Objectives
The study aim to investigate whether elderly patients with resectable pancreatic ductal adenocarcinoma (PDAC) could benefit from postoperative chemotherapy.
Methods
This study selects the data of PDAC patients who were diagnosed between 2004 and 2014 from the Surveillance, Epidemiology, and End Results program. Median overall survival (mOS) is determined by Kaplan-Meier survival curves. Multivariate logistic regression analysis and hazard ratio are employed to assess the association among potential prognostic factors. Propensity score matching evaluation is used to reduce bias.
Results
In total, there are 11,865 PDAC patients selected from the Surveillance, Epidemiology, and End Results database. Elderly PDAC patients have poor prognoses compared with younger (mOS, 15 vs 21 months). The possible reason might be that the elderly patients are less likely to receive postoperative chemotherapy. After propensity score matching, it is found that, for those who receive postoperative chemotherapy, although the mOS of older group is not as good as that of the younger group (mOS, 20 vs 23 months; 18-month survival rate: 53.4% vs 61.3%), the mOS of older group prolonged by postoperative chemotherapy is similar to that of younger group (9 vs 9 months).
Conclusions
Elderly PDAC patients (≥70 years) might benefit from the currently used postoperative chemotherapy regimens.
Key Words: resectable pancreatic ductal adenocarcinoma, postoperative chemotherapy, SEER, cancer survival
Although surgical techniques and systemic therapies for treating malignant tumors have made remarkable progress during the past decades, pancreatic ductal adenocarcinoma (PDAC) remains one of the most challenging malignant diseases to be overcome in the world.1 Relevant studies have predicted that pancreatic cancer will become the second leading cause of cancer related death in the United States in the next 20 years. Approximately 85% of PDAC patients are in the position of either unresectable or metastatic when being diagnosed, and only 10% of them survived longer than 5 years in the United States. For those who have the opportunity to obtain surgical resection, the prognosis remains poor, with only 20% of the 5-year survival rate.2,3 The poor prognosis of pancreatic cancer is still a major problem that plagues humans.
Compared with younger counterparts, older patients are more likely to suffer from pancreatic cancer.4 The onset is most commonly observed from 60 to 80 years old.5 Because of poor health status or comorbidities, elderly patients with PDAC generally have a worse prognostic survival than younger patients.6 Even for the elderly patients who are in good physical condition and have few complications, factors, such as age and cancer-induced cachexia, might prevent them from receiving more active treatments.6 What is more, whether elderly PDAC patients should receive postoperative chemotherapy has not been specifically mentioned in the latest National Comprehensive Cancer Network guidelines yet.7 Clinicians usually use the Eastern Cooperative Oncology Group as standard to assess patients' physical conditions to determine whether postoperative chemotherapy is needed or not.8–11 Generally speaking, the best postoperative treatment regimen for elderly PDAC patients remains controversial.
The Surveillance, Epidemiology, and End Results (SEER) program is a long-established and publicly-available resource that allows for population-based surveillance and analysis of all cancers in the United States,12 and the statistical data is of large scale and good quality, which reveals the risk model and trend of tumor.13 This study explores whether elderly patients with PDAC could benefit from the currently used postoperative chemotherapy regimens based on SEER.
MATERIALS AND METHODS
Ethics Statement
Patients from the SEER database had previously consented to participate in any scientific research worldwide. This clinical research was approved by the Institutional Review Board of The Second Affiliated Hospital of Zhejiang University School of Medicine.
Patients
The data of patients with PDAC was selected from the SEER (2004–2014) database. All cases in SEER are identified by the topographical code of “pancreas” (International Classification of Disease for Oncology, third edition) using SEER*Stat software (version 8.3.8, NCI, Bethesda, Md). The histology codes 8140/3 and 8500 were used to identify the specific patients with PDAC. Participants were uniformly restaged according to the eighth edition of the American Joint Committee on Cancer Staging Manual. After the following exclusions, a total of 11,856 PDAC patients who have no distal metastasis and underwent surgical resection were collected for further analysis. Follow-up time ranged from 0 to 81 months. The principles for inclusion are as follows: (1) 18 years or older; (2) PDAC as the first malignant tumor with histology diagnosis; (3) without distant metastasis at the time when diagnosed; (4) received surgical resection; (5) specific number of positive regional lymph nodes; (6) specific size of tumor; (7) specific information about whether received chemotherapy or not; (8) died of PDAC or still alive.
Definition of Variables
Demographic characteristics include age, sex, race, insurance status, marital status at diagnosis. Age is treated as an ordinal variable: young (<50, 50–59, 60–69 years) and elderly (70–79, ≥80 years). Race is classified as White, Black, and others (including Asian/Pacific Islander and others). Insurance status is categorized as uninsured, insured, or Medicaid and unknown. Insured or Medicaid patients have lower financial burden, therefore they are able to get timely treatment, which might lead to a better survival.14 Marital status is classified as married, unmarried or widowed. Compared with those who are unmarried, married patients could receive more support from their partners and might have a healthier lifestyle, which could impact the survival.15,16
Tumor characteristics include the position of tumor (located at head, body, or tail of pancreas), differentiation grade and American Joint Committee on Cancer stage. Treatment characteristics include chemotherapy and radiotherapy.
Statistical Analysis
All data are analyzed by IBM (Armonk, NY) SPSS 26.0 software. Frequency of demographic and clinic categorical variables is calculated. An 18-month survival rate and median overall survival (mOS) months are estimated by log-rank tests. Multivariate analysis is performed by means of cox-regression to calculate hazard ratios (HRs) and 95% confidence interval so as to find independent prognostic factors. The oncological outcomes of different ages are analyzed by propensity score matching (PSM) analysis. Here, P < 0.05 is considered statistically significant.
RESULTS
Clinical Features and Univariate Analysis Calculated by Log-Rank Test
In this study, there are 11,856 PDAC and pancreatic adenocarcinoma patients (all received surgical resection) gathered from SEER. After the log-rank tests, it is found that age, sex, insurance status, marital status, tumor position, differentiation grade, stage, chemotherapy, radiotherapy are prognostic factors (Table 1). As for age, elderly patients (≥70 years) have an evidently worse prognosis. Apparent drop of mOS and 18-month survival rate for elderly patients are observed when compared with the younger (mOS: 15–17 vs 20–22 months; 18-month survival rate: 42.9%–46.5% vs 53.4%–57.5%; P < 0.01). Details of other clinical features and results of log-rank tests are also shown in Table 1.
TABLE 1.
Log-Rank Tests for OS of All Nonmetastatic Operated PDAC Patients (n = 11,856)
| Characteristics | No. Patients | mOS, mo | 18-Month Survival Rate, % | P |
|---|---|---|---|---|
| Overall | 11,856 | 19.0 | 51.1 | |
| Age, y | <0.01 | |||
| <50 | 743 | 21.0 | 56.0 | |
| 50–59 | 2280 | 22.0 | 57.5 | |
| 60–69 | 3921 | 20.0 | 53.4 | |
| 70–79 | 3680 | 17.0 | 46.5 | |
| ≥80 | 1232 | 15.0 | 42.9 | |
| Race | 0.22 | |||
| White | 9772 | 19.0 | 51.3 | |
| Black | 1186 | 18.0 | 47.9 | |
| Other | 898 | 20.0 | 53.3 | |
| Sex | <0.01 | |||
| Male | 5955 | 18.0 | 49.8 | |
| Female | 5901 | 20.0 | 52.4 | |
| Insurance status | <0.01 | |||
| Insured or any Medicaid | 9197 | 20.0 | 52.4 | |
| Uninsured | 201 | 20.0 | 54.3 | |
| Unknown | 2458 | 17.0 | 45.9 | |
| Marital status | <0.01 | |||
| Married | 7472 | 20.0 | 52.5 | |
| Divorced/widowed | 4384 | 18.0 | 48.7 | |
| Tumor site | <0.01 | |||
| Head of pancreas | 8901 | 19.0 | 51.0 | |
| Body of pancreas | 788 | 21.0 | 55.2 | |
| Tail of pancreas | 1074 | 19.0 | 50.5 | |
| Other | 1093 | 18.0 | 49.1 | |
| Grade | <0.01 | |||
| Well-differentiated | 1133 | 27.0 | 64.8 | |
| Moderately differentiated | 5889 | 21.0 | 55.2 | |
| Poorly or undifferentiated | 4167 | 15.0 | 40.3 | |
| Unknown | 667 | 24.0 | 59.3 | |
| Stage | <0.01 | |||
| IA | 954 | 38.0 | 73.5 | |
| IB | 2209 | 26.0 | 61.8 | |
| IIA | 784 | 18.0 | 49.7 | |
| IIB | 4678 | 18.0 | 49.3 | |
| III | 3231 | 15.0 | 40.0 | |
| Chemotherapy | <0.01 | |||
| No chemotherapy | 3798 | 11.0 | 35.0 | |
| Yes | 8058 | 22.0 | 58.6 | |
| Radiotherapy | <0.01 | |||
| No radiotherapy | 7442 | 17.0 | 46.4 | |
| Yes, postoperative | 3934 | 22.0 | 58.9 | |
| Yes, preoperative | 480 | 24.0 | 59.9 |
OS, overall survival.
Multivariate OS Cox Regression Analysis
The statistically significant parameters in log-rank tests are further analyzed in the Cox regression. As shown in Table 2, the results of multivariate Cox regression analysis prove that age, marital status, differentiation grade, stage, chemotherapy, radiotherapy are significantly associated with mOS in PDAC patients (P < 0.05) and are independent factors affecting the mOS of PDAC patients. Older PDAC patients have higher HRs than younger patients.
TABLE 2.
Multivariate Analysis for OS of All Patients (n = 11,856)
| Characteristics | HR (95% CI) | P |
|---|---|---|
| Age, y | ||
| <50 | Reference | |
| 50–59 | 0.927 (0.845–1.016) | 0.105 |
| 60–69 | 1.039 (0.953–1.134) | 0.384 |
| 70–79 | 1.190 (1.091–1.299) | <0.001 |
| ≥80 | 1.206 (1.089–1.334) | <0.001 |
| Sex | ||
| Male | Reference | |
| Female | 0.893 (0.858–0.93) | <0.001 |
| Insurance status | ||
| Insured or any Medicaid | Reference | |
| Uninsured | 0.925 (0.784–1.090) | 0.351 |
| Unknown | 1.126 (1.073–1.182) | <0.001 |
| Marital status | ||
| Married | Reference | |
| Divorced/widowed | 1.077 (1.032–1.123) | 0.001 |
| Tumor site | ||
| Head of pancreas | Reference | |
| Body of pancreas | 0.971 (0.894–1.055) | 0.488 |
| Tail of pancreas | 0.984 (0.916–1.056) | 0.647 |
| Other | 0.988 (0.922–1.060) | 0.739 |
| Grade | ||
| Well-differentiated | Reference | |
| Moderately differentiated | 1.291 (1.199–1.390) | <0.001 |
| Poorly or undifferentiated | 1.706 (1.582–1.841) | <0.001 |
| Unknown | 1.288 (1.152–1.439) | <0.001 |
| Stage | ||
| IA | Reference | |
| IB | 1.540 (1.402–1.691) | <0.001 |
| IIA | 1.929 (1.724–2.158) | <0.001 |
| IIB | 2.320 (2.127–2.530) | <0.001 |
| III | 3.058 (2.796–3.345) | <0.001 |
| Chemotherapy | ||
| No chemotherapy | Reference | |
| Yes | 0.585 (0.558–0.614) | <0.001 |
| Radiotherapy | ||
| No radiotherapy | Reference | |
| Yes, postoperative | 0.912 (0.869–0.956) | <0.001 |
| Yes, preoperative | 0.918 (0.825–1.023) | 0.121 |
CI indicates confidence interval.
No Chemotherapy Rates of PDAC Patients Among Different Ages
The PDAC patients are divided into five groups by age and the no chemotherapy rates of different groups are computed. We discover that the no chemotherapy rates continue to rise by the age (from 19.0% to 60.5%). In addition, the 18-month survival rates got worse (from 56.0% to 42.9%). The results are presented in Table 3 and Figure 1. Overall, according to Table 2 and Table 3, the survival of the first 3 age groups (<50, 50–59, 60–69 years) are similar. Thus, in the further analysis, the first 3 age groups are merged into one group (age, <70 years).
TABLE 3.
No Chemotherapy Rates and Postoperative Survival Rates in Different Ages
| Age, y | No Chemotherapy Rate, % | 18-mo Survival Rate, % | mOs, mo |
|---|---|---|---|
| <50 | 19.10 | 56.00 | 21.0 |
| 50–59 | 21.40 | 57.50 | 22.0 |
| 60–69 | 26.00 | 53.40 | 20.0 |
| 70–79 | 38.20 | 46.50 | 17.0 |
| ≥80 | 60.50 | 42.90 | 15.0 |
FIGURE 1.
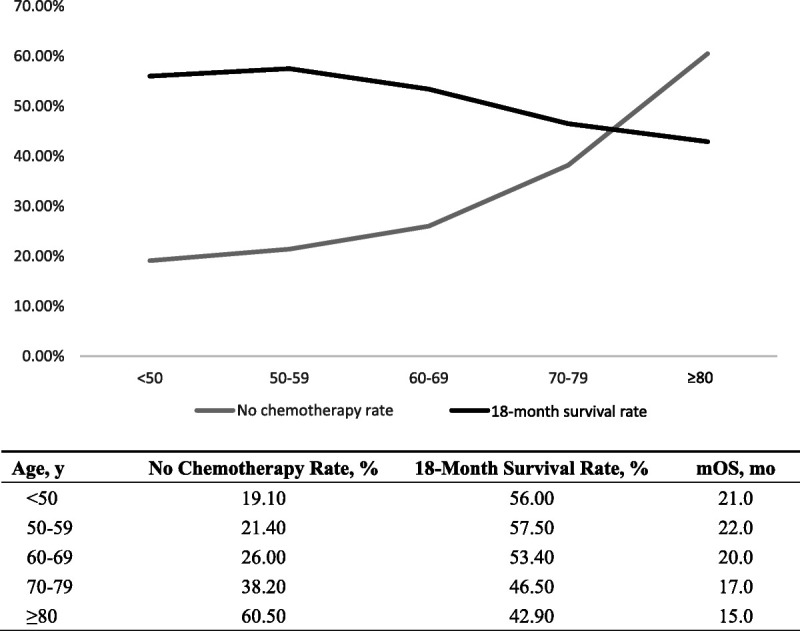
No chemotherapy rates and postoperation survival rates in different ages.
mOS of PDAC Patients Among Different Age Groups Treated With or Without Chemotherapy Before PSM
According to the results in the previous section, a question arises. Do the lower chemotherapy rates among elderly PDAC patients lead to worse overall survival? To solve this question, a subgroup analysis is designed. As shown in Table 4 and Figure 2, PDAC patients are divided into three groups according to their ages (<70, 70–79, ≥80 years). Compared with younger patients, although the elderly patients have worse survival whether they received postoperative chemotherapy or not (chemotherapy: mOS, 23.0, 21.0, 20.0 months, respectively, P < 0.01; no chemotherapy: mOS: 13.0, 10.0, 11.0 months, respectively, P < 0.01), the mOS improved by chemotherapy are almost the same (10.0, 11.0, 9.0 months, respectively). In other words, postoperative chemotherapy can improve the survival for PDAC patients regardless of age differences. The results in Figure 3 also demonstrate this point.
TABLE 4.
mOS and 18-Month Survival Rates of PDAC Patients Treated With or Without Chemotherapy Before PSM
| Age Group | P | |||
|---|---|---|---|---|
| Treatment | <70 y | 70–79 y | ≥80 y | |
| Treated with chemotherapy | <0.01 | |||
| No. patients | 5296 | 2275 | 487 | |
| mOS, mo | 23.0 | 21.0 | 20.0 | |
| 18-mo survival rate, % | 60.50 | 55.40 | 53.40 | |
| No chemotherapy | <0.01 | |||
| No. patients | 1648 | 1405 | 745 | |
| mOS, mo | 13.0 | 10.0 | 11.0 | |
| 18-mo survival rate, % | 37.30 | 31.90 | 36.00 | |
| mOS increased, mo | 10.0 | 11.0 | 9.0 | |
FIGURE 2.
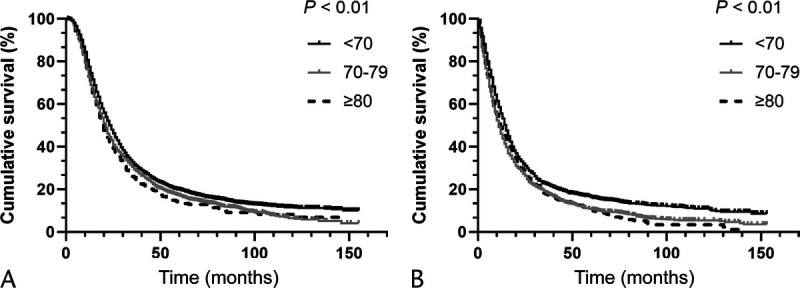
A, OS analysis for PDAC patients who received chemotherapy before PSM. B, OS analysis for PDAC patients who did not receive chemotherapy before PSM.
FIGURE 3.
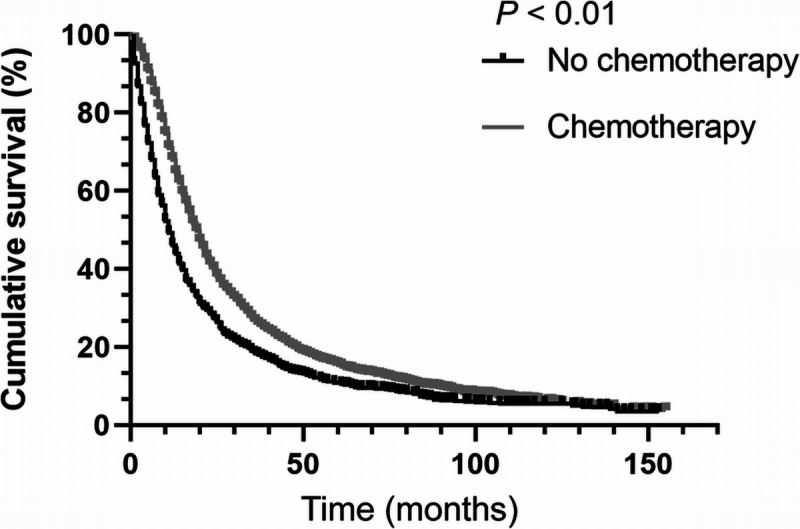
OS Analysis for elderly PDAC patients (≥70 years).
Intergroup Differences of All Independent Prognosis Factors Among Age Groups
The previous analysis has preliminarily shown that older patients could benefit from postoperative chemotherapy, but some other factors still need to be considered. In other words, the intergroup differences of other independent prognosis factors are neglected. Thus, the intergroup differences of all independent prognosis factors among age groups should be calculated (Table 5). It is found that the rates of divorced and widowed have an obvious increase with age (from 34.9% to 47.2%, P < 0.01). Similar results could be observed in the no-radiotherapy rate (from 56.0% to 82.3%, P < 0.01). These intergroup differences may affect the accuracy of the conclusion, so a further PSM analysis is carried out to reduce these biases.
TABLE 5.
Differences in Number of Patients in Different Age Groups Before PSM
| Age Group, n (%) | |||||
|---|---|---|---|---|---|
| Characteristics | <70 y | 70–79 y | ≥80 y | Overall, n (%) | P |
| Marital status | <0.01 | ||||
| Married | 4519 (65.1) | 2302 (62.6) | 651 (52.8) | 7472 (63.0) | |
| Divorced/widowed | 2425 (34.9) | 1378 (37.4) | 581 (47.2) | 4384 (37.0) | |
| Grade | |||||
| Well-differentiated | 644 (9.3) | 360 (9.8) | 129 (10.5) | 1133 (9.6) | |
| Moderately differentiated | 3474 (50.0) | 1819 (49.4) | 596 (48.4) | 5889 (49.7) | |
| Poorly or undifferentiated | 2394 (34.5) | 1310 (35.6) | 463 (37.6) | 4167 (35.1) | |
| Unknown | 432 (6.2) | 191 (5.2) | 44 (3.6) | 667 (5.6) | |
| Stage | |||||
| IA | 557 (8.0) | 283 (7.7) | 114 (9.3) | 954 (8.0) | |
| IB | 1207 (17.4) | 758 (20.6) | 244 (19.8) | 2209 (18.6) | |
| IIA | 451 (6.5) | 244 (6.6) | 89 (7.2) | 784 (6.6) | |
| IIB | 2742 (39.5) | 1426 (38.8) | 510 (41.4) | 4678 (39.5) | |
| III | 1987 (28.6) | 969 (26.3) | 275 (22.3) | 3231 (27.3) | |
| Chemotherapy | |||||
| No chemotherapy | 1648 (23.7) | 1405 (38.2) | 745 (60.5) | 3798 (32.0) | <0.01 |
| Yes | 5296 (76.3) | 2275 (61.8) | 487 (39.5) | 8058 (68.0) | |
| Radiotherapy | |||||
| No radiotherapy | 3886 (56.0) | 2542 (69.1) | 1014 (82.3) | 7442 (62.8) | <0.01 |
| Yes, postoperative | 2705 (39.0) | 1025 (27.9) | 204 (16.6) | 3934 (33.2) | |
| Yes, preoperative | 353 (5.1) | 113 (3.1) | 14 (1.1) | 480 (4.0) | |
mOS of PDAC Patients Among Different Age Groups Treated With or Without Chemotherapy After PSM
The PDAC patients younger than 70 years are set as cohort 1, 70 to 79 years old as cohort 2, and older than 80 years as cohort 3. The PSM evaluation is performed between cohort 1 and cohort 2 (2275 pairs), cohort 1 and cohort 3 (487 pairs), respectively. After PSM, compared with the younger group, the elderly patients aged 70 to 79 years have a worse survival whether they received postoperative chemotherapy or not (chemotherapy: mOS, 21.0 vs 25.0 months; 18 months survival rate: 55.4% vs 63.2%, respectively, P < 0.01 and no chemotherapy: mOS: 10.0 vs 13.0 months; 18-month survival rate: 31.9% vs 37.0%; respectively, P < 0.01). However, the mOS prolonged by chemotherapy are virtually the same (12.0 vs 11.0 months). Similar results could be observed between cohort 1 (age, <70 years) and cohort 3 (age, ≥80 years) (Table 6, Figs. 4 and 5).
TABLE 6.
mOS and 18-Month Survival Rates of PDAC Patients Treated With or Without Chemotherapy After PSM
| Age Group | Age Group | |||||
|---|---|---|---|---|---|---|
| <70 y | 70–79, y | P | <70 y | ≥80 y | P | |
| Chemotherapy | <0.01 | |||||
| No. patients | 2275 | 2275 | 487 | 487 | ||
| mOS, mo | 25 | 21 | <0.01 | 23 | 20 | |
| 18-mo survival rate, % | 63.20 | 55.40 | 61.30 | 53.40 | ||
| No chemotherapy | <0.01 | |||||
| No. patients | 1405 | 1405 | 745 | 745 | ||
| mOS, mo | 13 | 10 | <0.01 | 14 | 11 | |
| 18-month survival rate, % | 37.00 | 31.90 | 40.30 | 36.00 | ||
| mOS increased, mo | 12 | 11 | 9 | 9 | ||
FIGURE 4.
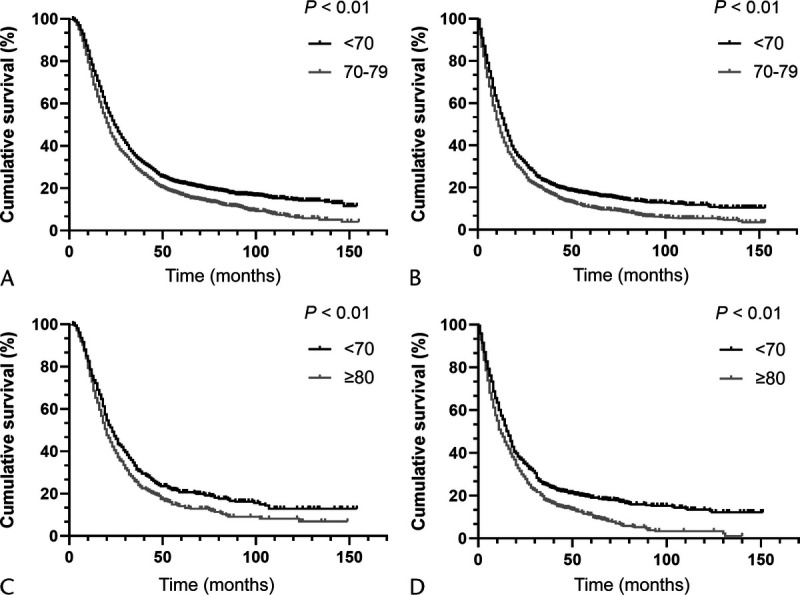
A, OS analysis for PDAC patients (age, <70 vs 70–79 years) who received chemotherapy after PSM. B, OS analysis for PDAC patients (age, <70 vs 70–79 years) who did not receive chemotherapy after PSM. C, OS analysis for PDAC patients (age, <70 vs ≥80 years) who received chemotherapy after PSM. D, OS analysis for PDAC patients (age, <70 vs ≥80 years) who did not receive chemotherapy after PSM.
FIGURE 5.
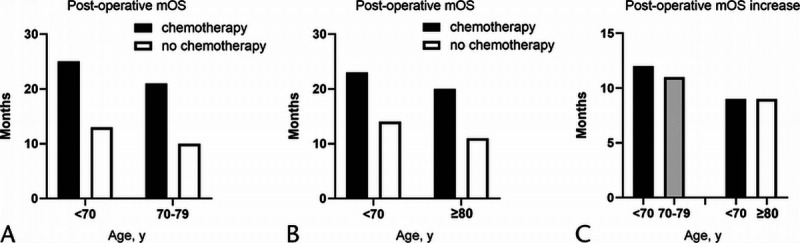
A, Postoperation mOS for PDAC patients (age, <70 vs 70–79 years). B, Postoperation mOS for PDAC patients (age, <70 vs ≥80 years). C, Postoperation mOS increased by chemotherapy (the first and second columns in panel C are from OS analysis after the first PSM, as shown in panel A. The third and fourth columns in panel C are from OS analysis after the second PSM, as shown in panel B).
DISCUSSION
Pancreatic cancer, with a 5-year survival rate of 7.2%, is considered one of the most deadly cancers in China's cancer registry.17 At the time of diagnosis, less than 20% of PDAC patients have the opportunity to receive radical operation. Also, more than 60% of the patients suffer from tumor recurrence after surgery.2,3 Therefore, to prolong postoperative survival, perioperative chemotherapy is of vital importance for PDAC patients. As the National Comprehensive Cancer Network guideline proposes, postoperative chemotherapy is regarded as the main adjuvant treatment modality for PDAC patients.7
Because of its features, such as rapid progression and late detection, pancreatic cancer frequently assaults the elderly.18 Because of the high morbidity of the elderly, the number of elderly PDAC patients will continue to increase with the aging trend of the population. Dr. Smith and his fellows19 concluded that approximately 70% of PDAC patients will have been diagnosed in older adults by 2030. Despite recent advances in PDAC therapy, PDAC is still a challenge for aged patients. The reason might be the lack of information about the safety and efficiency of chemotherapy in recent clinical trials in which few elderly patients participated.6 Clinicians usually use Eastern Cooperative Oncology Group standards to assess a patient's competence to decide whether or not postoperative chemotherapy is needed.8–11 However, it is still not clear whether elderly patients can benefit from it. Therefore, it is difficult to locate a balance between appropriate age and the adjuvant postoperative chemotherapy for elderly PDAC patients.
In this study, it is identified that age, marital status, differentiation grade, stage, chemotherapy, and radiotherapy are independent prognostic factors for PDAC patients. Elderly patients have a conspicuously poor prognosis after surgical resection when compared with the younger patients (mOS, 15.0–17.0 vs 20.0–22.0 months, respectively). Further subgroup analysis reveals that, with age, the rate of divorce has an evident increase (from 34.9% to 47.2%, P < 0.01). Separated patients may receive less support from their families and have unhealthy lifestyles, which may affect their survival. Similar results could be observed in the no chemotherapy rate and the no-radiotherapy rate (from 19.1% to 60.5%, P < 0.01; from 56.0% to 82.3%, P < 0.01; respectively). All of the factors mentioned above can lead to poor prognosis in elderly PDAC patients. Therefore, whether the low rate of postoperative chemotherapy is responsible for the poor prognosis of elderly PDAC patients is still contentious. Hence, a PSM analysis is carried out to reduce the biases of marital status and radiotherapy. After that, a subgroup analysis reveals that elderly patients have relatively poor prognosis, but the prolongation of postoperative survival months in elderly patients who received postoperative chemotherapy is similar to younger patients. This means that older PDAC patients could benefit from postoperative chemotherapy.
The results of some clinical trials also support this conclusion. The CONKO-001 clinical trial confirmed that gemcitabine could improve OS compared with surgery alone for resectable PDAC patients.20 The JASPAC-01 trial showed the superiority of the fluoropyrimidine prodrug S1 over gemcitabine in the Asian population.21 The ESPAC-4 trials indicated that the gemcitabine plus capecitabine may offer more survival benefits when compared with single gemcitabine regimen (median OS, 28 vs 25.5 months).22 In those studies, PDAC patients older than 65 years have similar survival when compared with younger patients, but no detailed information for elderly patients (age, >80 years) was provided.
Although elderly PDAC patients could benefit from postoperative chemotherapy, the low chemotherapy rate leads to poor overall survival. What factors lead to a lower rate of postoperative chemotherapy in elderly PDAC patients? First, aged patients have a higher rate of getting postoperative complications. Chen et al23 showed in their retrospective analysis that elderly patients had more severe postoperative complications after pancreatic resection. They found that aging is an independent risk factor for severe postoperative complications after pancreatic resection. Merkow et al24 revealed that postoperative complications are associated with adjuvant chemotherapy omission and treatment delays. Another study also showed that avoiding postoperative complications after pancreatectomy can contribute to the long-term survival of PDAC patients after pancreatectomy.25 Second, the physical conditions of elderly patients may be relatively poor. Weight loss and changes in body composition are common in PDAC patients, especially in the elderly.26 A Japanese retrospective study demonstrated that low body mass index was associated with an increased risk of death (normal weight: HR, 0.58; P = 0.038; overweight/obese: HR, 0.54; P = 0.059) in Japanese PDAC patients who received surgical resection.27 Another study also demonstrated that aging is a risk factor for increased toxicity and decreased tolerance to chemotherapy because of poor physical conditions.28 Third, because of conservative attitudes, older PDAC patients themselves tend to refuse postoperative chemotherapy. This study indicates that elderly PDAC patients do benefit from postoperative chemotherapy, which makes chemotherapy a reasonable option for elderly PDAC patients.
The highlights of this research are follows. First, 11,856 PDAC participants are selected from SEER between the years of 2004 to 2014. With such a large number of samples, the accuracy and reliability of the results can be guaranteed. Second, the relationship between age and chemotherapy is systematically analyzed. It has been shown that elderly PDAC patients might benefit from postoperative chemotherapy, which provides reference basis for postoperative adjuvant treatments for elderly PDAC patients.
However, there are some inevitable limitations. Although the data from SEER has an overwhelming advantage in quantity, the details of surgical margins, radiation dose, chemotherapy regimens, and chemotherapy sequence are not recorded. In addition, SEER lacks some key clinical information such as carbohydrate antigen 19–9 levels and body mass index, which might be vital for prognostic analysis.29,30 What is more, PSM analysis cannot balance unobservable confounding factors, and therefore residual deviation still exists. Finally, because of the lack of detailed chemotherapy information, the effects of specific chemotherapy regimens on the survival of elderly PDAC patients cannot be analyzed. More prospective studies are required for further exploration and verification in the future.
CONCLUSION
Elderly PDAC patients (≥70 years) could benefit from the currently used postoperative chemotherapy regimens. More prospective studies are required to verify the conclusion of this study.
Footnotes
B.X., J.S., and W.L. contributed equally.
The authors declare no conflict of interest.
This work was supported by the National Natural Science Foundation of China (81772562).
We warrant that the manuscript is original work, has not been published, and is not under consideration for publication elsewhere. All authors have approved the final version of the manuscript being submitted.
Contributor Information
Bin Xu, Email: 2194028@zju.edu.cn.
Jinbo Shi, Email: 3130101948@zju.edu.cn.
Wenjie Lu, Email: luwenjie@zju.edu.cn.
Yulian Wu, Email: yulianwu@zju.edu.cn.
REFERENCES
- 1.Vincent A Herman J Schulick R, et al. Pancreatic cancer. Lancet. 2011;378:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Viale PH. The American Cancer Society's Facts & Figures: 2020 edition. J Adv Pract Oncol. 2020;11:135–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohal DP, Mangu PB, Laheru D. Metastatic pancreatic cancer: american society of clinical oncology clinical practice guideline summary. J Oncol Pract. 2017;13:261–264. [DOI] [PubMed] [Google Scholar]
- 5.Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10:10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macchini M Chiaravalli M Zanon S, et al. Chemotherapy in elderly patients with pancreatic cancer: efficacy, feasibility and future perspectives. Cancer Treat Rev. 2019;72:1–6. [DOI] [PubMed] [Google Scholar]
- 7.Tempero MA. NCCN guidelines updates: pancreatic cancer. J Natl Compr Cancer Netw. 2019;17:603–605. [DOI] [PubMed] [Google Scholar]
- 8.Zukin M Barrios CH Pereira JR, et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol. 2013;31:2849–2853. [DOI] [PubMed] [Google Scholar]
- 9.Sandler A Gray R Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Rahman O. ECOG performance score 0 versus 1: impact on efficacy and safety of first-line 5-FU-based chemotherapy among patients with metastatic colorectal cancer included in five randomized trials. Int J Color Dis. 2019;34:2143–2150. [DOI] [PubMed] [Google Scholar]
- 11.Singh RR, O'Reilly EM. New treatment strategies for metastatic pancreatic ductal adenocarcinoma. Drugs. 2020;80:647–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doll KM, Rademaker A, Sosa JA. Practical guide to surgical data sets: Surveillance, Epidemiology, and End Results (SEER) database. JAMA Surg. 2018;153:588–589. [DOI] [PubMed] [Google Scholar]
- 13.Gordon-Dseagu VL Devesa SS Goggins M, et al. Pancreatic cancer incidence trends: evidence from the Surveillance, Epidemiology and End Results (SEER) population-based data. Int J Epidemiol. 2018;47:427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole AP Lu C Krimphove MJ, et al. Comparing the association between insurance and mortality in ovarian, pancreatic, lung, colorectal, prostate, and breast cancers. J Natl Compr Cancer Netw. 2019;17:1049–1058. [DOI] [PubMed] [Google Scholar]
- 15.Wang XD Qian JJ Bai DS, et al. Marital status independently predicts pancreatic cancer survival in patients treated with surgical resection: an analysis of the SEER database. Oncotarget. 2016;7:24880–24887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reyngold M Winter KA Regine WF, et al. Marital status and overall survival in patients with resectable pancreatic cancer: results of an ancillary analysis of NRG oncology/RTOG 9704. Oncologist. 2020;25:e477–e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei W Zeng H Zheng R, et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. 2020;21:e342–e349. [DOI] [PubMed] [Google Scholar]
- 18.Lee SY, Sissoko M, Hartshorn KL. Update on the management of pancreatic cancer in older adults. Curr Oncol Rep. 2016;18:60. [DOI] [PubMed] [Google Scholar]
- 19.Smith BD Smith GL Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. [DOI] [PubMed] [Google Scholar]
- 20.Oettle H Neuhaus P Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. [DOI] [PubMed] [Google Scholar]
- 21.Uesaka K Boku N Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248–257. [DOI] [PubMed] [Google Scholar]
- 22.Neoptolemos JP Palmer DH Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. [DOI] [PubMed] [Google Scholar]
- 23.Chen YT Ma FH Wang CF, et al. Elderly patients had more severe postoperative complications after pancreatic resection: a retrospective analysis of 727 patients. World J Gastroenterol. 2018;24:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merkow RP Bilimoria KY Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260:372–377. [DOI] [PubMed] [Google Scholar]
- 25.Kasahara N Noda H Kakizawa N, et al. A lack of postoperative complications after pancreatectomy contributes to the long-term survival of patients with pancreatic cancer. Pancreatology. 2019;19:686–694. [DOI] [PubMed] [Google Scholar]
- 26.Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9(suppl 2):S51–S63. [DOI] [PubMed] [Google Scholar]
- 27.Okura T Fujii M Shiode J, et al. Impact of body mass index on survival of pancreatic cancer patients in Japan. Acta Med Okayama. 2018;72:129–135. [DOI] [PubMed] [Google Scholar]
- 28.Wedding U Honecker F Bokemeyer C, et al. Tolerance to chemotherapy in elderly patients with cancer. Cancer Control. 2007;14:44–56. [DOI] [PubMed] [Google Scholar]
- 29.Nishida K Kaneko T Yoneda M, et al. Doubling time of serum CA 19-9 in the clinical course of patients with pancreatic cancer and its significant association with prognosis. J Surg Oncol. 1999;71:140–146. [DOI] [PubMed] [Google Scholar]
- 30.Santucci N Facy O Ortega-Deballon P, et al. CA 19-9 predicts resectability of pancreatic cancer even in jaundiced patients. Pancreatology. 2018;18:666–670. [DOI] [PubMed] [Google Scholar]


