Abstract
Background:
when using post-transplant cyclophosphamide (PTCy) graft-versus-host-disease (GVHD) prophylaxis for lymphoma patients, it is currently unknown whether a matched unrelated donor (MUD) or a haploidentical related donor is preferable if both are available.
Objective:
In this study we wanted to test if using a haploidentical donor has the same results of a MUD.
Study design:
a total of 2140 adults (34% CIBMTR, 66% EBMT registry) aged ≥18 years who received their first haploidentical hematopoietic cell transplant (haplo-HCT) or MUD-HCT (8/8 match at HLA-loci A, B, C, and DRB1) for lymphoma using PTCy-based GVHD prophylaxis from 2010–2019 were retrospectively analyzed.
Results:
The majority of both MUD and haploidentical HCTs received reduced intensity/non-myeloablative conditioning (74% and 77%, respectively), used a peripheral blood stem cell graft (91% and 60%, respectively) and a three-drug GVHD prophylaxis (PTCy + calcineurin inhibitor + MMF in 54% and 90%, respectively). Haploidentical HCT has less favorable results versus MUD cohort in terms of overall mortality (HR=1.69, 95%CI=1.30–2.27, p<0.001), progression-free survival (HR=1.39, 95%CI=1.10 – 1.79, p=0.008), non-relapse mortality (HR=1.93, 95% CI=1.21 – 3.07, p=0.006), platelets engraftment (HR=0.69, 95%CI=0.59 – 0.80, p<0.001), acute grade 2–4 GVHD incidence (HR=1.65, 95%CI=1.28 – 2.14, p<0.001) and chronic GVHD (HR=1.79, 95%CI=1.30 – 2.48, p<0.001). No significant differences were observed in terms of relapse and neutrophil engraftment. Adjusting for propensity score yielded similar results.
Conclusion:
whenever MUD is available in a timely manner, it should be preferred over a haploidentical donor when using PTCy-based GVHD prophylaxis for patients with lymphoma.
Keywords: Lymphoma, haploidentical donor, matched unrelated donor, post-transplant cyclophosphamide
INTRODUCTION
The use of post-transplant cyclophosphamide (PTCy) based graft-versus-host-disease (GVHD) prophylaxis rapidly expanded due to its promising results in the setting of haploidentical hematopoietic cell transplantation (haplo-HCT) 1. Initial studies showed acceptable long-term survival and a strikingly low chronic GVHD incidence when using a reduced-intensity conditioning (RIC) and a marrow graft source for both myeloid and lymphoid malignancies.2–4. Considering the promising and consistent results obtained in the haploidentical setting, retrospective comparisons between haplo-HCT with PTCy and matched-unrelated donor (MUD) HCT with standard GVHD prophylaxis (calcineurin inhibitor [CNI] + mycophenolate [MMF] or methotrexate with or without antithymocyte globulin [ATG]) were made in the setting of both myeloid and lymphoid malignancies 5–8. Taking into consideration that international guidelines advise use of MUDs as the preferred donor type in the absence of an HLA-matched related donor 7, it is fundamental to understand the real impact of donor type when using PTCY-based GVHD prophylaxis. While there are ongoing prospective trials to identify the best GVHD prophylaxis in the HLA-matched setting (e.g., PROGRESS 3 trial, NCT03959241), it is difficult to conduct randomized trials based on donor type. Recently, Gooptu et al. performed a comparison between MUD and haploidentical donor transplantation, both using PTCy GVHD prophylaxis, for myeloid diseases 8. That study demonstrated a substantial survival benefit with MUDs for allogeneic HCT with reduced-intensity conditioning regimen. The aim of our study was to explore the same question in lymphomas.
MATERIALS and METHODS
Data sources
The study was performed through collaboration between the European Society for Blood and Marrow Transplantation (EBMT) and the Center for International Blood and Marrow Transplant Research (CIBMTR) as described elsewhere.9
Patients
Included in this analysis are adult (≥18 years) patients with Hodgkin (HL) or non-Hodgkin lymphoma (NHL) treated with haplo-HCT or MUD-HCT using PTCy-based GVHD prophylaxis between 2010 and 2019 and reported to either the CIBMTR or EBMT. Recipients of haplo-HCT were mismatched at 2 or more HLA loci, whereas MUD transplants were matched at the allele level at HLA-A, -B, -C, and -DRB1 (8/8). GVHD prophylaxis in both groups included PTCy-based regimens, most commonly in combination with CNI + MMF. Both peripheral blood and bone marrow grafts were included. Myeloablative (MAC) and non-myeloablative/reduced intensity conditioning (NMA/RIC) were included.
Definitions
The intensity of conditioning regimens was determined using consensus criteria.10 Response to last line of therapy before allo-HCT was defined as per Lugano criteria.11
Study Endpoints
The primary endpoint overall survival (OS) and secondary endpoints non-relapse mortality (NRM), progression/relapse, progression-free survival (PFS), neutrophil and platelet recovery, acute GVHD and chronic GVHD were calculated using standard criteria.12
Statistical analysis
The haplo-HCT cohort was compared against the MUD-HCT cohort. Probabilities of PFS and OS were calculated as described previously.13 Cumulative incidences of NRM, lymphoma progression/relapse and hematopoietic recovery were calculated to accommodate for competing risks.14 The primary analysis evaluated associations among patient-, disease-, and transplantation-related variables and outcomes of interest using Cox proportional hazards regression. Backward elimination was used to identify covariates associated with outcomes. Covariates with a p<0.05 were retained in the models. To adjust for association testing of multiple endpoints, a statistically significant difference was considered when p<0.01. The proportional hazards assumption for Cox regression was tested by adding a time-dependent covariate for each risk factor and each outcome. Covariates violating the proportional hazards assumption were adjusted via stratification in the Cox regression model. Interactions between the main effect and significant covariates were examined. Center effect was adjusted as clusters using GEE approach for all the endpoints.15 Relative risks were expressed as hazard ratios (HR). Variables considered in the multivariate analyses are shown in supplementary file 1 of the supplemental appendix. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Due to concerns about potential imbalance of significant risk factors between the haplo-HCT and MUD-HCT cohorts, a sensitivity analysis based on propensity score was also conducted (supplementary file 2). The propensity score was based on disease, disease stage, donor/recipient CMV status, HCT-CI, Karnofsky performance status, registry (EBMT vs. CIBMTR), patient age and donor age. Because the maximum unrelated donor age was 55 years old, these additional analyses, including calculation of propensity scores, excluded 260 patients receiving transplants from haploidentical donors older than 55 years old.
RESULTS
Baseline Characteristics
The baseline patient-, disease-, and transplantation-related characteristics are shown in Table 1. Lymphoma subtypes in haploidentical transplant recipients were 44% HL and 56% NHL; in MUD recipients they were 28% HL and 72% NHL (P<0.001). GVHD prophylaxis consisted of PTCy + CNI + MMF in 90% of the haploidentical group and 54% of the MUD group (P<0.001). The graft source was peripheral blood in 60% (n = 1089) of haplo-HCT and 91% (n = 283) of MUD transplants (P<0.001). The donor age was <40 in 51% (n=927) of haplo-HCT and 76% (n=236) of MUD (P<0.001). The maximum MUD age was 55 years.
Table 1.
Baseline characteristics of CIBMTR and EBMT cohorts of lymphoma patients undergoing haploidentical related donor or 8/8 matched unrelated donor transplantation
| Haploidentical, N (%) |
Matched unrelated, N (%) | ||
|---|---|---|---|
| Variable | N (%) | N (%) | p-valuea |
| Number of recipients | 1830 | 310 | |
| Number of centers | 277 | 103 | |
| Recipient age at transplant | <0.001 | ||
| 18–29 years | 429 (23) | 45 (15) | |
| 30–39 years | 316 (17) | 48 (15) | |
| 40–49 years | 298 (16) | 40 (13) | |
| 50–59 years | 443 (24) | 89 (29) | |
| 60 years and older | 344 (19) | 88 (28) | |
| Median (Range) | 46 (18–71) | 53 (19–71) | <0.001 |
| Sex | 0.07 | ||
| Male | 1169 (64) | 214 (69) | |
| Female | 660 (36) | 95 (31) | |
| missing | 1 (<1) | 1 (<1) | |
| Karnofsky performance score | 0.09 | ||
| 90–100 | 1282 (70) | 200 (65) | |
| 10–80 | 497 (27) | 103 (33) | |
| Missing | 51 (3) | 7 (2) | |
| HCT-CI | <0.001 | ||
| <=2 | 1050 (57) | 157 (51) | |
| >2 | 362 (20) | 94 (30) | |
| missing | 418 (23) | 59 (19) | |
| Lymphoma subtype | <0.001 | ||
| Follicular Lymphoma | 137 (7) | 31 (10) | |
| DLBCL | 447 (24) | 75 (24) | |
| Mantle cell Lymphoma | 178 (10) | 53 (17) | |
| Classical Hodgkin lymphoma | 805 (44) | 86 (28) | |
| T-cell lymphoma | 263 (14) | 65 (21) | |
| NHL Disease status prior to HCT | 0.54 | ||
| Complete remission | 535 (52) | 122 (54) | |
| Partial remission | 317 (31) | 61 (27) | |
| Chemoresistant | 173 (17) | 41 (18) | |
| HD Disease status prior to HCT | 0.07 | ||
| Complete remission | 397 (49) | 52 (60) | |
| Partial remission | 264 (33) | 18 (21) | |
| Chemoresistant | 144 (18) | 16 (19) | |
| Prior auto-HCT | 0.05 | ||
| No | 736 (40) | 143 (46) | |
| Yes | 1094 (60) | 167 (54) | |
| Graft type | <0.001 | ||
| Marrow | 741 (40) | 27 (9) | |
| PBSC | 1089 (60) | 283 (91) | |
| Conditioning regimen intensity | 0.23 | ||
| Myeloablative | 415 (23) | 80 (26) | |
| Non-myeloablative/RIC | 1415 (77) | 230 (74) | |
| GVHD prophylaxis | <0.001 | ||
| PTCy + CNI + MMF | 1647 (90) | 167 (54) | |
| PTCy + othersc | 183 (10) | 143 (46) | |
| Time from diagnosis to HCT, months | |||
| Median (Range) | 29 (3–421) | 32 (3–369) | 0.88 |
| N Eval | 1822 | 309 | |
| Donor age | <0.001 | ||
| Less than 20 years | 112 (6) | 13 (4) | |
| 20–29 years | 392 (21) | 155 (50) | |
| 30–39 years | 423 (23) | 68 (22) | |
| 40–49 years | 307 (17) | 30 (10) | |
| 50+ years | 402 (22) | 7 (2) | |
| Missing | 194 (11) | 37 (12) | |
| Median (Range) | 37 (12–76) | 28 (18–55) | <0.001 |
| Donor/recipient sex match | <0.001 | ||
| Male-to-male | 706 (39) | 166 (54) | |
| Male-to-female | 341 (19) | 55 (18) | |
| Female-to-male | 461 (25) | 44 (14) | |
| Female-to-female | 317 (17) | 39 (13) | |
| Missing | 5 (<1) | 6 (2) | |
| Donor/recipient CMV match status | <0.001 | ||
| Donor +/ recipient + | 843 (46) | 95 (31) | |
| Donor +/ recipient − | 228 (12) | 36 (12) | |
| Donor – / recipient + | 310 (17) | 77 (25) | |
| Donor − / recipient − | 406 (22) | 94 (30) | |
| Missing | 43 (2) | 8 (3) | |
| Year of transplant | 0.002 | ||
| 2010–2014 | 529 (29) | 63 (20) | |
| 2015–2019 | 1301 (71) | 247 (80) | |
| Follow-up among survivors, Months | |||
| N Eval | 1146 | 231 | |
| Median (25th-75th quartiles) | 33 (14–55) | 21 (12–35) | <0.001 |
Abbreviations: CIBMTR, center for international blood and marrow research; EBMT, European Society for Blood and Marrow Transplantation; KPS, Karnofsky performance score; HCT hematopoietic cell transplantation; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; RIC, reduced intensity conditioning; GVHD, graft-versus-host disease; PTCy, post-transplant cyclophosphamide; CNI, calcineurin inhibitors; MMF, mycophenolate; CMV, cytomegalovirus
The Pearson chi-square test was used for comparing discrete variables; the Kruskal-Wallis test was used for comparing continuous variables
evaluable patients in haploidentical group = 1146 and MUD group = 231
PTCy + others: CNI only, sirolimus, methotrexate
Overall Survival
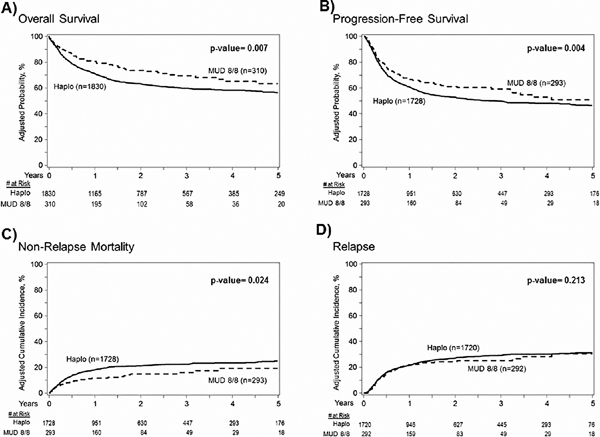
The estimated 2-year OS rates were 63% (95%CI 61–66) and 73% (95%CI 67–79) in the haplo-HCT and MUD groups, respectively (overall P=0.007) (Table 2, Figure 1A). In multivariate analysis (Table 3, Figure 2), haplo-HCT was associated with higher overall mortality (inverse of OS) compared to MUD-HCT, (HR=1.69, 95%CI 1.30–2.27, P<0.001).
Table 2.
Univariate analysis of patient outcomes by donor type
| Haploidentical, N (%) | Matched unrelated, N (%) | ||||
|---|---|---|---|---|---|
| Outcomes | N | Prob (95% CI) | N | Prob (95% CI) | Overall P Value |
| Neutrophil engraftment | 1753 | 292 | <0.001 | ||
| 28-day | 90 (89–92)% | 93 (90–96)% | |||
| 100-day | 96 (95–97)% | 98 (96–99)% | |||
| Platelet recovery | 1653 | 259 | <0.001 | ||
| 28-day | 54 (51–56)% | 66 (60–71)% | |||
| 100-day | 86 (84–87)% | 92 (88–95)% | |||
| Acute GVHD II-IV | 1690 | 279 | 0.004 | ||
| 100-day | 33 (31–35)% | 24 (20–30)% | |||
| Acute GVHD III-IV | 1707 | 286 | 0.018 | ||
| 100-day | 10 (8–11)% | 5 (3–8)% | |||
| Chronic GVHD | 1717 | 277 | 0.124 | ||
| 6 months | 14 (12–16)% | 10 (7–14)% | |||
| 1-year | 24 (22–26)% | 17 (13–22)% | |||
| 2-year | 28 (26–30)% | 23 (18–29)% | |||
| Relapse | 1728 | 293 | 0.213 | ||
| 100-day | 9 (7–10)% | 7 (5–10)% | |||
| 1-year | 21 (20–23)% | 20 (15–25)% | |||
| 2-year | 27 (25–29)% | 22 (17–27)% | |||
| Non-relapse mortality | 1728 | 293 | 0.024 | ||
| 100-day | 9 (7–10)% | 6 (4–10)% | |||
| 1-year | 18 (16–20)% | 12 (8–16)% | |||
| 2-year | 21 (19–23)% | 15 (10–19)% | |||
| Progression-free survival | 1728 | 293 | 0.004 | ||
| 100-day | 83 (81–85)% | 86 (82–90)% | |||
| 1-year | 61 (58–63)% | 69 (63–74)% | |||
| 2-year | 53 (50–55)% | 63 (57–69)% | |||
| Overall Survival | 1830 | 310 | 0.007 | ||
| 1-year | 72 (70–74)% | 80 (75–85)% | |||
| 2-year | 63 (61–66)% | 73 (67–79)% | |||
Abbreviations: GVHD, graft-versus-host-disease; CI, confidence interval
Figure 1:
Kaplan-Meier estimates and cumulative incidence. (A) Overall survival: 2-year OS was 63% (95% CI 61–66%) and 73% (95%CI 67–79%) in the haploidentical and matched unrelated groups, P=0.007. (B) Progression free survival: 2-year PFS was 53% (95% CI 50–55%) and 63% (95%CI 57–69%) in the haploidentical and matched unrelated groups, P=0.004. (C) Non-relapse mortality: 2-year NRM was 21% (95% CI 19–23%) and 15% (95%CI 10–19%) in the haploidentical and matched unrelated groups, P=0.024. (D) Relapse: 2-year risk of lymphoma relapse was 27% (95% CI 25–29%) and 22% (95%CI 17–27%) in the haploidentical and matched unrelated groups, P=0.21.
Table 3.
Multivariate analysis of transplant outcomes by donor type
| Outcome by donor type | Evaluable | Events | HR (95% CI) | P-value |
|---|---|---|---|---|
| Neutrophil engraftment | ||||
| Haploidentical | 1753 | 1681 | 0.82 (0.68 – 0.97) | 0.025 |
| Matched unrelated | 292 | 288 | 1.00 | |
| Platelet recovery | ||||
| Haploidentical | 1645 | 1423 | 0.69 (0.59 – 0.80) | <0.001 |
| Matched unrelated | 258 | 243 | 1.00 | |
| Acute GVHD II-IV | ||||
| Haploidentical | 1690 | 590 | 1.65 (1.28 – 2.14) | <0.001 |
| Matched unrelated | 279 | 71 | 1.00 | |
| Acute GVHD III-IV | ||||
| Haploidentical | 1707 | 179 | 2.04 (1.28 – 3.25) | 0.003 |
| Matched unrelated | 286 | 17 | 1.00 | |
| Chronic GVHD | ||||
| Haploidentical | 1721 | 464 | 1.79 (1.30 – 2.48) | <0.001 |
| Matched unrelated | 277 | 58 | 1.00 | |
| Relapse | ||||
| Haploidentical | 1720 | 465 | 1.04 (0.78 – 1.38) | 0.805 |
| Matched unrelated | 292 | 63 | 1.00 | |
| Non-relapse mortality | ||||
| Haploidentical | 1728 | 367 | 1.93 (1.21 – 3.07) | 0.006 |
| Matched unrelated | 293 | 41 | 1.00 | |
| Progression or death | ||||
| Haploidentical | 1728 | 838 | 1.39 (1.10 – 1.79) | 0.008 |
| Matched unrelated | 294 | 104 | 1.00 | |
| Overall mortality | ||||
| Haploidentical | 1830 | 684 | 1.69 (1.30–2.27) | <0.001 |
| Matched unrelated | 310 | 79 | 1.00 |
Abbreviations: GVHD, graft-versus-host-disease; CI, confidence interval
Neutrophil engraftment: adjusted by patient age, HCT CI, sex match; stratified by population resources, GVHD prophylaxis, graft type and conditioning regimen.
Platelet recovery: adjusted by Patient age, HCT CI, CMV match, disease stage, time from diagnosis to transplant; stratified by population resources, GVHD prophylaxis, graft type and conditioning regimen.
Acute GVHD II-IV: adjusted by donor age, HCT CI; stratified by population resources, GVHD prophylaxis, graft type and conditioning regimen. Acute GVHD III-IV: adjusted by previous auto HCT; stratified by population resources, GVHD prophylaxis, graft type and conditioning regimen. cGVHD: adjusted by previous auto HCT; stratified by population resources, GVHD prophylaxis, graft type and conditioning regimen.
Relapse: adjusted by year of transplant; stratified by disease stage, time from diagnosis to transplant, population resources, GVHD prophylaxis, graft type and conditioning regimen.
Non-relapse mortality: adjusted by patient age, disease stage, donor age, CMV match, Karnofsky score; stratified by population resources, GVHD prophylaxis, graft type and conditioning regimen.
Progression or death: Inverse of progression-free survival; adjusted by disease stage, CMV match; stratified by disease type, donor age, population resources, GVHD prophylaxis, graft type and conditioning regimen.
Overall mortality: inverse of overall survival; djusted by patient age, disease stage, HCT CI, CMV match, Karnofsky score; stratified by disease type, donor age, population resources, GVHD prophylaxis, graft type and conditioning regimen.
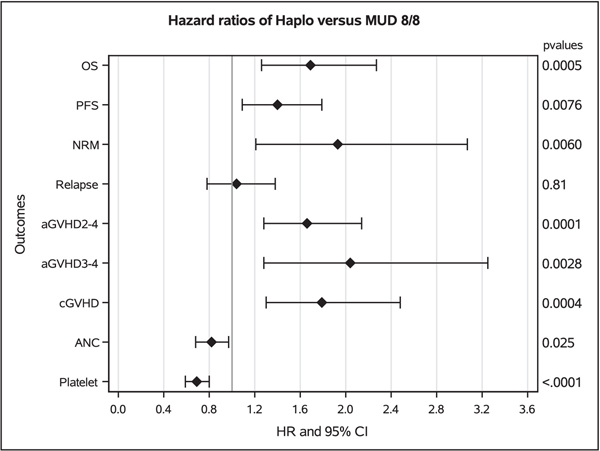
Figure 2:
Forest plot showing the results of multivariate analysis of patients with lymphomas undergoing haploidentical donor versus matched unrelated donor allogeneic transplantation using post-transplant cyclophosphamide based graft-versus-host disease prophylaxis. HR to the right of 1.0 favor MUD for all outcomes except ANC and platelets. For ANC and platelets, HR to the left of 1.0 favor MUD.
Independent of donor type, pre-HCT disease status being PR or chemoresistant (overall P<0.001), HCT-CI ≥3 (HR=1.47, 95%CI 1.17–1.86) and KPS <90 (HR=1.46, 95%CI 1.13–1.88, P=0.004) were associated with poorer survival (supplementary file 1)
Progression-free Survival
The estimated 2-year PFS was 53% (95%CI, 50–55) and 63% (95% CI, 57–69) in the haplo-HCT and MUD groups, respectively (overall P=0.004) (Table 2, Figure 1B). Multivariate analysis (Table 3, Figure 2) showed that haplo-HCT was associated with higher rates of progression or death (inverse of PFS) compared to MUD-HCT, (HR=1.39, 95%CI 1.10–1.79, P=0.008).
Independent of donor type, pre-HCT disease status being PR or chemoresistant (overall P<0.001), and KPS <90 (HR=1.40, 95%CI 1.17–1.68, P<0.001) were associated with poorer survival.
Relapse and NRM
The cumulative incidences of relapse at 2-years were 27% (95%CI 25–29) and 22% (95%CI 17–27) in the haploidentical and MUD groups, respectively (overall P=0.213, Table 2, Figure 1D). Multivariate analysis (Table 3, Figure 2), showed no significant difference in the risk of relapse/progression with haplo-HCT vs. MUD (HR=1.04, 95%CI 0.78–1.38, P=0.805). Independent of donor type, HCT done between 2017–2019 (compared to 2010–2013 and 2014–2016) was associated with a lower risk for lymphoma relapse, (HR=0.70, 95%CI 0.56–0.88, P<0.001) (supplementary file 1).
Among recipients of haplo-HCT, the 1-year NRM was 18% (95%CI, 16–20) compared with 12% (95%CI, 8–16) in MUD recipients. The corresponding 2-year NRM was 21% (95%CI, 19–23) and 15% (95%CI, 10–19), respectively (overall P= 0.024; Table 2, Figure 1C). Multivariate analysis showed a significantly higher risk of TRM with haplo-HCT versus MUD HCT (HR=1.93, 95%CI 1.21–3.07, P=0.006).
Independent of donor type, older donor age (cutoff for statistical significance ≥50, P=0.007) and older recipient age ≥50 (overall P<0.001) were associated with inferior NRM.
Hematopoietic Recovery
The cumulative incidence of neutrophil recovery at day-28 was 90% (95% confidence interval [CI], 89–92) in the haploidentical group compared with 93% (95%CI, 90–96) in the MUD group (overall P<0.001). The day-28 and day-100 cumulative incidences of platelet recovery in similar order were 54% (95%CI 51–56) and 66% (95%CI 60–71) and 86% (95%CI 84–87) and 92% (95% CI 88–95) (overall P<0.001; Table 2). Multivariate analysis (Table 3, Figure 2) revealed a slower rate of platelet engraftment in haploidentical compared to MUD transplant recipients (HR=0.69; 95%CI 0.59–0.80, P<0.001); rates of neutrophil recovery were not statistically different (HR=0.82; 95%CI 0.68–0.97, P=0.025).
Independent of donor type, sex mismatched transplants from female donor to male recipient (HR=0.86, 95%CI 0.77–0.95, P=0.003) and recipient age ≥60 (overall P<0.001) were associated with poorer neutrophil recovery (supplementary file 1). Similarly, platelet recovery was negatively associated with chemoresistant disease status (HR=0.81, 95%CI 0.71–0.92, P=0.001), HCT-CI score ≥2 (overall P=<0.001), time from diagnosis to transplant of 6–12 months (HR 0=61, 95%CI 0.44–0.84, P=0.003) and recipient age ≥40 (overall P<0.001), whereas donor positive/patient negative cytomegalovirus (CMV) serological status was associated with better platelet recovery, (HR=1.27, 95%CI 1.09–1.47, P=0.002) (supplementary file 1).
Acute and Chronic GVHD
Univariate analysis showed the cumulative incidence of grade II-IV acute GVHD at day 100 (Table 2) in the haplo-HCT cohort was 33% (95%CI 31–35) compared with 24% (95%CI 20–30) in the MUD group, (overall P=0.004). The corresponding rates of grade III-IV acute GVHD were 10% (95%CI 8–11) and 5% (95%CI 3–8), overall P=0.018. Multivariate analysis (Table 3) showed a higher risk of both grade II-IV acute GVHD (HR=1.65, 95%CI 1.28–2.14, P<0.001) and grade III-IV acute GVHD (HR=2.04, 95%CI 1.28–3.25, P=0.003) in haploidentical compared to MUD transplant recipients.
The cumulative incidences of chronic GVHD at 1-year (Table 2) in the haplo-HCT and MUD groups were 24% (95%CI 22–26) and 17% (95%CI 13–22) respectively, overall P=0.124. However, multivariate analysis indicated the risk of chronic GVHD was significantly higher after haploidentical transplantation (HR=1.79, 95%CI 1.30–2.48, P<0.001) relative to MUD allo-HCT (Table 3, Figure 2). In the haplo-HCT group, a peripheral blood stem cells graft was associated to higher-risk of acute grade 2–4 GVHD, acute grade 3–4 GVHD, and chronic GVHD.
Sensitivity analysis for the primary outcome of overall mortality: Inverse Probability Treatment Weighting (IPTW) Regression Using Propensity Score (PS) Weighting and Propensity Score Matching Analysis
Considering the heterogeneity of the study population, particularly with regard to patient and donor age, two sensitivity analyses using propensity scores on a restricted sub-cohort of patients receiving transplants from donors age ≤ 55 years old were conducted. The PS was based on disease, disease stage, donor CMV status, HCT-CI, Karnofsky performance status, registry, patient age and donor age (supplementary file 2). Both sensitivity analyses confirmed multivariate results, whether patient age and donor age were considered as continuous or categorical variables in the PS modeling. Considering ages as continuous variables, the IPTW weighted Cox model for overall mortality (1 ‒OS) with haploidentical vs. MUD HCT show a HR of 1.38 (95% CI 1.03–1.85, p=0.03). Considering age as categorical variables, the HR was 1.36 (95% CI 1.01–1.83, p=0.04).
A propensity score matched pair analysis was also performed. We were able to match 273 pairs. Treating age as a continuous variable, there was no difference in propensity scores (p=0.96), patient age (p=0.48) or donor age (p=0.26) between haplo-HCT and MUD HCT recipients in the matched cohort. The HR for overall mortality with haplo versus MUD was 1.49 (95% CI 1.09–2.04), p=0.012. Considering age as a categorical variables, there was again no difference in propensity scores or donor or patient age between haplo and HCT recipients in the matched cohort, and the HR for mortality was 1.58 (95% CI 1.16–2.16, p<0.01)
Causes of Death
With a median follow up of 33 months (range 0–123) in the haplo-HCT and 21 months (range 0–108) in the MUD group, numbers of deaths in both groups at last follow up were 684 and 79, respectively. As reported by the treating institution, lymphoma relapse/progression was the cause of death in 244 (36%) and 29 (37%) of patients in the haplo-HCT and MUD recipients, respectively, making it the most common cause of death in either group (Table 4). Other leading causes of deaths were infection followed by GVHD in both haplo-HCT and MUD recipients. It should be noted that cause of death data was missing in 23 (3%) of haplo-HCT and 5 (6%) of MUD patients.
Table 4.
Causes of death
| Cause of death | Haploidentical, N (%) | Matched unrelated, N (%) |
|---|---|---|
| No. of deaths = 684 | No. of deaths = 79 | |
| Primary Disease | 244 (36) | 29 (37) |
| GVHD | 95 (14) | 9 (11) |
| Infection | 197 (29) | 22 (28) |
| Other Causes | 125 (18) | 14 (18) |
| Missing | 23 (3) | 5 (6) |
Abbreviations: GVHD, graft-versus-host-disease.
DISCUSSION
The advent and widespread use of PTCy-based GVHD prophylaxis to successfully perform HLA-mismatched transplantation has made haploidentical donors an acceptable graft source that is rapidly available for the vast majority of patients, even those underrepresented in international donor registries. 16 Nevertheless, the existing evidence did not allow us to know whether this is the best approach if using PTCy-based GVHD prophylaxis for MUD HCT.
In the current study, where all transplantations were done using the PTCy platform, the use of MUDs was associated with a significant advantage in OS. This effect was explained by lower NRM and GVHD incidences. No significant differences were observed in relapse. These results are in line with a recent study from Gooptu et al.8 where a survival advantage of MUDs over haploidentical donors in the myeloid RIC setting was explained by a higher NRM, driven in part by more frequent acute GVHD. In our study, almost 70% of patients in both cohorts received RIC, which is expected in a lymphoma cohort. Compared to the Gooptu study, we also observed an association between a higher chronic GVHD incidence and the use of a haploidentical donor. A second difference between the two groups was the shorter platelet engraftment time of the MUD cohort. This could be explained by the more prevalent use of PBSC graft in this group. This difference has been previously documented both in the use of standard CNI-based GVHD prophylaxis and HLA-matched donors 17 and in the haploidentical setting with PTCy.18However, other studies reported in literature are describing different results when comparing haplo-HCT to MUD in lymphomas. In a recent metanalysis by Gagelmann et al., showed that haplo-HCT with PTCy had increased relapse than MUD for lymphoma patients. However, in that large metanalysis, GVHD prophylaxis for the MUD cohort was ATG-based in most cases.19
This analysis also shows that PTCy can be used for MUD HCT. We previously demonstrated 3-year OS rates of 62% and 50% for MUDs with standard calcineurin inhibitor GVHD prophylaxis, without or with ATG, respectively.6 The 72% 2-year OS after MUD HCT cohort in the current study, using PTCy, compares well with these previously published results. The same is true for relapse incidence (28% and 36% at 3 years versus 22% at 2 years), NRM (13% and 20% versus 12% at 1 year), PFS (49% and 28% at 3 years versus 63% at 2 years), grade 2–4 acute GVHD (40% and 49% versus 24% at day +100) and chronic GVHD (51% and 33% versus 17% at 1 year). Prospective trials, including the CTN PROGRESS 3 study (NCT03959241), are addressing this question in a randomized fashion. Although the use of PTCy should not yet be considered standard, a recent prospective phase 2 study from Shaw et al. shows how such a platform could be safely used for HLA mismatched unrelated donor HCT (with bone marrow graft),20 making it an attractive platform to expand the donor pool to races and ethnicities underrepresented in donor registries. However, other differences such as infections incidence (e.g. viral reactivations, fungal infections) should also be considered while comparing different GVHD prophylaxis.21 In our study, causes of death related to infectious complications were similar between the two types of donor. This was in line with previous reports.22
A third significant observation in our study is related to the HL subset analysis. No significant differences between haploidentical or MUD donors were confirmed in the HL population (Supplementary file 3). Probably, the low number of patients and short follow-up of the MUD cohort did not allow the study to have sufficient statistical power.
This study has some limitations. The inclusion of PTCy as GVHD prophylaxis platform in the MUD setting is quite recent. The number of reported patients is limited, and this fact might compromise the statistical power to detect small differences in outcomes. To overcome this issue, a joint study between CIBMTR and EBMT, the two largest HCT registries in the world was made. Secondly, we do not know how centers performed donor selection. It is possible that a few centers preferred haploidentical donor over MUD donor based on institutional preference or because of time restrictions while others deferred use of a haploidentical donor until a MUD search was unsuccessful, which could introduce bias. However, we believe that a prospective randomized study between MUD and haploidentical donors would be extremely difficult to conduct because both types of donors may not be available for all patients. Another issue is the heterogeneity of the study population in terms of donor registry, GVHD prophylaxis, graft source, conditioning regimen and donor age. All these factors were included and adjusted for in the multivariate analyses. Results were also confirmed independently in two sensitivity analyses that incorporated propensity scores to further adjust for population differences. Regarding the use of different GVHD prophylaxis, we can observe that the MUD cohort received more heterogeneous prophylaxis regimens instead of the classic PTCy + CNI + MMF. Specifically, in the MUD cohort there was a higher percentage of patients who received a 2-drug (PTCy + CNI) instead of classic 3-drug GVHD prophylaxis (0.7% versus <0.1%). We know from a previous study from the EBMT that a 3-drug PTCy-based GVHD prophylaxis has a better GVHD and relapse-free survival.23 Despite this, the MUD cohort had a lower GVHD incidence, possibly suggesting that a 2-drug PTCy-based GVHD prophylaxis can be sufficient in this setting. This has been reported in a recent study by Mehta and colleagues.24 Of note, in a recent prospective randomized study made in HLA-identical donors, the sole use of PTCy without additional drugs had same results that standard CNI-based GVHD prophylaxis.25 Another critique could be related to the higher use of BM graft in the haploidentical cohort. To compensate for different use of graft sources, we performed an analysis restricted to PBSC population (supplementary file 4) confirming general results. Also, the study population is quite heterogeneous in terms of disease type between the two groups. This could limit the analysis of the graft-versus-lymphoma effect which is different depending on lymphoma subtype. Finally, median donor age was higher in the haploidentical group (37 years versus 29 years). Adjustments for age were performed in two separate analyses. In addition, in a recent retrospective study from Perales et al. comparing MUD with standard GVHD prophylaxis versus haplo-HCT with PTCy for acute myeloid leukemia, donor age had no significant impact on survival.26The same results were confirmed on a large retrospective analysis on haplo-HCT with PTCy where donor age did adversely affect survival despite being associated with higher acute GVHD and NRM incidence. The latter was counterbalance by less relapse.27 Prospective data could help to further address this question.
In conclusion, our results suggest that utilization of a MUD over a haploidentical donor when using PTCy-based GVHD prophylaxis could be preferable for non-Hodgkin lymphoma patients. The data do not support favoring a haploidentical donor if a MUD is available in a timely manner. MUD donors are unfortunately less of an option for the majority of patients/recipients of non-European Caucasian descent who either do not have quick access to an 8/8 MUD donor or have no 8/8 MUD donor prospect. For this large group, haploidentical transplants result in acceptable outcomes for this high-risk disease population. Moreover, PTCy seems to be a valid alternative to standard CNI-based GVHD prophylaxis for MUD HCT. Clinical differences between the MUD and haplo cohorts such as a different use of graft sources (bone marrow was mostly used in the haplo cohort) or type of registry (CIBMTR versus EBMT) could have biased these results and prospective trials are awaited in this setting.
Supplementary Material
HIGHLIGHTS.
MUD donor has higher survival than haplo for lymphoma patients using PTCy
Such results are explained by a decreased NRM
ACKOWLEDGEMENTS
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2832 and N00014-21-1-2954 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies Corporation; Adienne SA; Allogene; Allovir, Inc.; Amgen, Inc.; Anthem; Astellas Pharma US; Atara Biotherapeutics; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx Inc; CRISPR; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Fate Therapeutics; Gamida-Cell, Ltd.; Gilead; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Kadmon; Karius; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Medac GmbH; Medexus; Merck & Co.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc.; Ossium Health, Inc; Pfizer, Inc.; Pharmacyclics, LLC; Priothera; Sanofi Genzyme; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Terumo Blood and Cell Technologies; TG Therapeutics; Tscan; Vertex; Xenikos BV.
Footnotes
Declaration of interests
We declare no other competing interests for the execution of this study
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data sharing
The study data belong to the CIBMTR and EBMT and could be requested through previous authorization.
The study has been presented as oral presentation at the following congresses:
American society of Hematology Congress 2021, Atlanta, US
European Society for Bone Marrow Transplantation Research congress 2022, Prague, Czech Republic
Tandem Meeting 2022, Salt Lake City, US
REFERENCES
- 1.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008; 14: 641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashey A, Zhang M-J, McCurdy SR, et al. Mobilized Peripheral Blood Stem Cells Versus Unstimulated Bone Marrow As a Graft Source for T-Cell-Replete Haploidentical Donor Transplantation Using Post-Transplant Cyclophosphamide. J Clin Oncol 2017; 35: 3002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon SR, St. Martin A, Shah NN, et al. Myeloablative vs reduced intensity T-cell–replete haploidentical transplantation for hematologic malignancy. Blood Advances 2019; 3: 2836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.on behalf of the Japan Study Group for Cell Therapy and Transplantation (JSCT), Sugita J, Kagaya Y, et al. Myeloablative and reduced-intensity conditioning in HLA-haploidentical peripheral blood stem cell transplantation using post-transplant cyclophosphamide. Bone Marrow Transplant 2019; 54: 432–41. [DOI] [PubMed] [Google Scholar]
- 5.Ciurea SO, Zhang M-J, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood 2015; 126: 1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanate AS, Mussetti A, Kharfan-Dabaja MA, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood 2016; 127: 938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dehn J, Spellman S, Hurley CK, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: guidelines from the NMDP/CIBMTR. Blood 2019; 134: 924–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gooptu M, Romee R, St Martin A, et al. HLA Haploidentical versus Matched Unrelated Donor Transplants with Post-Transplant Cyclophosphamide based prophylaxis. Blood 2021; published online April 13. DOI: 10.1182/blood.2021011281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamadani M, Ngoya M, Sureda A, et al. Outcome of allogeneic transplantation for mature T-cell lymphomas: impact of donor source and disease characteristics. Blood Adv 2022; 6: 920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the Intensity of Conditioning Regimens: Working Definitions. Biology of Blood and Marrow Transplantation 2009; 15: 1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. JCO 2014; 32: 3059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iacobelli S, On behalf of the EBMT Statistical Committee. Suggestions on the use of statistical methodologies in studies of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2013; 48: S1–37. [DOI] [PubMed] [Google Scholar]
- 13.Hu Z-H, Wang H-L, Gale RP, Zhang M-J. A SAS macro for estimating direct adjusted survival functions for time-to-event data with or without left truncation. Bone Marrow Transplant 2021; : s41409-021-01435–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Zhang M-J. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Computer Methods and Programs in Biomedicine 2011; 101: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Commenges D, Andersen PK. Score test of homogeneity for survival data. Lifetime Data Anal 1995; 1: 145–56. [DOI] [PubMed] [Google Scholar]
- 16.Gragert L, Eapen M, Williams E, et al. HLA Match Likelihoods for Hematopoietic Stem-Cell Grafts in the U.S. Registry. N Engl J Med 2014; 371: 339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 2012; 367: 1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu X, Liu L, Xie Z, et al. Bone marrow versus peripheral blood as a graft source for haploidentical donor transplantation in adults using post-transplant cyclophosphamide—A systematic review and meta-analysis. Critical Reviews in Oncology/Hematology 2019; 133: 120–8. [DOI] [PubMed] [Google Scholar]
- 19.Gagelmann N, Bacigalupo A, Rambaldi A, et al. Haploidentical Stem Cell Transplantation With Posttransplant Cyclophosphamide Therapy vs Other Donor Transplantations in Adults With Hematologic Cancers: A Systematic Review and Meta-analysis. JAMA Oncol 2019; published online Oct 17. DOI: 10.1001/jamaoncol.2019.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw BE, Jimenez-Jimenez AM, Burns LJ, et al. National Marrow Donor Program-Sponsored Multicenter, Phase II Trial of HLA-Mismatched Unrelated Donor Bone Marrow Transplantation Using Post-Transplant Cyclophosphamide. J Clin Oncol 2021; : JCO2003502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crocchiolo R, Bramanti S, Vai A, et al. Infections after T-replete haploidentical transplantation and high-dose cyclophosphamide as graft-versus-host disease prophylaxis. Transpl Infect Dis 2015; 17: 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oltolini C, Greco R, Galli L, et al. Infections after Allogenic Transplant with Post-Transplant Cyclophosphamide: Impact of Donor HLA Matching. Biology of Blood and Marrow Transplantation 2020; 26: 1179–88. [DOI] [PubMed] [Google Scholar]
- 23.Ruggeri A, Labopin M, Bacigalupo A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol 2018; 11: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta RS, Saliba RM, Hayase E, et al. Mycophenolate Mofetil: A Friend or a Foe with PostTransplantation Cyclophosphamide and Tacrolimus Prophylaxis in HLA-Matched Donors? Transplantation and Cellular Therapy 2022; 28: 500.e1–500.e10. [DOI] [PubMed] [Google Scholar]
- 25.Luznik L, Pasquini MC, Logan B, et al. Randomized Phase III BMT CTN Trial of Calcineurin Inhibitor–Free Chronic Graft-Versus-Host Disease Interventions in Myeloablative Hematopoietic Cell Transplantation for Hematologic Malignancies. JCO 2022; 40: 356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perales M-A, Tomlinson B, Zhang M-J, et al. Alternative donor transplantation for acute myeloid leukemia in patients aged ≥50 years: young HLA-matched unrelated or haploidentical donor? Haematologica 2020; 105: 407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariotti J, Raiola AM, Evangelista A, et al. Impact of donor age and kinship on clinical outcomes after T-cell–replete haploidentical transplantation with PT-Cy. Blood Advances 2020; 4: 3900–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study data belong to the CIBMTR and EBMT and could be requested through previous authorization.
The study has been presented as oral presentation at the following congresses:
American society of Hematology Congress 2021, Atlanta, US
European Society for Bone Marrow Transplantation Research congress 2022, Prague, Czech Republic
Tandem Meeting 2022, Salt Lake City, US