Abstract
Background and Aims:
The type of fat consumed in animal-based western diets, typically rich in the saturated fat palmitate, has been implicated in cardiometabolic disease risk. In contrast, the most abundant mono- and polyunsaturated fats, more typical in a vegetarian or plant-based diet, potentiate less deleterious effects. This study determined differences in plasma and urine metabolites when switching from omnivorous to vegetarian diet, including metabolites involved in fatty acid utilization.
Methods and Results:
A prospective cohort of 38 European (EA) and African American (AA) omnivorous females were matched by age (25.7±5.3y) and BMI (22.4±1.9kg/m2). Pre-intervention samples were collected while subjects consumed habitual animal-based diet. Changes in metabolites were assessed by ultra-high-performance liquid chromatography-tandem mass spectroscopy (Metabolon, Inc.) upon completing four days of novel vegetarian diet provided by the Vanderbilt Metabolic Kitchen. Changes in several diet-derived metabolites were observed, including increases in compounds derived from soy food metabolism along with decreases in metabolites of xanthine and histidine. Significant changes occurred in metabolites of saturated, monounsaturated and polyunsaturated fatty acids along with significant differences between EA and AA women in changes in plasma concentrations of acylcarnitines, which reflect the completeness of fatty acid oxidation (versus storage).
Conclusion:
These data suggest improvements in fatty acid metabolism (oxidation vs storage), a key factor in energy homeostasis, may be promoted rapidly by adoption of a vegetarian (plant-based) diet. Mechanistic differences in response to diet interventions must be understood to effectively provide protection against the widespread development of obesity and cardiometabolic disease in population subgroups, such as AA women.
INTRODUCTION
In the United States and globally, obesity has become a public health epidemic across all population subgroups. Yet, the propensity to develop obesity, and its associated cardiometabolic diseases, differs by sex and race. African American women are at highest risk, despite having less visceral fat and less atherogenic lipid profiles compared to European Americans (1). The type of fat consumed in animal-based “western” diets, typically rich in the saturated fatty acid (SFA) palmitate, has been implicated in increased risk for obesity and cardiometabolic disease. This is likely due to the different biological and physiological functions of fats based on their carbon chain length and saturation status. Replacing SFA with monounsaturated (MUFA) and polyunsaturated (PUFA) fats, which may be more available in a typical vegetarian or plant-based diet, can be cardiometabolic protective (2). Indeed, replacement of SFAs with PUFAs is associated with a 10% reduced risk for cardiovascular disease (CVD) (3). Further, increasing consumption of the most widely available MUFA, oleic acid, promotes reduced fat mass and improved blood pressure (4). Moreover, consuming a vegetarian diet has been effective in decreasing risk for high body mass (BMI), type 2 diabetes (T2DM), coronary heart disease, and all-cause mortality (5–11).
The effects of improving dietary intake on cardiometabolic disease risk by modulating the type of fat consumed may be mediated through alterations in the levels of plasma and urine metabolites involved in fatty acid oxidation, storage as triglyceride, or incorporation into plasma phospholipid and lipoprotein particles. Global metabolic profiling (metabolomics) is a novel “meet-in the-middle” analytical approach that identifies intermediary molecules of metabolism including those associated with macronutrient metabolism and energy homeostasis. Thus, metabolomics can provide mechanistic insights regarding the link between changes in dietary intake exposure and biomarkers of cardiometabolic disease (12). Prior metabolomics work has already identified metabolites linked to onset of prediabetes, T2DM and CVD, such as the branch chained amino acids and the aromatic amino acids, as well as metabolites involved in fatty acid oxidation including α-hydroxybutyric acid, ketones, and other lipids. Further, metabolomics is a reliable tool to discriminate profiles associated with omnivore, vegetarian, or vegan diet (13–15). When compared to omnivores, habitual consumption of a vegetarian diet is associated with lower concentrations of glycerophospholipids and sphingolipids, which may promote reduced CVD risk in vegetarians via alterations in lipoprotein synthesis (16). Moreover, habitual vegetarians show significantly different concentrations of branched chain amino acids and medium-chain acylcarnitines, which may affect the availability of fuel and efficiency of energy metabolism (17).
In the present study, we used global metabolomics profiling to assess the acute effects of switching from animal-based to plant-based diet on plasma and urine metabolites in European American and African American women. The central hypothesis was that there would be significant alterations in plasma and urine metabolites associated with several metabolic pathways in response to new exposure to a vegetarian diet. Considering, that our prior work showed a significant difference by race in the circulating lipid and lipoprotein response to changes in the type of fat consumed (18) and that we also observed a difference by race in the interaction between cannabinoid receptor 1 (CNR1) gene haplotypes and HDL-cholesterol response to dietary fat (19), we further hypothesized that there would be differences in the response by race, particularly regarding metabolites involved in fatty acid utilization.
MATERIALS AND METHODS
Subjects
European American (EA) and African American (AA) women were recruited from the metropolitan Nashville community by posting of Institutional Review Board (IRB) approved study flyers at college campuses, public libraries, community parks, and community agency offices. Potential subjects were excluded if they did not meet age (18–40 years) or BMI criteria (18.5–24.9 kg/m2), were not weight stable during the three months prior to enrollment, had a history of bariatric surgery or took medications for weight loss, had food allergies or dietary restrictions, or were currently consuming herbal or dietary supplements (amino acids, omega-3 fatty acids, vitamins, minerals) other than a daily multivitamin. Exclusion criteria also included any prior or current smoking, vaping, drug or alcohol abuse, medications for chronic disease management, current illness, infection or inflammatory state, or if women were pregnant or lactating. At enrollment, subjects confirmed they were born in the U.S. and self-identified race by confirming that both parents originated from the same racial group. Genetic ancestry was verified from DNA analysis performed at the Vanderbilt Technologies for Advanced Genomics (VANTAGE) Core lab. The study was approved by the Vanderbilt University IRB (#171170), registered at ClinicalTrials.gov (ID: NCT03314194), and all subjects provided written informed consent prior to participation.
Study Design
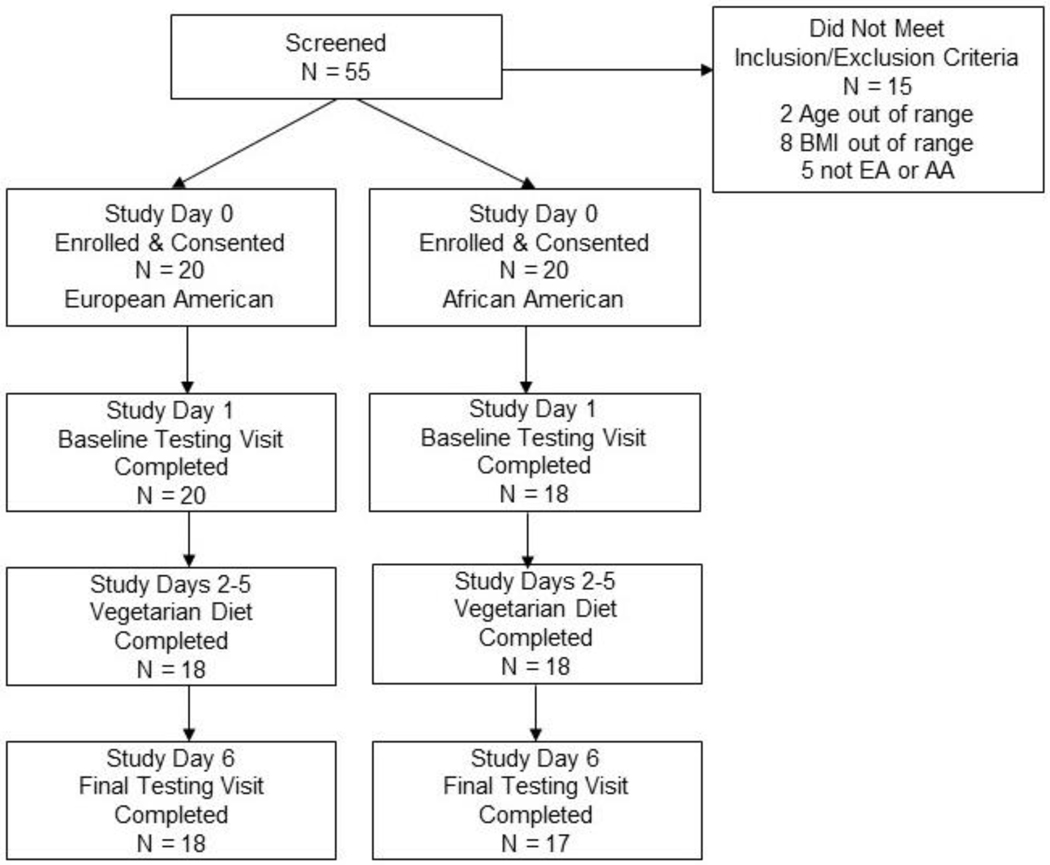
The study was designed to evaluate acute effects of novel exposure to consuming a vegetarian diet under eucaloric conditions. Upon enrollment and signing of consent (study day 1), EA and AA women were matched by age, BMI, physical activity level and energy requirement. Each subject then served as their own control to compare pre- versus post-intervention metabolic outcomes (Figure 1). To control for seasonal variation in food availability and habitual dietary intake, enrollment occurred in two phases with the first 20 women recruited between October 2017 through February 2018 and the next group of women between November 2018 to February 2019.
Figure 1:
Flow Diagram of Recruitment and Retention of EA and AA Women
Diet Intervention
Menus for study days 2–5 were designed to be a major shift from habitual omnivorous intakes by eliminating all meat, fish, poultry, eggs, and dairy products. Vegetarian menus encompassing four days of 3 meals and 2 snacks per day were developed using Nutrition Data System for Research (NDS-R) 2017 software (Nutrition Coordinating Center, Minn., MN) by research dietitians at the Vanderbilt Diet, Body Composition and Human Metabolism Core (Supplemental Table 1). Menus were designed to have an average daily macronutrient composition of 35% fat, 50% carbohydrate and 15% protein. Menus were adjusted to be eucaloric for weight maintenance of each subject based on individual energy (kcal) goal calculated using the Harris-Benedict equation multiplied by an activity factor derived from results on the International Physical Activity Questionnaire (IPAQ) questionnaire (20). Caloric distribution over each day was 19% of kcal at breakfast, 29.5% at lunch, 39% at dinner, and 12.5% from snacks. All meals and snacks on the daily menus were pre-weighed, prepared and packaged for subject carry-out at the Vanderbilt Metabolic Kitchen. Compliance with the vegetarian diet was assessed by having subjects complete a checklist for each menu day indicating the amount consumed of each food item. In addition, 24-hour diet recall interviews were conducted each morning when subjects came to the Vanderbilt Center for Human Nutrition for physical assessments (study days 2–5).
Diet, Anthropometric and Questionnaire Assessments
Habitual dietary intakes were assessed on study days 0 and 1 using a semi-quantitative food frequency questionnaire and 24-hour diet recall interviews (21). All recalls were performed using the validated U.S. Department of Agriculture five-step multi-pass methodology, a standardized questionnaire, and computer-generated prompts from NDS-R version 2017 (22, 23). Direct entry into NDS-R allowed dietitians to identify foods and beverages consumed by name, brand and preparation method from a database of 18,000 items. Subjects indicated the portion sizes of all foods and beverages consumed during the 24-hour periods using standard measuring utensils (plates, cups, bowls and spoons of various sizes). Anthropometric measures were acquired each morning of the study period (days 1–6) with subjects in the fasted state and after voiding. Height (±0.1cm), weight (±0.1kg), and waist and hip circumferences (±0.1cm) were measured in triplicate using standardized procedures. Questionnaires included the IPAQ on study day 1 and a gastrointestinal symptom questionnaire administered on study days 1–6 to assess for changes in bowel habits from consuming the vegetarian diet. As part of the consent process, subjects agreed to refrain from alcohol consumption and vigorous physical activity during the diet intervention.
Specimen Collection and Analysis
Whole blood (5 mL EDTA tube) and midstream urine samples were collected in the early mornings of study days 1 and 6 with subjects in a 10-hour fasted state. A urine β-hCG test confirmed non-pregnancy. Blood samples were immediately placed on ice and centrifuged (3,000g for 15 minutes at 4°C). Plasma and buffy coat aliquots were stored at −80°C. Urine samples were centrifuged (2,000g for 10 minutes at 4°C) to remove possible cells or other solids and aliquots of supernatant were stored at −80°C. Concentration of plasma C-reactive protein (CRP) was measured by ELISA (Quantikine CRP Immunoassay, R&D Systems, Minneapolis, MN). Plasma and urine samples were batch mailed to Metabolon, Inc. (Morrisville, NC) for global metabolomics profiling pre- and post-intervention. Following receipt, each sample was inventoried, assigned a unique identifier in the Metabolon LIMS system, and stored at −80°C until processing. Proteins were precipitated with methanol under vigorous shaking for 2 minutes (Glen Mills GenoGrinder 2000) followed by centrifugation and removal of organic solvent (TurboVap®, Zymark). Extracts were divided into fractions for analysis: two for analysis by two separate reverse phase (RP) / ultra-high-performance liquid chromatography-tandem mass spectroscopy (UPLC-MS/MS) methods with positive ion mode electrospray ionization (ESI), one for analysis by RP/UPLC-MS/MS with negative ion mode ESI, and one for analysis by HILIC/UPLC-MS/MS with negative ion mode ESI. Sample extracts were stored overnight under nitrogen prior to preparation for analysis. Raw data were extracted, peak-identified, and quality control processed using Metabolon’s hardware and software. All methods utilized a Waters ACQUITY ultra-performance liquid chromatography and a Thermo Scientific Q-Extractive high-resolution/accurate mass spectrometer interfaced with a heated electrospray ionization source and Orbitrap mass analyzer operated at 35,000 mass resolution. Compounds were identified by comparison to Metabolon’s library of purified standards or recurrent unknown biochemicals. Peaks were quantified using area-under-the-curve. Biochemical identifications are based on three criteria: retention index (RI) within a narrow RI window of the proposed identification, accurate mass match to the library +/− 10 ppm, and the MS/MS forward and reverse scores between the experimental data and authenticated standards. The MS/MS scores are based on a comparison of the ions present in the experimental spectrum to the ions present in the library spectrum. For each metabolite passing thresholds, the raw peak intensity was rescaled to set the median across all samples equal to 1 to establish a normal distribution and values below the limit of detection were imputed with the lowest observed value in the dataset.
Statistical Analysis
Analysis for energy and nutrient composition of habitual and vegetarian dietary intakes was determined based on the NDS-R intake properties output file which provides the average daily intake for 178 nutrients, nutrient ratios/indices, and other food components. Secondly, the NDS-R serving count totals output file, which incorporates 168 food and beverage subgroups, and the component/ingredient output file were used to determine the amounts of animal-based and plant-based items that provided sources of fats and oils. Demographic, anthropometric and diet variables were summarized using means with standard deviations. Using raw metabolite data log transformed to have a normal distribution, paired t-tests were used to compare dietary and metabolic changes within groups and independent t-tests to compare changes between EA and AA women. Multivariable linear regression modeling was used to determine diet by race interactions. To account for false positives with the large number of plasma and urine metabolic compounds being analyzed, we estimated the false discovery rate using the Benjamin-Hochberg test with a q-value cutoff for significance of <0.05 indicating high confidence (24). Principle component analysis provided visualization of plasma and urine samples to determine whether samples segregated by type of diet exposure, thereby, reflecting metabolic differences pre- and post-vegetarian diet (Supplemental Figure). Statistical analyses were performed using SPSS software version 26 (IBM, Armonk, N.Y) and R: a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria URL https://www.R-project.org/), with a P-value for significance set at 0.05.
RESULTS
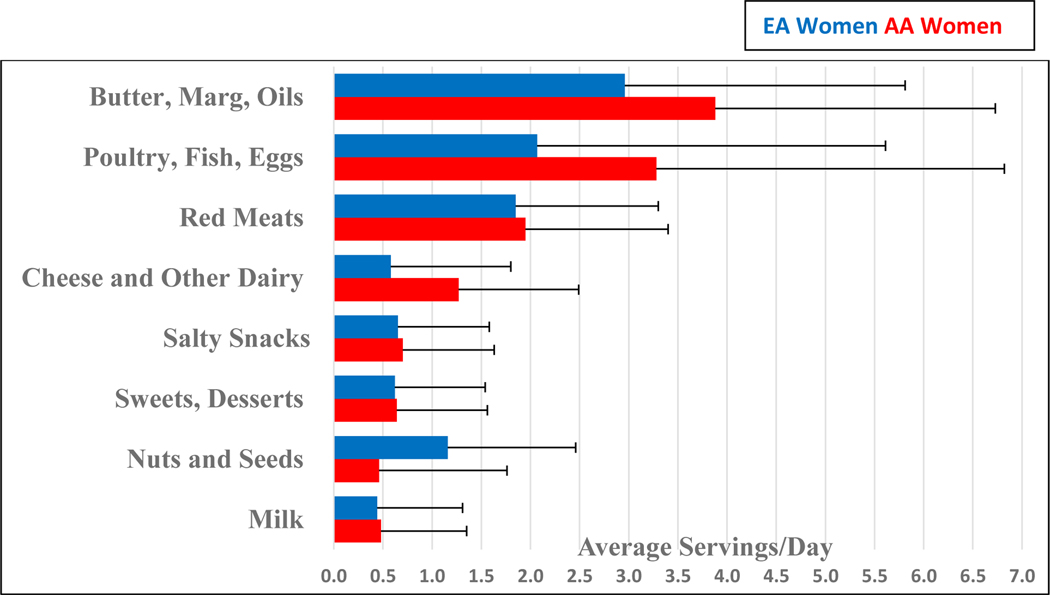
Of the 38 women, 20 (52.6%) were of European ancestry (EA) and 18 (47.4%) were of African ancestry (AA). Consistent with the rigorous matching of subjects, there were no significant differences by study enrollment phase (2017/18 vs 2018/19) for age, height, weight, BMI, habitual physical activity level, or habitual energy intake and macronutrient distribution. Likewise, there were no significant differences between EA and AA women for age, height, weight, BMI or habitual physical activity level (Table 1). Baseline diet recalls of habitual omnivorous intakes showed no significant differences for energy or macronutrient composition (percentage of energy from protein, carbohydrate or fat) (Table 2). There were also no differences at baseline in the animal or plant-based food items being consumed that provided sources of fats and oils (Figure 2). A difference at baseline between groups was detected for AA women consuming sugary sweet foods more frequently (P = 0.04) and EA women consuming water >32 ounces/day more frequently (P = 0.01). No differences were detected between groups in the frequency of experiencing gastrointestinal symptoms. PCA analysis showed segregation of the plasma and urine samples by habitual omnivorous vs vegetarian diet (Supplemental Figure).
Table 1.
Baseline Characteristics of Women by Race
| European American | African American | ||
|---|---|---|---|
| N = 20 | N = 18 | P Value | |
| Age (y) | 26.90 ± 6.18 | 24.30 ± 3.67 | 0.19 |
| Height (m) | 166.15 ± 6.29 | 167.51 ± 6.37 | 0.53 |
| Weight (kg) | 59.85 ± 6.28 | 64.56 ± 6.56 | 0.25 |
| BMI (kg/m2) | 21.68 ± 1.95 | 22.91 ± 1.56 | 0.48 |
| Energy Goal (kcal)* | 1904.76 ± 249.95 | 1929.42 ± 172.35 | 0.73 |
| Activity Factor* | 3.02 ± 0.89 | 2.59 ± 1.10 | 0.44 |
| C-Reactive Protein (mg/dl) | 1.44 ± 1.37 | 1.35 ± 1.64 | 0.70 |
Harris-Benedict equation (655 + [9.563 x weight in kg] + [1.850 x height in cm] − [4.676 x age in years]) x Activity factor (1.0 sedentary; 2.0 low active; 3.0 active; 4.0 very active) (46).
Table 2.
Comparison of Changes in Dietary Nutrient Intakes from Habitual Omnivorous Diet to Vegetarian Diet in European American and African American Women
| European American | African American | ||||||
|---|---|---|---|---|---|---|---|
| Habitual Animal-Based Diet | Study Vegetarian Diet | P value within group | Habitual Animal-Based Diet | Study Vegetarian Diet | P value within group | P value for change between groups | |
| Energy (kcal) | 1917.08 ± 402.06 | 1874.34 ± 236.61 | 0.39 | 1933.41 ± 547.44 | 1901.79 ± 163.61 | 0.73 | 0.40 |
| Protein (% kcal) | 18.44 ± 6.68 | 11.45 ± 0.76 | <0.001 | 17.59 ± 3.09 | 11.35 ± 0.65 | <0.001 | 0.64 |
| Protein (g) | 84.14 ± 27.64 | 48.09 ± 9.73 | <0.001 | 86.04 ± 29.79 | 46.64 ± 10.94 | <0.001 | 0.36 |
| Animal protein (g) | 53.85 ± 29.94 | 3.97 ± 0.86 | <0.001 | 58.56 ± 27.94 | 3.94 ± 0.52 | <0.001 | 0.33 |
| Vegetable protein (g) | 31.86 ±11.05 | 42.64 ± 9.19 | 0.002 | 26.83 ± 8.36 | 41.19 ± 10.67 | 0.002 | 0.61 |
| Tryptophan (g) | 0.88 ± 0.35 | 0.49 ± 0.11 | <0.001 | 1.01 ± 0.37 | 0.47 ± 0.11 | <0.001 | 0.19 |
| Threonine (g) | 2.85 ± 1.25 | 1.53 ± 0.34 | <0.001 | 3.35 ± 1.27 | 1.46 ± 0.33 | <0.001 | 0.17 |
| Isoleucine (g) | 3.28 ± 1.40 | 1.74 ± 0.38 | <0.001 | 3.86 ± 1.41 | 1.65 ± 0.37 | <0.001 | 0.15 |
| Leucine (g) | 5.71 ± 2.29 | 3.16 ± 0.69 | <0.001 | 6.61 ± 2.38 | 3.00 ± 0.67 | <0.001 | 0.17 |
| Lysine (g) | 4.91 ± 2.59 | 1.99 ± 0.46 | <0.001 | 5.86 ± 2.62 | 1.90 ± 0.40 | <0.001 | 0.22 |
| Methionine (g) | 1.61 ± 0.79 | 0.65 ± 0.14 | <0.001 | 1.96 ± 0.79 | 0.63 ± 0.13 | <0.001 | 0.15 |
| Cystine (g) | 0.95 ± 0.38 | 0.65 ± 0.14 | 0.002 | 1.09 ± 0.38 | 0.61 ± 0.12 | <0.001 | 0.15 |
| Phenylalanine (g) | 3.29 ± 1.15 | 2.13 ± 0.47 | <0.001 | 3.69 ± 1.22 | 2.00 ± 0.45 | <0.001 | 0.18 |
| Tyrosine (g) | 2.49 ± 1.00 | 1.29 ± 0.29 | <0.001 | 2.91 ± 1.02 | 1.23 ± 0.27 | <0.001 | 0.17 |
| Valine (g) | 3.71 ± 1.49 | 2.09 ± 0.45 | <0.001 | 4.28 ± 1.49 | 1.99 ± 0.43 | <0.001 | 0.18 |
| Arginine (g) | 4.32 ± 1.75 | 3.21 ± 0.82 | 0.006 | 4.71 ± 2.09 | 3.00 ± 0.89 | 0.02 | 0.37 |
| Histidine (g) | 2.10 ± 0.92 | 1.06 ± 0.24 | <0.001 | 2.35 ± 0.95 | 1.00 ± 0.24 | <0.001 | 0.32 |
| Alanine (g) | 3.52 ± 1.58 | 1.92 ± 0.43 | <0.001 | 4.16 ± 1.72 | 1.80 ± 0.42 | <0.001 | 0.17 |
| Aspartic Acid (g) | 6.78 ± 2.73 | 4.48 ± 1.07 | 0.001 | 7.55 ± 3.16 | 4.16 ± 1.04 | 0.002 | 0.24 |
| Glutamic Acid (g) | 14.50 ± 4.87 | 10.35 ± 2.13 | 0.002 | 16.09 ± 4.64 | 9.80 ± 2.21 | 0.001 | 0.17 |
| Glycine (g) | 3.13 ± 1.29 | 1.89 ± 0.44 | <0.001 | 3.62 ± 1.42 | 1.74 ± 0.46 | 0.001 | 0.17 |
| Proline (g) | 4.66 ± 1.39 | 2.89 ± 0.56 | <0.001 | 5.23 ± 1.31 | 2.83 ± 0.53 | <0.001 | 0.13 |
| Serine (g) | 3.35 ± 1.17 | 2.20 ± 0.48 | <0.001 | 3.70 ± 1.24 | 2.10 ± 0.46 | <0.001 | 0.25 |
| Carbohydrate (% kcal) | 45.47 ± 11.26 | 53.51 ± 3.88 | 0.005 | 44.53 ± 8.76 | 54.83 ± 4.81 | 0.002 | 0.49 |
| Carbohydrate (g) | 200.25 ± 58.95 | 202.54 ± 38.33 | 0.71 | 213.15 ± 45.16 | 203.92 ± 34.93 | 0.53 | 0.48 |
| Starch (g) | 93.82 ± 41.37 | 95.78 ± 17.44 | 0.44 | 109.64 ± 36.72 | 95.27 ± 14.97 | 0.22 | 0.15 |
| Sucrose (g) | 27.96 ± 15.58 | 29.84 ± 8.76 | 0.77 | 25.29 ± 13.43 | 30.44 ± 7.18 | 0.09 | 0.44 |
| Fructose (g) | 13.09 ± 9.78 | 15.32 ± 4.56 | 0.42 | 15.33 ± 8.75 | 16.76 ± 3.56 | 0.53 | 0.91 |
| Galactose (g) | 0.30 ± 0.46 | 0.48 ± 0.10 | 0.12 | 0.27 ± 0.51 | 0.51 ± 0.07 | 0.07 | 0.70 |
| Glucose (g) | 13.21 ± 10.58 | 15.86 ± 5.06 | 0.35 | 15.58 ± 8.29 | 16.71 ± 4.00 | 0.80 | 0.64 |
| Lactose (g) | 7.85 ± 9.30 | 3.16 ± 0.74 | 0.03 | 8.99 ± 11.92 | 3.27 ± 0.29 | 0.07 | 0.81 |
| Maltose (g) | 2.70 ± 3.15 | 1.23 ± 0.49 | 0.08 | 3.45 ± 3.97 | 1.25 ± 0.47 | 0.04 | 0.41 |
| Fiber (g) | 24.46 ± 7.64 | 24.58 ± 5.29 | 0.99 | 19.69 ± 8.08 | 23.45 ± 5.45 | 0.28 | 0.35 |
| Soluble Fiber (g) | 7.15 ± 2.78 | 5.96 ± 1.31 | 0.13 | 5.38 ± 2.31 | 5.89 ± 1.23 | 0.63 | 0.16 |
| Insoluble Fiber (g) | 17.34 ± 6.62 | 18.53 ± 4.06 | 0.54 | 14.25 ± 6.66 | 17.55 ± 4.29 | 0.22 | 0.49 |
| Pectins (g) | 3.00 ± 1.37 | 3.40 ± 0.86 | 0.19 | 1.94 ± 1.42 | 3.59 ± 0.84 | 0.006 | 0.07 |
| Fat (% kcal) | 34.86 ± 7.86 | 35.39 ± 3.88 | 0.69 | 36.39 ± 8.25 | 34.12 ± 4.86 | 0.37 | 0.33 |
| Fat (g) | 69.59 ± 27.46 | 62.49 ± 17.38 | 0.46 | 72.39 ± 41.11 | 59.91 ± 20.99 | 0.11 | 0.22 |
| Saturated Fatty Acids (g) | 20.34 ± 8.38 | 10.21 ± 2.79 | <0.001 | 26.02 ± 10.59 | 10.31 ± 2.88 | <0.001 | 0.08 |
| Monounsaturated Fatty Acids (g) | 25.05 ± 11.61 | 30.35 ± 9.54 | 0.09 | 27.07 ± 15.97 | 28.56 ± 11.89 | 0.89 | 0.47 |
| Polyunsaturated Fatty Acids (g) | 17.89 ± 9.25 | 17.72 ± 4.44 | 0.84 | 22.35 ± 15.10 | 17.10 ± 5.48 | 0.25 | 0.22 |
| SFA 4:0 (butyric acid) (g) | 0.38 ± 0.30 | 0.13 ± 0.06 | 0.003 | 0.54 ± 0.30 | 0.14 ± 0.03 | 0.001 | 0.14 |
| SFA 6:0 (caproic acid) (g) | 0.23 ± 0.18 | 0.09 ± 0.04 | 0.004 | 0.34 ± 0.22 | 0.09 ± 0.02 | <0.001 | 0.10 |
| SFA 14:0 (myristic acid) (g) | 1.61 ± 0.96 | 0.43 ± 0.17 | <0.001 | 2.22 ± 1.13 | 0.45 ± 0.09 | <0.001 | 0.07 |
| SFA 16:0 (palmitic acid) (g) | 10.75 ± 4.56 | 6.38 ± 1.65 | 0.001 | 14.34 ± 6.32 | 6.35 ± 1.85 | 0.001 | 0.05 |
| SFA 18:0 (stearic acid) (g) | 4.65 ± 2.59 | 2.12 ± 0.65 | 0.001 | 6.11 ± 3.02 | 2.19 ± 0.71 | <0.001 | 0.10 |
| MUFA 14:1 (myristoleic acid) (g) | 0.08 ± 0.06 | 0.02 ± 0.01 | 0.001 | 0.15 ± 0.07 | 0.02 ± 0.01 | <0.001 | 0.002 |
| MUFA 16:1 (palmitoleic acid) (g) | 0.88 ± 0.61 | 0.27 ± 0.06 | <0.001 | 1.24 ± 0.89 | 0.25 ± 0.06 | 0.001 | 0.10 |
| MUFA 18:1 (oleic acid) (g) | 23.56 ± 11.24 | 29.46 ± 9.49 | 0.06 | 25.21 ± 15.01 | 27.59 ± 11.73 | 0.76 | 0.49 |
| PUFA 18:2 (linoleic acid) (g) | 15.59 ± 8.69 | 16.08 ± 4.29 | 0.62 | 19.49 ± 13.48 | 15.25 ± 5.14 | 0.30 | 0.22 |
| PUFA 18:3 (linolenic acid) (g) | 1.63 ± 0.87 | 1.13 ± 0.26 | 0.05 | 2.35 ± 1.43 | 1.15 ± 0.25 | 0.007 | 0.06 |
| PUFA 18:3 n-3 (ALA) (g) | 1.59 ± 0.85 | 1.11 ± 0.26 | 0.05 | 2.31 ± 1.39 | 1.12 ± 0.25 | 0.006 | 0.05 |
| PUFA 20:4 (arachidonic acid) (g) | 0.19 ± 0.48 | 0.02 ± 0.00 | 0.10 | 0.15 ± 0.11 | 0.02 ± 0.01 | 0.001 | 0.60 |
| 16:0/16:1 (ratio) | 0.08 ± 0.04 | 0.04 ± 0.01 | 0.001 | 0.08 ± 0.02 | 0.04 ± 0.00 | <0.001 | 0.76 |
| 18:0/18:1 (ratio) | 13.97 ± 1.99 | 6.04 ± 2.95 | <0.001 | 12.33 ± 2.57 | 4.20 ± 1.12 | <0.001 | 0.39 |
| Unsaturated/Saturated (ratio) | 2.31 ± 0.94 | 4.75 ± 0.61 | <0.001 | 1.93 ± 0.84 | 4.34 ± 0.69 | <0.001 | 0.95 |
Figure 2.
Sources of Fatty Acids in the Habitual Omnivorous Diet of European American and African American Women*
*No significant differences observed for baseline sources of fats (all Ps > 0.05).
Energy and Nutrient Intake Changes in Response to Vegetarian Diet
Consuming the vegetarian diet did not significantly change the energy intakes of EA and AA women (Table 2) and no significant changes occurred during the study in daily measured body weights for any women. Daily diet assessments showed vegetarian diet significantly altered the proportion of energy intake from animal-based vs plant-based protein sources, with no difference by race in total protein (grams) consumed or the intakes of individual amino acids (Table 2). The percentage of energy from carbohydrates increased ~10%, with no difference by race (P=0.49). Among the individual carbohydrates, there were significant reductions in lactose and maltose intakes, which also did not differ by race (P=0.81 and P=0.41, respectively). Intakes for total, soluble and insoluble fibers did not change in either group. The intake of pectin increased significantly in AA women (P=0.006), but the change only trended toward a significant difference by race (P=0.07). There was no change in the percentage of energy from total fat intakes and no differences by race in the consumption of the 33 foods that contributed to total fat intake (Table 2, Figure 2). However, SFA intakes decreased ~50% in both groups (Ps<0.001) and the ratio of saturated to unsaturated fat intakes reduced 50% in both groups (Ps<0.001). In addition, there were significant changes in dietary indicators of desaturase activity with decreased ratio of 16:0 SFA to 16:1 MUFA and 18:0 SFA to 18:1 MUFA in both groups (Ps<0.001).
Overall Physiologic/Metabolic Changes in Response to Vegetarian Diet
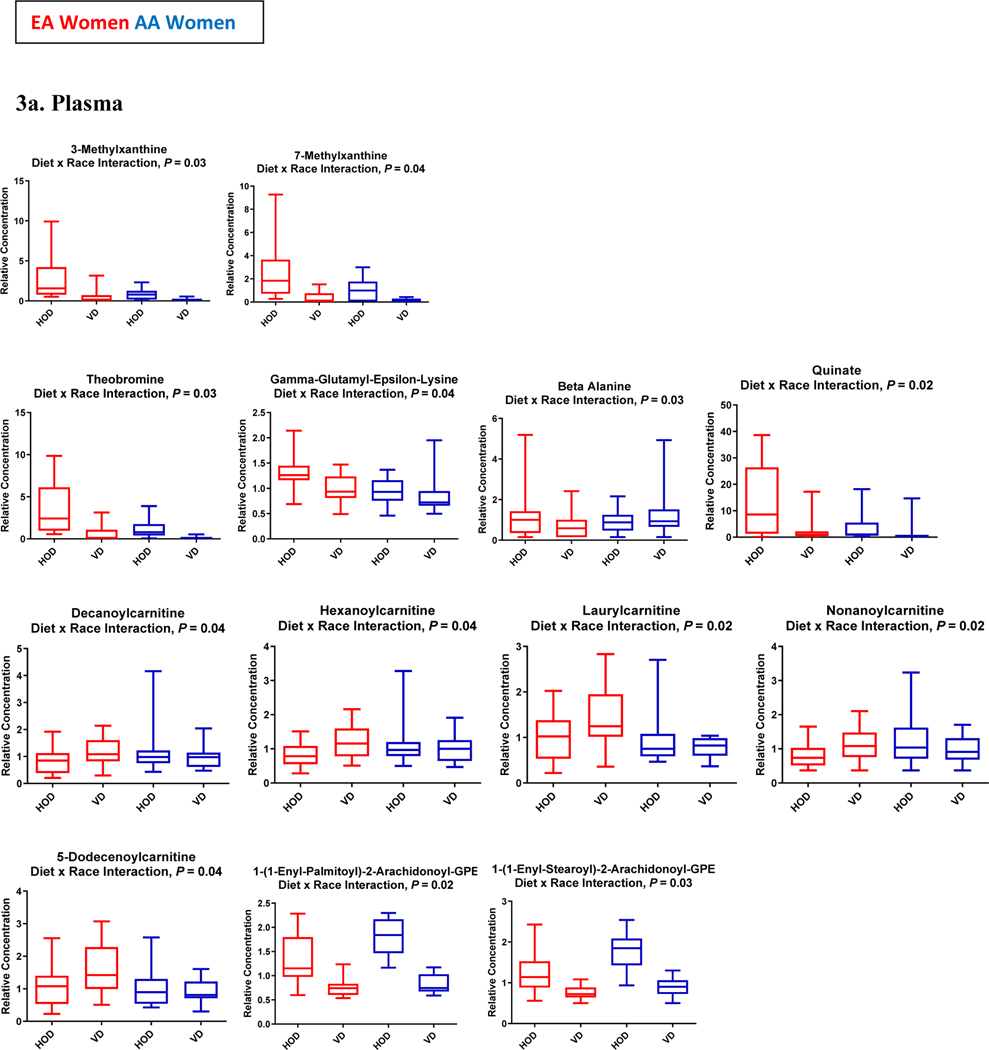
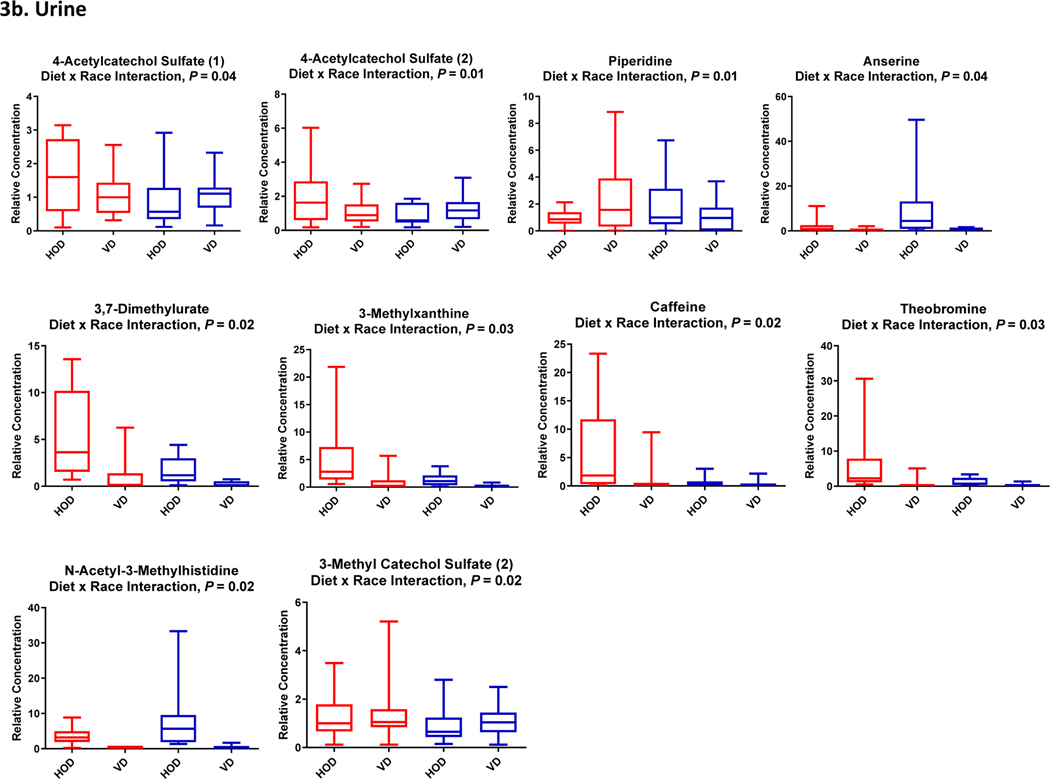
Both EA and AA women reported an increase in the frequency of experiencing “excessive flatulence” (2.0 ± 0.2 vs 5.0 ± 0.3 episodes/day, P = 0.01) and in the number of bowel movements during vegetarian diet study days (1.0 ± 0.4 vs 1.5 ± 0.6 /day, P = 0.02). Upon completion of the vegetarian diet, plasma CRP levels decreased by 21.7% (P=0.03), with no difference in reduced levels by race (P=0.70). Of 736 named plasma metabolites, 101 were significantly up-regulated and 180 were significantly down-regulated (Supplemental Table 2). Of 835 named urine metabolites, 306 were significantly up-regulated and 98 significantly down-regulated. In the adjusted analyses, there were significant diet by race interactions (Ps < 0.05) for response to the vegetarian diet for 23 metabolites (Figure 3) and a trend toward significance (0.5 ≥ P ≤ 0.10) for 25 other metabolites.
Figure 3:
Boxplots of Changes in Plasma (3a) and Urine (3b) Metabolites that Significantly Differed by Race
Metabolic Changes Confirming Adherence to the Vegetarian Diet
Consistent with the reduced intake of caffeine from the diet intervention, there was a significant down-regulation of 1,3,7-trimethylxanthine (caffeine) metabolism (P=3.46E-06, q=2.42E-05) along with significant reductions in the concentrations of all plasma and urine metabolites involved in xanthine metabolism including theobromine (↓87%), theophylline (↓73%) and paraxanthine (↓57%). There were significant diet by race interactions for the reductions in plasma and urine concentrations of several xanthine metabolites, including theobromine, 2-methyl xanthine and 3-methyl xanthine (Figure 3).
The increased consumption of plant-based protein sources from the vegetarian diet was demonstrated in the significant up-regulation of metabolites derived from dietary soybean and other soy-based food items including plasma genistein sulfate which showed a 10-fold increase and plasma equol sulfate which showed a 3-fold increase (Figure 4). The soy-derived metabolites showed similar up-regulation in urine samples. Concurrently, there was significant down-regulation of plasma metabolites associated with animal-based protein intake (meats and milk) including lysine (↓16%), pyralline (↓34%), and alanine (↓12%) along with significant reductions in the concentrations of plasma metabolites of histidine metabolism including a 21% decrease in plasma 1-methyl histidine (P=0.0002, q=0.0006) and a 93% decrease in plasma 3-methyl histidine (P=1.00E-15, q=3.86E-13). A significant diet by race (Ps < 0.05) was observed for the reductions in some histidine metabolites, including plasma pyralline and urinary N-acetyl-3-methylhistidine (Figure 3).
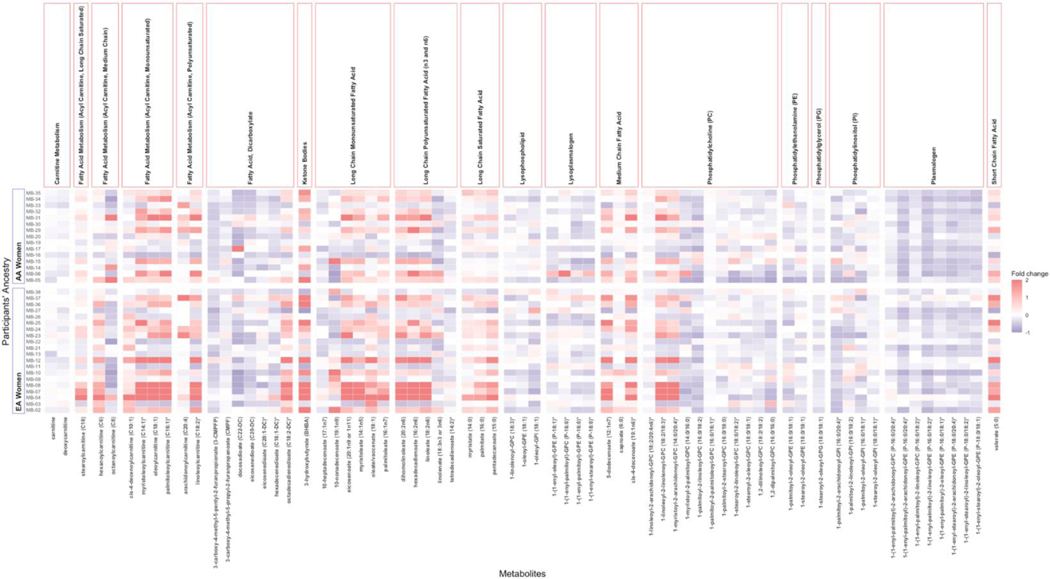
Figure 4:
Heatmap to Compare Changes in Concentrations of Plasma and Urine Fatty Acid Metabolites by Race
Adherence to the increased portions of fruits, vegetables and whole grains on the vegetarian diet menu was also confirmed with significant up-regulation detected in the concentrations of plasma stachydrine (1.3-fold increase), thymol sulfate (1.5-fold increase), alliin (6.5-fold increase), N-acetylalliin (5.3-fold increase), S-allylcysteine (6.6-fold increase), 2-aminophenolsulfate (1.9-fold increase), and betaine (1.8-fold increase). No diet by race interactions were observed for these metabolites.
Metabolic Changes in Response to Vegetarian Diet Associated with Fatty Acid Utilization and Energy Metabolism
Consistent with the increase in food sources of dietary MUFA and PUFA (peanuts, tree nuts, soy-based margarine and oil, safflower oil, olive oil), there was significant upregulation of many fatty acids. This included a 25–30% upregulation of several long chain monounsaturated fatty acids (myristoleate, palmitoleate, 10-heptadecenoate, oleate, eicosanoate) and long chain polyunsaturated fatty acids (hexadecadienoate, linoleate, linolenate, dihomo-linoleate, docosadienoate) simultaneously with a 25–30% change in the plasma concentrations of long chain saturated fatty acids (myristate, pentadecanoate and palmitate) (Figure 4). These changes occurred concurrent with a significant decrease in the ratio of stearic to oleic acid, 18:0/18:1 (↓42%, P=0.002), and the ratio of 16:0/16:1 (↓27%, P=9.026e-05) suggesting reduced stearoyl-CoA desaturase (SCD-1) activity. There were also increases in circulating concentrations of the conjugated fatty acid metabolites of carnitine metabolism including acetylcarnitine and several acylcarnitines. The amount and direction of change in circulating concentrations of acylcarnitines differed by race for five plasma acylcarnitines: nonanoylcarnitine, hexanoylcarnitine, laurylcarnitine, decanoylcarnitine and 5-dodecenoylcarnitine (Figure 3). A trend toward a significant diet by race interaction was also observed for the changes in seven other fatty acid metabolites, including plasma myristoleoylcarnitine and octanoylcarnitine (Figures 3 and 4).
The changes in fatty acid metabolites occurred with an increase in the ketone, 3-hydroxybutyrate (P=0.02, q=0.02) and decrease in long chain dicarboxylate fatty acids. Other changes associated with fatty acid utilization included down-regulation of metabolites involved in branched chain amino acid metabolism (leucine, isoleucine and valine). At the same time, there was significant down-regulation of plasma concentrations of cell membrane lipids, including many phospholipids, lysophospholipids, lysoplasmalogens and plasmalogens. Significant differences by race were observed for two down-regulated plasmalogens: 1-(1-enyl-stearoyl)-2-arachidonoyl-GPE and 1-(1-enyl-palmitoyl)-2-arachidonoyl-GPE (Figure 4).
Other Metabolic Changes in Response to Vegetarian Diet Associated with Macronutrient and Energy Metabolism
In addition to the changes observed in circulating metabolites directly associated with diet-derived animal and soy proteins, there were significant changes in the circulating levels of metabolites that may be derived intrinsically from alterations in the gut microbiome including down-regulation of metabolites of the aromatic amino acids (AAA) phenylalanine, tyrosine and tryptophan along with plasma and urine changes in gut fermentation products involved in AAA metabolism such as indoleacetate (↓24%, P=1.28E-06, q=1.07E-05). Further, plasma concentration of trimethylamine-N-oxide (TMAO), a gut flora dependent metabolite of choline and L-carnitine metabolism was reduced by 33% after vegetarian diet (P=4.96e-05, q=0.0002). Alterations were also detected in metabolites of endocannabinoid metabolism with a 1.2-fold increase in circulating oleoyl ethanolamide (P=0.002, q=0.004) and 1.4-fold increase in circulating N-oleoyltaurine (P=0.0009, q=0.003). Changes in microbiome-modified metabolites were also detected in urine samples, including imidazole lactate, 2-hydroxyphenylacetate, serotonin, and indoleacetate. Moreover, there were significant changes in plasma and urine for metabolites of benzoate metabolism with altered levels of hippurate, 4ethylcatechol sulfate, 4-acetylphenol sulfate, 4-ethylphenyl sulfate, 4-vinylphenol sulfate, 3–3-hydroxyphenyl proprionate sulfate, and a significant diet by race interaction was observed for the change in 3-methyl catechol sulfate.
DISCUSSION
Many factors may explain variance observed in the response to diet interventions aimed at improving health status and preventing (or treating) cardiometabolic disease including sex, genetics, and ancestry. The present study was designed to examine acute alterations in plasma and urine metabolites in response to new exposure to a vegetarian diet. Based on our prior findings showing differences in the impact of diet intervention between European and African American women (19, 25), particularly with respect to altering dietary fat intake, we also assessed differences in the response to new onset vegetarian diet by race.
Principle component analysis, a method of reducing dimensionality but retaining variation in a dataset, showed the plasma and urine samples could be segregated by diet type (habitual omnivorous vs new-onset vegetarian), reflecting metabolic differences among the samples. Computerized nutritional analysis using software based on standardized comprehensive food composition databases showed switching from omnivorous to vegetarian consumption differed in caffeine, the types of high protein foods, total carbohydrate content, and the vegetarian diet reduced the ratio of saturated to unsaturated fats. Adherence to the vegetarian diet menus was further confirmed by the alterations observed in known metabolic products derived from meat, fish, dairy, fruit and vegetable consumption including the plasma and urine concentrations of 1- and 3-methyl histidine, stachydrine, pyralline and betaine. (26)
Confirming the reduction in caffeine consumption, significant alterations occurred in the concentrations of plasma and urine metabolites of xanthine metabolism, in particular paraxanthine, theobromine, theophylline, and quinine. These metabolites were previously shown to be derived from constituents of habitual coffee intake in the European Prospective Investigation on Cancer and Nutrition (EPIC) study (27). Thus, the significant difference in xanthine metabolism observed between EA and AA women indicates differences in baseline consumption. While the cumulative evidence does not support increased risk for type 2 diabetes or cardiovascular disease with moderate intake, when taking into account genetic predisposition, high coffee intake has been associated with high body mass, blood pressure, triglycerides, total cholesterol, and low HDL-cholesterol, and increased risk for coronary heart disease (28, 29).
The replacement of high protein animal-based foods in the diet with soybean and soy-based foods was accompanied by significant changes in known metabolites of soy, which has a distinct isoflavone content, particularly for genistein and daidzein. In the present data, the increased consumption of soy products resulted in greater than a 5-fold change in plasma and urine concentrations of isoflavones. Many studies have investigated links between soy food consumption and human health. While there has been concern raised whether increased consumption is beneficial or harmful with regard to risk for some types of cancer, the evidence has been limited primarily to epidemiological data (30). In contrast, a review of 114 meta-analyses and systematic reviews indicates soy-derived isoflavones are particularly beneficial for reducing cancer and CVD risk in women (31). Further, a meta-analysis of 14 trials revealed that soy isoflavones may significantly reduce blood levels of C-reactive protein, an established risk factor for cardiovascular disease (32). Moreover, data from the Nurses’ Health Study showed that higher red meat intake is associated with higher plasma CRP as well as higher fasting insulin and HbA1c in non-diabetic women (33). The replacement of animal-based foods with soy-based foods in the vegetarian diet may explain the >20% reduction in CRP observed in EA and AA women.
A large impact of the vegetarian diet was the alterations in fatty acid metabolites. Overall, long-chain fatty acids were increased by ~30%. There were significant reductions in the ratio of dietary saturated to unsaturated fats, which may increase the abundance of unsaturated fats in plasma, cell membranes, and adipose tissue. Indeed, the ratios of both 16 and 18 carbon saturated to unsaturated fatty acids was reduced which may be consistent with reduced systemic SCD-1 activity, a desaturase enzyme that converts palmitoyl to palmitoleoyl and stearoyl to oleoyl (34), the most abundant fatty acids in triglycerides, cholesterol esters and phospholipids. Beyond reduced need to convert dietary saturated to unsaturated fats, decreased activity of SCD-1 may indicate increased partitioning of lipids to fatty acid oxidation rather than triglyceride storage.(35) Notably, increased activity of SCD-1 has been associated with increased overall and visceral adiposity, insulin resistance, and the metabolic syndrome.(36)
An intriguing finding was a significant difference by race in the amount and direction of change in concentrations of several acylcarnitines in response to the vegetarian diet, despite no differences in the amount or type of fats consumed during study days. Carnitines are essential for the complete β-oxidation of long-chain fatty acids and higher concentrations of carnitines are typically detected in high protein, high saturated fat animal-based foods such as meats, milk and dairy products. Consistent with the present findings, another study showed that 14-days of a high soy / high cruciferous vegetable and citrus fruit diet (compared to high dairy / low fruit and vegetable diet) resulted in significant changes in the urinary concentrations of acylcarnitines and branched chain amino acids.(37) Of concern is that a few prior studies showed that a metabolic profile high in short-chain acylcarnitines, branched chain amino acids, and some aromatic amino acids is associated with obesity and insulin resistance (38, 39). In a study of 56 AA women, levels of plasma carnitines were significantly higher in those with type 2 diabetes compared to non-diabetics, suggesting that the greater propensity for cardiometabolic disease in AA women may be related to incomplete long-chain fatty acid oxidation (and reduced citric acid cycle activity) yielding plasma accumulation of shorter-chain molecules that would activate pro-inflammatory pathways (40). Indeed, preclinical evidence shows that both diet and genetically driven insulin resistance is associated with both accumulation of fatty acylcarnitine intermediates in skeletal muscle and incomplete fatty acid oxidation.(41) It is plausible that the greater changes observed in several carnitine metabolites in response to the vegetarian diet by EA women indicates a greater propensity to oxidize fatty acids rather than a preference for fat storage in hepatocyte, adipocytes and skeletal muscle depots, which may help elucidate differences observed between EA and AA women with regard to the development of obesity, type 2 diabetes, and CVD (42).
Another mechanism for diet affecting cardiometabolic risk is by providing substrate that alters the composition and diversity of the gut microbiome, which may subsequently modify the metabolome. One example is the changes observed in plasma and urine metabolites of benzoate metabolism, which are derived from polyphenols in the vegetarian diet foods. Dietary choline and L-carnitine can also be converted by gut microbiota, to trimethylamine, which undergoes oxidation in the liver yielding trimethylamine N-oxide (TMAO). We observed a significant reduction in TMAO in response to the vegetarian diet, suggesting therapeutic potential of the diet for reducing systemic inflammation, atherosclerosis and adverse cardiovascular events (43). Also mediated by gut microbiota, the upregulation of endocannibanoid metabolism related compounds observed would be involved in body weight and lipid regulation, thereby influencing cardiometabolic risk.
While the overall impact of the new onset vegetarian diet on the metabolome was substantial, the metabolite differences detected between EA and AA women in response to the vegetarian diet should be interpreted cautiously due to the small sample size and lack of random sampling of the populations represented. There remains need for replication of the study findings in larger groups and for longer intervention periods. Further, we recognize that the exploratory nature of an untargeted approach for metabolic profiling may provide false positives and negatives. To address the potential for such bias, we adjusted for the multiple comparisons performed using the Benjamin-Hochberg test. A strength of the study was the matching of EA and AA women at baseline by age and BMI as well as their similarity with regard to habitual dietary intakes and physical activity levels. While prior evidence has compared metabolic profiles between omnivores and vegetarians, the present study furthers the evidence base by prospectively recruiting subjects whose habitual diet was omnivorous and had never followed a vegetarian diet. Moreover, the choice of a short-term diet intervention enabled not only that the vegetarian diet be rigorously designed as a major shift from habitual diet by careful selection and calculation of plant-based menu items, but also that all menu items were prepared, portioned, and provided directly from the Vanderbilt Metabolic Kitchen to subjects for each study day. Finally, although short in duration, the 4-day intervention period was long enough to assess acute metabolite response upon digestion and absorption of the novel diet.(44, 45)
Investigating relationships between dietary intakes, especially consumption of specific foods or dietary patterns, and metabolic biomarkers aids our understanding of the effects of foods and nutrients at the cellular level and their role in the development of disease. Moreover, it helps elucidate differences in our response to particular foods or diet interventions. The present findings provide insight to potential mechanistic underpinnings of cardiometabolic disease risk and how the promise for diet interventions to be efficacious in reducing risk may differ across population subgroups necessitating more personalized nutrition strategies.
Supplementary Material
*Dark blue and dark purple circles represent samples after 4 days of vegetarian diet (study day 6), light blue and light purple circles represent samples from habitual (omnivorous) diet (study day 1).
FUNDING:
This study was supported by funding from the Vanderbilt University Office of the Provost Trans-Institutional Program to SRB, JFF and HJS, and American Heart Association grant #15SDG24890015 to JFF.
Footnotes
DISCLOSURE STATEMENT:
The authors have no conflicts with the present study to report.
REFERENCES
- 1.Robbins JM, Vaccarino V, Zhang H, Kasl SV. Socioeconomic status and type 2 diabetes in African American and non-Hispanic white women and men: evidence from the Third National Health and Nutrition Examination Survey. American journal of public health. 2001;91(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahleova H, Levin S, Barnard N. Cardio-Metabolic Benefits of Plant-Based Diets. Nutrients. 2017;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Micha R, Wallace S. Effects on Coronary Heart Disease of Increasing Polyunsaturated Fat in Place of Saturated Fat: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLOS Medicine. 2010;7(3):e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwingshackl L, Strasser B, Hoffmann G. Effects of Monounsaturated Fatty Acids on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Annals of Nutrition and Metabolism. 2011;59(2–4):176–86. [DOI] [PubMed] [Google Scholar]
- 5.Kwok CS, Umar S, Myint PK, Mamas MA, Loke YK. Vegetarian diet, Seventh Day Adventists and risk of cardiovascular mortality: A systematic review and meta-analysis. International Journal of Cardiology. 2014;176(3):680–6. [DOI] [PubMed] [Google Scholar]
- 6.Orlich MJ, Singh PN, Sabaté J, Jaceldo-Siegl K, Fan J, Knutsen S, et al. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA internal medicine. 2013;173(13):1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser GE. Vegetarian diets: what do we know of their effects on common chronic diseases? The American journal of clinical nutrition. 2009;89(5):1607S–12S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkow SE, Barnard N. Vegetarian Diets and Weight Status. Nutrition Reviews. 2006;64(4):175–88. [DOI] [PubMed] [Google Scholar]
- 9.Tonstad S, Butler T, Yan R, Fraser GE. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes care. 2009;32(5):791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutrition, Metabolism and Cardiovascular Diseases. 2013;23(4):292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakobsen MU, O’Reilly EJ, Heitmann BL, Pereira MA, Bälter K, Fraser GE, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. The American journal of clinical nutrition. 2009;89(5):1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chadeau-Hyam M, Athersuch TJ, Keun HC, De Iorio M, Ebbels TMD, Jenab M, et al. Meeting-in-the-middle using metabolic profiling – a strategy for the identification of intermediate biomarkers in cohort studies. Biomarkers. 2011;16(1):83–8. [DOI] [PubMed] [Google Scholar]
- 13.Playdon MC, Sampson JN, Cross AJ, Sinha R, Guertin KA, Moy KA, et al. Comparing metabolite profiles of habitual diet in serum and urine. The American Journal of Clinical Nutrition. 2016;104(3):776–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. 2016;65(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindqvist HM, Rådjursöga M, Malmodin D, Winkvist A, Ellegård L. Serum metabolite profiles of habitual diet: evaluation by 1H-nuclear magnetic resonance analysis. The American Journal of Clinical Nutrition. 2019;110(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt JA, Rinaldi S, Ferrari P, Carayol M, Achaintre D, Scalbert A, et al. Metabolic profiles of male meat eaters, fish eaters, vegetarians, and vegans from the EPIC-Oxford cohort. The American journal of clinical nutrition. 2015;102(6):1518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stella C, Beckwith-Hall B, Cloarec O, Holmes E, Lindon JC, Powell J, et al. Susceptibility of Human Metabolic Phenotypes to Dietary Modulation. Journal of Proteome Research. 2006;5(10):2780–8. [DOI] [PubMed] [Google Scholar]
- 18.Niswender KD, Fazio S, Gower BA, Silver HJ. Balanced high fat diet reduces cardiovascular risk in obese women although changes in adipose tissue, lipoproteins, and insulin resistance differ by race. Metabolism - Clinical and Experimental. 2018;82:125–34. [DOI] [PubMed] [Google Scholar]
- 19.Silver HJ, Niswender KD, Keil CD, Jiang L, Feng Q, Chiu S, et al. CNR1 genotype influences HDL-cholesterol response to change in dietary fat intake. PLoS One. 2012;7(5):e36166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallal PC, Victora CG, Wells JCK, Lima RC, Valle NJ. Comparison of Short and Full-Length International Physical Activity Questionnaires. 2004;1(3):227. [Google Scholar]
- 21.National Cancer Institute. Usual Dietary Intakes: NHANES Food Frequency Questionnaire (FFQ) 2020, July 24 [Available from: https://epi.grants.cancer.gov/diet/usualintakes/ffq.html.
- 22.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. The American Journal of Clinical Nutrition. 2003;77(5):1171–8. [DOI] [PubMed] [Google Scholar]
- 23.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88(2):324–32. [DOI] [PubMed] [Google Scholar]
- 24.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(16):9440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niswender KD, Fazio S, Gower BA, Silver HJ. Balanced high fat diet reduces cardiovascular risk in obese women although changes in adipose tissue, lipoproteins, and insulin resistance differ by race. Metabolism. 2018. [DOI] [PubMed] [Google Scholar]
- 26.Kochlik B, Gerbracht C, Grune T, Weber D. The Influence of Dietary Habits and Meat Consumption on Plasma 3-Methylhistidine-A Potential Marker for Muscle Protein Turnover. Molecular nutrition & food research. 2018;62(9):e1701062-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothwell JA, Keski-Rahkonen P, Robinot N, Assi N, Casagrande C, Jenab M, et al. A Metabolomic Study of Biomarkers of Habitual Coffee Intake in Four European Countries. Molecular Nutrition & Food Research. 2019;63(22):1900659. [DOI] [PubMed] [Google Scholar]
- 28.Nordestgaard AT, Thomsen M, Nordestgaard BG. Coffee intake and risk of obesity, metabolic syndrome and type 2 diabetes: a Mendelian randomization study. Int J Epidemiol. 2015;44(2):551–65. [DOI] [PubMed] [Google Scholar]
- 29.Cornelis MC, El-Sohemy A. Coffee, caffeine, and coronary heart disease. Curr Opin Lipidol. 2007;18(1):13–9. [DOI] [PubMed] [Google Scholar]
- 30.Messina MJ. Legumes and soybeans: overview of their nutritional profiles and health effects. Am J Clin Nutr. 1999;70(3 Suppl):439s–50s. [DOI] [PubMed] [Google Scholar]
- 31.Li N, Wu X, Zhuang W, Xia L, Chen Y, Zhao R, et al. Soy and Isoflavone Consumption and Multiple Health Outcomes: Umbrella Review of Systematic Reviews and Meta-Analyses of Observational Studies and Randomized Trials in Humans. Mol Nutr Food Res. 2020;64(4):e1900751. [DOI] [PubMed] [Google Scholar]
- 32.Dong J-Y, Wang P, He K, Qin L-Q. Effect of soy isoflavones on circulating C-reactive protein in postmenopausal women: meta-analysis of randomized controlled trials. Menopause. 2011;18(11). [DOI] [PubMed] [Google Scholar]
- 33.Ley SH, Sun Q, Willett WC, Eliassen AH, Wu K, Pan A, et al. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. The American Journal of Clinical Nutrition. 2013;99(2):352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodson L, Fielding BA. Stearoyl-CoA desaturase: rogue or innocent bystander? Prog Lipid Res. 2013;52(1):15–42. [DOI] [PubMed] [Google Scholar]
- 35.Sampath H, Miyazaki M, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J Biol Chem. 2007;282(4):2483–93. [DOI] [PubMed] [Google Scholar]
- 36.Sampath H, Ntambi JM. The role of stearoyl-CoA desaturase in obesity, insulin resistance, and inflammation. Ann N Y Acad Sci. 2011;1243:47–53. [DOI] [PubMed] [Google Scholar]
- 37.May DH, Navarro SL, Ruczinski I, Hogan J, Ogata Y, Schwarz Y, et al. Metabolomic profiling of urine: response to a randomised, controlled feeding study of select fruits and vegetables, and application to an observational study. British Journal of Nutrition. 2013;110(10):1760–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laferrère B, Reilly D, Arias S, Swerdlow N, Gorroochurn P, Bawa B, et al. Differential Metabolic Impact of Gastric Bypass Surgery Versus Dietary Intervention in Obese Diabetic Subjects Despite Identical Weight Loss. Science Translational Medicine. 2011;3(80):80re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metabolism. 2009;9(4):311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139(6):1073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7(1):45–56. [DOI] [PubMed] [Google Scholar]
- 42.Guasch-Ferré M, Ruiz-Canela M, Li J, Zheng Y, Bulló M, Wang DD, et al. Plasma Acylcarnitines and Risk of Type 2 Diabetes in a Mediterranean Population at High Cardiovascular Risk. J Clin Endocrinol Metab. 2019;104(5):1508–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA, Solas M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients. 2018;10(10):1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen MB, Kristensen M, Manach C, Pujos-Guillot E, Poulsen SK, Larsen TM, et al. Discovery and validation of urinary exposure markers for different plant foods by untargeted metabolomics. Anal Bioanal Chem. 2014;406(7):1829–44. [DOI] [PubMed] [Google Scholar]
- 45.Draper CF, Vassallo I, Di Cara A, Milone C, Comminetti O, Monnard I, et al. A 48-Hour Vegan Diet Challenge in Healthy Women and Men Induces a BRANCH-Chain Amino Acid Related, Health Associated, Metabolic Signature. Mol Nutr Food Res. 2018;62(3). [DOI] [PubMed] [Google Scholar]
- 46.Harris JA, Benedict FG. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci U S A. 1918;4(12):370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
*Dark blue and dark purple circles represent samples after 4 days of vegetarian diet (study day 6), light blue and light purple circles represent samples from habitual (omnivorous) diet (study day 1).