Abstract
Engagement in cognitively stimulating activities is gaining prominence as a potential strategy to maintain cognitive functioning in old age. In a population-based cohort of individuals aged 65+ years, we examined patterns of change in frequency of engagement in total cognitive activity (TCA), higher cognitive activity, (HCA) and frequent cognitive activity (FCA) based on the Florida Cognitive Activities Scale over an average of 3.62 years, and whether these patterns were associated with incident mild cognitive impairment (MCI) during this same period. Among 867 cognitively normal participants, 129 (15%) progressed to MCI. Latent class trajectory modeling identified high and stable, slowly, and quickly declining patterns for TCA; high and stable, slowly declining, and slowing increasing patterns for FCA; and high and stable, and slowly declining patterns for HCA. Separate, adjusted Cox proportional hazard models, revealed that compared to the high, stable pattern, both slow decline (HR = 2.5, 95% CI: 1.5-4.0) and quick decline (HR = 11.0, 95% CI: 6.3-19.2) in TCA, and slow decline in the FCA (HR = 8.7, 95% CI: 5.3-14.3) and HCA (HR= 3.4, 95% CI: 2.0-5.6) subscales increased risk for incident MCI. Maintaining engagement in cognitive activities may be protective against progression to MCI, alternatively, declining engagement may be a marker for impending cognitive impairment.
Keywords: Mild cognitive impairment, cognitive activity, epidemiology
Introduction
Engagement in cognitively stimulating activities is gaining prominence as a potential behavioral strategy to maintain cognitive functioning in old age. Observational studies suggest that those who report participating in mentally effortful activities, with both higher frequency and greater variety, have slower age-related cognitive decline1-3 and a lower risk of mild cognitive impairment4-6 and dementia7-9. In the same vein, a variety of intervention trials that provide cognitive stimulation; ranging from training specific cognitive domains such as the Advanced Cognitive Training for Vital Elderly (ACTIVE) study10, to programs that provide the opportunity for activities that may stimulate multiple cognitive domains through activities such as volunteering11, video games12-14, computer games15, creative problem solving16, and creative expression17 have shown to benefit cognitive performance and/or to lower risk for cognitive impairment in older adults. However, results from both observational studies and interventions have produced inconclusive results18-20, which are likely explained by differences in study methodology19, 21-23.
Longitudinal studies that examine patterns of change in activity, rather than only initial level of activity, may help clarify whether modifying (i.e., changing) the level of engagement in activities would influence the development of cognitive impairment over time, and thus would support interventional approaches to increase everyday cognitive stimulation. Further, examining predictors of change in engagement in cognitive activity may help identify those with declining engagement that may benefit from such interventions. For example, we would hypothesize that older participants, men, those with lower education, and are unmarried or living alone would be less likely to remain engaged in activities. Poor sensory and health functioning would also be expected to negatively influence frequency of engagement in cognitive activity with aging.
The purpose of the present study is to examine patterns of change in cognitive activity over time, predictors of these patterns, and whether these patterns are associated with risk of developing mild cognitive impairment (MCI) in initially cognitively normal participants. A limitation of this line of research has been the variety of measurements of everyday engagement in cognitive activities that have been employed in different studies21. This has limited not only comparisons across studies, but also our understanding of the parameters of cognitive activity for intervention trials. We therefore utilized the Florida Cognitive Activities Scale (FCAS) that was developed and validated by Schinka and colleagues24 as a psychometrically sound measure of cognitive engagement. We hypothesized that cognitively normal older adults who experience a decline in activities, compared to those maintaining their levels of engagement, are more likely to develop subsequent cognitive impairment.
Methods
Participants
Participants were members of a prospective cohort study designed to investigate MCI in the community, locally known as Monongahela-Youghiogheny Healthy Aging Team (MYHAT). Details of the study design and methods are published elsewhere25. Briefly, community-dwelling elders aged 65 years or older were drawn as an age-stratified random sample from voter registration lists of select towns in a geographically defined region of Southwestern Pennsylvania between 2006 and 2008. A total of 2,036 participants were initially recruited; based on an age-education adjusted Mini-Mental Status Examination (MMSE) score < 21/3026, 27, 54 were considered too cognitively impaired for a study of MCI. The remaining 1,982 had a mean age of 77.6 years, were 61% female, and 94.8% White. They had a median education level of high school graduate; 41.1% had more than a high school education. Their average MMSE score was 26.9 (SD=2.4; Range 15-30). These participants were assessed in detail at study entry and reassessed in annual cycles. The MYHAT study protocol was reviewed and approved by the Institutional Review Board of the University of Pittsburgh and all participants provided written informed consent.
To be included in the present analyses, participants needed to have complete data on the FCAS, which was added to the MYHAT assessment battery in January 2010, at which time participants were in their 3rd, 4th, or 5th annual follow-up cycle. A total of 1322 participants were assessed on the FCAS for the first time at cycle 3 (n = 322), cycle 4 (n = 703), or cycle 5 (n = 297), which was considered his/her “baseline” for the present analyses. The survey has since been repeated at annual assessments. In addition, participants had to be cognitively normal, i.e., to have a Clinical Dementia Rating (CDR; described below)=028, 29 at the “baseline” assessment, which further excluded 380 participants. Finally, 75 participants were excluded because they did not have at least one year of follow-up, resulting a final sample of 867 participants.
Measures
Incident MCI.
The Clinical Dementia Rating scale28 is a used to rate cognitive function based on everyday performance. A summary CDR rating of 0 (no dementia), 0.5 (very mild dementia), and 1.0 through 3.0 (mild, moderate, and severe dementia) is generated using an algorithm that is weighted towards memory. Incident MCI was defined as a CDR rating of 0.5 occurring for the first time in an individual with a previous annual rating of CDR=0, with the time of onset defined as the midpoint between the two ratings.
Cognitive Activity.
The FCAS24 is a 25-item, reliable and valid scale designed to measure self-reported frequency of engagement across a spectrum of activities varying in cognitive demand. Participants report the frequency of engagement in each activity during the past year, with response choices being “ never or used to do, but not in the past year”, “less than 1 time per month”, “1-4 times per month”, “5 or more times per month, but not every day”, and “every day.” A total cognitive activity (TCA) score is calculated by summing scores on all 25-tems. Two, non-mutually exclusive subscales are calculated to assess activities with higher cognitive demand and engaged in frequently, referred to as higher cognitive activity (HCA) and frequent cognitive activity (FCA), respectively. The HCA subscale score is calculated by summing ten items found to be correlated with a cognitive composite score, and the FCA subscale score is calculated by summing eight items found to be engaged in at least weekly in the initial scale development.24 The FCAS score was not calculated if a participant was missing item-level data, which occurred in only 9 participants at their own “baselines.”
Since the FCAS was developed as a self-administered survey, and the MYHAT study assessment is interviewer-administered, we implemented additional guidelines to assist interviewers in coding the “average” frequency over the past year since frequency can vary over the course of the year. We also provided guidelines regarding acceptable activities not explicitly listed on the survey, e.g., “writing emails” was acceptable under the original survey question “writing letters to friends or relatives.” The adapted FCAS survey can be viewed as Supplemental Digital Content 1, with activities contributing to the HCA and FCA subscales denoted.
Other variables.
The following baseline variables were considered for inclusion in the statistical models. Sociodemographic characteristics included age (continuous), gender, race (White vs. non-White), apolipoprotein E allele status (absence vs. presence of e4 allele), education (< high school vs. ≥ high school), marital status (not married vs. married or living as married), living arrangement (alone vs. with others) and employment status (not working vs. working (full- or part-time)). Physical health included subjective rating of health (poor/fair/good vs. very good/excellent), number of prescription medications (0-3 vs. ≥ 4), vision and hearing (no correction needed vs. correction needed), the timed Get-Up-and-Go test measured in seconds (continuous), and waist-to-hip ratio (continuous). Vascular health included history of self-report s (yes vs. no) medical diagnosis of stroke, hypertension, hypercholesterolemia, diabetes mellitus, heart attack, and congestive heart failure, as well as objective measures of systolic and diastolic blood pressure (continuous). Depression was assessed by the number of depressive symptoms on the modified Center for Epidemiologic Scale (mCES-D)30 (0 vs. ≥ 1 symptom based on median score). Cognitive ability was measured with the Mini-Mental State Examination26 (MMSE; continuous), and subjective cognitive concerns were based on a 21item scale (0 vs. ≥ 2 complaints based on median score) and a self-report of memory being worse than it was one year ago (yes vs. no)31. Lifestyle behaviors included physical activity of at least moderate intensity in the past year (yes vs. no), smoking (current vs. not current), and drinking (never/previous use vs. in past year). Self-report of medical history and lifestyle behaviors is standard in population –based surveys and sufficiently reliable in individuals without dementia32, 33.
Statistical Analyses
We first summarized the baseline characteristics of the sample. Next, we used latent class trajectory modeling (LCTM)34 to identify participants with homogeneous longitudinal trajectories (i.e., stable, increasing, or decreasing) of TCA, FCA, and HCA scores. Bayesian information criteria (BIC) and likelihood ratio tests were combined to determine the number of components in a latent trajectory model and the appropriate functional form of each component (linear, quadratic, etc.). The model with the smallest BIC value among all the candidate models with a significant likelihood ratio test statistic was used to select the number of underlying trajectory groups. Each participant was then assigned to the trajectory group with the largest estimated posterior probability of his/her belonging to that group. Intercepts and slopes were estimated for each trajectory group.
After determining the trajectory group, we further explored the association between baseline characteristics and trajectory group membership by fitting univariable multinomial logistic regression models.
Finally, we used weighted Cox proportional hazards regression models to estimate the association between trajectory patterns of each cognitive activity scale and time to MCI incidence. To account for differences between those who were still in the study at wave 3, 4, or 5 and those who had been lost prior to the addition of FCAS, inverse probability weights were estimated for each participant using multiple logistic regression models. This method estimates the probability of being in the study at the cycle when FCAS was first asked, with the weights adjusting for potential attrition bias. 35 In these models, we also adjusted for covariates that were associated with risk for MCI in the univariable models (p < 0.15). (Supplemental Digital Content 2 shows the univariable model results).
LCTM analysis was performed using lcmm package in R v3.1.0, and all other analyses were performed in SAS 9.336.
Results
A total of 867 participants were included in the present study and were followed from cycle 3, 4 or 5 (depending on the cycle when FCAS was first assessed) through cycle 8, with an average of 3.6 years (maximum = 5 years) of follow-up. Table 1 shows the characteristics of the participants at baseline.
Table 1.
Characteristics of the study participants at baseline (N=867)
| Continuous Variables | Mean | Standard Deviation |
|---|---|---|
| Age | 79.76 | 6.73 |
| Total Cognitive Activity | 44.00 | 9.21 |
| Frequent Cognitive Activity | 25.25 | 3.69 |
| Higher Cognitive Activity | 14.12 | 5.48 |
| MMSE | 28.00 | 1.75 |
| Systolic Blood Pressure | 130.69 | 15.03 |
| Diastolic Blood Pressure | 73.06 | 8.24 |
| Timed Get Up and Go (sec) | 12.73 | 3.62 |
| Waist-hip Ratio | 0.90 | 0.09 |
| Categorical Variables | N | % |
| Female | 550 | 63.44 |
| Education ≥ high school | 406 | 46.83 |
| White | 832 | 95.96 |
| ApoE4 (+) | 162 | 20.05 |
| Married or living as married | 425 | 49.02 |
| Living alone | 356 | 41.06 |
| Working full or part time | 109 | 12.57 |
| Stroke | 99 | 11.42 |
| Hypertension | 560 | 64.59 |
| Hypercholesterolemia | 473 | 54.56 |
| Heart attack | 7 | 0.81 |
| Diabetes mellitus | 184 | 21.22 |
| Congestive heart failure | 25 | 2.88 |
| ≥ 1 Depressive symptoms | 125 | 14.43 |
| Physically active | 600 | 69.20 |
| Currently smoking | 50 | 5.77 |
| ≥ 2 Subjective memory concerns | 250 | 18.84 |
| Memory worse than 1 year ago | 102 | 11.79 |
| Alcohol in past year | 137 | 15.80 |
| Subjective Health (very good/excellent) | 371 | 42.84 |
| ≥4 of Prescription medications | 481 | 55.48 |
| Vision correction | 809 | 93.31 |
Note: MMSE = Mini-Mental State Exam
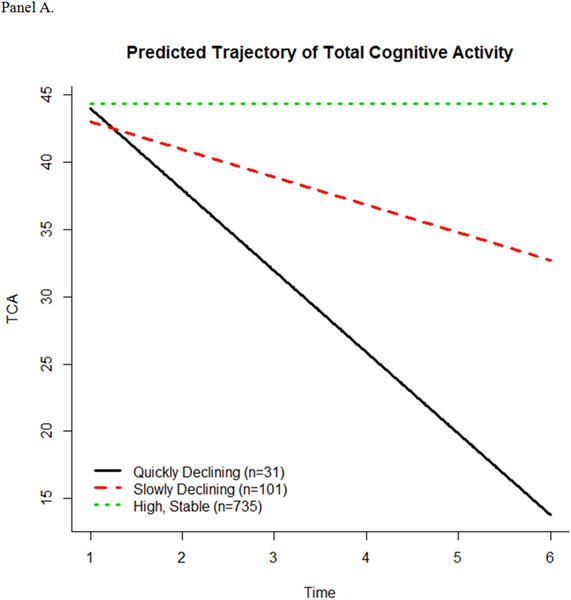
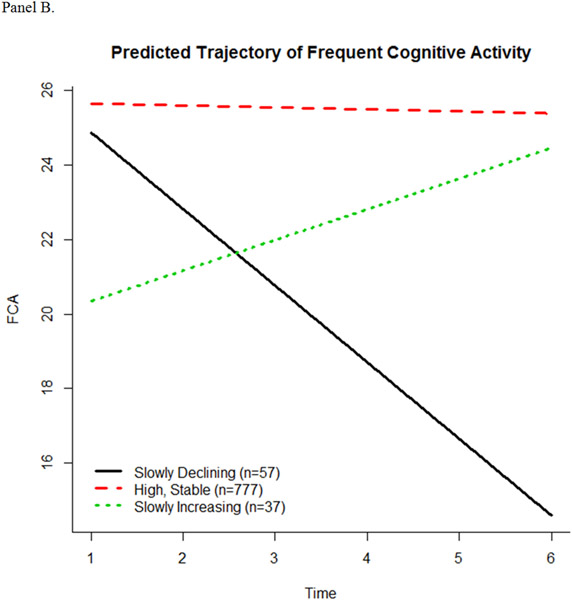
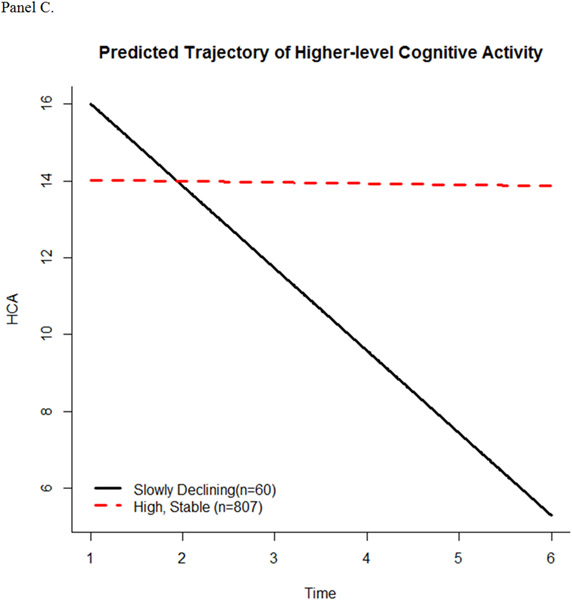
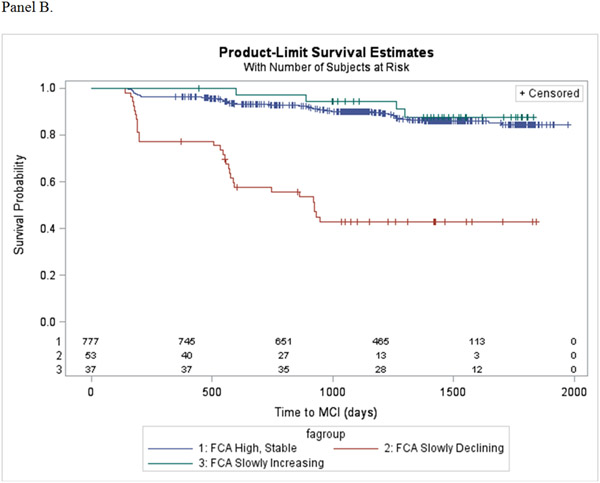
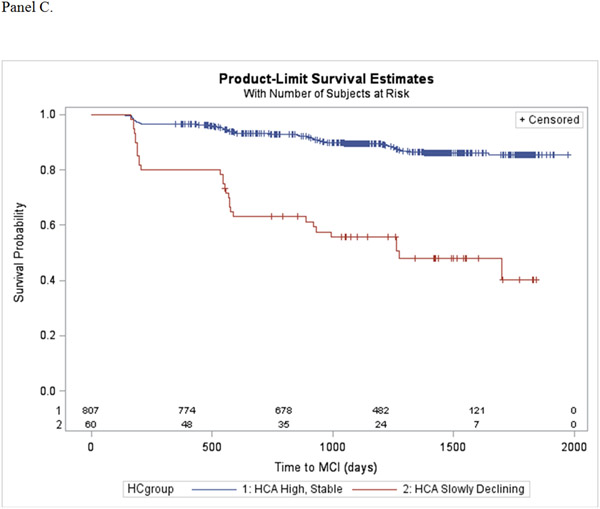
The latent class trajectory models predicted 3 trajectory patterns for TCA, 3 trajectory patterns for FCA, and 2 trajectory patterns for HCA (Figure 1, panels A-C). The intercept and slope estimates of fitted trajectories for each scale are shown in Table 2. For the TCA scale, participants started out with a similar high level of engagement. The majority (n = 735) continued this level of engagement over time (high, stable), while some slowly declined (n = 101) or quickly declined (n = 31). For the FCA subscale, the majority of participants (n = 777) had a high, stable pattern of activity in engagement, while others started high and then slowly declined over time (n = 57). A small number of participants (n = 37) began at a significantly lower level of engagement in FCA compared to the high, stable and slowly declining groups, but had a slow increase in engagement. For the HCA subscale, most (n = 807) participants showed a high, stable pattern of activity, while 60 participants had significantly higher baseline level of engagement compared to the high, stable pattern, but slowly declined over time.
Figure 1.
Predicted trajectories of cognitive activity over time. (A) Total Cognitive Activity, (B) Frequent Cognitive Activity, and (C) Higher Cognitive Activity.
Table 2.
Intercept and slope estimates of fitted trajectories of cognitive activity (n = 867)
| Total Cognitive Activity |
Frequent Cognitive Activity |
Higher Cognitive Activity |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Intercept | Slope | n | Intercept | Slope | n | Intercept | Slope | |
| High, stable | 735 | 44.31 | 0.01 | 777 | 25.69 | −0.05 | 807 | 14.05 | −0.03 |
| Slowly Declining | 101 | 45.08 | −2.06 | 57 | 25.92 | −2.05 | 60 | 18.16 | −2.14 |
| Quickly Declining | 31 | 50.07 | −6.05 | --- | --- | --- | --- | --- | --- |
| Slowly Increasing | --- | --- | --- | 37 | 19.52 | 0.82 | --- | --- | --- |
Table 3 shows baseline predictors of the cognitive activity patterns. Older participants, those with lower education, lower MMSE, more subjective cognitive concerns, slower gait speed, and who were physically inactive were more likely to decline in all three cognitive activity scales. Slow decline in FCA was associated with being unmarried, living alone, never or previous alcohol consumption, and taking 4 or more prescription medications; whereas being a current smoker was associated with the increasing pattern of activity in FCA. Congestive heart failure was associated with slow decline in TCA and HCA only, more depressive symptoms were associated with slow and quick decline in TCA only, and hypercholesterolemia was associated with lower risk for slow decline in HCA only.
Table 3.
Predictors of trajectories of cognitive activity based on univariable multinomial logistic regression model with the high, stable group as the reference for each cognitive activity scale
| Total Cognitive Activity |
Frequent Cognitive Activity |
Higher Cognitive Activity |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quickly Declining |
Slowly Declining |
Slowly Declining |
Slowly Increasing |
Slowly Declining |
||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| TCA Intercept | 1.028 | 0.988-1.071 | 1.001 | 0.979-1.025 | 0.958 | 0.929-0.989 | 0.896 | 0.862-0.931 | 1.025 | 0.966-1.056 |
| FCA Intercept | 1.059 | 0.950-1.182 | 0.980 | 0.926-1.037 | 1.018 | 0.934-1.109 | 0.691 | 0.632-0.755 | 1.071 | 0.988-1.160 |
| HCA Intercept | 1.067 | 1.000-1.139 | 1.002 | 0.964-1.040 | 0.952 | 0.905-1.003 | 0.894 | 0.839-0.953 | 1.112 | 1.058-1.167 |
| TCA Baseline | 0.992 | 0.954-1.032 | 0.986 | 0.964-1.009 | 0.939 | 0.911-0.969 | 0.898 | 0.865-0.932 | 0.997 | 0.969-1.026 |
| FCA Baseline | 1.034 | 0.968-1.104 | 0.985 | 0.948-1.023 | 0.935 | 0.888-0.986 | 0.896 | 0.840-0.955 | 1.015 | 0.944-1.091 |
| HCA Baseline | 1.001 | 0.908-1.105 | 0.965 | 0.914-1.019 | 0.963 | 0.891-1.041 | 0.705 | 0.648-0.768 | 1.070 | 1.019-1.123 |
| Age | 1.141 | 1.077-1.209 | 1.070 | 1.037-1.104 | 1.166 | 1.111-1.223 | 1.010 | 0.961-1.062 | 1.111 | 1.066-1.157 |
| Female | 2.002 | 0.851-4.409 | 0.928 | 0.605-1.124 | 1.483 | 0.802-2.744 | 0.859 | 0.439-1.683 | 1.794 | 0.983-3.274 |
| Education ≥ high school | 0.199 | 0.076-0.524 | 0.679 | 0.445-1.038 | 0.214 | 0.103-0.444 | 0.888 | 0.458-1.721 | 0.389 | 0.216-0.700 |
| White | 0.757 | 0.100-5.733 | 0.696 | 0.209-2.318 | 1.011 | 0.235-4.359 | 3.126 | 1.039-9.411 | 0.809 | 0.189-3.455 |
| ApoE4 (+) | 1.089 | 0.435-2.728 | 1.392 | 0.845-2.293 | 0.868 | 0.412-1.826 | 0.988 | 0.423-2.307 | 1.194 | 0.627-2.273 |
| Married or living as married | 0.555 | 0.262-1.174 | 0.913 | 0.602-1.385 | 0.483 | 0.267-0.875 | 1.889 | 0.948-3.765 | 0.675 | 0.396-1.152 |
| Living alone | 1.821 | 0.884-3.752 | 1.205 | 0.793-1.833 | 2.259 | 1.279-3.990 | 0.627 | 0.305-1.288 | 1.278 | 0.755-2.163 |
| Working full- or part-time | 0.438 | 0.103-1.864 | 0.473 | 0.213-1.049 | − | − | 1.011 | 0.385-2.654 | 0.478 | 0.170-1.344 |
| Systolic blood pressure | 1.018 | 0.995-1.041 | 1.000 | 0.986-1.014 | 1.002 | 0.984-1.021 | 1.013 | 0.992-1.035 | 1.011 | 0.944-1.029 |
| Diastolic blood pressure | 0.989 | 0.947-1.033 | 0.990 | 0.965-1.015 | 0.980 | 0.948-1.013 | 1.014 | 0.974-1.056 | 0.990 | 0.959-1.022 |
| Stroke | 1.196 | 0.408-3.505 | 1.299 | 0.706-2.391 | 1.192 | 0.522-2.720 | 0.949 | 0.328-2.742 | 1.406 | 0.670-2.952 |
| Hypertension | 0.872 | 0.417-1.824 | 1.085 | 0.699-1.684 | 1.587 | 0.847-2.974 | 1.347 | 0.655-2.766 | 1.302 | 0.736-2.303 |
| Hypercholesterolemia | 0.446 | 0.211-0.943 | 1.008 | 0.664-1.532 | 0.661 | 0.378-1.156 | 0.679 | 0.350-1.316 | 0.493 | 0.288-0.845 |
| Heart attack | 4.050 | 0.473-34.708 | − | − | ||||||
| Diabetes mellitus | 0.849 | 0.342-2.105 | 0.666 | 0.380-1.168 | 0.863 | 0.424-1.753 | 1.192 | .0552-2.576 | 0.728 | 0.362-1.465 |
| Congestive heart failure | 4.526 | 1.253-16.345 | 2.200 | 0.794-6.098 | 2.059 | 0.596-7.114 | − | − | 3.577 | 1.293-9.895 |
| ≥ 1 Depressive symptoms | 1.263 | 0.474-3.367 | 1.936 | 1.161-3.230 | 1.772 | 0.903-3.478 | 0.534 | 0.161-1.769 | 1.050 | 0.503-2.190 |
| MMSE | 0.775 | 0.645-0.932 | 0.855 | 0.760-0.962 | 0.745 | 0.647-0.859 | 0.953 | 0.775-1.173 | 0.828 | 0.717-0.957 |
| ≥ 2 Subjective memory concerns | 1.216 | 0.958-1.543 | 1.285 | 1.121-1.474 | 1.116 | 0.918-1.357 | 1.362 | 1.120-1.655 | 1.252 | 1.061-1.478 |
| Memory worse than 1 year ago | 1.506 | 0.563-4.029 | 1.260 | 0.686-2.317 | 1.188 | 0.520-2.712 | 1.822 | 0.777-4.271 | 1.352 | 0.644-2.835 |
| Timed Get Up and Go | 1.137 | 1.055-1.227 | 1.129 | 1.071-1.190 | 1.116 | 1.050-1.186 | 0.929 | 0.821-1.050 | 1.095 | 1.034-1.159 |
| Waist-hip ratio | 0.385 | 0.005-27.847 | 1.664 | 0.149-18.531 | 0.744 | 0.029-19.369 | 2.588 | 0.051-131.760 | 0.305 | 0.014-6.621 |
| Physically active | 0.543 | 0.261-1.128 | 0.450 | 0.295-0.687 | 0.545 | 0.310-0.959 | 0.613 | 0.312-1.203 | 0.416 | 0.245-0.705 |
| Currently smoking | 1.167 | 0.269-5.063 | 1.261 | 0.550-2.891 | 0.686 | 0.162-2.916 | 3.387 | 1.339-8.566 | 1.927 | 0.786-4.723 |
| Alcohol in past year | 0.548 | 0.222-1.353 | 0.673 | 0.412-1.099 | 0.353 | 0.157-0.794 | 0.988 | 0.423-2.307 | 0.797 | 0.436-1.457 |
| Subjective health (very good/excellent) | 0.712 | 0.336-1.507 | 0.848 | 0.555-1.297 | 0.622 | 0.343-1.126 | 1.390 | 0.718-2.689 | 0.950 | 0.558-1.616 |
| ≥4 of Prescription medications | 1.006 | 0.489-2.071 | 1.317 | 0.860-2.016 | 1.925 | 1.053-3.519 | 0.979 | 0.505-1.898 | 1.315 | 0.768-2.254 |
| Vision correction | 1.058 | 0.245-4.564 | 1.156 | 0.482-2.768 | 0.825 | 0.286-2.379 | 0.431 | 0.161-1.154 | 0.773 | 0.297-2.012 |
Note: TCA = Total Cognitive Activity, FCA = Frequent Cognitive Activity, HCA = Higher Cognitive Activity, MMSE = Mini-Mental State Exam.
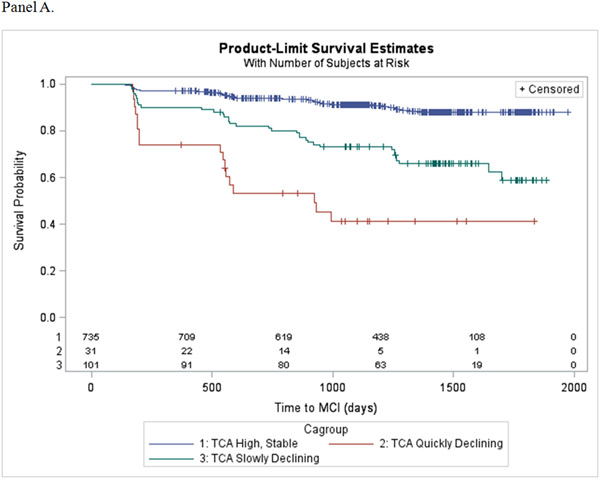
During the follow-up period, 129 (15%) participants progressed from normal cognitive status to mild cognitive impairment. Table 4 show the results of the Cox proportional hazards regression models estimating the association of the trajectory patterns for each cognitive activity scale, compared to the high, stable group, and incident MCI after adjusting for covariates and different baseline levels of engagement. Decline in TCA was found to increase risk for incident MCI, ranging from 3 to 11-fold for the slowly and quickly declining patterns, respectively. Slow decline in FCA also significantly increased risk for incident MCI by over 8-fold, while the slowly increasing pattern was not associated with risk for MCI. Finally, slowly declining engagement in HCA was found to increase risk for incident MCI over 3-fold. Kaplan-Meier estimators display the probability of progressing to MCI for each cognitive activity trajectory pattern (Figure 2, panels A-C).
Table 4.
The association between cognitive activity and risk for incident mild cognitive impairment (CDR = 0.5) (n = 867)
| Total Cognitive Activity |
Frequent Cognitive Activity |
Higher Cognitive Activity |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio |
95% CI | Hazard Ratio |
95% CI | Hazard Ratio |
95% CI | |
| TCA Quickly Declining | 10.953 | 6.265, 19.150 | ||||
| TCA Slowly Declining | 2.470 | 1.505, 4.054 | ||||
| FCA Slowly Declining | 8.671 | 5.264, 14.285 | ||||
| FCA Slowly Increasing | 0.373 | 0.059, 2.339 | ||||
| HCA Slowly Declining | 3.395 | 2.039, 5.654 | ||||
| Baseline age | 1.037 | 1.003, 1.071 | ||||
| ≥ High school education | 0.609 | 0.385, 0.962 | 0.514 | 0.329, 0.804 | ||
| Living alone | 2.185 | 1.443, 3.309 | 1.775 | 1.158, 2.720 | 2.056 | 1.353, 3.123 |
| MMSE | 0.879 | 0.808, 0.956 | 0.881 | 0.804, 0.965 | 0.882 | 0.807, 0.964 |
| Systolic blood pressure | 0.987 | 0.975, 0.999 | ||||
| Diabetes mellitus | 1.859 | 1.221, 2.828 | 1.563 | 1.039, 2.353 | 1.871 | 1.230, 2.846 |
| Alcohol drinking (ever) | 0.535 | 0.325, 0.880 | ||||
| ≥ 4 prescription medications | 0.852 | 0.581, 1.248 | ||||
| Memory worse than 1 year ago | 2.045 | 1.234, 3.389 | 2.258 | 1.354, 3.767 | 1.830 | 1.112, 3.011 |
| ≥ 2 Subjective memory concerns | 1.388 | 1.233, 1.561 | 1.348 | 1.189, 1.529 | 1.376 | 1.215, 1.559 |
| Hearing correction | 0.438 | 0.261, 0.736 | 0.558 | 0.348, 0.893 | ||
| Subjective Health: very good/excellent | 0.562 | 0.372, 0.847 | ||||
Note: CI = Confidence Interval, TCA = Total Cognitive Activity, FCA = Frequent Cognitive Activity, HCA = Higher Cognitive Activity, MMSE = Mini-Mental Status Exam. Multivariable model adjusted for covariates significantly associated with risk for incident MCI in univariable models (p < 0.15; Supplemental Digital Content 2) and different “baseline” intercepts. High, stable pattern is reference for each activity scale.
Figure 2.
Kaplan-Meier survival plots showing the probability of progressing to MCI as a function of (A) Total Cognitive Activity, (B) Frequent Cognitive Activity, and (C) Higher Cognitive Activity.
Discussion
This study examined how changing patterns of engagement in everyday cognitive activities are associated with risk for mild cognitive impairment over a period of up to 5 years in a population-based cohort of older adults. The results suggest that declining frequency of engagement in cognitive activities over time is associated with an increased risk of progressing from normal cognitive status to mildly impaired status. The effect of declining activity was most pronounced for quick decline in total cognitive activity with a 11-fold increased risk. Slower decline in total cognitive activity and in the subscales of frequent and higher cognitive activities were also associated with elevated risk for progressing to MCI, with risk elevation ranging from 3 to 8-fold.
One interpretation of these results is that maintaining engagement in cognitive activities over time may reduce the risk for cognitive impairment, while reducing time engaged in cognitive activities increases risk. Thus, intervention studies may be warranted that encourage participants to maintain or increase everyday engagement in cognitive activities. The alternative interpretation of our findings is that declining engagement in cognitive activity could be a marker for impending cognitive impairment. There is a long pre-symptomatic phase prior to the onset of detectable cognitive impairment37 during which underlying aging and disease processes could begin to affect engagement in cognitive activities. To examine this possibility further, we conducted sensitivity analyses excluding participants classified as CDR > 0 at any cycle prior to their own “baselines”, i.e., fluctuated between normal and MCI prior to the first FCAS assessment. The associations were slightly attenuated, but remained significant (Supplemental Digital Content 3 shows the Cox proportional hazards models of the associations between cognitive activity and incident MCI in this restricted sample), hinting that the association of decline in cognitive activity with increased risk for incident MCI could be driven by those who are already experiencing cognitive decline. However, given that the results remained statistically significant after excluding these fluctuating participants, the conclusion that maintaining engagement in activities offers protection against the development of MCI remains plausible.
We also examined factors that may influence engagement in cognitive activity over time and found associations with demographic and health factors in the expected direction. Our results suggest that there is a greater likelihood of declining frequency of engagement in cognitive activities among those with increasing age, who are male, have a lower education, live alone or are unmarried, and are physically inactive. In addition, lower objective and subjective cognitive functioning and the presence of health problems as indicated by slow gait speed, more prescription medications, and possibly heart failure or depressive symptoms also are associated with less frequent engagement in cognitive activities over time. If engagement in cognitive activity lowers risk for cognitive impairment, older adults with these characteristics may be the appropriate target for everyday cognitive activity interventions. In the final models examining the association between patterns of change in cognitive activity and risk for MCI, we found that some of these factors also remained significantly associated with risk for MCI and could perhaps partially explain the association between cognitive activity and incident MCI. Taken together, the results suggest that factors that influence the opportunity for (i.e., living with others), and interest in (i.e., educational attainment) engagement, as well as cognitive ability (objective and subjective) to participate in cognitive activities may play an important role in both maintaining engagement in cognitive activities and cognitive function with aging.
The mechanisms underlying the benefits of cognitive activity on brain and cognitive health currently being investigated38 may explain the lower risk of progression to MCI found in this study. According to the STAC-r model39, engaging in cognitively stimulating activities may be a strategy to enhance brain structure and function and contribute to the process of “neural resource enrichment.” Cognitive activity may reduce risk of cognitive impairment by improving indicators of brain health (e.g., connectivity, greater synaptic density and cortical thickness, and higher levels of brain-derived neurotropic factor), increasing the capacity for compensatory scaffolding, or by directly lowering the level of neuropathology associated with Alzheimer’s disease40, 41. While there is growing evidence from both observational and intervention trials to support these potential pathways, additional evidence from neuroimaging and brain biomarker studies are needed to fully understand the mechanisms.
The FCAS provides an overall picture of engagement in older adults’ activities requiring cognitive demand, with some requiring more mental effort than others. Previous studies examining the association between cognitive activity and cognitive outcomes suggest that activities that are novel and more cognitively demanding have the greatest likelihood of reducing the risk for cognitive decline and impairment among older adults42. We did not find that decline in engagement in HCA was associated with the greatest risk for MCI. A limitation of the FCAS is that activities included in the higher cognition subscale are based on exploratory factor analysis and expert opinion, and not on participants’ subjective ratings of the level of cognitive demand of these activities. The finding that declining overall engagement is associated with the greatest risk for MCI may reflect that activities with cognitive as well as social (e.g., playing board games, talking on the phone, going to social clubs or events) and physical (e.g., gardening, walking in unfamiliar places, shopping) aspects offer the greatest benefit to cognition rather than cognitive stimulation alone.
This study was conducted in a large population–based sample of community-dwelling older adults with a focus on measuring cognitive outcomes over time. We enhanced the generalizability of the study findings by adjusting for potential attrition bias that may have been present due to different study “baselines.” Cognitive activity was measured annually using a validated scale, allowing us to model and examine patterns of change in activity in relation to incident MCI. Limitations of the FCAS have been previously noted. The outcome of MCI was defined as CDR=0.5, which was assigned by trained study interviewers based on the assessment of cognitively-driven everyday function at each annual visit. Participants were not required to have an informant in this study, which may have underestimated the degree of impairment and the strength of the association with cognitive activity; however, all were cognitively normal (CDR=0) at baseline. Also, for analytic purposes we treated MCI as a categorical variable with a distinct date of onset; however, cognition declines on a continuum with the threshold for MCI being somewhat arbitrary. Therefore, the exact date of onset is less important than the fact that a participant’s level of cognitive had declined. Finally, the follow-up period of this study is relatively short in the context of the development and progression of cognitive decline leading to MCI and dementia, which is believed to occur over 20 years or more37. The benefits of engagement in cognitive activity for cognitive health would be more convincing if assessed over a longer period of time.
In conclusion, the majority of older adults in our sample maintained a relatively stable level of engagement in cognitive activities, but those whose level of engagement declined over the study period experienced a significantly elevated risk for developing MCI. This suggests that either maintaining engagement in cognitive activities may offer protection for older adults against developing cognitive impairment, or that declining engagement is an early warning sign for cognitive problems to come. Older adults should be encouraged to remain actively engaged in leisure and purposeful activities for successful aging as additional research is conducted to clarify the specific association between cognitive activities and cognitive outcomes.
Supplementary Material
Supplemental Digital Content 1. Adapted Florida Cognitive Activities Scale. (Microsoft Word Document)
Supplemental Digital Content 2. Univariable models of the association between covariates and risk of incident MCI (Microsoft Word Document - Table)
Supplemental Digital Content 3. The association between cognitive activity and risk for incident mild cognitive impairment (CDR = 0.5) excluding participants who had been classified as CDR > 0 at any cycle prior to “baseline” (n = 757) (Microsoft Word Document- Table)
Conflicts of Interest and Source of Funding:
Supported in part by grants # R01AG023651 and K07AG44395, from the National Institute on Aging, NIH, US, DHHS.
Dr. Mary Ganguli is a member of the scientific advisory committee for Biogen Inc., 2016-2017.
References
- 1.Hertzog C, Kramer AF, Wilson RS, et al. Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychol Sci 2009; 9: 1–65. [DOI] [PubMed] [Google Scholar]
- 2.Small B, Dixon RA, McArdle JJ, et al. Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria Longitudinal Study. Neuropsychology 2012; 26(2): 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howard EP, Morris JN, Steel K, et al. Short-term lifestyle strategies for sustaining cognitive status. BioMed Research International 2016; Volume 2016, Article ID 7405748, 8 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verghese J, LeValley A, Derby C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology 2006; 66, 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geda YE, Topazian HM, Roberts LA, et al. Engaging in cognitive activities, aging, and mild cognitive impairment: a population-based study. J Neuropsychiatry Clin Neurosci 2011; 23: 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes TF, Becker JT, Lee CW, et al. Independent and combined effects of cognitive and physical activity on incident MCI. Alzheimer Dement 2015; 11(11): 1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes TF, Chang C-CH, Vander Bilt J, et al. Cognitive activity and incident dementia in the community: The MoVIES Project. Am J Alzheimers Dis Other Demen 2010; 25: 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer’s disease. Neurology 2007; 69: 1911–1920. [DOI] [PubMed] [Google Scholar]
- 9.Valenzuela M, Sachdev P. Can cognitive exercise prevent the onset of dementia? Systematic review of randomized clinical trials with longitudinal follow-up. Am J Geriatr Psychiatry 2009; 17: 179–187. [DOI] [PubMed] [Google Scholar]
- 10.Ball K, Berch D, Helmers KF, et al. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA 2002; 288(18: 2271–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson MC, Saczynski JS, Rebok GW, et al. Exploring the effects of an “everyday” activity program on executive function and memory in older adults: Experience Corps®. Gerontologist 2008; 48(6), 793–801. [DOI] [PubMed] [Google Scholar]
- 12.Hughes TF, Flatt JD, Fu B, et al. Interactive video games compared with health education in older adults with mild cognitive impairment: a feasibility study. Int J Geriatr Psychiatry 2014; 29(9): 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson-Hanley C, Maloney MM, Barcelos N, et al. Neuropsychological benefits of neuro-exergaming for older adults: a pilot study of an interactive physical and cognitive exercise system (iPaces™). J Aging Phys Act 2016; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14.Toril P, Reales JM, Ballesteros S. Video game training enhances cognition of older adults: a meta-analytic study. Psychol Aging 2014; 29(3): 706–716. [DOI] [PubMed] [Google Scholar]
- 15.Lampit A, Hallk H, Valenzuela M. Computerized cognitive training in cognitively healthy older adults: A systematic review and meta-analysis of effect modifiers. PLoS Med. 2014; [eEpub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stine-Morrow EAL, Parisi JM, Morrow D, et al. The effects of an engaged lifestyle on cognitive vitality: a field experiment. Psychol Aging 2008; 23, 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park DC, Lodi-Smith J, Drew L, et al. The impact of sustained engagement on cognitive function in older adults: The Synapse Project Psychol Sci 2014; 25(1): 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simons DJ, Boot WR, Charness N, et al. Do “brain-training” programs work? Psychol Sci Public Interest 2016; 17(3): 103–186. [DOI] [PubMed] [Google Scholar]
- 19.Martin M, Clare L, Altgassen AM, Cameron MH, Zehnder F. Cognition-based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database Syst Rev. 2011;(1): CD006220. [DOI] [PubMed] [Google Scholar]
- 20.Plassman BL, Williams JW, Burke JR, et al. Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med 2010; 153: 182–193. [DOI] [PubMed] [Google Scholar]
- 21.Bielak AA. Different perspectives on measuring lifestyle engagement: a comparison of activity measures and their relation with cognitive performance in older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2016l [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22.Ghisletta P, Bickle J-F, Lövdén M. Does activity engagement protect against cognitive decline in old age? Methodological and analytical considerations. J Gerontol B Psychol Sci Soc Sci (2006) 61 (5): P253–P261. [DOI] [PubMed] [Google Scholar]
- 23.Papp KV, Walsh SJ, Snyder PJ. Immediate and delayed effects of cognitive interventions in healthy elderly: a review of current literature and future directions. Alzheimers Dement. 2009. Jan;5(1):50–60. [DOI] [PubMed] [Google Scholar]
- 24.Schinka JA, McBride VA, Vanderploeg RD, et al. Florida Cognitive Activities Scale: initial development and validation. J Int Neuropsychol Soc 2005; 11: 108–116. [DOI] [PubMed] [Google Scholar]
- 25.Ganguli M, Snitz BE, Vander Bilt J, et al. How much do depressive symptoms affect cognition at the population level? The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) study. Int J Geriatr Psychiatry 2009; 24: 1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 27.Mungas D, Marshall SC, Weldon M, et al. Age and education correction of Mini-Mental State Examination for English and Spanish-speaking elderly. Neurology 1996; 46: 700–706. [DOI] [PubMed] [Google Scholar]
- 28.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993; 43(11): 2412–2414. [DOI] [PubMed] [Google Scholar]
- 29.Ganguli M, Change CC, Snitz BE, et al. Prevalence of mild cognitive impairment by multiple classifications: The Monongahela-Youghiogheny Healthy Aging Team (MYAT) project. Am J Geriatr Psychiatry. 2010; 18(8): 674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganguli M, Gilby J, Seaberg E, et al. Depressive symptoms and associated factors in a rural elderly population. Am J Geriatr Psychiatry. 1995; 3: 144–160. [DOI] [PubMed] [Google Scholar]
- 31.Snitz BE, Morrow LA, Rodriguez EG, Huber KA, Saxton JA. Subjective memory complaints and concurrent memory performance in older patients of primary care providers. J Int Neuropsychol Soc. 2008; 14(06): 1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis PB, Robins LN. History-taking in the elderly with and without cognitive impairment: how useful is it? J Am Geriatr Soc 1989; 37: 249–255. [DOI] [PubMed] [Google Scholar]
- 33.Oksanen T, Kivimaki M, Pentti J, et al. Self-report as an indicator of incident disease. Ann Epidemiol 2010; 20: 547–554. [DOI] [PubMed] [Google Scholar]
- 34.Proust-Lima C, Phillips V, Liquet B. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm, Arxiv. 2015. [Google Scholar]
- 35.Ganguli M, Lee C-W, Hughes TF, et al. Who wants a free brain scan? Assessing and correcting for recruitment biases in a population-based sMRI pilot study. Brain Imaging Behav 2015; 9(2): 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SAS System for Microsoft Windows [computer software]. Version 9.3. Cary, NC, SAS Institute Inc, 2002-2010. [Google Scholar]
- 37.Rajan KB, Wilson RS, Weuve J, et al. Cognitive impairment 18 years before clinical diagnosis of Alzheimer’s disease dementia. Neurology 2015; 85: 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nithianantharajah J, Hannan AJ. The neurobiology of brain and cognitive reserve: Mental and physical activity as modulators of brain disorders. Prog Neurobiol 2009; 89: 369–382. [DOI] [PubMed] [Google Scholar]
- 39.Reuter-Lorenz PA, Park DC. How Does it STAC Up? Revisiting the Scaffolding Theory of Aging and Cognition. Neuropsychology Review 2014; 24 (3): 355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landau SM, Marks SM, Morimino EC, et al. Association of lifetime cognitive engagement and low β-amyloid deposition. Arch Neurol. 2012; 69: 623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vemuri P, Lesnick TG, Przybelski SA, et al. Effects of lifestyle activities on Alzheimer disease biomarkers and cognition. Ann Neural. 2012; 72; 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dolcos S, MacDonald SW, Braslavsky A, et al. Mild cognitive impairment is associated with selected functional markers: integrating concurrent, longitudinal, and stability effects. Neuropsychology. 2012; 26(2): 209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Adapted Florida Cognitive Activities Scale. (Microsoft Word Document)
Supplemental Digital Content 2. Univariable models of the association between covariates and risk of incident MCI (Microsoft Word Document - Table)
Supplemental Digital Content 3. The association between cognitive activity and risk for incident mild cognitive impairment (CDR = 0.5) excluding participants who had been classified as CDR > 0 at any cycle prior to “baseline” (n = 757) (Microsoft Word Document- Table)