Abstract
Background
The effects of body mass index (BMI) on mortality of sepsis remain unknown, since previous meta-analyses have reported conflicting results. Several observational studies published recently have provided new evidence. Thus, we performed this updated meta-analysis.
Methods
PubMed, Embase, Web of Science, and Cochran Library were searched for articles published before February 10, 2023. Observational studies that assessed the association of BMIs with mortality of sepsis patients aged > 18 years were selected. We excluded studies of which data were unavailable for quantitative synthesis. Odds ratios (OR) with 95% confidence interval (CI) were the effect measure, which were combined using fixed-effect or random-effect models. The Newcastle–Ottawa Scale was applied for quality assessment. Subgroups analyses were conducted according to potential confounders.
Results
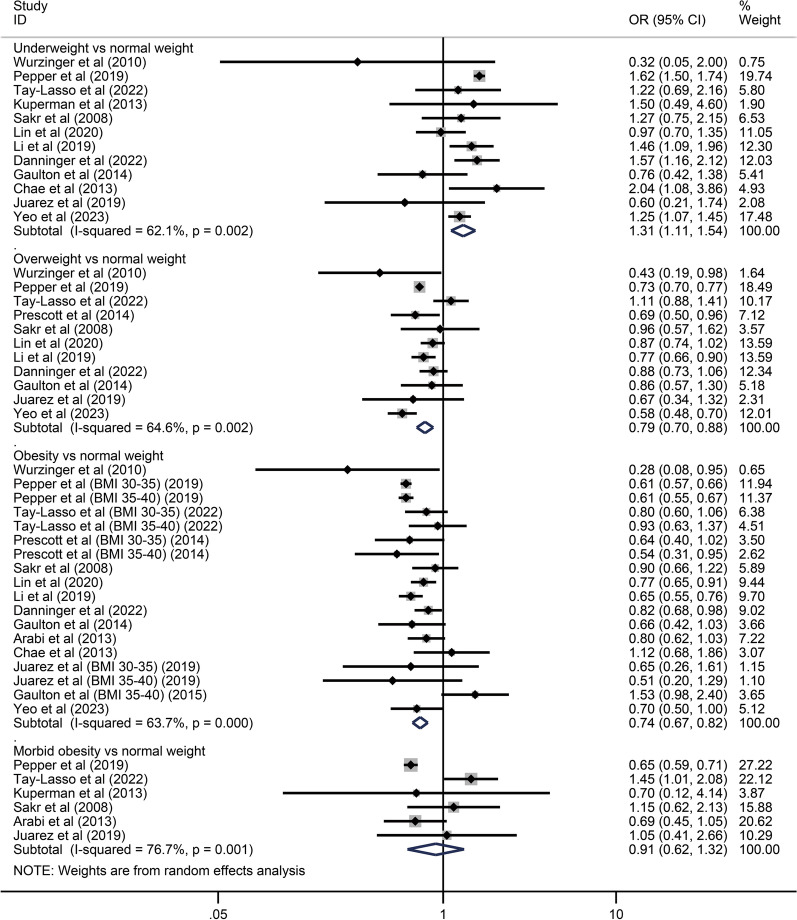
Fifteen studies (105,159 patients) were included in the overall analysis, which indicated that overweight and obese BMIs were associated with lower mortality (OR: 0.79, 95% CI 0.70–0.88 and OR: 0.74, 95% CI 0.67–0.82, respectively). The association was not significant in patients aged ≤ 50 years (OR: 0.89, 95% CI 0.68–1.14 and OR: 0.77, 95% CI 0.50–1.18, respectively). In addition, the relationship between morbidly obesity and mortality was not significant (OR: 0.91, 95% CI 0.62–1.32).
Conclusions
Overweight and obese BMIs (25.0–39.9 kg/m2) are associated with reduced mortality of patients with sepsis or septic shock, although such survival advantage was not found in all crowds.
Trial registration The protocol of this study was registered in PROSPERO (registration number CRD42023399559).
Supplementary Information
The online version contains supplementary material available at 10.1186/s40560-023-00677-0.
Keywords: Sepsis, Mortality, Body mass index, Obesity, Meta-analysis
Background
Sepsis is characterized clinically by life-threatening organ dysfunction, resulting from a dysregulated host response to severe systemic infection [1]. In 2017, the sepsis-related deaths were estimated 11.0 million, accounting for approximately 20% of global deaths, and it has become the leading cause of in-hospital mortality [2].
Obesity was reported to be associated with increased risk of sepsis [3], and more than one-quarter of adults admitted to intensive care units (ICU) have obese or overweight body mass indexes (BMI). In animal models, it was demonstrated that obesity could enhance sepsis-induced organ injury [4–6]. Thus, it is reasonable to speculate that obese BMI could worsen clinical outcomes. Surprisingly, clinical studies have reported mixed results. A systematic review revealed that obesity may decrease, increase, or not affect the survival [7]. Based on this, Pepper et al. undertook a meta-analysis and found that overweight and obese patients with sepsis were at a decreased risk of death [8]. Nevertheless, another meta-analysis conducted by Wang et al. suggested that overweight, rather than obesity or morbid obesity could reduce the mortality of patients with sepsis [9].
Consecutive meta-analyses and systematic reviews have failed to reach a consensus. An obvious limitation of these previously published studies is that evidence from prospective studies was insufficient. Moreover, it was discovered that the association between obesity and outcomes of interest could be confounded [10]. However, bias analyses were not performed in prior meta-analyses due to insufficient included studies, and the issues remain unsolved till now. Therefore, we conducted an updated meta-analysis, including current available studies, to summarize the latest and most comprehensive evidence. We also performed subgroup analyses according to possible biases, intending to better clarify the association between obesity and mortality of sepsis.
Methods
We undertook and reported the meta-analysis and systematic review in accordance with Meta-analysis of Observational Studies in Epidemiology (MOOSE) proposal [11]. Please see the MOOSE checklist in Additional file 1.
Two researchers (Le Bai and Jingyi Huang) were in charge of literature retrieval, data extraction, and quality assessment of eligible studies independently. A third researcher (Dan Wang) would be consulted if the disputation could not be resolved by discussion.
Literature search and study selection
We performed the literature search without any language restriction in the following electronic databases: PubMed, Embase, Cochrane Library, and Web of Science. Articles published before February 10, 2023 were retrieved. Please see the detailed search strategy in Additional file 2.
Observational studies that evaluated the association between obesity and mortality of patients diagnosed with sepsis or septic shock were included. The outcomes should be short-term (˂ 30 days) mortality. BMI was utilized as the measure of obesity in our study, which was calculated as the following formula: BMI = weight (kg)/height × height (m2). The control groups were patients with normal BMIs (18.5–24.9 kg/m2) while the exposure groups consisted of patients with abnormal BMIs. The following studies were excluded: (1) studies that recruited patients aged ˂ 18 years; (2) studies with incomplete data for the systematic review and meta-analysis; (3) studies that did not select patients with normal weight as control groups; (4) studies in which BMI was considered as a continuous variable rather than a categorical variable; (5) studies published in the forms of letter, comment or conference abstract. In addition, we selected odds ratio (OR) with 95% confidence interval (CI) as the effect measure, since most individual studies have reported ORs. Thus, studies that reported relative risk (RR) or hazard ratio (HR) were not included in the quantitative synthesis in case of potential bias.
Data extraction and risk of bias assessment
Extracted data included author, study design, country or region, publication year, population characteristics of included study, diagnostic criteria of sepsis (or septic shock), definition of exposure and control groups, outcome, and adjusted covariates in each study.
The Newcastle–Ottawa Scale (NOS) was employed for quality evaluation of eligible studies [12]. The details of the scale and standards of grading are provided in Additional file 3. It was designed to evaluate the quality of non-randomized controlled trials and has been the most widely used tool for observational studies in systematic reviews [13]. The scale consisted of three parts, which were aimed to evaluated the risk of selection bias (comparability between control and exposure groups), information bias (ascertainment of exposure and outcome), and confounding bias.
Several other factors (e.g., age, study design) were also reported to be clinically relevant to the mortality of sepsis [8, 10], which could confound the real effects of obesity on mortality of sepsis. These issues have not been addressed in previous meta-analyses [8, 9]. Thus, in the present meta-analysis, subgroup analyses were undertaken to further investigate whether obesity and sepsis are truly associated. In addition, publication bias was evaluated using the funnel plot.
Data synthesis and statistical analysis
In the quantitative synthesis, comparisons were made between patients with normal BMIs versus those with abnormal BMIs. OR with 95% CI was selected as the effect measure. Forest plots were employed to show the results for effects of obesity on mortality of sepsis. I-squared (I2) statistics was used for assessment of the heterogeneity. Random-effect model was selected if the heterogeneity was high (I2 > 50%). Otherwise, the fixed-effect model would be selected. We conducted all statistical analyses using Stata 15.0 (Stata Corp, College Station, Texas, USA).
Results
Study selection and characteristics of eligible studies
A total of 9633 records were yielded from all databases, according to the initial retrieval. After removing the duplicates, we screened titles and abstracts of rest literature, and obtained 32 potentially eligible articles. We then reviewed the full text and included 15 studies in the quantitative synthesis. The process of study selection is presented in Fig. 1.
Fig. 1.
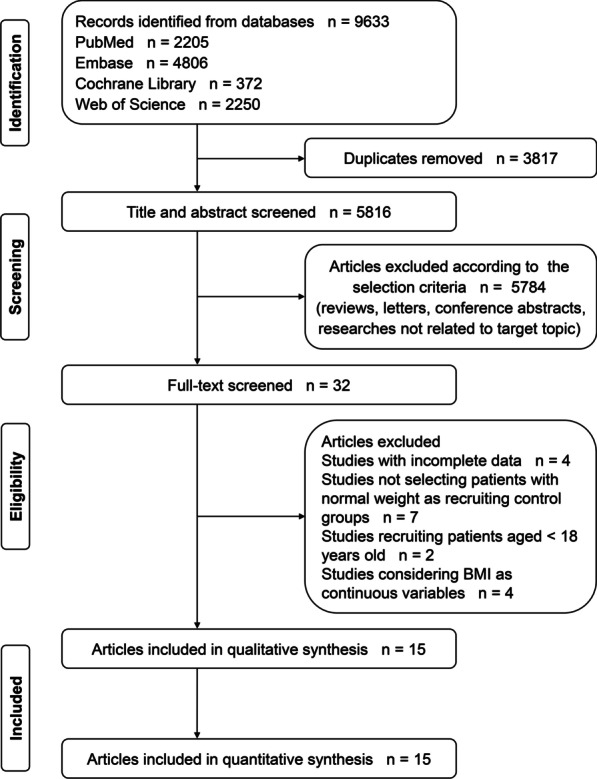
The flow chart of study selection
Three prospective studies [14–16] and 12 retrospective studies [17–28] were included in the present meta-analysis. Ten studies were from the United States [17–26], two were from Korea [14, 15], one was from Austria [28], and the other two were multicenter studies [16, 27]. These studies were published between 2008 and 2023. The subjects were patients with sepsis, severe sepsis or septic shock, and the sample size ranged from 301 to 55,038. In most studies (12 of all) [14–17, 19, 20, 22–25, 27, 28], the diagnoses were based on the criteria defined on the three international conferences (i.e., Sepsis 1.0, Sepsis 2.0, and Sepsis 3.0 criteria) [1, 29, 30]. In two studies [18, 21], sepsis and septic shock were diagnosed according to the diagnostic codes in databases, and the diagnostic criteria were not available in one study[26]. The outcomes included ICU, in-hospital, and 28-day (or 30-day) mortality. In addition, multiple covariates were adjusted in each study and the details are summarized in Table 1.
Table 1.
Characteristics of eligible studies
| Author | Study design | Publish year | Region | Age | Study population | Sample size | Diagnosis of sepsis | BMI category, kg/m2 | Outcome | Adjusted covariates |
|---|---|---|---|---|---|---|---|---|---|---|
| Wurzinger et al. [28] | Retrospective cohort study | 2010 | Austria | ≥ 18 years | Patients with septic shock | 301 | Sepsis 2.0 | Underweight (< 18.5); normal weight (18.5–24.9, ref); overweight (25.0–29.9); obesity (≥ 30.0) | ICU mortality | Admission year, sex, age, presence of heart disease or chronic renal insufficiency, the number of pre-morbidities, origin of sepsis and the simplified acute physiology score II |
| Pepper et al. [20] | Retrospective cohort study | 2019 | United States | ≥ 20 years | Patients with sepsis | 55,038 | Sepsis 3.0 | Underweight (< 18.5); normal weight (18.5–24.9, ref); overweight (25.0–29.9); obesity Class-I (30.0–34.9); obesity Class-II (35.0–39.9); obesity Class-III (≥ 40.0) | Short-term mortality | Variables which are considered clinically relevant (details are NA) |
| Tay-Lasso et al. [17] | Retrospective cohort study | 2022 | United States | ≥ 18 years | Patients with severe sepsis after trauma | 1,246 | Sepsis 2.0 (sepsis + organ dysfunction, hypotension, or hypoperfusion to 1 or more organs) | Underweight (< 18.5); normal weight (18.5–24.9, ref); overweight (25.0–29.9); obesity (30.0–34.9); severe obesity (35.0–39.9); morbid obesity (≥ 40.0) | In-hospital mortality | Age, alcohol use, hypertension, congestive heart failure, diabetes mellitus, injury severity score, respiratory rate, pulse rate, systolic blood pressure, intensive care unit days, Glasgow coma scale |
| Kuperman et al. [26] | Retrospective cohort study | 2013 | United States | ≥ 18 years | Patients with sepsis | 792 | NA | Underweight (< 18.5); normal weight (18.5–24.9, ref); overweight (25.0–29.9); obesity (30.0–39.9); morbid obesity (40.0–49.9) | In-hospital mortality | Age, race, gender, length of stay, comorbidities, APACHE II score |
| Prescott et al. [24] | Retrospective cohort study | 2014 | United States | Older patients (> 50 years) | Patients with severe sepsis | 1,404 | Sepsis 2.0 | Normal weight (18.5–24.9, ref); overweight (25.0–29.9); obesity (30.0–34.9); morbid obesity (≥ 40.0) | In-hospital, 90-day, and 1-year mortality | Age, race, gender, marital status, wealth, acute organ dysfunctions, mechanical ventilation, comorbidities, baseline cognitive status, functional limitations |
| Sakr et al. [16] | Prospective cohort study | 2008 | European multicenter | ≥ 15 years | Patients with sepsis | 2,878 | Sepsis 1.0 | Underweight (< 18.5); normal weight (18.5–24.9, ref); overweight (25.0–29.9); obesity (30.0–39.9); morbid obesity (≥ 40.0) | In-hospital, 60-day mortality | Age, gender, comorbidities, SAPS II score and SOFA score, the type of admission, mechanical ventilation, renal replacement therapy |
| Lin et al. [19] | Retrospective cohort study | 2020 | United States | ≥ 18 years | Patients with sepsis | 7,967 | Sepsis 3.0 | Underweight (< 18.5); normal weight (18.5–24.9, ref); overweight (25.0–29.9); obesity (≥ 30.0) | 28-day mortality | Age, sex, SOFA, mechanical ventilation, renal replacement therapy, comorbidities, alcohol abuse, drug abuse, depression |
| Danninger et al. [18] | Retrospective cohort study | 2022 | United States | NA | Patients with sepsis | 16,612 | Diagnostic code in the eICU Collaborative Research database | Underweight (< 18.5); normal weight (18.5–24.9, ref); overweight (25.0–29.9); obesity (≥ 30.0) | ICU mortality | Age, sex, creatinine concentration, ethnics, heart rate, infection focus, lactate concentration, SOFA score, mechanical ventilation, vasopressor use |
| Gaulton et al. [23] | Retrospective cohort study | 2014 | United States | ≥ 18 years | Patients with severe sepsis | 1,191 | Sepsis 2.0 | Underweight (< 18.5); normal weight (18.5–24.9, ref); overweight (25.0–29.9); obesity (30.0–39.9); morbid obesity (≥ 40.0) | 28-day mortality | Age, APACHE II, intubation, oncology service, initiation of early goal directed therapy, creatinine clearance |
| Gaulton et al. [25] | Retrospective cohort study | 2015 | United States | ≥ 18 years | Patients with sepsis | 1,779 | Similar to sepsis 1.0* | Non-obesity (18.5–29.9); obesity (≥ 30.0) | 28-day mortality | Age, gender, race, year and hospital of admission, length of stay, drug use, positive blood cultures, severity, comorbidities |
| Arabi et al. [27] | Retrospective cohort study | 2013 | Multicenter | Adult patients | Patients with septic shock | 2,882 | Sepsis 1.0 | Underweight (< 18.5); normal weight (18.5–24.9, ref); overweight (25.0–29.9); obesity (30.0–39.9); morbid obesity (≥ 40.0) | In-hospital mortality | Age, gender, mechanical ventilation, APACHE II score, chronic co-morbidities, infections, creatinine clearance |
| Chae et al. [15] | Prospective cohort study | 2013 | Korea | ≥ 18 years | Patients with severe sepsis or septic shock | 770 | Sepsis 1.0 | Underweight (< 18.5); normal weight (18.5–24.9, ref); obese (≥ 25.0) | In-hospital mortality | Age, gender, comorbidities, infection focus, SOFA score, serum lactate |
| Li et al. [21] | Retrospective cohort study | 2019 | United States | ≥ 18 years | Patients with sepsis, severe sepsis or septic shock | 5,563 | Diagnostic code in the MIMIC-III database | Underweight (< 18.5); normal weight (18.5–24.9, ref); overweight (25.0–29.9); obesity (≥ 30.0) | 30-day, and 1-year mortality | Age, gender, race, marriage status, admission ICU type, MAP, SAPS II, SOFA, surgery |
| Juarez et al. [22] | Retrospective cohort study | 2019 | United States | 18–89 years | Patients with sepsis or septic shock | 312 | Sepsis 3.0 | Underweight (< 18.5); normal weight (18.5–24.9, ref); overweight (25.0–29.9); obesity Class-I (30.0–34.9); obesity Class-II (35.0–39.9); obesity Class-III (≥ 40.0) | In-hospital mortality | Age, gender, comorbidities, APACHE II |
| Yeo et al. [14] | Prospective cohort study | 2023 | Korea | ≥ 18 years | Patients with sepsis | 6424 | Sepsis 3.0 | Underweight (< 18.5); normal weight (18.5–24.9, ref); overweight (25.0–29.9); obesity (≥ 30.0) | In-hospital mortality | Age, sex, comorbidities, SOFA score, presence of septic shock, site of infection, type of infection |
BMI body mass index, Ref reference, NA not available, APACHE II acute physiology and chronic health evaluation II, SOFA sequential organ failure assessment, MAP mean arterial pressure, SAPS II simplified acute physiology score II
*About 94.5% positive predictive value for sepsis 1.0
Risk of bias assessment
According to the NOS scale, all studies are considered as high quality. Details are provided in Additional file 4. We also focused on the potential confounders as follows: (1) age; Abbate et al. found that the association between obesity and mortality of sepsis was significant in patients aged 50–89 years but not in those aged 20–49 years [10]; (2) study design; retrospective cohort studies are easily subject to bias (e.g., recall bias, data integrity) and prospective studies could better clarify the relationship between obesity and outcomes of interest; (3) diagnostic criteria of sepsis; the specificity and sensitivity of different criteria may be different; (4) severity of sepsis; concerns about hypoventilation and insufficient care in obese patients on general wards may lead to ICU admission of patients with mild infection [8].
Meta-analysis and bias analysis
Fifteen studies [14–28] containing 105,159 patients were included in the meta-analysis. The reference groups were patients with normal BMIs (18.5–24.9 kg/m2) while the exposure groups were patients with underweight (< 18.5 kg/m2), overweight (25.0–29.9 kg/m2), obese (30.0–39.9 kg/m2), and morbidly obese BMIs (≥ 40.0 kg/m2).
The primary analysis indicated that overweight and obesity were associated with decreased mortality (OR: 0.79, 95% CI 0.70–0.88 and OR: 0.74, 95% CI 0.67–0.82, respectively) while underweight BMIs were associated with increased mortality (OR: 1.31, 95% CI 1.11–1.54). The association between morbid obesity and mortality was not significant (OR: 0.91, 95% CI 0.62–1.32). Please see Fig. 2.
Fig. 2.
Individual and pooled results of the association of underweight, overweight, obese, and morbidly obese BMIs with mortality in patients with sepsis
We then conducted subgroup analyses for patients with underweight, overweight and obese BMIs, according to age, study design, diagnosis and severity of sepsis. Since only six studies [16, 17, 20, 22, 26, 27] reported results including a measure between morbid obesity and mortality, secondary analyses for these patients were not performed. Results of subgroup analyses are displayed in Table 2. In patients aged > 50 years, underweight BMIs were associated with higher mortality (OR: 1.58, 95% CI 1.46–1.71) while overweight and obese BMIs were associated with lower mortality (OR: 0.77, 95% CI 0.67–0.89 and OR: 0.62, 95% CI 0.59–0.65, respectively). In patients aged ≤ 50 years, underweight BMIs were associated with higher mortality (OR: 1.72, 95% CI 1.35–2.19) while the association of overweight and obese BMIs with mortality was not significant (OR: 0.89, 95% CI 0.68–1.14 and OR: 0.77, 95% CI 0.50–1.18, respectively). Please see Additional file 5: Fig. S1A–F. In retrospective studies [17–28], underweight BMIs were associated with increased mortality (OR: 1.26, 95% CI 1.01–1.56) while overweight and obese BMIs were associated with decreased mortality (OR: 0.81, 95% CI 0.73–0.91 and OR: 0.72, 95% CI 0.64–0.80, respectively). In prospective studies [14–16], underweight BMIs were associated with higher mortality (OR: 1.28, 95% CI 1.11–1.47) while overweight and obese BMIs had no impact on mortality (OR: 0.70, 95% CI 0.43–1.13 and OR: 0.85, 95% CI 0.69–1.05, respectively). Please see Additional file 5: Fig. S2A–F. In studies where the diagnoses of sepsis were based on Sepsis 1.0 or 2.0 criteria [15–17, 23–25, 27, 28], underweight and overweight BMIs were not associated with mortality (OR: 1.19, 95% CI 0.89–1.58 and OR: 0.84, 95% CI 0.65–1.10, respectively) while obese BMIs were related to decreased mortality (OR: 0.83, 95% CI 0.74–0.94). In studies where sepsis was diagnosed according to Sepsis 3.0 criteria [14, 19, 20, 22], underweight did not influence the mortality (OR: 1.24, 95% CI 0.95–1.62) while overweight and obese BMIs were associated with lower mortality (OR: 0.72, 95% CI 0.62–0.84 and OR: 0.63, 95% CI 0.59–0.66, respectively). Please see Additional file 5: Fig. S3A–F. In patients with sepsis, underweight BMIs were associated with increased mortality (OR: 1.34, 95% CI 1.13–1.59) while overweight and obese BMIs were associated with decreased mortality (OR: 0.77, 95% CI 0.69–0.86 and OR: 0.72, 95% CI 0.64–0.82, respectively). In patients with severe sepsis or septic shock, underweight and overweight BMIs were not related to mortality (OR: 1.17, 95% CI 0.84–1.61 and OR: 0.80, 95% CI 0.61–1.05, respectively) while obese BMIs were associated with decreased mortality (OR: 0.79, 95% CI 0.69–0.91). Please see Additional file 5: Fig. S4A–F.
Table 2.
Subgroup analyses according to age, study design, diagnosis and severity of sepsis
| Underweight | Overweight | Obesity | |
|---|---|---|---|
| Age | |||
| > 50 years | 1.58 (1.46–1.71) | 0.77 (0.67–0.89) | 0.62 (0.59–0.65) |
| ≤ 50 years | 1.72 (1.35–2.19) | 0.89 (0.68–1.14) | 0.77 (0.50–1.18) |
| Study design | |||
| Retrospective study | 1.26 (1.01–1.56) | 0.81 (0.73–0.91) | 0.72 (0.64–0.80) |
| Prospective study | 1.28 (1.11–1.47) | 0.70 (0.43–1.13) | 0.85 (0.69–1.05) |
| Diagnostic criteria | |||
| Sepsis 1.0 or 2.0 | 1.19 (0.89–1.58) | 0.84 (0.65–1.10) | 0.83 (0.74–0.94) |
| Sepsis 3.0 | 1.24 (0.95–1.62) | 0.72 (0.62–0.84) | 0.63 (0.59–0.66) |
| Severity | |||
| Patients with sepsis | 1.34 (1.13–1.59) | 0.77 (0.69–0.86) | 0.72 (0.64–0.82) |
| Patients with severe sepsis or septic shock | 1.17 (0.84–1.61) | 0.80 (0.61–1.05) | 0.79 (0.69–0.91) |
In addition, the funnel plot for obese BMI groups indicated significant publication bias. Please see Fig. 3.
Fig. 3.
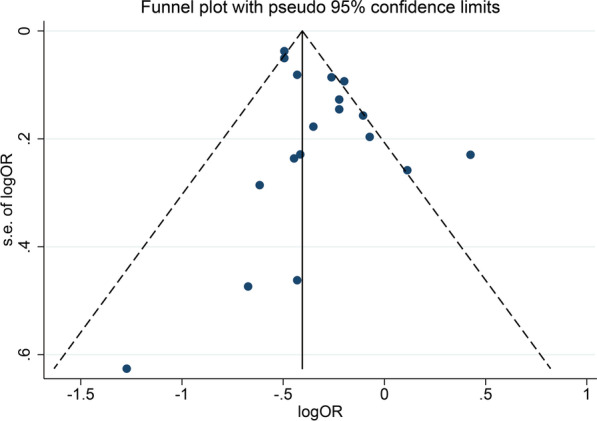
The funnel plot of the publication bias assessment for obese BMI (30.0–39.9 kg/m2) groups. BMI body mass index
Discussion
This is an updated meta-analysis, which was designed to evaluate the effects of BMI on mortality of sepsis. Consistent with Pepper et al.’s meta-analysis [8], the present one indicated that overweight and obese BMIs were associated with decreased mortality. However, we included more studies and the results were thereby more convincing with improved statistical power. On the other hand, stricter standards were adopted for study selection in our meta-analysis to control bias. In previous analyses, outcomes including in-hospital, 28-day and 60-day mortality were pooled. In comparison, we only included studies that reported short-term mortality (˂ 30 days). Meanwhile, we excluded studies that used HR as the effect measure (these studies were included in previous pooled analyses), given that HR involves a time factor which could be the possible source of bias.
Concerns were raised that significant reduction of mortality may result from methodology in studies rather than increased BMIs themselves [8]. Similarly, Robinson et al. [31] emphasized the importance of stratifying risk factors and controlling for covariates, which are conducive to describe the phenotype of sepsis survivors. Thereby, in the present meta-analysis, subgroup analyses were performed in accordance to potential confounders. Abbate et al. [10] discovered that age may modify the association of BMIs with mortality. We obtained the similar conclusion which suggested that only patients aged over 50 years could benefit from higher BMIs. Pepper et al. [8] also raised concerns about ICU admission of obese and overweight patients with mild infection, which could cause selection bias. Accordingly, we conducted secondary analyses for those diagnosed with severe sepsis or septic shock. Although overweight BMIs did not decrease the risk of death, these patients could still benefit from obese BMIs.
Pepper et al.’s [8] pooled analysis of three studies suggested that underweight had no impact on the mortality of sepsis. Our meta-analysis included 12 studies and found that underweight patients, compared with patients with normal BMIs, were at higher risk of death. Similarly, several cohort studies suggested that underweight BMIs was associated with increased mortality, compared with non-underweight BMIs [32–34]. In addition to protective effects of higher BMIs, possible pathophysiological reasons include poor nutritional status, persistent inflammation and catabolism syndrome [35]. It should be noted that the association between underweight BMIs and mortality was not significant in studies, where diagnoses of sepsis were based on the consensus reached in the several international conferences (Sepsis 3.0 or Sepsis 1.0/2.0) [1, 29, 30]. The association between overweight BMIs and mortality are subject to different diagnosis criteria as well. As yet it is unclear whether the diagnosis of sepsis confounded the association of BMI with mortality. However, specificity and sensitivity of other criteria were inferior to those of consensus criteria, and early and rapid identification of sepsis could help to improve clinical outcomes [36]. Therefore, the diagnostic criteria which are not recognized should be avoided in future studies.
Two prospective, multicenter studies (Sakr et al. [16] and Yeo et al. [14]) were included in the present meta-analysis, but the results were inconsistent with each other. On one hand, Sakr et al.’s study [16] was conducted during 2002 while Yeo et al.’s study [14] was conducted between 2019 and 2020; the management of sepsis and critical care support in 2002 differed a lot from those nowadays, which could lead to different results. On the other hand, Sakr et al. recruited patients from 24 European countries while Yeo et al.’s study was conducted in multiple centers in Korea. Thus, racial difference may be a possible explanation. We noticed that another prospective single-center study (Chae et al. [15]) from Korea concluded that increased BMIs were not associated with mortality. However, different from Yeo et al.'s study, Chae et al. recruited patients with severe sepsis or septic shock (Yeo et al. recruited patients with sepsis). The association of BMI with mortality was subject to severity of sepsis, which could be a reason for the conflicting results of these two Korean studies.
In addition, there remain some limitations, and issues that need be addressed in future researches. First, whether it is reasonable that BMI is used as the only measure of obesity? Although it is calculated according to weight and height measured in ICU settings, biases are still inevitable, especially considering that patients suspected of sepsis may have received fluid resuscitation treatment before ICU admission. Thus, better measure of adiposity such as BMI combined with waist-to-hip ratio, computed tomography/magnetic resonance or bioelectrical impedance should be considered in future studies. Second, the mechanisms behind effects of obesity on mortality have not been fully understood. As summarized in previous systematic review, increased energy stores, beneficial immunoregulation, inactivation of harmful bacterial products and improved renin–angiotensin–aldosterone system function could play roles [8]. However, for the moment, these are not enough to explain the contradictory results of prospective studies. Third, frailty, which affects both weight and mortality, was not considered in most of included studies. It should be regarded as a confounder and be adjusted for in future studies. Finally, we found significant publication bias, which has never been evaluated in previous meta-analyses. This suggested that studies reporting positive results are easier to be published which may overestimate the effects of increased BMIs.
Conclusions
Our study indicated that overweight and obese BMIs (25.0–39.9 kg/m2) are associated with reduced mortality of patients with sepsis or septic shock, although such survival advantage was not found in all crowds (e.g., patients ≤ 50 years). Considering the protective association of BMI with clinical outcomes found in multiple critical diseases, it seems illogical to negate the discovery of the present meta-analysis. However, the possible BMI measurement bias should not be neglected, and inconsistent results of prospective studies need more reasonable explanations as well. Whether specific factors or potential biases influence the association of BMI with sepsis should be further clarified.
Supplementary Information
Additional file 1. MOOSE checklist for meta-analyses of observational studies.
Additional file 2. Search strategies for all databases.
Additional file 3. Newcastle-Ottawa Quality Assessment Scale.
Additional file 4. The Newcastle-Ottawa Quality Assessment Scale of Included Case-Control or Cohort Studies.
Additional file 5. Supplementary figures.
Acknowledgements
Not applicable.
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- HR
Hazard ratio
- ICU
Intensive care units
- I2
I-squared
- NOS
The Newcastle–Ottawa Scale
- OR
Odds ratio
- MOOSE
Meta-analysis of Observational Studies in Epidemiology
- RR
Relative risk
Author contributions
XZ and YX were responsible for the whole study including the design, implementation, and quality control. LB, JH and DW contributed to literature search, data extraction and risk of bias assessment. QZ and TL performed data analyses. LB and DZ were in charge of manuscript writing. All authors have read and approved the final manuscript for submission.
Funding
None.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [14–28].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Le Bai, Jingyi Huang and Dan Wang contributed equally to this work.
Contributor Information
Xianmei Zhou, Email: zhouxianmeijsszyy@163.com.
Yong Xu, Email: njzyyxuyong@163.com.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet (London, England) 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang HE, Griffin R, Judd S, Shapiro NI, Safford MM. Obesity and risk of sepsis: a population-based cohort study. Obesity (Silver Spring, Md) 2013;21(12):E762–769. doi: 10.1002/oby.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan JM, Nowell M, Lahni P, Shen H, Shanmukhappa SK, Zingarelli B. Obesity enhances sepsis-induced liver inflammation and injury in mice. Obesity (Silver Spring, Md) 2016;24(7):1480–1488. doi: 10.1002/oby.21504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vachharajani V, Russell JM, Scott KL, Conrad S, Stokes KY, Tallam L, Hall J, Granger DN. Obesity exacerbates sepsis-induced inflammation and microvascular dysfunction in mouse brain. Microcirculation. 2005;12(2):183–194. doi: 10.1080/10739680590904982. [DOI] [PubMed] [Google Scholar]
- 6.Petronilho F, Giustina AD, Nascimento DZ, Zarbato GF, Vieira AA, Florentino D, Danielski LG, Goldim MP, Rezin GT, Barichello T. Obesity exacerbates sepsis-induced oxidative damage in organs. Inflammation. 2016;39(6):2062–2071. doi: 10.1007/s10753-016-0444-x. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi V, Bavishi C, Jean R. Impact of obesity on sepsis mortality: a systematic review. J Crit Care. 2015;30(3):518–524. doi: 10.1016/j.jcrc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Pepper DJ, Sun J, Welsh J, Cui X, Suffredini AF, Eichacker PQ. Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: a systematic review and meta-analysis. Crit Care (London, England) 2016;20(1):181. doi: 10.1186/s13054-016-1360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Liu X, Chen Q, Liu C, Huang C, Fang X. The role of increased body mass index in outcomes of sepsis: a systematic review and meta-analysis. BMC Anesthesiol. 2017;17(1):118. doi: 10.1186/s12871-017-0405-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbate LM, Perman SM, Clambey ET, Van Pelt RE, Ginde AA. Age modifies the association between obesity and mortality in individuals hospitalized with severe sepsis. J Am Geriatr Soc. 2016;64(4):882–883. doi: 10.1111/jgs.14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 12.GA Wells BS, D O'Connell, J Peterson, V Welch, M Losos, P Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohrica/programs/clinical_epidemiology/oxfordasp.
- 13.Farrah K, Young K, Tunis MC, Zhao L. Risk of bias tools in systematic reviews of health interventions: an analysis of PROSPERO-registered protocols. Syst Rev. 2019;8(1):280. doi: 10.1186/s13643-019-1172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeo HJ, Kim TH, Jang JH, Jeon K, Oh DK, Park MH, Lim CM, Kim K, Cho WH. Obesity paradox and functional outcomes in sepsis: a multicenter prospective study. Crit Care Med. 2023;51(6):742–752. doi: 10.1097/CCM.0000000000005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chae MKCD, Shin TG, Jeon K, Suh GY, Sim MS, Song KJ, Jeong YK, Jo IJ. Body Mass index and outcomes in patients with severe sepsis or septic shock. Korean J Crit Care Med. 2013;28(4):266–271. doi: 10.4266/kjccm.2013.28.4.266. [DOI] [Google Scholar]
- 16.Sakr Y, Madl C, Filipescu D, Moreno R, Groeneveld J, Artigas A, Reinhart K, Vincent JL. Obesity is associated with increased morbidity but not mortality in critically ill patients. Intensive Care Med. 2008;34(11):1999–2009. doi: 10.1007/s00134-008-1243-0. [DOI] [PubMed] [Google Scholar]
- 17.Tay-Lasso E, Grigorian A, Lekawa M, Dolich M, Schubl S, Barrios C, Nguyen N, Nahmias J. Obesity does not increase risk for mortality in severe sepsis trauma patients. Am Surg. 2022:31348221078986. [DOI] [PubMed]
- 18.Danninger T, Rezar R, Mamandipoor B, Dankl D, Koköfer A, Jung C, Wernly B, Osmani V. Underweight but not overweight is associated with excess mortality in septic ICU patients. Wien Klin Wochenschr. 2022;134(3–4):139–147. doi: 10.1007/s00508-021-01912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin S, Ge S, He W, Zeng M. Association between body mass index and short-term clinical outcomes in critically ill patients with sepsis: a real-world study. Biomed Res Int. 2020;2020:5781913. doi: 10.1155/2020/5781913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pepper DJ, Demirkale CY, Sun J, Rhee C, Fram D, Eichacker P, Klompas M, Suffredini AF, Kadri SS. Does obesity protect against death in sepsis? A retrospective cohort study of 55,038 adult patients. Crit Care Med. 2019;47(5):643–650. doi: 10.1097/CCM.0000000000003692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Hu X, Xu J, Huang F, Guo Z, Tong L, Lui KY, Cao L, Zhu Y, Yao J, et al. Increased body mass index linked to greater short- and long-term survival in sepsis patients: a retrospective analysis of a large clinical database. Int J Infect Dis. 2019;87:109–116. doi: 10.1016/j.ijid.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Juarez EEH, Lear M, Sanchez A, Yang S, Nugent K. The association between body mass index and outcomes in patient with sepsis and acute respiratory failure. Southwest Respir Crit Care Chronicles. 2019;7(31):13–23. doi: 10.12746/swrccc.v7i31.615. [DOI] [Google Scholar]
- 23.Gaulton TG, Marshall MacNabb C, Mikkelsen ME, Agarwal AK, Cham Sante S, Shah CV, Gaieski DF. A retrospective cohort study examining the association between body mass index and mortality in severe sepsis. Intern Emerg Med. 2015;10(4):471–479. doi: 10.1007/s11739-015-1200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prescott HC, Chang VW, O'Brien JM, Jr, Langa KM, Iwashyna TJ. Obesity and 1-year outcomes in older Americans with severe sepsis. Crit Care Med. 2014;42(8):1766–1774. doi: 10.1097/CCM.0000000000000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaulton TG, Weiner MG, Morales KH, Gaieski DF, Mehta J, Lautenbach E. The effect of obesity on clinical outcomes in presumed sepsis: a retrospective cohort study. Intern Emerg Med. 2014;9(2):213–221. doi: 10.1007/s11739-013-1002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuperman EF, Showalter JW, Lehman EB, Leib AE, Kraschnewski JL. The impact of obesity on sepsis mortality: a retrospective review. BMC Infect Dis. 2013;13:377. doi: 10.1186/1471-2334-13-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arabi YM, Dara SI, Tamim HM, Rishu AH, Bouchama A, Khedr MK, Feinstein D, Parrillo JE, Wood KE, Keenan SP, et al. Clinical characteristics, sepsis interventions and outcomes in the obese patients with septic shock: an international multicenter cohort study. Crit Care (London, England) 2013;17(2):R72. doi: 10.1186/cc12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wurzinger B, Dünser MW, Wohlmuth C, Deutinger MC, Ulmer H, Torgersen C, Schmittinger CA, Grander W, Hasibeder WR. The association between body-mass index and patient outcome in septic shock: a retrospective cohort study. Wien Klin Wochenschr. 2010;122(1–2):31–36. doi: 10.1007/s00508-009-1241-4. [DOI] [PubMed] [Google Scholar]
- 29.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 30.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 31.Robinson J, Swift-Scanlan T, Salyer J. Obesity and 1-year mortality in adults after sepsis: a systematic review. Biol Res Nurs. 2020;22(1):103–113. doi: 10.1177/1099800419876070. [DOI] [PubMed] [Google Scholar]
- 32.Roh J, Jo EJ, Eom JS, Mok J, Kim MH, Kim KU, Park HK, Lee MK, Yeom S, Lee K. Factors predicting long-term survival of patients with sepsis on arrival at the emergency department: a single-center, observational study. Medicine. 2019;98(33):e16871. doi: 10.1097/MD.0000000000016871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato T, Kudo D, Kushimoto S, Hasegawa M, Ito F, Yamanouchi S, Honda H, Andoh K, Furukawa H, Yamada Y, et al. Associations between low body mass index and mortality in patients with sepsis: a retrospective analysis of a cohort study in Japan. PLoS ONE. 2021;16(6):e0252955. doi: 10.1371/journal.pone.0252955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oami T, Karasawa S, Shimada T, Nakada TA, Abe T, Ogura H, Shiraishi A, Kushimoto S, Saitoh D, Fujishima S, et al. Association between low body mass index and increased 28-day mortality of severe sepsis in Japanese cohorts. Sci Rep. 2021;11(1):1615. doi: 10.1038/s41598-020-80284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim M, Watase T, Jablonowski KD, Gatewood MO, Henning DJ. A sepsis-related diagnosis impacts interventions and predicts outcomes for emergency patients with severe sepsis. West J Emerg Med. 2017;18(6):1098–1107. doi: 10.5811/westjem.2017.7.34770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. MOOSE checklist for meta-analyses of observational studies.
Additional file 2. Search strategies for all databases.
Additional file 3. Newcastle-Ottawa Quality Assessment Scale.
Additional file 4. The Newcastle-Ottawa Quality Assessment Scale of Included Case-Control or Cohort Studies.
Additional file 5. Supplementary figures.
Data Availability Statement
All data generated or analyzed during this study are included in this published article [14–28].