Abstract
Fortification of human milk is the standard of care for very low birth weight (VLBW) infants and is required to support adequate postnatal growth and development. Achieving adequate growth velocity and preventing growth faltering is critical for the developing neonatal brain and optimizing long-term neurodevelopmental outcomes. Mother’s milk is the gold standard nutrition to feed preterm infants, however, it does not provide the nutrients needed to support the growth of VLBW infants. After the decision is made to use mother’s milk (if available) or alternatively, donor human milk, many dilemmas exist with regards to additional treatment decisions surrounding the type of fortification to use, when to fortify, and the duration of fortification. In this article, we will review the differences in mother’s milk compared to donor milk, the different types of human milk fortifiers, the optimal timing of fortification, and discuss when to discontinue human milk fortification.
INTRODUCTION
Human milk has coevolved with humans for millions of years to provide nutrition for infants and is considered the ideal source of nutrition for infants born at all gestational ages [1–4]. However, human milk evolved for the nourishment of term and later preterm infants, not very preterm infants, as these infants previously did not survive. The nutritional needs of very low birth weight infants (VLBWs; <1500 g birth weight), are greater than term infants, driven by growth targets that are traditionally based on in utero growth rates [5]. While the goal of replicating intrauterine growth rates has been evolving [6], particularly with regard to weight, with greater recognition of variability among VLBW infants, the benchmark of achieving intrauterine growth velocities in weight, length, and head circumference remains [7]. Likewise, what constitutes optimal growth in VLBW infants is an area of active investigation [8].
Based on in utero growth velocity targets, the macro- and micronutrient content of human milk, whether preterm, term, or donor milk, is not sufficient to meet the nutritional needs of VLBW infants when fed in usual volumes without macro- and micronutrient fortification [9, 10]. This shortfall is complicated by the variable nutrient content of human milk [11]. Challenges with achieving adequate growth velocity can be more acute with donor human milk; there is a tradeoff between bioactive components tailored to human infants (such as immunoglobulins and human milk oligosaccharides) and less robust growth compared to the alternative of bovine milk-based nutrition [12]. With these realities, fortifying human milk feeding is essential to provide sufficient macronutrients to achieve goal growth [13–15]. Unfortified human milk at usual feeding volumes (160 mL/kg/day) does not meet recommended energy provision (110–130 kcal/kg/ day) or enteral protein intake of 3.5–4.5 g/kg/day for VLBW infants [9, 11, 13, 16].
The micronutrient composition of human milk does not provide the recommended daily amounts of vitamins and minerals, such as iron, calcium, phosphorus, zinc, vitamins A and D. While iron and vitamin D are frequently supplemented to preterm infants to prevent deficiency, VLBW infants are at particular risk for calcium and phosphorus deficiency due to a significant mineral deposition occurring during the last trimester of gestation [17, 18]. The lack of mineral deposition leaves VLBW infants at risk for metabolic bone disease of prematurity with lasting consequences [18]. In the setting of a human milk diet, fortification with additional calcium, and phosphorus can meet the needs of VLBW infants. Similarly, vitamin A can be deficient in human milk, and levels are commonly low in VLBWs [19]. Vitamin A deficiency has been linked to a higher risk for bronchopulmonary dysplasia (BPD) and retinopathy of prematurity [20–22]. Human milk fortifiers include vitamin A to prevent deficiency, but it is unclear if the amount in fortifiers is sufficient to reduce the risk for these conditions.
As discussed, preterm infants do not receive the full in utero transfer of nutrients during the 3rd trimester and postnatally require additional energy, macronutrients, and minerals to maintain extrauterine growth similar to intrauterine growth [23]. Despite its advantages, human milk does not provide sufficient amounts of nutrients to meet this demand within the feeding volumes tolerated by preterm infants. Mother’s own milk is always the preferred nutrition, but it is not designed to solely feed a preterm infant [24]. Pasteurized donor milk is frequently used as a supplement to mother’s own milk when it is unavailable [25, 26]. While donor milk has shown benefits over preterm formula in reducing the incidence of necrotizing enterocolitis (NEC), the milk is often donated from mothers of term infants [12, 27]. Therefore, donor milk has been shown to be lower in protein, fat, and sodium than preterm milk. Pasteurization and milk banking processes, including multiple plastic container transfers, also decrease the fat content present in donor milk [28]. In addition, the pasteurization process lessens many immune factors and important enzymes that preterm infants require for digestion [27]. Preterm infants have delayed endogenous production of enzymes such as lipase which are key to the digestion of protein and long-chain fatty acids [29]. All these factors combined create challenges in feeding VLBW infants. The goal is to optimize nutrition and avoid postnatal growth failure while not increasing the risk of NEC to an immature gut [29, 30]. This balance of enteral nutrition, NEC risk, and avoidance of postnatal growth failure remains a major challenge in neonatal nutrition.
Dilemmas in human milk fortification
There are different types of fortifiers that can be added to human milk to provide supplemental protein, calcium, and phosphorus to preterm infants [31]. However, even with contemporary nutrition strategies including the use of human milk fortifiers, it is difficult to achieve recommended energy and nutrient intakes [31]. A limitation to providing goal enteral nutrition is the restriction of fluid intake for preterm infants. This is a common practice in Neonatology meant to limit fluid intake to decrease the risk of BPD, however, evidence is lacking for this practice [32]. In addition, the current approach to enteral nutrition for VLBW infants has changed in the last 10 years with the widespread adoption of the use of pasteurized donor human milk which is supported by the American Academy of Pediatrics and the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition [24, 25]. Newer generation human milk fortifiers (HMF) include liquid bovine-based HMF and donor human milk-based HMF. There are important considerations when choosing how to increase the energy content and minerals in human milk.
Type of human milk fortifiers
With the invention of multicomponent bovine-based fortifiers and the adoption of use in NICUs about 30 years ago, clinicians were given the option to fortify mothers’ own milk instead of using supplemental preterm formula. The initial bovine-based fortifiers were powder-based, however, newer generation fortifiers in the U.S. are now liquid-based. The addition of a liquid bovine-based fortifier allows for the avoidance of powder and associated infection risk and provides higher amounts of protein to meet the needs of preterm infants [30, 31]. Bovine-based products were the only option available until the late 2000s when a commercial pasteurized donor human milk-based fortifier became available for use. These products, derived from human milk, provide human milk protein, calcium, and phosphorus. The majority of the studies of an exclusive human milk (EHM) diet (mother’s own milk, donor human milk, and donor human milk-based fortifier) have compared outcomes related to a mixed bovine-based diet, meaning the bovine groups received mother’s own milk with bovine-based fortifier and/or formula [33, 34]. The two study groups reflected the nutritional practice during the time when pasteurized donor human milk was not widely used. When an EHM diet is compared to a mixed bovine-based diet, studies have shown an association with decreased risk of NEC, BPD, retinopathy of prematurity (ROP), sepsis, and mortality [33–36]. However, only one randomized controlled trial by O’Connor et al. has compared donor human milk-based fortifier vs. bovine milk-based fortifier with mother’s own milk and donor human milk as the base diet [37]. In this trial, infants were fed mothers’ own milk, supplemented with donor human milk, and then randomized to the type of fortifier. There was no difference in NEC or feeding intolerance between the two groups, however, there was decreased incidence of ROP in the EHM diet group [37]. The lack of a large-scale randomized controlled trial directly comparing donor human milk-based fortifiers to bovine-based fortifiers causes a quandary for clinicians who want to provide adequate enteral nutrition to VLBW infants without increasing their risk of NEC.
Clinicians must consider a variety of factors when deciding which fortification strategy to use (Fig. 1). The choice of fortifier also depends on the type of human milk infants are receiving as there is a range of variability in macronutrients. Depending on which base milk and fortifier are used, there can be variability in energy content and macronutrients [38]. Pasteurized DHM provides the least amount of protein [27, 39]. Preterm mother’s milk in the first 1–2 weeks of life is high in protein (1.5–2.2 g/dL) compared to pasteurized DHM which is considered term milk (0.8–0.9 g/dL protein) [11, 27]. However, without daily macronutrient analysis using a human milk analyzer, it is difficult to assume that all mothers’ own milk is higher in protein exceeding the average value in mature human milk [11]. This is important because when calculating the energy and macronutrient intakes delivered with fortified human milk, they are dependent on the values that are assumed for human milk. The challenge with the use of human milk analyzers is that they are expensive and labor-intensive for staff and require close monitoring of the feeding recipe for the infant [40]. Until targeted fortification is more streamlined for clinical practice, the clinician must make assumptions when calculating the nutrients in fortified human milk.
Fig. 1. Dilemmas clinicians face when choosing which fortifier to use for very low birth weight infants.
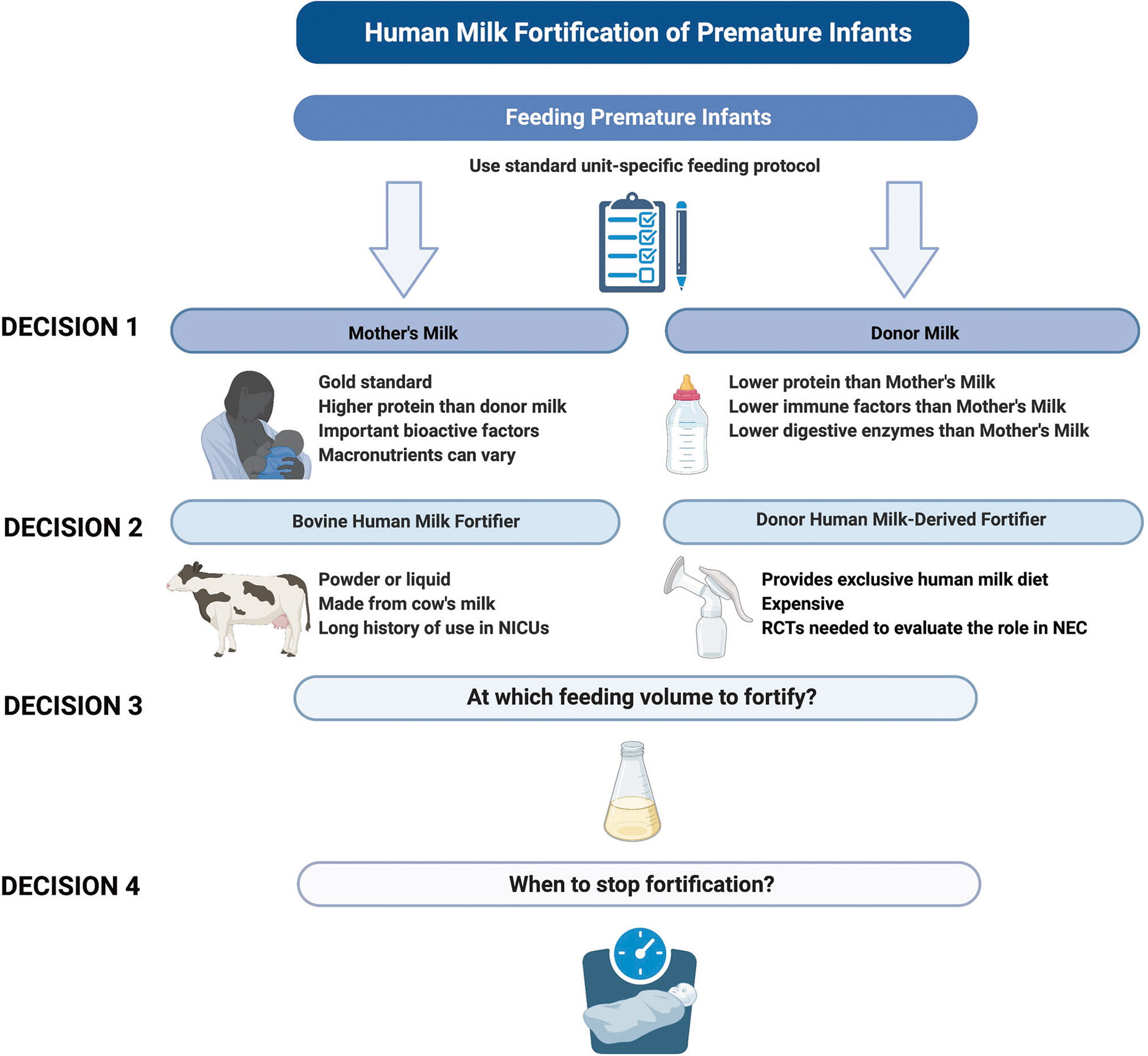
Abbreviations: Neonatal intensive care units (NICUs); Randomized controlled trials (RCTs). Figure created with BioRender.com.
Studies have shown that mother’s own milk has a dose-dependent effect with outcomes emphasizing the importance of limiting the amount of milk displaced with the addition of fortifiers [41]. Powdered bovine-HMF causes the least amount of milk to be displaced, however, there are risks associated with nonsterile powder. In 2022, the Food and Drug Administration recalled powdered formula products from the market due to concerns about contamination with Cronobacter and Salmonella [42]. In addition, powdered HMF provides less total protein. Newer generation liquid bovine-HMFs provide a sterile product with higher protein delivery, however, there is some displacement of human milk since more liquid fortifier needs to be added to the milk to reach targeted nutrient goals [30]. Recent liquid bovine-HMFs provide partially digested protein that needs further study but may be easier for the digestion issues common in the immature GI tracts of preterm infants. Donor human milk-based fortifiers provide an EHM diet allowing for avoidance of bovine products until infants reach ~34 weeks postmenstrual age (PMA), the time at which NEC incidence decreases [43, 44]. However, this fortifier is much more expensive than bovine fortifiers and displaces more mothers’ own milk [37, 45, 46]. One advantage of donor human milk-based fortifiers is that there is a range of fortification levels that provide up to 30 kcal/oz of energy and higher amounts of protein above 4.5 g/kg/day depending on the fluid intake of the infant [43]. Because the fortifier is made from human milk, the total osmolarity of the fortified milk remains lower than liquid bovine-based fortifiers [30]. It is important to note that often decisions about the type of fortifiers available are driven by hospital systems rather than individual clinicians in a particular unit. Figure 1 depicts the challenges clinicians face when choosing which fortifier to use for VLBW infants.
Early versus late fortification of human Milk
In addition to which type of fortifier to use, the decision of when to add fortifier to human milk can be challenging and is a topic of debate in the field. A recent Cochrane Review aimed to evaluate the effects of growth and the safety of early (enteral feeding volume of <100 mL/kg/day) versus late (enteral feeding volume of ≥100 mL/kg/day or ≥7 days after birth) fortification in different gestational age categories [47]. The meta-analysis included two trials and found there was no effect on growth (linear, head circumference, or regaining birth weight) between the early or late fortified group of premature infants [47]. However, due to the limited published data on this topic, the authors concluded that the available evidence to support early versus late fortification was not available [47]. One randomized controlled trial (RCT) demonstrated that there was no difference in the time to reach full feeding volumes, episodes of feeding intolerance, and NEC incidence between two fortification groups (20 mL/kg/day compared to 100 mL/kg/day) [48]. A literature review demonstrated that early fortification was tolerated by infants and was safe, but did not improve growth outcomes or decrease the infants’ length of stay [49]. In the absence of data from RCT demonstrating significant differences in early versus late fortification, premature infant feeding protocols still vary significantly across neonatal intensive care units (NICUs) and growth faltering remains an important morbidity in the NICU [50].
Rather than a dichotomous approach to early or late fortification, several studies have evaluated whether there is an optimal time for HMF in the transition from parenteral to enteral feeds. One study utilized a nutrition modeling approach to provide the optimal concentration of amino acids in standardized parental nutrition to ensure maximal protein intake during this transition [51]. The authors found that from a modeling perspective, the optimal amino acid concentration was 3.5 g/100 mL of standardized parenteral nutrition, while also providing early HMF at 80 mL/kg/day [51]. Other investigators found that to optimize protein and energy intakes, HMF could be targeted once an infant achieved 33–67% of enteral intake [52]. The parenteral to enteral transition phase is critical for premature infants as one study demonstrated up to 46% incidence of poor growth during this transition, which can also be an early indicator of extrauterine growth failure at discharge [53].
Length of fortification
Fortification of human milk helps meet the recommended enteral requirements of protein, minerals, and vitamins that premature infants need to reduce the nutritional gaps that are inherently present. Fortification also is critical to prevent growth faltering and provide premature infants with the nutritional support to maintain bone mineralization. How long to fortify the human milk of VLBW infants is of growing importance as more VLBWs are discharged home with a human milk-based diet [54]. Fetal growth velocity, in terms of change per kilogram per day, tapers in the mid-third trimester (34–36 week gestation), and therefore, needs for macronutrients and energy also decrease. Whether and when to adjust, or stop human milk fortification for VLBWs at 34–36 weeks PMA in recognition of this change in fetal growth trajectory remains ill-defined, as there may be in-hospital growth deficits, as well as macro- and micronutrient deficits that call for continuing milk fortification [16].
Consideration of whether to continue human milk fortification beyond term PMA, or discharge, is similarly in need of further definition, and may take into account the in-hospital growth, clinical course, current and projected nutritional needs, and the capability of caregivers to successfully carry out a prescribed feeding plan [16]. If fortification is to be continued past term PMA, the options for outpatient fortification include 1) bovine-based post-discharge transitional powdered formula (“sprinkles”) mixed into pumped human milk, 2) 1–2 bottles per day of post-discharge transitional formula daily in concert with breastfeeding and pumped human milk feeds, an alternative to “sprinkles”, [55] and 3) continuing fortification of human milk with bovine HMF, which is now possible in some areas for outpatient use through the Special Supplemental Nutrition Program for Women, Infants, and Children [56]. The choices depend on the assessment of individual patient characteristics and clinical needs, for example, the choice of extended HMF fortification as an outpatient to address large protein, calcium, and phosphorus deficits past term PMA that would be difficult to address with post-discharge transitional formula powder-based fortification, because of the significantly higher protein and mineral content per gram available from HMF [16].
CONCLUSION
In conclusion, there are many factors to consider when evaluating the current fortifiers available for use in preterm infants. The prioritization and use of mothers’ own milk should be emphasized for all infants. In addition, a standardized feeding protocol should be adopted as studies have shown that a feeding protocol is associated with improved outcomes [57]. While feeding protocols and standardized fortification of human milk should apply to most preterm infants, it is important to individualize nutrition based on the unique needs and growth trajectories of the patient. In the past 10 years, there have been many advances in neonatal nutrition including pasteurized donor human milk and newer generation fortifiers. Future thoughtfully designed feeding studies are needed to answer the numerous unanswered questions surrounding enteral feeding and fortification in preterm infants.
FUNDING
BS is supported by Gerber Foundation grant #21-6234 and a grant from Evolve Biosystems. ABH and MG are supported by the National Institutes of Health (NIH) grant R01DK124614, and MG is also supported by NIH grants R01DK118568 and R01HD105301. None of the funding sources had any role in this manuscript.
Footnotes
COMPETING INTERESTS
The authors declare no competing interests.
ADDITIONAL INFORMATION
Reprints and permission information is available at http://www.nature.com/reprints
REFERENCES
- 1.Eidelman AI. Breastfeeding and the use of human milk: an analysis of the American Academy of Pediatrics 2012 Breastfeeding Policy Statement. Breastfeed Med: Off J Acad Breastfeed Med. 2012;7:323–4. [DOI] [PubMed] [Google Scholar]
- 2.Vohr BR, Poindexter BB, Dusick AM, McKinley LT, Wright LL, Langer JC, et al. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics. 2006;118:e115–123. [DOI] [PubMed] [Google Scholar]
- 3.Vohr BR, Poindexter BB, Dusick AM, McKinley LT, Higgins RD, Langer JC, et al. Persistent beneficial effects of breast milk ingested in the neonatal intensive care unit on outcomes of extremely low birth weight infants at 30 months of age. Pediatrics. 2007;120:e953–9. [DOI] [PubMed] [Google Scholar]
- 4.Brown JVE, Walsh V, McGuire W. Formula versus maternal breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2019;8:Cd002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schanler RJ. Evaluation of the evidence to support current recommendations to meet the needs of premature infants: the role of human milk. Am J Clin Nutr. 2007;85:625s–8s. [DOI] [PubMed] [Google Scholar]
- 6.Fenton TR, Cormack B, Goldberg D, Nasser R, Alshaikh B, Eliasziw M, et al. “Extrauterine growth restriction” and “postnatal growth failure” are misnomers for preterm infants. J Perinatol. 2020;40:704–14. [DOI] [PubMed] [Google Scholar]
- 7.Chou FS, Pandey V, Yeh HW. Postnatal growth in extremely low birth weight newborns: nature or nurture? J Perinatol. 2021;41:648–9. [DOI] [PubMed] [Google Scholar]
- 8.Belfort MB, Ramel SE. NICU diet, physical growth and nutrient accretion, and preterm infant brain development. Neoreviews. 2019;20:e385–96. [DOI] [PubMed] [Google Scholar]
- 9.Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50:85–91. [DOI] [PubMed] [Google Scholar]
- 10.Fenton TR, Griffin IJ, Groh-Wargo S, Gura K, Martin CR, Taylor SN, et al. Very low birthweight preterm infants: a 2020 evidence analysis center evidence-based nutrition practice guideline. J Acad Nutr Diet. 2022;122:182–206. [DOI] [PubMed] [Google Scholar]
- 11.Gidrewicz DA, Fenton TR. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014;14:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2019;7:Cd002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg DL, Becker PJ, Brigham K, Carlson S, Fleck L, Gollins L, et al. Identifying malnutrition in preterm and neonatal populations: recommended indicators. J Acad Nutr Diet. 2018;118:1571–82. [DOI] [PubMed] [Google Scholar]
- 14.Brown JV, Embleton ND, Harding JE, McGuire W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database Syst Rev. 2016;5:Cd000343. [DOI] [PubMed] [Google Scholar]
- 15.Fenton TR, Groh-Wargo S, Gura K, Martin CR, Taylor SN, Griffin IJ, et al. Effect of enteral protein amount on growth and health outcomes in very-low-birth-weight preterm infants: phase II of the pre-B project and an evidence analysis center systematic review. J Acad Nutr Diet. 2021;121:2287–2300.e2212. [DOI] [PubMed] [Google Scholar]
- 16.Taylor SN, Martin CR. Evidence-based discharge nutrition to optimize preterm infant outcomes. Neoreviews. 2022;23:e108–e116. [DOI] [PubMed] [Google Scholar]
- 17.Rustico SE, Calabria AC, Garber SJ. Metabolic bone disease of prematurity. J Clin Transl Endocrinol. 2014;1:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faienza MF, D’Amato E, Natale MP, Grano M, Chiarito M, Brunetti G. et al. Metabolic bone disease of prematurity: diagnosis and management. Front Pediatr. 2019;7:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kositamongkol S, Suthutvoravut U, Chongviriyaphan N, Feungpean B, Nuntnarumit P. Vitamin A and E status in very low birth weight infants. J Perinatol. 2011;31:471–6. [DOI] [PubMed] [Google Scholar]
- 20.Mactier H, Weaver LT. Vitamin A and preterm infants: what we know, what we don’t know, and what we need to know. Arch Dis Child Fetal Neonatal Ed. 2005;90:F103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mactier H, McCulloch DL, Hamilton R, Galloway P, Bradnam MS, Young D, et al. Vitamin A supplementation improves retinal function in infants at risk of retinopathy of prematurity. J Pediatr. 2012;160:954–9.e951. [DOI] [PubMed] [Google Scholar]
- 22.Shenai JP. Vitamin A supplementation in very low birth weight neonates: rationale and evidence. Pediatrics. 1999;104:1369–74. [DOI] [PubMed] [Google Scholar]
- 23.Greer FR. Feeding the premature infant in the 20th century. J Nutr. 2001;131:426s–30s. [DOI] [PubMed] [Google Scholar]
- 24.American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and theuse of human milk. Pediatrics. 2012;129:e827–841.22371471 [Google Scholar]
- 25.Arslanoglu S, Corpeleijn W, Moro G, Braegger C, Campoy C, Colomb V, et al. Donor human milk for preterm infants: current evidence and research directions. J Pediatr Gastroenterol Nutr. 2013;57:535–42. [DOI] [PubMed] [Google Scholar]
- 26.American Academy of Pediatrics Committee on Nutrition, Section on Breastfeeding, Committee on Fetus and Newborn. Donor human milk for the high-risk infant: preparation, safety, and usage options in the united states. Pediatrics. 2017;139:e20163440. [DOI] [PubMed] [Google Scholar]
- 27.Meier P, Patel A, Esquerra-Zwiers A. Donor human milk update: evidence, mechanisms, and priorities for research and practice. J Pediatr. 2017;180:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friend LL, Perrin MT. Fat and protein variability in donor human milk and associations with milk banking processes. Breastfeed Med: Off J Acad Breastfeed Med. 2020;15:370–6. [DOI] [PubMed] [Google Scholar]
- 29.Burge K, Vieira F, Eckert J, Chaaban H. Lipid composition, digestion, and absorption differences among neonatal feeding strategies: potential implications for intestinal inflammation in preterm infants. Nutrients. 2021;13:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arslanoglu S, Boquien CY, King C, Lamireau D, Tonetto P, Barnett D, et al. Fortification of human milk for preterm infants: update and recommendations of the European Milk Bank Association (EMBA) Working Group on human milk fortification. Front Pediatr. 2019;7:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koo W, Tice H. Human milk fortifiers do not meet the current recommendation for nutrients in very low birth weight infants. J Parenter Enter Nutr. 2018;42:813–20. [DOI] [PubMed] [Google Scholar]
- 32.Barrington KJ, Fortin-Pellerin E, Pennaforte T. Fluid restriction for treatment of preterm infants with chronic lung disease. Cochrane Database Syst Rev. 2017;2:Cd005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawoger R, Kiechl-Kohlendorfer U, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156:562–7.e561. [DOI] [PubMed] [Google Scholar]
- 34.Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl-Kohlendorfer U, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013;163:1592–5.e1. [DOI] [PubMed] [Google Scholar]
- 35.Hair AB, Rechtman DJ, Lee ML, Niklas V. Beyond necrotizing enterocolitis: other clinical advantages of an exclusive human milk diet. Breastfeed Med: Off J Acad Breastfeed Med. 2018;13:408–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Assad M, Elliott MJ, Abraham JH. Decreased cost and improved feeding tolerance in VLBW infants fed an exclusive human milk diet. J Perinatol. 2016;36:216–20. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor DL, Kiss A, Tomlinson C, Bando N, Bayliss A, Campbell DM, et al. Nutrient enrichment of human milk with human and bovine milk-based fortifiers for infants born weighing <1250 g: a randomized clinical trial. Am J Clin Nutr. 2018;108:108–16. [DOI] [PubMed] [Google Scholar]
- 38.Bergner EM, Taylor SN, Gollins LA, Hair AB. Human milk fortification: a practical analysis of current evidence. Clin Perinatol. 2022;49:447–60. [DOI] [PubMed] [Google Scholar]
- 39.Meredith-Dennis L, Xu G, Goonatilleke E, Lebrilla CB, Underwood MA, Smilowitz JT. Composition and variation of macronutrients, immune proteins, and human milk oligosaccharides in human milk from nonprofit and commercial milk banks. J Hum Lactation. 2018;34:120–9. [DOI] [PubMed] [Google Scholar]
- 40.Ramey SR, Merlino Barr S, Moore KA, Groh-Wargo S. Exploring innovations inhuman milk analysis in the neonatal intensive care unit: a survey of the United States. Front Nutr. 2021;8:692600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J Perinatol. 2009;29:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.https://www.fda.gov/consumers/infant-formula-recall-what-know. Accessed 26 Feb 2022.
- 43.Hair AB, Hawthorne KM, Chetta KE, Abrams SA. Human milk feeding supports adequate growth in infants ≤1250 grams birth weight. BMC Res Notes. 2013;6:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK, et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012;129:e298–304. [DOI] [PubMed] [Google Scholar]
- 45.Hampson G, Roberts SLE, Lucas A, Parkin D. An economic analysis of human milk supplementation for very low birth weight babies in the USA. BMC Pediatr. 2019;19:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radmacher PG, Adamkin DH. Fortification of human milk for preterm infants. Semin Fetal Neonatal Med. 2017;22:30–35. [DOI] [PubMed] [Google Scholar]
- 47.Thanigainathan S, Abiramalatha T. Early fortification of human milk versus late fortification to promote growth in preterm infants. Cochrane Database Syst Rev. 2020;7:CD013392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah SD, Dereddy N, Jones TL, Dhanireddy R, Talati AJ. Early versus delayed human milk fortification in very low birth weight infants-a randomized controlled trial. J Pediatr. 2016;174:126–31.e121. [DOI] [PubMed] [Google Scholar]
- 49.Godden B, Collins CT, Hilditch C, McLeod G, Keir A. Does early compared to late fortification of human milk for preterm infants improve clinical outcomes? J Paediatr Child Health. 2019;55:867–72. [DOI] [PubMed] [Google Scholar]
- 50.Poindexter BB, Cormack BE, Bloomfield FH. Approaches to growth faltering. World Rev Nutr Diet. 2021;122:312–24. [DOI] [PubMed] [Google Scholar]
- 51.Brennan AM, Kiely ME, Fenton S, Murphy BP. Standardized parenteral nutrition for the transition phase in preterm infants: a bag that fits. Nutrients. 2018;10:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Falciglia GH, Murthy K, Holl JL, Palac HL, Oumarbaeva Y, Woods DM, et al. Energy and protein intake during the transition from parenteral to enteral nutrition in infants of very low birth weight. J Pediatr. 2018;202:38–43.e31. [DOI] [PubMed] [Google Scholar]
- 53.Miller M, Vaidya R, Rastogi D, Bhutada A, Rastogi S. From parenteral to enteral nutrition: a nutrition-based approach for evaluating postnatal growth failure in preterm infants. J Parenter Enter Nutr. 2014;38:489–97. [DOI] [PubMed] [Google Scholar]
- 54.Parker MG, Greenberg LT, Edwards EM, Ehret D, Belfort MB, Horbar JD. National trends in the provision of human milk at hospital discharge among very low-birth-weight infants. JAMA Pediatr. 2019;173:961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Groh-Wargo S, Thompson M. Managing the human-milk-fed, preterm, VLBW infant at NICU discharge: the sprinkles dilemma. Infant, Child, Adolesc Nutr. 2014;6:262–9. [Google Scholar]
- 56.Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). 2021. https://www.fns.usda.gov/wic/about-wic. Accessed 1 May 2022. [PubMed]
- 57.McCallie KR, Lee HC, Mayer O, Cohen RS, Hintz SR, Rhine WD. Improved outcomes with a standardized feeding protocol for very low birth weight infants. J Perinatol. 2011;31:S61–7. [DOI] [PubMed] [Google Scholar]


