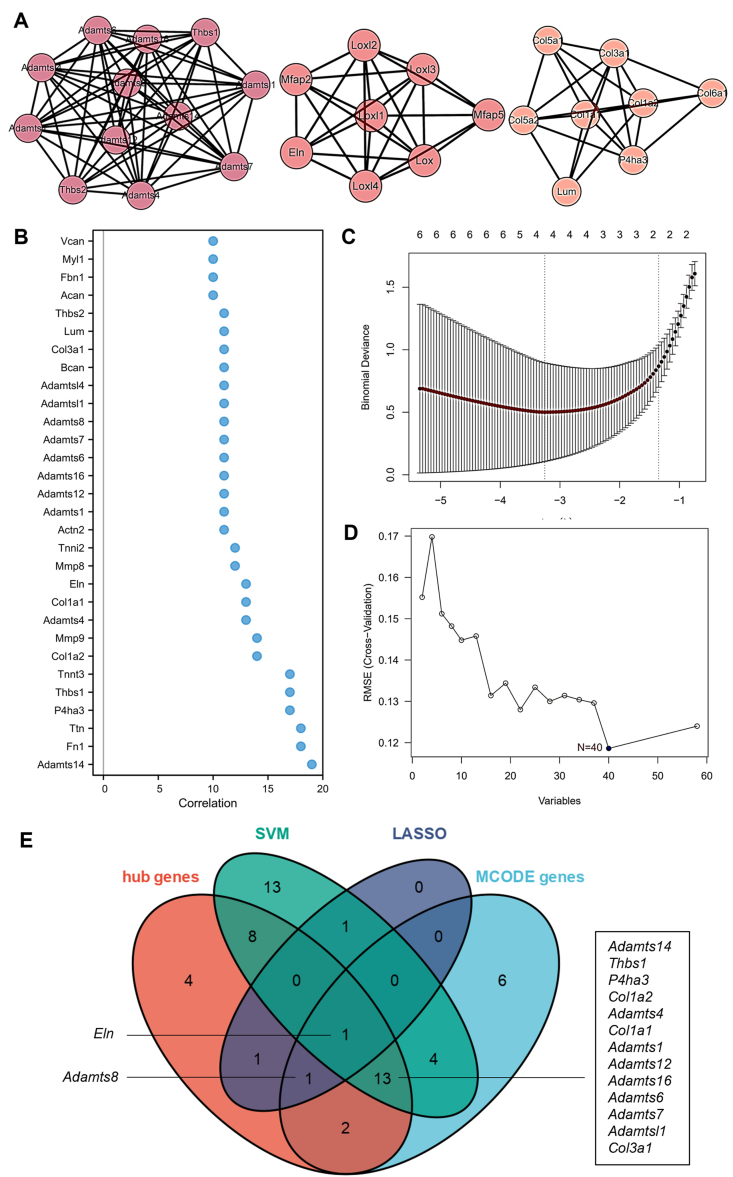
Then, we constructed a protein-protein interaction analysis by STRING database to determine the interaction relationship between DEGs. A close interaction among DEGs was shown with the assistance of Cytoscape software (Fig. 2F). In order to find a small part of the PPI network with a highly connected area, which has a higher probability of participating in biological regulation, the MCODE plugin was used. The MCODE plugin analysis showed that there existed three key modules containing 27 nodes (Fig. 3A). CytoHubba plugin extracted 30 hub genes (Fig. 3B). To further screen for diagnostic molecular markers, we used two machine learning algorithms. By using the Lasso logistic regression, we have successfully screened 4 markers (Fig. 3C), including Mmp9, Eln, Adamts8, and Ccl4. We used a support vector machine (SVM) as the classifier, screening 40 markers (Fig. 3D). The Veen diagram showed the overlap of predicted key genes (Fig. 3E). Fifteen genes, including Adamts14, Thbs1, P4ha3, Col1a2, Adamts4, Col1a1, Eln, Adamts1, Adamts12, Adamts16, Adamts6, Adamts7, Adamts8, Adamtsl1, and Col3a1, were selected as the most important diagnostic molecular markers.
Fig. 3.
Comprehensive identification of candidate biomarkers of SG fibrosis. (A) Key modules from the PPI network identified by MCODE including 27 genes. (B) Top 30 hub genes extracted by CytoHubba plugin from the PPI network. (C) Features selected using LASSO logistic regression. The two dotted vertical lines were drawn at the optimal scores by minimum criteria and 1-standard-error criteria. (D) Cross-validation showing the relationship between the number of feature variables and root-mean-square deviation in the SVM analysis. (E) Venn diagram representing candidate biomarkers identified by CytoHubba plugin (red), SVM (green), LASSO (blue), MCODE (turquoise). The intersection part represents more robust biomarkers. PPI, perform protein-protein interaction; DEGs, differentially expressed genes; SVM, support vector machine; MCODE, molecular complex detection.
Thbs1 and P4ha3 were identified as novel biomarkers of SG fibrosis
As the Collagen family and ADAMTS family were well-recognized protein families in tissue fibrosis, we further explored the role of Thbs1 and P4ha3. We first verified the expression of Thbs1 and P4ha3 in SGs. We performed IHC staining and found that THBS1 was strongly stained in the acinar cells of ligated SGs, whereas it was rarely stained in untreated SGs (Fig. 4A). P4HA3 was expressed in the intracellular matrix in the control SG, while its staining was evidently strengthened in the ducts of ligated SGs (Fig. 4B). Quantitative analysis showed a significantly improved positive area of both THBS1 and P4HA3. Next, we found that the mRNA expression of both Thbs1 and P4ha3 was upregulated (Fig. 4D and E).
Fig. 4.
THBS1 and P4HA3 are involved in multi-organ fibrosis and immune regulation. (A) The representative immunohistochemistry staining of THBS1 and P4HA3 on SG sections on day 7. bar = 100 μm. (B) The ratio of the positive area on immunohistochemistry staining of THBS1 on SG sections. (C) The ratio of the positive area on immunohistochemistry staining of P4HA3 on SG sections. (D) The mRNA expression of THBS1 per GAPDH in SGs on day 7. (E) The mRNA expression of P4HA3 per GAPDH in SGs on day 7. (F) The heatmap of the AUC of ROC in the heart, liver, lung, and kidney fibrosis in homo sapiens and mus musculus. (G) Correlation between THBS1 or P4HA3 and immune cell infiltration. ∗∗, P < 0.01; ∗∗∗, P < 0.001.
Thbs1 and P4ha3 are also biomarkers of other organ fibrosis
To further ensure the specificity and applicability of SG fibrosis markers in other organs, we tested the AUC of 15 key genes in the heart, liver, lung, and kidney (Fig. 4F). In homo sapiens, the AUC of Thbs1 reached 0.847 (95%CI 0.748 to 0.934) in the kidney and 0.778 (95%CI 0.333 to 1.000) in the heart; the AUC of P4ha3 reached 0.900 (95%CI 0.600 to 1.000) in the lung and even 1.000 (95%CI 1.000 to 1.000) in the liver. In the models of mus musculus, the AUC of Thbs1 reached 0.956 (95%CI 0.930 to 0.978) in the liver, 0.833 (95%CI 0.500 to 1.000) in the lung, and even 1.000 (95%CI 1.000 to 1.000) in the kidney; the AUC of P4ha3 reached 0.767 (5% CI 0.400 to 1.000) in the lung.
Discussion
In this study, we successfully constructed a mouse model of SG fibrosis by ligating the excretory main ducts of mice. By MCODE, CytoHubba, Lasso, and SVM algorithms, we identified 15 candidate key genes closely associated with SG fibrosis. Herein, the ADAMTS family is involved in multiple processes in organism development and disease, including fibrosis,11, 12, 13 and the COL family encodes collagen, which is a major component of the extracellular matrix, and Eln, which encodes elastic fibers.14 Hence, we selected Thbs1 and P4ha3 for further validation.
Despite SG fibrosis, THBS1 is involved in the fibrotic process in several organs. Cardiac myocytes have elevated levels of THBS1 in response to stress stimulation15 and THBS1 can release active TGF-β and trigger fibrosis.16 This trial demonstrates that THBS1 is also involved in the fibrosis process in the SG, and therefore THBS1 could be a new biomarker for fibrosis in the SG. P4HA3 is involved in collagen synthesis, a gene downstream of TGF-β that is significantly increased in idiopathic pulmonary fibrosis, atheroscl, and atrial fibrosis.17, 18, 19, 20
We also performed a biomimetic analysis of the heart, liver, kidney, and lung. Interestingly, P4ha3 was highly expressed in hepatic fibrosis; while Thbs1 was expressed higher in renal fibrosis This result suggests that THBS1 and P4HA3 may vary independently without evident upstream or downstream relationship. THBS1 may receive mechanical pressure regulation.21 Kidney and SG are structurally similar in that internal pressure is elevated during ductal obstruction, which may explain why THBS1 was upregulated similarly in the SG and kidney. Due to limited information, it may be difficult to explain why P4HA3 changes similarly in the SG and liver.
This study provides two potential biomarkers of SG fibrosis, THBS1 and P4HA3, which may be useful for the construction of a complete regulatory network of fibrosis. This study still has several limitations. First, we did not address the molecular mechanism of THBS1 and P4HA3 in SG fibrosis. Second, we did not validate other biomarkers (e.g., Adamts14, Adamts4, Eln, etc.). However, as ADAMTS family and elastin are well-recognized biomarkers or components in the fibrous tissue, their expression in SG fibrosis may be predictable. Third, we did not validate their expression in other SG fibrosis models, including radiation-induced or aging-related fibrosis. However, we validated them in multiple organs of humans and mice.
In conclusion, THBS1 and P4HA3 may be potential biomarkers of SG fibrosis. They may be also applicable in the diagnosis of multi-organ fibrosis.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant number: 81870782, 82270994) and Sichuan University Innovation Spark Library (Grant number: 0082604151090).
Contributor Information
Chunjie Li, Email: lichunjie@scu.edu.cn.
Yubin Cao, Email: yubin.cao@scu.edu.cn.
References
- 1.Wynn T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarino M., Tosoni A., Nebuloni M. Direct contribution of epithelium to organ fibrosis: epithelial-mesenchymal transition. Hum Pathol. 2009;40:1365–1376. doi: 10.1016/j.humpath.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Muddu A.K., Guha I.N., Elsharkawy A.M., Mann D.A. Resolving fibrosis in the diseased liver: translating the scientific promise to the clinic. Int J Biochem Cell Biol. 2007;39:695–714. doi: 10.1016/j.biocel.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Meyer K.C. Pulmonary fibrosis, part I: epidemiology, pathogenesis, and diagnosis. Expet Rev Respir Med. 2017;11:343–359. doi: 10.1080/17476348.2017.1312346. [DOI] [PubMed] [Google Scholar]
- 5.Kang H.M., Ahn S.H., Choi P., et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X., Yun J.S., Han D., Yook J.I., Kim H.S., Cho E.S. TGF-β pathway in salivary gland gibrosis. Int J Mol Sci. 2020;21:9138. doi: 10.3390/ijms21239138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson K.F., Meier J.D., Ward P.D. Salivary gland disorders. Am Fam Physician. 2014;89:882–888. [PubMed] [Google Scholar]
- 8.Brito-Zerón P., Baldini C., Bootsma H., et al. Sjögren syndrome. Nat Rev Dis Prim. 2016;2 doi: 10.1038/nrdp.2016.47. [DOI] [PubMed] [Google Scholar]
- 9.Hall B.E., Zheng C., Swaim W.D., et al. Conditional overexpression of TGF-beta1 disrupts mouse salivary gland development and function. Lab Invest. 2010;90:543–555. doi: 10.1038/labinvest.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu M., Liu W., Ma P., et al. Smad7 attenuates TGF-β-mediated aging-related hypofunction of submandibular glands. Exp Biol Med. 2021;246:1269–1273. doi: 10.1177/1535370221993430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang N., Zhang Q., Ye S., et al. Adamts18 deficiency causes spontaneous salivary gland fibrogenesis in adult mice. J Dent Res. 2022;101:226–234. doi: 10.1177/00220345211029270. [DOI] [PubMed] [Google Scholar]
- 12.Mead T.J., Apte S.S. ADAMTS proteins in human disorders. Matrix Biol. 2018;7172:225–239. doi: 10.1016/j.matbio.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao Y., Hu C., Song Q., et al. ADAMTS16 activates latent TGF-β, accentuating fibrosis and dysfunction of the pressure-overloaded heart. Cardiovasc Res. 2020;116:956–969. doi: 10.1093/cvr/cvz187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Procknow S.S., Kozel B.A. Emerging mechanisms of elastin transcriptional regulation. Am J Physiol Cell Physiol. 2022;323:C666–C677. doi: 10.1152/ajpcell.00228.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X., Yassouf Y., Huang K., et al. Ex vivo hydrostatic pressure loading of atrial tissues activates profibrotic transcription via TGF-β signal pathway. Int Heart J. 2022;63:367–374. doi: 10.1536/ihj.21-481. [DOI] [PubMed] [Google Scholar]
- 16.Atanasova V.S., Russell R.J., Webster T.G., et al. Thrombospondin-1 is a major activator of TGF-β signaling in recessive dystrophic epidermolysis bullosa fibroblasts. J Invest Dermatol. 2019;139:1497–1505.e5. doi: 10.1016/j.jid.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Dorotheou D., Farsadaki V., Bochaton-Piallat M.L., Giannopoulou C., Halazonetis T.D., Kiliaridis S. Increased cell proliferation and gene expression of genes related to bone remodeling, cell adhesion and collagen metabolism in the periodontal ligament of unopposed molars in growing rats. Front Physiol. 2017;8:75. doi: 10.3389/fphys.2017.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo Y., Xu W., Chen H., et al. A novel profibrotic mechanism mediated by TGFβ-stimulated collagen prolyl hydroxylase expression in fibrotic lung mesenchymal cells. J Pathol. 2015;236:384–394. doi: 10.1002/path.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu C., You M., Zhang P., Zhang S., Yin Y., Zhang X. A five-gene signature is a prognostic biomarker in pan-cancer and related with immunologically associated extracellular matrix. Cancer Med. 2021;10:4629–4643. doi: 10.1002/cam4.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao Z., Reddy D.P.K., Xue C., et al. Profiling of miR-205/P4HA3 following angiotensin II-induced atrial fibrosis: implications for atrial fibrillation. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.609300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneshige A., Kaji T., Zhang L., et al. Relayed signaling between mesenchymal progenitors and muscle stem cells ensures adaptive stem cell response to increased mechanical load. Cell Stem Cell. 2022;29:265–280.e6. doi: 10.1016/j.stem.2021.11.003. [DOI] [PubMed] [Google Scholar]