Abstract
Naked mole-rats (NMR) and Damaraland mole-rats (DMR) exhibit extraordinary longevity for their body size, high tolerance to hypoxia and oxidative stress and high reproductive output; these collectively defy the concept that life-history traits should be negatively correlated. However, when life-history traits share similar underlying physiological mechanisms, these may be positively associated with each other. We propose that one such potential common mechanism might be the bioenergetic properties of mole-rats. Here, we aim to characterize the bioenergetic properties of two African mole-rats. We adopted a top-down perspective measuring the bioenergetic properties at the organismal, cellular, and molecular level in both species and the biological significance of these properties were compared with the same measures in Siberian hamsters and C57BL/6 mice, chosen for their similar body size to the mole-rat species. We found mole-rats shared several bioenergetic properties that differed from their comparison species, including low basal metabolic rates, a high dependence on glycolysis rather than on oxidative phosphorylation for ATP production, and low proton conductance across the mitochondrial inner membrane. These shared mole-rat features could be a result of evolutionary adaptation to tolerating variable oxygen atmospheres, in particular hypoxia, and may in turn be one of the molecular mechanisms underlying their extremely long lifespans.
Keywords: Basal metabolic rate, Respiration, Glycolysis, Mitochondria, Hypoxia, Longevity
1. Introduction
Energy is the currency of life [1]. As the theoretical biologist Alfred Lotka famously stated, “In the struggle for existence, the advantage must go to those organisms whose energy-capturing devices are most efficient in directing available energies into channels favorable to the preservation of the species” [2]. Life-histories of animals should therefore reflect the allocation of metabolic energy to those traits that determine fitness [3-6]. Ironically, although metabolic energy is the fundamental currency of fitness, few life-history studies directly focus on bioenergetics [7].
A key premise of life-history theory posits that certain life-history traits are negatively associated with each other and are considered “trade-offs” [8]. The two commonly accepted eusocial mammals, the naked mole-rats (NMR; Heterocephalus glaber) and the Damaraland mole-rats (DMR, Fukomys damarensis) appear to challenge this concept of life-history trade-offs [9,10]. Both species exhibit extreme longevity on the basis of their body size and a delayed/retarded aging phenotype [11,12]. Although less is known about the DMR age-related biology than that of the NMR [12,13], breeders of both species show no menopause and demonstrate enormous reproductive outputs (60–140 times greater than other rodents [14]), yet are often the most long-lived individuals in their colonies [15,16]. More impressively, both these species also share other characteristics such as hypoxia tolerance [17] and tolerance of oxidative stress [18], features considered evolved traits in response to life below ground for millenia [19]. In spite of the similarities of these exceptional life-history traits within this monophyletic clade [20], NMR and DMR exhibit fundamental differences in their evolutionary history: studies suggest they evolved eusociality independently [21,22], and have divergent physiological responses to different stressors [22]. Consequently, by studying these two similar but evolutionarily distinct species, we can better understand the effects of the evolutionary force and avoid biased from the life-history of a single species [23].
The two mole-rat species, especially the NMR, have been intensively studied as model species for aging and cancer research [24,25]. However, the bioenergetic properties of these mole-rats attract less attention. Previous studies in mammals found that animals with low metabolic rate and energy expenditure tend to have longer lifespan [26,27]. Several empirical studies in invertebrates have also found that experimental downregulation of metabolism at the mitochondrial level extended lifespan of animals [28]. Hence, could the extreme longevity of both mole-rat species be explained by their bioenergetic properties at the organismal, cellular, and mitochondrial levels? Lovegrove [29] and McNab [30] have pioneered the bioenergetic measurements in these mole-rats species where they found that the basal metabolic rate (BMR) in both NMR and DMR was >40 % lower compared to a size-matched rodent. Moreover, BMR also did not show any detectable age-related changes in NMR, whereas age-related decline of BMR is often observed in laboratory rodents [31]. Recently, using isolated mitochondria from various tissues, researchers found conflicting results on the respiratory rates between NMR and laboratory mouse, Mus musculus. The rates of state 4 respiration (uncoupled respiration) of mitochondria from various tissues had been shown to be either lower [32-34] or higher [35-37], suggesting that NMR mitochondria are either more coupled or less coupled when compared to mouse counterparts. At the cellular level, Swovick, et al. [38] employed primary dermal fibroblast and documented that quiescent fibroblast from NMR had lower basal respiration and glycolytic rates, resulted in lower ATP production rate than mouse fibroblast at baseline levels. In conclusion, not only are studies focusing on the bioenergetic characteristics of mole-rats are very limited, inconclusive and biased toward NMR, the comprehensive evaluation of bioenergetics of these two mole-rat species also remains largely unexplored.
Mode-of-life theory suggests that ecological traits, such as fossoriality, are positively correlated with lifespan [39]. Fossorial animals are protected from predation, airborne contagious agents and unfavorable climatic conditions and this affords protection from extrinsic mortality and contributes to a longer lifespan [14]. However, it is possible that aside from the ecological benefits of subterranean lifestyle, fossoriality also led to a suite of evolutionary and physiological adaptations to hypoxia. We proposed that these adaptations could provide a causal mechanism of extended lifespan not explained by difference in extrinsic mortality. Adaptation to hypoxic environment includes decline in BMR at the organismal level [40], higher reliance on glycolysis than OXPHOS for ATP production at the cellular level [41], and decreased mitochondrial proton conductance at the organelle level [42]. These bioenergetic adaptations that may further contribute to the prolonged lifespans of underground dwelling species.
In the present study, we employed an uprecedented integrative approach to concurrently study bioenergetic properties of DMR and NMR at the organismal, cellular and organelle level. This integrative approach allowed us to investigate compensatory mechanisms and synergistic interactions between different physiological levels that would otherwise be masked if only one biological level (e.g. whole organism) was explored. We also investigate the significance of these properties by comparing these findings with shorter-lived, above-ground dwelling laboratory rodents, such as the Siberian hamster (Phodopus sungorus) and the C57BL/6 mouse. We used the hamster and C57BL/6 mouse as comparisons since these species were housed in the same animal facility as DMR and NMR respectively. We believe this integrative approach will enable us to obtain synergistic feedback and valuable insights to understand the biological mechanisms governing the aging process and longevity.
2. Methods
2.1. Animals
The husbandry of DMR and Siberian hamsters and the study procedures were conducted under University of Memphis IACUC permits (#0862; #0797). The husbandry of NMR and C57BL/6 mice and the study procedures were conducted at Calico Life Sciences [43]. All experimental procedures were approved by the Calico IACUC committee (B-2-2020). Please see the electronic supplementary material, methods for more information on the husbandary of these animals.
2.2. Whole organism basal metabolic rate measurement
A total of ten young adult hamsters (4 months old; 5 male and 5 female), eleven young DMR (4–5 years old; 6 male and 5 female), nine middle-aged DMR (9–10 years old; 4 male and 5 female), and eight old DMR (16–20 years old; 4 male and 4 female) were used for whole organismal BMR measurements. Only non-breeding animals were used in the study. All BMR measurements were conducted using a standard flow-through respirometry system (Sable Systems International, Las Vegas, NV, USA) following that described in Yap et al. [44] (electronic supplementary material, methods). BMR was measured between 9: 00 and 17:00 at 30 °C, which is within the thermoneutral zone for both DMR and hamster [29,45]. BMR calculations were done based on the lowest averaged 3 min of oxygen consumption per measurement sequence after 4 h according to Lighton's Eqs. (10.6) and (10.7) [46] with ExpeData software, version 1.2.6 (Sable Systems International). The measurement of age-related changes in BMR were previously undertaken for NMR using the identical methodology [31] and similarly considerable data is available for the BMR of mice [47].
2.3. Primary fibroblast isolation and culture
Primary dermal fibroblasts from NMR and C57BL/6 mice were obtained from Buffenstein lab as previous described [48]. NMR cells were initially propagated at 32 °C with 5 % CO2 and 3 % O2 before acclimation at 37 °C overnight. The measurements of cellular respiration were then conducted according to Swovick, et al. [49]. Primary dermal fibroblasts of mice were isolated using identical procedures but maintained at 37 °C. Primary lung fibroblasts and primary dermal fibroblasts from DMR and hamsters were isolated from two three-year-old DMR and two four-month-old hamsters according to Seluanov et al. [50] and Zhang and Wong [51].
2.4. Cellular respiration measurements
The rates of respiration in cells were measured using Seahorse XFe96 Analyzers (Agilent, Santa Clara, CA) according to Wong et al. [52] (electronic supplementary material, methods). Rates of oxygen consumption and extracellular acidification were simutamously measured and expressed relative to the protein contents of the appropriate wells. Rates of ATP production during baseline from glycolysis and oxidative phosphorylation were calculated according to Mookerjee et al. [53]. Glycolytic ATP production comes from two parts. The first is ATP produced by glycolysis to pyruvate and then converted to lactate. This is calculated from extracellular acidification rate (ECAR) as PPRglyc (Proton Production Rateglyc) × ATP/lactate. The second part is ATP produced by glycolysis to pyruvate that is subsequently transported into mitochondria which eventually converted to CO2 and then bicarbonate. This is calculated from the oxygen consumption rate (OCR) by multiplying the mitochondrial OCR by the P/O ratio when pyruvate is fully oxidized. We used a conversion factor of 2 to account for oxygen atoms ([O]) in P/O ratio to oxygen molecules (O2) in OCR. As a result, As a result, Total ATP production rate from glycolysis is therefore PPRglyc × ATP/lactate + OCRmito × 2P/Oglyc. Here, we used P/Oglyc value of 0.167 when cells were supplied with glucose according to Mookerjee et al. [53]. The rate of ATP production from oxidative phosphorylation (OXPHOS) is calculated from the mitochondrial oxygen consumption rate. Briefly, the ATP production rate attributable to NADH/FADH2 oxidation is equal to the coupled OCR multiplied by the P/O ratio of oxidative phosphorylation: OCRcoupled × 2P/Ooxphos. The rate of ATP production from substrate level phosphorylation is determined by multiplying the mitochondrial oxygen consumption rate by the P/O ratio attributable to tricarboxylic acid cycle flux (OCRmito × 2P/OTCA). The total rate of ATP production during OXPHOS = (OCRcoupled × 2P/Ooxphos) + (OCRmito × 2P/OTCA) [53].
2.5. Mitochondria isolation
Lung (DMR and hamsters) and heart (NMR and C57BL/6 mice) tissues were immediately excised upon euthanasia. Lung mitochondria were isolated from lung tissue of DMR (n = 5; 4 years old) and hamster (n = 5; 4 month old) according to Spear and Lumeng [54]. Heart mitochondria were isolated from hearts of NMR and C57BL/6J mice in ice-cold heart sucrose buffer (electronic supplementary material, methods). Regardless of species, tissues were homogenized and subjected to differential centrifugations. The resultant supernatant was discarded, the final mitochondria pellets were suspended in ice-cold Mitochondrial Assay Solution (MAS-1) and were kept at high concentration (~20 mg protein/mL) on ice until use according to Mookerjee et al. [55].
2.6. Mitochondria respiration measurement
Body temperature varies for the various species used in this study: NMR (32–34 °C), DMR (~35.2 °C), mouse (36.2–38 °C) and hamster (36.1–38 °C) [56]. In order to have a fair comparison of the intrinsic functionality of mitochondria, respiration chamber temperature was standardized to avoid the influence of temperature on mitochondrial respiration. Munro et al. [34] previously showed the respiration of isolated mitochondria did not vary significantly between 30 °C and 37 °C for NMR but differed significantly for mouse. As a result, we used 37 °C as respiratory chamber temperature for all four species. Lung mitochondria (0.35 mg/ml) respiration was measured in MAS-1 at 37 °C using high resolution respirometry (Oroboros O2k, Innsbruck, Austria). The rates of respiration of heart mitochondria isolated from NMR and C56BL/6J mice were assessed according to Rogers et al. [57] by Seahorse XFe96 extracellular flux analyzer (electronic supplementary material, methods).
2.7. Statistical analyses
All statistical tests were carried out using IBM SPSS, version 26.0. BMR and body mass were log10 transformed prior to analyses to conform to normality. We first used general linear models (GLM) including the factor sex to compare BMR between DMR and hamster. However, adding or removing sex as covariate yielded similar results, so it was removed from statistical analyses. We tested the effect of species on BMR using GLM with BMR as dependent variable, species as main effect, and body mass as covariate [58]. To investigate if BMR differed between DMR from different age groups, we tested the effect of age group on BMR of DMR using GLM with BMR as dependent variable, age group as main effect, and body mass as covariate. F- and t-statistics and P values were reported. Student t-test was used for comparison in cellular and mitochondrial levels between NMR and DMR with their counterparts. We considered P < 0.05 as statistically significant.
3. Results
3.1. Whole body BMR
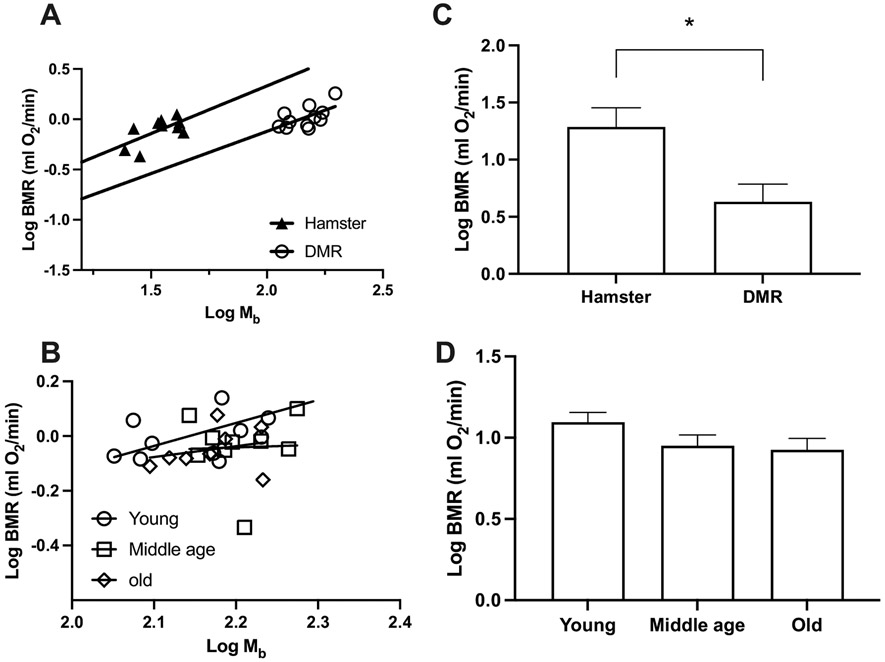
Our results of BMR in captive DMR are consistent with the reported BMR of this species captured in the wild [29] and in captivity [59]. The measured BMR of hamsters was also consistent with previous studies [45] (Supplementary Table 1). Both DMR (P = 0.029) and hamsters (P = 0.046) demonstrated a statistically significant positive correlation between their body mass and BMR. We therefore included body mass as a covariate when performing comparisons of metabolic rates. Based on the comparison, DMR demonstrated a 50 % lower BMR than hamsters (F1,18 = 4.55, P = 0.047; Fig. 1A,C), which aligned with predictions by Lovegrove [29]. We detected no significant differences between young, middle-aged and old DMR (F2,24 = 2.15, P = 0.138; Fig. 1B,D). Body mass also stayed constant between age groups in DMR (F2,25 = 0.99, P = 0.386; Supplementary Table 1). This is consistent with previous reports on NMR, where no age-related changes in body mass and BMR were found [31].
Fig. 1.
Basal metabolic rate (BMR) in Damaraland mole-rats (DMR) and Siberian hamsters. Least-squares linear regressions of log10-transformed BMR against log10 body mass (Mb) for (A) DMR and hamsters, (B) young, middle age, and old DMR. Least-squares means (±s.e.m.) controlling for body mass for each metabolic rate (C) DMR and hamsters, (D) young, middle age, and old DMR. * indicates P < 0.05.
3.2. Primary fibroblasts respiration
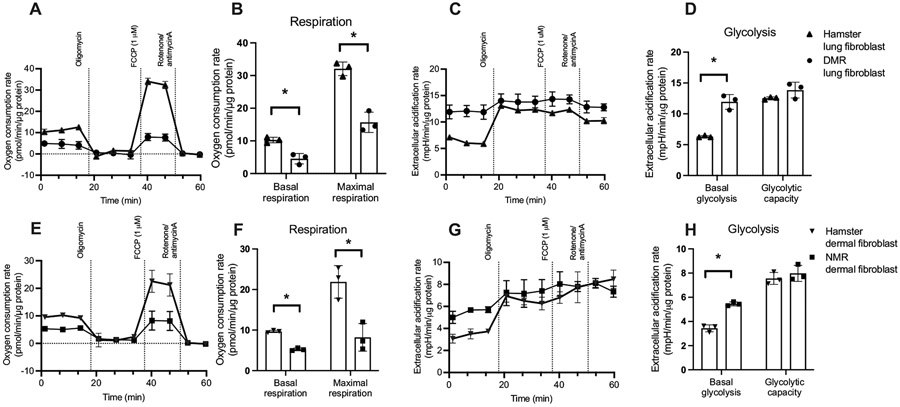
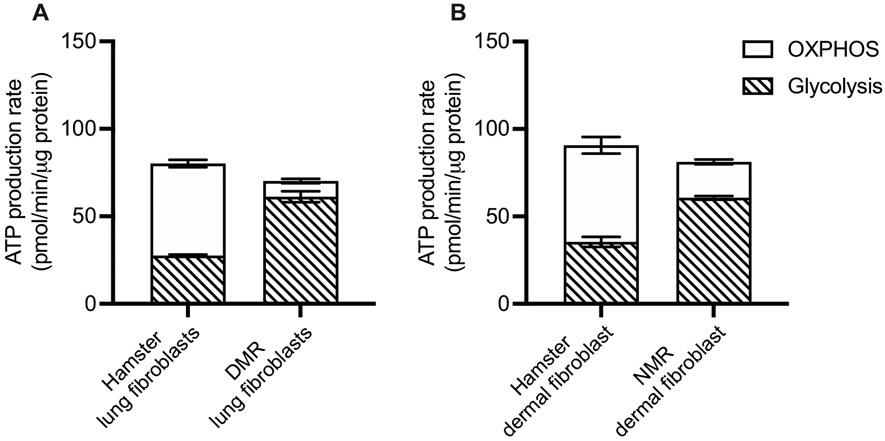
To better understand our observations, we moved on to explore cellular bioenergetics of both species. We did so by measuring the rates of cellular respiration and the rates of glycolysis, the two main pathways to generate energy, using an extracellular flux analyzer. Primary lung fibroblasts of DMR showed significantly lower rates of basal (t4 = 5.47, P = 0.005; Fig. 2A, B) and FCCP-induced maximal (t4 = 7.61, P = 0.002; Fig. 2A, B) respiration when compared with hamster lung fibroblasts. Oligomycin-induced state 4O respiration was not different between DMR and hamsters (P = 0.60), which indicated proton leak across mitochondrial inner membrane might not be different. However, proton motive force was not controlled in these measurements. Interestingly, basal extracellular acidification rates, which reflect basal glycolytic rates, were significantly higher in lung fibroblasts of DMR than of hamsters' (t4 = 8.06, P = 0.001; Fig. 2C, D). The maximal capacity of glycolysis of these cells showed no differences between DMR and hamsters (P = 0.15). We also calculated the rates of ATP production based on the rates of respiration and glycolysis and observed no statistically significant differences of ATP production rates in lung fibroblasts of DMR and hamsters under basal condition (P = 0.230; Fig. 3A).
Fig. 2.
Respiration and glycolysis for (A-D) Damaraland mole-rat (DMR) and Siberian hamster lung fibroblast, and (E-H) naked mole-rat (NMR) and Siberian hamster dermal fibroblast. Seahorse traces for oxygen consumption (A, E) and extracellular acidification (glycolysis; C, G) rate in fibroblasts. B, F: calculated basal and maximal respiration. Basal and Maximal respirations for hamster lung fibroblast were indicated by shaded area in A as an example. D, H: calculated basal glycolysis and glycolytic capacity. Basal glycolysis and glycolytic capacity for hamster lung fibroblast were indicated by shaded area in C as an example. N = 3 independent experiments, Data are shown as means ± s.e.m., * indicates P < 0.05.
Fig. 3.
ATP production rates during baseline from oxidative phosphorylation (OXPHOS) and glycolysis in (A) Damaraland mole-rat (DMR) and hamster lung fibroblast, (B) naked mole-rat (NMR) and hamster dermal fibroblast. N = 3 independent experiments, Data are shown as means ± s.e.m., * indicates P < 0.05.
We extended our investigation to also cover primary dermal fibroblasts of NMR. Intriguingly, similar patterns were observed between the comparisons between NMR and hamster dermal fibroblasts. Dermal fibroblasts of NMR demonstrated significantly lower rates of basal (t4 = 17.49, P < 0.001; Fig. 2E, F) and maximal respiration (t4 = 4.49, P = 0.011; Fig. 2E, F), whereas the rates of state 4 respiration were not different (t4 = 0.31, P = 0.77). While the maximal glycolytic rates were similar between dermal fibroblasts of NMR and hamster (P = 0.43), we again observed a significantly higher basal glycolytic rates in NMR dermal fibroblasts (t4 = 10.44, P < 0.001; Fig. 2G, H). No differences in ATP production rates were detected between dermal fibroblasts of NMR and hamster (P = 0.904; Fig. 3B).
3.3. Mitochondrial respiration
Investigation using isolated mitochondria allows us to better understand the bioenergetic machinery employed during respiration and may provide some explanations for their disparate bioenergetic characteristics observed at either the cellular or organismal level (Supplementary Figs. 1, 2).
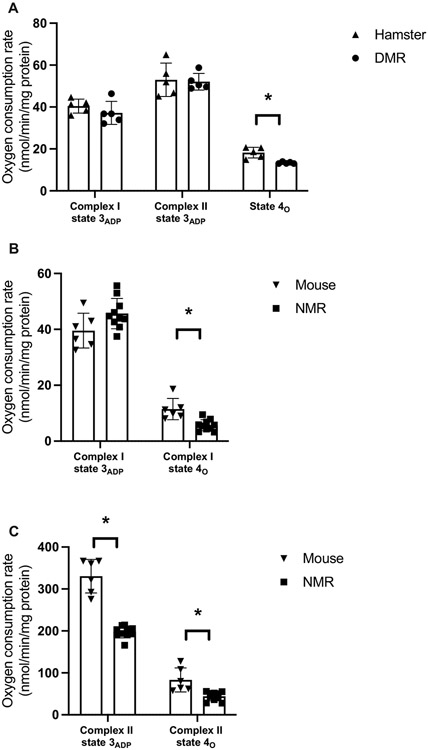
The rates of mitochondrial respiration were examined using both complex I-linked and complex II-linked substrates. In general, regardless of the tissues of interest (lungs: hamster and DMR; hearts: NMR and mice) mitochondria of DMR and NMR showed similar patterns that differed from those of hamsters and mice respectively. While the rates of Complex I-driven mitochondrial state 3ADP respiration were found to be similar when comparing the mole-rats to their laboratory counterparts (DMR vs. hamsters: P > 0.288; Fig. 4A; NMR vs. mice: t14 = 2.06, P = 0.058; Fig. 4B), the rates of state 4o respiration were found to be significantly lower for both DMR and NMR than those of hamsters or mice (DMR vs. hamsters: t8 = 4.21, P = 0.003; Fig. 4A; NMR vs. mice: t14 = 2.96, P = 0.010; Fig. 4B). Collectively, this gives rise to the idea that mole-rats have significantly higher respiratory control ratios (RCR) (P < 0.01; Supplementary Fig. 3B). Respiratory control ratio, while commonly used as an indicator of mitochondrial integrity, is in fact a reflection of the ADP-mediated stimulation of mitochondrial respiration. The discrepant RCR observed in mitochondria isolated from different species may be explained by the availability of ADP [60], which in this case was governed by the kinetics of ADP transport by adenine nucleotide transporter (ANT) as ADP was provided in excess during our measurements, or the molecular interaction between ADP/ATP and mitochondrial respiratory machineries [61]. The higher RCR values observed in mitochondria isolated from DMR and NMR therefore suggest the intrinsic differences of mitochondrial energetic machineries between mole-rats and hamster or mouse.
Fig. 4.
Respiration rate for (A) isolated lung mitochondria from Damaraland mole-rat (DMR; N = 5) and Siberian hamster (N = 5) measured using Oroboros O2k, (B) isolated heart mitochondria from naked mole-rat (NMR; N = 10) and C57BL/6 mouse (N = 6) measured using Seahorse XF96 Analyzers. Data are shown as means ± s.e.m., * indicates P < 0.05.
We also observed some degree of species-specific characteristics. When comparing DMR and hamsters, no differences were observed for Complex II-driven state 3ADP respiration in lung mitochondria. However, we observed a significant decrease in the rate of Complex II-driven state 3ADP respiration in heart mitochondria of NMR when compared to that of mice. The slower Complex II-driven state 3ADP respiration observed here were consistent with previous findings [32,62]. Such low levels of respiration by complex II substrates in NMR mitochondria were tied to decreased ROS production rates from complex I [62,63].
4. Discussion
Here we report that both DMR and NMR exhibited significantly different bioenergetic properties at the organismal, cellular and organelle levels compared to their above-ground dwelling, shorter-lived laboratory counterparts. Most notably both species showed lower whole body BMR and cellular respiration than above-ground dwelling animals while RCR of isolated mitochondria were found to be higher. These findings suggest convergent evolutionary processes most likely had arisen in response to social living under the variable oxygen atmospheres encountered below ground.
BMR of the two mole-rat species were estimated to be about 30 % - 60 % lower than that predicted by body mass [12,29-31,56,64]. The comparison between these two mole-rats species and other subterranean rodents, other partial fossorial mole-rat species, also shows a reduced BMR by >29 % [29]. The observed lower BMR might be explained by their fully fossorial lifestyle and eusocial colony organization which likely results in repeated exposure to severe hypoxia [17]. Interestingly, while age-related declines in BMR due to decreased lean mass in disease-free individuals were extensively documented in human and laboratory rodent species [65-67], we did not observe significant age-related declines in body mass and BMR in both NMR and DMR. This is likely indicative of well-maintained body composition, most notably lean mass, tissue function, and bioenergetic properties with increasing age [68].
BMR was calculated based on oxygen consumption measurements. To understand the mechanisms underlying lower BMR in mole-rats, we extended our investigation to measure cellular respiration using primary fibroblasts. We found that fibroblasts from both mole-rat species exhibit lower rates of basal and maximal cellular respiration than that of hamsters and mice, with associated elevations in basal glycolytic rates. We further calculated the rates of total ATP production based on the rates of basal cellular respiration and glycolysis. We observed that the rates of ATP production of primary fibroblasts isolated from the two mole-rat species were comparable to those of hamster in spite of reduced basal cellular respiration rates in the two mole-rat species (Fig. 3). This intriguing finding may be explained by the compensatory mechanism between mitochondrial and glycolytic ATP production during which either one of the pathways can be upregulated to compensate for the slowdown of the other. Our observations suggest a higher reliance of mole-rat fibroblasts on glycolysis instead of oxidative phosphorylation (OXPHOS) for ATP production. Although Swovick et al. [38] also showed lower basal respiration levels in NMR compared to mice; inconsistent with our findings, that study reported lower rates of basal glycolysis with concomitant lower total ATP production compared to that of mouse cells. The exact reason for this interstudy discrepancy is still unclear. However, Swovick et al. [38] conducted their measurements in confluent, quiescent cells, whereas our measurements were performed using proliferating cells. It is possible that proliferating cells may have a higher ATP demand which was met by an increased basal glycolysis in mole-rat cells. Despite this discrepancy, our findings are consistent with Swovick et al. [38] that a higher percentage of ATP production from glycolysis than OXPHOS in NMR compared to mouse cells, in keeping with the Warburg effect [69]. Gene expression analyses at transcriptional and translational levels demonstrated upregulated expressions of glycolytic enzymes, ketohexokinase and glucose transporter 5 [41], suggesting an increased glycolytic capacity than other laboratory rodents. The significant role of glycolysis in NMR metabolism was also supported by cardiac metabolomic profiling in NMR [70]. Interestingly, while lactate accumulation and metabolic acidosis due to high reliance on glycolysis can be detrimental, previous studies showed an improved lactate metabolism, and pH buffering capacity in NMR tissues [71]. It has been shown that NMR possesses tissue and/or blood buffering capacity that masks typical markers of metabolic acidosis and prioritize the synthesis of glucose from lactate during recovery in the liver [71]. Overall, multiple lines of evidence demonstrate an upregulated reliance on glycolysis for ATP production in mole-rat species.
This preference for glycolysis over OXPHOS reportedly elicits a broad range of beneficial healthspan and/or lifespan consequences [69]. For example, pharmacological and genetic manipulations that moderately inhibit mitochondrial respiration, such as metformin, TPP-thiazole, reportedly extend lifespan [72-74] by shifting ATP production from mitochondrial OXPHOS to glycolysis. The observed similar bioenergetic pattern favoring ATP generation from glycolysis is beneficial under hypoxic conditions where oxygen is limited for OXPHOS and may indirectly contribute to the extremely long lifespan of these mole rats.
Isolated mitochondria from both mole-rat species had lower state 4O respiration relative to comparison species. State 4O respiration is used as an indirect measure of the proton conductance across the mitochondrial inner membrane and serves as an indicator of mitochondrial coupling. Increased mitochondrial coupling is suggestive of enhanced efficiency of mitochondrial ATP production per unit of oxygen consumed, findings consistent with previous reports on isolated mitochondria from liver, heart and skeletal muscle of NMR [32-34]. The mitochondria isolated from the two mole-rat species in this current study, were much more coupled than those of hamsters and mice.
Lau et al. [32] observed a lower state 4O respiration and a concomitant increase in mitochondrial membrane potential (ΔΨ) in NMR heart mitochondria compared to mouse counterparts, these indicated that mitochondria were hyperpolarized during state 4O respiration. The observed lower mitochondrial proton conductance may therefore be explained by differences in either trans-membrane transporters (such as uncoupling proteins (UCPs), adenine nucleotide transport) or inner membrane phospholipid composition [75]. Upregulation in NMR uncoupling proteins [76] or a lower proportion of the polyunsaturated fatty acid docosahexaenoic acid in the mitochondrial inner membrane [77] would reduce proton conductance across the inner membrane and therefore contribute to the observed lower state 4o respiration measured in NMR in both this and Lau's study. It is worth noting that some previous studies had reported contradictory results [35-37]; those studies suggest that NMR mitochondria are more uncoupled than other laboratory rodents, as indicated by their findings of a lower ΔΨ during state 4 respiration when compared to mice. Their observations support the “Uncoupling to Survive” hypothesis which proposes that mitochondrial uncoupling would result in decreased reactive oxygen species production, reducing oxidative stress with concomitant beneficial effects on longevity [78]. Further studies are warranted to explain interstudy variability and if this rather reflects disparate acclimation or other laboratory-specific experimental conditions. Inevitably, uncoupled mitochondria would produce more heat than those that are well-coupled, facilitating better maintenance of body temperature. Given that NMR are extremely thermolabile, unable to defend body temperature at ambient temperatures even a few degrees below thermoneutrality [30,79], we believe our observation is more consistent with their physiology.
One of the strengths of the present study is the use of an integrative approach to concurrently measure respiration at the organismal, cellular and organelle levels. Such an approach enables synergistic feedback of insights from observations at multiple levels of organization, and is more informative than conducting such studies at a single biological level. In this context, we observed a mismatch between results from isolated mitochondria and the findings obtained from cellular respiration. Most notably, we observed lower rates of state 4 and similar rates of state 3 respiration in isolated mitochondria, but lower rates of state 3 and similar rates of state 4 were seen in cells from mole-rats compared to their counterparts. This may be explained by the fact that in isolated mitochondria assays, excessive substrates were provided during state 3 respiration. As a result, the slower state 3 respiration in cells may suggest a limitation/slower supply of respiratory substrates between mole-rats fibroblasts compared to their counterparts. The activation of UCPs and other factors might influence mitochondrial proton conductance in cells and could explain the differences between state 4 respiration in mitochondria and cells [80]. These results suggest that mitochondria could behave differently in cells compared to when they are isolated and supplied with unlimited substrates. Consequently, we need to be cautious when extrapolating data solely obtained from isolated mitochondria when attemping to explain physiological phenomena.
It has also been hypothesized that mitochondria basal proton leak (represented by state 4 respiration) significantly contributes to BMR in vivo [81]. This is consistent with this study that significantly lowers mitochondrial basal proton leak, cellular respiration and BMR. However, it is important to note that other factors may also contribute to the magnitude of species differences in BMR; mitochondrial basal proton leak was 39 % - 43 % lower depending on both the substrates and species, basal respiration in isolated primary dermal fibroblast cultures were reduced by 45 %–55 % although rates of ATP production were similar, and BMR was 50 %–60 % lower for two mole-rat species compared to their counterparts [82].
Our study employed both NMR and DMR to evaluate bioenergetic profiles of subterranean species. Although these two species have similar life-history traits, they are fundamentally different in their evolutionary history. Phenotypic convergence could be the result of similar adaptation to shared environments [83]. Consequently, convergent evolution can serve as a valuable proxy for repeated evolutionary experiments in nature. Moreover, understanding how convergent traits evolve, especially at different biological levels, has the potential to inform general rules about adaptation [84]. Mode-of-life theory suggests that ecological traits, such as fossoriality, are positively correlated with lifespan [39]. Strictly subterranean animals can escape predation and unfavorable above-ground conditions, these reductions in extrinsic mortality also may contribute to longer lifespan [14]. But in the physiological context, it is possible that the adaptations for life below ground where low oxygen atmospheres may commonly contribute to the unique bioenergetic properties shared by these two mole-rat species and as a byproduct thereof also could contribute to their longevity. Adaptation to hypoxic environment includes decline in BMR at the organismal level [40], higher reliance on glycolysis than OXPHOS for ATP production at the cellular level [41], and decreased mitochondrial proton conductance at the organelle level [42] traits observed in both mole-rat species.
Although the exact physiological pathways underlying these bioenergetic adaptations are unknown, a top candidate may be HIF-1. HIF-1, or its alpha subunit (HIF-1α), is considered as the master transcriptional regulator of cellular response to hypoxia [85]. In most mammals, HIF-1α is only activated during hypoxia and is constantly being degraded during normoxia. In NMR, genomic analysis indicated several mutations can prevent HIF-1α from degradation during normoxia, which would chronically upregulate HIF-1α mediated signaling pathways [76]. In DMR, HIF-1α protein level in lungs was much higher than in hamsters (Supplementary Fig. 4), and similarly NMR have higher HIF-1α than observed in mice [76]. Interestingly, HIF-1 activation could also delay cellular senescence [86], and extend lifespan in many different model species [87,88]. It is likely that adaptation to the hypoxic environment upregulate HIF-1 regulated pathways resulted in these unique bioenergetic properties. The persistent upregulation of HIF-1 pathways and the unique bioenergetics may elicit beneficial effects to promote the extremely long lifespan of these mole-rat species. Such hypothesis is in line with recent studies that indicated that instead of trade-offs, different life-history traits could be positively associated with each other, when these traits share similar underpinning physiological mechanisms [89,90].
Admittedly, our study only involved 4 species (two pairs of two-species comparisions). However, two- or few-species comparisons are still informative and relevant especially in a purely mechanistic context [91]. Despite certain degree of discrepancies between findings from isolated mitochondria, primary cells, and whole organismal measurements, all data come to a general conclusion that NMR and DMR exhibited lower levels of basal respiration. However, future study directly testing on the bioenergetic properties at these biological levels with lifespan is needed. For example, primary fibroblasts, especially lung and dermal fibroblasts are widely used in cellular senescence research [92,93]. In summary, our study shows similar bioenergetic properties shared by NMR and DMR at organismal, cellular, and organelle levels. These bioenergetic characteristics, such as lower but relatively constant BMR throughout the life, dependence on glycolysis rather than mitochondrial OXPHOS for ATP production in cells, lower basal proton conductance through mitochondria inner membrane, could result from adaptation to hypoxic environments. These metabolic adaptations, on the other hand, might be beneficial for other life-history traits such as longevity. Future studies directly testing these underlying mechanisms, together with studies that involve other fossorial or semi-fossorial rodents would provide important insights for changes in metabolism in hypoxic environments, and its effects on aging. More importantly, these studies can reveal mechanistically how different life-history traits interact and influence each other.
Supplementary Material
Acknowledgement
We would like to thank the vivarium staff from both University of Memphis and Calico Life Sciences for their excellent animal care. We also thank Matthew Butawan, Megan Smith, Mary McMahon, and Wendy Craft for their technical support. Y.Z. was supported by US National Science Foundation grant IOS – 2037735, while Calico Life Sciences funded work undertaken there.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbabio.2022.148582.
References
- [1].Lane N, The Vital Question: Energy, Evolution, and the Origins of Complex Life, WW Norton & Company, 2015. [Google Scholar]
- [2].Lotka AJ, Contribution to the energetics of evolution, Proc. Natl. Acad. Sci. U. S. A 8 (1922) 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stearns SC, Trade-offs in life-history evolution, Funct. Ecol 3 (1989) 259–268. [Google Scholar]
- [4].Monaghan P, Metcalfe NB, Torres R, Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation, Ecol. Lett 12 (2009) 75–92. [DOI] [PubMed] [Google Scholar]
- [5].Speakman JR, Selman C, The free-radical damage theory: accumulating evidence against a simple link of oxidative stress to ageing and lifespan, Bioessays 33 (2011) 255–259. [DOI] [PubMed] [Google Scholar]
- [6].Speakman JR, Blount JD, Bronikowski AM, Buffenstein R, Isaksson C, B. Kirkwood T, Monaghan P, Ozanne SE, Beaulieu M, Briga M, Oxidative stress and life histories: unresolved issues and current needs, Ecol. Evol 5 (2015) 5745–5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Burger JR, Hou C, Brown JH, Toward a metabolic theory of life history, Proc. Natl. Acad. Sci 116 (2019) 26653–26661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zera AJ, Harshman LG, The physiology of life history trade-offs in animals, Annu. Rev. Ecol. Syst 32 (2001) 95–126. [Google Scholar]
- [9].Schulze-Makuch D, The naked mole-rat: an unusual organism with an unexpected latent potential for increased Intelligence? Life 9 (2019) 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Saldmann F, Viltard M, Leroy C, Friedlander G, The naked mole rat: a unique example of positive oxidative stress, Oxidative Med. Cell. Longev 2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lewis KN, Soifer I, Melamud E, Roy M, McIsaac RS, Hibbs M, Buffenstein R, Unraveling the message: insights into comparative genomics of the naked mole-rat, Mamm. Genome 27 (2016) 259–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Buffenstein R, Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species, J. Comp. Physiol. B 178 (2008) 439–445. [DOI] [PubMed] [Google Scholar]
- [13].Lewis KN, Buffenstein R, The naked mole-rat: a resilient rodent model of aging, longevity, and healthspan, in: Handbook of the Biology of Aging, Elsevier, 2016, pp. 179–204. [Google Scholar]
- [14].Buffenstein R, Jarvis J, The naked mole rat–a new record for the oldest living rodent, in: Science of Aging Knowledge Environment 2002, SAGE KE, 2002. pe7–pe7. [DOI] [PubMed] [Google Scholar]
- [15].Schmidt CM, Jarvis JU, Bennett NC, The long-lived queen: reproduction and longevity in female eusocial Damaraland mole-rats (Fukomys damarensis), Afr. Zool 48 (2013) 193–196. [Google Scholar]
- [16].Ruby JG, Smith M, Buffenstein R, Naked mole-rat mortality rates defy gompertzian laws by not increasing with age, elife 7 (2018), e31157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ivy CM, Sprenger RJ, Bennett NC, van Jaarsveld B, Hart DW, Kirby AM, Yaghoubi D, Storey KB, Milsom WK, Pamenter ME, The hypoxia tolerance of eight related african mole-rat species rivals that of naked mole-rats, despite divergent ventilatory and metabolic strategies in severe hypoxia, Acta Physiol. 228 (2020), e13436. [DOI] [PubMed] [Google Scholar]
- [18].Lewis KN, Andziak B, Yang T, Buffenstein R, The naked mole-rat response to oxidative stress: just deal with it, Antioxid. Redox Signal 19 (2013) 1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Buffenstein R, Craft W, The idiosyncratic physiological traits of the naked mole-rat; a resilient animal model of aging, longevity, and healthspan, in: T.P.A.M.H. Buffenstein R (Ed.), The Extraordinary Biology of the Naked Mole-Rat, Springer, 2021, pp. 221–254. [DOI] [PubMed] [Google Scholar]
- [20].Buffenstein R, Amoroso V, Andziak B, Avdieiev S, Azpurua J, Barker AJ, C. Bennett N, Brieño-Enríquez MA, Bronner GN, Coen C, The naked truth: a comprehensive clarification and classification of current ‘myths’ in naked mole-rat biology, Biol. Rev (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jarvis J, Bennett N, Eusociality has evolved independently in two genera of bathyergid mole-rats—but occurs in no other subterranean mammal, Behav. Ecol. Sociobiol 33 (1993) 253–260. [Google Scholar]
- [22].Zhang SY, Pamenter ME, Ventilatory, metabolic, and thermoregulatory responses of Damaraland mole rats to acute and chronic hypoxia, J. Comp. Physiol. B 189 (2019) 319–334. [DOI] [PubMed] [Google Scholar]
- [23].Sackton TB, Clark N, Convergent evolution in the genomics era: new insights and directions, in: The Royal Society, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Buffenstein R, The naked mole-rat: a new long-living model for human aging research, J. Gerontol. A Biol. Sci. Med. Sci 60 (2005) 1369–1377. [DOI] [PubMed] [Google Scholar]
- [25].Shepard A, Kissil JL, The use of non-traditional models in the study of cancer resistance—the case of the naked mole rat, Oncogene 39 (2020) 5083–5097. [DOI] [PubMed] [Google Scholar]
- [26].Zhang L, Yang F, Zhu W-L, Evidence for the ‘rate-of-living’ hypothesis between mammals and lizards, but not in birds, with field metabolic rate, Comp. Biochem. Physiol. A Mol. Integr. Physiol 253 (2021), 110867. [DOI] [PubMed] [Google Scholar]
- [27].Lints FA, The rate of living theory revisited, Gerontology 35 (1989) 36–57. [DOI] [PubMed] [Google Scholar]
- [28].Austad SN, The comparative biology of mitochondrial function and the rate of aging, Integr. Comp. Biol 58 (2018) 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lovegrove B, The metabolism of social subterranean rodents: adaptation to aridity, Oecologia 69 (1986) 551–555. [DOI] [PubMed] [Google Scholar]
- [30].McNab BK, The metabolism of fossorial rodents: a study of convergence, Ecology 47 (1966) 712–733. [Google Scholar]
- [31].O'Connor TP, Lee A, Jarvis JU, Buffenstein R, Prolonged longevity in naked mole-rats: age-related changes in metabolism, body composition and gastrointestinal function, Comp. Biochem. Physiol. A Mol. Integr. Physiol 133 (2002) 835–842. [DOI] [PubMed] [Google Scholar]
- [32].Lau G, Milsom W, Richards J, Pamenter M, Heart mitochondria from naked mole-rats (Heterocephalus glaber) are more coupled, but similarly susceptible to anoxia-reoxygenation stress than in laboratory mice (Mus musculus), Comp. Biochem. Physiol. B: Biochem. Mol. Biol 240 (2020), 110375. [DOI] [PubMed] [Google Scholar]
- [33].Heinze I, Bens M, Calzia E, Holtze S, Dakhovnik O, Sahm A, Kirkpatrick JM, Szafranski K, Romanov N, Sama SN, Species comparison of liver proteomes reveals links to naked mole-rat longevity and human aging, BMC Biol. 16 (2018) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Munro D, Baldy C, Pamenter ME, Treberg JR, The exceptional longevity of the naked mole-rat may be explained by mitochondrial antioxidant defenses, Aging Cell 18 (2019), e12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Skulachev VP, Holtze S, Vyssokikh MY, Bakeeva LE, Skulachev MV, Markov AV, Hildebrandt TB, Sadovnichii VA, Neoteny, prolongation of youth: from naked mole rats to “naked apes”(humans), Physiol. Rev 97 (2) (2017) 699–720. [DOI] [PubMed] [Google Scholar]
- [36].Vyssokikh MY, Holtze S, Averina OA, Lyamzaev KG, Panteleeva AA, Marey MV, Zinovkin RA, Severin FF, Skulachev MV, Fasel N, Mild depolarization of the inner mitochondrial membrane is a crucial component of an anti-aging program, Proc. Natl. Acad. Sci 117 (2020) 6491–6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Holtze S, Eldarov C, Vays V, Vangeli I, Vysokikh MY, Bakeeva L, Skulachev V, Hildebrandt T, Study of age-dependent structural and functional changes of mitochondria in skeletal muscles and heart of naked mole rats (Heterocephalus glaber), Biochem. Mosc 81 (2016) 1429–1437. [DOI] [PubMed] [Google Scholar]
- [38].Swovick K, Firsanov D, Welle KA, Hryhorenko JR, Wise JP Sr., George C, Sformo TL, Seluanov A, Gorbunova V, Ghaemmaghami S, Interspecies differences in proteome turnover kinetics are correlated with lifespans and energetic demands, Mol. Cell. Proteomics 100041 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Healy K, Guillerme T, Finlay S, Kane A, Kelly SB, McClean D, Kelly DJ, Donohue I, Jackson AL, Cooper N, Ecology and mode-of-life explain lifespan variation in birds and mammals, Proc. R. Soc. B Biol. Sci 281 (2014), 20140298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Singer D, Metabolic adaptation to hypoxia: cost and benefit of being small, Respir. Physiol. Neurobiol 141 (2004) 215–228. [DOI] [PubMed] [Google Scholar]
- [41].Park TJ, Reznick J, Peterson BL, Blass G, Omerbašić D, Bennett NC, J Kuich PH, Zasada C, Browe BM, Hamann W, Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat, Science 356 (2017) 307–311. [DOI] [PubMed] [Google Scholar]
- [42].Gnaiger E, Méndez G, Hand SC, High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia, Proc. Natl. Acad. Sci 97 (2000) 11080–11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Smith M, Buffenstein R, Managed care of naked mole-rats, in: The Extraordinary Biology of the Naked Mole-Rat, Springer, 2021, pp. 381–407. [DOI] [PubMed] [Google Scholar]
- [44].Yap KN, Kim OR, Harris KC, Williams TD, Physiological effects of increased foraging effort in a small passerine, J. Exp. Biol 220 (2017) 4282–4291. [DOI] [PubMed] [Google Scholar]
- [45].Weiner J, Heldmaier G, Metabolism and thermoregulation in two races of Djungarian hamsters: Phodopus sungorus sungorus and P. s. campbelli, Comp. Biochem. Physiol. A Comp. Physiol 86 (1987) 639–642. [DOI] [PubMed] [Google Scholar]
- [46].Lighton JR, Measuring Metabolic Rates: A Manual for Scientists, Oxford University Press, 2018. [Google Scholar]
- [47].Joly-Amado A, Serraneau KS, Brownlow M, de Evsikova CM, Speakman JR, Gordon MN, Morgan D, Metabolic changes over the course of aging in a mouse model of tau deposition, Neurobiol. Aging 44 (2016) 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Liang S, Mele J, Wu Y, Buffenstein R, Hornsby PJ, Resistance to experimental tumorigenesis in cells of a long-lived mammal, the naked mole-rat (Heterocephalus glaber), Aging Cell 9 (2010) 626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Swovick K, Firsanov D, Welle KA, Hryhorenko J, Wise JP, George C, Sformo TL, Seluanov A, Gorbunova V, Ghaemmaghami S, Interspecies differences in proteome turnover kinetics are correlated with lifespans and energetic demands, Mol. Cell. Proteomics 20 (100041) (2020) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Seluanov A, Vaidya A, Gorbunova V, Establishing primary adult fibroblast cultures from rodents, JoVE (Journal of Visualized Experiments) 44 (e2033) (2010), e2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang Y, Wong HS, Are mitochondria the main contributor of reactive oxygen species in cells? J. Exp. Biol 224 (2021). [DOI] [PubMed] [Google Scholar]
- [52].Wong H-S, Benoit B, Brand MD, Mitochondrial and cytosolic sources of hydrogen peroxide in resting C2C12 myoblasts, Free Radic. Biol. Med 130 (2019) 140–150. [DOI] [PubMed] [Google Scholar]
- [53].Mookerjee SA, Gerencser AA, Nicholls DG, Brand MD, Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements, J. Biol. Chem 292 (2017) 7189–7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Spear RK, Lumeng L, A method for isolating lung mitochondria from rabbits, rats, and mice with improved respiratory characteristics, Anal. Biochem 90 (1978) 211–219. [DOI] [PubMed] [Google Scholar]
- [55].Mookerjee SA, Quinlan CL, Wong H-S, Dighe P, Brand MD, Plate-based measurement of respiration by isolated mitochondria, in: Mitochondrial Bioenergetics, Springer, 2018, pp. 301–313. [DOI] [PubMed] [Google Scholar]
- [56].White CR, Seymour RS, Mammalian basal metabolic rate is proportional to body mass2/3, Proc. Natl. Acad. Sci 100 (2003) 4046–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rogers GW, Brand MD, Petrosyan S, Ashok D, Elorza AA, Ferrick DA, Murphy AN, High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria, PloS one 6 (2011), e21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Packard GC, Boardman TJ, The use of percentages and size-specific indices to normalize physiological data for variation in body size: wasted time, wasted effort? Comp. Biochem. Physiol. A Mol. Integr. Physiol 122 (1999) 37–44. [Google Scholar]
- [59].Bennett N, Clarke B, Jarvis J, A comparison of metabolic acclimation in two species of social mole-rats (Rodentia, Bathyergidae) in southern Africa, J. Arid Environ 23 (1992) 189–198. [Google Scholar]
- [60].Jacobus W, Moreadith R, Vandegaer K, Mitochondrial respiratory control. Evidence against the regulation of respiration by extramitochondrial phosphorylation potentials or by [ATP]/[ADP] ratios, J. Biol. Chem 257 (1982) 2397–2402. [PubMed] [Google Scholar]
- [61].Kadenbach B, Arnold S, A second mechanism of respiratory control, FEBS Lett. 447 (1999) 131–134. [DOI] [PubMed] [Google Scholar]
- [62].Lambert AJ, Boysen HM, Buckingham JA, Yang T, Podlutsky A, Austad SN, Kunz TH, Buffenstein R, Brand MD, Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms, Aging Cell 6 (2007) 607–618. [DOI] [PubMed] [Google Scholar]
- [63].Lambert AJ, Buckingham JA, Boysen HM, Brand MD, Low complex I content explains the low hydrogen peroxide production rate of heart mitochondria from the long-lived pigeon, Columba livia, Aging Cell 9 (2010) 78–91. [DOI] [PubMed] [Google Scholar]
- [64].Withers P, Jarvis J, The effect of huddling on thermoregulation and oxygen consumption for the naked mole-rat, Comp. Biochem. Physiol. A Physiol 66 (1980) 215–219. [Google Scholar]
- [65].Poehlman ET, Goran MI, Gardner AW, Ades PA, Arciero PJ, Katzman-Rooks SM, Montgomery SM, Toth MJ, Sutherland PT, Determinants of decline in resting metabolic rate in aging females, Am. J. Physiol. Endocrinol. Metab 264 (1993) E450–E455. [DOI] [PubMed] [Google Scholar]
- [66].McCarter R, Palmer J, Energy metabolism and aging: a lifelong study of Fischer 344 rats, Am. J. Physiol. Endocrinol. Metab 263 (1992) E448–E452. [DOI] [PubMed] [Google Scholar]
- [67].Pontzer H, Yamada Y, Sagayama H, Ainslie PN, Andersen LF, Anderson LJ, L. Arab I Baddou, K. Bedu-Addo, E.E. Blaak, Daily energy expenditure through the human life course, Science 373 (2021) 808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Stoll EA, Karapavlovic N, Rosa H, Woodmass M, Rygiel K, White K, Turnbull DM, Faulkes CG, Naked mole-rats maintain healthy skeletal muscle and complex IV mitochondrial enzyme function into old age, Aging (Albany NY) 8 (2016) 3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].DeBerardinis RJ, Chandel NS, We need to talk about the Warburg effect, Nat. Metab 2 (2020) 127–129. [DOI] [PubMed] [Google Scholar]
- [70].Faulkes CG, Eykyn TR, Aksentijevic D, Cardiac metabolomic profile of the naked mole-rat—glycogen to the rescue, Biol. Lett 15 (2019), 20190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Pamenter ME, Dzal YA, Thompson WA, Milsom WK, Do naked mole rats accumulate a metabolic acidosis or an oxygen debt in severe hypoxia? J. Exp. Biol 222 (3) (2019), jeb191197. [DOI] [PubMed] [Google Scholar]
- [72].De Haes W, Frooninckx L, Van Assche R, Smolders A, Depuydt G, Billen J, Braeckman BP, Schools L, Temmerman L, Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2, Proc. Natl. Acad. Sci 111 (2014) E2501–E2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Tavallaie M, Voshtani R, Deng X, Qiao Y, Jiang F, Collman JP, Fu L, Moderation of mitochondrial respiration mitigates metabolic syndrome of aging, Proc. Natl. Acad. Sci 117 (2020) 9840–9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Cristina D, Cary M, Lunceford A, Clarke C, Kenyon C, A regulated response to impaired respiration slows behavioral rates and increases lifespan in Caenorhabditis elegans, PLoS Genet. 5 (2009), e1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Brown GC, Murphy MP, Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD, Mitochondrial proton and electron leaks, Essays Biochem. 47 (2010) 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kim EB, Fang X, Fushan AA, Huang Z, Lobanov AV, Han L, Marino SM, Sun X, Turanov AA, Yang P, Genome sequencing reveals insights into physiology and longevity of the naked mole rat, Nature 479 (2011) 223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mitchell TW, Buffenstein R, Hulbert A, Membrane phospholipid composition may contribute to exceptional longevity of the naked mole-rat (Heterocephalus glaber): a comparative study using shotgun lipidomics, Exp. Gerontol 42 (2007) 1053–1062. [DOI] [PubMed] [Google Scholar]
- [78].Brand M, Uncoupling to survive? The role of mitochondrial inefficiency in ageing, Exp. Gerontol 35 (2000) 811–820. [DOI] [PubMed] [Google Scholar]
- [79].Buffenstein R, Yahav S, Is the naked mole-rat hererocephalus glaber an endothermic yet poikilothermic mammal? J. Therm. Biol 16 (1991) 227–232. [Google Scholar]
- [80].Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Superoxide activates mitochondrial uncoupling proteins, Nature 415 (2002) 9699. [DOI] [PubMed] [Google Scholar]
- [81].Porter RK, Brand MD, Body mass dependence of H+ leak in mitochondria and its relevance to metabolic rate, Nature 362 (1993) 628–630. [DOI] [PubMed] [Google Scholar]
- [82].Polymeropoulos ET, Heldmaier G, Frappell PB, McAllan BM, Withers KW, Klingenspor M, White C, Jastroch M, Phylogenetic differences of mammalian basal metabolic rate are not explained by mitochondrial basal proton leak, Proc. R. Soc. B Biol. Sci 279 (2012) 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chen L, DeVries AL, Cheng C-HC, Convergent evolution of antifreeze glycoproteins in Antarctic notothenioid fish and Arctic cod, Proc. Natl. Acad. Sci 94 (1997) 3817–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Losos JB, Convergence, adaptation, and constraint, evolution: international journal of organic, Evolution 65 (2011) 1827–1840. [DOI] [PubMed] [Google Scholar]
- [85].Semenza GL, Targeting HIF-1 for cancer therapy, Nat. Rev. Cancer 3 (2003) 721–732. [DOI] [PubMed] [Google Scholar]
- [86].Welford SM, Bedogni B, Gradin K, Poellinger L, Powell MB, Giaccia AJ, HIF1α delays premature senescence through the activation of MIF, Genes Dev. 20 (2006) 3366–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zhong L, D'Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1α, Cell 140 (2010) 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chen D, Thomas EL, Kapahi P, HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans, PLoS Genet. 5 (2009), e1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhang Y, Hood WR, Current versus future reproduction and longevity: a re-evaluation of predictions and mechanisms, J. Exp. Biol 219 (2016) 3177–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zhang Y, Brasher AL, Park NR, Taylor HA, Kavazis AN, Hood WR, High activity before breeding improves reproductive performance by enhancing mitochondrial function and biogenesis, J. Exp. Biol 221 (7) (2018) jeb. 177469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Goymann W, Schwabl H, The tyranny of phylogeny—a plea for a less dogmatic stance on two-species comparisons: funding bodies, journals and referees discourage two-or few-species comparisons, but such studies provide essential insights complementary to phylogenetic comparative studies, BioEssays 43 (2021), 2100071. [DOI] [PubMed] [Google Scholar]
- [92].Zhao Y, Tyshkovskiy A, Muñoz-Espín D, Tian X, Serrano M, De Magalhaes JP, Nevo E, Gladyshev VN, Seluanov A, Gorbunova V, Naked mole rats can undergo developmental, oncogene-induced and DNA damage-induced cellular senescence, Proc. Natl. Acad. Sci 115 (2018) 1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Odeh A, Dronina M, Domankevich V, Shams I, Manov I, Downregulation of the inflammatory network in senescent fibroblasts and aging tissues of the long-lived and cancer-resistant subterranean wild rodent, Spalax, Aging Cell 19 (2020), e13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.