SUMMARY
The melanocortin pathway is well established to be critical for body-weight regulation in both rodents and humans. Despite extensive studies focusing on this pathway, the downstream brain sites that mediate its action are not clear. Here, we found that, among the known paraventricular hypothalamic (PVH) neuron groups, those expressing melanocortin receptors 4 (PVHMc4R) preferably project to the ventral part of the lateral septum (LSv), a brain region known to be involved in emotional behaviors. Photostimulation of PVHMc4R neuron terminals in the LSv reduces feeding and causes aversion, whereas deletion of Mc4Rs or disruption of glutamate release from LSv-projecting PVH neurons causes obesity. In addition, disruption of AMPA receptor function in PVH-projected LSv neurons causes obesity. Importantly, chronic inhibition of PVH- or PVHMc4R-projected LSv neurons causes obesity associated with reduced energy expenditure. Thus, the LSv functions as an important node in mediating melanocortin action on body-weight regulation.
Graphical Abstract
In brief
Xu et al. demonstrate the function of the neurocircuit from paraventricular hypothalamic neurons expressing melanocortin receptor 4, an important human obesity gene, to the ventral lateral septum (LSv), an emotion-related brain region, in co-regulating body weight and emotion-related behaviors, identifying LSv as an important downstream mediator of the melanocortin pathway.
INTRODUCTION
Extensive studies in the last decades have identified the hypothalamus as a key regulator of feeding and energy expenditure. In particular, the melanocortin pathway has been well established to play a critical role in body-weight homeostasis.1,2 This pathway involves proopiomelanocortin (POMC)-expressing and agouti-related protein (AgRP)-expressing neurons in the arcuate nucleus (Arc) and their downstream neurons that express melanocortin receptor (mainly melanocortin receptor 4 [Mc4R]). Within this pathway, POMC and AgRP neurons release respective α-melanocyte-releasing hormone (α-MSH, a POMC-derived peptide) and AgRP, which function as respective agonist and antagonist (or inverse agonist) of Mc4Rs to inhibit and promote feeding.1 Mutations in just a few key genes involved in the melanocortin pathway account for a sizable human population with obesity, with Mc4R representing the most monogenetic human obesity gene,3-7 suggesting the melanocortin pathway as a conserved pathway in feeding and body-weight regulation shared between rodents and humans.
Despite diffuse projection patterns of both POMC and AgRP neurons as well as the broad expression pattern of the Mc4R gene, the paraventricular hypothalamus (PVH) represents a major site that mediates the melanocortin action on body weight.8 Targeted deletion of Mc4Rs from the PVH leads to obesity, and selective restoration of Mc4Rs in the PVH of Mc4R null mice reduces the major part of Mc4R obesity.9,10 Consistently, activation or inhibition of PVH Mc4R neurons (denoted as PVHMc4R neurons) leads to a potent effect on inhibiting or promoting feeding, respectively.9 While inhibition of the whole PVH projections to the periaqueductal gray (PAG) promotes feeding more potently than those to the parabrachial nucleus (PBN) or the nucleus of solitary tract (NTS), inhibition of PVHMc4R neuron projections to the PBN produces stronger effects on feeding than those to the PAG,9,11 suggesting a unique role of PVHMc4R neuron projections in feeding regulation. These studies based on optogenetic or chemogenetic manipulation of the activity of PVHMc4R neuron projections clearly demonstrate a potent effect of PVHMc4R neurons in acute feeding behavior. However, as shown previously for both hindbrain POMC neurons12 and arcuate AgRP neurons,13,14 acute feeding alterations may not necessarily translate into body-weight changes. In particular, the downstream sites that mediate the action of PVHMc4R neurons on body weight remain to be established. It is important to mention that, in addition to feeding regulation, Mc4Rs are also involved in anxiety-related behaviors.15,16 Notably, double knockout of Mc4R and SAP90/PSD95-associated protein 3 (SAPAP3) completely rescues the obesity phenotype of Mc4R single knockout and the anxiety-related self-grooming phenotype in SAPAP3 single-knockout mice,17 suggesting a shared pathway underlying both Mc4R-regulated obesity and SAPAP3-regulated anxiety-related behaviors. Thus, we reasoned that a potential PVHMc4R neuron downstream brain region known to regulate anxiety-related behaviors also mediates the effect of PVHMc4R neurons in feeding and body weight. Given our preliminary studies suggesting that PVHMc4R neurons send abundant projections to the ventral part of the lateral septum (LSv), we hypothesize that the PVHMc4R-to-LSv projection co-regulates body weight and anxiety-related behaviors.
The lateral septum represents a basal forebrain structure involved in a wide variety of functions, including emotional, motivational, and spatial navigation behaviors.18 Activation and inhibition of lateral septum (LS) corticotropin-releasing hormone receptor 2 (CRFR2)-expressing neurons promotes and reduces anxiety-like behaviors, respectively.19 LS lesion causes raging behaviors, and activation of LS CRFR2 neuron terminals in the anterior hypothalamic area promotes social aggression.19 Recent results also implicate LS neurons in feeding and emotionally related feeding behaviors, especially with respect to pathways involving the known feeding-regulation hormones that include glucagon-like peptide 1 (GLP1), neurotensin, and ghrelin.20-24 In addition, the LS has emerged as a site that mediates the interaction between stress-related emotions and feeding control via connections with the hippocampus and/or the hypothalamus.25 However, the relevance of LS neurons to obesity development is not clear. In addition, the LS is generally divided into dorsal, intermediate, and ventral subdivisions,26 and the relevance of the LSv to feeding and body-weight regulation remains largely unknown.
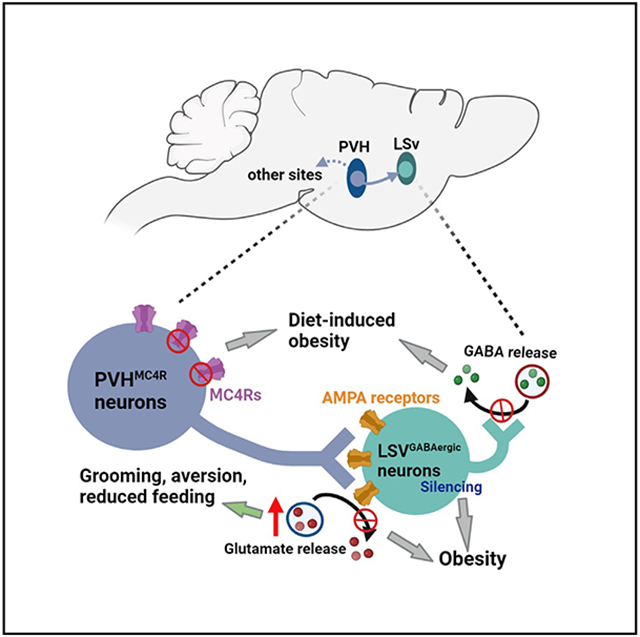
Here we show that the LSv represents a major downstream projection site of PVHMc4R neurons. Activation of PVHMc4R→LSv projections promoted stress-related self-grooming and inhibited feeding. Importantly, mouse models with loss of function in this pathway, including disruption of glutamate release from or deletion of Mc4Rs in LSv-projecting PVH neurons, or disruption of glutamate-AMPA receptors in PVHMc4R-projected LSv neurons, all develop obesity. Furthermore, chronic inhibition of PVH- or PVHMc4R-projected LSv neurons result in severe obesity associated with reduced anxiety-like behaviors. Together, these results reveal the LSv as an important downstream mediator of the melanocortin pathway in co-regulating obesity and stress-related behaviors.
RESULTS
Stimulation of PVHMc4R→LSv projections reduces feeding and promotes aversion
PVHMC4R projections have been studied in the hindbrain area including the PAG and PBN.11,27 To examine whether these neurons also project to forebrain sites, we stereotactically injected an AAV-Flex-ChR2-EYFP virus to the PVH of Mc4R-Cre mice (Figures 1A and 1B, left). In addition to the known projections found in the hindbrain regions (Figure S1), we identified strong ChR2-positive fibers in the LSv (Figure 1B, middle and right) but not in other subregions of the LS (Figure S1). The body-weight-regulating neurons in the PVH can be broadly divided into PVHMc4R and PVHPdyn neurons (neurons expressing prodynorphin).28 To examine whether PVHPdyn neurons also project to the LSv, we performed similar viral injections in Pdyn-Cre mice and found only minor projections to the LSv. Consistent with the minor projection pattern, photostimulation of PVHPdyn→LSv projections caused no obvious changes in feeding or place preference behaviors (Figure S2). These results, combined with the previous data showing that PVH neurons expressing oxytocin or arginine vasopressin (AVP) do not contribute to the projections to the LSv,29 suggest that PVHMC4R neurons represent the major contributing subset of PVH neurons that send projections to the LSv.
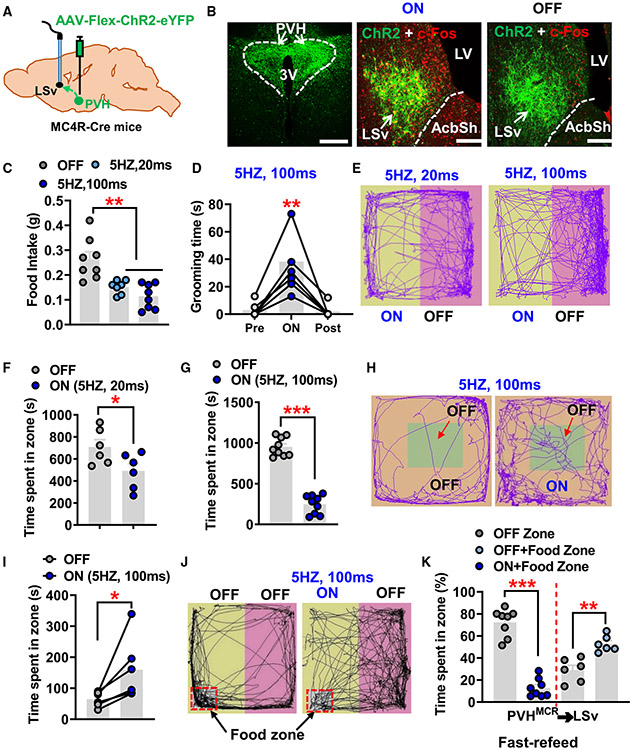
Figure 1. Photostimulation of PVHMc4R→LSv fibers reduces feeding and induces aversion.
(A) Diagram depicting injections of the AAV-Felx-ChR2-eYFP virus to the PVH of MC4R-Cre mice.
(B) Viral expression pattern in PVHMc4R neurons within the PVH (left) and PVHMc4R→LSv fibers with c-Fos immunostaining (red) with photostimulation (middle) or without photostimulation (right).
(C) Food intake within 20 min after fasting with or without photostimulation at the indicated stimulation protocol (n = 8 mice; one-way ANOVA, **p < 0.01).
(D) Time spent in self-grooming during a 2-min period with photostimulation, 2 min immediately before and after photostimulation (n = 6 mice; one-way ANOVA, **p < 0.01).
(E–G) Mouse movement tracks in the place preference testing arena with half of the arena paired with photostimulation at 20 ms (E and F, n = 6 mice) and 100 ms (E and G) stimulation duration (n = 9 mice; paired t test, *p < 0.05, ***p < 0.001).
(H and I) Mouse movement tracks in the open-field arena with the peripheral area of the arena paired without photostimulation (H, left panel) or with photostimulation (H, right panel), and comparison of time spent in the central area between two conditions (I, n = 6 mice; paired t test, *p < 0.05).
(J and K) Movement tracks of fasted mice in the place preference testing arena with food placed in one corner of the half arena paired without photostimulation (J, left) or with photostimulation (J, right), and quantified comparisons of time spent in each zone (K, n = 6 mice for OFF and n = 8 mice for ON; paired t test, **p < 0.01, ***p< 0.001).
AcbSh, accumbens nucleus shell; LV, lateral ventricle; LSv, ventral lateral septum; PVH, paraventricular nucleus of hypothalamus; 3V, third ventricle. All data presented as mean ± SEM. Scale bars in (B) represent 100 μm in the right panel and 50 μm in the middle and left panels. See also Figures S1-S3.
Photostimulation of PVHMc4R terminals in the LSv induced c-Fos expression in numerous LSv neurons (Figure 1B, middle) compared with controls (Figure 1B, right), suggesting an excitatory effect of the projection. This observation is consistent with the fact that PVHMc4R neurons are glutamatergic.30 Given the strong implication of Mc4R neurons in feeding, we first examined the effect of targeted stimulation on feeding. In overnight fasted animals, photostimulation of PVHMc4R terminals in the LSv induced a strong inhibitory effect on fasting-induced refeeding (Figure 1C). Interestingly, however, in fed mice the stimulation elicited repetitive self-grooming behaviors (Figure 1D). To probe the emotional valence associated with the observed self-grooming behavior, we next subjected these mice to real-time place preference (RTPP) tests. The same photostimulation protocol (5 Hz, 100 ms) caused an obvious avoidance to the stimulation (Figures 1E-1G), which led to a stronger avoidance phenotype (Figure 1G) than that induced by a lower-strength photostimulation protocol (5 Hz, 20 ms) (Figure 1F), suggesting a scalable effect of stimulating PVHMc4R neuron terminals in the LSv toward eliciting an avoidance behavior. To further assess the effect on natural avoidance behavior, we paired photostimulation with mouse stay in the periphery of the open-field arena (Figure 1H). Interestingly, compared with the control non-stimulation condition, photostimulation resulted in a longer time spent in the non-paired center zone in all mice (Figures 1H and 1I), suggesting that photostimulation caused an avoidance that dominates over the innate anxiety associated with the center stay in an open field.
To more precisely examine the avoidance behavior elicited by photostimulation on feeding behavior, we fasted mice overnight and placed food in the corner of the testing arena that was paired with or without photostimulation (Figure 1J). Under this condition, mice could choose to stay in the unstimulated side with hunger or in the stimulated side with food consumption. While mice spent the majority of time in the food zone when not paired with photostimulation, they spent much less time in the food zone when paired (Figures 1J and 1K). Associated with this, mice ate much less food with photostimulation (Figure 1K). These results demonstrate that stimulation of PVHMC4R terminals in the LSv causes a strong avoidance that effectively reduces hunger-driven feeding.
To determine whether LSv-projecting PVHMc4R neurons also send collateral projections to other downstream brain targets, we next performed experiments in Mc4R-Cre mice by delivering a retrogradely trafficked AAVrg-FlpO-mCherry virus into the LSv followed by injecting AAV-con/fon-EYFP viral vectors into the PVH. We observed EYFP-positive fibers in a few other brain regions, including median eminence, PAG area, and PBN (Figure S3), suggesting that LSv-projecting PVHMc4R neurons indeed send collaterals to these brain regions.
LSv-projecting PVH neurons in obesity development
Given the effect on feeding by photostimulation of PVHMc4R terminals in the LSv, we next examined the role of LSv-projecting PVH neurons in body-weight regulation. PVH neurons express vesicular glutamate transporter 2 (Vglut2, also named Slc17a6), a marker for glutamatergic neurons that is required for presynaptic glutamate release in PVH neurons.30To selectively target LSv-projecting PVH neurons, we stereotactically delivered a retrograde AAVrg-FlpO-mCherry virus into the LSv, which is able to trace LSv-projecting PVH neurons.31 We then delivered AAV-fDIO-Cre mixed with AAV-DIO-GFP (to report the Cre expression) vectors into the PVH of either Vglut2flox/flox or Mc4Rflox/flox mice to facilitate FlpO-mediated Cre expression and subsequent deletion of Vglut2 or Mc4R expression exclusively in LSv-projecting PVH neurons (Figure 2A). The specific delivery of AAVrg-FlpO-mCherry to the LSv was confirmed post hoc via viral expression (Figure 2B, left). As expected, we observed AAV-fDIO-Cre expression in a subset of PVH neurons (Figure 2B, right), suggesting selective targeting of LSv-projecting PVH neurons. Of note, we also observed GFP-positive fibers in the LSv (Figure 2B, left), confirming the projection from GFP-expressing PVH neurons. To confirm Cre-mediated deletion of Vglut2, we performed in situ hybridization (ISH) experiments. Compared with control mice injected with AAV-fDIO-GFP virus in the PVH, there was an obvious reduction of Vglut2 mRNA expression in the PVH of Cre-injected mice (Figure 2C). Of note, since the viral GFP signal will be completely quenched during the pretreatment steps of the ISH procedure, the ISH signal of Vglut2 can be reliably visualized with green fluorescence. These results suggest effective deletion of Vglut2 by FlpO-mediated Cre expression. Cre-mediated deletion of Vglut2 and the resultant loss of glutamate release using the same floxed allele have also been documented previously.29,30 Interestingly, compared with controls, mice with specific Vglut2 deletion in LSv-projecting PVH neurons develop obesity when fed a chow diet (Figure 2D). The body-weight gain was about 15 g at 8 weeks after the viral injection, suggesting rapid obesity development.
Figure 2. Disruption of glutamate release or deletion of Mc4R from LSv-projecting PVH neurons causes obesity.
(A) Diagram depicting the experimental strategy.
(B) Example expression patterns of AAVrg-FlpO-mCherry in the LSv (left) and Cre expression reported by co-injected Cre-dependent GFP reporter fluorescence (AAV-Flex-GFP) in the retrogradely traced PVH neurons (right).
(C) In situ hybridization shows expression patterns of Vglut2 in the PVH of Vglut2flox/flox mice at 4 weeks after delivery of GFP (left panel) or Cre (right panel) vectors.
(D) Weekly body weight of Vglut2flox/flox mice fed with chow for 8 weeks after viral delivery (n = 10 for GFP group and n = 8 for Cre group; two-way ANOVA, **p < 0.01, ***p < 0.001).
(E) Expression patterns of Vglut2 (right panels) and Mc4r (left panels) in the PVH of Mc4Rflox/flox mice at 4 weeks after delivery of GFP (top, n = 7 mice) or Cre (bottom, n = 10 mice) vectors.
(F) Weekly body weight of control and Mc4Rflox/flox mice fed with chow for 8 weeks followed by 8 weeks fed with HFD (two-way ANOVA, **p < 0.01, ***p < 0.001).
AcbSh, accumbens nucleus shell; LV, lateral ventricle; LSv, ventral lateral septum; PVH, paraventricular nucleus of hypothalamus; 3V, third ventricle. All data presented as mean ± SEM. Scale bars represent 100 μm in the right panel of (B), 50 μm in the left panel of (B) and in (C), and 25 μm in (E). See also Figure S4.
We also performed ISH and immunohistochemical experiments to validate Cre-mediated deletion of Mc4R. Although no difference in Vglut2 ISH signal was observed between groups (Figure 2E, left) compared with controls (Figure 2E, top), Cre-injected Mc4Rflox/flox mice showed a significant reduction of Mc4r ISH signal in the PVH (Figure 2E, bottom). In addition, compared with the abundant co-expression of Mc4R and control GFP viral vectors, a low level of Mc4R immunostaining was observed in the PVH of Cre-injected Mc4Rflox/flox mice (Figure S4). Altogether, these results suggested effective deletion of Mc4R expression by FlpO-mediated Cre expression. In these mice, we measured their weekly body weight on chow for 8 weeks followed by another 8 weeks on a high-fat diet (HFD). Although Mc4R knockout (KO) mice showed a trend in obesity development only on normal chow, these mice exhibited sensitivity to HFD-induced obesity. After 8 weeks on HFD, they gained up to 10 g of body weight (Figure 2F). The milder effect on chow toward obesity development in Mc4R KO mice relative to Vglut2 KO mice may reflect a smaller number of neurons targeted in Mc4R KO models, since the Mc4R-expressing neurons only account for a subset of PVH neurons32 or reflect the Mc4R as only one of many upstream signals that affect body weight through these neurons. Nevertheless, these results strongly support that glutamatergic PVHMC4R projections to the LSv are required for body-weight regulation.
Glutamate receptors in PVH-projected LSv neurons in body-weight regulation
Although previous studies have implicated LS neurons, especially dorsal LS neurons, in feeding regulation,20-24,33 the role of LS neurons in body-weight regulation is unknown. Given that LSv-projecting PVHMc4R neurons also send collaterals to other brain sites, it is imperative to determine the role of LSv neurons in mediating the observed effects. To this end, we aimed to selectively target those LSv neurons that receive inputs from PVH glutamatergic neurons. We first examined the role of glutamate receptors in LSv neurons that receive direct PVH inputs (hereafter defined as PVH-projected LSv neurons). α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartic acid (NMDA) receptors represent the major classes of postsynaptic receptors that mediate glutamate action. To selectively manipulate AMPA receptors, we took advantage of a C-terminal fragment of the GluR1 (hereafter defined as ctGluR1) subunit that can be used to disrupt the receptor trafficking from cytosol to postsynaptic membranes and consequent glutamatergic neurotransmission.34 We generated a conditional AAV-DIO-ctGluR1-mCherry vector, which expresses the ctGluR1 fragment in a Cre-dependent manner. We delivered anterograde AAV1-Cre-GFP viral particles to the PVH to trace direct downstream LSv neurons, and at the same time we delivered the AAV-DIO-ctGluR1-mCherry or control AAV-DIO-mCherry viral particles to the LSv (Figure 3A). Targeted delivery of AAV1-Cre-GFP was confirmed in the PVH (Figure 3B, left), as was Cre-mediated viral expression of mCherry in PVH-projected LSv neurons (Figure 3B, middle and right). To validate ctGluR1 expression, we performed immunostaining with the antibody raised against ctGluR1. Compared with control mice (Figure 3C, top), we found that PVH-projected LSv neurons exhibited a higher level of ctGluR1 signal in ctGluR1-injected mice (Figure 3C, arrows in bottom panels), confirming ctGluR1 overexpression in these neurons. Interestingly, we also noted that mice with ctGluR1 overexpression in PVH-projected LSv neurons rapidly developed obesity on the chow diet (Figure 3D). Given the known role of LS neurons in emotional modalities, we also tested these mice for anxiety-like behaviors. In fact these mice spent more time in the center of open-field tests (OFTs) (Figures 3E and 3F), suggesting a reduced level of anxiety. These findings support that normal function of AMPA receptors in PVH-projected LSv neurons is required for body-weight regulation.
Figure 3. Disruption of AMPA receptor function in PVH-projected LSv neurons causes obesity.
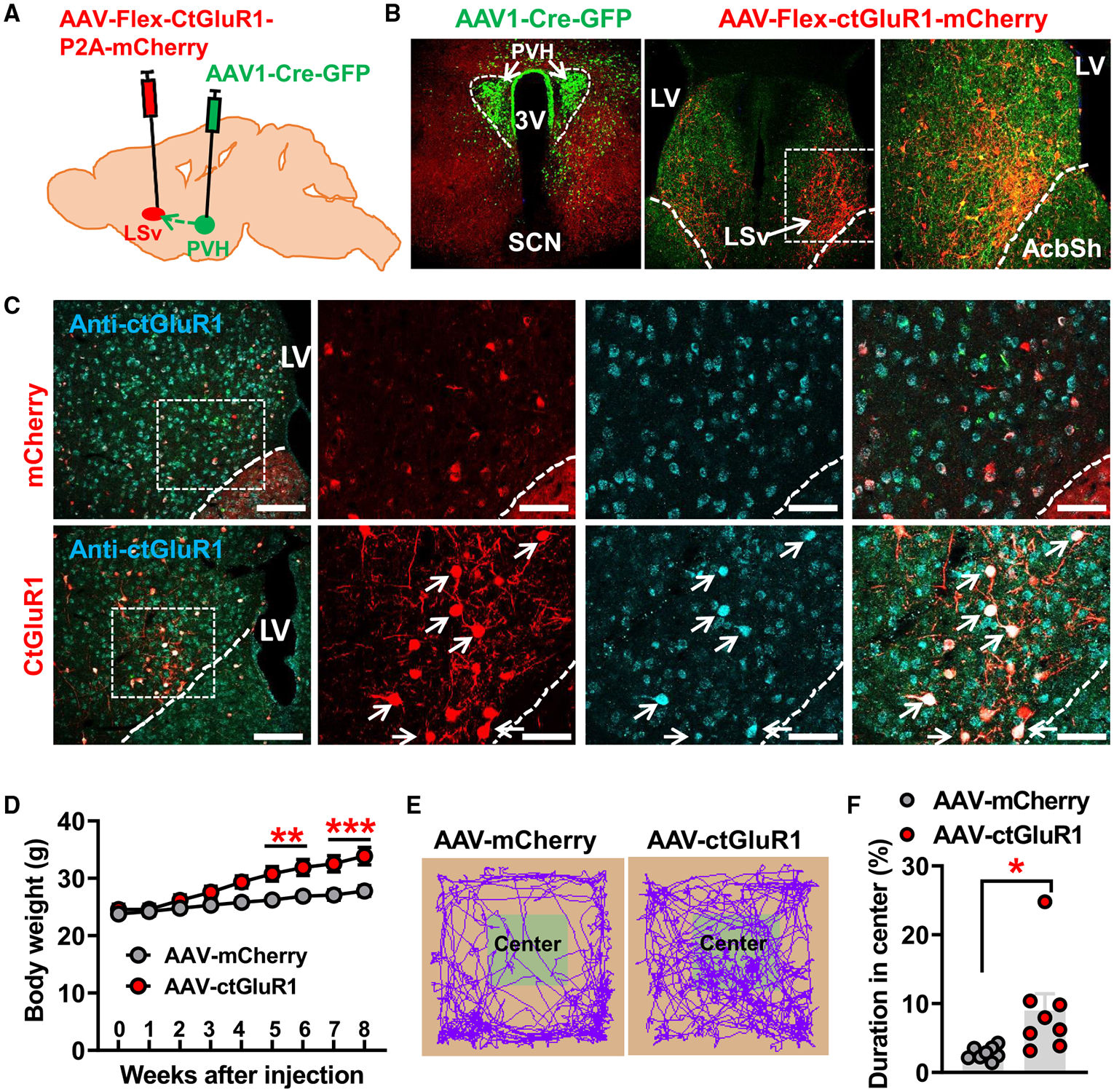
(A) Diagram depicting the experimental strategy.
(B) AAV1-Cre-GFP expression patterns in the PVH (left) and the expression pattern of AAV-Flex-ctGluR1-P2A-mCherry viral vectors in the LSv mediated by Cre expression in traced neurons (middle and right; the right panel is an amplified image of the boxed area in the middle panel).
(C) Expression patterns of immunolabeled GluR1 using a specific antibody against the C-terminal peptides of GluR1 (ctGluR1, blue) in the LSv of mice injected with control AAV-Flex-mCherry (red, n = 8 mice) or AAV-Flex-ctGluR1-P2A-mCherry (red, n = 6 mice). The three panels on the right show the magnified areas of the boxed areas in the left panel. Arrows point to the overexpressed ctGluR1 subunit in LSv neurons.
(D) Comparisons in weekly body weight between control- and ctGluR1-injected mice fed with chow for 8 weeks after viral delivery (two-way ANOVA, **p < 0.01, ***p < 0.001).
(E) Representative movement tracks of control-injected (left) and ctGluR1-injected (right) mice in an OFT arena.
(F) Statistical comparisons of time spent in the center zone of OFT chamber between control- and ctGluR1-injected mice (paired t test, *p < 0.05).
AcbSh, accumbens nucleus shell; LV, lateral ventricle; LSv, ventral lateral septum; PVH, paraventricular nucleus of hypothalamus; SCN, suprachiasmatic nucleus; 3V, third ventricle. All data presented as mean ± SEM. Scale bars represent 100 μm in the left and middle panels of (B) and left panel of (C), 50 μm in the right panel of (B), and 25 μm in right three panels of (C). See also Figure S5.
NMDA receptors have been previously shown to be involved in feeding and body-weight regulation.34 To directly examine the role of NMDA receptors in PVH-projected LSv neurons, we bilaterally injected the AAV1-FlpO-mCherry vectors to the PVH of Grin1flox/flox mice (the Grin1 gene encodes the NR1 subunit of NMDA receptors) while also delivering AAV-fDIO-Cre or control AAV-fDIO-GFP vectors to the LSv, which allows us to express Cre or GFP in FlpO-expressing LSv neurons (Figure S5A). In Cre-injected Grin1flox/flox mice (Figure S5B, right), compared with the control GFP group (Figure S5B, top) NR1 expression was reduced in the LSv as demonstrated by NR1 immunostaining (Figure S5B, bottom), and electrically evoked currents showed a significant decrease in AMPA receptor/NMDA receptor ratio (Figures S5C and S5D), suggesting effective Cre-mediated deletion of the NR1 subunit. We next monitored weekly body weight in these mice on chow for 8 weeks after viral injections, and somewhat surprisingly found no differences in body weight between NR1 KO and control mice in both males (Figure S5E) and females (Figure S5F). Together, these data collectively suggest that LSv AMPA receptors but not NMDA receptors mediate the PVH glutamatergic action on body-weight regulation.
Chronic inhibition of PVH-projected LSv neurons leads to massive obesity
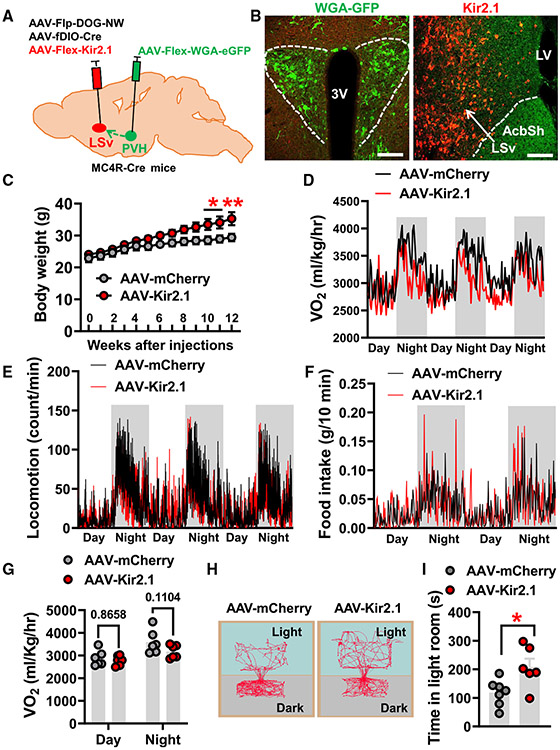
One of the consequences of disrupting glutamatergic inputs is reduced activity of targeted neurons. Thus, we next examined the consequence of chronic inhibition of PVH-projected LSv neurons. To this end, we delivered a conditional AAV-Flex-Kir2.1-dTomato virus to the LSv of wild-type mice that also received injections of anterograde AAV1-Cre-GFP vectors in the PVH (Figure 4A). The Kir2.1 virus expresses a mutated K+ channel that has been shown to achieve chronic inhibition of neurons.14 As evidenced by Cre-dependent expression of Kir2.1-dTomato (Figure 4B, right), AAV1-Cre-GFP injection in the PVH (Figure 4B, left) resulted in the expression of Cre-GFP in a subset of LSv neurons. To functionally evaluate Kir2.1 expression, given PVH neurons known to be predominantly glutamatergic and LSv neurons known to be mainly GABAergic,18,29,30 we also delivered AAV-DIO-hM3Gq-mCherry vectors in the PVH and followed this by injecting a mixture of AAV-Flex-Kir2.1-dTomato and AAV-fDIO-Cre vectors or control AAV-fDIO-mCherry to the LSv in compound Vglut2-Ires-Cre::Vgat-FlpO mice (Figure S6A). The expression of hM3Gq in the PVH and the expression of control mCherry or Kir2.1-dTomato in the LSv were confirmed in both groups (Figures S6B and S6C, left). We then examined c-Fos expression in LSv neurons to evaluate their overall activity upon activation of upstream PVH neurons with clozapine N-oxide (CNO). While control mice showed intense c-Fos expression in the LSv, Kir2.1-injected mice exhibited much less c-Fos expression, which did not co-localize with Kir2.1-dTomato expression (Figures S6B and S6C, right), confirming the effect of Kir2.1 expression in reducing PVH-projected LSv neuron activity.
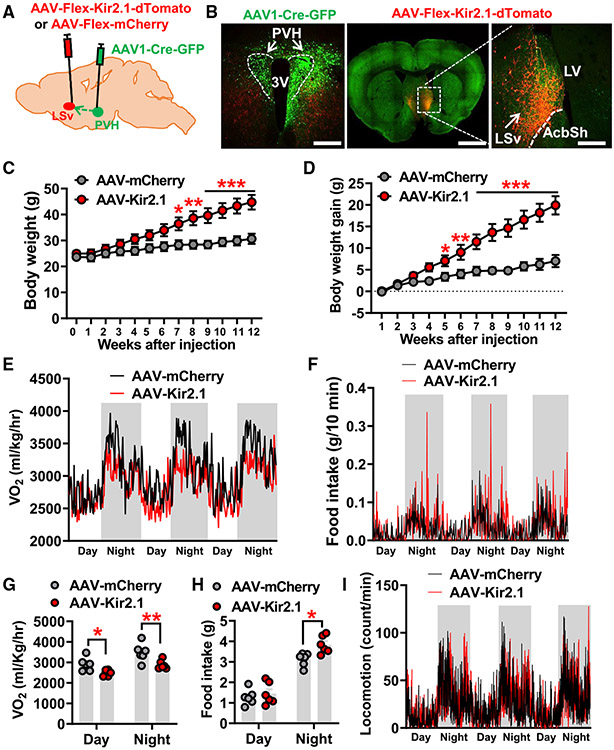
Figure 4. Chronic inhibition of PVH-projected LSv neurons causes massive obesity.
(A) Diagram depicting the experimental strategy.
(B) Representative expression patterns AAV1-Cre-GFP in the PVH (green) and projection fibers from PVH-projected Kir2.1 expressing LSv neurons in the peri-PVH area but not in the PVH (left) and expression patterns of AAV-Flex-Kir2.1-dTomato viral particles in LSv neurons mediated by antegrade transported Cre recombinase from PVH (middle and right; the right panel is an amplified image of boxed area in the middle panel.
(C and D) Comparison of weekly body weight (C) and net body weight gain (D) between mice with control mCherry (n = 8 mice) or Kir2.1 (n = 6 mice) expression in PVH-projected LSv neurons (two-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001).
(E and F) Traces of energy expenditure (E) and food intake (F) measured by CLAMS at 1 week after viral delivery when there was no diverged body weight between groups (n = 6 mice/group).
(G and H) Statistical comparison of O2 consumption
(G) and food intake (H) between mCherry- and Kir2.1-injected mice (paired t test, *p < 0.05, **p < 0.01).
(I) Locomotion (bream breaks) traces of the two groups of mice in (G) and (H).
AcbSh, accumbens nucleus shell; LV, lateral ventricle; LSv, ventral lateral septum; PVH, paraventricular nucleus of hypothalamus; 3V, third ventricle. All data presented asmean ± SEM. Scale bars in (B) represent 100 μm in the left panel, 800 μm in the middle panel, and 50 μm in the right panel. See also Figure S6.
Interestingly, chronic inhibition of PVH-projected LSv neurons led to massive obesity when fed normal chow (Figure 4C), and the average body-weight gain was up to 15 g by 12 weeks after Kir2.1 expression (Figure 4D). The observed obesity development was associated with both reduced energy expenditure (Figures 4E and 4G) and increased food intake (Figures 4F and 4H). Notably, the differences in feeding between controls and Kir2.1-injected mice were more obvious during the night period compared with the daytime (Figure 4E), suggesting a potential effect of the PVH→LSv projection in diurnal pattern regulation. However, we did not observe any difference in locomotion between control and Kir2.1 groups (Figure 4I). Of note, there was no body-weight difference between groups when metabolic parameters were measured, so any observed phenotype was not due to a secondary effect of data normalization. Collectively, these results demonstrate the importance of PVH-projected LSv neurons in body-weight regulation.
Chronic inhibition of PVH-projected LSv neurons induces behavioral signs of reduced anxiety level
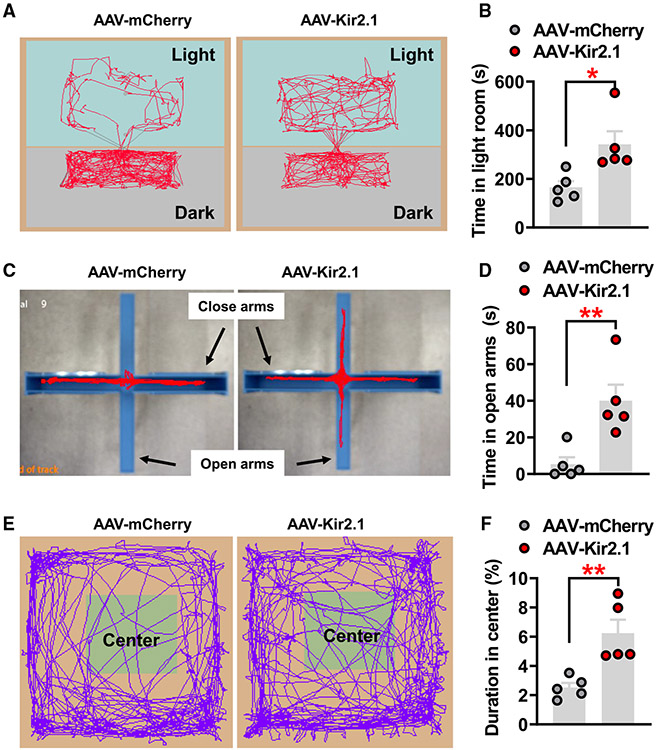
Given the known role of LS neurons in emotional control, we also subjected mice with chronic inhibition of PVH-projected neurons to various tests for behavioral signs of anxiety, including light dark box tests (LDT), elevated-plus maze (EPM) tests, and OFTs. In all assays, we performed the behavioral tests prior to body-weight divergence to eliminate potential secondary effects of obesity. Consistently, mice with chronic inhibition of PVH-projected LSv neurons exhibited increased time spent in the lit room of LDTs (Figures 5A and 5B), increased time in exploring the open arm of EPM tests (Figures 5C and 5D), and increased time staying in the center during OFTs (Figures 5E and 5E), suggesting that chronic inhibition of PVH-projected LSv neurons effectively reduces anxiety-like emotional modality in mice.
Figure 5. Chronic inhibition of PVH-projected LSv neurons leads to behavioral signs of reduced anxiety level.
(A and B) Representative movement traces of LDT in control-injected (mCherry) and Kir2.1-injected mice (n = 5 mice/each group) (A), and statistical comparison between these two groups (B, paired t test, *p < 0.05).
(C and D) Representative movement traces of EPM tests in control and Kir2.1 mice (C) and statistical comparison between these two groups (D, paired t test, **p < 0.01).
(E and F) Representative movement traces of OFT in control and Kir2.1 mice (E) and statistical comparison between these two groups (F, paired t test, **p < 0.01).
All data presented as mean ± SEM.
GABA release from PVH-projected LSv neurons in body-weight regulation
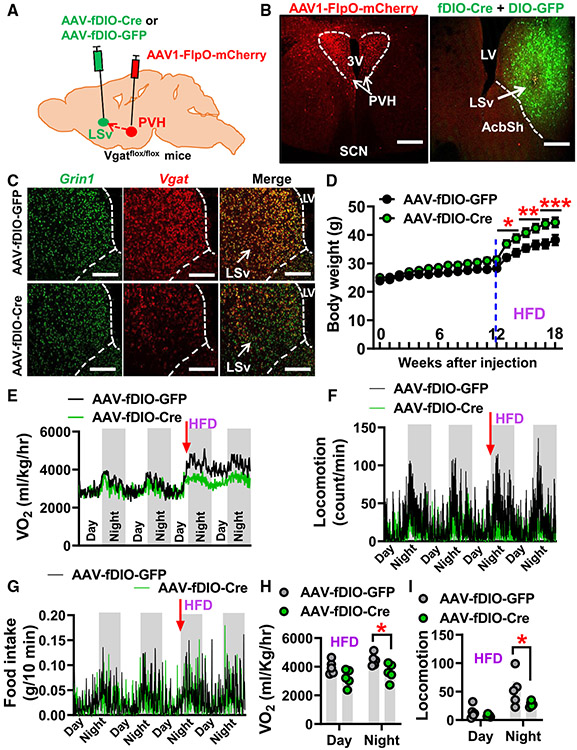
The LS mainly consists of GABAergic neurons and expresses abundant vesicular GABA transporters (Vgat, also named Slc32a1).18 To examine the role of GABA release from PVH-projected LSv neurons, we delivered AAV1-FlpO-mCherry to the PVH and followed this by injecting either AAV-fDIO-Cre mixed with AAV-DIO-GFP vectors or control AAV-fDIO-GFP virus to the LSv of Vgatflox/flox mice (Figure 6A). The expression of AAV1-FlpO-mCherry was confirmed in the PVH (Figure 6B, left) and, as expected, the expression of FlpO-mediated expression of Cre recombinase (reported by co-injected Cre-dependent GFP) was also found in the LSv (Figure 6B, right). We then performed dual ISH of Grin1 and Vgat to gene deletion. Despite comparable expression levels of Grin1 (Figure 6C, left), the ISH signal of Vgat mRNA in the Cre group was obviously reduced compared with the GFP vector-injected controls (Figure 6C, right). Consistent with this Vgat mRNA reduction, our electrophysiological recording data further showed that laser-evoked inhibitory postsynaptic currents in LSv neurons was significantly lower in Cre-injected Vgatflox/flox mice compared with the GFP group following photostimulation of LSv-projecting PVH neurons (Figures S7A-S7C). These results collectively suggest the effective Cre-mediated deletion of Vgat in PVH-projected LSv neurons. We next subjected these mice to normal chow diet feeding for 12 weeks followed by another 8 weeks on HFD feeding. Although mice with Vgat deletion in PVH-projected LSv neurons exhibited a trend toward altered feeding with no significant changes in body weight on chow, they indeed rapidly developed obesity on HFD (Figure 6D). When Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH) measurements were performed prior to body-weight divergence and during the chow-HFD transition, we found that mice with Vgat deletion exhibited a reduction in energy expenditure (Figures 6E and 6H) and locomotion (Figures 6F and 6I) in response to HFD. However, food intake measurements at this early time point exhibited no significant difference between groups (Figure 6G). These results collectively suggest an obligatory role for GABA release from PVH-projected LSv neurons in diet-induced obesity.
Figure 6. Disruption of GABA release from PVH-projected LSv neurons results in susceptibility to diet-induced obesity.
(A) Diagram depicting the experimental strategy.
(B) Representative expression patterns of AAV1-FlpO-mCherry in the PVH (left panel) and expression patterns of FlpO-mediated AAV-fDIO-Cre reported by co-injected Cre-dependent GFP reporter fluorescence in PVH-projected LSv neurons (right panel).
(C) ISH expression patterns of NMDA receptor subunit Grin1 (left, green), Vgat (middle, red), and their co-localization (right) in Vgatflox/flox mice of the LSv injected with GFP (control, top, n = 8 mice) and Cre (bottom, n = 10 mice).
(D) Comparison in body weight of Vgatflox/flox mice fed with chow for first 12 weeks and followed by another 6 weeks of HFD feeding after viral delivery (n = 8–10; two-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001).
(E–G) Traces of energy expenditure (E), locomotion (F), and food intake (G) measured with CLAMS at the time of chow-to-HFD transition (12 weeks after viral delivery, n = 6 mice).
(H and I) Statistical comparison of O2 consumption (H; paired t test, *p < 0.05) and locomotion (I; paired t test, *p < 0.05) on the first 2 days of HFD feeding.
AcbSh, accumbens nucleus shell; LV, lateral ventricle; LSv, ventral lateral septum; PVH, paraventricular nucleus of hypothalamus; SCN, suprachiasmatic nucleus; 3V, third ventricle. All data presented as mean ± SEM. Scale bars represent 100 μm in (B) and 50 μm in (C). See also Figure S7.
In addition, a subset of LSv neurons also express vesicular monoamine transporter 2 (VMAT2, also named Slc18a2)(Figure S7D), a gene known to be required for presynaptic release of monoamine, although the role of its expression in this area has never been explored. Similarly, we also adopted a comparable viral injection strategy in VMAT2flox/flox mice to facilitate conditional deletion of VMAT2 in PVH-projected LSv neurons (Figure S7E). While VMAT2 deletion was validated in the LSv (Figure S7F), mice lacking VMAT2 in PVH-projected LSv neurons exhibited no difference in body weight compared with control animals (Figure S7G), suggesting that VMAT2 expression in the PVH-projected LSv neurons is not required for body-weight regulation.
Chronic inhibition of PVHMc4R-projected LSv neurons results in obesity
Our anatomic data suggest that PVHMc4R neurons provide the majority of PVH inputs to the LSv. To specifically examine the role of PVHMc4R neuron projections to the LSv, we employed the previously established trans-synaptic tracer WGA-GFP, which is effectively transported to immediate downstream neurons but can also be transported in an anterograde fashion to presynaptic neurons.35,36 Since the LSv does not project to the PVH (Figure S8), we reasoned that WGA-GFP can be used to directly target LSv neurons that receive direct PVHMc4R neuron inputs (i.e., PVHMc4R-projected LSv neurons). To this end, we bilaterally injected AAV-Flex-WGA-GFP viral vectors to the PVH of Mc4R-Cre mice, while at the same time a mixture of AAV-FlpO-DOG-NW, AAV-fDIO-Cre, and AAV-Flex-Kir2.1-dTomato particles was injected into the LSv (Figure 7A). In this design, the AAV-FlpO-DOG-NW virus will express FlpO with the presence of GFP,37 which will then facilitate the conditional expression of Cre recombinase. Thus, Kir2.1 will be only expressed in PVHMc4R-projected LSv neurons with the presence of Cre. As shown in Figure 7B, the expression of GFP and dTomato was confirmed in a subset of LSv neurons, suggesting the specific expression of Kir2.1 in PVHMc4R-projected LSv neurons. Interestingly, compared with the control group (LSv injected with the mixture of AAV-FlpO-DOG-NW and AAV-fDIO-mCherry) these mice developed obesity when fed normal chow (Figure 7C). When measured with CLAMS prior to body-weight divergence, these mice showed a clear trend of reduction in energy expenditure, especially during the dark period (Figures 7D and 7G). However, we did not observe any obvious difference in locomotion (Figure 7E) or food intake (Figure 7F). Interestingly, compared with controls these mice showed more exploration time in the lit room of the LDT (Figures 7H and 7I). These results collectively demonstrate that the PVHMc4R→LSv circuit co-regulates both body weight and anxiety.
Figure 7. Chronic inhibition of PVHMc4R-projected LSv neurons leads to obesity.
(A) Diagram showing the surgical strategy.
(B) Representative images showing WGA-GFP expression in PVHMC4R neurons (left panel) and specific Kir2.1 expression in PVHMc4R-projected LSv neurons (right panel).
(C) Comparison of weekly body weight between Mc4R-Cre mice injected with mCherry (control) or Kir2.1 vectors and fed with chow diet for 12 weeks (n = 6 mice/group; two-way ANOVA, *p < 0.05, **p < 0.01).
(D–F) Traces of energy expenditure (D), locomotion
(E), and food intake (F) measured with CLAMS at 4 weeks after viral delivery when there was no divergent body weight between groups (n = 6).
(G) Statistical comparisons of O2 consumption between mCherry- and Kir2.1-injected mice.
(H and I) Representative movement traces of LDT in control (n = 7) and Kir2.1 (n = 6) mice (H) and comparison of time spent in lit room during the test between these two groups (I; unpaired t test, *p < 0.05). AcbSh, accumbens nucleus shell; LV, lateral ventricle; LSv, ventral lateral septum; 3V, third ventricle. All data presented as mean ± SEM. Scale bars in (B) represent 50 μm. See also Figure S8.
DISCUSSION
Despite extensive studies on the Mc4R function in obesity development in both rodents and humans, the neural pathway that mediates its action remains largely unexplored. Previous studies mostly focus on brain neurons that express Mc4Rs, but little is known regarding the function of their downstream neurons.8 In addition, the function of Mc4Rs has been primarily investigated in the context of homeostatic energy balance, yet their functional relevance to behavioral adaptation is unclear. An appropriate decision of whether or not to engage in feeding in a threatening environment is an important adaptive behavior for survival. Emerging studies have suggested that hypothalamic neurons that regulate feeding are also implicated in orchestrating feeding in response to environmental cues.25 Activation of AgRP neurons promotes feeding, suppresses feeding inhibition induced by anxiogenic cues,38-40 and prioritizes feeding behaviors over social interactions such as mating.41 These observations support the notion that the melanocortin pathway has the capacity to orchestrate feeding behaviors in response to ever-changing environmental cues.
Our results presented here reveal that PVHMc4R neurons send abundant projections to the LSv, the activation of which reduced feeding associated with behavioral signs of increased anxiety. Importantly, disruption of this pathway increases body weight associated with behavioral signs of reduced anxiety. Thus, the PVHMc4R→LSv projection bridges a key feeding brain area and an important emotion-related brain area, and co-regulates feeding and stress-related behaviors. Our results support that an increased activity level of this projection causes reduction in feeding and promotes stress-related behaviors, whereas a reduced level causes an opposite effect. Although stress-induced hypophagia has been well studied,42 the underlying neural pathways remain unclear. Our previous study has demonstrated that the activity of PVH and LS neurons increases in response to stress stimuli and decreases during feeding.29 Along these lines, the PVHMc4R→LSv projections may be involved in stress-induced hypophagia. Previous studies have implicated a role for AgRP neurons and Mc4R action in stress responses.16,38,39 Our current results on the PVHMc4R→LSv projection suggest that the LSv may be part of the downstream pathway mediating the effect. Given the well-documented correlation between eating disorders and psychiatric illnesses,43,44 our current results suggest a common PVHMc4R→LSv pathway as a potential neural basis for the correlation between psychiatric illness and eating disorders. Given the stress-related self-grooming induced by activating PVHMc4R→LSv projections, this pathway may potentially contribute to the previously described reciprocal rescuing effects observed between obesity with Mc4R deficiency and uncontrolled self-grooming from SAPAP3 KO in Mc4R and SAPAP3 double-KO models.17
One striking finding in the current study is the implication of LSv neurons in obesity development. Although previous studies have shown that LS neurons, especially those located in the dorsal part of the LS, play a role in feeding, and more specifically in coordinating stress-induced hypophagia,21,24 our results provide evidence that, beyond stress-induced hypophagia, the PVH→LSv projection also represents a bona fide body-weight-regulating pathway. Moreover, our data showed that defects in the glutamate-AMPA action within the pathway led to obesity development. In particular, chronic inhibition of PVH-projected LSv neurons caused a profound impact on obesity development. Thus, we reveal that LSv neurons represent an important key downstream site that mediates the PVH function in body-weight regulation.
Deletion of Vglut2 from LSv-projecting PVH neurons led to obesity in mice fed a chow diet, which is comparable with the obese phenotype we have previously observed with Vglut2 deletion directly from PVH neurons,30 suggesting a major role for the LSv as a downstream node in mediating PVH action on body weight. Previous studies on PVH were mostly focused on projections in the midbrain, brainstem, and spinal cord.11,27 Given the fact that LSv-projecting PVH neurons also send collateral projections to those brain sites, it is possible that a common subset of PVH neurons regulates body weight through a divergent projection to several downstream brain sites. However, given that our anatomic data show partial Vglut2 loss in the PVH as a result of targeted Vglut2 deletion from LSv-projecting PVH neurons and that PVH oxytocin- and AVP-expressing neurons do not project to the LSv,29 there should be a significant number of PVH neurons that do not project to the LSv. Notably, previous results suggest that PVH neurons that regulate body weight can be largely divided into two groups: Mc4R-expressing and prodynorphin-expressing neurons.28 Our current results demonstrate that PVHMC4R but not PVHPdyn neurons provide major projections to the LSv. Thus, the LSv may represent an important downstream site mediating the role of PVHMC4R action in feeding and body-weight regulation.
Altogether, our data show that disruption of glutamate release from LSv-projecting PVH neurons produces a much more robust obesity than Mc4R deletion from the same group of neurons, indicating a significant number of non-Mc4R-expressing neurons that contribute to the PVH→LSv projection. Consistent with this, direct photostimulation of PVH neuron projections in the LSv produces a much stronger behavioral effect that includes jumping and self-grooming,29 while the current study on PVHMc4R projections only produces relatively mild self-grooming. Moreover, chronic inhibition of PVH-projected LSv neurons with Kir2.1 expression causes greater obesity development than that of PVHMc4R-projected LSv neurons, indicating that a smaller number of downstream LSv neurons are targeted from PVHMc4R neurons. Thus, it appears that the PVHMc4R→LSv projection only represents a component of the PVH→LSv projection. An alternative explanation for more robust obesity from disruption of glutamate release than Mc4R deletion is that the Mc4R-mediated signaling only represents one of many upstream signals regarding body-weight regulation, whereas glutamate is the major mediating neurotransmitter of these neurons.30 Notably, we compared food intake and energy expenditure before body-weight divergence with an aim to identify the initiating factor for the observed obesity. For this reason the measured difference, especially in food intake known to be highly variable according to the measurement method, can be small. In this sense, caution should be exercised in interpreting the data regarding no difference in food intake in some of our obesity models examined, as there exists the potential that small differences in feeding may be masked by variations in measurement. In line with this, previous studies on PVHMc4R neurons also showed no difference in food intake when feeding is measured prior to obesity development.30,45,46 Nevertheless, we indeed observed consistent reduction in energy expenditure associated with the observed obesity, arguing that the initiating factor in obesity associated with the PVHMc4R→LSv projection is reduced energy expenditure, which is consistent with the previous observations on a primary role of PVHMc4R neurons in energy expenditure.30,45
To date, the identity of PVH-projected LSv neurons remains unknown. Mice with disruption of GABA release from PVH-projected LSv neurons exhibit diet-induced obesity, suggesting a role of GABA release in mediating the body-weight effect, which is consistent with the fact that LS neurons are GABAergic.18 We also found that some of these LSv neurons express VMAT2, a marker for monoaminergic neurons. However, VMAT2 deletion in these neurons caused no obvious changes in body weight, arguing against a potential role for VMAT2-mediated neurotransmission. A subset of LS neurons has been demonstrated to express other markers including CRFR2, GLP1 receptors, and neurotensin.20-22,24,47,48 However, since these neurons are not limited to the LSv,21,22,48 it is thus less likely that these neurons contribute significantly to the observed effects. Further single-cell RNA sequencing can be used to identify potential novel markers that specifically identify this subset of neurons. LS neurons have been involved in various behaviors including stress, defense, aggression, and memory.18 Our results on the susceptibility to diet-induced obesity in mice, either with Mc4R deletion in LSv-projecting PVH neurons or with disruption of GABA release from PVH-projected LSv neurons, suggest a potential role of PVH-projected LSv neurons in hedonic feeding.49 It will be interesting for future studies to investigate the underlying mechanism and pathway for the observed susceptibility to diet-induced obesity.
Limitations of the study
There are some limitations in the present study. The activities of both PVH and LSv neurons are known to be sensitive to external stressors and respond to feeding,29 and mutations in the Mc4R gene are associated with obesity and emotional changes,15 suggesting relevance to human physiology. Since extra-physiological optogenetics and mouse genetics were used to achieve gain or loss of functions of neurons and circuits in this study, one main limitation is the relevance to normal physiology. Further physiological studies are required to ascertain the level of involvement of the PVHMc4R→LSv projection in normal physiological feeding and anxiety-related behaviors. Technically, direct functional documentation of the effect of the ctGluR1 peptide on AMPA receptor trafficking is not presented, which, however, given the compelling data on the expression of the peptide and associated phenotypes, is unlikely to affect the main conclusion drawn from this study.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Qingchun Tong Qingchun.tong@uth.tmc.edu.
Materials availability
This study did not generate new or unique reagents.
Data and code availability
All data that support the findings of this study are available from the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice were housed at 21°C–22°C with a 12 h light/12 h dark cycle with standard pellet chow and water provided ad libitum unless otherwise noted for fasting experiments. Animal care and procedures were approved by the University of Texas Health Science Center at Houston Institutional Animal Care and Use Committee. MC4R-Cre, Pdyn-Cre, Mc4Rflox/flox, Vglut2flox/flox, Vgatflox/flox, VMAT2flox/flox, Grin1flox/flox, Vglut2-Ires-Cre, and Vgat-FlpO mice were described previously28,30,34,52-54 and were all obtained from the Jax lab. Both male and female mice were used as study subjects, except otherwise noted. All mice that were used for stereotaxic injections were at least 8–10 weeks old.
METHOD DETAILS
Surgeries and viral constructs
All mice that were used for stereotaxic injections were at least 8–10 weeks old. Briefly,55 mice were anesthetized with a ketamine/xylazine cocktail (100 mg/kg and 10 mg/kg, respectively), and their heads affixed to a stereotaxic apparatus. Viral vectors were delivered through a 0.5 μL syringe (Neuros Model 7000.5 kH, point style 3; Hamilton, Reno, NV, USA) mounted on a motorized stereotaxic injector (Quintessential Stereotaxic Injector; Stoelting, Wood Dale, IL, USA) at a rate of 30 nL/min. Viral preparations were titered at ~1012 particles/mL. Viral delivery was targeted to the PVH (bilateral, 150 nL/side; anteroposterior (AP): −0.9 mm; mediolateral (ML): ±0.1 mm; dorsoventral (DV): −4.8 mm) or LSv (200–300 nL/side; AP: +1.1 mm; ML: ±0.5 mm; DV: −3.5 mm). Uncleaved fiber optic cannulae (Ø1.25 mm Stainless Ferrule, Ø200 μm Core, 0.39 NA; ThorLabs, Newton, New Jersey, USA) were precut to 3.0 mm and implanted above the LSv. All study subjects were littermates and randomly distributed between study groups. Group size was estimated based on relevant literature reports and differences in average between groups from pilot studies. All behavioral tests were conducted during the light cycle following a minimum 4 weeks recovery period post-surgery.
For blue-light dependent activation of PVH→LSv fibers, AAV-EF1α-FLEX-hChR2(H134R)-EYFP (Addgene, plasmid # 20298)56 was injected bilaterally into the PVH (150 nL/each side) of Mc4R-Cre or Pdyn-Cre mice. The AAV-EF1α-DIO-GFP (Neuroconnectivity Core, Baylor College of Medicine) vector was also injected into the PVH (150 nL/each side) of Mc4R-Cre or Pdyn-Cre mice and used as an opsin-negative control for behavioral experiments.
For specific deletion of Vglut2 or Mc4R from LSv-projecting PVH neurons, the retrograde AAVrg-EF1α-IRES-FlpO-mCherry virus (Addgene, plasmid # 55634) was injected into bilateral LSv of Vglut2flox/flox or Mc4Rflox/flox mice (200nL/each side) and followed by injecting a cocktail of AAV-EF1α-fDIO-Cre (Addgene, plasmid # 121675) and AAV-EF1α-DIO-EGFP vectors (200 nL/each side, mixture at a 3:1 ratio) or control AAV-EF1α-fDIO-EGFP vectors (Neuroconnectivity Core, Baylor College of Medicine). The AAV-EF1α-DIO-EGFP was co-injected to report the activity of FlpO recombinase in the PVH.
For specific blocking of AMPA receptor signaling pathway in PVH-projected LSv neurons, the AAV-Flex-CtGluR1-P2A-mCherry vector was cloned by inserting the coding sequence of the C terminal fragment of the AMPA receptor GluR1 subunit, which was previously used to block GluR1 subunit trafficking,57 into the AAV-Flex-GFP by replacing the GFP coding sequence (Vectorbuilder Inc., Chicago, IL, USA). In wild type mice, the AAV1-CMV-Cre-eGFP virus (Addgene, Plasmid #105545) was injected into the PVH (bilateral,150 nL/each) to achieve anterograde tracing,58 and followed by bilateral LSv injection with 200 nL AAV-Flex-CtGluR1-P2A-mCherry or control AAV-hSyn-DIO-mCherry vectors (Addgene, Plasmid #50459).
For specific deletion of VMAT2, Mc4R or NR1 subunit of NMDA receptors from PVH-projected LSv neurons, the AAV1-CMV-Cre-eGFP vectors was injected into the PVH (bilateral, 150 nL/each) of Vgatflox/flox, VMAT2flox/flox or Grin1flox/flox mice and followed by the delivery of a cocktail of AAV-EF1α-fDIO-Cre mixed with AAV-EF1α-DIO-EGFP (3:1) into the bilateral LSv (200 nL/each).
For chronic inhibition of PVH-projected LSv neurons, the AAV1-CMV-Cre-eGFP virus was bilaterally injected into the PVH (150 nL/each) of wild type mice, and followed by LSv injection of the AAV-EF1α-Flex-Kir2.1-P2A-dTomato viruses (150 nL/each), which were the same as that used in our previous study,14 or control AAV-hSyn-DIO-mCherry vectors.
For specific evaluation the inhibitory effect of Kir2.1 expression on neuronal activity, the AAV-DIO-hM3Gq-mCherry was injected into the bilateral PVH (150nL/each, Addgene, Plasmid # 44361) and followed by injecting a mixture of AAV- EF1α-Flex-Kir2.1-dTomato and AAV- EF1α-fDIO-Cre vectors (1:1, 300nL/each) or AAV-EF1α-fDIO-mCherry (200nL/each, Addgene, Plasmid # 114471) alone to the bilateral LSv in Vglut2-Ires-CreVgat-FlpO mice. After 4 weeks, we examined c-Fos expression in LSv neurons at 90 min following activation of upstream PVH neurons with intraperitoneal injection of Clozapine N-oxide (CNO, C8032, Sigma, St. Louis, Missouri, USA; 1 mg/kg body weight).
For specific silencing of PVHMC4R-projected LSv neurons, AAV2/DJ8-CGA-Flex-WGA-eGFP vectors (Canadian Neurophotonics Platform Viral Vector Core Facility, SCR_016477) was injected into the PVH (bilateral, 150 nL/each) of Mc4R-Cre mice to achieve input specific transsynaptic tracing, and followed by bilateral LSv injection with the cocktail of GFP-dependent AAV-EF1α-FlpO-DOG-NW (Addgene, Plasmid #75469), FlpO-dependent AAV-EF1α-fDIO-Cre and Cre-dependent AAV-EF1α-DIO-Kir2.1-P2A-dTomato vectors (1:1:1, 300nL/each) or a control mixture of GFP-dependent AAV-EF1α-FlpO-DOG-NW and FlpO-dependent AAV-EF1α-fDIO-mCherry (1:1, 200nL/each).
Feeding assays
For in vivo experiments, an integrated rotary joint patch cable connected the ferrule end of optic fiber cannula with a Ø1.25 mm ferrule end of the patch cable via a mating ceramic sleeve (ThorLabs, Newton, New Jersey, USA). At the other end of the rotary joint, an FC/PC connector was connected to a 473 nm diode-pumped solid state (DPSS) laser (Opto Engine LLC, Midvale, Utah, USA). Light pulses were controlled by Master-8 pulse stimulator (A.M.P.I., Jerusalem, Israel). For optogenetic stimulation-feeding experiments, mice were ad libitum fed prior to testing (excluding competition experiments; see below). Mice after 20hr fasting underwent 15-min trials of refeeding with laser on or off. During the light-on period, blue light (473 nm, ~5 mW/mm2) was pulsed at 5 Hz, with each pulse-width duration lasting 20 or 100 ms. Food intake was measured and recorded after the completion of each epoch during the 15-min trial. Mice after 20hr fasting were also tested for feeding during alternating epoch of laser on and off. A video was recorded for each trial. An observer blind to the experimental conditions watched the videos and manually calculated the time spent in feeding with a stopwatch. Feeding time was counted when mice were actively engaged in biting, chewing, swallowing, or licking food.
Grooming assays
For optogenetic stimulation grooming experiments, mice were ad libitum fed prior to testing. Mice underwent 6-min trials consisting of three consecutive 2-min epochs (pre-light, light-on, and post-light). During pre-light and post-light periods, the laser was turned off. During the light-on period, blue light (473 nm, ~5–10 mW/mm2) was pulsed at 5 Hz, with each pulse-width duration lasting 100 m. An observer blind to the experimental condition watched the 6 min videos and manually quantified the time spent grooming during each epoch (2 min each) with a stop watch. Grooming time was counted when the mouse engaged in paw strokes made near the nose, eyes, and head and during paw, body, tail, and genital licking. To assess whether grooming induced by light activation results in abnormal patterning, an observer watched 5Hz, 100 ms videos of mice and quantified the number of grooming bouts, bout interruptions, and changes in grooming transitions using a grooming analysis algorithm described59 during pre-light and light-on conditions. Time spent in grooming times were averaged for each epoch (pre-light, light-on and post-light) and compared between epochs.
Real time place preference (RTPP) assays
For RTPP assays, a commutator (rotary joint; Doric, QC, Canada) was attached to a patch cable via FC/PC adaptor. The patch cable was then attached to the optic fiber cannula ferrule end via a ceramic mating sleeve. Another patch cable containing FC/PC connections at both ends allowed the connection between the commutator and the 473 nm laser, which was controlled by the Master-8 pulse stimulator. MC4R-Cre mice injected with Cre-dependent ChR2 or GFP viruses, respectively, and implanted with optic fibers above the LSv, were placed in a clean 45 cm × 45 cm X 50 cm chamber equipped with a camera mounted on top of the chamber and optical fiber patch cable attached to a commutator. The testing chamber was wiped down with 70% isopropyl alcohol between tests. Prior to starting experiments, the patch cable was attached to optic fiber ferrule end of the mouse’s cannula. At the start of the experiment, mice were placed in the light-off zone, in which no light was applied. Then, for 20 min, the mice were allowed to freely roam the enclosure, which was divided into two equal zones containing the light-off zone and a light-on zone, in which 5Hz, 20 or 100 ms (473nm, ~5 mW/mm2) light pulses were delivered. The side paired with photostimulation was counterbalanced between mice. For some experiments, instead of paring with one side, photostimulation was paired with the peripheral zone (with light off at the central zone of the arena). For experiments to test the competitive effects of photostimulation on feeding-suppression vs. aversion, 20 h fasted mice were used and photostimulation was paired with the side where chow food pellets were placed. Tracking data, including time spent in each zone, were collected and analyzed by Ethovision XT software (version 11.5; Noldus, Wageningen, Netherlands). Preference for side zones or center versus peripheral zones was determined by comparing the percentage of time spent in each zone.
Body weight and metabolic assays
Body weight studies: Weekly body weight was monitored on all genotype mice fed standard mouse chow (Teklad F6 Rodent Diet 8664, 4.05 kcal/g, 3.3 kcal/g metabolizable energy, 12.5% kcal from fat, Harlan Teklad, Madison, WI) for 8–12 weeks after viral delivery. In some experiments, weekly body weight was also monitored on all genotype mice after switched from normal chow to HFD (Research Diets D12492; 20% protein, 60% fat, 20% carbohydrate, 5.21 kcal/g) for 6–8 weeks,
CLAMS analysis: Mice were placed at room temperature (22°C–24°C) in chambers of CLAMS with capacity of simultaneous measurement of food intake, O2 consumption and locomotion (beam breaks). Energy expenditure was measured by O2 consumption by indirect calorimetry. Food and water were provided ad libitum. Mice were acclimatized in the chambers for at least 48 h prior to data collection. Readings of O2 consumption, locomotion and food intake were ploted and compared between groups. Individual housed mice maintained on chow diet were tested when there no significant difference in body weight between groups. Body weight were measured before and after CLAMS measurements. For experimental mice with chronic inhibition of PVH-projected LSv neurons, all animals were single housed and performed CLAMS tests after 1 week of viral delivery. For experimental mice with disruption of GABA release from PVH-projected LSv neurons, all animals were single housed and performed CLAMS tests after 12 weeks of viral delivery, and during the measurement period all mice were fed with chow diet for the first 4 days and then switched to HFD for the next 3 days. For experimental mice with chronic inhibition of PVHMC4R-projected LSv neurons, all animals were single housed and performed CLAMS tests after 4 weeks of viral delivery.
Behavioral assays
All behavioral assays were performed before body weight became different between groups fed on chow, and the animal ID was blinded when behavior tests were conducted.
Open field test (OFT):
The apparatus consists of a white Plexiglas box and brightly illuminated (120 lx). Each mouse was placed in the corner of the apparatus to initiate a 20-min test session. A camera (Noldus, Leesburg, VA, USA) mounted above the apparatus monitor the mice and the track of its movements was recorded, which were analyzed by EthoVision XT software. Time spent in the center recorded and subsequently analyzed, longer times spent exploring the arena in the center seen as less anxiety-like behaviors.
Elevated-plus maze (EPM):
Elevated-plus maze that consists of two open and two closed arms positioned 50 cm above the floor (Kinder Scientific, Poway, CA, USA). Mice were placed on the central facing an open arm, to initiate a 10-min session test. The time spent in the open or closed arms were recorded and analyzed by Kinder Scientific Motor Monitor software.
Light/Dark box test (LDT):
The light/dark room test apparatus consists of two same space parts, light room and the dark room (Kinder Scientific, Poway, CA, USA). Mice were placed in the center of apparatus with an opening to the both side and initiate a 10-min session test. The time spent in the light room were recorded and analyzed by Kinder Scientific Motor Monitor software.
Brain slices preparation and electrophysiology recordings
Electrophysiological recordings were performed as same as our previous work.60 Briefly, mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg), and transcardially perfused with ice-cold cutting solutions containing (in mM): 75 sucrose, 73 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 15 glucose, 7 MgCl2, and 0.5 CaCl2, saturated with 95% O2/5% CO2. The brains were quickly removed from the skull, blocked, and glued to a specimen plate, and submerged into the cutting solution. Coronal slices (300μm) containing LS or PVN were sectioned with a Leica VT 1000S vibratome, and transferred to a holding chamber with artificial CSF (aCSF) containing (in mM): 123 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 10 glucose, 1.3 MgCl2, and 2.5 CaCl2, saturated with 95% O2/5% CO2, held at 31–33°C for 30 min, then maintained at room temperature for at least 1h to allow for recovery.
Individual slices were transferred from the holding chamber to a recording chamber, in which they were submerged and continuously perfused with oxygenated aCSF, warmed to 31–33°C by passing through an in-line heater (TC-324B, Warner Instruments). Whole-cell patch-clamp recordings were performed under infrared-differential interference contrast visualization with a water-immersion 40× objective. For spontaneous postsynaptic currents recordings, pipettes (3-5MΩ) were filled with a K+-based internal solution containing (in mM): 142 K-gluconate, 10 HEPES, 1 EGTA, 2.5 MgCl2, 0.25 CaCl2, 4 Mg-ATP, 0.3 Na-GTP, and 10 Na2-phosphocreatine, adjusted to pH 7.30, osmolality 300 with KOH. For recordings of electrically or optogenetically evoked currents, pipettes were filled with a Cs+-based internal solution containing (in mM): 115 Cs-MeSO4, 20 CsCl, 10 HEPES, 1 EGTA, 2.5 MgCl2, 0.25 CaCl2, 10 Na2-phosphocreatine, 4 Mg-ATP, 0.3 Na-GTP, 0.1 spermine, and 5 QX-314, adjusted to PH 7.30, osmolality 300 with CsOH. Liquid junction potential was not corrected, and series resistance (Rs) was bridge balanced.
For AMPAR- and NMDAR-mediated currents, 20μM of bicuculline, a GABAA receptor antagonist, was included in the perfusate. To evoke currents, a concentric bipolar microelectrode (FHC) was placed lateral to the recording site. The intensity (0.05–0.3mA) of the stimulus (200μs, 0.05Hz) was adjusted to get 50–70% of maximal responses. Neurons were voltage-clamped at −70mV to record AMPARs-mediated currents, and at +40mV to record AMPARs- and NMDARs-mediated currents. Results were averaged from at least 6 sweeps. NMDAR/AMPAR ratios were calculated by dividing the value of the NMDARs eEPSC 50ms post stimulation at +40mV by the peak of AMPARs eEPSC at −70mV.57 To activate ChR2 in the brain slices, an optic fiber was placed close to the recording electrode above the slice to deliver laser pulses (473nm, 1 ms). Neurons were voltage-clamped at −70mV to record glutamatergic receptors-medicated currents, and at +40mV to record GABA receptors-mediated currents.29
Brain tissue preparation, imaging, and post-hoc analysis
After all behavioral experiments were completed, study subjects were anesthetized with a ketamine/xylazine cocktail (100 mg/kg and 10 mg/kg, respectively) and subjected to transcardial perfusion. During perfusion, animals were flushed with 20 mL of saline prior to fixation with 20 mL of 10% buffered formalin. Freshly fixed brains were then extracted and placed in 10% buffered formalin in 4°C overnight for post-fixation. The next day, brains were transferred to 30% sucrose solution and allowed to rock at room temperature for 24 h prior to sectioning. Brains were frozen and sectioned into 30 μm slices with a sliding microtome and mounted onto slides for post-hoc visualization of injection sites and cannula placements. Mice with missed injections to the P or misplaced optic fibers over the LSv were excluded from behavioral analysis. Representative pictures of LSv and PVH injection sites and cannula placements were visualized with confocal microscopy (Leica TCS SP5; Leica Microsystems, Wetzlar, Germany).
In situ hybridization (ISH) assays
RNAscope Fluorescent Multiplex ISH Kit (Advanced Cell Diagnostics, INC., Newark, CA, USA) was used to detect the transcription signals of Grin1, Vglut2, Vgat and Mc4r. ISH was performed as we previously described with modification and following the manufacture protocol of Fixed-frozen tissues.30 Briefly, the freshly prepared brain tissues perfused with 10% formalin were sectioned at 15 μm thickness and mounted to Super Plus Gold glass slices. The slides were then immersed in chilled 10% buffered formalin for 15 min at 4 °C. Thereafter, the sections were dehydrated by immersing in 50% EtOH, 70% EtOH, and 100% EtOH for 5 min each at room temperature. After incubated with RNAscope Hydrogen Peroxide, all sections were performed target retrieval for 15 min at 100°C. Then, a hydrophobic barrier was drawn around tissue with a hydrophobic barrier pen after allowing slides to air dry. The slides were then placed on a hybridization humidifying rack and treated with protease pretreatment solution for 30 min at room temperature. After pretreatment, slides were washed twice with fresh 1X PBS in a slide rack. PBS was gently tapped away from slides prior to applying the hybridization probe for Grin1 (Mm-Grin1-C1 probe), Vglut2 (Mm-Slc17a6-E1-E3 probe), Vgat (Mm-Slc32a1-C2 probe) and Mc4r (Mm-Mc4r-C3 probe). The slides were placed in the humidifying rack and allowed to incubate for 2 h at 40 °C in a hybridization incubator. After hybridization, slides went through a series of washes with 1X RNAscope wash buffer, followed by 4 amplification steps of the hybridization signal. After the wash and amplification step, slides were counterstained with DAPI and cover-slipped with Prolong Gold Anti-fade mounting medium (Life Technologies). Vglut2, Vgat and Mc4r signals in matched sections of control and knockout groups were visualized with confocal microscopy. Of note, viral GFP reporter fluorescence were completely bleached out during ISH procedures, which allows us to visualize in situ signals only from target genes.
Immunohistochemistry assays
All animals were perfused and collected brain tissues as same as mentioned above. Brain sections at 30 μm thickness were sliced for immunostaining studies, primary antibodies against ctGluR1(Rabbit, Invitrogen, PA5-99527), c-Fos (rabbit, Cell Signaling Technology, #2250), NR1(rabbit, ABclonal, A7677), VMAT2 (rabbit, Phoenix Pharmaceuticals, H-V008), or Mc4R (rabbit, Alomone labs, Cat#AMR-024). All brain sections were incubated with the primary antibodies overnight at room temperature following 1 h blocking in 10% normal donkey serum. After visualized with secondary donkey IgG serum conjugated with Alexa Fluor 488 or Alexa Fluor 647 (Jackson ImmunoResearch, West Grove, PA, USA), sections were photographed with a TCS SP5 confocal microscopy.
QUANTIFICATION AND STATISTICAL ANALYSIS
All data were presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism.9 (GraphPad Software, La Jolla, CA). Two-tailed unpaired Student’s t tests, one-way or two-way ANOVA followed by Tukey’s multiple comparison post-hoc tests were used. Statistical significance was set at *p < 0.05; **p < 0.01, and ***p < 0.001. The statistical details were shown in the respective figure legends.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| rabbit anti-c-Fos | Cell Signaling Technology | Cat# 2250s |
| rabbit anti-ctGluR1 | Invetrogen | Cat#PA5-99527 |
| rabbit anti-NR1 | ABclonal | Cat#A7677 |
| rabbit anti-VMAT2 | Phoenix Pharmaceuticals | Cat#H-V008 |
| rabbit anti-Mc4R | Alomone labs | Cat#AMR-024 |
| Bacterial and virus strains | ||
| AAV-EF1α-FLEX-hChR2(H134R)-EYFP | Karl Deisseroth Lab | RRID: Addgene_20298 |
| AAV-EF1α-DIO-GFP | Neuroconnectivity Core, Baylor College of Medicine | N/A |
| AAVrg-EF1α-IRES-FlpO-mCherry | Fenno et al.50 | RRID: Addgene_55634 |
| AAV1-CMV-Cre-eGFP | James M. Wilson Lab | RRID: Addgene_105545 |
| AAV-hSyn-DIO-mCherry | Bryan Roth Lab | RRID: Addgene_50459 |
| AAV-Flex-CtGluR1-P2A-mCherry | Vector builder | N/A |
| AAV-EF1α-fDIO-Cre | Schneeberger et al.51 | RRID: Addgene_121675 |
| AAV-DIO-hM3Gq-mCherry | Krashes et al.13 | RRID: Addgene_44361 |
| AAV2/DJ8-CGA-Flex-WGA-eGFP | Canadian Neurophotonics Platform Viral Vector Core | Cat#SCR_016477 |
| AAV-EF1α-DIO-Kir2.1-P2A-dTomato | Zhu et al.14 | N/A |
| AAV-EF1α-FlpO-DOG-NW | Tang et al.37 | RRID: Addgene_75469 |
| AAV-hSyn-Con/Fon EYFP-WPRE | UNC Vector Core | Cat# UNC AV-2404 |
| Chemicals, peptides, and recombinant proteins | ||
| Clozapine N-oxide (CNO) | Sigma-Aldrich | Cat# C8032 |
| Formalin | Fisher | Cat# SF100-4 |
| Critical commercial assays | ||
| RNAscope® Multiplex Fluorescent Detection Kit | ACD | Cat# 323110 |
| Experimental models: Organisms/strains | ||
| Mouse: MC4R-cre | The Jackson Laboratory | JAX:030759 |
| Mouse: Pdyn-cre | The Jackson Laboratory | JAX: 027958 |
| Mouse: MC4Rflox/flox | The Jackson Laboratory | JAX: 023720 |
| Mouse: VGlut2flox/flox | The Jackson Laboratory | JAX: 012898 |
| Mouse: Vgatflox/flox | The Jackson Laboratory | JAX: 012897 |
| Mouse: VMAT2flox/flox | Xu et al.52 | N/A |
| Mouse: Grin1flox/flox | The Jackson Laboratory | JAX: 005246 |
| Mouse: VGlut2-Ires-Cre | The Jackson Laboratory | JAX: 016963 |
| Mouse: Vgat-FlpO | The Jackson Laboratory | JAX: 029591 |
| Oligonucleotides | ||
| RNAscope® Probe - Mm-Mc4r-C2 | ACD | Cat#319181-C2 |
| RNAscope® Probe - Mm-Slc17a6-E1-E3 | ACD | Cat#456751 |
| RNAscope® Probe - Mm-Grin1 | ACD | Cat#431611 |
| RNAscope® Probe - Mm-Slc32a1-C2 | ACD | Cat#319191-C2 |
| Software and algorithms | ||
| pClamp | Molecular Devices | RRID:SCR_011323; http://www.moleculardevices.com/products/software/pclamp.html |
| GraphPad Prism (9.1.2) | GraphPad | RRID:SCR_002798; http://www.graphpad.com/ |
| Ethovision XT XT 11.5 | Noldus | https://www.noldus.com/ethovision-xt |
| OxyMax | Columbus Instruments | https://www.colinst.com/products/clams-comprehensive-lab-animal-monitoring-system |
Highlights.
The ventral lateral septum (LSv) receives projections from PVHMC4R neurons
Deletion of Mc4Rs in LSv-projecting PVH neurons causes obesity
Inhibition of PVHMc4R-projected neurons in the LSv causes obesity
The PVHMc4R→LSv circuit co-regulates body weight and emotion
ACKNOWLEDGMENTS
This study was supported by NIH R01 DK 114279, R01 DK136284, and R01 DK 131446 (Q.T.); NIH R01DK109934 and DOD W81XWH-19-1-0429 (Q.T. and B.R.A.); NIH R01 DK120858 (Q.T. and Yong Xu); Brain & Behavior Research Foundation 2019 Young Investigator Research Award (grant no. 28791 to Yuanzhong Xu); and NIH R01DK117281 and R01 DK101379 (Yong Xu). We also acknowledge the Neuroconnectivity Core funded by NIH IDDRC grant 1 U54 HD083092 and Baylor College of Medicine Gene Vector Core for providing AAV vectors. We would like to acknowledge the Tong lab members for helpful discussion and Dr. Zhengmei Mao for help with microscopy. Q.T. is holder of the Cullen Chair in Molecular Medicine at McGovern Medical School.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2023.112502.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Cone RD (2005). Anatomy and regulation of the central melanocortin system. Nat. Neurosci 8, 571–578. 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 2.Liu T, Xu Y, Yi CX, Tong Q, and Cai D (2022). The hypothalamus for whole-body physiology: from metabolism to aging. Protein Cell 13, 394–421. 10.1007/s13238-021-00834-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N, Ramanathan V, Strochlic DE, Ferket P, Linhart K, et al. (2013). Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science 341, 275–278. 10.1126/science.1233000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron M, Maillet J, Huyvaert M, Dechaume A, Boutry R, Loiselle H, Durand E, Toussaint B, Vaillant E, Philippe J, et al. (2019). Loss-of-function mutations in MRAP2 are pathogenic in hyperphagic obesity with hyperglycemia and hypertension. Nat. Med 25, 1733–1738. 10.1038/s41591-019-0622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biebermann H, Castañeda TR, van Landeghem F, von Deimling A, Escher F, Brabant G, Hebebrand J, Hinney A, Tschöp MH, Grüters A, and Krude H (2006). A role for beta-melanocyte-stimulating hormone in human body-weight regulation. Cell Metabol. 3, 141–146. 10.1016/j.cmet.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Coll AP, Farooqi IS, Challis BG, Yeo GSH, and O’Rahilly S (2004). Proopiomelanocortin and energy balance: insights from human and murine genetics. J. Clin. Endocrinol. Metab 89, 2557–2562. 10.1210/jc.2004-0428. [DOI] [PubMed] [Google Scholar]
- 7.O’Rahilly S, Farooqi IS, Yeo GSH, and Challis BG (2003). Minireview: human obesity-lessons from monogenic disorders. Endocrinology 144, 3757–3764. 10.1210/en.2003-0373. [DOI] [PubMed] [Google Scholar]
- 8.Krashes MJ, Lowell BB, and Garfield AS (2016). Melanocortin-4 receptor-regulated energy homeostasis. Nat. Neurosci 19, 206–219. 10.1038/nn.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garfield AS, Shah BP, Madara JC, Burke LK, Patterson CM, Flak J, Neve RL, Evans ML, Lowell BB, Myers MG, and Heisler LK (2014). A parabrachial-hypothalamic cholecystokinin neurocircuits controls counterregulatory responses to hypoglycemia. Cell Metabol. 20, 1030–1037. 10.1016/j.cmet.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al. (2005). Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123, 493–505. 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 11.Stachniak TJ, Ghosh A, and Sternson SM (2014). Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus–>midbrain pathway for feeding behavior. Neuron 82, 797–808. 10.1016/j.neuron.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, and Luo M (2013). Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J. Neurosci 33, 3624–3632. 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, and Lowell BB (2011). Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest 121, 1424–1428. 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu C, Jiang Z, Xu Y, Cai ZL, Jiang Q, Xu Y, Xue M, Arenkiel BR, Wu Q, Shu G, and Tong Q (2020). Profound and redundant functions of arcuate neurons in obesity development. Nat. Metab 2, 763–774. 10.1038/s42255-020-0229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S, Daily JW, Zhang X, Jin HS, Lee HJ, and Lee YH (2016). Interactions with the MC4R rs17782313 variant, mental stress and energy intake and the risk of obesity in Genome Epidemiology Study. Nutr. Metab 13, 38. 10.1186/s12986-016-0096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Garza JC, Li W, and Lu XY (2013). Melanocortin-4 receptor in the medial amygdala regulates emotional stress-induced anxiety-like behaviour, anorexia and corticosterone secretion. Int. J. Neuropsychopharmacol 16, 105–120. 10.1017/S146114571100174X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu P, Grueter BA, Britt JK, McDaniel L, Huntington PJ, Hodge R, Tran S, Mason BL, Lee C, Vong L, et al. (2013). Double deletion of melanocortin 4 receptors and SAPAP3 corrects compulsive behavior and obesity in mice. Proc. Natl. Acad. Sci. USA 110, 10759–10764. 10.1073/pnas.1308195110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizzi-Wise CA, and Wang DV (2021). Putting together pieces of the lateral septum: multifaceted functions and its neural pathways. eNeuro 8, ENEURO.0315, 21.2021, 8. 10.1523/ENEURO.0315-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anthony TE, Dee N, Bernard A, Lerchner W, Heintz N, and Anderson DJ (2014). Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit. Cell 156, 522–536. 10.1016/j.cell.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terrill SJ, Jackson CM, Greene HE, Lilly N, Maske CB, Vallejo S, and Williams DL (2016). Role of lateral septum glucagon-like peptide 1 receptors in food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol 311, R124–R132. 10.1152/ajpregu.00460.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terrill SJ, Maske CB, and Williams DL (2018). Endogenous GLP-1 in lateral septum contributes to stress-induced hypophagia. Physiol. Behav 192, 17–22. 10.1016/j.physbeh.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terrill SJ, Wall KD, Medina ND, Maske CB, and Williams DL (2018). Lateral septum growth hormone secretagogue receptor affects food intake and motivation for sucrose reinforcement. Am. J. Physiol. Regul. Integr. Comp. Physiol 315, R76–R83. 10.1152/ajpregu.00339.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calderwood MT, Tseng A, and Glenn Stanley B (2020). Lateral septum mu opioid receptors in stimulation of feeding. Brain Res. 1734, 146648. 10.1016/j.brainres.2020.146648. [DOI] [PubMed] [Google Scholar]
- 24.Azevedo EP, Tan B, Pomeranz LE, Ivan V, Fetcho R, Schneeberger M, Doerig KR, Liston C, Friedman JM, and Stern SA (2020). A limbic circuit selectively links active escape to food suppression. Elife 9, e58894. 10.7554/eLife.58894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sweeney P, and Yang Y (2017). Neural circuit mechanisms underlying emotional regulation of homeostatic feeding. Trends Endocrinol. Metabol 28, 437–48. 10.1016/j.tem.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Risold PY, and Swanson LW (1997). Chemoarchitecture of the rat lateral septal nucleus. Brain Res. Brain Res. Rev 24, 91–113. 10.1016/s0165-0173(97)00008-8. [DOI] [PubMed] [Google Scholar]
- 27.Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, et al. (2015). A neural basis for melanocortin-4 receptor-regulated appetite. Nat. Neurosci 18, 863–871. 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li MM, Madara JC, Steger JS, Krashes MJ, Balthasar N, Campbell JN, Resch JM, Conley NJ, Garfield AS, and Lowell BB (2019). The paraventricular hypothalamus regulates satiety and prevents obesity via two genetically distinct circuits. Neuron 102, 653–667.e6. 10.1016/j.neuron.2019.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y, Lu Y, Cassidy RM, Mangieri LR, Zhu C, Huang X, Jiang Z, Justice NJ, Xu Y, Arenkiel BR, and Tong Q (2019). Identification of a neurocircuits underlying regulation of feeding by stress-related emotional responses. Nat. Commun 10, 3446. 10.1038/s41467-019-11399-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Y, Wu Z, Sun H, Zhu Y, Kim ER, Lowell BB, Arenkiel BR, Xu Y, and Tong Q (2013). Glutamate mediates the function of melanocortin receptor 4 on Sim1 neurons in body weight regulation. Cell Metabol. 18, 860–870. 10.1016/j.cmet.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tervo DGR, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Ritola KD, Lindo S, Michael S, Kuleshova E, Ojala D, et al. (2016). A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92, 372–382. 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, et al. (2014). An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507, 238–242. 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweeney P, and Yang Y (2016). An inhibitory septum to lateral hypothalamus circuit that suppresses feeding. J. Neurosci 36, 11185–11195, 2042-16.2016. 10.1523/JNEUROSCI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, and Lowell BB (2012). Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron 73, 511–522. 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, et al. (2011). Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metabol. 14, 313–323. 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu P, He Y, Cao X, Valencia-Torres L, Yan X, Saito K, Wang C, Yang Y, Hinton A Jr., Zhu L, et al. (2017). Activation of serotonin 2C receptors in dopamine neurons inhibits binge-like eating in mice. Biol. Psychiatr 81, 737–747. 10.1016/j.biopsych.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang JC, Drokhlyansky E, Etemad B, Rudolph S, Guo B, Wang S, Ellis EG, Li JZ, and Cepko CL (2016). Detection and manipulation of live antigen-expressing cells using conditionally stable nanobodies. Elife 5, e15312, 5. 10.7554/eLife.15312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burnett CJ, Li C, Webber E, Tsaousidou E, Xue SY, Brüning JC, and Krashes MJ (2016). Hunger-driven motivational state competition. Neuron 92, 187–201. 10.1016/j.neuron.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dietrich MO, Zimmer MR, Bober J, and Horvath TL (2015). Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell 160, 1222–1232. 10.1016/j.cell.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padilla SL, Qiu J, Soden ME, Sanz E, Nestor CC, Barker FD, Quintana A, Zweifel LS, Rønnekleiv OK, Kelly MJ, and Palmiter RD (2016). Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat. Neurosci 19, 734–741. 10.1038/nn.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burnett CJ, Funderburk SC, Navarrete J, Sabol A, Liang-Guallpa J, Desrochers TM, and Krashes MJ (2019). Need-based prioritization of behavior. Elife 8, e44527, 8. 10.7554/eLife.44527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maniscalco JW, Kreisler AD, and Rinaman L (2012). Satiation and stress-induced hypophagia: examining the role of hindbrain neurons expressing prolactin-releasing Peptide or glucagon-like Peptide 1. Front. Neurosci 6, 199. 10.3389/fnins.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Momen NC, Plana-Ripoll O, Yilmaz Z, Thornton LM, McGrath JJ, Bulik CM, and Petersen LV (2022). Comorbidity between eating disorders and psychiatric disorders. Int.J. Eat. Disord 55, 505–517. 10.1002/eat.23687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ulfvebrand S, Birgegård A, Norring C, Högdahl L, and von Hausswolff-Juhlin Y (2015). Psychiatric comorbidity in women and men with eating disorders results from a large clinical database. Psychiatr. Res 230, 294–299. 10.1016/j.psychres.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, and Palmiter RD (2000). A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc. Natl. Acad. Sci. USA 97, 12339–12344. 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vella KR, Ramadoss P, Lam FS, Harris JC, Ye FD, Same PD, O’Neill NF, Maratos-Flier E, and Hollenberg AN (2011). NPY and MC4R signaling regulate thyroid hormone levels during fasting through both central and peripheral pathways. Cell Metabol. 14, 780–790. 10.1016/j.cmet.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheehan TP, Chambers RA, and Russell DS (2004). Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res. Brain Res. Rev 46, 71–117. 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Chen Z, Chen G, Zhong J, Jiang S, Lai S, Xu H, Deng X, Li F, Lu S, Zhou K, et al. (2022). A circuit from lateral septum neurotensin neurons to tuberal nucleus controls hedonic feeding. Mol. Psychiatr 27, 4843–4860. 10.1038/s41380-022-01742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu O, Iwai Y, Narukawa M, Ishikawa AW, Ishii KK, Murata K, Yoshimura Y, Touhara K, Misaka T, Minokoshi Y, and Nakajima KI (2019). Hypothalamic neuronal circuits regulating hunger-induced taste modification. Nat. Commun 10, 4560. 10.1038/s41467-019-12478-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fenno LE, Mattis J, Ramakrishnan C, Hyun M, Lee SY, He M, Tucciarone J, Selimbeyoglu A, Berndt A, Grosenick L, et al. (2014). Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods 11, 763–772. 10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneeberger M, Parolari L, Das Banerjee T, Bhave V, Wang P, Patel B, Topilko T, Wu Z, Choi CHJ, Yu X, et al. (2019). Regulation of energy expenditure by brainstem GABA neurons. Cell 178, 672–685.e12. 10.1016/j.cell.2019.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Y, Lu Y, Xu P, Mangieri LR, Isingrini E, Xu Y, Giros B, and Tong Q (2017). VMAT2-Mediated neurotransmission from midbrain leptin receptor neurons in feeding regulation. eNeuro 4, ENEURO.0083, 17. 2017. 10.1523/ENEURO.0083-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah BP, Vong L, Olson DP, Koda S, Krashes MJ, Ye C, Yang Z, Fuller PM, Elmquist JK, and Lowell BB (2014). MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc. Natl. Acad. Sci. USA 111, 13193–13198. 10.1073/pnas.1407843111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tong Q, Ye CP, Jones JE, Elmquist JK, and Lowell BB (2008). Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci 11, 998–1000. 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ung K, and Arenkiel BR (2012). Fiber-optic implantation for chronic optogenetic stimulation of brain tissue. J. Vis. Exp, e50004. 10.3791/50004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herman AM, Ortiz-Guzman J, Kochukov M, Herman I, Quast KB, Patel JM, Tepe B, Carlson JC, Ung K, Selever J, et al. (2016). A cholinergic basal forebrain feeding circuit modulates appetite suppression. Nature 538, 253–256. 10.1038/nature19789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J, Conde K, Zhang P, Lilascharoen V, Xu Z, Lim BK, Seeley RJ, Zhu JJ, Scott MM, and Pang ZP (2017). Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon-like peptide-1 in the paraventricular hypothalamus. Neuron 96, 897–909.e5. 10.1016/j.neuron.2017.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW, and Zhang LI (2017). AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93, 33–47. 10.1016/j.neuron.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalueff AV, Aldridge JW, LaPorte JL, Murphy DL, and Tuohimaa P (2007). Analyzing grooming microstructure in neurobehavioral experiments. Nat. Protoc 2, 2538–2544. 10.1038/nprot.2007.367. [DOI] [PubMed] [Google Scholar]
- 60.Jiang Z, Rajamanickam S, and Justice NJ (2018). Local corticotropin-releasing factor signaling in the hypothalamic paraventricular nucleus. J. Neurosci 38, 1874–1890. 10.1523/JNEUROSCI.1492-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings of this study are available from the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.