Abstract
Introduction
The purpose of this study was to compare bone mineral density (BMD) and bone turnover markers between combined oral contraceptive (COC) and non-COC users over 12 months.
Materials and methods
COC users (n = 34, age = 19.2 ± 0.5) and non-COC users (n = 28, age = 19.3 ± 0.6) provided serum at baseline, 6 months, and 12 months. C-terminal telopepetides (CTX) and pro-collagen type 1 N-terminal propeptides (P1NP) were determined using ELISA. BMD was measured at the three time points using dual-energy x-ray absorptiometry (DXA).
Results
COC users had greater CTX than non-COC users at baseline (18.6 ± 8.2 vs. 13.8 ± 5.3 ng/mL, P = 0.021) and 6 months (20.4 ± 10.3 vs. 14.2 ± 8.5 ng/mL, P = 0.018). Controlling for lean mass, groups were similar in BMD. Over 12 months, non-COC users maintained BMD at the spine, while the COC users declined 2.2% in lateral spine BMD (0.773 ± 0.014 to 0.756 ± 0.014 g/cm2, P = 0.03) and 0.7% in anterior–posterior spine BMD (1.005 ± 0.015 to 0.998 ± 0.015 g/cm2, P = 0.069). Non-COC users increased in BMD of the whole body over 12 months (P < 0.001) while COC users had no change. Women who began COCs within 4 years after menarche had lower BMD at the hip and whole body. Women taking very low dose COCs (20 mcg ethinyl estradiol, EE) significantly declined in CTX, P1NP, and lateral spine BMD in comparison to participants using low dose COCs (30/35 mcg EE).
Conclusion
College-aged women who did not use COCs increased BMD of the whole body, while COC users had elevated bone turnover, declines in spinal BMD, and lack of bone acquisition of the whole body over 12 months. Young females who initiate COC use early after menarche may experience skeletal detriments.
Keywords: Peak bone mass, Menarche, Premenopausal, CTX, P1NP
Introduction
Worldwide almost 650 million women used oral contraceptives in 2015 [1]. Among sexually active women between the ages of 15–44 years, 79% had “ever used” oral contraceptives while 16% were current users in 2015 [2]. Previous research shows that among American women specifically between the ages of 15 and 19 years, 56% have ever used oral contraceptives [2].
Combined oral contraceptives (COCs) contain ethinyl estradiol (EE) paired with a form of progestin. Estrogen positively affects the formation and proliferation of bone-forming osteoblasts, while simultaneously inhibiting apoptosis of bone-resorbing osteoclasts [3]. However, with the low doses of EE in modern COCs, the beneficial effects of estrogen may not be applicable [4]. The EE of COCs suppresses endogenous production of estradiol; therefore, serum estradiol levels in COC users are lower than in non-COC users [5–7]. Previous research demonstrates associations between serum estradiol, bone turnover markers (BTM), and bone mineral density (BMD) in COC and non-COC users [5, 6]. Further, hormones from COCs may lower insulin-like growth factor 1 (IGF-1), an important hormone in skeletal growth and development [8, 9]. Although the influence of progestogens cannot be completely overlooked [10], in comparison to estradiol, progestin components of COCs have minor effects on bone metabolism [11]. Therefore, COCs may affect bone acquisition in skeletally immature females directly through influence of osteoblast and osteoclast activity while also exerting an indirect influence on bone acquisition through the growth hormone-IGF-1 axis [8].
Osteoporosis is a chronic disease featuring low bone mass and increased risk for fracture affecting over 200 million women worldwide [12]. One method of preventing osteoporosis, is to establish an optimal peak bone mass during young adult years. Peak bone mass (PBM) is achieved when age-related alterations in BMD are no longer positive and have plateaued at a maximal value [13]. Timing of PBM varies between individuals and may depend on a variety of factors such as genetics, race/ethnicity, diet, activity level, and specific bone site [14, 15]. In females, a preponderance of PBM is likely accumulated in the 3–4 years after menarche; a time when lifestyle factors have a profound effect on life-long BMD [13]. Some research states PBM is attained by the age of 17 years [16], yet other studies report gains in bone mass of 5–12% during the second and third decade of life [14, 17], especially at the lumbar spine [18]. Of all the bone sites typically assessed, bone mass at the hip may reach PBM earliest, between the ages of 16 and 19, while bone mass at the spine and whole body continues to accrue and consolidate well into the third decade [14, 18].
Research examining the influence of COC use on bone health is mixed, with some studies showing benefit to skeletal health while others report harmful or no effects. The differing hormone milieu in pre, peri, or post-menopausal women may explain discrepancies in research literature. Investigations with postmenopausal [19] and perimenopausal [20] women have shown that exogenous hormones positively influence bone health. However, investigations of premenopausal women are complicated by inclusion of participants across a wide age range who may or may not have already achieved PBM at some or all sites. Studies which include women who began COC use after likely achieving PBM at all bone sites are positive [21] or neutral [22, 23]. Other research among adult women, early in the fourth decade of life, who had been using COCs for about 5 years, found skeletal benefit for previous but not current COC use [9].
Evidence suggests the skeletal response to COC use may depend on timing of medication use in combination with age of menarche and achievement of PBM [24, 25]. Of the studies that have investigated COC use and bone health in adolescents who have not likely achieved PBM at all sites, several report less bone acquisition in COC users compared to controls in the first 1–2 years of medication use, especially when COCs with 20 mcg of EE or less were investigated [5, 26–28]. A randomized controlled trial of adolescent females reported lower bone accrual at the spine in participants who received COCs with 20 mcg of EE for 12 months, in comparison to a reference group of non-COC users [28]. Assignment to use of a COC with 30 mcg of EE did not significantly influence spinal bone acquisition. However, a 4-year longitudinal study by Pikkarainen et al. [29] also reported blunted gains in bone mineral content at the lumbar spine and femoral neck in adolescent females who used COCs with 30 mcg EE for more than two years. In particular, COCs containing very low doses of EE may be especially detrimental to development of PBM in young females [5, 26, 30].
Attaining an optimal PBM during adolescence and young adult years is an effective way to delay or even prevent the onset of osteoporosis. Failure to reach optimal PBM could lead to an increased risk of skeletal disease [13]. Considering the widespread use of COCs among adolescent girls and young women, further research is needed on their potential influence on PBM development. Therefore, the aim of this study was to compare BTM and BMD according to COC use in college-age women over 12 months. A secondary aim was to examine BMD of females who initiated COC use soon after menarche. Last, among the COC users, we sought to explore changes in BTM and BMD according to the dose of EE.
Materials and methods
Participants
Analysis of oral contraceptive use and bone health was performed as part of a larger study examining lifestyle choices, alcohol consumption, and skeletal health in first and second-year college students [31]. All the procedures performed in this study were in accordance with the ethical standards of the Human Subject’s Institutional Review Board at Loyola Marymount University and with the 1964 Helsinki Declaration and its later amendments. All volunteers provided informed consent for procedures and scientific data use before initiating participation, as is standard at our University.
Volunteers were recruited through the study website, classroom broadcasts, at Greek life meetings, and via announcements on social media. Inclusion criteria required status as a first- or second-year college student, a self-reported body mass index (BMI) between 18.5 and 30, and lack of pregnancy, which was confirmed with a urine sample during each lab visit. In total, 90 women (ages 18–20) met the study criteria, volunteered to participate, and provided informed consent. A health and menstrual history questionnaire was used to collect data on hormonal contraceptive use, menarche, and menstrual regularity. All women were currently healthy and without any medical condition known to affect bone metabolism. Women included in this analysis (n = 62), provided blood samples and underwent bone scans at study entry, 6 months, and 12 months. Women were labeled as COC users (n = 34) at study entry if they reported using a COC medication continuously for the previous 5 months or longer. Women were considered non-COC users (n = 28) if they were not currently using a COC and had not used any form of hormonal contraception in the past year. When questioned about all of the possible “reasons for initiating oral contraceptive use”, 82.4% (n = 28) reported taking the medication specifically for contraception. Additionally, 73.5% (n = 25) reported using COCs to provide menstrual predictability, 50% (n = 17) to control acne, and 29.4% (n = 10) to reduce symptoms of premenstrual syndrome like headaches, cramping, and breast tenderness.
Of the 90 women who enrolled in the study at large, 10 women were not included in this analysis because they used another form of contraception such as the progestogen-only pill (n = 2), intrauterine device (n = 5), implant (n = 1), medroxyprogesterone acetate injection (n = 1), or vaginal ring (n = 1). Five women were removed from the analysis because they had used COCs for 3 months or less. Another 13 women were excluded from consideration because they failed to provide one or more serum samples.
Bone mineral density and body composition
Bone mineral density (g/cm2) of the anterior–posterior (AP) spine, lateral spine, femoral neck, total hip, and whole body were measured at three time points by dual-energy x-ray absorptiometry (DXA; Hologic Delphi A, Waltham, MA, USA). DXA scans took place at study entry, then 6 months and 12 months later. Three trained technicians performed scans after daily calibration via the spine phantom. Our laboratory exhibits a 1.0% test–retest reliability for BMD measurements of the hip and spine. The whole body scan was also used for analysis of body composition including percent body fat and bone-free lean mass. Further discussion of body composition in this paper will describe the bone-free lean mass derived from the whole body DXA scan as lean body mass (LBM).
Bone turnover markers
Participants provided fasting blood samples early in the morning at study entry, then again at 6 months and 12 months. Serum samples were processed and placed in long-term storage at − 80 °C before analyses. Serum levels of procollagen-type I N-terminal propeptide (P1NP, ng/mL), a measure of bone formation, and C-terminal telopeptides (CTX, ng/mL), a marker of bone resorption, were assessed by enzyme-linked immunosorbent assay (ELISA) kits from Cloud Clone Corp (Houston, TX, USA). All assays were run simultaneously after completion of the 12-month data collection using the SpectraMAX190 microplate reader (Molecular Devices, CA, USA). Each sample was run in duplicate demonstrating average inter-assay coefficients of variation (CV) of 5.3% for P1NP and 6.1% for CTX.
Dietary intake and physical activity
Dietary intake of calcium and vitamin D was assessed at study entry using the full-length Block 2014 food frequency questionnaire. Participants completed this web-based questionnaire at their own leisure on their personal computers. The Block questionnaire accounts for seasonal produce consumption and is designed to assess typical intake of nutrients over the previous year [32]. Regular physical activity was assessed using the Aerobics Center Longitudinal Questionnaire, which asks participants to detail duration and intensity of activity, more than 10 min in length, performed at least once per week over the previous three months [33]. Metabolic equivalents (METs) from the compendium of physical activity was used in combination with hours of exercise at various intensities to calculate MET·hours per week of activity [34].
Statistical approach
IBM SPSS Statistics for Windows version 27 (IBM Corp., Armonk, N.Y., USA) was used to analyze data. A repeated measures ANOVA evaluated changes in CTX and P1NP between COC and non-COC users. Differences in BMD between groups and over time were evaluated with a repeated measures ANCOVA controlling for LBM or BMI with application of a Bonferroni correction.
Results
COC and non-COC users were similar in age, height, weight, BMI, LBM, percent body fat, calcium intake, vitamin D consumption, physical activity levels, and years since menarche at study entry (Table 1). Percent body fat among COC users declined significantly over the 12-month study. None of the participants reported current or previous use of tobacco. COC users were taking medications containing 20–35 mcg of EE, combined with various types of progestin, such as norethindrone acetate (n = 10, 29%), norgestimate (n = 10, 29%), drospirenone (n = 6, 18%), levonorgestrel (n = 4, 13%), desogestrel (n = 3, 9%), and ethynodiol diacetate (n = 1, 3%). Most women were taking a 20-mcg dose (n = 16, 47%) of EE, while 32% (n = 11) were taking 35 mcg, and 21% (n = 7) taking 30 mcg. The average length of OC use was 1.9 ± 1.4 years, with a range of 0.4 to 6.1 years. Using date of birth and reported onset of menstruation, the number of years after menarche in which COC use began was calculated to be an average of 4.9 ± 1.5 years, with a range of 1.5 to 8.1 years.
Table 1.
Participant characteristics at study entry and 12 months
| Variable | COC users n = 34 Mean + SD or N and % |
Non-COC users n = 28 Mean + SD or N and % |
Comparisons between groups at study entry | ||
|---|---|---|---|---|---|
| Study Entry | 12 Months | Study entry | 12 months | P | |
|
| |||||
| Age (years) | 19.2 ± 0.5 | 20.2 ± 0.5* | 19.3 ± 0.6 | 20.2 ± 0.6* | 0.455 |
| Height (cm) | 165.5 ± 6.4 | 165.8 ± 6.5 | 163.7 ± 5.7 | 164.0 ± 5.8 | 0.250 |
| Weight (kg) | 64.5 ± 10.0 | 63.7 ± 8.4 | 61.0 ± 9.7 | 61.0 ± 9.7 | 0.170 |
| Body mass index (kg/m2) | 23.5 ± 3.0 | 23.2 ± 2.9 | 22.8 ± 3.5 | 22.7 ± 3.7 | 0.375 |
| Lean body mass (kg) | 42.0 ± 4.6 | 42.5 ± 4.4 | 39.9 ± 4.6 | 40.1 ± 4.5 | 0.079 |
| Percent body fat (%) | 31.6 ± 4.6 | 30.3 ± 4.7* | 31.3 ± 5.2 | 30.7 ± 5.2 | 0.786 |
| Calcium intake (mg/day) | 884.5 ± 315.1 | 796.3 ± 302.8 | 876.6 ± 430.1 | 767.2 ± 293.9 | 0.934 |
| Vitamin D intake (mcg/day) | 13.4 ± 25.4 | 10.6 ± 20.7 | 5.2 ± 3.9 | 5.6 ± 7.0 | 0.096 |
| Physical activity (MET·h/week) | 44.6 ± 42.8 | 43.8 ± 48.3 | 53.3 ± 61.6 | 45.1 ± 36.8 | 0.515 |
| Years since menarche | 6.7 ± 1.3 | 7.7 ± 1.3* | 6.9 ± 1.4 | 7.8 ± 1.3* | 0.531 |
| Years of COC use | 1.91 ± 1.4 | 2.89 ± 1.4* | NA | NA | NA |
No significant differences between groups at study entry
SD standard deviation, N number, % percent, COC combined oral contraceptive, MET metabolic equivalent
Significant change within group from study entry to 12 months, P < 0.05
Participants in this study showed no significant change in body weight or BMI during the 12 months (Table 1). However, COC users had a small, but significant, 1.3% decrease in percent body fat (31.6 ± 0.8% vs. 30.3 ± 0.8%, P = 0.001) while non-COC users displayed no significant change in adiposity. The change in body composition among COC users was driven by a 0.5-kg gain of LBM (42.0 ± 0.8 kg to 42.5 ± 0.8 kg, P = 0.05) in conjunction with a 1.2-kg reduction in fat mass (20.9 ± 1.1 kg vs. 19.7 ± 1.0 kg, P = 0.015).
COC users had greater CTX than non-COC users at study entry (18.6 ± 8.2 ng/mL vs. 13.8 ± 5.3 ng/mL, P = 0.021) and 6 months (20.4 ± 10.3 ng/mL vs. 14.2 ± 8.5 ng/mL, P = 0.018) but group means became more similar at the 12-month measurement (Fig. 1). Non-COC users had a trend for greater P1NP than COC users at study entry (95.7 ± 30.4 ng/mL vs. 82.3 ± 28.3 ng/mL, P = 0.12); however, these differences were not statistically different (Fig. 2).
Fig. 1.
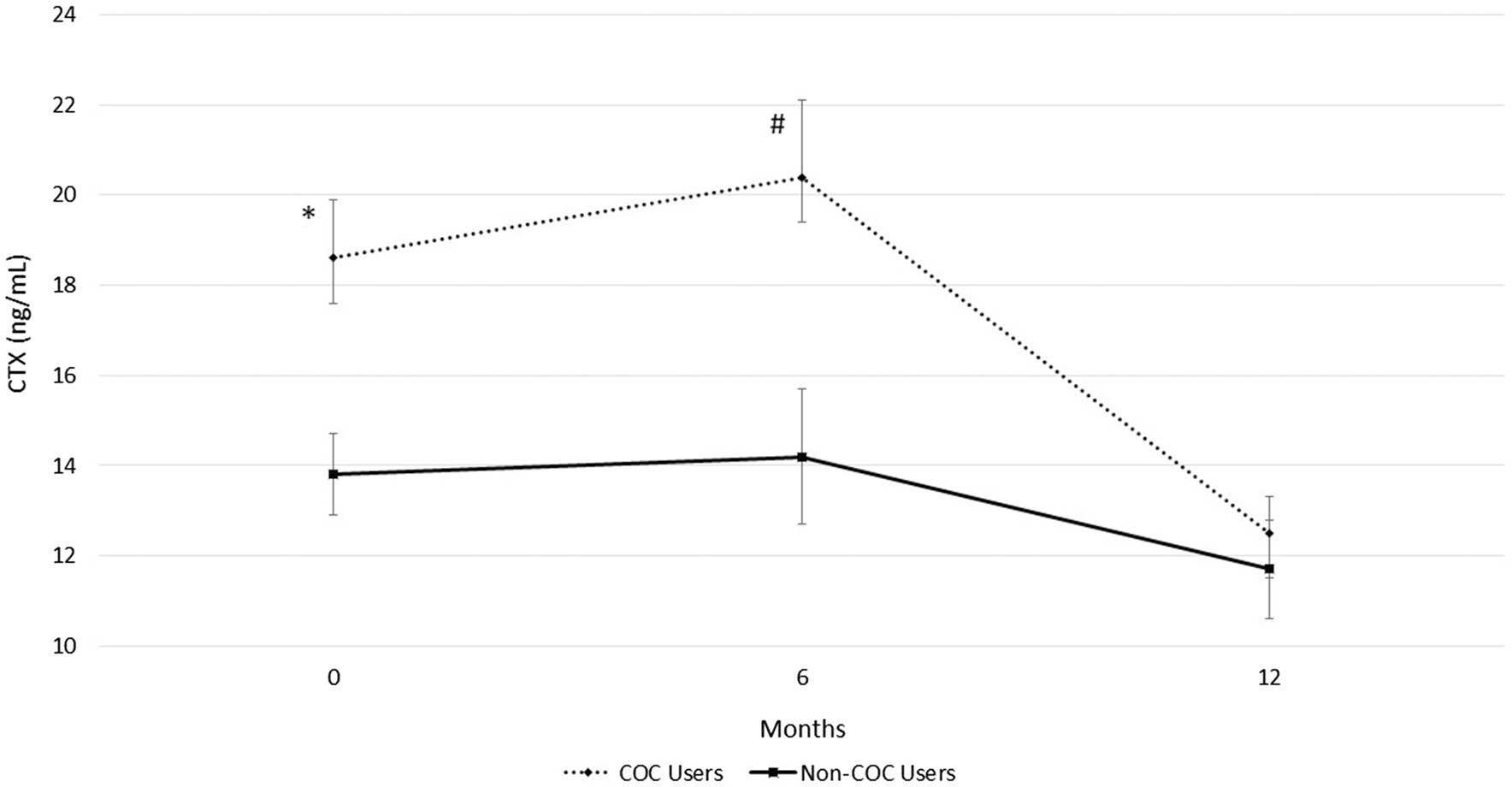
Resorption bone turnover marker in combined oral contraceptive (COC) users and non-COC users. Data are presented for study entry (0 months), 6, and 12 months in young females. CTX is C-terminal telopeptides. COC users have significantly greater CTX at study entry (*P = 0.02) and 6 months (#P = 0.018) compared to non-COC users
Fig. 2.
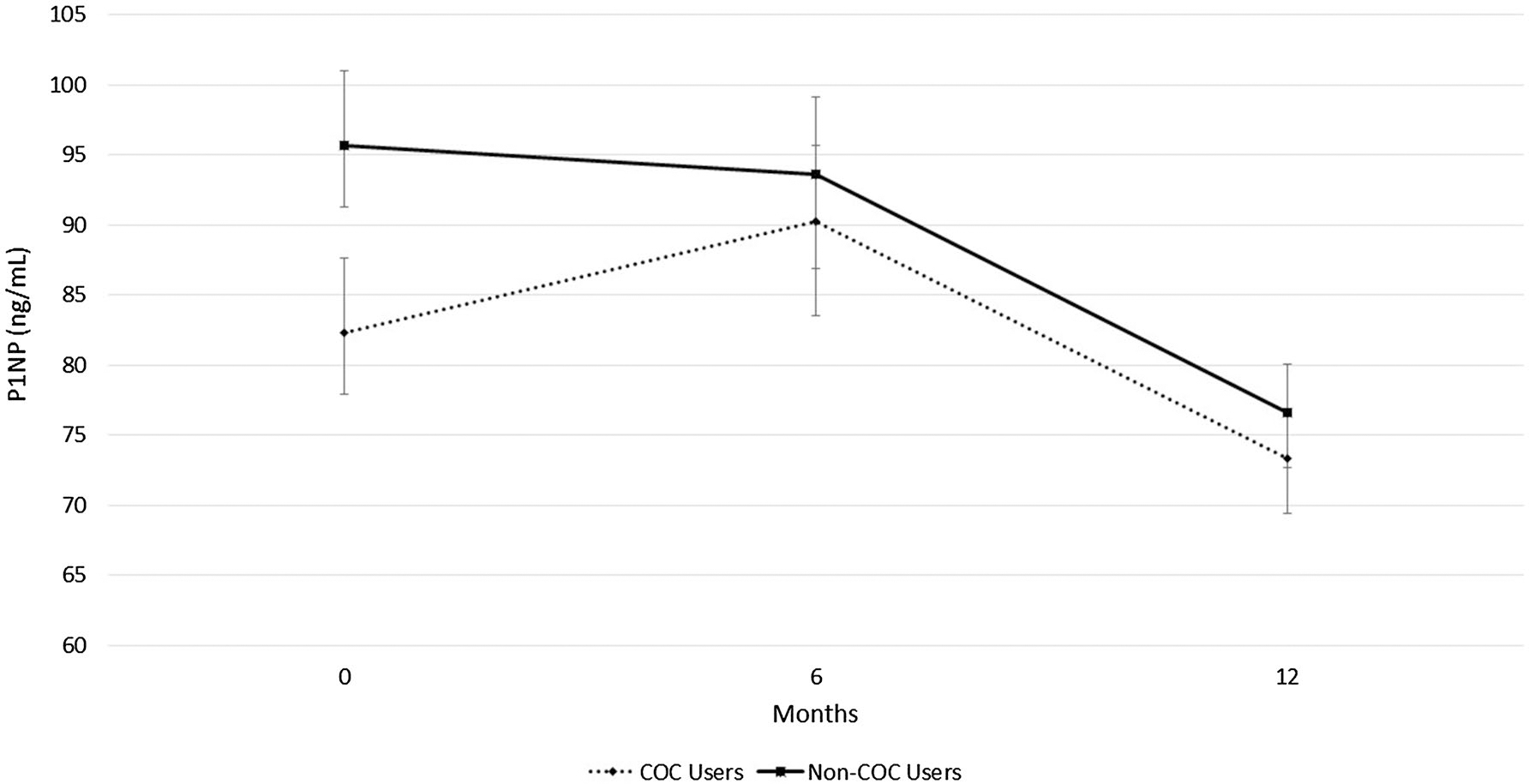
Formation bone turnover marker in combined oral contraceptive (COC) users and non-COC users. Data are presented for study entry (0 months), 6, and 12 months in young females. P1NP is procollagen type 1 N-terminal propeptides
While controlling for LBM, COC users and non-COC users exhibited similar BMD at the hip, spine, or whole body. However, while non-COC users maintained BMD at the spine during the 12-month study, the COC users had a significant decline at the lateral spine (0.772 ± 0.014 g/cm2 to 0.756 ± 0.014 g/cm2, P = 0.005) (Fig. 3). COC-users showed a trend for bone loss at the AP spine (1.006 ± 0.015 g/cm2 to 0.999 ± 0.016 g/cm2, P = 0.06) between 6- and 12-month measurements (Fig. 4). Non-COC users exhibited significant increases in BMD of the whole body during the 12-month study (1.043 ± 0.013 g/cm2 to 1.055 ± 0.012 g/cm2, P < 0.001) while the COC users maintained whole body BMD (1.037 ± 0.011 g/cm2 to 1.041 ± 0.011 g/cm2, P = 0.29) (Fig. 5).
Fig. 3.
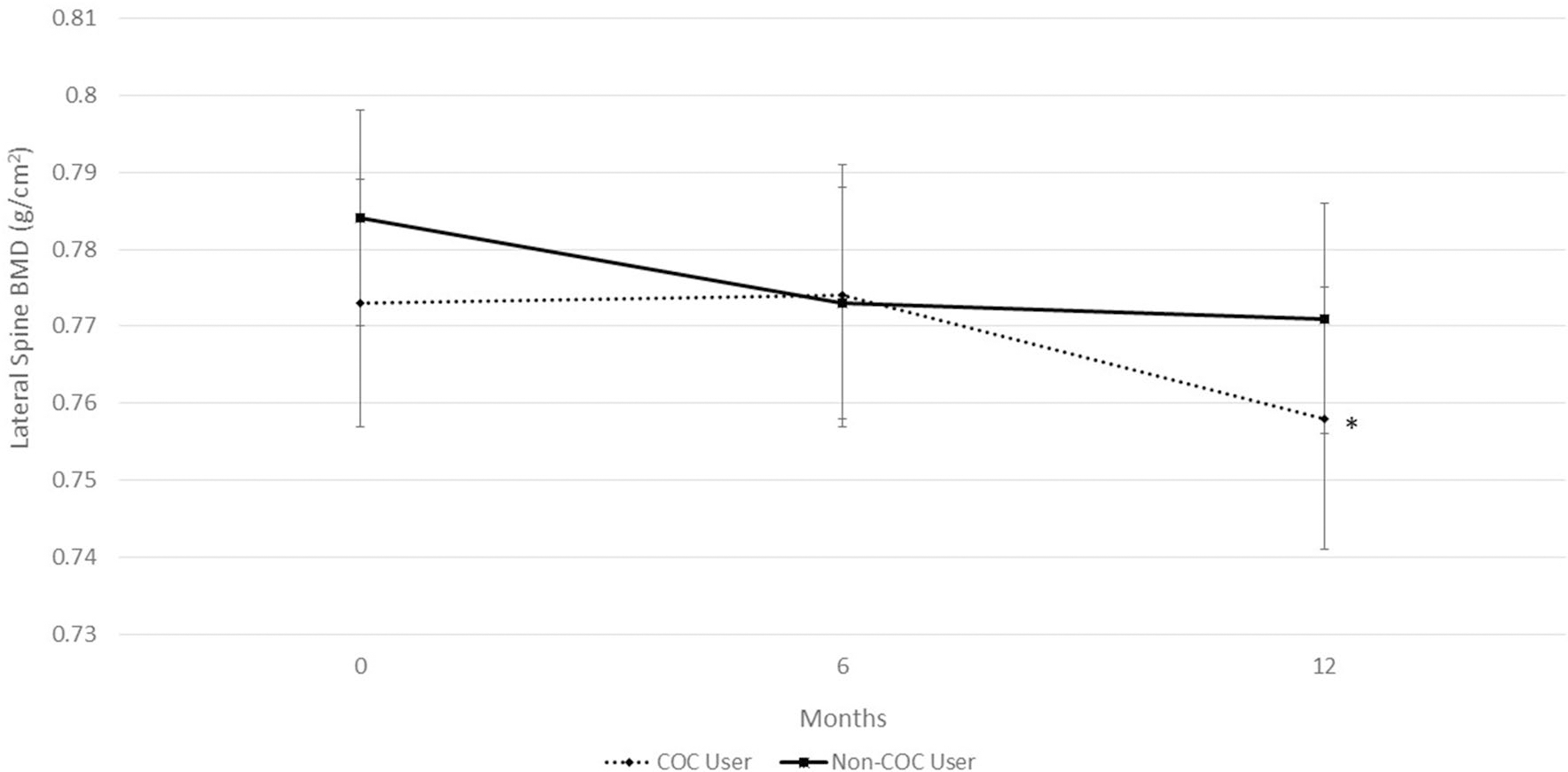
Bone mineral density (BMD) at the lateral spine in combined oral contraceptive (COC) users and non-COC users. Data are presented for study entry (0 months), 6, and 12 months. COC users show a significant decline in BMD from study entry to 12 months, *P = 0.005
Fig. 4.
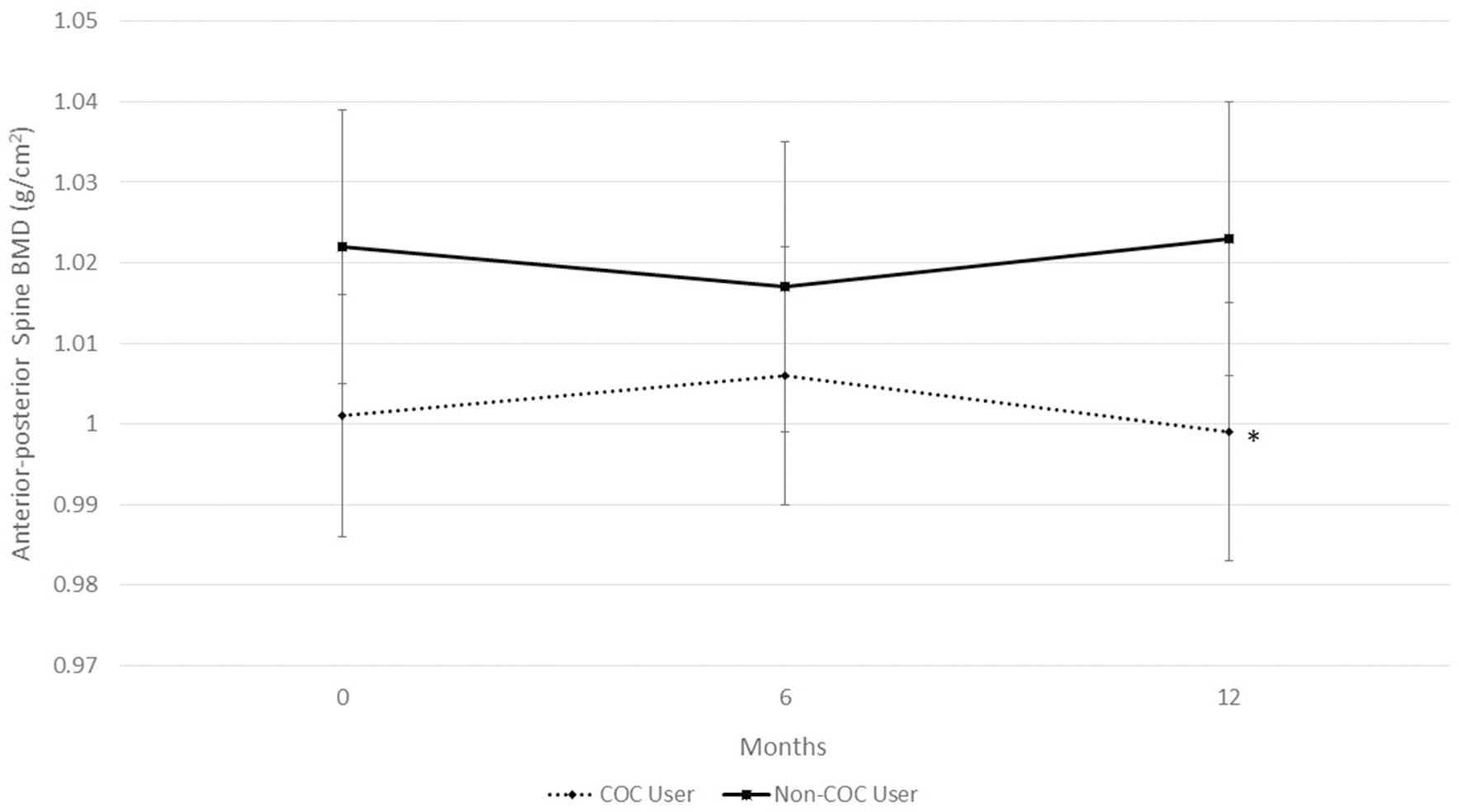
Bone mineral density (BMD) at the anterior–posterior (AP) spine in combined oral contraceptive (COC) users and non-COC users. Data are presented for study entry (0 months), 6, and 12 months. COC users have a declining trend in BMD from 6- to 12-month measurements, *P = 0.069
Fig. 5.
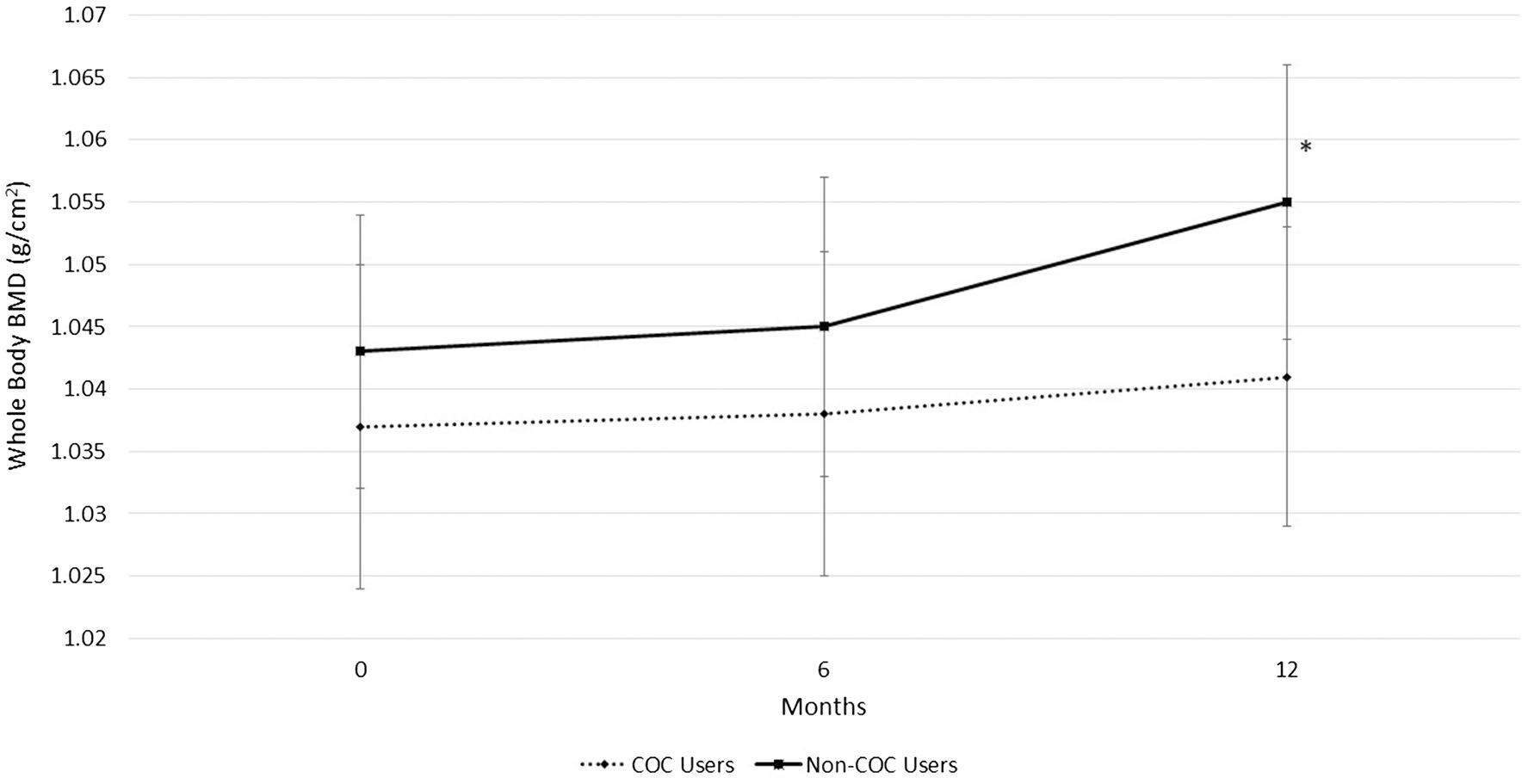
Bone mineral density (BMD) of the whole body in combined oral contraceptive (COC) users and non-COC users. Data are presented for study entry (0 months), 6, and 12 months. Non-COC users show a significant increase in BMD from study entry to 12 months, *P < 0.001
Years of COC use was not related to BTM or BMD in this group. At study entry, CTX (R = 0.24, P = 0.18) and P1NP (R = − 0.04, P = 0.83) were unrelated to years of medication use among COC users. Further, BMD was not correlated to years of COC use at study entry for any of the five bone sites assessed (R-values ranged from − 0.21 to 0.04, P > 0.05, depending on the bone site). To investigate how timing of COC use affects BMD in young females before PBM, a subset analysis was performed comparing BMD at study entry of early COC users, who began medication use within a 4-year window after age of menarche (n = 8), with females who began COC use more than 4 years after menarche (n = 26). This subset analysis revealed that early COC users had significantly lower BMD of the femoral neck, total hip, and whole body at study entry, but similar BMD at the AP and lateral spine to women who began using COCs more than 4 years after menarche (Table 2).
Table 2.
Comparison of bone health at study entry in early COC users and other COC users
| Variables at study entry | Early COC use n = 8 | COC use n = 26 | P |
|---|---|---|---|
|
| |||
| Age (years)a | 19.5 ± 0.7 | 19.2 ± 0.5 | 0.173 |
| Body mass index (kg/m2)a | 24.2 ± 3.8 | 23.2 ± 2.7 | 0.434 |
| Years after menarche began COCa Bone sitesb (g/cm2) | 2.9 ± 0.9 | 5.5 ± 1.0 | < 0.001 |
| Anterior–posterior spine | 0.972 ± 0.029 | 1.020 ± 0.016 | 0.151 |
| Lateral spine | 0.745 ± 0.024 | 0.790 ± 0.013 | 0.105 |
| Femoral neck | 0.835 ± 0.038 | 0.925 ± 0.021 | 0.048 |
| Total hip | 0.934 ± 0.030 | 1.009 ± 0.017 | 0.035 |
| Whole body | 1.007 ± 0.020 | 1.053 ± 0.011 | 0.050 |
P value for MANCOVA controlling for lean body mass at study entry, with Bonferroni correction
COC combined oral contraceptives, Early COC User women who began using COC medication ≤ 4 years menarche, COC Use women who began using COCs > 4 years after menarche
Means with standard deviations
Adjusted means with standard error
To explore changes in BTM and BMD according to the dose of EE, COC users were divided into two groups based on the medication dose. Women (n = 16) who used a very low dose of COCs (20 mcg of EE) were compared with 18 women who used COCs containing a low dose of EE (30 or 35 mcg, Table 3). A repeated-measure ANOVA showed that very low and low-dose COC users were not different in CTX or P1NP at study entry or 12 months. However, women using the 20-mcg dose significantly decreased in CTX (P = 0.04) and P1NP (P = 0.014) over the 12-month study. Because very low dose COC users has significantly lower BMI at study entry, we employed a repeated measures ANCOVA, with BMI as the covariate, to compare BMD values between groups and over time. Very low dose COC users had greater BMD of the AP and lateral spine at study entry. BMD at the femoral neck, total hip, or whole body did not differ between very low and low-dose COC users. However, very low dose users showed a significant decline in BMD of the lateral spine (P = 0.046) over 12 months, while there were no significant changes at other bone sites for either group.
Table 3.
Comparison of bone health in very low (20 mcg EE) and low-dose (30/35 mcg EE) COC users
| Variables | Very low-dose COC users n = 16 |
Low dose COC users n = 18 |
Comparisons between groups at study entry | ||
|---|---|---|---|---|---|
| Study entry | 12 months | Study entry | 12 months | P | |
|
| |||||
| Age (years)a | 19.4 ± 0.7 | 20.4 ± 0.7* | 19.1 ± 0.4 | 20.2 ± 0.4* | 0.131 |
| Body Mass Index (kg/m2)a | 22.2 ± 2.1 | 21.9 ± 2.4 | 24.7 ± 3.7 | 24.2 ± 3.2 | 0.046 |
| Years of COC usea | 1.5 ± 0.9 | 2.4 ± 0.9* | 2.1 ± 1.6 | 3.1 ± 1.6* | 0.199 |
| Years after menarche began COCa | 5.6 ± 1.3 | 6.5 ± 1.2* | 4.2 ± 1.4 | 5.2 ± 1.3* | 0.014 |
| Bone turnover markersa | |||||
| CTX (ng/mL) | 18.0 ± 2.5 | 12.4 ± 1.5* | 16.5 ± 2.3 | 12.9 ± 1.3 | 0.657 |
| P1NP (ng/mL) | 87.2 ± 9.2 | 67.7 ± 7.4* | 80.7 ± 8.5 | 75.8 ± 6.9 | 0.609 |
| Bone sitesb (g/cm2) | |||||
| Anterior–posterior spine | 1.058 ± 0.025 | 1.051 ± 0.023 | 0.987 ± 0.023 | 0.991 ± 0.021 | 0.054 |
| Lateral spine | 0.826 ± 0.019 | 0.799 ± 0.018* | 0.760 ± 0.018 | 0.751 ± 0.017 | 0.023 |
| Femoral neck | 0.903 ± 0.031 | 0.903 ± 0.030 | 0.899 ± 0.029 | 0.899 ± 0.028 | 0.930 |
| Total hip | 1.004 ± 0.028 | 1.005 ± 0.029 | 0.987 ± 0.026 | 0.980 ± 0.026 | 0.669 |
| Whole body | 1.047 ± 0.017 | 1.054 ± 0.017 | 1.034 ± 0.015 | 1.039 ± 0.016 | 0.593 |
P value for MANCOVA controlling for body mass index at study entry, with Bonferroni correction
EE ethinyl estradiol, COC combined oral contraceptives, CTX C-terminal telopepetides, P1NP pro-collagen type 1 N-terminal propeptides
Significant change within group from study entry to 12 months, P < 0.05
Means with standard deviations
Adjusted means with standard error
Discussion
We found higher bone resorption markers in COC users compared to non-COC users at study entry and 6 months later. In addition, we report a trend for lower formation markers in COC users at study entry. CTX is a serum marker of bone resorption reflecting collagen type 1 breakdown, whereas P1NP is a serum measure of osteoblast proliferation [4]. If this persisted, the combination of greater bone resorption and slightly lower bone formation may be detrimental to long-term bone health; however, measurements at 6 and 12 months showed groups to become more similar over time. The significantly greater CTX values in COC users at the first two time points contradict previous research suggesting lower BTM with hormonal contraceptive use [4, 35]. The discrepant findings may be due to differences in age of participants, length of COC use, and type of BTM assessed. Cibula et al. [5] reported a sharp decrease in CTX in the first 9 months of COC use among women of similar age to our population; however, BTM of young women initiating COC use and those with nearly two years of COC use may not be comparable. Researchers who studied other bone resorption markers, like pyridinoline, deoxypyridinoline, and urinary N-terminal cross links, report reductions upon initiating COC use. Studies of other formation markers among COC users showed no changes in osteocalcin [21, 36, 37] or an increase in alkaline phosphatase [38]. De Papp et al. [39] reported 20% lower CTX and 38% lower P1NP after nearly 10 years of COC use when compared to non-COC users; however, their participants were 15–20 years older than ones in this study. Elevated levels of CTX in COC users compared to non-COC users at study entry and 6 months suggests greater bone resorption activity among young women using hormonal contraception. Perhaps a combination of lower IGF-1 levels among adolescent COC users [8], along with the low doses of EE in modern COCs, and their suppression of endogenous hormonal production [4], interfere with expected osteoblast and osteoclast activity. Further research is needed to elucidate the exact mechanism driving changes in BTM with COC use, especially in females who have not yet achieved PBM. The decline in both CTX and P1NP among all women in the study, with or without COC use, agrees with previous research showing that reductions in bone metabolism in late adolescence and early adult years are normal while the skeleton approaches PBM [4, 5, 35].
The 19-year-old women in this study, who were not using COCs, significantly increased BMD of the whole body, which suggests women of this age are still working towards achieving PBM. While their peers accumulated 1.2% more bone mass in the entire skeleton, COC users maintained BMD of the whole body and showed a significant decline in bone mass of nearly 2% at the lateral spine. Although these percent changes may seem small, over many years of COC use during PBM development, differences could become clinically relevant. A 10% lower PBM, achieved while a young adult, results in a 50% increased risk for osteoporotic fracture later in life [40]. One explanation for differences in bone accrual between COC and non-COC users is timing of menarche; however, these groups displayed a similar number of years since menarche. Groups were also not different in variables known to affect PBM development such as calcium intake and physical activity. The between group differences in BMD reported here suggest that COC use, especially with very low doses of EE, which is initiated in the few years after menarche, may limit bone accrual and lead to reduced BMD in college-aged women.
There seems to be a consensus of research among adolescents and young women finding a negative influence of COC use on bone health particularly at the lumbar spine [5, 26, 27]. Perhaps timing of PBM development and the type of bone at the differing skeletal sites explain the negative influence specifically in the lumbar vertebrae. The lumbar spine is composed of primarily trabecular bone, which is more metabolically active than cortical bone and, therefore, more susceptible to influence by hormonal medications [13]. While exact timing of PBM is debated and highly variable between individuals, it is generally accepted that PBM at the spine occurs several years after the hip [14, 17]. Therefore, COC use during a window of time when the spine is still accruing mass and consolidating may impair progress towards a genetic potential.
This study reports 5–10% lower BMD at the femoral neck, total hip, and whole body in young women who began using COCs within 4 years of menarche when compared to women who began medication use more than 4 years after menarche. Recent research has drawn attention to the potentially harmful impact of COC use on skeletal health when initiated early after the onset of menstruation [24, 41]. Hartard et al. found a negative influence on BMD of the spine and hip for young females who initiated use within three years after menarche [41]. Initiating COC use at an earlier age, perhaps pre-PBM at the hip, may explain the lower BMD among early COC users at this site in comparison to the spine.
There have been several reports of poor bone acquisition in adolescents using COCs with very low doses of EE, i.e. ≤ 20 mcg [5, 26–28]. Gersten et al. reported blunted bone acquisition at the spine in adolescents randomly assigned to a COC with 20 mcg EE in comparison to females taking COCs with 30 mcg EE or non-COC users [28]. However, others have reported no annual changes in spinal BMD of females using COCs containing 15 and 20 mcg of EE; however, their participants were slightly older (~ 29 years) when COC use began [23]. In our ancillary analysis of BTM, when COC users were grouped according to EE dose, we found significant reductions in CTX and P1NP in 20 mcg users while others displayed no change. Surprisingly, the very low dose COC users had significantly greater BMD at the AP and lateral spine at study entry; however, they also exhibited a significant 3.3% reduction in bone mass at the lateral spine, while the 30/35 mcg EE users had no significant change in BMD. The small sample size in this ancillary analysis likely explains why our results are not as convincing as previous reports [5, 26, 28].
In parallel to the work of others [42, 43], COC and non-COC users in this study had similar body weight, BMI, and percent body fat. Further, COC users did not change in BMI or body weight across the 12 months. Contrary to common misperceptions that oral contraceptives cause weight gain [44], the low-dose COCs regularly used today do not seem to significantly influence body weight or contribute to adiposity. In fact, COC users, in this study, showed a small 1.3% reduction in body fat over 12 months.
Strengths of this investigation include analysis of BTM in addition to BMD at multiple skeletal sites. Contrary to previous research [9, 21, 23, 29], the population studied here includes women within a narrow age range (ages 18–20, average 19.2 ± 0.6 years), who began using COCs during adolescent years. In fact, 41% of COC users in this study began medication use before the age of 17 years and 24% began COC use within 4 years after menarche, a window of time particularly important for PBM development. Limitations of this study include the small sample size, the heterogeneity in types of COC medications used, and the large range in duration of COC use (0.4–6.1 years). Further research is needed with a larger sample size to explore the impact of specific doses of EE, type of progestin, and duration of COC use on bone health in young women who have not yet achieved PBM at all skeletal sites.
Although not statistically different, vitamin D intake among COC users was higher than consumption among non-COC users. Vitamin D is an important nutrient for bone health and non-COC users displayed healthier bone status, despite lower consumption of the vitamin. This study could be improved by assessing vitamin D levels in the blood. Even though dietary intake of this nutrient was low in this population, it is possible that serum levels were adequate due to production of cholecalciferol during skin exposure to ultraviolet sunlight [45]. This study was conducted in southern California where year-round exposure to sunlight is ample and may diminish the likelihood of bone health problems from low dietary intake of vitamin D.
Many of the women in this study report using COC medications for non-contraceptive benefits (premenstrual syndrome, acne, migraines, etc.). Perhaps medical professionals can avoid negative effects on skeletal health by working with adolescents who do not need contraception to treat acne, headaches, cramping, and breast tenderness without use of COCs. In addition, further research is needed to explore whether performance of weight-bearing exercise is able to counter-act the possible negative effects of COC use on PBM development in adolescents and young women. Importantly, more investigation is required to determine if the small differences in bone accrual between young COC users and non-COC users observed here, and in several other studies, relates to clinically relevant measures such as fracture risk.
Conclusion
The college-aged women in this study who did not use COCs increased BMD of the whole body, while COC users displayed evidence of elevated bone turnover, declines in BMD of the spine, and lack of bone acquisition of the whole body over 12 months. Young females who initiate COC use early after menarche, especially with medication containing very low doses of EE, may experience more pronounced skeletal detriments. Further research is needed on the use of COCs near the age of menarche and their impact on peak bone mass in young women.
Acknowledgements
This research was funded by the National Institute of Alcohol Abuse and Alcoholism at the National Institute of Health with Grant number R21AA022942. Support was also provided by the Frank R. Seaver College of Science and Engineering, the Bellarmine College of Liberal Arts, and the Rains Research Assistance program at Loyola Marymount University. We are grateful for all of the participants who volunteered for the investigation. We acknowledge Sarah Boyle, Andrew Earle, Angie Flores, Nicole Froidevaux, Danielle Good, Carolyn Jackson, Caitlin Jennings, Shantay Pierre, Todd Shoepe, and Liam Shorrock for their valuable contributions to this project. We also appreciate the many people who worked as part of the SELFY research team including Olivia Abdoo, Kate Collins, Kelsey Crispeno, Christa Demos, Zoe Daily, Isabella Kuroyama, Stephanie Lee, Haley Loeffler, Allison Leggett, Diana Martinez, Sydnie Maltz, Grant Mello, Savannah Mersola, Nhandi Scott, Fiona Shorrock, Alejandra Silva, Daniel Smith, and Lauren Sutherlin.
Footnotes
Compliance with ethical standards
Conflict of interest All authors have no conflicts of interest.
Ethical approval All the procedures performed in this study were in accordance with the ethical standards of the Human Subject’s Institutional Review Board at Loyola Marymount University and with the 1964 Helsinki Declaration and its later amendments.
Informed consent All volunteers provided informed consent for procedures and scientific data use before initiating participation, as is standard at our University.
References
- 1.United Nations, Department of Economic and Social Affairs, Population Division (2015) Trends in Contraceptive Use Worldwide 2015 (ST/ESA/SER.A/349)
- 2.Abma JC, Martinez GM (2017) Sexual activity and contraceptive use among teenagers in the United States, 2011–2015. Natl Health Stat Report, pp 1–23 [PubMed] [Google Scholar]
- 3.Manolagas SC (2000) Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137. 10.1210/edrv.21.2.0395 [DOI] [PubMed] [Google Scholar]
- 4.Herrmann M, Seibel MJ (2010) The effects of hormonal contraceptives on bone turnover markers and bone health. Clin Endocrinol (Oxf) 72:571–583. 10.1111/j.1365-2265.2009.03688.x [DOI] [PubMed] [Google Scholar]
- 5.Cibula D, Skrenkova J, Hill M, Stepan JJ (2012) Low-dose estrogen combined oral contraceptives may negatively influence physiological bone mineral density acquisition during adolescence. Eur J Endocrinol 166:1003–1011. 10.1530/EJE-11-1047 [DOI] [PubMed] [Google Scholar]
- 6.Ackerman KE, Singhal V, Baskaran C, Slattery M, Campoverde Reyes KJ, Toth A, Eddy KT, Bouxsein ML, Lee H, Klibanski A, Misra M (2019) Oestrogen replacement improves bone mineral density in oligo-amenorrhoeic athletes: a randomised clinical trial. Br J Sports Med 53:229–236. 10.1136/bjsports-2018-099723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludicke F, Sullivan H, Spona J, Elstein M (2001) Dose finding in a low-dose 21-day combined oral contraceptive containing gestodene. Contraception 64:243–248 [DOI] [PubMed] [Google Scholar]
- 8.Southmayd EA, De Souza MJ (2017) A summary of the influence of exogenous estrogen administration across the lifespan on the GH/IGF-1 axis and implications for bone health. Growth Horm IGF Res 32:2–13. 10.1016/j.ghir.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 9.Elkazaz AY, Salama K (2015) The effect of oral contraceptive different patterns of use on circulating IGF-1 and bone mineral density in healthy premenopausal women. Endocrine 48:272–278. 10.1007/s12020-014-0290-2 [DOI] [PubMed] [Google Scholar]
- 10.Hartard M, Kleinmond C, Luppa P, Zelger O, Egger K, Wiseman M, Weissenbacher ER, Felsenberg D, Erben RG (2006) Comparison of the skeletal effects of the progestogens desogestrel and levonorgestrel in oral contraceptive preparations in young women: controlled, open, partly randomized investigation over 13 cycles. Contraception 74:367–375. 10.1016/j.contraception.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 11.Taraborrelli S (2015) Physiology, production and action of progesterone. Acta Obstet Gynecol Scand 94:8–16. 10.1111/aogs.12771 [DOI] [PubMed] [Google Scholar]
- 12.NIH Consensus Panel (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795 [DOI] [PubMed] [Google Scholar]
- 13.Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O’Karma M, Wallace TC, Zemel BS (2016) The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int 27:1281–1386. 10.1007/s00198-015-3440-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger C, Goltzman D, Langsetmo L, Joseph L, Jackson S, Kreiger N, Tenenhouse A, Davison KS, Josse RG, Prior JC, Hanley DA, CaMos Research G (2010) Peak bone mass from longitudinal data: implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J Bone Miner Res 25:1948–1957. 10.1002/jbmr.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon CM, Zemel BS, Wren TA, Leonard MB, Bachrach LK, Rauch F, Gilsanz V, Rosen CJ, Winer KK (2017) The determinants of peak bone mass. J Pediatr 180:261–269. 10.1016/j.jpeds.2016.09.056 [DOI] [PubMed] [Google Scholar]
- 16.Sabatier JP, Guaydier-Souquieres G, Laroche D, Benmalek A, Fournier L, Guillon-Metz F, Delavenne J, Denis AY (1996) Bone mineral acquisition during adolescence and early adulthood: a study in 574 healthy females 10–24 years of age. Osteoporos Int 6:141–148 [DOI] [PubMed] [Google Scholar]
- 17.Henry YM, Fatayerji D, Eastell R (2004) Attainment of peak bone mass at the lumbar spine, femoral neck and radius in men and women: Relative contributions of bone size and volumetric bone mineral density. Osteoporos Int 15:263–273 [DOI] [PubMed] [Google Scholar]
- 18.Walsh JS, Henry YM, Fatayerji D, Eastell R (2009) Lumbar spine peak bone mass and bone turnover in men and women: a longitudinal study. Osteoporos Int 20:355–362. 10.1007/s00198-008-0672-5 [DOI] [PubMed] [Google Scholar]
- 19.Gambacciani M, Levancini M (2014) Hormone replacement therapy and the prevention of postmenopausal osteoporosis. Prz Menopauzalny 13:213–220. 10.5114/pm.2014.44996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gambacciani M, Cappagli B, Lazzarini V, Ciaponi M, Fruzzetti F, Genazzani AR (2006) Longitudinal evaluation of perimenopausal bone loss: Effects of different low dose oral contraceptive preparations on bone mineral density. Maturitas 54:176–180. 10.1016/j.maturitas.2005.10.007 [DOI] [PubMed] [Google Scholar]
- 21.Nappi C, Di Spiezio SA, Greco E, Tommaselli GA, Giordano E, Guida M (2005) Effects of an oral contraceptive containing drospirenone on bone turnover and bone mineral density. Obstet Gynecol 105:53–60. 10.1097/01.AOG.0000148344.26475.fc [DOI] [PubMed] [Google Scholar]
- 22.Kuohung W, Borgatta L, Stubblefield P (2000) Low-dose oral contraceptives and bone mineral density: an evidence-based analysis. Contraception 61:77–82 [DOI] [PubMed] [Google Scholar]
- 23.Nappi C, Di Spiezio SA, Acunzo G, Bifulco G, Tommaselli GA, Guida M, Di Carlo C (2003) Effects of a low-dose and ultra-low-dose combined oral contraceptive use on bone turnover and bone mineral density in young fertile women: A prospective controlled randomized study. Contraception 67:355–359 [DOI] [PubMed] [Google Scholar]
- 24.Tremollieres F (2013) Impact of oral contraceptive on bone metabolism. Best Pract Res Clin Endocrinol Metab 27:47–53. 10.1016/j.beem.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 25.Almstedt Shoepe H, Snow CM (2005) Oral contraceptive use in young women is associated with lower bone mineral density than that of controls. Osteoporos Int 16:1538–1544 [DOI] [PubMed] [Google Scholar]
- 26.Biason TP, Goldberg TB, Kurokawa CS, Moretto MR, Teixeira AS, Nunes HR (2015) Low-dose combined oral contraceptive use is associated with lower bone mineral content variation in adolescents over a 1-year period. BMC Endocr Disord 15:15. 10.1186/s12902-015-0012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gai L, Jia Y, Zhang M, Gai P, Wang S, Shi H, Yu X, Liu Y (2012) Effect of two kinds of different combined oral contraceptives use on bone mineral density in adolescent women. Contraception 86:332–336. 10.1016/j.contraception.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 28.Gersten J, Hsieh J, Weiss H, Ricciotti NA (2016) Effect of extended 30 ug ethinyl estradiol with continuous low-dose ethinyl estradiol and cyclic 20 ug ethinyl estradiol oral contraception on adolescent bone density: a randomized trial. J Pediatr Adolesc Gynecol 29:635–642. 10.1016/j.jpag.2016.05.012 [DOI] [PubMed] [Google Scholar]
- 29.Pikkarainen E, Lehtonen-Veromaa M, Mottonen T, Kautiainen H, Viikari J (2008) Estrogen-progestin contraceptive use during adolescence prevents bone mass acquisition: a 4-year follow-up study. Contraception 78:226–231. 10.1016/j.contraception.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 30.Scholes D, Ichikawa L, LaCroix AZ, Spangler L, Beasley JM, Reed S, Ott SM (2010) Oral contraceptive use and bone density in adolescent and young adult women. Contraception 81:35–40. 10.1016/j.contraception.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaBrie JW, Boyle S, Earle A, Almstedt HC (2018) Heavy episodic drinking is associated with poorer bone health in adolescent and young adult women. J Stud Alcohol Drugs 79:391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Block G, Subar AF (1992) Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. J Am Diet Assoc 92:969–977 [PubMed] [Google Scholar]
- 33.Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Utter AC, Zmuda JM (1997) A collection of physical activity questionnaires for health-related research. Med Sci Sports Exerc 29:S1–205 [PubMed] [Google Scholar]
- 34.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS (2011) 2011 Compendium of physical cctivities: a second update of codes and MET values. Med Sci Sports Exerc 43:1575–1581. 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 35.Adami S, Bianchi G, Brandi ML, Giannini S, Ortolani S, DiMunno O, Frediani B, Rossini M (2008) Determinants of bone turnover markers in healthy premenopausal women. Calcif Tissue Int 82:341–347. 10.1007/s00223-008-9126-5 [DOI] [PubMed] [Google Scholar]
- 36.Gargano V, Massaro M, Morra I, Formisano C, Di Carlo C, Nappi C (2008) Effects of two low-dose combined oral contraceptives containing drospirenone on bone turnover and bone mineral density in young fertile women: a prospective controlled randomized study. Contraception 78:10–15. 10.1016/j.contraception.2008.01.016 [DOI] [PubMed] [Google Scholar]
- 37.Lattakova M, Borovsky M, Payer J, Killinger Z (2009) Oral contraception usage in relation to bone mineral density and bone turnover in adolescent girls. Eur J Contracept Reprod Health Care 14:207–214. 10.1080/13625180902838828 [DOI] [PubMed] [Google Scholar]
- 38.Endrikat J, Mih E, Dusterberg B, Land K, Gerlinger C, Schmidt W, Felsenberg D (2004) A 3-year double-blind, randomized, controlled study on the influence of two oral contraceptives containing either 20 microg or 30 microg ethinylestradiol in combination with levonorgestrel on bone mineral density. Contraception 69:179–187. 10.1016/j.contraception.2003.10.002 [DOI] [PubMed] [Google Scholar]
- 39.de Papp AE, Bone HG, Caulfield MP, Kagan R, Buinewicz A, Chen E, Rosenberg E, Reitz RE (2007) A cross-sectional study of bone turnover markers in healthy premenopausal women. Bone 40:1222–1230. 10.1016/j.bone.2007.01.008 [DOI] [PubMed] [Google Scholar]
- 40.WHO (1994) Assessment of Fracture Risk and its Application to Screening for Postmenopausal Osteoporosis, vol 843. World Health Organisation Tehnical Report Series, World Health Organisation, Geneva: [PubMed] [Google Scholar]
- 41.Hartard M, Kleinmond C, Wiseman M, Weissenbacher ER, Felsenberg D, Erben RG (2007) Detrimental effect of oral contraceptives on parameters of bone mass and geometry in a cohort of 248 young women. Bone 40:444–450. 10.1016/j.bone.2006.08.001 [DOI] [PubMed] [Google Scholar]
- 42.de Melo NR, Aldrighi JM, Faggion D Jr, Reyes VR, Souza JB, Fernandes CE, Larson E (2004) A prospective open-label study to evaluate the effects of the oral contraceptive Harmonet (gestodene75/EE20) on body fat. Contraception 70:65–71. 10.1016/j.contraception.2003.10.016 [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg M (1998) Weight change with oral contraceptive use and during the menstrual cycle. Results of daily measurements. Contraception 58:345–349 [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg MJ, Waugh MS, Meehan TE (1995) Use and misuse of oral contraceptives: risk indicators for poor pill taking and discontinuation. Contraception 51:283–288 [DOI] [PubMed] [Google Scholar]
- 45.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine S (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930. 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]


