Abstract
Objective:
Death anxiety, represented by the HDQLIFE™ Concern with Death and Dying (CwDD) patient-reported outcome (PRO) questionnaire, captures a person's worry about the death and dying process. Previous work suggests that death anxiety remains an unremitting burden throughout all stages of Huntington disease (HD). Although palliative interventions have lessened death anxiety among people with advanced cancer, none has yet to undergo testing in the HD population. An account of how death anxiety is associated with longitudinal changes to aspects of health-related quality of life (HRQoL) would help optimize neuropalliative interventions for people with HD.
Methods:
HDQLIFE collected PROs concerning physical, mental, social, and cognitive HRQoL domains and clinician-rated assessments from people with HD at baseline and 12 and 24 months. Linear mixed-effects models were created to determine how baseline death anxiety was associated with follow-up changes in HRQoL PROs after controlling for baseline death anxiety and other disease and sociodemographic covariates.
Results:
Higher baseline HDQLIFE CwDD is associated with 12- and 24-month declines in HDQLIFE Speech Difficulties, neurology quality of life (NeuroQoL) Depression, Suicidality, HDQLIFE Meaning and Purpose, and NeuroQoL Positive Affect and Well-being.
Interpretation:
Death anxiety may be a risk factor for worsening mental health and speech difficulty. A further prospective study is required to evaluate whether interventions on death anxiety or mental health generally can reduce declines in HRQoL for people with HD over time.
Keywords: death anxiety, Huntington disease, neuropalliative, quality of life
Introduction
Huntington disease (HD) is a neurodegenerative disease inherited through an autosomal dominant genetic mutation in the HTT gene, often manifesting in middle adulthood between ages 30 and 50 and affecting around 37,000 people in the United States.1,2 HD is associated with high burdens of existential and spiritual concerns (e.g., suicidality, loss of meaning and purpose, hopelessness, and concern with death and dying [CwDD]).3–5 “Death anxiety,” also known as the CwDD, poses a considerable challenge in treating people with HD.
Examples of death anxiety include thoughts about hastening death, dread about the death and dying process, rumination about death, and fear of the future and the illness's impact on it.6 At present, there remains limited evidence as to whether a particular palliative intervention or pharmacotherapy, which may successfully lessen death anxiety for other serious illnesses, may also do so for people with HD.7 Furthermore, data governing the relationships between death anxiety and health-related quality of life (HRQoL) might assist clinicians throughout the trajectory of the disease.
Previous bivariate analyses suggest that depression strongly correlates with death anxiety in an HD cohort.3 However, in the previous study, longitudinal information was lacking on the association between baseline death anxiety and 12- and 24-month changes over time in HRQoL patient-reported outcomes (PROs). To close these knowledge gaps, we examined the relationship between death anxiety and HRQoL outcomes over time in HD using the HDQLIFE™ cohort.8 We sought to determine how baseline reports of death anxiety were associated with physical, mental, social, and cognitive HRQoL PROs over a 12- and 24-month period. We hypothesized that higher baseline death anxiety would relate to worse mental and social health outcomes (PROs and clinician-rated aspects of mental health) at both 12 and 24 months. We specifically anticipated that baseline reports of death anxiety would relate to worsening measures of anger, depression, suicidality, meaning and purpose, and positive affect and well-being.
Methods
Study design/setting and participants
Our analysis included 322 people with either 36 or more CAG repeats for the HD genetic mutation or a clinical diagnosis of HD (n = 50 prodromal; n = 171 early-stage manifest; and n = 101 late-stage manifests). These individuals were recruited as part of a national, multicenter longitudinal study designed to develop high-quality PROs responsive to the issues important to people with HD (entitled “HDQLIFE”).6,8 HDQLIFE was intended to extend the work of neurology quality of life (Neuro-QoL) and Patient-Reported Outcomes Measurement Information System (PROMIS),9–11 augmenting universal HRQoL domains and validating existing PROs relevant to the needs of people with the HD genetic mutation.4,8,12–17
Participants for HDQLIFE were recruited from several sources, including the PREDICT-HD study,18 the national HD roster, skilled nursing facilities, web-based advertisements, and academic clinics across the United States. Inclusion criteria included, but were not limited to, the HD genetic mutation with at least 36 or more CAG repeats, 18 years of age or older, English speaking, and able to provide informed consent (obtained at the baseline visit). All HDQLIFE study sites received Institutional Review Board approval to collect data. The study design, setting, and participants are found elsewhere.8
Assessment battery
PROs and clinician-rated assessments were administered at baseline and 12 and 24 months. The study period spanned from 2012 to 2016. The assessments, collected in-person or remotely, lasted approximately two hours. Clinician-rated measurements were collected in person.
PROs in HD participants are scored in reference to a specific normative population (i.e., for HDQLIFE, the population comparison is those with prodromal, early-stage, and late-stage manifest HD). The average score in the reference population is T = 50, with a standard deviation (SD) = 10. Higher scores on a given PRO indicate more of the stated domain (i.e., higher HDQLIFE CwDD scores indicate worse mental HRQoL and elevated death anxiety, whereas higher Neuro-QoL Positive Affect and Well-Being indicate higher well-being/affect and better mental HRQoL). Generally speaking, group T score differences >2 points are considered meaningful.16 Details of the validation of PROMIS/Neuro-QoL/HDQLIFE HRQoL PROs scales have been published.6,8,13,15–17,19–23
Clinician-rated assessments included the Problem Behavior Assessment-short (PBAs) and Unified Huntington Disease Rating Scales (UHDRS®).24 The PBAs consist of 11 items, including suicidality with input from a collateral source25,26 when available.27 Scores are computed by multiplying the frequency by severity. Each variable is rated from 0 to 4 (with higher scores indicating the more significant frequency or severity); scores for a single item, suicidality, were also assessed. The UHDRS has a clinician-rated section entitled the “Diagnostic Confidence Level (DCL),” scored based on confidence that the hallmark motor manifestations are present.
Scores range from 0 to 4, with a score of 4 representing 99% confidence in manifesting HD motor features and scores <4 and >1 suggest premanifest or prodromal HD (Note: the distinction from prodromal to manifest is the appearance of motor symptoms, even though neuropsychiatric features may be present in people with the HD genetic mutation 15 years before the motor symptoms appear.) “Total Functional Capacity” ascertains the degree to which a person with the HD mutation carries out daily living activities. The maximum score is 13, and the minimum is 0, with higher scores representing greater independence. Scores from 7 to 13 (i.e., Stages I and II) with a DCL of 4 suggest early-stage manifest HD, and scores less than 7 (i.e., Stages III and IV) with a DCL of 4 indicate late-stage manifest HD.8
Definition of study variables
We created several separate models to determine how baseline reports of death anxiety are associated with physical, mental, social, and cognitive HRQoL PROs over a 12- and 24-month period. We defined HDQLIFE CwDD as the predictor variable collected at baseline enrollment in the HDQLIFE study.
We defined an outcome variable as a given HD-validated HRQoL PRO from the physical (HDQLIFE Chorea, HDQLIFE Speech difficulties, HDQLIFE Swallowing difficulties, Neuro-QoL Upper Extremity Function, and Neuro-QoL Lower Extremity Function), mental (PROMIS Anger, HDQLIFE Meaning and Purpose, HDQLIFE End of Life Planning, Neuro-QoL Anxiety, Neuro-QoL Depression, Neuro-QoL Emotional and Behavioral Dyscontrol, Neuro-QoL Positive Affect and Well-Being, and the clinician-rated Suicide from the PBAs), social (Neuro-QoL Ability to Participate in Social Roles and Activities, Neuro-QoL Satisfaction with Social Roles and Activities, and Neuro-QoL Stigma), or cognitive (Neuro-QoL Applied Cognition—Executive Function and NeuroQoL Applied Cognition—General Concerns) HRQoL domain at the 12- and 24-month follow-up visits.
Statistical analysis plan
Baseline characteristics were described using means (SDs) and frequencies for continuous and categorical variables. Based on the normal distribution for HDQLIFE CwDD and other HDQLIFE PROs scaled to the HD population, baseline HDQLIFE CwDD was stratified as follows: low (t ≤ 40) versus medium (t between 40 and 60) versus high (t ≥ 60), and by baseline HD disease stage. We employed two statistical tests appropriate for the data type under consideration to examine group differences: Kruskal–Wallis and chi-square tests.
A series of linear mixed-effects models determined the impact of baseline HDQLIFE CwDD on HRQoL PROs at 12 and 24 months. For these models, scores at 12 and 24 months were modeled as a function of baseline HDQLIFE CwDD and baseline levels of the HRQoL PRO. Covariates included age, education, sex, and baseline stage. We used two-sided statistical tests, defined a presignificance level of alpha = 0.05, and then corrected our several linear-mixed effects models for multiple comparisons through the Benjamini–Hochberg procedure.28 Statistical investigations were performed through SAS V9.4 (SAS Institute, Inc., Cary, NC).
Results
Among the HDQLIFE cohort (n = 322), who completed the HRQoL measures, 318 people with the HD genetic mutation finished the HDQLIFE CwDD PRO (n = 49 prodromal HD, n = 170 early-stage manifest HD, and n = 99 late-stage manifest HD). There was no significant difference in the magnitude of symptom burden of HDQLIFE CwDD among the stages of HD (T score = 50.0 for prodromal; T score = 50.6 for early-stage manifesting HD, and T score = 49.8 for late-stage manifesting HD). Between 51% and 56% of people's scores were above average for HDQLIFE CwDD. Some (17%) people with HD scored in the high range in the HDQLIFE CwDD (i.e., one SD above the HD population mean), and 19% exhibited low HDQLIFE CwDD (i.e., one SD below the HD population mean); the remaining were classified in the medium range.
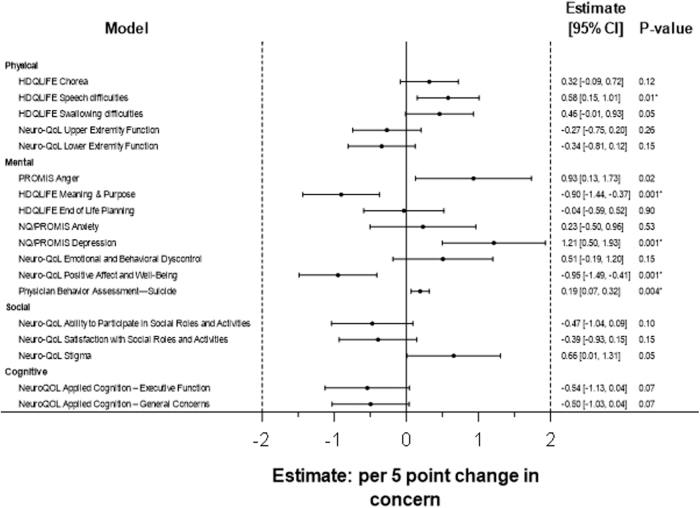
Additional descriptive characteristics for our cohort may be found in Table 1. After correcting for multiple comparisons, HDQLIFE CwDD at baseline related to 12- and 24-month worsening HDQLIFE Speech difficulties, HDQLIFE Meaning and Purpose, Neuro-QoL Depression, Neuro-QoL Positive Affect and Well-Being, and PBA-Suicide, even after controlling for relevant covariates (Figs. 1 and 2). HDQLIFE CwDD had the most substantial effect on Neuro-QoL Depression. For every 5-point increase in baseline HDQLIFE CwDD, Neuro-QoL Depression worsened by 1.21 T score units at follow-up (Supplementary Table S1).
Table 1.
Demographic Data by Baseline Concern With Death and Dying
| Characteristic | Baseline concern with death and dying |
Overall | p | ||
|---|---|---|---|---|---|
| Low (≤40) | Medium (40–60) | High (≥60) | |||
| Demographics | |||||
| Age (years)a | 55.7 (11.17) | 51.3 (13.01) | 47.7 (12.34) | 51.5 (12.76) | 0.0027 |
| Femaleb | 29 (48) | 99 (49) | 17 (31) | 145 (46) | 0.0733 |
| Hispanic or Latinob | 1 (2) | 5 (2) | 2 (4) | 9 (3) | 0.6094 |
| Raceb | 0.1110 | ||||
| African American | 1 (2) | 3 (1) | 5 (9) | 9 (3) | |
| Caucasian | 58 (97) | 196 (96) | 48 (89) | 302 (95) | |
| Other | 1 (2) | 4 (2) | 1 (2) | 6 (2) | |
| Unknown | 0 (0) | 1 (0) | 0 (0) | 1 (0) | |
| Education (years)a | 14.3 (2.58) | 14.9 (2.80) | 14.5 (2.60) | 14.7 (2.73) | 0.2531 |
| Marital statusb | 0.0580 | ||||
| Single, never married | 4 (7) | 28 (14) | 11 (20) | 43 (14) | |
| Married | 31 (52) | 126 (62) | 27 (50) | 184 (58) | |
| Separated/divorced | 21 (35) | 37 (18) | 15 (28) | 73 (23) | |
| Living with partner | 3 (5) | 6 (3) | 1 (2) | 10 (3) | |
| Widowed | 1 (2) | 7 (3) | 0 (0) | 8 (3) | |
| CAG repeatsa | 42.1 (2.15) | 43.3 (5.14) | 44.3 (4.83) | 43.3 (4.73) | 0.0189 |
| HDQLIFE concern with death and dyinga | 36.1 (2.82) | 50.4 (5.17) | 65.4 (4.88) | 50.3 (9.96) | <0.0001 |
| Physical | |||||
| HDQLIFE Choreaa | 50.1 (8.35) | 52.7 (8.26) | 56.4 (9.05) | 52.9 (8.60) | 0.0002 |
| HDQLIFE Speech difficultiesa | 49.2 (9.15) | 51.1 (7.64) | 54.3 (8.36) | 51.3 (8.19) | 0.0046 |
| HDQLIFE Swallowing difficultiesa | 49.6 (7.92) | 51.7 (8.13) | 56.5 (9.00) | 52.1 (8.49) | <0.0001 |
| Neuro-QoL Upper Extremity Functiona | 40.9 (10.95) | 41.6 (9.88) | 37.9 (10.62) | 40.8 (10.27) | 0.0388 |
| Neuro-QoL Lower Extremity Functiona | 46.4 (9.80) | 45.7 (10.00) | 41.0 (9.33) | 45.1 (9.99) | 0.0045 |
| Mental | |||||
| PROMIS Angera | 38.8 (9.73) | 48.1 (11.01) | 58.7 (11.47) | 48.1 (12.37) | <0.0001 |
| HDQLIFE Meaning and Purposea | 54.0 (8.72) | 49.5 (8.95) | 45.3 (9.64) | 49.6 (9.37) | <0.0001 |
| HDQLIFE End of Life Planninga | 51.5 (10.04) | 50.5 (10.02) | 48.8 (8.56) | 50.4 (9.80) | 0.2103 |
| PROMIS Anxietya | 44.7 (9.39) | 53.5 (8.35) | 63.7 (9.02) | 53.6 (10.32) | <0.0001 |
| PROMIS Depressiona | 42.4 (8.40) | 50.8 (9.11) | 62.0 (9.43) | 51.1 (10.75) | <0.0001 |
| Neuro-QoL Emotionala and Behavioral Dyscontrola | 39.0 (9.48) | 47.2 (9.83) | 55.5 (9.01) | 47.1 (10.81) | <0.0001 |
| Neuro-QoL Positive Affect and Well-Beinga | 60.8 (7.95) | 54.2 (7.97) | 50.2 (7.65) | 54.7 (8.54) | <0.0001 |
| Problem Behavior Assessment—Suicidea | 0.1 (0.79) | 0.4 (1.68) | 0.9 (1.95) | 0.4 (1.62) | 0.0050 |
| Social | |||||
| Neuro-QoL Ability to Participate in Social Roles and Activitiesa | 50.4 (9.00) | 46.1 (7.98) | 41.9 (6.66) | 46.2 (8.36) | <0.0001 |
| Neuro-QoL Satisfaction with Social Roles and Activitiesa | 52.0 (9.00) | 47.3 (7.94) | 42.8 (5.76) | 47.4 (8.29) | <0.0001 |
| Neuro-QoL Stigmaa | 43.4 (7.84) | 51.9 (7.19) | 58.1 (8.61) | 51.3 (8.75) | <0.0001 |
| Cognitive | |||||
| NeuroQOL Applied Cognition—Executive Functiona | 38.7 (12.12) | 36.9 (9.65) | 31.6 (9.68) | 36.4 (10.39) | 0.0002 |
| NeuroQOL Applied Cognition—General Concernsa | 45.5 (10.20) | 39.2 (8.43) | 35.3 (7.99) | 39.7 (9.25) | <0.0001 |
Mean (SD).
Frequency (%).
NeuroQOL, neurology quality of life; PROMIS, Patient-Reported Outcomes Measurement Information System; SD, standard deviation.
FIG. 1.
Mixed-model results of baseline HDQLIFE CwDD predicting symptoms at follow-up (adjusted for baseline levels of outcome, age, sex, education, and stage). CwDD, Concern with Death and Dying.
FIG. 2.
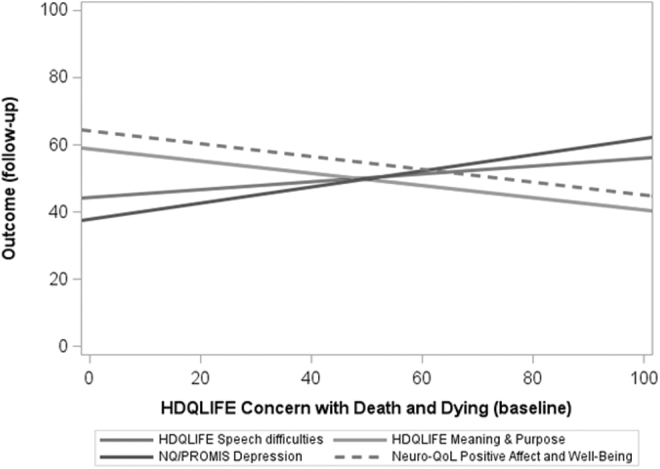
Significant relationships from Figure 1.
Discussion
Our study contributes to the HD-neuropalliative literature, in that death anxiety is associated with worsening speech difficulties, meaning and purpose, depression, positive affect and well-being, and suicidality at follow-up. Although from a new patient population, this finding further adds to the existing data on the role of death anxiety and health-related quality of life. While our study, a retrospective observational analysis, did not intervene in death anxiety, these data may serve as the preliminary basis for designing future clinical trials to mitigate death anxiety symptoms.
Indeed, randomized controlled existential palliative interventions in people with advanced cancer25–27,29 have addressed and intervened upon symptoms of “death anxiety.”30 Meaning-Centered Psychotherapy (“MCP”) is a multisession intervention that improves spiritual well-being and overall HRQoL.25–27 Similarly, Cancer and Living Meaningfully (“CALM”), another palliative psychotherapeutic intervention rooted in existential theory, reduced depression,29 which the study authors posited as the “final common pathway” of existential concerns.31 Studies,32–35 which laid the foundation for which MCP and CALM were built, suggest a reconnection to various sources of meaning (based on the works of the late neurologist/psychiatrist Dr. Viktor Frankl) decreased the influence of death anxiety. Furthermore, they suggested that a sense of meaning may also have mediated death anxiety.36
In contrast to our initial hypotheses, one unexpected piece of data surrounded the direct relationship between death anxiety and speech difficulties. We believe that, while this deserves further prospective study with adequate controlling of all relevant genetic and environmental factors, several underlying causes could explain this association. One possibility is that a certain endophenotype governs those with death anxiety. Those with a worsening sense of death anxiety may concomitantly have speech difficulties or a self-rated (heightened) feeling of these difficulties, independent of disease stage and other confounders, explained perhaps from an overlapping (convergent) neurobiological substrate. A spurious association could likely (and equally) explain this result, emanating from reverse causation or residual confounding. Our study may have failed to account for other genetic measurements associated with more precise staging.
Despite this unexpected result in the analysis of death anxiety and a particular physical PRO, our data indicate that death anxiety was associated with declines in the sense of meaning and purpose over time. Notably, previously published work from our group found that baseline meaning and purpose were not associated with death anxiety at 12 and 24 months.5 In HD, there appears to be one-way directionality in the temporal relationship between death anxiety and a later sense of meaning and purpose. Death anxiety was associated with a sense of meaning and purpose in the future; however, the sense of meaning and purpose was not associated with death anxiety at follow-up. Thus, previously successful existential palliative interventions may have targeted not just a sense of meaning and purpose but rather death anxiety itself, which contributed to increases in meaning and purpose and overall HRQoL.
Based on our data from people with the HD genetic mutation, lower death anxiety was associated with future increases in the sense of meaning and purpose (or, stated otherwise, elevated death anxiety relates to future decreases in the sense of meaning and purpose). Currently, in contrast to the psycho-oncology field,31,37 neither the HD nor neuropalliative (psychoneurology38) arenas have a fundamental biopsychosocial-spiritual model for how existential factors (e.g., death anxiety, meaning, and purpose) may influence psychiatric diagnoses (e.g., depression, anxiety, and suicidality) or markers of well-being (e.g., positive affect and well-being); and how these entities could reciprocally predict or mediate, one another—especially in the face of a deteriorating neurological substrate, which subserves these critical functions.
A model would be a helpful contribution to the field of neuropalliative care, HD, and other movement disorders in helping determine what patient-reported aspects ought to be targeted during neuropalliative intervention refinement and help clarify inclusion/exclusion criteria for future trials to predict and demonstrate a specific effect.
Our project's objectives and approach follow the spirit of Stage 0 data analysis (National Institutes of Health [NIH] Stage Model of Development),39 and thus is an essential, mechanistic element to optimize and develop neuropalliative interventions that target death anxiety for this population in the future. Our findings are not without limitations. For example, participants were primarily recruited from academic centers, introducing possible selection bias.
In addition, our prodromal group achieved further education than all groups by approximately one year. While unclear as to what factor might govern this difference, we again see the possibility of a selection bias, as those who may have enrolled at that juncture may have had personal experience caring for family members with the disease. Furthermore, no information is available in our dataset to determine the utilization of nonpharmacological (e.g., exercise) or pharmacological therapies (e.g., antidepressants) throughout the study period, which may confound some of our results.
Our study also did not execute any latent modeling. Further work should explore, using cross-sectional and longitudinal data, a model to better understand the direct and indirect effects of existential and psychiatric factors coalescing to inform the mental (and global) HRQoL in HD and other related dementias. Specifically, does death anxiety cause depression, and is the converse also true? Which pathway is most potent, and might a sense of meaning and purpose mediate these effects? Utilizing existing models from psycho-oncology and assessing their generalizability and limitations to HD, while adding other legacy-making/coping patterns,40 religious, spiritual, and personality measurements into the model would be a sensible future direction to capture the entirety of other characteristics that could be exploited during intervention refinement.
In summary, death anxiety was associated with 12- and 24-month worsening physical and mental HRQoL. Overall, our data provide an essential step toward optimizing palliative interventions for this population, as no validated HD-neuropalliative intervention exists, despite HD's profound psychosocial disability on the self, care partners, and other related first-degree relatives.7
Supplementary Material
Authors' Contributions
L.L.S. conceived the project, wrote the first draft, devised the research questions, interpreted the work, revised the article for valuable intellectual content, and approved the final draft. All other authors oversaw the work, conception, and design, drafted the work for important intellectual content, and approved the final draft.
Funding Information
Data reported in this article were collected with support from the NIH, National Institute of Neurological Disorders and Stroke (R01NS077946: PI N.E.C.; R01NS0400068: PI J.S.P.), and the National Center for Advancing Translational Sciences (NCATS) (UL1TR000433). J.P.T. was supported, in part, by the NCATS for the Michigan Institute for Clinical and Health Research (UL1TR002240). L.L.S. is supported, in part, extramurally by the Huntington's disease Society of America (HDSA), SP0070054. B.M.K. is supported by the National Institute of Aging (K02 AG062745).
Author Disclosure Statement
L.L.S. receives financial support as a paid consultant from the HD Society of America, American Film Institute, and Tikvah for Parkinson; he has received research support from the Memorial Sloan Kettering MCP NCI R25 Training, and Northwestern Physician Scientist Training program. J.P.T. owns stocks in Procter & Gamble and General Electric and has received research funding through the University of Michigan with Complexa, Inc., Retrophin, Inc., and Goldfinch Bio, and the University of Michigan with Vertex Pharmaceuticals and Pfizer, Inc., D.B. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Speaker: Teva Pharmaceuticals, Acorda Therapeutics, Neurocrine Biosciences, Adamas Pharmaceuticals Consulting: Biogen Pharmaceuticals, Amgen Pharmaceuticals, Acadia Pharmaceuticals, Genentech, Inc., GE Healthcare, Gerson Lehrman Group, Guidepoint, and L.E.K.C., and has received personal compensation in an editorial capacity for Editor: Annals of Clinical and Translational Neurology. J.S.P. receives grant support from NIH (NS103475, NS105509, AG074608) and receives compensation for consultation from Wave Life Sciences. B.M.K. received research grant support from the National Institute of Aging, National Institute of Nursing Research, and Patient-Centered Outcomes Research Institute; he has received speaker honoraria from the Parkinson's Foundation. A.J.A. receives funding from the NIH, BlueNote Therapeutics, and the American Cancer Society. S.F. has received personal compensation in the range of $500−$4,999 for serving as a Consultant for uniQure. S.F. has received personal compensation in the range of $500−$4,999 for serving as a Consultant for MCG Health. The institution of S.F. has received research support from HDSA. The institution of S.F. has received research support from Michael J Fox Foundation. The institution of S.F. has received research support from Roche/Genentech. The institution of S.F. has received research support from CHDI Foundation. The institution of S.F. has received research support from Huntington Study Group (HSG). The institution of S.F. has received research support from Triplet Therapeutics. S.F. has received personal compensation in the range of $500−$4,999 for serving as a Consultant for Oscine Therapeutics. M.A.N. has received personal compensation in the range of $5,000−$9,999 for serving as a Consultant for Voyager. M.A.N. has received personal compensation in the range of $500−$4,999 for serving as a Consultant for Roche. M.A.N. has received personal compensation in the range of $5,000−$9,999 for serving on a Scientific Advisory or Data Safety Monitoring board for Roche. M.A.N. has received personal compensation in the range of $500−$4,999 for serving on a Scientific Advisory or Data Safety Monitoring board for uniQure. An immediate family member of M.A.N. has received stock or an ownership interest from Fresca. The institution of M.A.N. has received research support from HDSA. The institution of M.A.N. has received research support from Parkinson Foundation. M.A.N. has received research support from Parkinson Foundation. The institution of M.A.N. has received research support from Neuraly. M.A.N. has received personal compensation in the range of $500−$4,999 for serving as a speaker with AAN. K.E.A. received $2,000 payment for her involvement in the Enroll-HD Scientific Oversight Committee. She has also received travel funding to attend Enroll-HD SOC meetings, including where this article was planned and discussed. Through Georgetown University, K.E.A. also receives funding from CHDI as site principal investigator for the Georgetown MedStar Enroll-HD clinical site. J.S.P. has received research funding from the NIH (NS075321, NS103957, NS107281, NS092865, U10NS077384, NS097437, U54NS116025, U19 NS110456, AG050263, AG-64937, NS097799, NS075527, ES029524, NS109487, R61 AT010753 [NCATS, NINDS, NIA], RO1NS118146, R01AG065214), Department of Defense (DOD W81XWH-217-1-0393), Michael J Fox Foundation, Barnes-Jewish Hospital Foundation (Elliot Stein Family Fund and Parkinson disease research fund), American Parkinson Disease Association (APDA) Advanced Research Center at Washington University, Greater St. Louis Chapter of the APDA, Paula and Rodger Riney Fund, Jo Oertli Fund, HD Society of America, Murphy Fund, and CHDI; has received honoraria from CHDI, HD Study Group, Parkinson Study Group, Beth Israel Hospital (Harvard group), University of Pennsylvania, and Stanford University; is co-director for the Dystonia Coalition, which has received the majority of its support through the NIH (grants NS116025, NS065701 from the National Institutes of Neurological Disorders and Stroke and TR 001456 from the Office of Rare Diseases Research at the NCATS); serves as Director of Medical and Scientific Advisory Committee of the Dystonia Medical Research Foundation, Chair of the Scientific Advisory Committee of the Parkinson Study Group, Chair of the Interim Executive Membership Committee of the HSG, Chair of the Nominating Committee of the HSG, Chair of the Standards Committee of the HSG, member of the Scientific Advisory Board of the APDA, Chair of the Scientific and Publication Committee for ENROLL-HD, and member of the Education Committee of the HSG; and has provided medical legal consultation to Wood, Cooper and Peterson, LLC, and Simmons and Simmons LLP. C.A.D. receives support from the NIMH, VA, and NCATS. J.G. receives support from the NIH. D.C. reports consultant fees from AbbVie, Bristol Myers Squibb (BMS), Exelixis, Merck, Novartis, and Pfizer; reports licensing fees from FACIT.org; research grants (institutional) from AbbVie, Astellas, Aveo, BMS, GlaxoSmithKline, Merck, Novartis, and Pfizer; and is an officer of FACIT.org. N.E.C. reports research grants from the NIH, the Neilsen Foundation, and CHDI, as well as a contract from Teva Pharmaceuticals. She is also supported by research funding from the Alzheimer's Association, the Food and Drug Administration (FDA), as well as the Department of Health and Human Services—Centers for Medicare and Medicaid Services. She receives honoraria for her role on the CHDI scientific advisory board and is a consultant on the TBI Congressionally mandated study.
Supplementary Material
References
- 1. Ross CA, Aylward EH, Wild EJ, et al. . Huntington disease: Natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol 2014;10(4):204–216. [DOI] [PubMed] [Google Scholar]
- 2. McColgan P, Tabrizi SJ. Huntington's disease: A clinical review. Eur J Neurol 2018;25(1):24–34. [DOI] [PubMed] [Google Scholar]
- 3. Carlozzi NE, Boileau NR, Paulsen JS, et al. . End-of-life measures in Huntington disease: HDQLIFE meaning and purpose, concern with death and dying, and end of life planning. J Neurol 2019;266(10):2406–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carlozzi NE, Downing NR, McCormack MK, et al. . New measures to capture end of life concerns in Huntington disease: Meaning and purpose and concern with death and dying from HDQLIFE (a patient-reported outcomes measurement system). Qual Life Res 2016;25(10):2403–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sokol LL, Troost JP, Kluger BM, et al. . Meaning and purpose in Huntington's disease: A longitudinal study of its impact on quality of life. Ann Clin Transl Neur 2021;8(8):1668–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carlozzi NE, Tulsky DS. Identification of health-related quality of life (HRQOL) issues relevant to individuals with Huntington disease. J Health Psychol 2013;18(2):212–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zarotti N, Dale M, Eccles F, et al. . Psychological interventions for people with Huntington's disease: A call to arms. J Huntingtons Dis 2020;9(3):231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carlozzi NE, Schilling SG, Lai J-S, et al. . HDQLIFE: Development and assessment of health-related quality of life in Huntington disease (HD). Qual Life Res 2016;25(10):2441–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cella D, Yount S, Rothrock N, et al. . The Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care 2007;45(5):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cella D, Riley W, Stone A, et al. . The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63(11):1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cella D, Nowinski C, Peterman A, et al. . The neurology quality-of-life measurement initiative. Arch Phys Med Rehab 2011;92(10):S28–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carlozzi NE, Victorson D, Sung V, et al. . HD-PRO-TRIADTM validation: A patient-reported instrument for the symptom triad of Huntington's disease. Tremor Other Hyperkinetic Movements 2014;4:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carlozzi NE, Boileau NR, Chou KL, et al. . HDQLIFE and neuro-QoL physical function measures: Responsiveness in persons with Huntington's disease. Movement Disord 2020;35(2):326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carlozzi NE, Ready RE, Frank S, et al. . Patient-reported outcomes in Huntington's disease: Quality of life in neurological disorders (Neuro-QoL) and Huntington's disease health-related quality of life (HDQLIFE) physical function measures. Movement Disord 2017;32(7):1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carlozzi NE, Boileau NR, Paulsen JS, et al. . Psychometric properties and responsiveness of Neuro-QoL Cognitive Function in persons with Huntington disease (HD). Qual Life Res 2020;29(5):1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carlozzi NE, Boileau NR, Roché MW, et al. Responsiveness to change over time and test-retest reliability of the PROMIS and Neuro-QoL mental health measures in persons with Huntington disease (HD). Qual Life Res 2020;29(12):3419–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carlozzi NE, Goodnight S, Kratz AL, et al. . Validation of neuro-QoL and PROMIS mental health patient reported outcome measures in persons with Huntington disease. J Huntingtons Dis 2019;8(4):467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paulsen JS, Langbehn DR, Stout JC, et al. . Detection of Huntington's disease decades before diagnosis: The Predict-HD study. J Neurology Neurosurg Psychiatry 2008;79(8):874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salsman JM, Victorson D, Choi SW, et al. . Development and validation of the positive affect and well-being scale for the neurology quality of life (Neuro-QOL) measurement system. Qual Life Res 2013;22(9):2569–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lai J-S, Goodnight S, Downing NR, et al. . Evaluating cognition in individuals with Huntington disease: Neuro-QoL cognitive functioning measures. Qual Life Res 2018;27(3):811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carlozzi NE, Schilling SG, Lai J-S, et al. . HDQLIFE: The development of two new computer adaptive tests for use in Huntington disease, Speech Difficulties, and Swallowing Difficulties. Qual Life Res 2016;25(10):2417–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ho AK, Gilbert AS, Mason SL, et al. . Health-related quality of life in Huntington's disease: Which factors matter most? Movement Disord 2009;24(4):574–578. [DOI] [PubMed] [Google Scholar]
- 23. Mestre TA, Carlozzi NE, Ho AK, et al. . Quality of life in Huntington's disease: Critique and recommendations for measures assessing patient health-related quality of life and caregiver quality of life. Movement Disord 2018;33(5):742–749. [DOI] [PubMed] [Google Scholar]
- 24. Unified Huntington's disease rating scale: Reliability and consistency. Movement Disord 1996;11(2):136–142. [DOI] [PubMed] [Google Scholar]
- 25. Breitbart W, Pessin H, Rosenfeld B, et al. . Individual meaning-centered psychotherapy for the treatment of psychological and existential distress: A randomized controlled trial in patients with advanced cancer. Cancer 2018;124(15):3231–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Breitbart W, Poppito S, Rosenfeld B, et al. . Pilot randomized controlled trial of individual meaning-centered psychotherapy for patients with advanced cancer. J Clin Oncol 2012;30(12):1304–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Breitbart W, Rosenfeld B, Gibson C, et al. . Meaning-centered group psychotherapy for patients with advanced cancer: A pilot randomized controlled trial. Psycho Oncol 2010;19(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Statistical Soc Ser B Methodol 1995;57(1):289–300. [Google Scholar]
- 29. Rodin G, Lo C, Rydall A, et al. . Managing cancer and living meaningfully (CALM): A randomized controlled trial of a psychological intervention for patients with advanced cancer. J Clin Oncol 2018;36(23):JCO..2017.77.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grossman CH, Brooker J, Michael N, et al. . Death anxiety interventions in patients with advanced cancer: A systematic review. Palliative Med 2018;32(1):172–184. [DOI] [PubMed] [Google Scholar]
- 31. Rodin G, Lo C, Mikulincer M, et al. . Pathways to distress: The multiple determinants of depression, hopelessness, and the desire for hastened death in metastatic cancer patients. Soc Sci Med 2009;68(3):562–569. [DOI] [PubMed] [Google Scholar]
- 32. Breitbart W, Rosenfeld B, Gibson C, et al. . Impact of treatment for depression on desire for hastened death in patients with advanced AIDS. Psychosomatics 2010;51(2):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McClain CS, Rosenfeld B, Breitbart W. Effect of spiritual well-being on end-of-life despair in terminally-ill cancer patients. Lancet 2003;361(9369):1603–1607. [DOI] [PubMed] [Google Scholar]
- 34. O'Mahony S, Goulet J, Kornblith A, et al. . Desire for hastened death, cancer pain and depression: Report of a longitudinal observational study. J Pain Symptom Manag 2005;29(5):446–457. [DOI] [PubMed] [Google Scholar]
- 35. Breitbart W, Rosenfeld B, Pessin H, et al. . Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA 2000;284(22):2907–2911. [DOI] [PubMed] [Google Scholar]
- 36. Rosenfeld B, Cham H, Pessin H, et al. . Why is Meaning-Centered Group Psychotherapy (MCGP) effective? Enhanced sense of meaning as the mechanism of change for advanced cancer patients. Psycho Oncol 2018;27(2):654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edmondson D, Park CL, Blank TO, et al. . Deconstructing spiritual well-being: Existential well-being and HRQOL in cancer survivors. Psycho Oncol 2008;17(2):161–169. [DOI] [PubMed] [Google Scholar]
- 38. Sokol LL, Lum HD, Creutzfeldt CJ, et al. . Meaning and dignity therapies for psychoneurology in neuropalliative care: A vision for the future. J Palliat Med 2020;23(9):1155–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Onken LS, Carroll KM, Shoham V, et al. . Reenvisioning clinical science. Clin Psychol Sci 2013;2(1):22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sokol LL, Jordan SR, Applebaum AJ, et al. . Social media perceptions of legacy-making: A qualitative analysis. Palliat Med Rep 2020;1(1):326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.