Abstract
Interest in amyotrophic lateral sclerosis (ALS) biomarkers has grown exponentially over the course of the last 25 years, with great hope that they might serve as tools to facilitate the development of meaningful therapies for this otherwise inexorably progressive and invariably fatal disease. Effective use of biomarkers, however, requires an understanding of what it means for them to be ‘fit-for-purpose’ as well as an appreciation of the nuances of the clinical context(s) in which they will be applied.
Neurofilament light chain (NfL) has emerged as a leading candidate with enormous potential to aid ALS therapy development; it is, however, also profoundly misunderstood. Within the conceptual framework of the BEST (Biomarkers, EndpointS, and other Tools) Resource, developed by the National Institutes of Health and the Food and Drug Administration in the USA, we consider the evidence supporting the use of NfL for a variety of purposes in different clinical contexts.
We conclude that: (i) it may serve as a susceptibility/risk biomarker in populations at elevated risk for ALS; (ii) it has value as a prognostic biomarker when measured early in the course of established disease, empowering stratification or dynamic randomization to amplify the signal-to-noise ratio of promising therapeutics; and (iii) there is sufficient evidence to support the use of a reduction in NfL in response to an experimental therapeutic as a pharmacodynamic biomarker that may aid in phase 2 trial go/no-go decisions. Moreover, the basis for expecting that a reduction in NfL is a reasonably likely surrogate end-point (i.e. reasonably likely to predict clinical benefit—which may be more than simply survival) is nuanced, and depends on when in the course of disease the experimental therapeutic is administered.
Keywords: neurofilament, biomarker, surrogate, therapy development
NfL has great potential to aid ALS therapy development, but its utility is often misunderstood. Benatar et al. discuss the value of NfL as a risk, prognostic or pharmacodynamic biomarker, depending on when in the disease course it is measured. It may even predict clinical benefit, depending on when treatment is initiated.
Introduction
Almost half a century of trials for the neurodegenerative disorder amyotrophic lateral sclerosis (ALS) has, to date, resulted in the licensing of just three drugs based on different outcome measures: riluzole, on the basis of modestly extended tracheostomy-free survival at 12 months; and edaravone (Japan and North America only) and AMX0035 (North America), both on the basis of a modest reduction in functional disability after 6 months follow-up. The Food and Drug Administration (FDA) in the USA recently approved AMX0035 (Amylyx Pharmaceuticals), and is currently reviewing a new drug application (NDA) for tofersen (Biogen) as a disease-modifying therapy. In both cases, the importance (or not) of a reduction in the level of neurofilaments in response to the experimental therapeutic, has been specifically highlighted. In seeking approval for their SOD1 antisense oligonucleotide, tofersen, under the FDA’s accelerated approval pathway, Biogen’s application refers to their study’s ‘use of neurofilament as a surrogate biomarker that is reasonably likely to predict clinical benefit’.1 Conversely, an FDA advisory board meeting about AMX0035 questioned whether the lack of a reported difference in neurofilament concentrations between placebo and active treatment cast doubt on the other putative signals of efficacy.2 To that end, the authors of a recent editorial arguing in favour of AMX0035’s approval addressed this by stating that ‘there is at present no evidence that changes in neurofilament levels predict the efficacy of an agent.’3
In light of the interest and excitement around neurofilament light chain (NfL) and the dismal record to date of successfully identifying effective treatments for ALS, this is an opportune time to appraise the evidence for the utility of NfL as a biomarker in ALS therapy development. The discussion about NfL, however, has often either neglected the critically important concept that biomarkers should be fit-for-purpose4 or failed to adequately consider the clinical context in which the biomarker will be used. Overcoming these blind spots requires a nuanced understanding of the temporal dynamics of changes in NfL in ALS, as well as the relationship between levels of NfL and clinically meaningful end-points. We begin, therefore, by briefly revisiting the existing and emerging framework for biomarker conceptualization.
Biomarker framework
In an effort to harmonize the terms used in translational science and medical product development, the FDA and NIH have developed the BEST (Biomarkers, EndpointS, and other Tools) Resource.5 A clinical trial end-point is an event or outcome that can be measured to determine whether the intervention being studied is beneficial. The most reliable end-points are clinical outcomes that directly measure what matters most to patients—how they feel, how they function, and whether they live longer. In certain circumstances, biomarkers may be used as surrogate end-points, which are further explained below. The BEST framework emphasizes the need to conceptualize biomarkers as being fit-for-purpose—meaning that biomarkers might have different utility or clinical application and that the performance characteristics of the biomarker may well vary based on the intended used of the biomarker. Equally important, however, albeit less emphasized by the BEST Resource, is the clinical context in which the biomarker will be used. The following FDA biomarker categories are most relevant to the potential utility of NfL in ALS.
Susceptibility or risk biomarkers
Susceptibility or risk biomarkers indicate the potential for developing a disease or medical condition in an individual who does not currently have clinically apparent disease or the medical condition. A high serum cholesterol, for example, indicates elevated future risk of a myocardial infarction or stroke. In the context of ALS this currently has relevance to studies of asymptomatic carriers of genetic variants with potential value in predicting when clinically manifest ALS will emerge. Indeed, an increase in NfL above a prespecified threshold (along with a minimum increase in concentration compared to prior assessment) is being pioneered as an eligibility criterion to enroll select presymptomatic SOD1 variant carriers into the randomized, double-blind, placebo-controlled phase of the ongoing ‘ATLAS’ trial of tofersen.6
Prognostic biomarkers
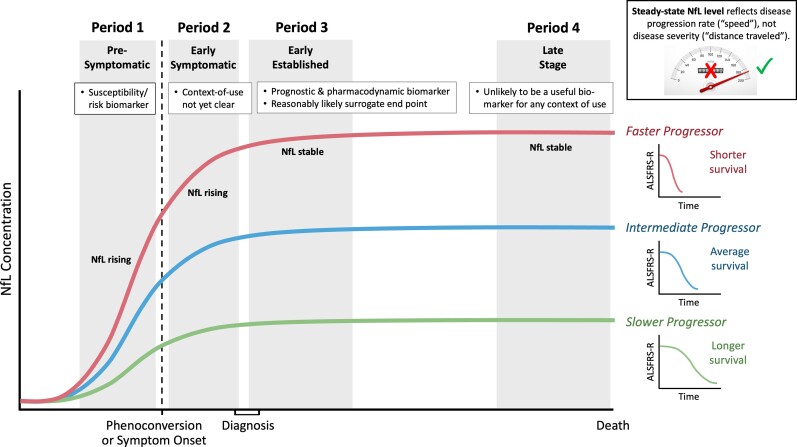
Prognostic biomarkers are used in individuals who have the disease or medical condition of interest, to predict the likelihood of a clinical event, disease recurrence or progression. For example, in males who have undergone radical prostatectomy, a rising concentration of prostate-specific antigen (PSA) may indicate residual or recurrent disease.5,7 In the context of ALS, a prognostic biomarker might be used to facilitate dynamic randomization or to stratify patients based on expected prognosis to better discern heterogeneity of outcome that is attributable to an experimental treatment rather than variability in natural history. The prognostic value of NfL will, of course, be greatest when measured early in established disease (Period 3 in Fig. 1) when much of the course of symptomatic disease still lies ahead. At end stage disease, by contrast, there is unfortunately little future prognosis left to predict.
Figure 1.
Temporal trajectory of neurofilame NfL, and its utility as a biomarker, through the course of pre-symptomatic and symptomatic ALS. During the pre-symptomatic stage of disease (Period 1), NfL generally increases ahead of the emergence of weakness. A rise in NfL during this stage of disease may serve as a susceptibility/risk biomarker, predicting the impending emergence of clinically manifest ALS. During early symptomatic disease (Period 2), NfL continues to rise, before reaching a plateau as disease becomes established (Period 3) and a diagnosis is made. Since the average latency from symptom onset to diagnosis is ∼12 months, we conceptualize early symptomatic versus established disease as within versus after ∼12 months following symptom onset, respectively. The utility of once-off measurement of NfL during Period 2 is unclear, but longitudinal measures that reveal the rate of change in NfL might have prognostic value. By contrast, the steady state level of NfL measured during Period 3 has established value in predicting prognosis (future rate of disease progression and survival duration). NfL also has pharmacodynamic value during this stage of disease, as a reduction in NfL plausibly predicts a future clinical benefit. NfL levels are projected to remain stable into advanced or late-stage disease (Period 4). Although NfL is stable during Periods 3 and 4, the level differs between individuals, with higher levels being reached among those with faster progressing disease. It is, however, far less clear that a reduction in NfL during Period 4 is likely to translate into a clinical benefit since significant pathology and disability has already accrued. Of note, during Periods 3 and 4, the level of NfL serves a marker of disease aggressivity (speed or rate of progression) and not of clinical stage (cumulative disability) (inset).
Predictive biomarkers
Predictive biomarkers, which should be distinguished from prognostic biomarkers, may be used to identify individuals who are more likely than others to experience a benefit (or harm) from an investigational product. In ALS, gene mutation status (e.g. presence of an SOD1 mutation) is expected to be predictive of which patients would most likely benefit from an anti-sense oligonucleotide or gene therapy approach that targets SOD1, for example. NfL, on the other hand, is a non-specific marker of axonal injury; it is, therefore, not expected to be useful as a predictive biomarker.
Response biomarkers
Response biomarkers may be used to show that a biological response, potentially beneficial (or harmful), has occurred in an individual who has been exposed to a medical product. In ALS the current ‘gold standard’ clinical outcome measures are survival or significant slowing of the rate of disability accrual according to the ALS Functional Rating Sscale-revised (ALSFRS-R), or a composite of the two such as the Combined Assessment of Function and Survival (CAFS).8 Of note, steady-state NfL concentrations do not correlate with contemporaneously collected ALSFRS-R scores (which may continue to decrease), but there is a robust association between higher steady-state NfL concentrations and faster future rates of progression as measured by the change in ALSFRS-R over time and shorter survival time.9
Pharmacodynamic biomarkers
Pharmacodynamic biomarkers are a type of response biomarker that indicate biological activity of a medical product without necessarily drawing conclusions about clinical efficacy. The international normalized ratio (INR), for example, may serve as a pharmacodynamic biomarker when evaluating response to treatment with the anti-coagulant, warfarin.5,10
Surrogate end-point biomarkers
Surrogate end-point biomarkers are another type of response biomarker that serve as a substitute for a direct measure of how a patient feels, functions, or survives. Importantly, surrogate end-points do not directly measure the clinical benefit (or harm) but, rather, are expected to predict it. Reduction in HIV viral load, for example, is a validated surrogate end-point for HIV clinical disease control.5 From a regulatory standpoint, depending on the level of clinical validation, the FDA recognizes: (i) validated surrogate end-points; (ii) reasonably likely surrogate end-points; and (iii) candidate surrogate end-points.
Diagnostic biomarkers
Diagnostic biomarkers are used to detect or confirm the presence of a disease or condition of interest or to identify individuals with a subtype of the disease. In this context, the sensitivity and specificity of the biomarker for the condition of interest are of paramount importance. Much has been written about the potential value of neurofilaments, measured in CSF or blood, as tools to facilitate the diagnosis of ALS.11-14 The concentration of NfL is generally elevated among patients with ALS compared to controls and even disease mimics. In current clinical practice, however, there is typically a long latency from symptom onset to evaluation by a specialist. By then, disease is sufficiently progressed that a diagnosis can readily be made by a specialist based on clinical findings alone, thereby limiting the need for a diagnostic biomarker to atypical phenotypes.15 The frustratingly long diagnostic delay reflects the fact that ALS is a relatively rare disorder that is characterized by insidious onset and progression of largely painless weakness. Failure to identify early symptoms as potentially representing ALS and to initiate appropriate referral16 are ‘health system’ problems, requiring an educational solution. Whether or not NfL may become a useful screening tool for use in the primary care setting, for example, to prompt referral to an ALS specialist, is yet to be determined. This does, however, require focused investigation as the potential for both false positive and false negative results may limit the predictive value of NfL as a screening tool.
Neurofilament light chain in the context of ALS
Neurofilaments are neuron-specific cytoskeletal intermediate filaments that comprise heteropolymers of NfL, neurofilament medium chain (NfM) and neurofilament heavy chain (NfH).17 These polymers, which also contain α-internexin in the CNS or peripherin in the PNS, are major structural components of axons.18 CSF levels were first noted to be significantly raised in ALS patients, compared to both healthy and Alzheimer’s disease controls, more than 25 years ago.19 Catalysed by the development of sensitive assays capable of measuring blood levels (which correlate very closely with CSF levels20,21), NfL has emerged most consistently as a non-specific marker of axonal loss across a wide range of mainly CNS neurological disorders.22 Older age remains a confound to be unravelled more fully, but ALS is among the diseases with the highest levels of NfL in most cases.23
Approximately 15% of ALS cases are linked to a highly-penetrant, dominantly inherited, monogenetic pathological variant. From studies of asymptomatic carriers of these variants, blood NfL concentrations appear to significantly increase pre-symptomatically in ALS,24 with the temporal course of this rise dependent, at least in part, on underlying genotype.25 Among carriers of highly penetrant SOD1 mutations that are associated with rapidly progressive disease, this rise in NfL occurs 6–12 months before phenoconversion and predicts the emergence of clinically manifest ALS with high sensitivity and specificity.6 We do recognize, however, that ALS is biologically and phenotypically heterogeneous and that observations from this most aggressive forms of SOD1 ALS may not always be generalizable to other forms of disease.26
NfL concentrations continue to increase to some extent during, on average, the first year or so following phenoconversion in mutation carriers (or following symptom onset in patients with apparently sporadic ALS), after which the levels reach a plateau and then remain relatively stable through most of the course of symptomatic disease9,20,24,27 (Fig. 1). The biochemical reasons why NfL concentrations stabilize are unclear; nonetheless, the stable levels indicate that the rate of release of this relatively soluble and small molecule from what is an essentially inexhaustible pool of degenerating axons (even in late-stage ALS) reaches an equilibrium with blood clearance mechanisms. The concentration at which NfL reaches a steady state (i.e. plateau) varies widely from patient to patient. Higher steady state concentration is associated with more aggressive disease that is characterized by more rapid rate of functional decline and shorter survival from initial symptom onset9,20,27 (Fig. 1). As such, NfL has emerged as a clinically validated prognostic biomarker.9
Perhaps the most important question, however, is the extent to which NfL might be used as a response biomarker—either pharmacodynamic or surrogate end-point in ALS. Most would probably agree that NfL would have value as a pharmacodynamic biomarker if there were a consistent reduction in concentration following administration of an experimental therapeutic. Indeed, NfL has been shown to be a pharmacodynamic biomarker in other neurological disorders, including HIV-related neurodegeneration,28 multiple sclerosis29 and spinal muscular atrophy.30 Moreover, there is strong face validity in the idea that a reduction in NfL, a marker of axonal damage, should be favourably regarded. Similarly, a rise in NfL following administration of an experimental therapeutic, would intuitively be considered a potential sign of harm.
Since there is currently no published evidence that NfL is a substitute for a direct measure of how patients with clinically manifest ALS feel or function, or how long they survive, NfL cannot yet be considered a validated surrogate end-point. Whether or not NfL might be regarded as a reasonably likely surrogate end-point (specifically, that its reduction will predict some future clinical benefit) is, however, much less clear. To understand why, it is helpful to think of NfL as a marker of the rate with which disease is progressing—akin to the speed at which a runaway train is moving towards a cliff. (Notably, a steady state level of NfL, as shown in Periods 3 and 4 in Fig. 1, is not a marker of how many motor neurons have been lost, how much disability has accrued, or how far advanced the disease has become—all of which may be considered ‘distance travelled’, in the speeding train analogy.) Whether or not reducing NfL will predict meaningful preservation of function depends on when in the course of disease, the reduction occurs. In the runaway train analogy, reducing the train’s speed means that it will take longer before reaching the edge of the cliff. This is a benefit (because there is more time for rescue); and this benefit can be predicted. When train is near the cliff’s edge, however, there is little to no time left for rescue. And in the absence of a benefit, there is nothing to predict. Similarly, a reduction in NfL occurring earlier (rather than later) in the course of disease is more plausibly a reasonably likely surrogate end-point.
According to the BEST framework, a reasonably likely surrogate end-point is an ‘endpoint supported by strong mechanistic … rationale such that an effect on the surrogate endpoint is expected to be correlated with an endpoint intended to assess clinical benefit in clinical trials, but without sufficient clinical data to show that it is a validated surrogate endpoint’.5 For NfL, there is indeed a strong mechanistic rationale to expect that its reduction might correlate with a clinical benefit. Whether or not NfL can be considered a reasonably likely surrogate, however, depends to a significant extent on when in the course of disease NfL is reduced by the therapeutic intervention.
An immediate role for neurofilament light chain in ALS therapy development
Considering what is already known about NfL in ALS and other neurological disorders, there is something circular in the reasoning that, in order to consider it a potential biomarker of pharmacodynamic response, we must first evaluate it in the context of a highly effective disease-modifying drug for ALS. After all, no such therapy currently exists; and once we have a highly effective therapy, the need for a pharmacodynamic biomarker may be less pressing. Parenthetically, riluzole and edaravone both have very small absolute effects, neither of which are quantifiable in, or perceptible to, the treated individual; and neither drug is expected to make a patient ‘feel’ better. It is perhaps not surprising that NfL concentrations do not appear to change in response to treatment with riluzole.31 (The impact of edaravone on NfL is currently unknown.) Interestingly, a recent phase 2 futility trial of guanabenz, a selective inhibitor of endoplasmic reticulum stress-induced eIF2a-phosphatase, met its primary end-point of non-futility but did not show a change in NfL over a 6-month treatment period.32 The authors concluded that a larger trial of a molecule targeting the unfolded protein response pathway, but without the alpha-2 adrenergic side effects of guanabenz, is warranted. Whether the lack of an NfL response in phase 2 should have led to a different conclusion will only be revealed by the results of the proposed follow-up trial.
As we contemplate the challenges of ALS therapy development, there appear to be a wide range of candidate therapeutics now jostling for priority testing in phase 3 clinical trials. Many of these have emerged from the development of high-throughput screening methodologies, with ‘promising signals’ in cellular and rodent models. Importantly, compounds with ‘safety and tolerability’ data from small phase 2 human subject studies must not be portrayed as having evidence of therapeutic efficacy. [Incidentally, those living with ALS, and the clinicians advising them, must objectively navigate the interests (including commercial) of an increasing number of entities purporting to have the most promising candidate.] A crucial rate-limiting step in assessing the aforementioned plethora of drug candidates is the availability of financial resources for and the logistical feasibility of large (>200 patients) and lengthy (12–18 month) phase 3 randomized placebo-controlled trials that meet the currently acceptable end-points for licensing.
An immediate solution might be to use NfL as a pharmacodynamic biomarker, with a significant reduction in NfL concentration in response to an experimental therapeutic as a mechanism of triage. This could be achieved through studies involving a relatively small group of ALS patients, and undertaken over a shorter period (e.g. 6 months). Candidate drugs achieving a pre-specified threshold for group mean reduction in NfL could be prioritized for the necessarily longer phase 3 randomized placebo-controlled trials. Smaller triage studies would lend themselves to being conducted at fewer centers, allowing faster screening of more candidates and supported by existing international platforms for trial registration to minimize duplication. Despite the accepted risk of overlooking a highly effective drug that, for some reason, did not impact NfL at all, the gains in terms of accelerating screening to clear the current backlog of candidates remains a strong appeal.
Concluding remarks
NfL, despite its lack of specificity for ALS, is currently the most accessible, quantitative, and robust marker of disease ‘aggressivity’ in ALS, a concept that warrants consideration for incorporation into the BEST framework. As a marker of the speed with which ALS is progressing, NfL holds immediate value as a prognostic biomarker, especially in early established disease. It has the additional value of predicting phenoconversion to clinically manifest ALS in those at high genetic risk, offering the first opportunity to administer potentially preventative therapeutics. A nuanced understanding of the temporal dynamics of NfL in ALS, however, only partially support the notion that NfL is a reasonably likely surrogate end-point—because the correlative clinical effects of lowering NfL depend on the timing of treatment. Nevertheless, NfL has enormous face validity as a pharmacodynamic biomarker and is worthy of immediate consideration for its utility in making go/no-go decisions in phase 2 clinical trials.
Contributor Information
Michael Benatar, Department of Neurology, University of Miami, Miami, FL 33136, USA.
Joanne Wuu, Department of Neurology, University of Miami, Miami, FL 33136, USA.
Martin R Turner, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford OX3 9DU, UK.
Funding
This work was supported by funding from the National Institutes of Health - United States for the Pre-fALS study and CReATe Consortium (R01-NS105479, U54-NS092091, and U01-NS107027) and from Motor Neurone Disease Association - United Kingdom.
Competing interests
M.B. reports remuneration for ad hoc consulting for Alector, Annexon, Arrowhead, Biogen, Novartis, Orphazyme and UniQure. He serves as the global academic lead for the ATLAS study (Biogen). The University of Miami has licensed intellectual property to Biogen for the design of the ATLAS study. He reports grants from the ALS Association, the Muscular Dystrophy Association, and the National Institutes of Health. He has a provisional patient pending (Determining Onset of Amyotrophic Lateral Sclerosis), and he serves as a member of the Board of Trustees for the ALS Association. J.W. reports reports grants from the National Institutes of Health. M.R.T. reports remuneration for ad hoc clinical advice as a member of the Endpoint Adjudication Committee for the ATLAS study (Biogen).
References
- 1. FDA Accepts Biogen’s New Drug Application and Grants Priority Review of Tofersen for a Rare, Genetic Form of ALS. News release. Biogen. Published 26 July 2022. https://investors.biogen.com/news-releases/news-release-details/fda-accepts-biogens-new-drug-application-and-grants-priority
- 2. Combined FDA and Applicant Briefing Document. Published 30 March 2022. https://www.fda.gov/media/157186/download
- 3. Cudkowicz ME, Shefner JM. Regulatory Paproval in ALS; When is a single study enough? Ann Neurol. 2022;91:737–739. [DOI] [PubMed] [Google Scholar]
- 4. Benatar M, Boylan K, Jeromin A, et al. ALS biomarkers for therapy development: State of the field and future directions. Muscle Nerve. 2016;53:169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.https://www.ncbi.nlm.nih.gov/books/NBK326791/ FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource. In: Silver Spring (MD): Food and Drug Administration (US). Co-published by National Institutes of Health (US), Bethesda (MD); 2016. [PubMed]
- 6. Benatar M, Wuu J, Andersen PM, et al. Design of a randomized, placebo-controlled. phase;3, trial of tofersen initiated in clinically presymptomatic SOD1 variant carriers: The ATLAS study. Neurotherapeutics. 2022;19:1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roberts SG, Blute ML, Bergstralh EJ, Slezak JM, Zincke H. PSA doubling time as a predictor of clinical progression after biochemical failure following radical prostatectomy for prostate cancer. Mayo Clin Proc. 2001;76:576–581. [DOI] [PubMed] [Google Scholar]
- 8. Berry JD, Miller R, Moore DH, et al. The combined assessment of function and survival (CAFS): A new endpoint for ALS clinical trials. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:162–168. [DOI] [PubMed] [Google Scholar]
- 9. Benatar M, Zhang L, Wang L, et al. Validation of serum neurofilaments as prognostic and potential pharmacodynamic biomarkers for ALS. Neurology. 2020;95:e59–e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e152S–e184S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feneberg E, Oeckl P, Steinacker P, et al. Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology. 2018;90:e22–e30. [DOI] [PubMed] [Google Scholar]
- 12. Poesen K, De Schaepdryver M, Stubendorff B, et al. Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology. 2017;88:2302–2309. [DOI] [PubMed] [Google Scholar]
- 13. Steinacker P, Feneberg E, Weishaupt J, et al. Neurofilaments in the diagnosis of motoneuron diseases: A prospective study on 455 patients. J Neurol Neurosurg Psychiatry. 2016;87:12–20. [DOI] [PubMed] [Google Scholar]
- 14. Verde F, Steinacker P, Weishaupt JH, et al. Neurofilament light chain in serum for the diagnosis of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2019;90:157–164. [DOI] [PubMed] [Google Scholar]
- 15. Turner MR, Benatar M. Ensuring continued progress in biomarkers for amyotrophic lateral sclerosis. Muscle Nerve. 2015;51:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Turner MR, Talbot K. Mimics and chameleons in motor neurone disease. Pract Neurol. 2013;13:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fuchs E, Cleveland DW. A structural scaffolding of intermediate filaments in health and disease. Science. 1998;279:514–519. [DOI] [PubMed] [Google Scholar]
- 18. Zhao J, Liem RK. α-Internexin and peripherin: Expression, assembly, functions, and roles in disease. Methods Enzymol. 2016;568:477–507. [DOI] [PubMed] [Google Scholar]
- 19. Rosengren LE, Karlsson JE, Karlsson JO, Persson LI, Wikkelso C. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem. 1996;67:2013–2018. [DOI] [PubMed] [Google Scholar]
- 20. Lu CH, Macdonald-Wallis C, Gray E, et al. Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology. 2015;84:2247–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gray E, Oeckl P, Amador MDM, et al. A multi-center study of neurofilament assay reliability and inter-laboratory variability. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(5-6):452–458. [DOI] [PubMed] [Google Scholar]
- 22. Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14:577–589. [DOI] [PubMed] [Google Scholar]
- 23. Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90:870–881. [DOI] [PubMed] [Google Scholar]
- 24. Benatar M, Wuu J, Andersen PM, Lombardi V, Malaspina A. Neurofilament light: A candidate biomarker of pre-symptomatic ALS and phenoconversion. Ann Neurol. 2018;84:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benatar M, Wuu J, Lombardi V, et al. Neurofilaments in pre-symptomatic ALS and the impact of genotype. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(7-8):538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benatar M, Granit V, Andersen PM, et al. Mild motor impairment as prodromal state in amyotrophic lateral sclerosis: A new diagnostic entity. Brain. 2022;145:3500–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thompson AG, Gray E, Verber N, et al. Multicentre appraisal of amyotrophic lateral sclerosis biofluid biomarkers shows primacy of blood neurofilament light chain. Brain Commun. 2022;4:fcac029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abdulle S, Mellgren A, Brew BJ, et al. CSF neurofilament protein (NFL) – a marker of active HIV-related neurodegeneration. J Neurol. 2007;254:1026–1032. [DOI] [PubMed] [Google Scholar]
- 29. Sormani MP, Haering DA, Kropshofer H, et al. Blood neurofilament light as a potential endpoint in phase 2 studies in MS. Ann Clin Transl Neurol. 2019;6:1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Darras BT, Crawford TO, Finkel RS, et al. Neurofilament as a potential biomarker for spinal muscular atrophy. Ann Clin Transl Neurol. 2019;6:932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Esselin F, De la Cruz E, Hirtz C, et al. Repeated neurofilament light chain measurements did not capture riluzole therapeutic effect in amyotrophic lateral sclerosis patients. CNS Neurosci Ther. 2022;28:1532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dalla Bella E, Bersano E, Antonini G, et al. The unfolded protein response in amyotrophic later sclerosis: Results of a phase 2 trial. Brain. 2021;144:2635–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]