Abstract
An increase in the efficiency of clinical trial conduct has been successfully demonstrated in the oncology field, by the use of multi-arm, multi-stage trials allowing the evaluation of multiple therapeutic candidates simultaneously, and seamless recruitment to phase 3 for those candidates passing an interim signal of efficacy. Replicating this complex innovative trial design in diseases such as Parkinson’s disease is appealing, but in addition to the challenges associated with any trial assessing a single potentially disease modifying intervention in Parkinson’s disease, a multi-arm platform trial must also specifically consider the heterogeneous nature of the disease, alongside the desire to potentially test multiple treatments with different mechanisms of action.
In a multi-arm trial, there is a need to appropriately stratify treatment arms to ensure each are comparable with a shared placebo/standard of care arm; however, in Parkinson’s disease there may be a preference to enrich an arm with a subgroup of patients that may be most likely to respond to a specific treatment approach. The solution to this conundrum lies in having clearly defined criteria for inclusion in each treatment arm as well as an analysis plan that takes account of predefined subgroups of interest, alongside evaluating the impact of each treatment on the broader population of Parkinson’s disease patients.
Beyond this, there must be robust processes of treatment selection, and consensus derived measures to confirm target engagement and interim assessments of efficacy, as well as consideration of the infrastructure needed to support recruitment, and the long-term funding and sustainability of the platform. This has to incorporate the diverse priorities of clinicians, triallists, regulatory authorities and above all the views of people with Parkinson’s disease.
Keywords: Parkinson’s disease, multi-arm, multi-stage, platform trial, complex innovative trial design
Multi-arm, multi-stage platform designs have increased the efficiency of clinical trials in oncology. Foltynie et al. consider the issues that would need to be addressed in order to use this approach to assess potential disease-modifying treatments in progressive neurological conditions such as Parkinson's disease.
Introduction
Delaying or halting disease progression is a key aim for current research in Parkinson’s disease (PD). The process of setting up and running a clinical trial to assess whether a drug might slow down the rate of the progression of PD, as for any chronic neurodegenerative disorder of the brain, is hugely time and resource consuming. Most new interventions that have been evaluated in patients with PD have failed to provide improvements in outcomes often at the phase III stage. Thus, in setting up a phase III trial it seems sensible to simultaneously evaluate as many promising new interventions as possible, acknowledging that many may ‘fail’. This necessitates the involvement of large numbers of people with PD and potentially long term follow up requiring detailed planning to ensure successful recruitment and participant retention. This also needs the support of the appropriate statistical and methodological framework to provide clear and robust data to the community at large and thus contribute to improving outcomes for patients with PD.
Complex innovative trial designs including ‘Multi-Arm, Multi-Stage’ (MAMS) platform trials can simultaneously recruit to multiple active treatment arms, perform interim analyses to assess whether a drug/intervention is engaging its target or reaches a preliminary measure of activity. This then allows one to stop recruitment to futile treatment arms and replace these with different interventions, while continuing to recruit and evaluate those with the most encouraging data all the way to phase III. This adaptive approach therefore dispenses with the repeated cycle of dismantling and rebuilding the trial infrastructure, while allowing removal and addition of trial arms and adjustment of trial design simply through the process of substantial amendment. The oncology field has pioneered this approach and identified numerous agents that are now routinely incorporated into standard of care, e.g. the STAMPEDE trial, as well as promptly identifying futile interventions.1 The coronavirus disease 2019 (COVID-19) pandemic triggered the development of the RECOVERY trial, based on MAMS principles, which enabled the rapid identification of multiple effective and ineffective drugs to improve outcomes from COVID-19 infection.2
The efficiency provided by MAMS platform trials is scientifically superior, because it reduces the evaluation time for a large of number of treatments from many decades to less than one decade. However, it is also financially advantageous in terms of reducing the costs of repeated trial set-up and dismantling, as well as speeding up the process of identifying recruiting sites, is more popular with patients, as it results in fewer individuals being allocated to placebo arms, and with investigators, as it reduces the administrative burden associated with trial set-up and close-down.
Population to study
There are specific challenges inherent to disease-modifying trials in slowly progressive diseases like PD. The existence of ‘symptomatic’ approaches, whilst clearly welcome for clinical purposes, limits the assessment of disease progression, as they can effectively mask the extent to which the disease has progressed and therefore impede our ability to recognize whether a candidate therapy may usefully slow disease progression. The traditional approach of recruiting only untreated PD patients greatly restricts the possible duration of follow-up, as the majority of PD patients will require dopaminergic replacement therapy in the first 1–2 years post diagnosis. As a result, there is interest in identifying people who are at risk of developing PD based on genetic testing, or who have prodromal non-motor symptoms but have not yet manifested any of the typical motor symptoms of PD, as a strategy for assessing whether earlier long-term intervention may prevent or delay the motor symptoms of PD.3
Another approach is to instead target patients who have already started dopaminergic therapy (this represents the majority of prevalent individuals with PD), which offers the opportunity to continue sufficiently long follow-up to evaluate the emergence of disability despite dopaminergic replacement. In further support of this strategy, it is clear from patient input that there is a desire to participate in disease modifying trials by patients at all ages and stages of the disease, given the shared fear of the inevitable long-term outcomes of PD, i.e. falls, dementia and bulbar impairment that may not manifest until many years after the first symptoms of motor PD. It is this population that would demand access to any disease modifying treatment emerging from clinical trials.
The optimal trial would therefore accommodate the broader population of people with PD and also properly recognize and appeal to the diversity of the population affected by the disease, including gender, ethnicity and age. However, as the rate of progression of PD is not linear, the duration of disease and/or its severity must be considered at the time of recruitment, and other factors that potentially influence the subsequent rate of progression including age, gender, history of REM sleep behaviour disorder, coexistent diabetes and tremor dominant phenotype4 may need to be balanced appropriately across trial arms in order to allow for more inclusive participation.
Different treatments for different patients
The recognition of PD heterogeneity in terms of its underlying pathophysiology and differential rates of motor and non-motor progression needs further consideration, given the possibility of targeting therapeutic approaches at subgroups of participants most likely to benefit. Ideally, mechanistic stratification should precede the inclusion of patients in mechanistically defined treatment arms, e.g. subgroups of patients with PD due to GBA1 mutations (approximately 10% of PD patients).5
This subgroup has a faster rate of progression of PD motor and cognitive symptoms6 and can be readily identified through routine genotyping. However, rather than attempting to stratify these individuals across treatment arms, it is instead appealing to enrich a treatment arm (that might be testing an agent considered to specifically address lysosomal function) with GBA1 patients. To prevent compromising the overall benefits of the MAMS platform, i.e. its shared placebo group, the most simple solution is to deliberately enrich the relevant treatment arm with GBA1 patients to increase the likelihood of detection of an additional effect in this subgroup, and as a consequence to define preplanned subgroup analyses to compare this arm against an equivalent number of GBA1 positive and negative patients across all the other arms in the trial (making appropriate adjustment in the event that positive effects from other interventions are seen in other active arms as well). This would maintain the placebo group as a valid comparison for the other treatment arms, while maximizing power to detect any specific benefit on the GBA1 subgroup, in comparison to placebo, and to the non-GBA1 individuals in the relevant treatment arm. Other clearly defined subgroups that may have a greater likelihood of response to a specific treatment arm, e.g. patients with active neuroinflammation based on TSPO PET imaging or CSF analysis, can also be enriched (into, for example, a neuroinflammatory treatment arm), provided the subgroup can be adequately defined and is sufficiently prevalent to provide adequate power within the planned subgroup analysis.
This approach of trying to address the issue of PD heterogeneity in the context of a multi-arm platform trial of course has its limitations. There may be precision interventions that only have a chance of effectiveness in individuals with, for example, specific rare mutations in GBA1 that will require a precision approach, i.e. a trial with far stricter inclusion criteria. On the other hand, there will be treatment options that may have broader appeal than may first appear and may lend themselves to a multi-arm platform trial approach. For example, LRRK2 kinase activity is elevated in people both with and without LRRK2 mutations,7 and initial results of the DNL201 LRRK2 inhibitor indicate that this drug may have a role in people both with and without LRRK2 mutations.8 Furthermore, the consequences of LRRK2 mutations extend beyond the neurodegenerative processes of PD, indeed they can lead to tauopathies, amyloidopathies or TDP43 proteinopathies, such that even a basket trial approach9 may be successful. On balance, it remains likely that if there is any therapeutic overlap across widely differing phenotypes, any initial success will be small, and expansion of success will need to continue to consider the importance of precision approaches not only within PD but across the whole range of neurodegenerative diseases. The incorporation of genotyping, wet biomarkers and imaging biomarkers in the design and setup of a MAMS platform trial to allow specific a priori subgroup analyses will greatly improve the chances of its success.
Outcome measures
The choice of a uniform primary outcome measure for multiple arms of a disease-modifying trial is also challenging. Disease modification is easier to demonstrate when the clinical end-points are clear and not controversial, e.g. absolute and progression-free survival, metastatic spread, confirmed infection and need for ventilatory support. The choice of outcome is more difficult in a chronic neurodegenerative disease, especially when the rate of progression is slow and heterogeneous between patients. The standard measure for PD trials, the Movement Disorders Society Unified Parkinson’s disease Rating Scale (MDS-UPDRS), was developed for symptomatic treatment and other clinical studies but may not be sensitive and specific enough for disease modifying trials especially in early disease.10
In this context, some of the challenges of participant retention in long-term trials as well as outcome measurement may be partially mitigated by embracing advances in remote data capture/home based assessment, passive and continuous technological measurement of PD severity, as well as linkage to routine health and social care datasets. However, a consensus regarding the optimal phase III primary outcome for a disease modifying trial in PD has not yet been achieved. Different teams have adopted various approaches to the consideration of outcome measurement and licensing decisions, and it is increasingly recognized that the traditional objective assessments of motor symptom severity (MDS-UPDRS part 3) may be less relevant to people with PD and regulators than self-reported measures of function and ability to perform activities of daily living (as measured in parts 1b and part 2 of the MDS-UPDRS). Importantly, these do not necessarily require face to face assessment, greatly enhancing the convenience of long-term trial participation.
There is interest in creating a modified method of analysing data from the MDS-UPDRS, using a ‘milestones’11 or ‘emergent symptoms’12 based approach, rather than change in absolute scores. Participants could be scored according to whether they reach a predefined threshold for an important event, such as falls/cognitive impairment, or based on their reporting ‘emergent symptoms’ on part Ib or part II of the MDS UPDRS, rather than focusing on a change in symptom severity that had already been present at the baseline visit. Further validation of these approaches are needed, although early explorations suggest that these approaches could lower the sample size needed to demonstrate disease modifying effects of an intervention.12
In contrast, the earlier capture of interim measures needs to inform the decision whether to drop an arm or continue recruitment. Such interim measures can be tailored to the specific intervention and could comprise confirmation of target engagement (e.g. through blood or CSF measurement of drug level or its substrate) or preliminary signals of efficacy in any of a number of predefined clinical or imaging interim measures. A hybrid approach of remote data capture alongside intermittent in-person visits should optimize the beneficial effects of face to face interaction, quality and safety of participants, while minimizing inconvenience and any excessive burden of long-term trial participation.
Intervention choice and funding
With the advancement of in silico and artificial intelligence-based drug identification and development programmes, the number of candidate drugs that have preliminary credentials for disease modifying effects is growing.13 Therefore a process for prioritizing which drugs/interventions to include in the multiple arms of a PD MAMS trial is needed. A systematic approach for identifying the initial list of candidate interventions can be followed by a scoring system to include the strength and quality of the preclinical data and rationale, as well as any supporting epidemiological data, including the assessment of which preclinical models are most meaningful for supporting translation into human disease or to evidence mode of action. Other considerations include whether therapeutics targeting different mechanisms of action might work synergistically. Dosing considerations are also important—such as the mode and frequency of delivery, mindful of pill size and pill burden—and the patient voice is critical in these deliberations. It is also important to adequately attempt to double-blind the intervention to participants and raters. This can be an additional challenge for the trial design and delivery teams, given that multiple interventions may not have the same route or frequency of administration, and therefore careful consideration is needed to minimize any differential placebo effects, while avoiding over-burdening participants with a requirement to take multiple dummy preparations.
An alternative approach to treatment selection is a pragmatic one, inviting commercial involvement, on a ‘pay to play’ basis. While this may have clear economic advantages, there may be a struggle between maintaining an optimal trial design across multiple interventions against the inevitable commercial interest in prioritizing success for an individual arm.
Faced by all these complex decisions, a final challenge is to consider the position of non-commercial research funding bodies. While they may be enthusiastic about the broad approach and the financial efficiencies introduced by MAMS trials, the large costs and long-term nature of the funding required may fall outside the majority of research funders’ usual funding models. Discussions regarding approaches to funding and long-term project sustainability are at least as important as the scientific details themselves.
Rising to the challenge
Despite the difficulties that have to-date delayed the PD research field in embracing a platform design to assess disease modifying therapies in PD, the unmet burden of disease faced by patients, as well as the large societal impact, demands that a more efficient and novel process of drug assessment is developed. To address this demand, the Edmond J. Safra Foundation is supporting an initiative led by University College London, University of Plymouth and the MRC Clinical Trials Unit to develop a neuroprotective MAMS platform trial for Parkinson’s disease. This project is known as the Edmond J. Safra Accelerating Clinical trials in PD (EJS ACT-PD) initiative. Its main aim is to produce a protocol that addresses the major controversial trial design issues, indicated above, with solutions reached through transparent data-driven processes, with detailed considered input from all the relevant stakeholders, and importantly incorporating the patient voice at its core.
To this end, six working groups have been set up, each addressing a particular component of platform design and delivery: trial design, outcome measures, therapy selection, infrastructure, funding and sustainability and patient and public engagement. The consortium includes more than 75 individuals from across the UK, comprising patients and carers, neurologists, geriatricians, clinical triallists, statisticians, funders, methodologists, epidemiologists, health economists, trials pharmacists and a range of experience from clinical and preclinical researchers expert in disease modifying drug development and trial design. The patient perspective is central to the process, with patient/carer members embedded in each working group and thus involved in all decisions based on their collective discussions. Patient/carer consortium members are given training regarding all the technical issues and decisions as part of the process. Sustainability of the programme is supported by the inclusion of an early career researcher in each working group. An additional level of oversight, as well as an international perspective, is provided by panel of international advisors. Engagement with the Medicines and Healthcare products Regulatory Agency (MHRA) and reference to European and USA regulatory developments and requirements ensures that the regulatory perspective is incorporated into the design choices.
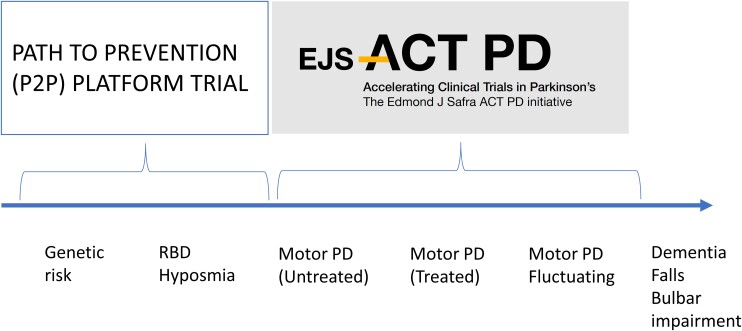
An additional approach that is in set-up is the Pathway to Prevention (P2P) platform.3 This project plans to recruit people at risk of PD, on the basis of confirmed genetic risk (e.g. LRRK2 or GBA1 mutation carriers), or with hyposmia or REM sleep behaviour disorder, but without any manifest symptoms or signs of motor PD. This has the intuitive advantage that people will be identified either before or very early on in the onset of any neurodegenerative process, i.e. at a time where there may be more salvageable neurons, and furthermore avoids the issue of symptomatic dopaminergic treatments confounding measures of disease progression. The additional challenges arising include the uncertainty of risk within the groups, given the incomplete penetrance of the LRRK2 and GBA1 genes, and thus the threshold for tolerability of adverse effects of an intervention may be lower. Furthermore, any positive results that emerge would almost certainly still need subsequent exploration among a population with manifest motor PD to assess the relevance to the larger prevalent population. The P2P and EJS ACT-PD initiatives are therefore highly complementary and will ensure shared knowledge and wisdom (Fig. 1).
Figure 1.
The P2P platform trial. The P2P platform trial plans to recruit people at risk of developing PD on the basis of known genetic risks and/or prodromal symptoms such as REM sleep behaviour disorder to identify treatments which prevent or delay the conversion to motor PD. The EJS ACT-PD platform plans to recruit people with established motor PD to identify treatments that will prevent or delay subsequent progression of motor and non-motor symptoms.
Parallel projects in other neurological diseases
In addressing the challenge of setting up a platform trial in PD, the EJS ACT-PD initiative will learn from the experience and developed expertise within the MRC clinical trials unit, who have pioneered the development and successful delivery of platform trials over the last 15 years, initially in oncology and more recently in other neurodegenerative conditions—such as motor neuron disease (MND-SMART)14 and progressive multiple sclerosis (OCTOPUS)15; their involvement is vital to ensure that the most appropriate design, conduct and analysis choices are made, and that this platform builds on invaluable lessons learnt to date.
Many of the issues emerging in the set-up of a platform trial for disease modification in PD are also relevant to Alzheimer’s disease. The Dominantly Inherited Alzheimer’s Network Trials Unit (DIAN-TU) trial16 pioneered the concept of preventing the emergence of neurodegeneration among people at risk of Alzheimer’s disease due to confirmed dominant genetic risks. Initial results from the first two tested drugs demonstrates that the platform can effectively recruit patients, and while early positive data regarding target engagement have not yet been followed by clinical advantage in cognitive decline in the first two treatment arms targeting amyloid-β,17 recruitment into subsequent treatment arms with different mechanisms of action is already underway (ClinicalTrials.gov Identifier: NCT05269394). The European Prevention of Alzheimer Dementia (EPAD), which also focuses on a prevention approach, has set up a longitudinal cohort study of non-demented individuals over the age of 50 years to try and better identify people at risk of developing Alzheimer’s disease, with a view to future recruitment to a multi-arm platform trial.18 The ACORD (A Collaboration Of groups, Running and Reporting multi-arm multi-stage trials in neurodegenerative Diseases) initiative19 also includes a team setting up a platform trial in people with established Alzheimer’s disease and helps disseminate ideas to overcome the shared challenges of these complex trials.
Conclusion
The need to identify agents that slow down, stop or reverse the progression of PD has never been higher: as our global population ages, the prevalence of PD rises, and the costs associated with the disability and care needs of people with PD become unaffordable.20 We need to create an infrastructure that allows participation in clinical trials by a far greater proportion of PD patients, lowers the burden of participation for patients, assessors and trial pharmacies, has well thought out analytic approaches that account for PD heterogeneity and evaluates therapies targeting precise pathophysiological mechanisms all the way to phase III evaluation. Such an approach will accelerate the discovery of treatments to address this major societal need.
Supplementary Material
Appendix 1
EJS ACT-PD consortium members
Further details are provided in the Supplementary material.
Yoav Ben Shlomo, Mark Edwards, Alan Whone, Carl Counsell, Caroline Clarke, Matthew Burnell, Dorothy Salathiel, Sue Whipps, Anna Jewell, Tom Barber, Rimona Weil, Caroline Williams Gray, Michele Hu, Lynn Rochester, Paola Piccini, Henrik Zetterberg, Alastair Noyce, Ray Chaudhuri, Michael Lawton, Ashwani Jha, Carroll Siu, Michèle Bartlett, Daniel van Wamelen, Simon Stott, George Tofaris, Esther Sammler, Heather Mortiboys, Li Wei, Alan Wong, Susan Duty, David Dexter, Paula Scurfield, Edwin Jabbari, Huw Morris, David Breen, Chris Lambert, Prasad Korlipara, Monty Silverdale, Kailash Bhatia, Alison Yarnall, Raj Khengar, Helen Collins, Fleur Hudson, Gareth Baxendale, Rebecca Croucher, Sandra Bartolomeur-Pires, Jennifer Allison, Antony Morgan, Sheila Wonnacott, Dilan Athauda, Emily Henderson, Shona Clegg, Karen Matthews, Eric Deeson, Laurel Miller, Joel Handley, Helen Matthews, Amit Batla, Nikul Bakshi, Beckie Port, Romy Ellis-Doyle, Sally L. Collins, Judith Rudiger, Rebecca Chapman, Jesse Cedarbaum, Anthony Lang, Brain Fiske, Richard Wyse, Adam Boxer, Denise Wilson, Jean Christophe Corvol, Jennifer Harris.
Contributor Information
Tom Foltynie, Department of Clinical & Movement Neurosciences, UCL Institute of Neurology, London WC1N 3BG, UK.
Sonia Gandhi, Department of Clinical & Movement Neurosciences, UCL Institute of Neurology, London WC1N 3BG, UK.
Cristina Gonzalez-Robles, Department of Clinical & Movement Neurosciences, UCL Institute of Neurology, London WC1N 3BG, UK.
Marie-Louise Zeissler, Faculty of Health, University of Plymouth, Plymouth PL4 9AA, UK.
Georgia Mills, Department of Clinical & Movement Neurosciences, UCL Institute of Neurology, London WC1N 3BG, UK.
Roger Barker, John van Geest Centre for Brain Repair, Department of Clinical Neurosciences, University of Cambridge, Cambridge CB2 0PY, UK.
James Carpenter, MRC Clinical Trials Unit at UCL, London WC1V 6LJ, UK.
Anette Schrag, Department of Clinical & Movement Neurosciences, UCL Institute of Neurology, London WC1N 3BG, UK.
Anthony Schapira, Department of Clinical & Movement Neurosciences, UCL Institute of Neurology, London WC1N 3BG, UK.
Oliver Bandmann, Sheffield Institute for Translational Neuroscience, University of Sheffield, Sheffield S10 2HQ, UK.
Stephen Mullin, Faculty of Health, University of Plymouth, Plymouth PL4 9AA, UK.
Joy Duffen, MRC Clinical Trials Unit at UCL, London WC1V 6LJ, UK.
Kevin McFarthing, Parkinson's Research Advocate, Oxford, UK.
Jeremy Chataway, Department of Clinical & Movement Neurosciences, UCL Institute of Neurology, London WC1N 3BG, UK.
Mahesh Parmar, MRC Clinical Trials Unit at UCL, London WC1V 6LJ, UK.
Camille Carroll, Faculty of Health, University of Plymouth, Plymouth PL4 9AA, UK.
the EJS ACT-PD Consortium:
Yoav Ben Shlomo, Mark Edwards, Alan Whone, Carl Counsell, Caroline Clarke, Matthew Burnell, Dorothy Salathiel, Sue Whipps, Anna Jewell, Tom Barber, Rimona Weil, Caroline Williams Gray, Michele Hu, Lynn Rochester, Paola Piccini, Henrik Zetterberg, Alastair Noyce, Ray Chaudhuri, Michael Lawton, Ashwani Jha, Carroll Siu, Michèle Bartlett, Daniel van Wamelen, Simon Stott, George Tofaris, Esther Sammler, Heather Mortiboys, Li Wei, Alan Wong, Susan Duty, David Dexter, Paula Scurfield, Edwin Jabbari, Huw Morris, David Breen, Chris Lambert, Prasad Korlipara, Monty Silverdale, Kailash Bhatia, Alison Yarnall, Raj Khengar, Helen Collins, Fleur Hudson, Gareth Baxendale, Rebecca Croucher, Sandra Bartolomeur-Pires, Jennifer Allison, Antony Morgan, Sheila Wonnacott, Dilan Athauda, Emily Henderson, Shona Clegg, Karen Matthews, Eric Deeson, Laurel Miller, Joel Handley, Helen Matthews, Amit Batla, Nikul Bakshi, Beckie Port, Romy Ellis-Doyle, Sally L Collins, Judith Rudiger, Rebecca Chapman, Jesse Cedarbaum, Anthony Lang, Brain Fiske, Richard Wyse, Adam Boxer, Denise Wilson, Jean Christophe Corvol, and Jennifer Harris
Funding
The Edmond J. Safra Accelerating Clinical Trials in Parkinson’s Initiative is funded by the Edmond J. Safra Foundation.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marek K, Siderowf A, Coffey C, et al. Path to prevention (P2P) – developing a prodromal PD progression biomarker program [abstract]. Mov Disord. 2019;34:S64-S64. Accessed 24 June 2022. https://www.mdsabstracts.org/abstract/path-to-prevention-p2p-developing-a-prodromal-pd-progression-biomarker-program/ [Google Scholar]
- 4. Fereshtehnejad S, Postuma RB. Subtypes of Parkinson's disease: What do they tell us about disease progression? Curr Neurol Neurosci Rep. 2017;17:34. [DOI] [PubMed] [Google Scholar]
- 5. Winder-Rhodes SE, Evans JR, Ban M, et al. Glucocerebrosidase mutations influence the natural history of Parkinson's disease in a community-based incident cohort. Brain. 2013;136:392–399. PMID: 23413260. [DOI] [PubMed] [Google Scholar]
- 6. Stoker TB, Camacho M, Winder-Rhodes S, et al. Impact of GBA1 variants on long-term clinical progression and mortality in incident Parkinson's disease. J Neurol Neurosurg Psychiatry. 2020;91:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Maio R, Hoffman EK, Rocha EM, et al. LRRK2 Activation in idiopathic Parkinson's disease. Sci Transl Med. 2018;10:eaar5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jennings D, Huntwork-Rodriguez S, Henry AG, et al. Preclinical and clinical evaluation of the LRRK2 inhibitor DNL201 for Parkinson's disease. Sci Transl Med. 2022;14:eabj2658. [DOI] [PubMed] [Google Scholar]
- 9. Cummings J, Montes A, Kamboj S, Cacho JF. The role of basket trials in drug development for neurodegenerative disorders. Alzheimers Res Ther. 2022;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Regnault A, Boroojerdi B, Meuneir A, et al. Does the MDS-UPDRS provide the precision to assess progression in early Parkinson's disease? Learnings from the Parkinson's progression marker initiative cohort. J Neurol. 2019;266:1927–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kieburtz K, Katz R, McGarry A, Olanow CW. A new approach to the development of disease-modifying therapies for PD; fighting another pandemic. Mov Disord. 2021;36:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tosin MHS, Simuni T, Stebbins GT, Cedarbaum JM. Tracking emergence of new motor and non-motor symptoms using the MDS-UPDRS: A novel outcome measure for early Parkinson's disease? J Parkinsons Dis. 2022;12:1345–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McFarthing K, Rafaloff G, Baptista M, et al. Parkinson's disease drug therapies in the clinical trial pipeline: 2021 update. J Parkinson’s Dis. 2021;11:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beswick E, Glasmacher SA, Dakin R, et al. Prospective observational cohort study of factors influencing trial participation in people with motor neuron disease (FIT-participation-MND): A protocol. BMJ Open. 2021;11:e044996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MS Society . Introducing Octopus – the world’s first multi-arm, multi-stage trial for MS. Published online 4 May 2021. Accessed 9 January 2023. https://www.mssociety.org.uk/research/latest-research/latest-research-news-and-blogs/introducing-octopus-worlds-first-multi-arm-multi-stage-trial-ms
- 16. Bateman RJ, Benzinger TL, Berry S, et al. DIAN-TU Pharma consortium for the dominantly inherited Alzheimer network. The DIAN-TU next generation Alzheimer's prevention trial: Adaptive design and disease progression model. Alzheimers Dement. 2017;13:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salloway S, Farlow M, McDade E, et al. Dominantly inherited Alzheimer network–trials unit. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer's disease. Nat Med. 2021;27:1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ritchie CW, Molinuevo JL, Truyen L, Satlin A, Van der Geyten S, Lovestone S. European Prevention of Alzheimer's dementia (EPAD) consortium. Development of interventions for the secondary prevention of Alzheimer's dementia: The European prevention of Alzheimer's dementia (EPAD) project. Lancet Psychiatry. 2016;3:179–186. [DOI] [PubMed] [Google Scholar]
- 19. MRC Clinical Trials Unit . Accessed 9 January 2023. https://www.mrcctu.ucl.ac.uk/studies/all-studies/a/acord/
- 20. Weir S, Samnaliev M, Kuo TC, et al. Short- and long-term cost and utilization of health care resources in Parkinson's disease in the UK. Mov Disord. 2018;33:974–981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.