Abstract
Skin is the largest organ of the human body, having the purpose of regulating temperature, protecting us from microbes or mechanical shocks, and allowing the sensations from touch. It is generally accepted that aging induces profound changes in the skin’s biochemical, structural and physical properties, which can lead to impaired biological functions and/or diverse diseases. So far, the effects of aging on these skin properties have been well documented. However, very few studies have focused exclusively on the relationship among these critical properties in the aging process, which is this review’s primary focus. Many in vivo, ex vivo, and in vitro techniques have been previously used to characterize these properties of the skin. This review aims to provide a comprehensive overview on the effects of aging on the changes in biochemical, structural, and physical properties, and explore the potential mechanisms of skin with the relation between these properties. First, we review different or contradictory results of aging-related changes in representative parameters of each property, including the interpretations of the findings. Next, we discuss the need for a standardized method to characterize aging-related changes in these properties, to improve the way of defining age-property relationship. Moreover, potential mechanisms based on the previous results are explored by linking the biochemical, structural, and physical properties. Finally, the need to study changes of various functional properties in the separate skin layers is addressed. This review can help understand the underlying mechanism of aging-related alterations, to improve the evaluation of the aging process and guide effective treatment strategies for aging-related diseases.
Keywords: Aging, Skin tissue, Biochemical property, Structural property, Physical property
Introduction
Human skin tissue, which is composed of the epidermis, dermis, and hypodermis, takes up one-sixth of a person’s total body weight, providing a functional barrier that protects the underlying organs or tissues from environmental pollution, chemicals, mechanical shocks, temperature changes and microorganisms (Biglari et al. 2019; Huan et al. 2019; Barros et al. 2021). The epidermis is the outermost layer of the three layers, containing several sublayers such as the stratum corneum (also known as a horny layer), stratum lucidum, stratum granulosum, stratum spinosum, stratum basale, and the basement membrane. The dermis is composed of two connective tissues, the papillary dermis and the reticular dermis (Haake and Holbrook 1999), where the main components include dermal fibroblasts, collagen/elastin fibers, a ground substance such as glycosaminoglycans (GAGs), and blood/lymphatic vessels. This layer is responsible for giving flexibility, elasticity, and firmness to the skin, while providing physical support to the epidermis, vascular networks, nerves, and appendages. The hypodermis (also known as the subcutaneous fat layer), mainly consisting of fat, blood vessels and nerves, provides a reservoir of progenitor cells, energy from fat, and insulation to the body. Moreover, it protects the skin from mechanical impact, links the dermis to the various organs, and is associated with major endocrine and paracrine signaling (Sepe et al. 2010; Tchkonia et al. 2010; Chang et al. 2017; Carrer et al. 2018; Schosserer et al. 2018).
Aging induces marked alterations in biochemical, structural and physical properties of the skin, which results in an impairment of biological functions and a diversity of diseases (Diridollou et al. 2001; Harn et al. 2019). Clear visual skin changes during aging include thinning, wrinkling, sagging, and also the greying of hair. In general, aging of human skin can be driven by two basic processes: intrinsic aging (also known as chronological or natural aging), a process that happens due to inherent genetics; and extrinsic aging, a process occurring due to environmental conditions, such as chronic sunlight exposure or smoking (Oh et al. 2011). Early studies have demonstrated that some of the aging-related alterations in the skin can differ depending on the aging process and these skin changes can even be contradictory. As an example, crosslinks in collagen were observed to increase in the photoaged skin (Kligman et al. 1989), while a decrease can be found in the chronologically aged skin (Kaur et al. 2019). Hence, it is essential to separate these two aging processes while analyzing and interpreting test results.
So far, numerous studies have been reported on the effects of aging on several functional properties such as biochemical (or molecular), structural (or morphological), and physical properties. However, no systematic study or review has been performed to explore how such functional properties relate to each other, which can be one of the main factors to understand the underlying mechanism of aging-related alterations and skin aging. Here we present a review of systematic and detailed studies on the biomechanical, structural and physical changes at different scales (nano, micro and macro), during aging of the human skin and how such properties are associated (Fig. 1). The goal of this review is to integrate the results of previous studies and reports of aging-related changes in the skin, so potential mechanisms based on the aging-related properties can be explored. In the first chapter, an attempt is made to delineate the aging-related changes in biochemical properties such as ground substance/water content, collagen density/synthesis/fragmentation/solubility, and collagen crosslinking. In the second chapter, aging-related changes in structural properties, such as extracellular matrix (ECM) fiber orientation and skin thickness, are reviewed. In the third chapter, we review aging-related changes in physical properties, including mechanical/viscoelastic properties and transport properties. Finally, we discuss potential mechanisms of aging-related alterations by relating all the properties described before.
Fig. 1.
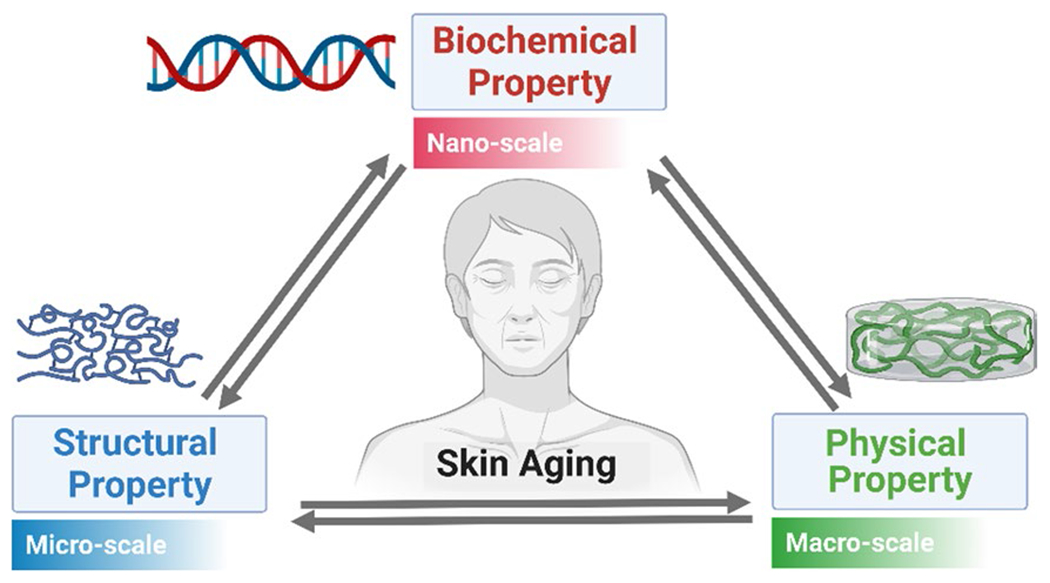
A graphical schematic for the relationship between aging-related biochemical, structural, and physical changes at the nano, micro, and macro-scale
Aging-related changes in biochemical/molecular properties
Water content and ground substance
Water is an essential component in the skin and its total amount and structure changes with the progression of age, which is still a poorly understood process (Gniadecka et al. 1998). GAGs, which account for 0.1–0.3% of the dry weight of the dermis, play a crucial role in maintaining the water content of skin tissue (Taylor and Gallo 2006; Stern and Maibach 2008), showing also critical capacity of holding water due to its negative charge that results by the presence of carboxyl and sulfate groups. GAGs are polysaccharide compounds, and can be of six different types: chondroitin sulfate (CS), dermatan sulfate (DS), keratan sulfate (KS), heparan sulfate (HS), heparin (HP), and hyaluronic acid (HA) (Oh et al. 2011). An early study showed that a change in water content or amount during the aging process could be estimated as a correlation of the change in the number of GAGs. Oh et al. measured the amount of HA, total sulfated GAG (tsGAG), and total uronic acid (tUA) in the skin tissue of both forearm and buttock of young (20–30 years) and aging (70–80 years) human subjects (Oh et al. 2011). In the photoaged forearm dermis, the content of HA, tsGAG, tUA, and tissue water showed an increase, while no change was observed in the photoaged epidermis. For the intrinsically aged buttock, however, HA in the epidermis, and tsGAG and tUA in the dermis reduced its values during aging. Despite these findings, the relationship between GAGs and water content is still controversial, needing further analysis. Other studies have also directly measured water content in aging/aged and young tissue. Russel et al. tested the skin of the back of the left hand from 16 adults aged between 24 and 63 years, suggesting that aged skin has a less amount of water compared to young skin (Potts et al. 1984). Another experimental investigation revealed that aged skin is less hydrated, showing a reduced lipid content compared to a young skin (Roskos et al. 1986).
In contrast, Nakagawa et al. observed that aged dermis has a much higher water content than young dermis, by using in vivo confocal Raman spectroscopy (Nakagawa et al. 2010). Magnetic resonance imaging (MRI) has also been widely used to measure water content (Richard et al. 1993; Querleux et al. 1994). For example, Richard et al. discovered that the upper part of the dermis (approximately 200 μm in thickness) contains more water protons and content in chronologically aged skin than young adult skin, using MRI technology (Richard et al. 1993). Kligman found an increased water content in the abdomen dermis with the increase of age (Kligman 1979). Pearce et al. observed that the water content of the dermis is enriched with an advanced age, however the total amount of lipid does not change, based on the collected data from seven women and six men aged 17–81 years (Pearce and Grimmer 1972). The increased water content with chronological age may be explained by concepts of bound water and mobile water (Richard et al. 1993; Gniadecka et al. 1998). While the bound water can be described as water that binds to diverse proteins (e.g., collagen), mobile water has its molecules bound to each other, but is not bound to other proteins (also known as bulk water or tetrahedron water). The mobile water content was found to be greater in elderly individuals than young individuals, possibly due to the presence of fewer proteins such as collagen. In the case of photoaged skin, mounting evidence also revealed that water content and hydration are elevated (Gniadecka et al. 1998). Studies have demonstrated that water tends to accumulate in the upper part of the dermis, and a plausible explanation is that, since the amount of GAG increases in photoaged skin, this could prevent the binding of water molecules to the proteins by holding the water. Moreover, the degree of protein folding such as collagen was found to increase in the photoaged skin, and thus this could promote movement from the bound water to mobile water.
Collagen density, synthesis, fragmentation, and solubility
Collagen constitutes approximately 70% of the skin dry mass, and collagen density is defined as the packing of fibrils or fibers in the skin dermis. It is generally accepted that collagen density decreases with age, in both papillary and reticular dermis layers (Diridollou et al. 2001; Panwar et al. 2015; Kaur et al. 2019; Blair et al. 2020). This decrease may happen due to the reduction in collagen synthesis and/or the increase in collagenase activity (Uitto 1970; Bailey et al. 1974). Marcos-Garces et al. found a decrease in the density of collagen bundles in the aging process, with a higher reduction in the reticular dermis, that goes from 58% in aged tissue to 81% in young tissue (Marcos-Garcés et al. 2014). Shuster et al. measured the collagen density of the forearm skin of 72 males and 76 females aged 15–93 years, and found out that skin collagen tends to decrease linearly by approximately 1% per year throughout the adult life, while the loss rate is the same across gender (Shuster et al. 1975). In the same study, they also confirmed that the decrease rate of skin collagen is higher than the skin thickness (Shuster et al. 1975). The other study by Want et al. exhibited that collagen fiber density decreases with age, contrarily to elastin fiber density, which increases with age (Wang et al. 2018).
The experimental observation by Varani et al. revealed that procollagen synthesis is significantly decreased in intrinsically aged skin due to the partially degraded collagen which is one of the hallmarks of skin aging (Varani et al. 2001). In this study, the number of collagen fragments in the hip skin samples was higher in old individuals aged 80 + years as compared to those aged 18–29 years (Varani et al. 2001). In other studies, they also concluded that collagen synthesis is significantly reduced by approximately 68–75% in chronologically aged skin (subjects with 80+ years), when compared to young skin (subjects with 18–29 years) (Varani et al. 2000, 2006). The plausible mechanism for the reduced collagen synthesis may be illustrated by the fact that collagen-degrading matrix metalloproteinase-1 (MMP-1) (Varani et al. 2000), which is produced by fibroblasts or macrophages, is highly upregulated during aging; thereby leading to an increase of fragmented collagen and thus to a defective mechanical stimulation. This abnormal mechanical tension does not properly stimulate the fibroblasts to produce enough collagen molecules. Another possible mechanism could be explained by aged fibroblasts. In vitro study performed by Verani et al. showed that fibroblasts from old individuals (80 + years) produce an average of 56 ng of type I procollagen per 5 × 104 cells, whereas those from young individuals (18–29 years) synthesize 82 ng per 5 × 104 cells (Varani et al. 2006). Furthermore, the number of fibroblasts in aged skin was observed to be approximately 35% lower than that in young skin, which may be related to the reduced type I procollagen synthesis.
Mature and stable collagen fibrils or bundles are insoluble in dilute acid or neutral salt solutions, whereas immature collagens (e.g., tropocollagens), which are the newly formed molecules, are soluble (Nimni et al. 1965). It has been well agreed that the presence of soluble collagen is essential to maintain a long-lasting hydrating effect, decreasing wrinkles or fine lines, and helping wound healing (because of microcirculation promotion) (Morganti et al. 1986). Since collagen solubility varies with age, the functional properties and state of the skin (e.g., stiffness, elasticity, and hydration) are changed during aging processes. Early studies exhibited that the change in collagen solubility varies depending on the type of aging processes—intrinsic and extrinsic aging. For intrinsic aging, numerous studies have shown that collagen fibrils in the non-photoaged dermis become more mature and insoluble with chronological age (Nimni et al. 1965) (Miyahara et al. 1982) This may happen due to an increase in the crosslinks such as histidinohydroxylysinonorleucine (HHL), where HHL is known to be the most mature and stable crosslink in skin tissue (Saito et al. 1997). Other studies have suggested that the relation of collagen solubility with age differs and is highly dependent on the solution type. For instance, a significant aging-related decrease in acetic acid-soluble collagen was observed, whereas no similar change was found in neutral salt-soluble collagen (Schnider and Kohn 1981). In addition, prior findings revealed that most of the insoluble collagen from older people was not solubilized by pepsin digestion while that from infants was mostly solubilized by pepsin digestion (Miyahara et al. 1982).
Extrinsic aging shows a different aspect of collagen solubility with age. Sams et al. demonstrated that the quantity of total soluble collagen increases in photoaged dermis compared to the non-photoaged dermis, whereas insoluble collagen decreases (Sams and Smith 1961). In particular, it was observed that the salt-soluble collagen fraction in the UV-irradiated skin primarily contributes to the increase in the total soluble collagen, although both pepsin-soluble and acid-soluble collagen slightly increase (Nomura et al. 2004). The decrease in insoluble collagen may be caused by the activation of proteolytic enzymes by sunlight (i.e., ultraviolet radiation) (Fisher et al. 1996), but further studies need to be completed to properly understand the aging-related changes in soluble and insoluble collagen in the photoaging process.
Collagen crosslinking in the dermal matrix
Collagen intermolecular and intramolecular crosslinking plays a central role in the stability and tensile strength of collagen matrices (Yamauchi et al. 1991). It is known that except for the skin, the crosslink practically does not appear in other major collagenous tissues like bone, dentin, tendon, and ligament. Aging-related covalent collagen crosslinks include HHL, deoxypyridinoline (DPyr), pyridinoline (Pyr), dehydrohydroxylysinonorleucine (deH-HLNL) and histidinohydroxymerodesmosine (HHMD) (Zhang et al. 2019). The effects of aging on collagen crosslinking are still controversial, although a lot of research in this topic has been carried out. The early findings showed that collagen crosslinks increase with chronologic age, possibly due to two mechanisms, such as an enzyme-controlled process that converts immature divalent crosslinks into mature trivalent crosslinks of HHL, Pyr and DPyr, and a non-enzymatic glycosylation leading to secretion of advanced glycosylation end (AGE) products, like pentosidine (Pen) (Wulf et al. 2004). Yamauchi et al. quantified a degree of the crosslink (e.g., HHL content) in aged human skin as well as bovine skin, and suggested a rapid increase in the crosslink from birth to maturation, but afterwards a gradual increase (Yamauchi et al. 1988). The increase in non-reducible crosslinks between molecules with the increase of age contributes to a higher collagen stability (Thakur et al. 2009). In contrast, there was compelling evidence that crosslinks decrease with age, due to loss of crosslinking protein such as hyaluronan and proteoglycan link protein 1 (HAPLN1) (Kaur et al. 2019).
Unlike the majority of cases in chronological aging, UV-driven photoaging was shown to decrease collagen crosslinks. Yamauchi et al. discovered that the crosslinks are significantly reduced in photoaged skin tissue, in contrast to chronologically aged skin, possibly due to either photolysis of HHL or hampering HHL formation by UV radiation (Yamauchi et al. 1991). Another collagen crosslink, Pyr, was also found to be vulnerable to UV light, thereby decreasing in photoaged skin, which implies reduced crosslinks (Sakura et al. 1982).
Aging-related changes in structural/morphological properties
Skin thickness
It has been agreed that skin thickness changes with age but results about the effect of aging on skin stiffness differ among studies (Waller and Maibach 2005). To date, a large number of studies have been performed to investigate changes in both the whole skin tissue and individual layers of the skin, such as the epidermis, the dermis, the stratum corneum, and the hypodermis. Early findings have shown that the whole skin thickness can either decrease or increase with chronological aging. Ishikawa et al. and Gniadecka et al. discovered thinning of forehead skin during the aging process using ultrasound techniques (Ishikawa et al. 1995; Gniadecka 1998). Escoffier et al. also found a negative correlation between age and skin thickness, showing a more profound reduction after 65 years of age (Escoffier et al. 1989). In contrast, Pellacani et al. found an increase in facial skin thickness with the increase of age, by testing 40 people with ages of 25–90 years using B-mode ultrasonography (Pellacani and Seidenari 1999). Some studies have shown that the tendency of changes in skin thickness varies depending on different ages. For example, the previous research concluded that the skin thickness increases at the age of 10–20 years, however it decreases after 50 years (Kalra and Lowe 2016). Other studies suggested that the relationship between age and skin thickness looks like a bell-shaped curve with an increase in the first 20 years of age and, after that, a gradual or marked decrease (Dykes and Marks 1977; Denda and Takahashi 1990; Seidenari et al. 1994).
Collagen fiber orientation
It is essential to understand collagen fiber orientation during aging, since the fiber orientation can be a potential biomarker for various diseases such as cancer (Majeed et al. 2017), myocardial infarction (Goergen et al. 2016), and aging/aged skin (Wu et al. 2011). Very few studies have been performed to examine the aging effect on the collagen fiber orientation in human skin tissue. The prior research performed by Eklouh-Molinier et al. found that collagen fiber orientation is notably altered with skin aging, by testing skin samples from two age groups (35–38 years and 60–66 years) (Eklouh-Molinier et al. 2015). Using polarized-FTIR imaging, they observed that collagen fiber orientation tends to be more aligned in the old skin samples than young ones while suggesting that this finding may be due to the fact that water/collagen interactions are weakened with age. Using the same method, Nguyen et al. revealed that type I collagen fibers run in a parallel orientation to the skin surface in aged skin tissue (Nguyen et al. 2014). Wang et al. harbored two-photon fluorescence (TPF) and second harmonic generation (SHG) microscopy to visualize the skin dermal fibers in vivo having demonstrated that elastin fibers, as well as collagen fibers, are highly anisotropic in terms of fiber orientation at the old age group (Wang et al. 2018). All these studies were performed using human skin, although in an animal model such as mice, aging collagen fibers were also observed to be parallelly aligned, exactly like human skin (Wu et al. 2011).
Aging-related changes in physical properties
Mechanical properties
Mechanical properties of the human skin are significantly altered during the aging process (Park et al. 2020). Such mechanical properties include elastic properties (e.g., elastic modulus, shear modulus, stiffness, complex modulus, extensibility, etc.), viscous properties (e.g., viscosity and relaxation time), or combined such as viscoelastic properties (Park and Chen 2019). Various measurement techniques have been invented to help the characterization of the mechanical properties of the human skin, such as torsion (Gosain et al. 2005; Ruvolo et al. 2007), stretching (tensile test) (Sepe et al. 2010; Schosserer et al. 2018), suction (Ishikawa et al. 1995; Machann et al. 2005), indentation/compression (Van Kuilenburg et al. 2013; Park et al. 2019; Thieulin et al. 2020; Escoffier et al. 1989), and wave propagation using optical coherence tomography (Liang and Boppart 2010). The magnitude of the mechanical properties is known to be substantially different, depending on the skin site. Prior findings revealed that the elastic modulus of skin tissue of the volar forearm (101 kPa) is higher than that of the dorsal forearm (69 kPa) and palm (25 kPa) (Liang and Boppart 2010) although other study, by Ishikawa et al., also found that Young’s modulus of the skin on the chest tends to be lower than that on the finger, forearm and hand (Ishikawa et al. 1995).
Many studies have shown aging-related changes in the skin elastic properties, but, as happens with other properties, the results can be contradictory among studies. Some of the studies have concluded that mechanical properties increase with age. Firooz et al. observed that Young’s modulus of human skin increases with age, while testing 8 body regions (forehead, cheek, nasolabial fold, neck, forearm, dorsal side of the hand, palm, and leg) of 5 age groups (10 subjects each, 5 from each sex), ranging from 10 to 60 years (Firooz et al. 2012). Agache et al. implemented a torsion test for the human dermis samples from 138 individuals of ages ranging from 3 years to 89 and according to it, Young’s modulus for older individuals was significantly increased compared to younger subjects (Agache et al. 1980). Grahame also suggested that the elastic modulus of skin tissue increases with aging using the suction method (Grahame 1970). Diridollou et al. confirmed through a 20 MHz scan echography that Young’s modulus linearly increases with the increase of age, by testing volar forearm skin of 206 male and female human subjects, aged 6 months-90 years (Diridollou et al. 2001). Boyer et al. measured the mechanical property of human skin tissues of 46 individuals aged from 18 to 70 years, using a dynamic indentation method, having suggested that the complex modulus of the forearm for the oldest individual group (10.7 kPa) is 43% higher than that of the youngest individual group (7.2 kPa) (Boyer et al. 2009).
On the other hand, many other studies have also shown opposite results (i.e., a negative correlation between elastic properties and age). Sanders et al. showed a continuous decrease in the elastic property of human skin of the forearm with age (using an age range of 6–61 years) applying the torsion method (Sanders 1973). With the same method, Leveque et al. discovered that the skin extensibility decreases with increasing aging, by testing the samples from the forearm skin of 141 individuals aged 3–89 years (Agache et al. 1980). Escoffier et al. concluded that aging induces a decrease in elastic recovery of skin tissues of the forearm (Escoffier et al. 1989). Boyer et al. employed a non-contact airflow device to test the forearm skin tissues of two age groups (healthy women aged 23.2 ± 1.6 and 60.4 ± 2.4), showing that Young’s modulus of the old group is substantially reduced compared to that of the young group (Boyer et al. 2012).
In addition to the results addressed above, it has been reported that these changes can have different behaviors depending on the specific age and the gender. Research by Alexander et al. revealed that Young’s modulus of the skin tissue tends to increase with age, except for to the initial 30 years, where Young’s modulus decreases with age. This test was performed in the back and forearm of 116 male and female subjects in age groups ranging from 2 to 67 years (Alexander and Cook 2006). Another study showed different trends of aging-related changes in the mechanical properties between men and women, where the elastic modulus of the skin for men tends to increase after 80 years, while for women tends to decrease (Kalra and Lowe 2016).
Noticeably, very few studies, have been carried out on the effect of aging and age-related changes on viscous or viscoelastic properties, when compared to the large number of studies on elastic properties (Moronkeji and Akhtar 2015). Computational investigations by Jayabal et al. revealed that both viscosity and relaxation time are elevated in the aged tissues, by using the Kelvin-Voigt viscoelastic model (Jayabal et al. 2019). In contrast, Escoffier et al. found that the creep relaxation time is linearly reduced with age while the viscous part of the deformation remains unchanged (Escoffier et al. 1989). Ryu et al. measured viscoelastic properties on the face, upper arm and back of 96 healthy women aged 20–75 years using the suction method, having showed that viscoelastic properties are markedly reduced during aging (Ryu et al. 2008). In the case of viscous properties, no study on the direct relationship between age and viscous properties has been found. Alternatively, it is possible to deduce the tendency of viscous changes through an aging-related change in plasma viscosity inside the blood vessel, since the plasma accounts for a large portion of the interstitial fluid. Experimental observations showed that plasma viscosity increases with age due to an increase of fibrinogen concentration, even though the underlying mechanism has been poorly established (Cavestri et al. 1992; Avellone et al. 1993; Hager et al. 1994; Coppola et al. 2000; Kovács et al. 2006; Simmonds et al. 2013). However, Carallo et al. found that plasma viscosity is not altered by aging, whereas blood viscosity increases with age (Carallo et al. 2011). Collectively, all these results are not consistent to make definitive statements for the relationship between aging and viscous properties/viscoelastic properties of skin tissue and thus, further investigation is still needed.
Transport properties
Aging significantly contributes to a change in transport properties in the skin, such as permeability/hydraulic conductivity or diffusivity of molecules and drugs. Unlike the mechanical properties, it is extremely difficult to directly measure the transport properties (Park 2017), particularly with in vivo scenarios. Therefore, the majority of the studies have been performed in vitro. Until now, various tools have been utilized to measure the transport properties of the skin, ECM, and other soft tissues, such as gravity-driven technique (Park et al. 2015), flow chamber (Chor and Li 2007), and permeability measurement with fluorescent molecules (Schmidt et al. 2020) for permeability/hydraulic conductivity; and fluorescence recovery after photobleaching (FRAP) (Cornelissen et al. 2008) and integrative optical imaging technique (IOI) (Thorne et al. 2004) for diffusivity.
Most studies for permeability/hydraulic conductivity have been focused on the stratum corneum. Experimental studies revealed that permeability of sodium fluorescein and tetrachlorosalicylanilide is elevated in the stratum corneum of aged skin, possibly due to a reduction in the sebaceous gland secretion with age, whereas permeability of testosterone in vivo study is decreased with age (Fenske and Lober 1986; Martini and Nath 2009; Thakur et al. 2009). However, in a separate study, in vitro permeability of water was observed not to change in the aging process (Thakur et al. 2009). Experimental results by Lesch et al. showed that permeability of tritiated water in the stratum corneum decreases in the elderly where necropsy specimens from 45 males and 28 females aged 16 to 96 years were tested (Lesch et al. 1989). In this study, they also observed that the degree of decreasing permeability is more prominent in males than females. Compared to the stratum corneum, data regarding other skin layers are extremely scarce. We recently found, using a numerical method, that hydraulic conductivity in the aging dermis is significantly higher than that in the young dermis. This conclusion was achieved by simulating aging and young ECM networks, with the posterior calculation of the hydraulic conductivity, which may be associated with diverse diseases such as lymphedema; more details will be published elsewhere. For the diffusion in the skin, possible theories include either no change or a decrease in drug or molecular diffusion with chronological age. Kaestli et al. found no significant difference in transdermal diffusion of drugs between young and old individuals (Kaestli et al. 2008). Other study found that drug absorption and transport are slowed down due to an increased skin stiffness during aging (Ferreira et al. 2020). However, more studies on the individual skin layers and transport properties need to be conducted to improve the analysis.
Discussion and conclusion
The biomechanical, structural, and physical changes of skin tissue are prominent in the aging process. However, numerous studies have shown different or contradictory results. The primary reason for the different results may be attributed to the use of different techniques (Neto et al. 2013), the considered skin site (Griffin et al. 2017), gender of subjects (Luebberding et al. 2014), aging type (e.g., intrinsic aging vs photoaging) (El-Domyati et al. 2002), sample state (e.g., in vivo, ex vivo, and in vitro) (Moronkeji and Akhtar 2015), anisotropy of the skin (Ní Annaidh et al. 2012), among others. As an example, most of the mechanical properties were measured based on assuming the isotropic material of the skin. However, it turned out that the skin has anisotropic properties due to the different orientations of collagen fibers in the dermis, thereby resulting in different mechanical properties within the same tissue (Kalra and Lowe 2016). In addition to the properties addressed above, there are other biophysical or biochemical parameters that can be used to evaluate the aging process (Firooz et al. 2012), such as dermal–epidermal junction (DEJ) (Lavker et al. 1987), cellular property (Dulińska-Molak et al. 2014), pH (Waller and Maibach 2005), sebum content (Pochi et al. 1979), transepidermal water loss (TEWL) (Wilhelm et al. 1991), microvasculature (Bentov and Reed 2015) or melanin index (Lee et al. 2002). The marked difference in the DEJ was observed between young and old skin, where the DEJ exhibits a reduced surface area and a flattened morphology in the process of age-related remodeling (Langton et al. 2016). Young’s modulus of aging fibroblasts was found to increase as people age, when testing fibroblasts isolated from donors of 30-, 40- and 60 years old using AFM (Dulińska-Molak et al. 2014). The experimental study by Waller et al. revealed that pH is reduced with age, particularly after the age of 70 years (Waller and Maibach 2005). Taken together, a standardized method considering all these factors is indispensable in future studies to reach a consensus about aging-related changes.
Based on changes in a diverse number of functional properties of the aging skin tissue, potential mechanisms for chronological skin aging-related alterations can be postulated by linking the biochemical, structural, and physical changes (Fig. 2). In the case of the positive correlation between aging and stiffness, three possible scenarios can be taken into account (Fig. 2a). The first scenario is that aged/aging fibroblasts has a poor production of collagen crosslinks, due to the loss of crosslinking agents such as HAPLN1. This can later lead to the change in the architecture of the collagen network toward the higher fiber alignment, which subsequently increases skin stiffness, particularly when force is applied parallel to the fibers. In the second scenario, crosslinks such as HHL are increased by aged/aging fibroblasts and thus, more robustly connect fibers and promote enhanced interaction between collagen fibers maintaining isotropic collagen networks, which results into higher skin stiffness (Lovell et al. 1987; Thakur et al. 2009). The last scenario is driven by the reduced water content, due to a decline in the function of GAGs or proteoglycans produced by aging/aged cells. The decreased water content induces a higher fiber-to-water ratio, and leading eventually to an elevated, skin stiffness (Park and Baddiel 1972). In addition, a decrease in the ratio of chondroitin-sulfate and keratin-sulfate in the aging skin dermis can result in difficulties in deforming collagen fibers or networks—high stiffness (Hall 1967).
Fig. 2.
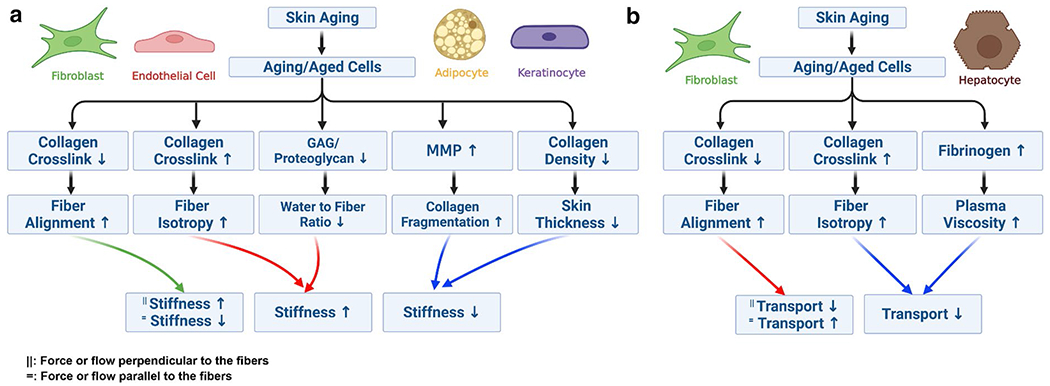
Potential mechanisms of chronological skin aging and aging-related changes based on biochemical, structural, and mechanical properties. a Potential mechanisms of aging and altered skin stiffness. b Potential mechanisms of aging and altered transport property
In the case of the negative correlation between aging and stiffness, two possible scenarios are suggested (Fig. 2a). First, MMP has a very strong production in aged/aging cells, such as activated macrophages or lymphocytes, where collagen fragmentation is accelerated, and thus, skin stiffness is reduced during aging. Besides, natural discontinuity or tear of the collagen network with age can also decrease mechanical properties such as the elastic modulus (Sanders 1973). The other scenario is that aged/aging fibroblasts show a functional decline in the secretion of collagen molecules, which cause a lower collagen density and thickness, subsequently leading to a lower stiffness of the skin (Shuster et al. 1975).
When it comes to the positive relationship between aging and transport properties (such as permeability/hydraulic conductivity and diffusivity), these are known to be increased due to the higher collagen alignment driven by aging, particularly at the parallel direction to the fibers, and in the skin dermis (Fig. 2b). In contrast, the negative correlation between aging and transport properties can be classified into two cases. The first case is that aging triggers lower collagen alignment (i.e., higher isotropic network) due to the increase of crosslinking, which leads to a lower permeability/hydraulic conductivity and diffusivity. In another case, hydraulic conductivity and diffusivity are significantly reduced by an aging-related increase in fibrinogen concentration and thus plasma viscosity. However, in this case, permeability remains unchanged irrespective of viscosity.
It should be noted that biochemical, structural, and mechanical changes from intrinsic aging are different from those from extrinsic aging resulting from sunlight, air pollution, or smoking, although there are quite a few common mechanisms between the two processes. Such differences include skin thickness (Shuster et al. 1975; Takema et al. 1994), crosslinks in collagen (Kligman et al. 1989; Yamauchi et al. 1991, 1988; Sakura et al. 1982; Kaur et al. 2019; Wulf et al. 2004), water content (Gniadecka et al. 1998), crosslinks in elastin (Yamauchi et al. 1991), collagen solubility (Sams and Smith 1961), HA content in the epidermis (Oh et al. 2011), and tsGAG and uTA content in the dermis (Oh et al. 2011) (Table 1). Hence, more systematic studies, which distinguish the two aging processes, should be performed to understand the exact underlying mechanism of skin aging and treat aging-related skin diseases.
Table 1.
Differences between photoaging and chronologically aging skin
| Factor | Photoaging skin | Chronologically aging skin |
|---|---|---|
| Skin thickness | Increase (Shuster et al. 1975) Decrease (Takema et al. 1994) |
Decrease (Shuster et al. 1975) |
| Crosslinks in collagen | Increase (Kligman et al. 1989) Decrease (Yamauchi et al. 1991; Sakura et al. 1982) |
Decrease (Kaur et al. 2019) Increase (Yamauchi et al. 1988; Wulf et al. 2004) |
| Water content | Increase (Gniadecka et al. 1998) | No change (Gniadecka et al. 1998) |
| Collagen solubility | Increase (Sams and Smith 1961) | Decrease (Sams and Smith 1961) |
| HAa in the epidermis | Increase (Oh et al. 2011) | Decrease (Oh et al. 2011) |
| tsGAGb and tUAc in the dermis | No change (Oh et al. 2011) | Decrease (Oh et al. 2011) |
| Crosslinks in elastin | Increase (Yamauchi et al. 1991) | Decrease (McCabe et al. 2020) |
HA hyaluronic acid
tsGAG total sulfated GAG
tUA total uronic acid; the aging-related change in photoaging or chronologically aging skin (i.e., increase, decrease, or no change) was evaluated relatively based on the sun-protected or healthy young skin, respectively.
It is agreed that the mechanical properties of skin differ according to the site of the skin. Our previous study also showed that the elastic modulus of the posterior wrist is greater than that of the anterior wrist and forearm by 2 to-3-fold (Park et al. 2019). However, the influence of body site on the changes in mechanical properties during aging processes could be subtle, due to the fact that there was no significant difference in molecular structure between different sites of skin (e.g., buttock vs forearm skin) (Gniadecka et al. 1998). Furthermore, little is known about the relationship between changes in mechanical properties of skin and gender. As addressed previously, a few studies have suggested that different trends of aging-related mechanical changes between men and women might occur. However, other studies have also demonstrated that no difference in skin hydration, elasticity and sebum between male and female skin is observed (Firooz et al. 2012), which indicates that gender has little effect on changes in mechanical properties with age. Thus, further studies should be warranted to elucidate the impact of the skin site and gender on the mechanical changes with age.
Currently, the measurement of mechanical properties of individual skin layers remains exceptionally challenging. There are limited studies on the quantitative mechanical properties of separate skin layers such as the stratum corneum, epidermis, dermis, and hypodermis using indentation (Van Kuilenburg et al. 2013), optical coherence tomography (OCT) (Liang and Boppart 2010), and suction method (Hara et al. 2013). In addition, there are very few studies on the effects of aging on the mechanical properties of the individual skin layers. Hara et al. showed that the elastic modulus of the skin epidermis layer and dermis layer have an different effect with age by testing the cheeks of 78 human subjects ranging from 20 to 68 years (Hara et al. 2013). While the first showed an increase with the increase of age, the second one did not show any variation with the increase of age.
In summary, aging induces significant changes in the biochemical, structural, and physical properties of human skin. Such changes are highly connected, but their relationship has been poorly established. Thus, additional work is required to elucidate the relationships between diverse functional properties, and the underlying biochemical and structural mechanisms. This future work enables us to unravel the complex relationships between aging, functional properties of skin tissue and aging-related diseases.
Acknowledgements
This study was supported by the National Institute on Aging of the National Institutes of Health under Award Number K25AG070286.
Footnotes
Conflict of interest None declared.
References
- Agache PG, Monneur C, Leveque JL, De Rigal J (1980) Mechanical properties and Young’s modulus of human skin in vivo. Arch Dermatol Res. 10.1007/BF00406415 [DOI] [PubMed] [Google Scholar]
- Alexander H, Cook T (2006) Variations with age in the mechanical properties of human skin in vivo. J Tissue Viability. 10.1016/S0965-206X(06)63002-7 [DOI] [PubMed] [Google Scholar]
- Avellone G, Di Garbo V, Panno AV et al. (1993) Haemorheological components in the pre-geriatric and geriatric age range in a randomly selected Western Sicily population sample (Casteldaccia study). Clin Hemorheol Microcirc 13:83–92. 10.3233/CH-1993-13111 [DOI] [Google Scholar]
- Bailey AJ, Robins SP, Balian G (1974) Biological significance of the intermolecular crosslinks of collagen. Nature. 10.1038/251105a0 [DOI] [PubMed] [Google Scholar]
- Barros NR, Kim HJ, Gouidie MJ et al. (2021) Biofabrication of endothelial cell, dermal fibroblast, and multilayered keratinocyte layers for skin tissue engineering. Biofabrication. 10.1088/1758-5090/aba503 [DOI] [PubMed] [Google Scholar]
- Bentov I, Reed MJ (2015) The effect of aging on the cutaneous microvasculature. Microvasc Res 100:25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biglari S, Le TYL, Tan RP et al. (2019) Simulating inflammation in a wound microenvironment using a dermal wound-on-a-chip model. Adv Healthc Mater. 10.1002/adhm.201801307 [DOI] [PubMed] [Google Scholar]
- Blair MJ, Jones JD, Woessner AE, Quinn KP (2020) Skin structure-function relationships and the wound healing response to intrinsic aging. Adv Wound Care. 10.1089/wound.2019.1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer G, Laquièze L, Le Bot A et al. (2009) Dynamic indentation on human skin in vivo: ageing effects. Ski Res Technol. 10.1111/j.1600-0846.2008.00324.x [DOI] [PubMed] [Google Scholar]
- Boyer G, Pailler Mattei C, Molimard J et al. (2012) Non contact method for in vivo assessment of skin mechanical properties for assessing effect of ageing. Med Eng Phys. 10.1016/j.medengphy.2011.07.007 [DOI] [PubMed] [Google Scholar]
- Carallo C, Irace C, De Franceschi MS et al. (2011) The effect of aging on blood and plasma viscosity. An 11.6 years follow-up study. Clin Hemorheol Microcirc 47:67–74. 10.3233/CH-2010-1367 [DOI] [PubMed] [Google Scholar]
- Carrer V, Alonso C, Oliver MA, Coderch L (2018) In vitro penetration through the skin layers of topically applied glucocorticoids. Drug Test Anal. 10.1002/dta.2412 [DOI] [PubMed] [Google Scholar]
- Cavestri R, Radice L, Ferrarini F et al. (1992) Influence of erythrocyte aggregability and plasma fibrinogen concentration on CBF with aging. Acta Neurol Scand 85:292–298. 10.1111/j.1600-0404.1992.tb04046.x [DOI] [PubMed] [Google Scholar]
- Chang AC, Liu BH, Shao PL, Liao JD (2017) Structure-dependent behaviours of skin layers studied by atomic force microscopy. J Microsc. 10.1111/jmi.12562 [DOI] [PubMed] [Google Scholar]
- Chor MV, Li W (2007) A permeability measurement system for tissue engineering scaffolds. Meas Sci Technol 18:208 [Google Scholar]
- Coppola L, Caserta F, De Lucia D et al. (2000) Blood viscosity and aging. Arch Gerontol Geriatr 31:35–42. 10.1016/S0167-4943(00)00063-7 [DOI] [PubMed] [Google Scholar]
- Cornelissen LH, Bronneberg D, Oomens CWJ, Baaijens FPT (2008) Diffusion measurements in epidermal tissues with fluorescent recovery after photobleaching. Skin Res Technol. 10.1111/j.1600-0846.2008.00313.x [DOI] [PubMed] [Google Scholar]
- Denda M, Takahashi M (1990) Measurement of facial skin thickness by ultrasound method. J Soc Cosmet Chem Jpn. 10.5107/sccj.23.316 [DOI] [Google Scholar]
- Diridollou S, Vabre V, Berson M et al. (2001) Skin ageing: Changes of physical properties of human skin in vivo. Int J Cosmet Sci. 10.1046/j.0412-5463.2001.00105.x [DOI] [PubMed] [Google Scholar]
- Dulińska-Molak I, Pasikowska M, Pogoda K et al. (2014) Age-related changes in the mechanical properties of human fibroblasts and its prospective reversal after anti-wrinkle tripeptide treatment. Int J Pept Res Ther. 10.1007/s10989-013-9370-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykes PJ, Marks R (1977) Measurement of skin thickness: a comparison of two in vivo techniques with a conventional histometric method. J Invest Dermatol. 10.1111/1523-1747.ep12507488 [DOI] [PubMed] [Google Scholar]
- Eklouh-Molinier C, Happillon T, Bouland N et al. (2015) Investigating the relationship between changes in collagen fiber orientation during skin aging and collagen/water interactions by polarized-FTIR microimaging. Analyst. 10.1039/c5an00278h [DOI] [PubMed] [Google Scholar]
- El-Domyati M, Attia S, Saleh F et al. (2002) Intrinsic aging vs photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 10.1034/j.1600-0625.2002.110502.x [DOI] [PubMed] [Google Scholar]
- Escoffier C, de Rigal J, Rochefort A et al. (1989) Age-related mechanical properties of human skin: an in vivo study. J Invest Dermatol. 10.1016/0022-202x(89)90058-4 [DOI] [PubMed] [Google Scholar]
- Fenske NA, Lober CW (1986) Structural and functional changes of normal aging skin. J Am Acad Dermatol. 10.1016/S0190-9622(86)70208-9 [DOI] [PubMed] [Google Scholar]
- Ferreira JA, De Oliveira P, Pinto L (2020) Aging effect on iontophoretic transdermal drug delivery. SIAM J Appl Math. 10.1137/19M1247188 [DOI] [Google Scholar]
- Firooz A, Sadr B, Babakoohi S et al. (2012) Variation of biophysical parameters of the skin with age, gender, and body region. Sci World J. 10.1100/2012/386936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS et al. (1996) Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 10.1038/379335a0 [DOI] [PubMed] [Google Scholar]
- Gniadecka M (1998) Quantitative evaluation of chronological aging and photoaging in vivo: studies on skin echogenicity and thickness. J Eur Acad Dermatol Venereol. 10.1016/s0926-9959(98)95063-2 [DOI] [PubMed] [Google Scholar]
- Gniadecka M, Nielsen OF, Wessel S et al. (1998) Water and protein structure in photoaged and chronically aged skin. J Invest Dermatol. 10.1046/j.1523-1747.1998.00430.x [DOI] [PubMed] [Google Scholar]
- Goergen CJ, Chen HH, Sakadžić S et al. (2016) Microstructural characterization of myocardial infarction with optical coherence tractography and two-photon microscopy. Physiol Rep. 10.14814/phy2.12894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosain AK, Klein MH, Sudhakar PV, Prost RW (2005) A volumetric analysis of soft-tissue changes in the aging midface using high-resolution MRI: Implications for facial rejuvenation. Plast Reconstr Surg. 10.1097/01.PRS.0000156333.57852.2F [DOI] [PubMed] [Google Scholar]
- Grahame R (1970) A method for measuring human skin elasticity in vivo with observations of the effects of age, sex and pregnancy. Clin Sci. 10.1042/cs0390223 [DOI] [PubMed] [Google Scholar]
- Griffin MF, Leung BC, Premakumar Y et al. (2017) Comparison of the mechanical properties of different skin sites for auricular and nasal reconstruction. J Otolaryngol - Head Neck Surg. 10.1186/s40463-017-0210-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haake AR, Holbrook K (1999) The structure and development of skin. In: Fitzpatrick’s dermatology in general medicine, 5 ed [Google Scholar]
- Hager K, Felicetti M, Seefried G, Platt D (1994) Fibrinogen and aging. Aging Clin Exp Res 6:133–138. 10.1007/BF03324226 [DOI] [PubMed] [Google Scholar]
- Hall DA (1967) The ageing of connective tissue. Symp Soc Exp Biol 21:101–125 [PubMed] [Google Scholar]
- Hara Y, Masuda Y, Hirao T, Yoshikawa N (2013) The relationship between the Young’s modulus of the stratum corneum and age: a pilot study. Skin Res Technol. 10.1111/srt.12054 [DOI] [PubMed] [Google Scholar]
- Harn HIC, Ogawa R, Hsu CK et al. (2019) The tension biology of wound healing. Exp Dermatol 28:464. [DOI] [PubMed] [Google Scholar]
- Huan S, Mattos BD, Ajdary R et al. (2019) Two-phase emulgels for direct ink writing of skin-bearing architectures. Adv Funct Mater. 10.1002/adfm.201902990 [DOI] [Google Scholar]
- Ishikawa T, Ishikawa O, Miyachi Y (1995) Measurement of skin elastic properties with a new suction device (I): relationship to age, sex and the degree of obesity in normal individuals. J Dermatol. 10.1111/j.1346-8138.1995.tb03907.x [DOI] [PubMed] [Google Scholar]
- Jayabal H, Dingari NN, Rai B (2019) A linear viscoelastic model to understand skin mechanical behaviour and for cosmetic formulation design. Int J Cosmet Sci. 10.1111/ics.12535 [DOI] [PubMed] [Google Scholar]
- Kaestli LZ, Wasilewski-Rasca AF, Bonnabry P, Vogt-Ferrier N (2008) Use of transdermal drug formulations in the elderly. Drugs Aging 25:269. [DOI] [PubMed] [Google Scholar]
- Kalra A, Lowe A (2016) An Overview of Factors Affecting the Skins Youngs Modulus. J Aging Sci. 10.4172/2329-8847.1000156 [DOI] [Google Scholar]
- Kaur A, Ecker BL, Douglass SM et al. (2019) Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 10.1158/2159-8290.CD-18-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligman AM (1979) Perspectives and problems in cutaneous gerontology. J Invest Dermatol. 10.1111/1523-1747.ep12532758 [DOI] [PubMed] [Google Scholar]
- Kligman LH, Gebre M, Alper R, Kefalides NA (1989) Collagen metabolism in ultraviolet irradiated hairless mouse skin and its correlation to histochemical observations. J Invest Dermatol. 10.1111/1523-1747.ep12277573 [DOI] [PubMed] [Google Scholar]
- Kovács Á, Szikszai Z, Várady É, Imre S (2006) Study on the hemorheological parameters of oldest-old residents in the East-Hungarian city, Debrecen. Clin Hemorheol Microcirc 35:83–88 [PubMed] [Google Scholar]
- Langton AK, Halai P, Griffiths CEM et al. (2016) The impact of intrinsic ageing on the protein composition of the dermal-epidermal junction. Mech Ageing Dev. 10.1016/j.mad.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Lavker RM, Zheng P, Dong G (1987) Aged skin: a study by light, transmission electron, and scanning electron microscopy. J Invest Dermatol. 10.1038/jid.1987.9 [DOI] [PubMed] [Google Scholar]
- Lee MH, Lim TW, Lee MH (2002) A study of skin color by melanin index according to sex, age, site and skin phototype in Koreans. Ann Dermatol. 10.5021/ad.2002.14.2.71 [DOI] [Google Scholar]
- Lesch CA, Squier CA, Cruchley A et al. (1989) The permeability of human oral mucosa and skin to water. J Dent Res. 10.1177/00220345890680091101 [DOI] [PubMed] [Google Scholar]
- Liang X, Boppart SA (2010) Biomechanical properties of in vivo human skin from dynamic optical coherence elastography. IEEE Trans Biomed Eng. 10.1109/TBME.2009.2033464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell CR, Smolenski KA, Duance VC et al. (1987) Type I and III collagen content and fibre distribution in normal human skin during ageing. Br J Dermatol. 10.1111/j.1365-2133.1987.tb04921.x [DOI] [PubMed] [Google Scholar]
- Luebberding S, Krueger N, Kerscher M (2014) Mechanical properties of human skin in vivo: a comparative evaluation in 300 men and women. Ski Res Technol. 10.1111/srt.12094 [DOI] [PubMed] [Google Scholar]
- Machann J, Thamer C, Schnoedt B et al. (2005) Age and gender related effects on adipose tissue compartments of subjects with increased risk for type 2 diabetes: a whole body MRI/MRS study. Magn Reson Mater Phys Biol Med. 10.1007/s10334-005-0104-x [DOI] [PubMed] [Google Scholar]
- Majeed H, Okoro C, Kajdacsy-Balla A et al. (2017) Quantifying collagen fiber orientation in breast cancer using quantitative phase imaging. J Biomed Opt. 10.1117/1.jbo.22.4.046004 [DOI] [PubMed] [Google Scholar]
- Marcos-Garcés V, Molina Aguilar P, Bea Serrano C et al. (2014) Age-related dermal collagen changes during development, maturation and ageing; a morphometric and comparative study. J Anat. 10.1111/joa.12186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini FH, Nath JL (2009) Fundamentals of anatomy and physiology. Pearson Educatio, San Francisco [Google Scholar]
- McCabe MC, Hill RC, Calderone K et al. (2020) Alterations in extracellular matrix composition during aging and photoaging of the skin. Matrix Biol plus. 10.1016/j.mbplus.2020.100041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara T, Murai A, Tanaka T et al. (1982) Age-related differences in human skin collagen: solubility in solvent, susceptibility to pepsin digestion, and the spectrum of the solubilized polymeric collagen molecules. J Gerontol. 10.1093/geronj/37.6.651 [DOI] [PubMed] [Google Scholar]
- Morganti P, Randazzo SD, Cardillo A (1986) Role of insoluble and soluble collagen as skin moisturizer. J Appl Cosmetol 4:141 [Google Scholar]
- Moronkeji K, Akhtar R (2015) Mechanical properties of aging human skin. Springer, Cham [Google Scholar]
- Nakagawa N, Matsumoto M, Sakai S (2010) In vivo measurement of the water content in the dermis by confocal Raman spectroscopy. Skin Res Technol. 10.1111/j.1600-0846.2009.00410.X [DOI] [PubMed] [Google Scholar]
- Neto P, Ferreira M, Bahia F, Costa P (2013) Improvement of the methods for skin mechanical properties evaluation through correlation between different techniques and factor analysis. Skin Res Technol. 10.1111/srt.12060 [DOI] [PubMed] [Google Scholar]
- Nguyen T, Eklouh-Molinier C, Sebiskveradze D et al. (2014) Changes of skin collagen orientation associated with chronological aging as probed by polarized-FTIR micro-imaging. Analyst. 10.1039/c3an00353a [DOI] [PubMed] [Google Scholar]
- Ní Annaidh A, Bruyère K, Destrade M et al. (2012) Characterization of the anisotropic mechanical properties of excised human skin. J Mech Behav Biomed Mater. 10.1016/j.jmbbm.2011.08.016 [DOI] [PubMed] [Google Scholar]
- Nimni ME, De Guia E, Bavetta LA (1965) Changes in the quantity and nature of collagen in rabbit skin as a function of age [27]. Nature 207:865. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Taniguchi M, Miyata M et al. (2004) Interferon-gamma liniment protects hairless mice against ultraviolet irradiation-induced skin damage. Biomed Res. 10.2220/biomedres.25.277 [DOI] [Google Scholar]
- Oh JH, Kim YK, Jung JY et al. (2011) Intrinsic aging- and photoaging-dependent level changes of glycosaminoglycans and their correlation with water content in human skin. J Dermatol Sci. 10.1016/j.jdermsci.2011.02.007 [DOI] [PubMed] [Google Scholar]
- Panwar P, Lamour G, Mackenzie NCW et al. (2015) Changes in structural-mechanical properties and degradability of collagen during aging-associated modifications. J Biol Chem. 10.1074/jbc.M115.644310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S (2017) Computational modeling for prediction of the shear stress of three-dimensional isotropic and aligned fiber networks. Comput Methods Programs Biomed. 10.1016/j.cmpb.2017.06.019 [DOI] [PubMed] [Google Scholar]
- Park AC, Baddiel CB (1972) Rheology of stratum corneum-I: a molecular interpretation of the stress-strain curve. J Soc Cosmet Chem 23:3–12 [Google Scholar]
- Park S, Chen Y (2019) Mechanics of biological systems. Morgon and Claypool Publisher, San Rafael [Google Scholar]
- Park S, Whittington C, Voytik-Harbin SL, Han B (2015) Microstructural parameter-based modeling for transport properties of collagen matrices. J Biomech Eng. 10.1115/1.4029920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Tao J, Sun L et al. (2019) An economic, modular, and portable skin viscoelasticity measurement device for in situ longitudinal studies. Molecules. 10.3390/molecules24050907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Jung WH, Pittman M et al. (2020) The effects of stiffness, fluid viscosity, and geometry of microenvironment in homeostasis, aging, and diseases: a brief review. J Biomech Eng 142:100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce RH, Grimmer BJ (1972) Age and the chemical constitution of normal human dermis. J Invest Dermatol. 10.1111/1523-1747.ep12540531 [DOI] [PubMed] [Google Scholar]
- Pellacani G, Seidenari S (1999) Variations in facial skin thickness and echogenicity with site and age. Acta Derm Venereol. 10.1080/000155599750010283 [DOI] [PubMed] [Google Scholar]
- Pochi PE, Strauss JS, Downing DT (1979) Age-related changes in sebaceous gland activity. J Invest Dermatol. 10.1111/1523-1747.ep12532792 [DOI] [PubMed] [Google Scholar]
- Potts RO, Buras EM, Chrisman DA (1984) Changes with age in the moisture content of human skin. J Invest Dermatol. 10.1111/1523-1747.ep12259203 [DOI] [PubMed] [Google Scholar]
- Querleux A, Richard S, Bittoun J et al. (1994) In vivo hydration profile in skin layers by high-resolution magnetic resonance imaging. Skin Pharmacol Physiol. 10.1159/000211296 [DOI] [PubMed] [Google Scholar]
- Richard S, Querleux B, Bittoun J et al. (1993) Characterization of the skin in vivo by high resolution magnetic resonance imaging: water behavior and age-related effects. J Invest Dermatol. 10.1111/1523-1747.ep12472356 [DOI] [PubMed] [Google Scholar]
- Roskos KV, Guy RH, Maibach HI (1986) Percutaneous absorption in the aged. Dermatol Clin 17:617. [PubMed] [Google Scholar]
- Ruvolo EC, Stamatas GN, Kollias N (2007) Skin viscoelasticity displays site- and age-dependent angular anisotropy. Skin Pharmacol Physiol. 10.1159/000108147 [DOI] [PubMed] [Google Scholar]
- Ryu HS, Joo YH, Kim SO et al. (2008) Influence of age and regional differences on skin elasticity as measured by the Cutometer®. Ski Res Technol. 10.1111/j.1600-0846.2008.00302.x [DOI] [PubMed] [Google Scholar]
- Saito M, Marumo K, Fujii K, Ishioka N (1997) Single-column high-performance liquid chromatographic-fluorescence detection of immature, mature, and senescent cross-links of collagen. Anal Biochem. 10.1006/abio.1997.2350 [DOI] [PubMed] [Google Scholar]
- Sakura S, Fujimoto D, Sakamoto K et al. (1982) Photolysis of pyridinoline, a cross-linking amino acid of collagen, by ultraviolet light. Can J Biochem. 10.1139/o82-064 [DOI] [PubMed] [Google Scholar]
- Sams WM, Smith JG (1961) The histochemistry of chronically sun-damaged skin. J Invest Dermatol. 10.1038/jid.1961.141 [DOI] [PubMed] [Google Scholar]
- Sanders R (1973) Torsional elasticity of human skin in vivo. Pflügers Arch Eur J Physiol. 10.1007/BF00591373 [DOI] [PubMed] [Google Scholar]
- Schmidt FF, Nowakowski S, Kluger PJ (2020) Improvement of a three-layered in vitro skin model for topical application of irritating substances. Front Bioeng Biotechnol. 10.3389/fbioe.2020.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnider SL, Kohn RR (1981) Effects of age and diabetes mellitus on the solubility and nonenzymatic glucosylation of human skin collagen. J Clin Invest. 10.1172/JCI110198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schosserer M, Grillari J, Wolfrum C, Scheideler M (2018) Age-induced changes in white, brite, and brown adipose depots: a mini-review. Gerontology 64:229. [DOI] [PubMed] [Google Scholar]
- Seidenari S, Pagnoni A, Di Nardo A, Giannetti A (1994) Echographic evaluation with image analysis of normal skin: variations according to age and sex. Skin Pharmacol Physiol. 10.1159/000211295 [DOI] [PubMed] [Google Scholar]
- Sepe A, Tchkonia T, Thomou T et al. (2010) Aging and regional differences in fat cell progenitors: a mini-review. Gerontology 57:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster S, Black MM, Mcvitie E (1975) The influence of age and sex on skin thickness, skin collagen and density. Br J Dermatol. 10.1111/j.1365-2133.1975.tb05113.x [DOI] [PubMed] [Google Scholar]
- Simmonds MJ, Meiselman HJ, Baskurt OK (2013) Blood rheology and aging. J Geriatr Cardiol 10:291–301. 10.3969/j.issn.1671-5411.2013.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R, Maibach HI (2008) Hyaluronan in skin: aspects of aging and its pharmacologic modulation. Clin Dermatol. 10.1016/j.clindermatol.2007.09.013 [DOI] [PubMed] [Google Scholar]
- Takema Y, Yorimoto Y, Kawai M, Imokawa G (1994) Age-related changes in the elastic properties and thickness of human facial skin. Br J Dermatol. 10.1111/j.1365-2133.1994.tb04975.x [DOI] [PubMed] [Google Scholar]
- Taylor KR, Gallo RL (2006) Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 10.1096/fj.05-4682rev [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T et al. (2010) Fat tissue, aging, and cellular senescence. Aging Cell 9:667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur R, Batheja P, Kaushik D, Michniak B (2009) Structural and biochemical changes in aging skin and their impact on skin permeability barrier. In: Skin aging handbook. Elsevier, Amsterdam [Google Scholar]
- Thieulin C, Pailler-Mattei C, Abdouni A et al. (2020) Mechanical and topographical anisotropy for human skin: ageing effect. J Mech Behav Biomed Mater. 10.1016/j.jmbbm.2019.103551 [DOI] [PubMed] [Google Scholar]
- Thorne RG, Hrabětová S, Nicholson C (2004) Diffusion of epidermal growth factor in rat brain extracellular space measured by integrative optical imaging. J Neurophysiol. 10.1152/jn.00352.2004 [DOI] [PubMed] [Google Scholar]
- Uitto J (1970) A method for studying collagen biosynthesis in human skin biopsies in vitro. BBA - Gen Subj. 10.1016/0304-4165(70)90163-7 [DOI] [PubMed] [Google Scholar]
- Van Kuilenburg J, Masen MA, Van Der Heide E (2013) Contact modelling of human skin: what value to use for the modulus of elasticity? Proc Inst Mech Eng Part J J Eng Tribol. 10.1177/1350650112463307 [DOI] [Google Scholar]
- Varani J, Warner RL, Gharaee-Kermani M et al. (2000) Vitamin A antagonizes decreased cell growth and elevated collagen- degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 10.1046/j.1523-1747.2000.00902.x [DOI] [PubMed] [Google Scholar]
- Varani J, Spearman D, Perone P et al. (2001) Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Am J Pathol. 10.1016/S0002-9440(10)64040-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Dame MK, Rittie L et al. (2006) Decreased collagen production in chronologically aged skin. Am J Pathol. 10.2353/ajpath.2006.051302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller JM, Maibach HI (2005) Age and skin structure and function, a quantitative approach (I): blood flow, pH, thickness, and ultrasound echogenicity. Skin Res Technol 11:221. [DOI] [PubMed] [Google Scholar]
- Wang H, Shyr T, Fevola MJ et al. (2018) Age-related morphological changes of the dermal matrix in human skin documented in vivo by multiphoton microscopy. J Biomed Opt. 10.1117/1.jbo.23.3.030501 [DOI] [PubMed] [Google Scholar]
- Wilhelm KP, Cua AB, Maibach HI (1991) Skin aging: effect on transepidermal water loss, stratum corneum hydration, skin surface pH, and casual sebum content. Arch Dermatol. 10.1001/archderm.127.12.1806 [DOI] [PubMed] [Google Scholar]
- Wu S, Li H, Yang H et al. (2011) Quantitative analysis on collagen morphology in aging skin based on multiphoton microscopy. J Biomed Opt Doi. 10.1117/1.3565439 [DOI] [PubMed] [Google Scholar]
- Wulf HC, Sandby-Møller J, Kobayasi T, Gniadecki R (2004) Skin aging and natural photoprotection. Micron 35:185. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Woodley DT, Mechanic GL (1988) Aging and cross-linking of skin collagen. Biochem Biophys Res Commun. 10.1016/S0006-291X(88)80124-4 [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Prisayanh P, Haque Z, Woodley DT (1991) Collagen cross-linking in sun-exposed and unexposed sites of aged human skin. J Invest Dermatol. 10.1111/1523-1747.ep12491727 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Naffa R, Garvey CJ et al. (2019) Quantitative and structural analysis of isotopically labelled natural crosslinks in type I skin collagen using LC-HRMS and SANS. J Leather Sci Eng. 10.1186/s42825-019-0012-x [DOI] [Google Scholar]


